- 1School of Public Health, Fudan University, Shanghai, China
- 2School of Public Health, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Department of Psychiatry, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 4Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5JC School of Public Health, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
Background: China’s National Essential Medicines Policy (NEMP) has been implemented for over 15 years; yet empirical evidence on its long-term impacts is lacking, particularly in remote and rural regions. This study aims to assess the short-and long-term effects of NEMP on the drug availability, price, and usage in a deprived rural county in southwestern China.
Methods: A quasi-experimental design was employed, featuring a single-group pre-and-post comparison. We gathered 74,436 procurement records spanning from 2009 to 2016 from the drug warehouses of local medical institutions. Pharmaceutical data were analyzed quarterly, considering various policy and therapeutic attributes. Fisher’s Drug Price Index (DPI-F) was calibrated for the retail and wholesale prices of a consistent collection of 405 medications. We conducted interrupted time-series analysis to examine the immediate and enduring impacts of NEMP’s initial (commencing in January 2011) and second (starting from December 2015) stages.
Results: After initiation of NEMP, the number of available essential medicines surged by 115 but subsequently faced a steady quarterly decline (−9.1) in township healthcare centers (THCs, primary care). Conversely, county hospitals (secondary care) initially saw a reduction of 40 in drug availability but later exhibited a steady increase (+4.2 per quarter) up to the second-stage NEMP. Regarding price, THCs encountered abrupt (−26.1%/−15.9% in retail/wholesale price) and sustained (−0.2%/−0.3% per quarter) price drops after NEMP. The immediate price change after NEMP in county hospitals were milder but significant in non-essential medicines, and long-term declines were also observed in all drugs. As for total sales, a significant long-term disparity emerged between THCs (+0.9% per quarter) and county hospitals (+3.3% per quarter). Following the second-stage NEMP, retail prices in county hospitals further decreased, although wholesale prices did not; however, following price upward trends were observed in both THCs and county hospitals. Lastly, the influences of NEMP varied across different therapeutical categories of medicines.
Conclusion: NEMP has successfully regulated drug prices in primary and secondary healthcare facilities in remote and rural areas, both short-term and long-term. However, a remarkable disparity in medicine availability and utilization was observed between different levels of facilities over time. Continuous monitoring is essential, with increased attention needed on the uneven impacts of the policy on diverse drugs, facilities, regions, and demographics.
Background
Access to safe, effective and affordable medicines is pivotal in improving health outcomes and achieving universal health coverage (1). In developing and resource-limited economies, low accessibility, availability, and utilization of medicines can be consistent and stemming from inadequate drug development, production and distribution, elevated prices and costs, along with poor information, prescription, and adherence practice (2, 3). Globally, the demands for accessible, available, and affordable medicines are also intensifying, due to the inevitably aging population, prevailing non-communicable diseases, and rapidly growing health needs and drug innovations (4–6). Since the introduction of essential medicines by the World Health Organization (WHO) in 1975, Essential Medicines Lists (EML) and related policies have been widely adopted as a powerful and cost-effective tool to promote population health and health equity in different countries (7, 8).
Over the past 15 years (2008–2022), China has witnessed a sixfold increase in per capital health spending, from US$117 (1 USD = 7.31 CNY) to US$843, with an annual growth rate of 16.4% (9). Despite a pullback after health system reforms, pharmaceutical expenses still count for a substantial 33% of China’s soaring health expenditure in China in 2018, significantly exceeding the OECD average of 17% (10, 11). Factors contributing to China’s high drug expenditures include a fragmented distribution system, lax regulation on advertising and promotions, distorted compensation mechanisms, and irrational medicine use (12, 13). The increases in both medicine and health spending also raise concerns about the accessibility and financial sustainability of health system.
In 2009, China launched the National Essential Medicines Policy (NEMP) as a cornerstone of healthcare system reform, aiming to ensure universal health coverage for all citizens (14). NEMP’s objectives are to enhance the availability, affordability, quality and rational use of medicines through the establishment of National Essential Medicines Lists (NEML), centralized tendering, procurement, distribution, and enforcement of essential medicine prescriptions and usage under zero-markup regulations (14, 15). The initial three-year implementation of NEMP in primary healthcare facilities (PHFs) has yielded positive outcomes, including declined drug prices and medical expenditure (16), increased access to medicines and basic health services (17), diminished financial incentives from drug sales for healthcare providers (14), and more rational medicine usage (18, 19).
While the NEMP in China has made strides, it faces significant hurdles. These include a scarcity of low-price and pediatric medicines (20–22), polypharmacy and the over-prescription of antibiotics and injections (18, 23). Primary care services under NEMP are also grappling with discontinuation, operational challenges, and financial constraints (24, 25). Particularly in the western, rural, and impoverished areas of China, these issues are more acute, due to the lower affordability and resources available to local residents and healthcare systems (25–27). Such policy shortcomings can be interpreted by the dynamic responses of stakeholders to the top-down implementation of NEMP within a complex adaptive system (28). For instant, suppliers might suspend the supply of medicines to rural and fragmented markets under low profits or even a financial loss; clinical practitioners might resort to inappropriate prescribing practice, due to insufficient information, fewer choices and altered financial incentives; and patients might changing their healthcare-seeking behavior, opting for higher-level facilities instead (25, 29, 30).
Besides the above challenges, NEMP in China requires further investigation as follows. First, the long-term impacts of NEMP vary from the short-term outcomes and exhibit considerable regional variations. Recent reviews have shown that despite a long-term decrease in median price ratios (31), the availability of essential medicines has not improve and remains low after a decade of NEMP implementation (32). This issue is even more pronounced in some western and rural areas, where the accessibility and affordability challenges have persisted or worsened (31–34). Given that, there is a necessity for more comprehensive and consistent long-term surveillance data from these areas (32).
Second, while NEMP has primarily focused on the primary care system, its spill-over effects on higher levels of facilities cannot be overlooked. Especially, several regulations have been expanded to encompass a broader scope over time. Studies from eastern and economically developed provinces reveal that the availability of essential medicines at secondary and tertiary care levels is significantly lower than at primary care facilities, suggesting a policy gap at these levels (35, 36). However, evidence from western and underdeveloped regions remain sparse.
Third, the implementation of NEMP may exert disproportionate impacts across various medicine categories. Notably, the availability of systematic hormonal preparations and nervous system medications was lower than other types, while the affordability of antineoplastic and immunomodulating agents remained less than optimal (31, 32). Prior research has often been limited to a narrow selection of medicines, leaving a gap in the understanding of effects on none-NEML pharmaceuticals and traditional Chinese medicines (TCMs).
Finally, while median price ratio—a comparison of local prices to median international reference prices—is a widely accepted method developed by WHO/HAI for evaluating drug prices (37), it relies heavily on the availability of international data and may not accurately represent a broad spectrum of medicines. It also fails to capture continuous fluctuations in drug prices and usage. Drug price index (DPI), which measures the ratio of prices of a fixed basket of goods between different periods, offers a viable alternative to address these shortcomings, and is increasingly used in drug price studies (16, 38, 39).
This study aims to evaluate both the short-and long-term impacts of NEMP on the drug availability, price and usage within a deprived rural county in southwestern China, covering the years 2009 to 2016. We further explore the varying impacts of NEMP across different healthcare facility levels, price types, and medicine classifications.
Methods
Research questions and hypotheses
Question 1: What are the short-and long-term impacts of NEMP on drug availability, price, and usage in a deprived rural county in southwestern China?
Hypothesis 1: NEMP has enhanced the availability, lowered prices and increased usage of essential medicines shortly after implementation.
Hypothesis 2: The initial benefits of NEMP has maintained over the long term.
Question 2: Do the impacts of NEMP remain uniform across PHFs and secondary care hospitals?
Hypothesis 3: NEMP has spillover impacts on the availability, prices and usage of medicines in higher-tier healthcare facilities.
Question 3: Are the impacts of NEMP consistent across different categories of medicines?
Hypothesis 4: Impacts of NEMP vary among different therapeutic classes of medicine.
Hypothesis 5: Impacts of NEMP differ between western medicines and TCMs.
Hypothesis 6: NEMP has spillover effects on the availability, prices and usage of non-essential medicines.
Question 4: Does selection of different price indicators affect the perceived impacts of NEMP on drug prices?
Hypothesis 7: Impacts of NEMP on retail prices are more pronounced than on wholesale prices.
Hypothesis 8: The Fisher Price Index reveals a greater price reduction of NEMP compared to the Laspeyres Price Index.
Study design, setting and policy
This study employed a quasi-experimental design to compare conditions before and after the implementation of NEMP in a rural, remote and poverty-stricken county in Yunnan Province, southwestern China. Yunnan province is characterized by its mountainous and plateau geography, and its residents are facing limited access to healthcare services. The county in selection is predominantly mountainous (94%); three-quarters of its population living in rural areas; its per capital gross domestic product per capita was only US$4,788 in 2020, 70% of the provincial average and 53% of the national average (40). In this economically and geographically isolated county, the three-tiered public healthcare system (73 village clinics – 7 township healthcare centers [THCs]—three county hospitals) severs as a primary healthcare provider for its residents. The county’s geographic and functional isolation makes it an exemplary site to study the impacts of NEMP as a complex social intervention at a micro-level.
NEMP’s rollout in the selected county occurred in two stages (Appendix 1). The initial stage policies (2010.9–2015.10) targeted all government-owned PHFs, including THCs and village clinics. This phase introduced centralized procurement, mandatory prescription of essential medicines, zero-markup retail pricing policy, and conditional financial support. The second stage started from November 2015, expanding to include secondary care facilities (county hospitals) with similar measures: centralized procurement, obligatory use and zero-markup price in essential drugs.
Data collection
Data were collected from two THCs and two government-operated county hospitals. The THCs, situated at the heart of two distinct townships, deliver primary care for 39.1% of the county’s population across 29.7% of its land. The county hospitals, as the highest-tier local medical institutions, provide secondary care for the entire population and guidance to all PHFs. Drug procurement records between January 2009 and December 2016 were compiled from both electronic systems or paper records in the pharmaceutical warehouses of these facilities.
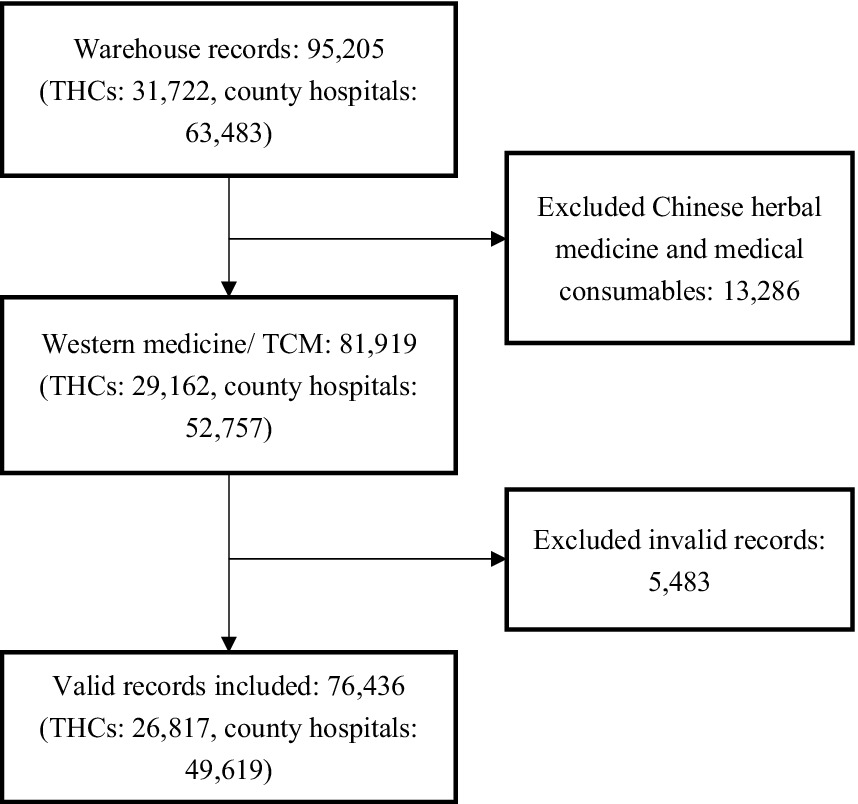
We presumed that all stocked medicines were ultimately dispensed to patients, equating drug purchases with drug utilization. Each procurement record contained details such as the purchase date, generic name, dosage form, specification, quantity, and both wholesale/manufacturer (i.e., medicines sold from manufacturers and distributors to hospitals) and retail/consumer prices (i.e., medicines prescribed from hospitals and doctors to patients). Hard-copy records were transcribed by two bachelor medical students and cross-validated by two research members independently (Z.H. and Z.S). Out of 95,205 purchase records, 76,436 were deemed suitable for analysis after discarding those of herbal medicine, medical consumables and invalid information (unmatched drug, extreme value, withdrawal orders; see Figure 1). The total sales from the analyzed records amounted to US$23.6 million, with 93.6% originated from county hospitals and 6.4% from THCs.
Drug categorization
Medicines were categorized by their policy properties (essential or non-essential drug; western or TCMs) and therapeutical attributes (Anatomical Therapeutic Chemical, ATC system) (41). The dataset encompassed 644 chemical and biological substances and 508 TCMs (see Appendices 2, 3). For standardized analysis of quantity and price, a “unique” medicine was defined by its generic name, dosage form, and specification (e.g., Abacavir-Oral-0.3 g). Different specifications of the same generic name and dosage would be converted to the minimum unit (e.g., Abacvir-Oral-0.6 g - > Abacvir-Oral-0.3 g*2). In total, 797 unique western medicines (520 essential, 422 non-essential) and 599 unique TCMs (272 essential, 327 non-essential) were identified.
Outcome measures
The availability of medicines was quantified by their presence in healthcare facilities. Drug usage was assessed by sales value. Defined daily doses (DDDs) were not used because over a half (52%) of western medicines and all TCMs did not correspond to a valid DDD value in the ATC system (41).
Regarding drug prices, both wholesale/manufacturer (medicines sold to hospitals) and retail/consumer prices (medicines prescribed to patients) were analyzed, for NEMP aimed to cut costs at these supply chain stages. Prices were tracked by the drug price index (DPI), an indicator of price ratios of a fixed basket of goods between the reporting period and the baseline period (38). Three commonly used DPIs were calibrated: Laspeyres (DPI-L), Paasche (DPI-P), and Fisher (DPI-F) (16, 42). In our analysis, DPIs were calculated for a fixed basket of 405 (29.0% among all 1,396) medicines with complete records from 2009 to 2016.
DPI-L measures price ratios over different periods, weighted by baseline consumption quantity ( ; where P0 and P1 denote prices of a good in baseline and reporting periods, and Q0 and Q1 denote the consumption quantities). It assumes constant consumption over time and reflects the complete price changes. Conversely, DPI-P is weighted by the consumption quantity in reporting periods ( ), assuming quantity changes follow after price changes. A DPI > 1 indicates increase costs to acquire the same basket of goods, < 1 indicates a decreased costs, and = 1 indicates stable costs.
Both DPI-L and DPI-P are biased due to their assumption of unchanged consumption. DPI-F addresses this by averaging changes in baseline and reporting periods using the geometric mean of DPI-L and DPI-P ( ) (43). Comparing DPI-L, DPI-P, and DPI-F further reflects relative consumption changes in different drugs, where DPI-F < DPI-L (or DPI-F > DPI-P) suggests increased use of low-price drugs, and vice versa.
Statistical analysis
Considering a policy lag-effect of 3 months, as determined by expert consultation and data observation, we established two intervention points (January 2011 and January 2016), and three policy periods (pre-implementation: January 2009–December 2010; first-stage implementation: January 2011—December 2015; and second-stage implementation: January 2016—December 2016). The analytical interval was set at 3 months, aligning with the purchase cycle of PHCs. The quarterly observation interval is also appropriate and stable to capture secular changes in drug price and usage at the county level. In total, 32 observation points were identified over the eight-year period.
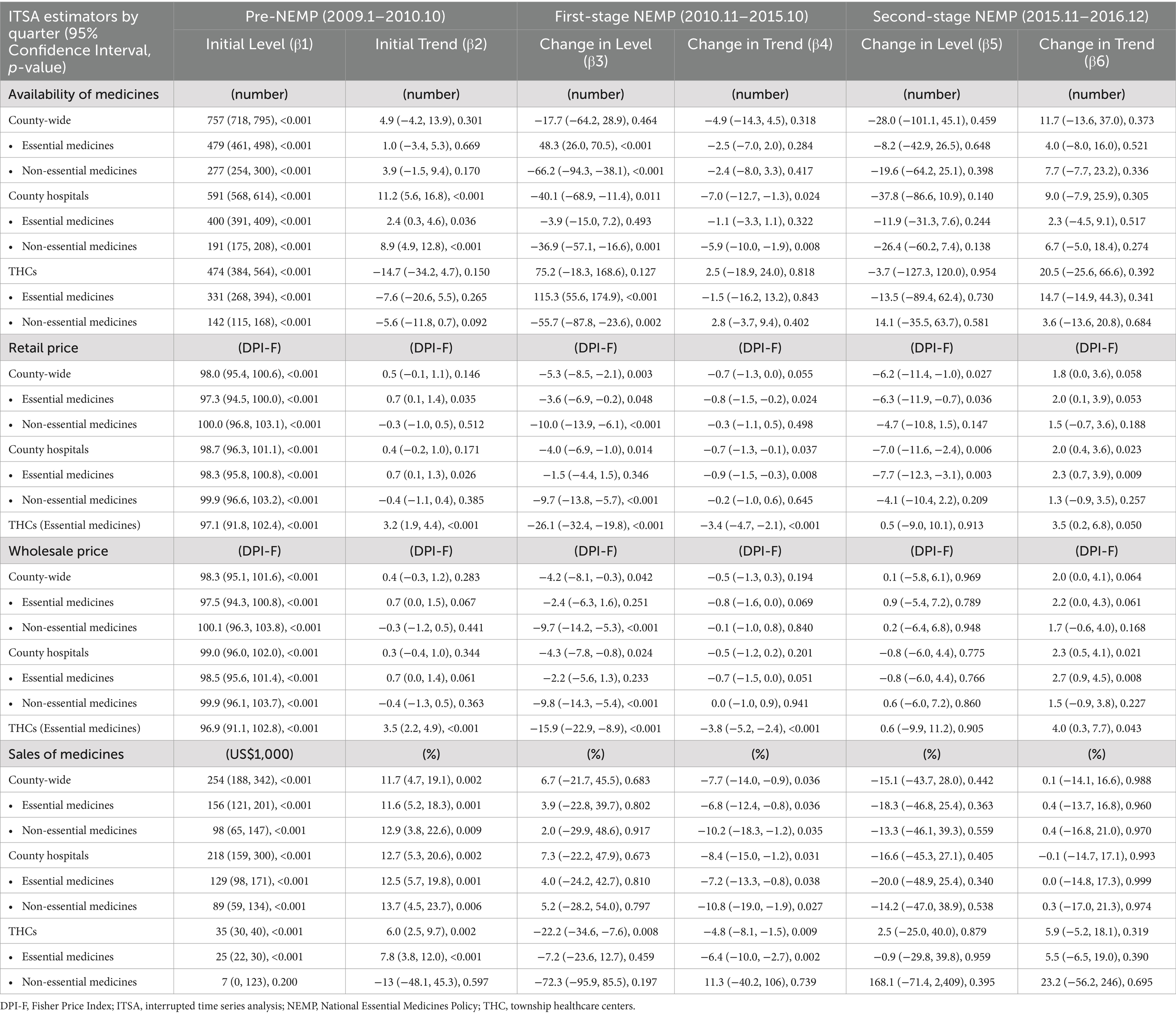
Descriptive analysis was performed on the number, sales, retail price and wholesale price of medicines, categorized by medicine types and facility levels of facilities (i.e., THCs, county hospitals, and overall facilities). Single-group interrupted time-series analysis (ITSA) was utilized to examine the immediate and sustained impacts of NEMP on these metrics (44–46). In ITSA, drug sales were log-transformed, and time interval was set quarterly, totaling 32 time points from 2009–2016: 8 for pre-NEMP, 20 for first stage NEMP, and 4 for second stage NEMP. Segmented linear regression models were constructed with two interruption points: , where Yt denotes the outcome in quarter t, T denotes the time point since observation, and X1_t and X2_t denote the implementation of first-stage and second-stage policies at T (coded 0 or 1). Coefficient β0 and β1 separately estimate the baseline level and trend of the outcome in pre-policy periods; β2 and β3 separately estimate changes in immediate level and long-term trend after the first-stage policy; and β4 and β5 separately estimates changes in level and trend after the second-stage policy. Εt measures the random error at time T. Autocorrelation in time-serial data was tested by Durbin–Watson statistics and adjusted with Prais-Winsten method (47).
In sensitivity analysis, inflation was accounted for in both retail (using consumer price index, CPI) and wholesale prices (using producer price index, PPI). All analyses were performed in R version 4.1, and a p-value <0.05 was considered statistically significant.
Results
Descriptive analysis
Over 8 years, the number of available medicines in the selected county increased modestly from 719 to 782 (+8.8%), while the DPI-F declined by 8.9% and the total medicine spending surged fivefold, from US$0.2 million to US$1.0 million (Appendix 4). By policy attributes, sales of essential medicines grew significantly more than non-essential drugs (5.4-fold vs. 3.0-fold); however, the price cut on essential medicines was less pronounced than on non-essential drugs, with DPI-F decreases of 1.9% versus 24.0%, respectively. By facility levels, county hospitals experienced a 26.4% increase in medicine numbers, a 383.5% surge in sales, and an 8.2% reduction in prices over the same period. Conversely, THCs saw a 46.2% decrease in medicine numbers, a 93.9% increase in sales, and a slight 2.1% price increase (Appendix 4).
Availability of medicines
Across the county, the number of available medicines remained steady at around 800 across 8 years, yet displaying a disparity between essential and non-essential medicines (Figure 2A). The number of essential medicines increased immediately after first-stage NEMP (ITSA estimate: +48 or 10.4%, p < 0.001), while the number of non-essential medicines decreased dramatically (−66 or − 23.6%, p < 0.001). No significant secular trend changes were detected in the number of either medicine types; however, the number of non-essential drugs underwent a brief dip after the second stage of NEMP (−20 or − 7.3%, p > 0.05).
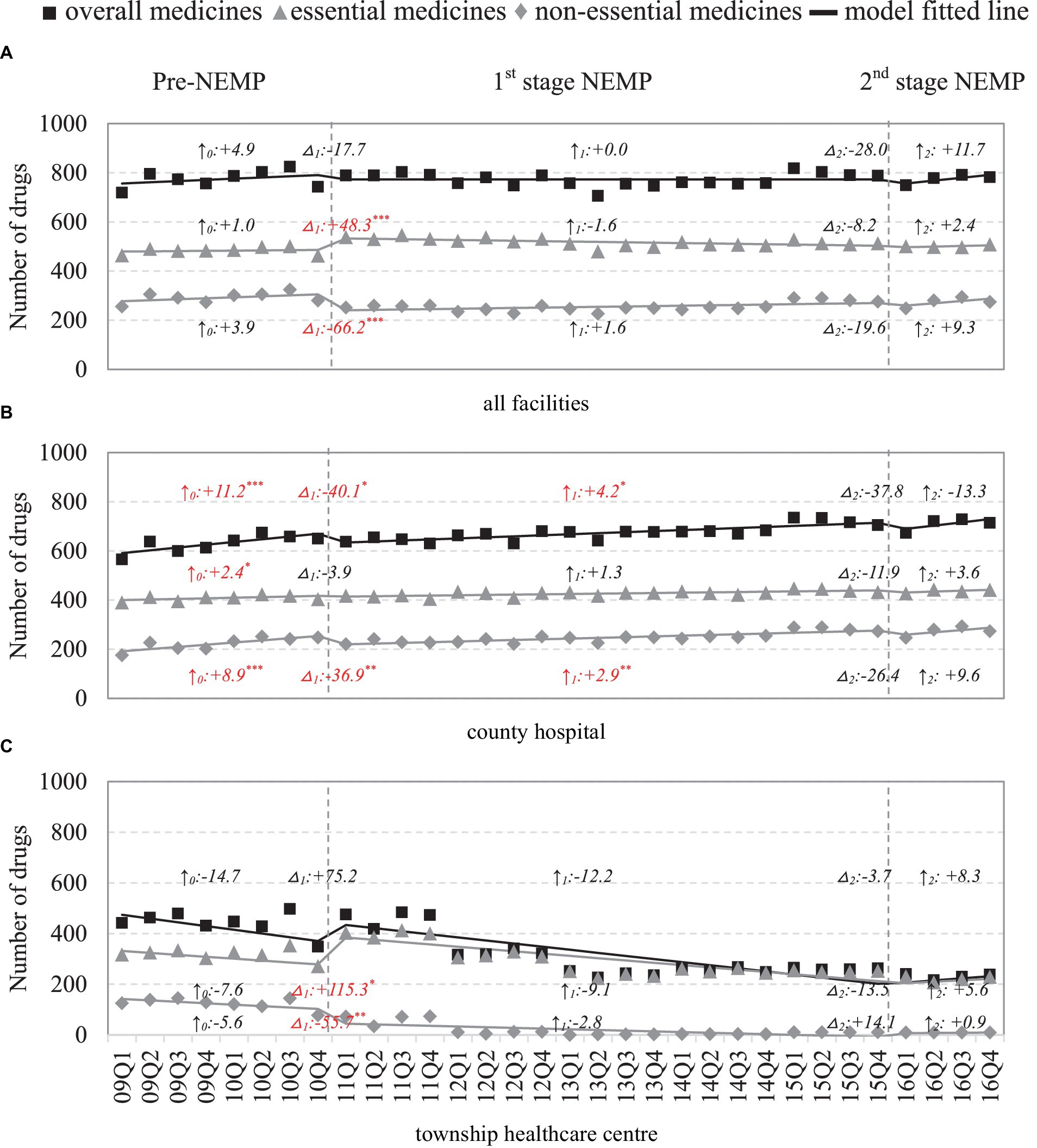
Figure 2. Number of medicines by facility level and essential medicine list. △1: immediate change after first-phase NEMP (estimator β3 in ITSA model); ↑0: sustained change per observation period before first-phase NEMP (β1); ↑1: sustained change per observation period after first-phase NEMP (β1 + β3); △2: immediate change after second-phase NEMP (β4); ↑2: sustained change per observation period after second-phase NEMP (β1 + β3 + β5); ITSA, interrupted time-series analysis; NEMP, National Essential Medicines Policy; (A) all facilities, (B) county hospital, (C) township healthcare center. *p < 0.05; **p < 0.01; ***p < 0.001.
Facility-wide, county hospitals noted a sharp drop in non-essential drugs (−37 or − 15.0%, p < 0.01) after first stage NEMP, followed by a deceleration in growth rate (+2.9 per period, p < 0.01; Figure 2B). In THCs, there was a marked increase in essential drugs (115 or 42.4%, p < 0.05) and an exit of non-essential drugs (−56 or 72.7%, p < 0.01) post the first stage NEMP (Figure 2C). Despite it, essential drugs in THCs continued to decline longitudinally, failing to recover even after the second-stage NEMP.
By medicine types, NEMP consistently influenced the availability of both western medicines and TCMs (Appendix 5). By ATC-classifications, most medicine categories in county hospitals initially dropped but then increased following the first-stage NEMP, except for genitourinary system and sex hormones drugs, which showed a sustained long-term decrease (Appendices 6, 7). In THCs, despite initial increases in most medicine categories after the first-stage NEMP, a long-term downward trend was common and persisted beyond the second-stage NEMP (Appendices 6, 7).
Sales of medicines
County-wide drug expenses were presented (Figure 3A). Drug expenses of county hospitals were a major driver in the pharmaceutical market, with increasing trends for both essential (ITSA estimate: +4.4% per quarter) and non-essential drugs (+1.4% per quarter) after first-stage NEMP (Figure 3B). However, following the second-stage NEMP, county hospitals encountered a 14.2–20.0% drop in drug expenditure of essential and non-essential drugs. In THCs, drug expenditure dropped significantly by 22.2% (essential: −7.2%; non-essential: −72.3%) yet kept increasing at a modest rate (+0.9% per quarter) after policy implementation (Figure 3C).
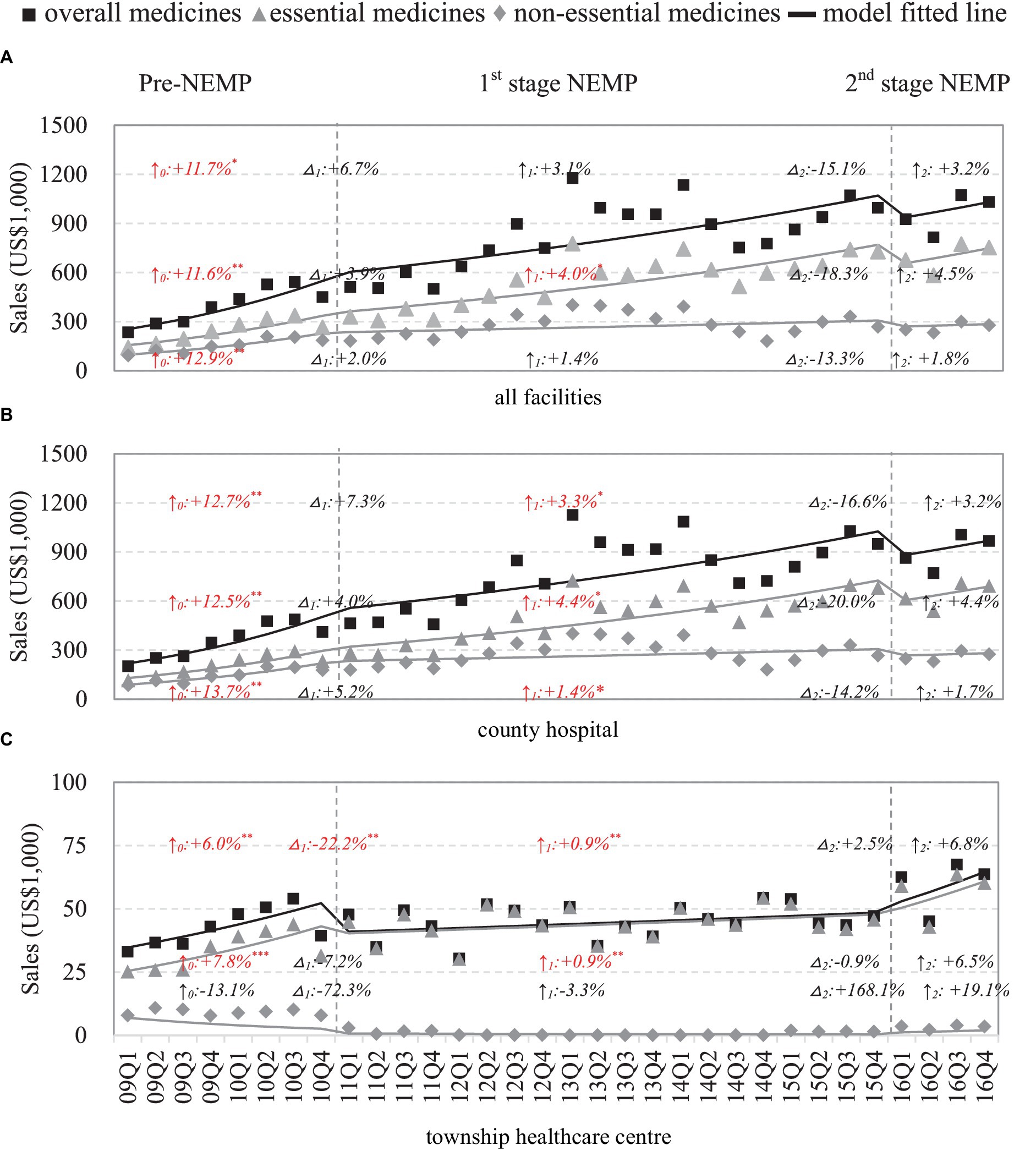
Figure 3. Sales of medicines by facility level and essential medicine list. △1: immediate change% after first-phase NEMP [exp(β3) in ITSA model]; ↑0: sustained change per observation period before first-phase NEMP (β1); ↑1: sustained change% per observation period after first-phase NEMP [exp(β1 + β3)]; △2: immediate change% after second-phase NEMP [exp(β4)]; ↑2: sustained change% per observation period after second-phase NEMP [exp(β1 + β3 + β5)]; ITSA, interrupted time-series analysis; NEMP, National Essential Medicines Policy; (A) all facilities, (B) county hospital, (C) township healthcare center. *p < 0.05; **p < 0.01; ***p < 0.001.
By medicine types, sales dynamics differed between western medicines (rebound after an initial drop) and TCMs (stable after a sharp rise) in THCs following NEMP (Appendix 8). Further by ATC-classifications, most medicine categories in county hospitals exhibited rising sales trends, except for (i) Genitourinary system and sex hormones, (ii) anti-infectives, (iii) antiparasitic, and (iv) TCMs of surgery, gynecology and orthopedics (Appendices 9, 10). In THCs, after the first-stage NEMP, rises in levels or secular trends were observed mainly in (i) systemic hormonal preparations, (ii) respiratory system and (iii) TCM for internal medicine (Appendices 9, 10).
Retail price of medicines
County-wide, immediate drops in DPIs were observed in both essential (ITSA estimates: first-stage −3.6%, p < 0.05; second-stage −6.3%, p < 0.05) and non-essential medicines (first-stage: -10.0% p < 0.001, second-stage: −4.7% p > 0.05) following the two stages of NEMP (Figure 4A). However, upward price trends were observed subsequently after the second-stage NEMP. By facility levels, in county hospitals, impacts of NEMP on prices mirrored the county-wide effects for both essential and non-essential medicines (Figure 4B). In THCs, a sharp decrease (−26.1%, p < 0.001) and a downward trend (−0.2% per quarter) were noted after the first-stage NEMP; however, prices began to climb after second stage NEMP (+3.3% per quarter, p < 0.05; Figure 4C).
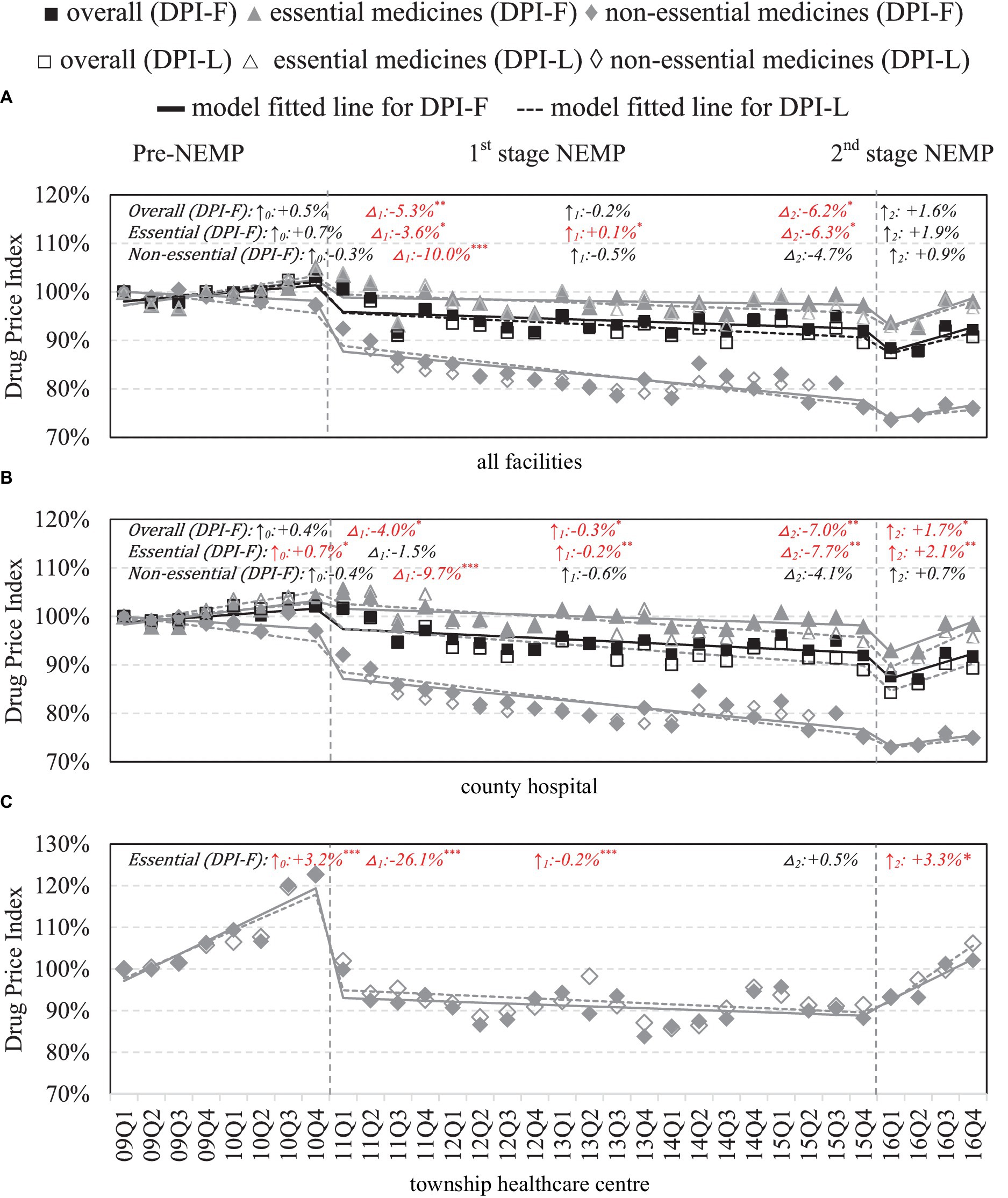
Figure 4. Retail price of medicines by facility level and essential medicine list. △1: immediate price change% after first-phase NEMP [exp(β3) in ITSA model]; ↑0: sustained change per observation period before first-phase NEMP (β1); ↑1: sustained price change% per observation period after first-phase NEMP [exp(β1 + β3)]; △2: immediate price change% after second-phase NEMP [exp(β4)]; ↑2: sustained price change% per observation period after second-phase NEMP [exp(β1 + β3 + β5)]; ITSA, interrupted time-series analysis; NEMP, National Essential Medicines Policy; (A) all facilities, (B) county hospital, (C) township healthcare center. *p < 0.05; **p < 0.01; ***p < 0.001.
By medicine types, in contrast to western medicines, retail prices of TCMs in county hospitals surged immediately after the first-stage NEMP (+4.4%, p < 0.05). Price cut in TCMs were also not as steep as western medicines in THCs (−15.3%, p < 0.01) following first-stage NEMP (Appendix 11). Further by ATC classifications, reverse price increases after NEMP were observed in county hospitals in (i) dermatological, (ii) genitourinary system and sex hormones, (iii) systemic hormonal preparations, (iv) Musculo-skeletal system, (v) antiparasitic, (vi) sensory organs and (vii) TCMs of surgical and orthopedic (Appendices 12, 13). In THCs, all medicines exhibited price declines post NEMP, except for (i) Musculo-skeletal and (ii) sensory organ drugs. Upward price trends were observed in various medicines after the second-stage NEMP, despite non-significant levels.
Comparative analyses using Fisher and Laspeyres indices showed similar results (Figure 4A–C), except for higher DPI-F in TCMs within county hospitals, suggesting a higher prescription rate of high-price TCMs (Appendix 11).
Wholesale price of medicines
County-wide, significant drops in wholesale prices were mainly observed in non-essential medicines (−9.7%, p < 0.001) after first-stage NEMP (Figure 5A). By facilities, in county hospitals, the first-stage NEMP brought a significant decrease in non-essential drug price (−9.8%, p < 0.001) (Figure 5B). In contrast, after the second-stage NEMP, essential drugs exhibited a significant upward trend (+2.6% per quarter, p < 0.01). In THCs, a sharp decline (−15.9%, p < 0.001) and a continued downward trend (−0.3% per quarter, p < 0.001) were observed in essential drug price after the first-stage NEMP. However, the trend reversed with an increase in prices after the second stage (+3.7%, p < 0.05; Figure 5C).
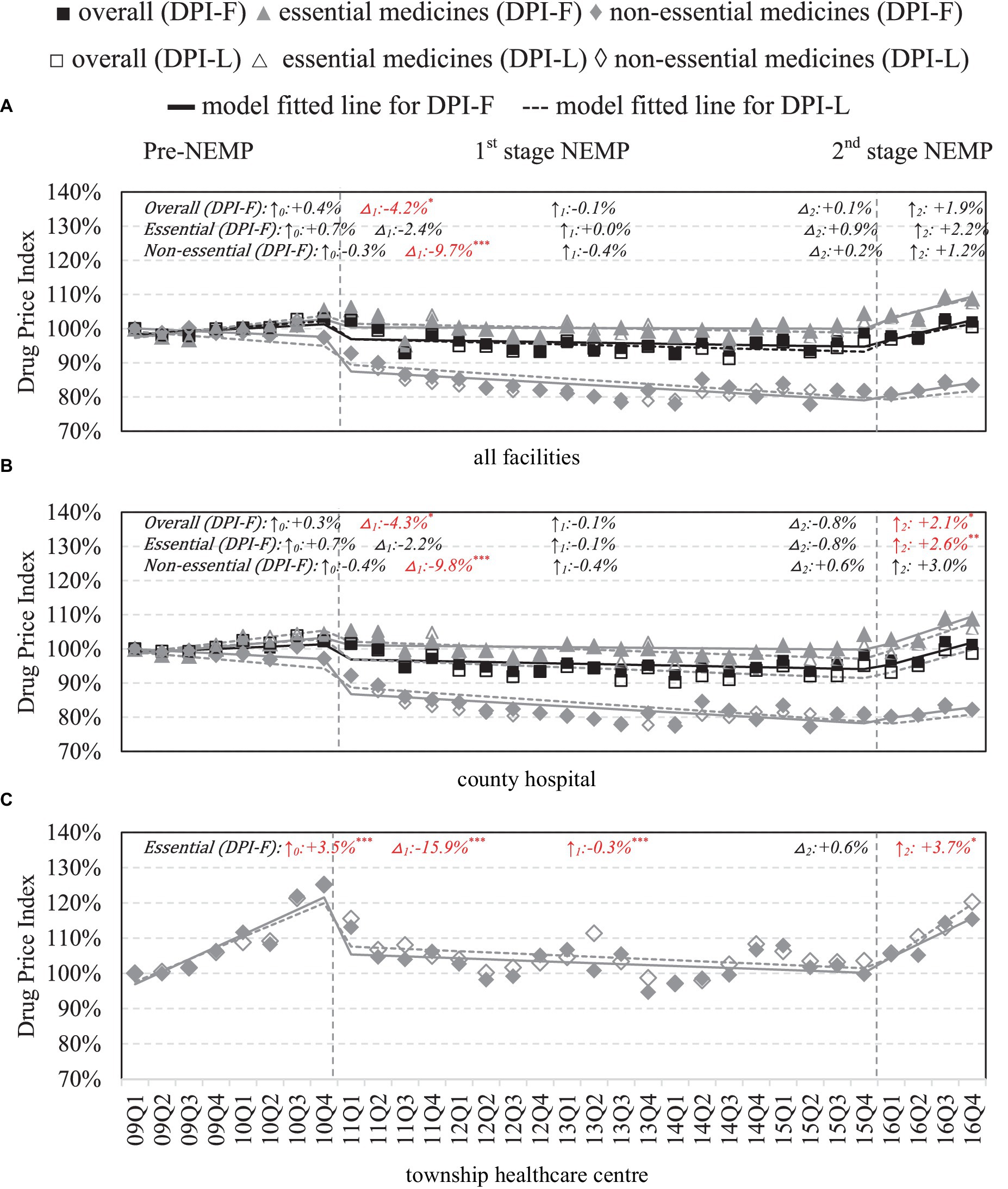
Figure 5. Wholesale price of medicines by facility level and essential medicine list. △1: immediate price change% after first-phase NEMP [exp(β3) in ITSA model]; ↑0: sustained change per observation period before first-phase NEMP (β1); ↑1: sustained price change% per observation period after first-phase NEMP [exp(β1 + β3)]; △2: immediate price change% after second-phase NEMP [exp(β4)]; ↑2: sustained price change% per observation period after second-phase NEMP [exp(β1 + β3 + β5)]; ITSA, interrupted time-series analysis; NEMP, National Essential Medicines Policy; (A) all facilities, (B) county hospital, (C) township healthcare center. *p < 0.05; **p < 0.01; ***p < 0.001.
By medicine types, TCMs saw increases in wholesale price after the first-stage and second-stage NEMP (Appendix 14). Further by ATC-classifications, NEMP’s impacts on wholesale prices in both county hospitals and THCs mirrored those in retail prices (Appendices 15, 16).
Sensitivity analysis
Main findings of adjusting retail and wholesale prices by CPI and PPI align with the base case analysis. There are only slight variations in the magnitude and statistical levels of impacts (Appendix 17).
Discussion
Main findings
The NEMP in China, a comprehensive and enduring medical reform, was evaluated in a deprived rural county in southwestern China. Our summary of findings (Table 1) reveals that NEMP initially improved the availability of essential drugs and curtailed the use of non-essential drugs in primary care facilities, in line with NEMP’s establishment and mandatory implementation. Unexpectedly, we observed a notable spillover effect where the number of non-essential drugs decreased in county hospitals (secondary care) after NEMP, possibly due to the substitution between drug types and new regulations on purchasing platforms. Nevertheless, consistent with previous evidence (31–34), we found the long-term availability of (essential) medicines deteriorated in the sample county, especially in primary care centers. This has become one of the nonnegligible concerns by the public (48), and could seriously hamper the sustainability of NEMP. Key obstacles to access essential medicines in undeveloped and remote areas are still to be overcome, including high transportation costs, low supplier profits, inadequate storage in PHFs, and a lack of motivation and confidence to provide medical or pharmaceutical services among primary care practitioners (24, 49).
Regarding drug prices, centralized procurement and zero-markup policies of NEMP led to a substantial decline by nearly 25% and a modest long-tern decrease in consumer price of essential medicines in THCs, aligning with prior studies (16, 31, 50). Also, in county hospitals (secondary care), we observed a modest decline as spillover effects in essential drugs after first-stage NEMP, as well as an abrupt drop of 8% after zero-markup policy in second-stage NEMP. Previous studies also revealed the availability and affordability of essential drugs are improved in secondary and tertiary hospitals (35, 36). Surprisingly as a new finding, prices of non-essential medicines also fell significantly in county hospitals, potentially due to the provincial centralized procurement for these medicines and manufacturers’ evolving pricing strategies (39). Despite these reductions, the upward price trends in all types of medicines and facilities after second-stage NEMP are noteworthy, possibly due to the price deregulations, warranting further observation (36).
As a pivotal link of medication cost, wholesale price changes from manufacturers to healthcare providers were also examined were also examined. We found that wholesale price post-NEMP showed identical trends of reduction with retail price, but with lower rates, suggesting room for additional measures to reduce procurement and distribution costs.
Concerning medicine sales, fluctuations continued after NEMP, with stark disparities between primary care and secondary care facilities. THCs saw a 22.2% drop in in total drug expenses experienced after NEMP due to the price reduction and mandatory use of essential drugs. Inversely, medicine sales in county hospitals experienced a 7.3% increase, despite the drop in prices. This indicates potentially larger usage of essential medicines in secondary care after NEMP. As a new finding, over the long term, THC drug sales exhibited only a modest increase of 0.9% quarterly, whereas drug sales in county hospitals grew at a high rate of 3.3% (even 4.4% in essential drugs). This widening gap suggests that price cut does not guarantee increased consumption in medicine (51). Except for the challenges of NEMP in rural areas listed above, patients migration from primary care to higher-level or private facilities was also an important reason of decreased medicine use, due to distrust of medicine quality and dissatisfaction with monotonous supply of generic medicines (28). These unexpected results could undermine PHFs’ role as health gatekeepers in rural and remote areas.
Lastly, NEMP’s impacts varied by medicine type. As for availability, we noted a declining number in genitourinary system and sex hormone medicines in rural and deprived areas, contrary to previous studies (32). Regarding prices, western medicines of (i) dermatological, (ii) genitourinary system and sex hormones, (iii) systemic hormonal preparations and (iv) Musculo-skeletal system, (v) antiparasitic, (vi) sensory organ, and TCMs of surgical and orthopedic were faced with upward trends and required further monitoring. Additionally, price cut in TCMs after NEMP was smaller than western medicines, suggesting opportunities for policy refinement. There was also a trend of prescribing higher-priced TCMs in county hospitals, likely influenced by the mark-up price policy and government subsidy standards based on drug sales at that moment (16). Total sales analysis revealed that the most significant increases in county hospitals were in cardiovascular, systemic hormonal, musculoskeletal, and metabolism medicines. In THCs, sales more than doubled for anti-infectives and TCMs for internal medicine, while sales for cardiovascular, sensory organs, and blood-forming organs decreased significantly, contradicting epidemiological trends and highlighting issues with polypharmacy and antibiotic overprescription (18, 23).
Strengths and limitations
This study is among the few to examine the long-term and secular impacts of NEMP over an eight-year period in southwestern China. It specifically focuses on a micro-context within a geographically isolated and economically underdeveloped county. The deliberate selection of an isolated county and the employment of a quasi-experimental design is expected to minimize potential confounding factors, such as other drug-related policies or interactions with surrounding areas. Leveraging comprehensive pharmaceutical records from major healthcare providers, we conducted multi-dimensional analyses encompassing a variety of outcomes, facility levels, medicine types, and prices. It also enables us to understand not only the policy’s intended effects but also its spillover effects on other healthcare system components. The use of price indices and ITSA serves as robust methods to address the limitations of WHO/HAI method (e.g., restrictions on dosage forms and specifications) (31, 32), and to more accurately reflect the temporal price changes of multiple medicines in the pharmaceutical market.
Undoubtedly, our study owns limitations. First, the quasi-experimental pre-post design lacking control groups cannot fully eliminate the influence of unobserved factors. We anticipate this limitation can be mitigated by selecting a geographically isolated region with a less dynamic social and healthcare system. Second, village clinics were excluded from our analysis due to incomplete drug records before 2012. These 73 clinics, typically managed by single physicians, play a vital role as foundational units in primary care. Their exclusion constrained the comprehensiveness of our findings. Also, we did not incorporate private sectors in our analysis. Since 2014, two private hospitals have been established in the studied county. Their presence could have significantly influenced patient flow and medicine consumption in the closed market. Notably, we detected fluctuations in medicine sales in county hospitals between 2014 and 2015, which merits further investigation.
Moreover, our calculation of Drug Price Index (DPI) only considered medicines with complete records across 8 years, omitting those newly introduced or discontinued during this time. Still and all, we analyzed prices of 405 pharmaceuticals, a substantially larger number than previous studies. Finally, data were only available for 1 year after the second-stage NEMP, insufficient to confirm trend changes afterwards. Extended surveillance is necessary. Moreover, using monetary sales, rather than DDDs, may be affected by the fluctuating drug prices and not accurately reflect the actual quantity of medicine usage. We advocate for more rigor methodologies to measure and amalgamate the quantities of various medicines.
Implication
This study illuminates the dynamic effects of the National Essential Medicines Policy (NEMP) on the local pharmaceutical system from a micro-perspective in a rural, remote, and economically challenged county in Southwestern China. Our findings offer insights in policy enhancements in similar contexts characterized by low economic development, scarce health resources, high transportation costs, and limited capacity in personnel’s, finances and services (31, 32).
First, despite establishment and mandatory implementation of NEML, the availability of essential medicines remained scarce in rural, remote and underdeveloped regions, especially in primary care settings. Continuous efforts are needed on (i) financial incentives for suppliers to counterbalance the high transportation costs and low profit margins in remote areas, (ii) an efficient drug bidding and distribution system, (iii) improved infrastructure and training for physicians to ensure the provision of essential medicines.
Second, medicine prices in remote and underdeveloped areas have been effectively controlled and maintained at lower levels over the long term, owing to both the zero-markup policy in retail price and centralized tendering and procurement. The significant price cut in non-essential medicines also enlighten future policies, suggesting that provincial and periodical centralized procurement (e.g., Volume-Based medicine Purchasing Policy, nationwide collective pharmaceutical procurement) can be potent tools for price regulation (33). Moreover, given the price fluctuation, routine monitoring systems for supply, price and usage are crucial for ongoing improvements, especially in regions with limited development and resources (31, 36).
Third, precise regulations targeted at specific categories of medicines may enhance NEMP’s effectiveness in rural and less developed counties. For instance, the availability of genitourinary and sex hormones and antiparasitic products was low in PHFs under NEMP, while prices of dermatological, genitourinary system and sex hormones, Musculo-skeletal, and sensory organ medicines kept rising. Optimal bidding procurement policies, pricing mechanisms, shortage warning systems, and administrative support are essential to meet patients’ needs for these medicines (32, 34). An affordable, efficient and accessible public-financed reimbursement system is also vital to address the emerging number of innovative and high-price medicines (e.g., oncology treatments) (52). Furthermore, the suboptimal price reduction of TCMs compared to western medicines, accompanied by their growing usage, suggests further regulations on TCMs can be potential directions for policy refinement.
Fourth, the fact that lower prices did not ensure higher consumption indicates that collaborative efforts are necessary to overcome barriers to the use of essential medicines in primary care in remote and rural areas. Safety and quality of essential medicines is a fundamental prerequisite. Regular and regional adjustments to essential medicine lists and centralized procurement and distribution system are needed to align with the needs of local pharmaceutical suppliers, healthcare providers and patients (34). Financial incentives (e.g., enhanced reimbursement rate) and education for practitioners and patients are also effective policy instruments to increase the use of essential drugs (31, 34). A robust and thriving market is key to fostering a virtuous cycle of population health and pharmaceutical development.
Finally, ongoing monitoring and evaluation of NEMP are imperative for future research. Considering the self-adaptive system and responses under top-down policies, it is important to focus on the disproportionate impacts across different regions (undeveloped, rural, and remote areas), populations (older adults), system levels (village, county, city, or province), medicine types, and sectors (public or private). Investigating the mechanisms underlying these expected or unintended outcomes is valuable from the perspectives of different stakeholders (i.e., regulators, public administrators, manufacturers, healthcare providers, patients and third-party payers). Additionally, exploring whether changes in medicine numbers, prices, and usage correlate with improvements in population health remains an essential yet unexplored area of study.
Conclusion
The NEMP represents a nationwide, systemic, and comprehensive medico-social reform in China. This study investigates the impacts of NEMP on the availability, price, and sales of medicines in a remote, rural and deprived county in Southwestern China. The policy has effectively capped drug prices in primary care in the short-and long-terms, while also exerting spill-over effects in curbing medication costs in secondary care institutions. However, over time, a widening gap in medicine availability and utilization has emerged between rural primary healthcare facilities and county hospitals. While the number of medicines has shown modest increases in county hospitals, it has been on a steady decline in THCs. Similarly, medicine consumption and sales have experienced moderate increases in county hospitals but have risen only marginally in THCs. Ongoing surveillance is necessary, particularly as a reversal of rising price was detected after the second-stage NEMP. Further research and policies are encouraged to address the uneven impacts of NEMP across various pharmaceuticals, care levels, regions and demographics.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XX: Formal analysis, Writing – original draft. ZH: Investigation, Formal analysis, Writing – original draft. SZ: Investigation, Writing – review & editing. GB: Project administration, Investigation, Writing – review & editing. SH: Investigation, Writing – review & editing. YZ: Investigation, Writing – review & editing. JJ: Writing – review & editing. JQ: Writing – review & editing. LL: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. National Natural Science Foundation of China (No. 72174041, LL, URL: https://www.nsfc.gov.cn/), and Key Projects of Philosophy and Social Sciences Research, Ministry of Education (No. 20JZD027, LL, URL: https://218.241.235.188/).
Acknowledgments
We sincerely appreciate all assistance provided by the administrative staffs and healthcare professionals from local health bureaus and healthcare facilities, for their help to collect data and promote the communication between different departments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1355239/full#supplementary-material
References
1. Wirtz, VJ, Hogerzeil, HV, Gray, AL, Bigdeli, M, and Reich, MR. Essential medicines for universal health coverage. Lancet. (2017) 389:403–76. doi: 10.1016/s0140-6736(16)31599-9
2. Haque, M. Essential medicine utilization and situation in selected ten developing countries: a compendious audit. J Int Soc Prev Community Dent. (2017) 7:147–60. doi: 10.4103/jispcd.JISPCD_224_17
3. Tharumia Jagadeesan, C, and Wirtz, VJ. Geographical accessibility of medicines: a systematic literature review of pharmacy mapping. J Pharm Policy Pract. (2021) 14:28. doi: 10.1186/s40545-020-00291-7
4. Organization WH. Global spending on health: rising to the pandemic’s challenges. (2022). Available at: https://iris.who.int/bitstream/handle/10665/365133/9789240064911-eng.pdf (Accessed November 11, 2023).
5. IQVIA. Global spending on medicines 2010-2027. The IQVIA Institute (2023). Available at: https://www.statista.com/statistics/280572/medicine-spending-worldwide/#statisticContainer (Accessed November 11, 2023).
6. Mela, A, Rdzanek, E, Poniatowski, ŁA, Jaroszyński, J, Furtak-Niczyporuk, M, Gałązka-Sobotka, M, et al. Economic costs of cardiovascular diseases in Poland estimates for 2015-2017 years. Front Pharmacol. (2020) 11:1231. doi: 10.3389/fphar.2020.01231
7. Organization WH. Promoting rational use of medicines: Core components. (2002). Available at: https://iris.who.int/bitstream/handle/10665/67438/WHO_EDM_2002.3.pdf (Accessed November 11, 2023).
8. Laing, R, Waning, B, Gray, A, Ford, N, and Hoen, E. 25 years of the WHO essential medicines lists: progress and challenges. Lancet. (2003) 361:1723–9. doi: 10.1016/s0140-6736(03)13375-2
9. Commission TNH. Statistical bulletin of China’s health development 2022. The National Health Commission. (2023). Available at: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=5d9a6423f2b74587ac9ca41ab0a75f66 (Accessed October 17, 2023).
10. Tie-min, Z, Yu-hui, Z, Quan, W, and Center, CNHDR. The results and analysis of National Health Accounts in China in 2018. Chinese Health Econ. (2020) 39:5–8.
12. Eggleston, K, Ling, L, Qingyue, M, Lindelow, M, and Wagstaff, A. Health service delivery in China: a literature review. Health Econ. (2008) 17:149–65. doi: 10.1002/hec.1306
13. Tse, E, Sun, S, Pan, P, and Ma, K. Changing landscape of China’s pharmaceutical distribution industry. (2012). Available at: http://www.strategyand.pwc.com/media/file/Changing_Landscape_of_China's_Pharmaceutical_Distribution_EN.pdf (Accessed November 11, 2023).
14. Yip, W, Fu, H, Chen, AT, Zhai, T, Jian, W, Xu, R, et al. 10 years of health-care reform in China: progress and gaps in universal health coverage. Lancet. (2019) 394:1192–204. doi: 10.1016/s0140-6736(19)32136-1
15. Hu, S. Essential medicine policy in China: pros and cons. J Med Econ. (2013) 16:289–94. doi: 10.3111/13696998.2012.751176
16. Song, Y, Bian, Y, Petzold, M, Li, L, and Yin, A. Effects of the National Essential Medicine System in reducing drug prices: an empirical study in four Chinese provinces. J Pharm Policy Pract. (2014) 7:12. doi: 10.1186/2052-3211-7-12
17. Zhou, Z, Su, Y, Campbell, B, Zhou, Z, Gao, J, Yu, Q, et al. The financial impact of the 'zero-markup policy for essential drugs' on patients in county hospitals in western rural China. PLoS One. (2015) 10:e0121630. doi: 10.1371/journal.pone.0121630
18. Song, Y, Bian, Y, Petzold, M, Li, L, and Yin, A. The impact of China's national essential medicine system on improving rational drug use in primary health care facilities: an empirical study in four provinces. BMC Health Serv Res. (2014) 14:507. doi: 10.1186/s12913-014-0507-3
19. Li, Y, Ying, C, Sufang, G, Brant, P, Bin, L, and Hipgrave, D. Evaluation, in three provinces, of the introduction and impact of China's National Essential Medicines Scheme. Bull World Health Organ. (2013) 91:184–94. doi: 10.2471/blt.11.097998
20. Fang, Y, Wagner, AK, Yang, S, Jiang, M, Zhang, F, and Ross-Degnan, D. Access to affordable medicines after health reform: evidence from two cross-sectional surveys in Shaanxi Province, western China. Lancet Glob Health. (2013) 1:e227–37. doi: 10.1016/s2214-109x(13)70072-x
21. Dai, Y, Li, ZP, and Xu, H. A multicenter survey of the accessibility of essential medicines for children in China. Zhonghua Er Ke Za Zhi. (2020) 58:301–7. doi: 10.3760/cma.j.cn112140-20190820-00527
22. Chen, Z, Li, S, Choonara, I, Zeng, L, Jia, ZJ, Cheng, G, et al. Accessibility of essential medicines for children in Sichuan Province of China: a cross-sectional study. Front Pharmacol. (2022) 13:828152. doi: 10.3389/fphar.2022.828152
23. Xiao, Y, Wang, J, Shen, P, Zheng, B, Zheng, Y, and Li, L. Retrospective survey of the efficacy of mandatory implementation of the essential medicine policy in the primary healthcare setting in China: failure to promote the rational use of antibiotics in clinics. Int J Antimicrob Agents. (2016) 48:409–14. doi: 10.1016/j.ijantimicag.2016.06.017
24. Yang, L, Liu, C, Ferrier, JA, and Zhang, X. Organizational barriers associated with the implementation of national essential medicines policy: a cross-sectional study of township hospitals in China. Soc Sci Med. (2015) 145:201–8. doi: 10.1016/j.socscimed.2015.08.044
25. Guo, Z, Guan, X, and Shi, L. The impacts of implementation of National Essential Medicines Policies on primary healthcare institutions: a cross-sectional study in China. BMC Health Serv Res. (2017) 17:1–7. doi: 10.1186/s12913-017-2698-x
26. Xu, R, Li, S, Lv, X, and Xie, X. Prices, availability, and affordability of national essential medicines in public primary hospitals: a cross-sectional survey in poverty-stricken rural areas in China. Int J Health Plann Manag. (2020) 35:545–57. doi: 10.1002/hpm.2963
27. Yao, Q, Liu, C, Ferrier, JA, Liu, Z, and Sun, J. Urban-rural inequality regarding drug prescriptions in primary care facilities - a pre-post comparison of the National Essential Medicines Scheme of China. Int J Equity Health. (2015) 14:58. doi: 10.1186/s12939-015-0186-7
28. Xiao, Y, Zhao, K, Bishai, DM, and Peters, DH. Essential drugs policy in three rural counties in China: what does a complexity lens add? Soc Sci Med. (2013) 93:220–8. doi: 10.1016/j.socscimed.2012.09.034
29. Zhou, X, Zhang, X, Yang, L, Hu, X, Shen, A, Huang, X, et al. Influencing factors of physicians' prescription behavior in selecting essential medicines: a cross-sectional survey in Chinese county hospitals. BMC Health Serv Res. (2019) 19:980. doi: 10.1186/s12913-019-4831-5
30. Wu, D, Lam, TP, Lam, KF, Sun, KS, and Zhou, XD. Patients' coping behaviors to unavailability of essential medicines in primary Care in Developed Urban China. Int J Health Policy Manag. (2021) 10:14–21. doi: 10.15171/ijhpm.2020.09
31. Liu, Z, Zou, K, Liu, D, Zhang, M, Shi, Y, Chen, Z, et al. The price and affordability of essential medicines, progress and regional distribution in China: a systematic review. Front Pharmacol. (2023) 14:1153972. doi: 10.3389/fphar.2023.1153972
32. Zhang, M, Zou, K, Liu, Z, Liu, D, Wang, X, Shi, Y, et al. Availability of essential medicines, progress and regional distribution in China: a systematic review and meta-analysis. Front Public Health. (2023) 11:1149838. doi: 10.3389/fpubh.2023.1149838
33. Yang, C, Hu, S, Ye, D, Jiang, M, Babar, ZU, and Fang, Y. Evaluating Price and availability of essential medicines in China: a mixed cross-sectional and longitudinal study. Front Pharmacol. (2020) 11:602421. doi: 10.3389/fphar.2020.602421
34. Wang, X, He, X, Ren, Y, Zhang, Z, Cai, L, Cao, Z, et al. Evaluating the Price, availability, and affordability of essential medicines in primary healthcare institutions: a mixed longitudinal and cross-sectional study in Jiangsu. China Front Public Health. (2022) 10:860471. doi: 10.3389/fpubh.2022.860471
35. Xi, X, Li, W, Li, J, Zhu, X, Fu, C, Wei, X, et al. A survey of the availability, prices and affordability of essential medicines in Jiangsu Province, China. BMC Health Serv Res. (2015) 15:345. doi: 10.1186/s12913-015-1008-8
36. Guan, X, Hu, H, Man, C, and Shi, L. A survey of availability, price and affordability of essential medicines from 2011 to 2016 in Chinese secondary and tertiary hospitals. Int J Equity Health. (2018) 17:158. doi: 10.1186/s12939-018-0870-5
37. Organization WH. Measuring medicine prices, availability, affordability and price components. World Health Organization. (2008).
38. Wynne, MA, and Sigalla, FD. The consumer price index. Federal Reserve Bank of Dallas Econ Rev. (1994) 2:1–22.
39. Wang, N, Yang, Y, Xu, L, Mao, Z, and Cui, D. Influence of Chinese National Centralized Drug Procurement on the price of policy-related drugs: an interrupted time series analysis. BMC Public Health. (2021) 21:1883. doi: 10.1186/s12889-021-11882-7
40. Xiong, X, Luo, L, Zhou, S, Li, VJ, Zhou, Y, and Huo, Z. A profile of patients' and doctors' perceptions, acceptance, and utilization of e-health in a deprived region in southwestern China. PLOS Digit Health. (2023) 2:e0000238. doi: 10.1371/journal.pdig.0000238
41. Methodology WCCFDS. ATC/DDD index (2023). WHO. Available at: https://www.whocc.no/atc_ddd_index/ (Accessed January 5, 2024).
42. Chen, L, Yang, Y, Luo, M, Hu, B, Yin, S, and Mao, Z. The impacts of National Centralized Drug Procurement Policy on drug utilization and drug expenditures: the case of Shenzhen, China. Int J Environ Res Public Health. (2020) 17:9415. doi: 10.3390/ijerph17249415
43. Balk, BM. Decompositions of fisher indexes. Econ Lett. (2004) 82:107–13. doi: 10.1016/j.econlet.2003.09.006
44. Lopez Bernal, J, Cummins, S, and Gasparrini, A. The use of controls in interrupted time series studies of public health interventions. Int J Epidemiol. (2018) 47:2082–93. doi: 10.1093/ije/dyy135
45. Wagner, AK, Soumerai, SB, Zhang, F, and Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. (2002) 27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x
46. Bernal, JL, Cummins, S, and Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. (2017) 46:dyw098–355. doi: 10.1093/ije/dyw098
47. Beck, N, and Katz, JN. What to do (and not to do) with time-series cross-section data. Am Polit Sci Rev. (1995) 89:634–47. doi: 10.2307/2082979
48. Ren, J, Huang, X, Zhang, T, Zhou, X, Liu, C, and Wang, X. Patient satisfaction with prescribed medicines in community health services in China: a cross-sectional survey 6 years after the implementation of the national essential medicines policy. Health Soc Care Community. (2018) 26:495–506. doi: 10.1111/hsc.12548
49. Li, Q, Chen, F, Yang, M, Lu, L, Pan, J, Li, X, et al. The effect of China's National Essential Medicine Policy on health expenses: evidence from a National Study. Inquiry. (2018) 55:46958018787057. doi: 10.1177/0046958018787057
50. Song, Y, Bian, Y, and Zhen, T. Making medicines more accessible in China: an empirical study investigating the early progress of essential medicine system. PLoS One. (2018) 13:e0201582. doi: 10.1371/journal.pone.0201582
51. Selvaraj, S, Farooqui, HH, Mehta, A, and Mathur, MR. Evaluating the impact of Price regulation (drug price control order 2013) on antibiotic sales in India: a quasi-experimental analysis, 2008-2018. J Pharm Policy Pract. (2022) 15:68. doi: 10.1186/s40545-022-00466-4
52. Mela, A, Poniatowski, ŁA, Drop, B, Furtak-Niczyporuk, M, Jaroszyński, J, Wrona, W, et al. Overview and analysis of the cost of drug programs in Poland: public payer expenditures and coverage of Cancer and non-neoplastic diseases related drug therapies from 2015-2018 years. Front Pharmacol. (2020) 11:1123. doi: 10.3389/fphar.2020.01123
Keywords: essential medicine, drug price, centralized procurement, rural, primary care
Citation: Xiong X, Huo Z, Zhou S, Bai G, He S, Zhou Y, Jia J, Quan J and Luo L (2024) Short- and long-term impacts of the National Essential Medicines Policy on drug availability, price, and usage in a deprived rural county in southwestern China: an interrupted time series analysis across 8 years. Front. Public Health. 12:1355239. doi: 10.3389/fpubh.2024.1355239
Edited by:
Nicolai Savaskan, District Office Neukölln of Berlin Neukölln, GermanyReviewed by:
Jing Wu, Tianjin University, ChinaŁukasz A. Poniatowski, Medical University of Warsaw, Poland
Copyright © 2024 Xiong, Huo, Zhou, Bai, He, Zhou, Jia, Quan and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Luo, bGlsdW9AZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work
 Xuechen Xiong1,2†
Xuechen Xiong1,2† Zhaohua Huo
Zhaohua Huo Jing Jia
Jing Jia Jianchao Quan
Jianchao Quan Li Luo
Li Luo