- 1Cochrane South Africa, South African Medical Research Council, Cape Town, South Africa
- 2Division of Epidemiology and Biostatistics, Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
- 3Department of Community Medicine, Bayero University/Aminu Kano Teaching Hospital, Kano, Nigeria
- 4Vaccine-Preventable Diseases Programme, World Health Organization Regional Office for Africa, Djoué, Brazzaville, Republic of Congo
The COVID-19 pandemic caused a surge in the number of unimmunized and under-immunized children in Africa. The majority of unimmunized (or zero-dose) children live in hard-to-reach rural areas, urban slums, and communities affected by conflict where health facilities are usually unavailable or difficult to access. In these settings, people mostly rely on the informal health sector for essential health services. Therefore, to reduce zero-dose children, it is critical to expand immunization services beyond health facilities to the informal health sector to meet the immunization needs of children in underserved places. In this perspective article, we propose a framework for the expansion of immunization services through the informal health sector as one of the pillars for the big catch-up plan to improve coverage and equity. In African countries like Nigeria, Ethiopia, Tanzania, and the Democratic Republic of Congo, patent medicine vendors serve as an important informal health sector provider group, and thus, they can be engaged to provide immunization services. A hub-and-spoke model can be used to integrate patent medicine vendors into the immunization system. A hub-and-spoke model is a framework for organization design where services that are provided by a central facility (hub) are complimented by secondary sites (spokes) to optimize access to care. Systems thinking approach should guide the design, implementation, and evaluation of this model.
Background
Immunization is effective in reducing the burden of common vaccine-preventative diseases that affect children, thereby improving their survival and overall development (1). To maximize the benefits of immunization for children, immunization systems worldwide have introduced vaccines such as pneumococcal conjugate vaccine (PCV), rotavirus vaccine, hepatitis B vaccine, and human papillomavirus (HPV) vaccine, among several others, indicating that countries have made progress since the launch of Expanded Program on Immunization (EPI) by the World Health Organization (WHO) 50 years ago (2, 3). It is estimated that immunization averted 39.5 million deaths worldwide (14.4 million in the WHO African Region) between 2011 and 2020, and can potentially prevent another 51 million deaths (23 million in the African region) between 2021 and 2030 (4).
However, the COVID-19 pandemic severely impacted primary health care systems and disrupted health services, and this affected the performance of immunization programs in Africa and globally in terms of coverage and functionality (5, 6). In the African region, coverage with three doses of the diphtheria-tetanus-pertussis containing vaccines (DTP3) was at 74% in 2016 and 2017 and increased to 77% in 2019 (7). But this marginal progress was lost during COVID-19 as DTP3 coverage declined to 72% in 2021, and still remained at that level in 2022 (7). In addition to decreased coverage levels, millions of children also dropped out from immunization services in Africa during COVID-19. An important measure of dropout, which is used by the immunization program, is the DTP1 – DTP3 dropout rate (8). The DTP1 – DTP3 dropout rate is the proportion of children who took DTP1 but did not complete their vaccination series with DTP3; i.e. under-immunized children (9). This index should not exceed 5% as it reflects the ability of the immunization programs in maintaining access to services (9). An analysis of the 2022 WHO – United Nations Children Fund (UNICEF) Estimates of National Immunization Coverage (WUENIC) data showed that the number of countries with DTP1 - DTP3 dropout rate greater than 5% progressively increased from 22 in 2020 to 23 in 2021 and then to 24 in 2022 (7). In 2022, countries such as Angola, Central African Republic, the Democratic Republic of Congo, Equatorial Guinea, and Guinea have DTP1-DTP3 dropout rate of 20% and above (7). The DTP1 – DTP3 dropout rate for all the countries in the African region from 2020 to 2022 is shown in Figure 1.
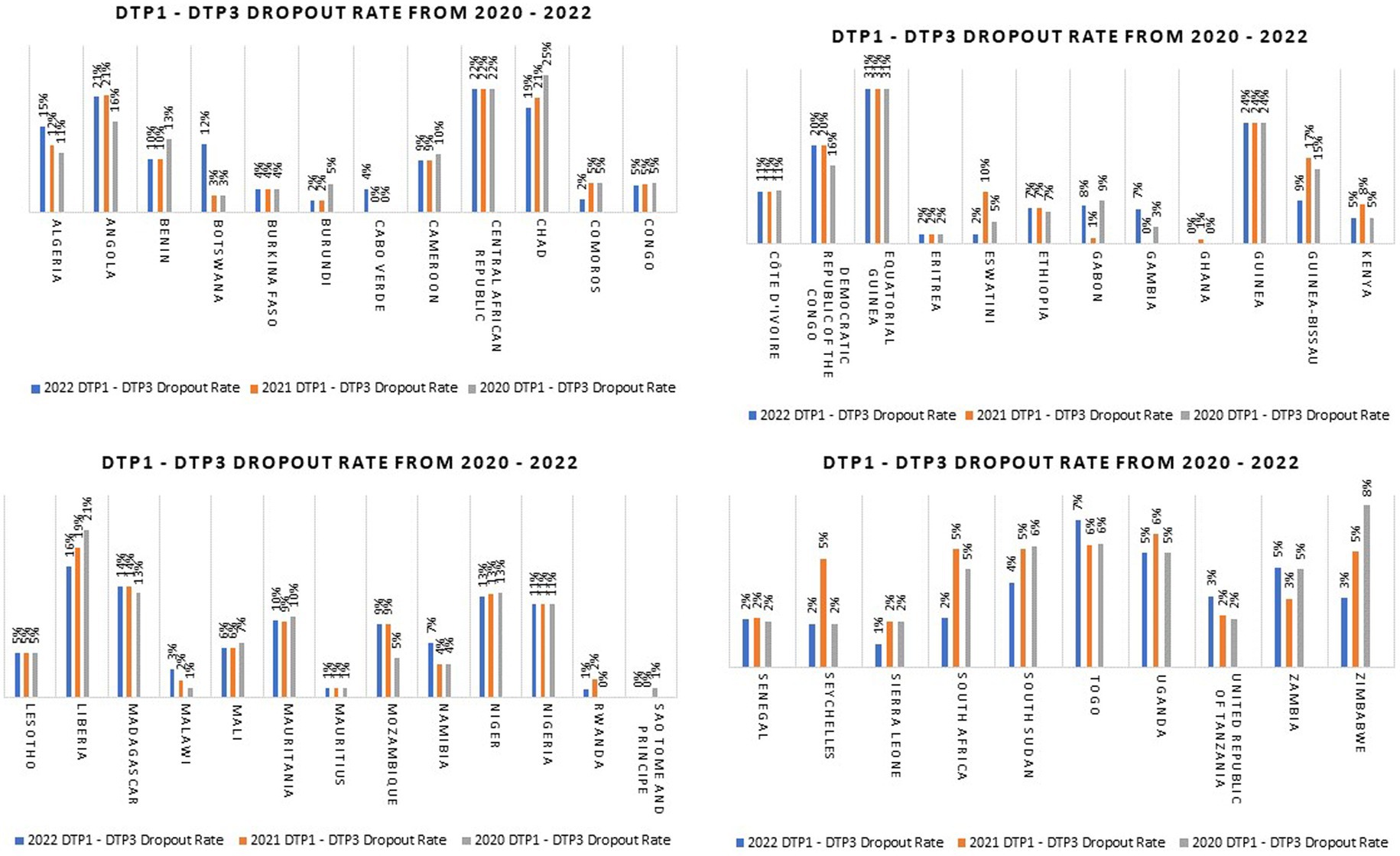
Figure 1. DTP1 – DTP3 dropout rates for countries in the WHO African Region from 2020 to 2022 (Data source: WUENIC 2022) (7).
The pandemic also affected the coverage level of other antigens (10). The first dose of measles containing vaccine (MCV1) coverage declined from 71% in 2019 to 69% in 2022 and the coverage of the third dose of PCV decreased from 72% in 2019 to 68% in 2022 (7). The disruption of routine immunization services resulted from multiple factors, including government movement restrictions, closure of health facilities, discontinuation of outreach services, and diversion of human resources from essential health care services to COVID-19 outbreak response, among other factors (5, 11, 12).
Zero-dose children in Africa
Perhaps, the most significant consequence of the COVID-19 pandemic on immunization programs especially in the WHO African Region is the surge in number of unimmunized children, otherwise known as zero-dose children, that it caused (13). Cumulatively, it is estimated that the number of zero-dose children that were added to the African region between 2019 and 2022 is about 28 million (7). These zero-dose children are spread across all countries in the region although the burden varies both between and within countries (14).
One of the immediate health systems implications for having such high numbers of zero-dose children is the occurrence of frequent outbreaks of vaccine-preventable diseases (VPDs) as already observed in some countries in the region (15). In the long term, it can derail progress toward the Immunization Agenda 2030 (IA2030) (16). IA2030 is the global strategic framework for immunization up to 2030 (16). It builds on lessons from the Global Vaccine Action Plan (GVAP) and ambitiously seeks to ensure equitable access to vaccines for all people regardless of their geographical location or age, to maximize the benefits of immunization (16). As part of its strategic priorities, this agenda has set a target to reduce the number of zero-dose children by 50% in 2030, compared to 2019 (13).
Leveraging the informal sector for immunization
Currently, routine immunization services are mainly provided through fixed sites (which include clinics and hospitals) and outreach stations (17). However, health care facilities are inequitably distributed with prominent disparities between rural and urban communities, and even when they are available, multiple structural factors make them difficult to access (18, 19). The informal health sector caters for the health care needs of people in socioeconomically disadvantaged settings but are rarely engaged to provide immunization services (20). Evidence suggests that zero-dose children are concentrated in such disadvantaged areas, especially rural hard-to-reach communities, urban slums, and communities affected by conflict (14). Therefore, ignoring this sector can delay progress toward reducing zero-dose children as efforts to enhance immunization using the traditional service delivery structures might not improve access where it is most needed.
One important informal health sector provider group that is gaining prominence in public health are patent medicine vendors (21). Patent medicine vendors are individuals who sell medicines in drug shops (21). They are widely distributed in some African countries, mainly in rural areas and urban slums where they serve as the main source of essential healthcare services for the people (22). Some patent medicine vendors have formal health training and even work in hospitals (23). They are often the sole point of contact with healthcare services for people in many underserved communities (21). It is estimated that patent medicine vendors provide 15–83% of child health services in some communities in Africa (24). In some communities in Kenya and Togo, patent medicine vendors treated 69 and 83% of childhood fevers, respectively (25, 26).
There are several reasons for this high patronage of patent medicine vendors. They are often located close to people’s residences, thus eliminating cost of transportation for seeking health care services (24, 25). For example, it is estimated that 87% of rural dwellers in some settings live within 1 km of a patent medicine vendor (25). Furthermore, people perceive services that are provided by patent medicine vendors as quick, and their opening and closing times are flexible, which is convenient (27). Also, they enjoy a high degree of trust from communities and their services are relatively inexpensive or free in some circumstances (28, 29).
Several public health programs are already using patent medicine vendors to improve access to essential services like malaria treatment, family planning including injectable contraceptives, and tuberculosis care (21, 30, 31). Given the spread and penetration of patent medicine vendors in rural communities and urban slums in some African countries, it might be worthwhile for immunization programs to explore opportunities for leveraging them.
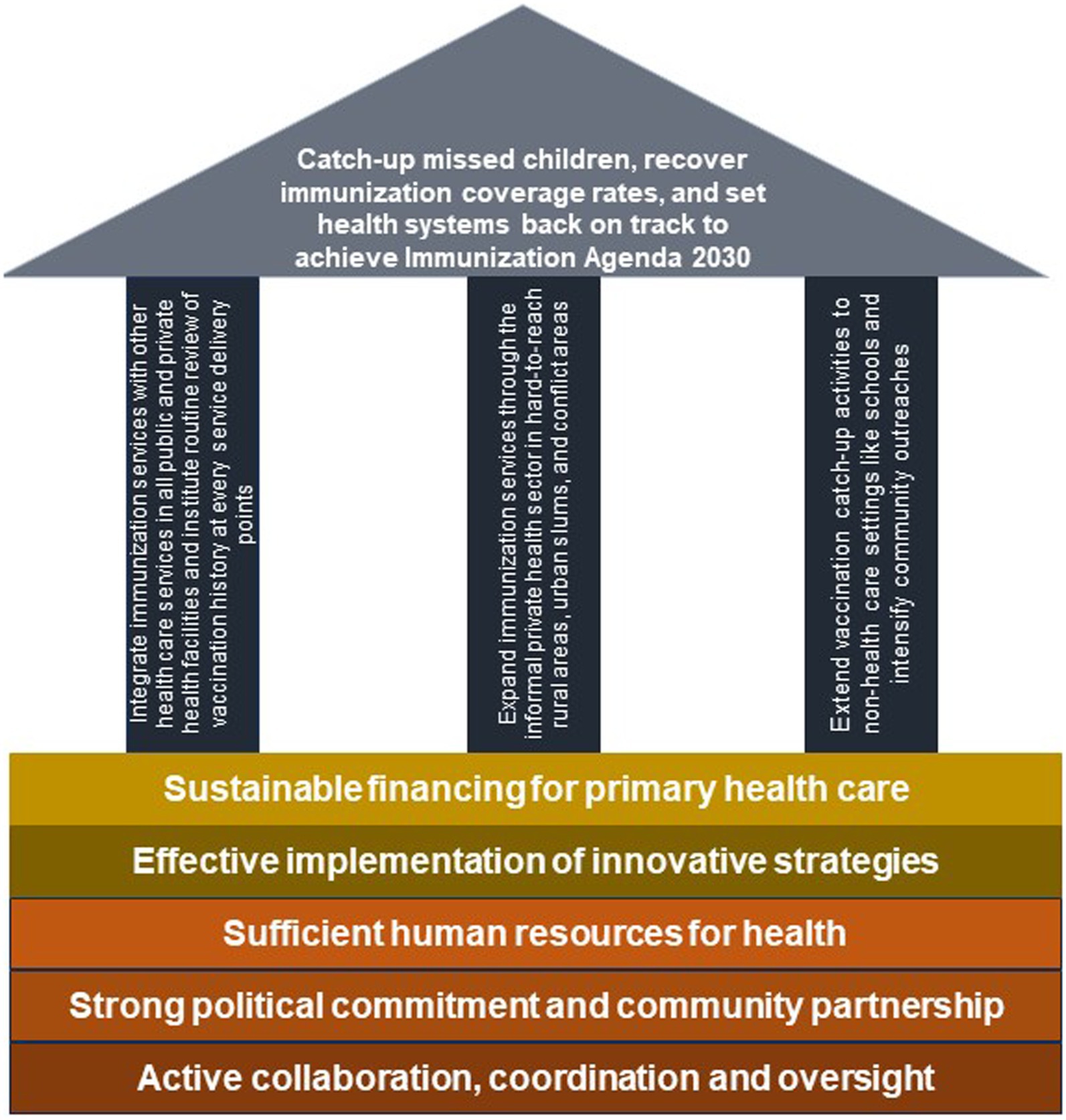
A framework for the big catch-up plan that engages the informal health sector
To address the impact of COVID-19 on immunization system performance, WHO and its partners have launched a recovery plan termed the “Big Catch-Up” (32). The WHO’s Big Catch-Up strategy has three main objectives: “reach children missed during the period 2019–2022 and provide all missing vaccinations; restore vaccination coverage in 2023 to at least 2019 levels; and strengthen immunization systems within primary health care approaches, to improve program resilience and accelerate toward reaching IA2030 and Gavi 5.1 goals and targets” (32).
A conceptual framework that aligns with these objectives and identifies the informal health sector as one of the priorities for the Big Catch-Up is proposed in Figure 2. This framework has three pillars. The first pillar focuses on immunization service integration in public and private facilities, the second pillar is on immunization service expansion through the informal private health sector, and the third pillar is on immunization activities outside the health sector. If immunization service is scaled up to all facilities (both public and private) within a particular area, and health workers at all service delivery points are trained to routinely review vaccination history and immediately vaccinate children who are under-immunized or un-immunized, more children can be reached. Furthermore, if catch up activities are extended to educational settings to target both pre-school children in daycare centers as well as school children, the immunization program is likely to improve in its performance. Additionally, if the informal health sector is integrated into the immunization system to provide immunization services, equity in coverage is likely to improve.
Although countries are expected to urgently initiate actions toward implementing the Big-Catch Up plan, it is important to take into cognizance the underlying complex problems that exist within health systems in the African region. Issues such as inadequate immunization financing, insufficient human resources for health, and weak coordination, among others, are commonly reported challenges (33–35). These constraints can hinder critical activities that are necessary for optimizing the function of immunization programs in the region. Efforts to close the immunization gaps caused by COVID-19 cannot be achieved without substantial financial investment in immunization as well as the other bedrocks highlighted in Figure 2.
A strategy for integrating patent medicine vendors into the immunization system
A hub-and-spoke model can be used to integrate informal health sector providers like patent medicine vendors into the immunization program to deliver some routine immunization services. The hub-and-spoke model is a framework for organization design where services that are provided by a central facility (hub) are complemented by secondary sites (spokes) to optimize access to care (36). Typically, the range of services that are delivered by the spokes are usually limited in scope, and people who require advanced care are referred to the hub (36, 37). Nevertheless, the spokes are able to leverage the skills and expertise of the hub, and this invariably improves the overall effectiveness of the services provided by the network (36). This model of organizing health care can be used to form a network of patent medicine stores (spokes) around a fixed immunization delivery facility (hub). If designed properly, this model can replicate routine immunization services across multiple hard-to-reach rural communities and urban slums that might not have functional health facilities thereby enhancing access equity (37).
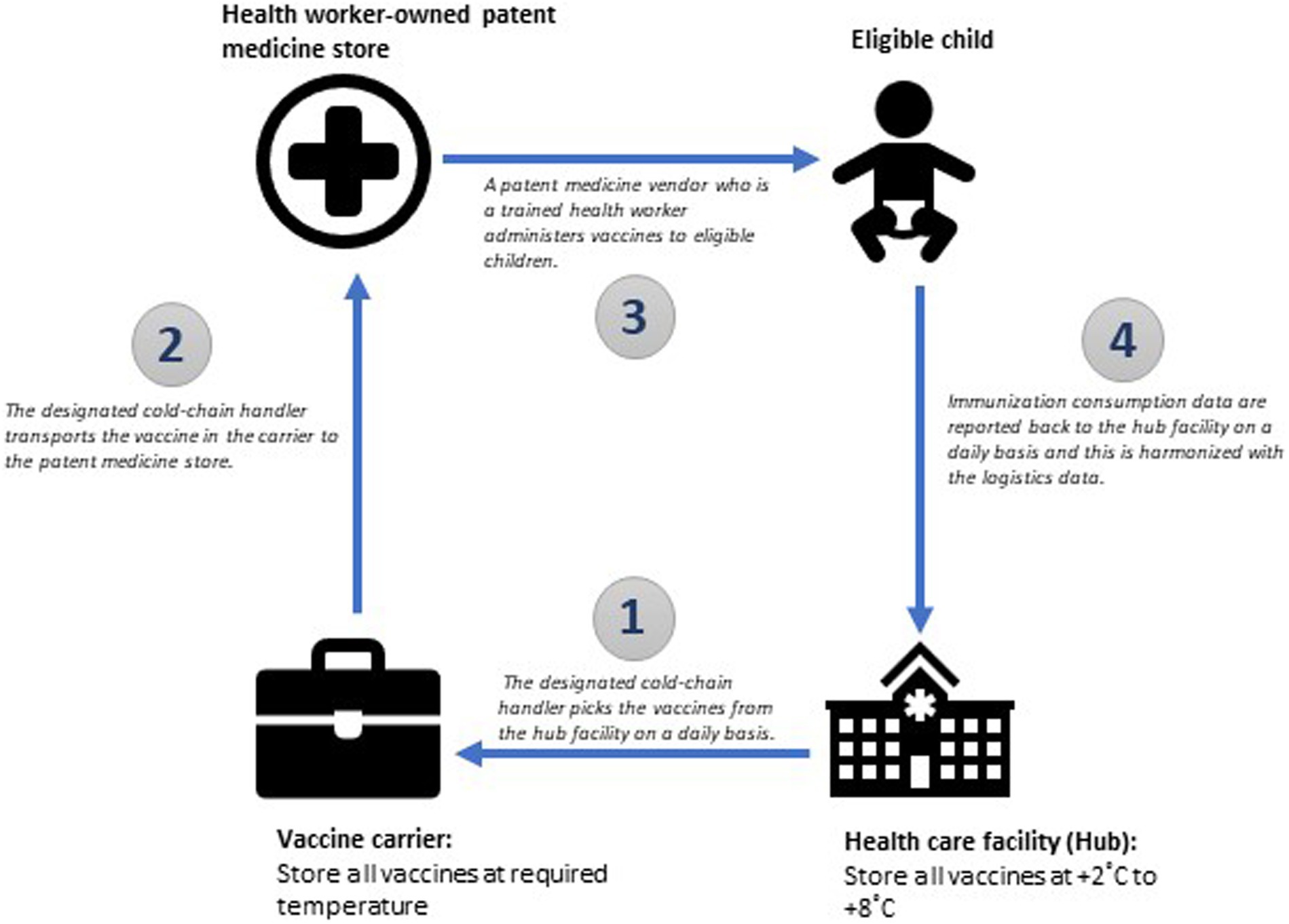
One key drawback that needs to be strongly considered when engaging patent medicine vendors is that managing vaccines is somewhat sophisticated. The medicines that patent medicine vendors typically sell do not have cold chain requirements like vaccines and are easier to store. Nonetheless, this can be addressed through thorough training on immunization including vaccine handling, and the provision of required equipment like vaccine carriers to maintain the cold chain outside the health facility. Another downside to engaging these providers for immunization services is that most vaccines are administered through injections. To overcome this, immunization programs need to be mindful of the roles that are assigned to different types of patent medicine vendors (differentiating those who are trained health workers from those who are not). On a broader level, the immunization programs need to ensure that the regulatory framework that guides the operation of patent medicine vendors is robust, and the quality of public health services that they provide is linked to their re-licensing. Figure 3 is a diagrammatic illustration of a hub-and-spoke network arrangement between a health care facility and patent medicine stores.

Figure 3. A hub-and-spoke model for integrating patent medicine stores into the existing routine immunization delivery system.
In this hub-and-spoke model, the role of a health worker owned patent medicine store (where the patent medicine vendor is a trained health personnel) and a non-health worker owned medicine store (where the patent medicine vendor is not a trained health personnel) is well differentiated as outlined in Table 1. Considering the technical complexity of immunization services, only patent medicine vendors who are qualified health personnel with local authorization to give injections and can recognize adverse events following immunization (AEFI) should be allowed to administer vaccines to children. Other patent medicine vendors who aren’t health personnel are also important as they can be engaged to conduct routine reviews of the vaccination history of children who seek services in their stores to identify under-immunized and un-immunized children, and escort (or refer) them with their caregivers to the nearest facility where they can access immunization services in the communities. This can be at a health worker owned patent medicine store or a health care facility, depending on which is closer to the child. These patent medicine vendors can sustain follow-up for such children until they are fully immunized.
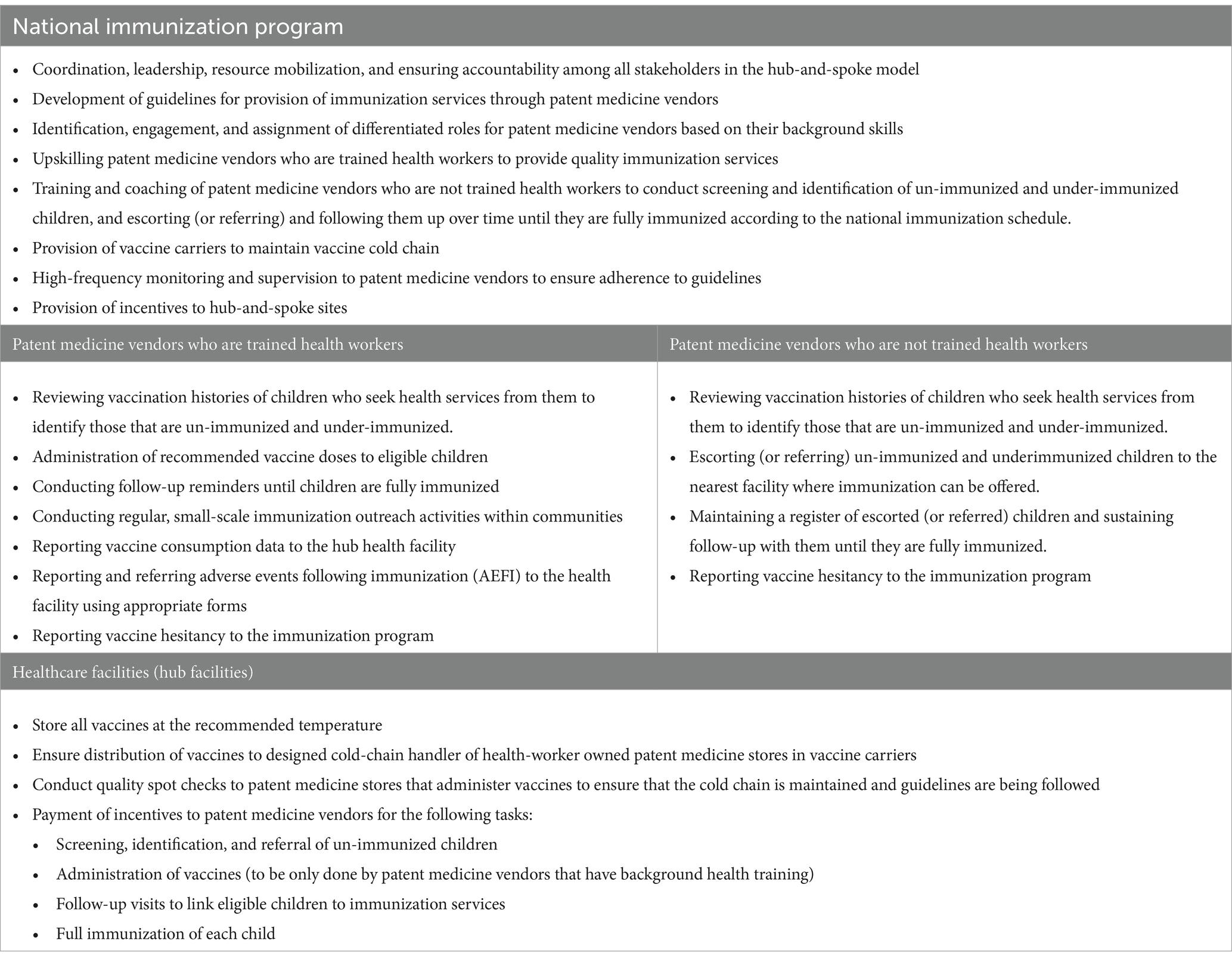
Table 1. Potential role of stakeholders involved in an integrated hub-and-spoke model to expand immunization services through patent medicine vendors.
To ensure optimal functioning and effectiveness of the hub-and-spoke model, the national immunization program has important roles to play. This includes coordination and oversight, and building in a clear accountability mechanism for all the stakeholders. A guideline is needed that clearly outlines the functions of all key actors. The immunization program can also develop standard operating procedures that summarize the expected task for each stakeholder. Importantly, all the patent medicine vendors need to be trained by the national immunization program on the role that they are expected to perform. Since patent medicine stores are for-profit entities, providing financial incentives can serve as an important motivator for quality immunization services. The hub facilities should conduct quality spot checks, which should then be used to determine whether a provider qualifies for incentives.
Vaccines must be stored and transported at the recommended temperature as they move along the pathway from the hub facility to the health worker-owned patent medicine store and finally to the eligible child. The hub facility should have advanced cold chain facilities that are capable of storing vaccines at the recommended temperature for a long period of time. The cold-chain handler at the health facility should be responsible for distributing vaccines to spoke sites through their handler. Once the vaccine is administered, data should be reported back promptly to the hub facility to ensure proper logistic management. The pathway is illustrated in Figure 4.
There are some anticipated benefits of strategically using a hub-and-spoke model in priority zero-dose countries with high volume of patent medicine vendors. They include immunization service expansion in hard-to-reach rural communities and urban slums to meet the needs of children residing in those areas, improvement in immunization knowledge and skills to provide services among patent medicine vendors who are often the first point of contact of health care for many people in underserved areas, and close monitoring and supervision from health workers in the hub facilities. Despite these benefits, some risks should be expected. On the side of the patent medicine vendors, possible risks can include poor quality reporting of immunization consumption data, high workload, poor quality of services, and unauthorized sale of vaccines among others. While on the health facility side, risks can include disruption of vaccine supply including consumables. Measure should be put in place to mitigate these risks in the design phase.
System-wide implications of integrating patent medicine vendors into the immunization system
Immunization program managers should bear in mind that health care is a complex adaptive system (CAS), thus integrating patent medicine vendors into the immunization sub-system using a hub-and-spoke model will naturally cause the emergence of new connections and the modification of existing ones (38). Typical of CAS is adaptation and self-organization (38). As the system is altered to expand provision of immunization services, these patent medicine vendors will mix into the existing immunization systems (39). Consequently, their behavior will be modulated by already existing agents in the system (such as individuals, health workers, and immunization program managers and policies) through formed dependencies and interconnections (39). Therefore, it is important to apply a systems thinking lens when approaching problems that arise from integrating patent medicine vendors into the immunization sub-system.
Systems thinking tools like causal loop diagrams are useful for exploring the interrelationships and interconnections, including feedback loops that can emerge if patent medicine vendors are integrated into the immunization sub-system (40–42). In a causal loop diagram, arrows are used to show the direction of influence and polarity is denotated using a (+) or (−) sign (41). The feedback loops can either be reinforcing or balancing (41).
Conclusion
Reducing un-immunized and under-immunized children, restoring immunization coverage to at least pre-pandemic levels, and setting immunization programs back on track from the disruptions caused by COVID-19 require contextualized and tailored approaches. In some communities, engagement of the informal health sector to provide immunization services might be the “magic bullet” that is needed to rapidly improve uptake and utilization of recommended vaccines. Patent medicine vendors are a potential asset within the health systems architecture but remain untapped for routine immunization. Engaging them to provide immunization services can improve access in underserved areas and invariably, improve equity in coverage. For this reason, program managers in countries with high patent medicine vendor activities should strongly consider leveraging them to expand services. However, there is a need to generate empirical evidence on the feasibility and effectiveness of integrating patent medicine vendors into the immunization sub-system using a hub-and-spoke model to reduce un-immunized and under-immunized children. To address this, implementation research in program settings, using type 2 hybrid design, is recommended (43). This type of research is suitable for examining system-wide change in real world implementation context as it allows the assessment of intervention effectiveness as well as implementation outcomes (43). It also enables experiential learning by both researchers and program implementers which allows course correction and can inform scale-up (43, 44).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AA: Conceptualization, Data curation, Investigation, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. RJ: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. DN: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. CW: Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author (s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ehreth, J . The value of vaccination: a global perspective. Vaccine. (2003) 21:4105–17. doi: 10.1016/S0264-410X(03)00377-3
2. Guignard, A, Praet, N, Jusot, V, Bakker, M, and Baril, L. Introducing new vaccines in low- and middle-income countries: challenges and approaches. Expert Rev Vaccines. (2019) 18:119–31. doi: 10.1080/14760584.2019.1574224
3. Bele, O, and Barakamfitiye, DG. The expanded program on immunization in the WHO African region: current situation and implementation constraints. Sante. (1994) 4:137–42.
4. Carter, A, Msemburi, W, Sim, SY, Gaythorpe, KA, Lambach, P, Lindstrand, A, et al. Modeling the impact of vaccination for the immunization agenda 2030: deaths averted due to vaccination against 14 pathogens in 194 countries from 2021 to 2030. Vaccine. (2023). doi: 10.1016/j.vaccine.2023.07.033
5. Buonsenso, D, Cinicola, B, Kallon, MN, and Iodice, F. Child healthcare and immunizations in sub-Saharan Africa during the COVID-19 pandemic. Front Pediatr. (2020) 8:517. doi: 10.3389/fped.2020.00517
6. Ota, MO, Badur, S, Romano-Mazzotti, L, and Friedland, LR. Impact of COVID-19 pandemic on routine immunization. Ann Med. (2021) 53:2286–97. doi: 10.1080/07853890.2021.2009128
7. World Heath Organization . GHO | Global Health Observatory data repository (African region) | diphtheria tetanus toxoid and pertussis (DTP3) - immunization coverage estimates by WHO region. WHO (2023). Available at: https://apps.who.int/gho/data/view.main-afro.81200?lang=en [Accessed September 16, 2023].
8. Organization WH . Global routine immunization strategies and practices (GRISP): A companion document to the global vaccine action plan (GVAP). World Health Organization (2016).
9. Feldstein, LR, Mariat, S, Gacic-Dobo, M, Diallo, MS, Conklin, LM, and Wallace, AS. Global routine vaccination coverage, 2016. MMWR Morb Mortal Wkly Rep. (2017) 66:1252–5. doi: 10.15585/mmwr.mm6645a3
10. Masresha, BG, Luce, R Jr, Shibeshi, ME, Ntsama, B, N’Diaye, A, Chakauya, J, et al. The performance of routine immunization in selected African countries during the first six months of the COVID-19 pandemic. Pan Afr Med J. (2020) 37:37. doi: 10.11604/pamj.supp.2020.37.1.26107
11. Aguinaga-Ontoso, I, Guillen-Aguinaga, S, Guillen-Aguinaga, L, Alas-Brun, R, Onambele, L, Aguinaga-Ontoso, E, et al. COVID-19 impact on DTP vaccination trends in Africa: a Joinpoint regression analysis. Vaccines (Basel). (2023) 11:1103. doi: 10.3390/vaccines11061103
12. Burkholder, B, Wadood, Z, Kassem, AM, Ehrhardt, D, and Zomahoun, D. The immediate impact of the COVID-19 pandemic on polio immunization and surveillance activities. Vaccine. (2023) 41:A2–A11. doi: 10.1016/j.vaccine.2021.10.028
13. O’Brien, KL, and Lemango, E. The big catch-up in immunisation coverage after the COVID-19 pandemic: progress and challenges to achieving equitable recovery. Lancet. (2023) 402:510–2. doi: 10.1016/S0140-6736(23)01468-X
14. Wigley, A, Lorin, J, Hogan, D, Utazi, CE, Hagedorn, B, Dansereau, E, et al. Estimates of the number and distribution of zero-dose and under-immunised children across remote-rural, urban, and conflict-affected settings in low and middle-income countries. PLOS Global Public Health. (2022) 2:e0001126. doi: 10.1371/journal.pgph.0001126
15. Medugu, N, Adegboro, B, Onipede, AO, Babazhitsu, M, and Amaza, R. A review of the current diphtheria outbreaks. Afr J Clin Exp Microbiol. (2023) 24:120–9. doi: 10.4314/ajcem.v24i2.2
16. O’Brien, KL, Lemango, E, Nandy, R, and Lindstrand, A. The immunization agenda 2030: a vision of global impact, reaching all, grounded in the realities of a changing world. Vaccine. (2022). doi: 10.1016/j.vaccine.2022.02.073
17. Ryman, T, Macauley, R, Nshimirimana, D, Taylor, P, Shimp, L, and Wilkins, K. Reaching every district (RED) approach to strengthen routine immunization services: evaluation in the African region, 2005. J Public Health. (2010) 32:18–25. doi: 10.1093/pubmed/fdp048
18. Wigley, AS, Tejedor-Garavito, N, Alegana, V, Carioli, A, Ruktanonchai, CW, Pezzulo, C, et al. Measuring the availability and geographical accessibility of maternal health services across sub-Saharan Africa. BMC Med. (2020) 18:237–10. doi: 10.1186/s12916-020-01707-6
19. Galadima, AN, Zulkefli, NAM, Said, SM, and Ahmad, N. Factors influencing childhood immunisation uptake in Africa: a systematic review. BMC Public Health. (2021) 21:1475–20. doi: 10.1186/s12889-021-11466-5
20. Bloom, G, Standing, H, Lucas, H, Bhuiya, A, Oladepo, O, and Peters, DH. Making health markets work better for poor people: the case of informal providers. Health Policy Plan. (2011) 26:i45–52. doi: 10.1093/heapol/czr025
21. Beyeler, N, Liu, J, and Sieverding, M. A systematic review of the role of proprietary and patent medicine vendors in healthcare provision in Nigeria. Plo S one. (2015) 10:e0117165. doi: 10.1371/journal.pone.0117165
22. Marriott, A . Blind optimism: Challenging the myths about private health care in poor countries - Oxfam Policy & Practice. Kenya: Oxfam International (2009). 55 p.
23. Adamu, AA, Gadanya, MA, Jalo, RI, Uthman, OA, Nnaji, CA, Bello, IW, et al. Assessing readiness to implement routine immunization among patent and proprietary medicine vendors in Kano, Nigeria: a theory-informed cross-sectional study. Expert Rev Vaccines. (2020) 19:395–405. doi: 10.1080/14760584.2020.1750379
24. Goodman, C, Brieger, W, Unwin, A, Mills, A, Meek, S, and Greer, G. Medicine sellers and malaria treatment in sub-Saharan Africa: what do they do and how can their practice be improved? Am J Trop Med Hyg. (2007) 77:203–18. doi: 10.4269/ajtmh.2007.77.203
25. Molyneux, CS, Mung'ala-Odera, V, Harpham, T, and Snow, RW. Maternal responses to childhood fevers: a comparison of rural and urban residents in coastal Kenya. Trop Med Int Health. (1999) 4:836–45. doi: 10.1046/j.1365-3156.1999.00489.x
26. Deming, MS, Gayibor, A, Murphy, K, Jones, TS, and Karsa, T. Home treatment of febrile children with antimalarial drugs in Togo. Bull World Health Organ. (1989) 67:695–700.
27. Goodman, CA . An economic analysis of the retail market for fever and malaria treatment in rural Tanzania. London: London School of Hygiene & Tropical Medicine (2005).
28. Amin, AA, Marsh, V, Noor, AM, Ochola, SA, and Snow, RW. The use of formal and informal curative services in the management of paediatric fevers in four districts in Kenya. Trop Med Int Health. (2003) 8:1143–52. doi: 10.1046/j.1360-2276.2003.01140.x
29. Brieger, WR, Sesay, HR, Adesina, H, Mosanya, ME, Ogunlade, PB, Ayodele, JO, et al. Urban malaria treatment behaviour in the context of low levels of malaria transmission in Lagos, Nigeria. Afr J Med Med Sci. (2001) 30:7–15.
30. Oluwasanu, MM, Adebayo, AM, Okunade, FT, Ajayi, O, Akindele, AO, Stanback, J, et al. Process evaluation of an intervention to improve access to injectable contraceptive services through patent medicine vendors in Nigeria: a mixed methods study. J Pharmaceutical Policy Prac. (2021) 14:1–17. doi: 10.1186/s40545-021-00336-5
31. Ochei, O, Ntaji, MI, Aduh, U, Okumagba, MT, and Awunor, NS. Effect of a training intervention for finding the missed cases of tuberculosis amongst patent medicine vendors in Delta state, Nigeria. Niger Postgrad Med J. (2023) 30:232–9. doi: 10.4103/npmj.npmj_50_23
32. World Health Organization . The big catch-up: An essential immunization recovery plan for 2023 and beyond. World Health Organization (2023).
33. Mihigo, R, Okeibunor, J, Anya, B, Mkanda, P, and Zawaira, F. Challenges of immunization in the African region. Pan Afr Med J. (2017) 27:27. doi: 10.11604/pamj.supp.2017.27.3.12127
34. Yawson, AE, Bonsu, G, Senaya, LK, Yawson, AO, Eleeza, JB, Awoonor-Williams, JK, et al. Regional disparities in immunization services in Ghana through a bottleneck analysis approach: implications for sustaining national gains in immunization. Arch Public Health. (2017) 75:10–08. doi: 10.1186/s13690-017-0179-7
35. Amponsah-Dacosta, E, Kagina, BM, and Olivier, J. Health systems constraints and facilitators of human papillomavirus immunization programmes in sub-Saharan Africa: a systematic review. Health Policy Plan. (2020) 35:701–17. doi: 10.1093/heapol/czaa017
36. Elrod, JK, and Fortenberry, JL. The hub-and-spoke organization design: an avenue for serving patients well. BMC Health Serv Res. (2017) 17:457. doi: 10.1186/s12913-017-2341-x
37. Devarakonda, S . Hub and spoke model: making rural healthcare in India affordable, available and accessible. Rural Remote Health. (2016) 16:1–8. doi: 10.22605/RRH3476
38. America I of M (US) C on Q of HC in . Redesigning health care with insights from the science of complex adaptive systems In: Crossing the quality chasm: A new health system for the 21st century. Ed. Geert Crombez - Ghent US: National Academies Press (2001).
39. Gomersall, T . Complex adaptive systems: a new approach for understanding health practices. Health Psychol Rev. (2018) 12:405–18. doi: 10.1080/17437199.2018.1488603
40. Peters, DH . The application of systems thinking in health: why use systems thinking? Health Res Policy Sys. (2014) 12:1–6. doi: 10.1186/1478-4505-12-51
41. Roxas, FMY, Rivera, JPR, and Gutierrez, ELM. Locating potential leverage points in a systems thinking causal loop diagram toward policy intervention. World Futures. (2019) 75:609–31. doi: 10.1080/02604027.2019.1654784
42. Tomoaia-Cotisel, A, Hyunjung, K, Allen, S, and Blanchet, K. Causal loop diagrams: a tool for visualizing emergent system behaviour In: Applied systems thinking for health systems research: A methodological handbook, Eds. Don de Savigny, Karl Blanchet, Taghreed Adam London: Open University Press. (2017). 97–114.
43. Landes, SJ, McBain, SA, and Curran, GM. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res. (2019) 280:112513. doi: 10.1016/j.psychres.2019.112513
Keywords: zero-dose children, patent medicine vendors, drug shops, hub and spoke, systems thinking, Africa, big catch-up, routine immunization
Citation: Adamu AA, Jalo RI, Ndwandwe D and Wiysonge CS (2024) Informal health sector and routine immunization: making the case for harnessing the potentials of patent medicine vendors for the big catch-up to reduce zero-dose children in sub-Saharan Africa. Front. Public Health. 12:1353902. doi: 10.3389/fpubh.2024.1353902
Edited by:
Marcelle Silva-Sales, Universidade Federal de Goiás, BrazilReviewed by:
Muhammad Zaheer Abbas, Queensland University of Technology, AustraliaCopyright © 2024 Adamu, Jalo, Ndwandwe and Wiysonge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdu A. Adamu, YWJkdS5hZGFtdUBnbWFpbC5jb20=
 Abdu A. Adamu
Abdu A. Adamu Rabiu I. Jalo3
Rabiu I. Jalo3 Duduzile Ndwandwe
Duduzile Ndwandwe