- 1School of Public Health, The Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, Guiyang, China
- 2Xishui County Center for Disease Control and Prevention, Xishui, Guizhou, China
- 3Key Laboratory of Karst Georesources and Environment, Ministry of Education, Guizhou University, Guiyang, China
- 4Guangdong Ecological and Environmental Monitoring Center, Guangzhou, China
Objective: Mercury (Hg) contamination in the environment around mercury mines is often accompanied by heavy metal contamination.
Methods: Here, we determined concentrations of chromium (Cr), zinc (Zn), strontium (Sr), barium (Ba), and lead (Pb) in duck eggs from a Hg mining area in Southwest China to assess the contamination and health risk.
Results: Duck eggs obtained from the mining area exhibit higher concentrations of Cr, Zn, Sr, Ba, and Pb compared to those from the background area, with egg yolks containing higher metal levels than egg whites. Specifically, the mean Cr, Zn, Sr, Ba, and Pb concentrations of duck eggs from the Hg mining area are 0.38, 63.06, 4.86, 10.08, and 0.05 μg/g, respectively, while those from the background area are only 0.21, 24.65, 1.43, 1.05, and 0.01 μg/g. Based on the single-factor contamination index and health risk assessment, heavy metal contamination in duck eggs poses an ecological risk and health risk.
Conclusion: This study provides important insight into heavy metal contamination in duck eggs from Hg mining areas.
1 Introduction
Heavy metal pollution can pose serious harm to wildlife and human beings. Mercury (Hg), a globally transported heavy metal with strong bioaccumulation, is found in excessive levels in the atmosphere, water, soil, vegetables, and rice in Hg mining areas (1). Hg pollution is often accompanied by other heavy metal contaminations in Hg mining areas (2). Soil, water and crops in Hg mining areas are contaminated with heavy metals to varying degrees (3–5). For instance, cadmium (Cd) and arsenic (As) levels in the soil exceed the standard limits in Wuchuan mining area, SW China (2). A previous study revealed high heavy metal (including Hg, As, Cd and Se) levels in eight types of vegetables in mining regions (6). Excessive ingestion of heavy metals can be toxic (7, 8). Cr has mutagenic, teratogenic, and carcinogenic properties (9). Pb and Hg are known to have neurotoxic effects, particularly harmful to the neurological development of children (10). Excessive amounts of Zn, Sr, and Ba have been found to induce genotoxic effects in cells (11). Given the potential harm heavy metals may pose to humans, heavy metal pollution in mining areas cannot be overlooked (6).
Consumption of poultry products could be an important exposure of heavy metals to humans. Poultry normally uptake heavy metals from various sources (e.g., feed, water), among which feed is the main source, and the females can transfer heavy metals to their eggs (12). Normally, farmed poultry eat a fixed recipe of feed, while free-range poultry mainly ingest local crops (12). However, most studies on health risk assessments of heavy metals focus on commercial (13), selenium-enriched (14), and free-range chicken eggs (15), with limited research on duck eggs. It is worth noting that China is the largest producer and consumer of duck eggs in the world, with an output of ~4 million tons annually (16, 17). A meta-analysis indicates that duck eggs contain higher levels of potentially toxic elements compared to chicken eggs (18). Due to the high Hg level in poultry eggs from Wuchuan compared to other areas, and the total Hg concentration in duck eggs exceeds that of chicken eggs (19), we hypothesize that concentrations of other heavy metals could be high in local poultry eggs (e.g., duck eggs). However, heavy metal (such as Zn, Cr, Sr, Ba and Pb) levels in local duck eggs and their potential harm to consumers are unclear so far. Therefore, it is crucial to assess the potential health risks associated with the consumption of duck eggs contaminated with heavy metals.
Here, we conducted the research on heavy metal concentrations in duck eggs from Wuchuan (a Hg mining area), to understand the heavy metal levels in duck eggs and their potential health risks in Hg mining area. This study could provide insights into the current of heavy metal levels in Wuchuan duck eggs and to assess the potential human health risks from consuming such duck eggs from the Hg mining area.
2 Materials and methods
2.1 Study area
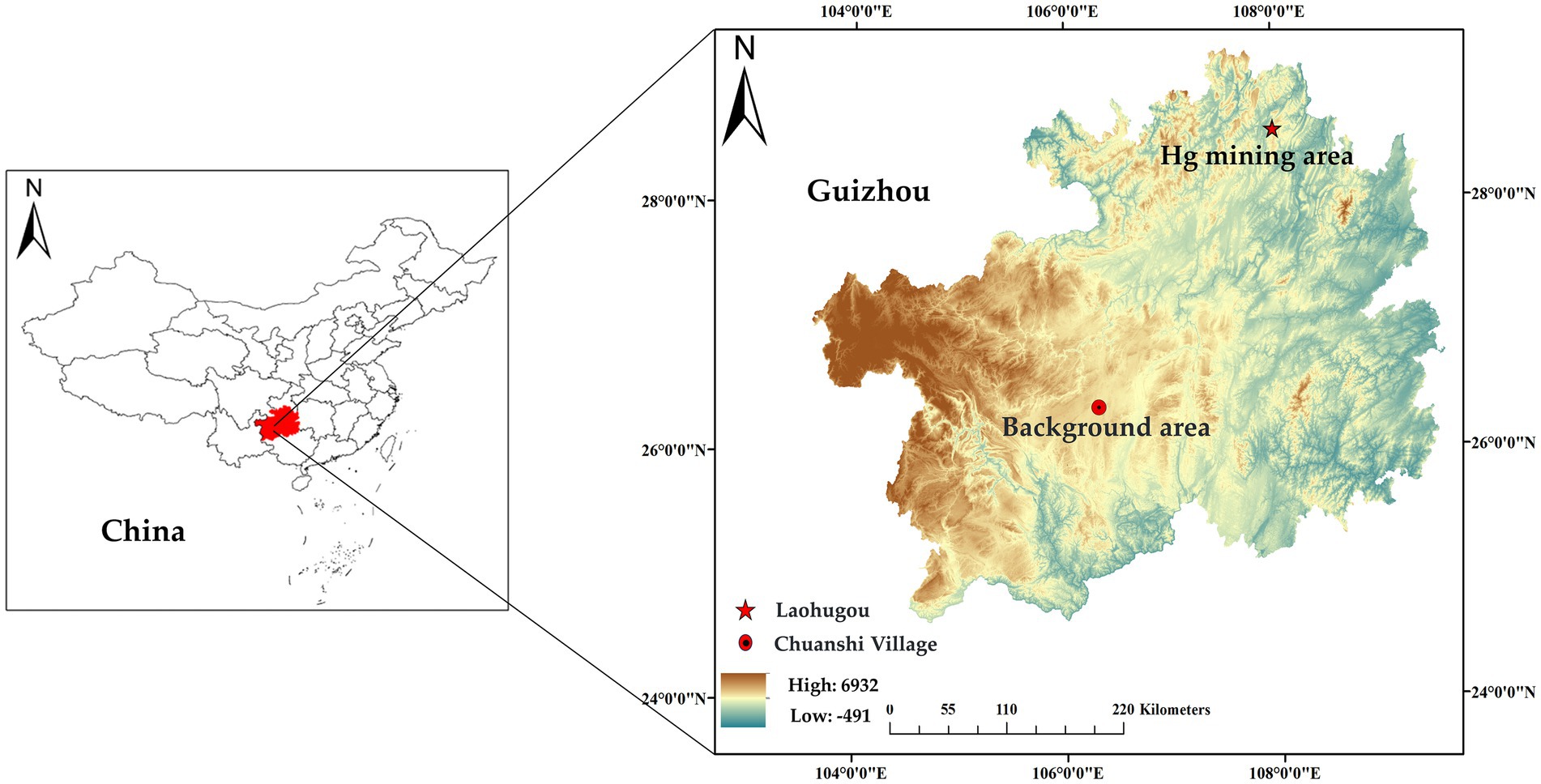
Wuchuan is located in Guizhou Province, southwestern China (Figure 1). Wuchuan Hg mine is one of the largest Hg mines in Guizhou Province, with a Hg production history of about 400 years (20, 21). Despite the cessation of mining for 20 years, local environmental Hg levels (in soil, water, and rice) are still evidently higher than the standard limitation (6). The concentrations of Hg are 1.3 ~ 360 mg/kg in local topsoil variation, 13 ~ 2,100 ng/L in water, and 6.0 ~ 113 in rice ng/g (22, 23). Different from Wuchuan, Hg and other heavy metals are within safe thresholds in Anshun (24, 25). In Anshun, Hg concentration in rice and other crops is about 0.01 mg/kg, significantly below the limit set by the “National Food Safety Standard for Contaminants in Foods” (24). Additionally, the level of heavy metal contamination in the soil is low (25).
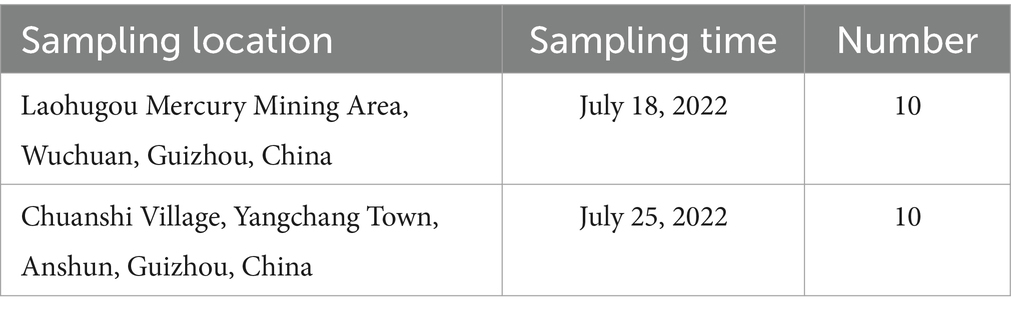
We collected ten duck eggs each from Laohugou, Wuchuan County, Zunyi (28°60′ N, 108°01′ E) and Chuanshi Village, Yangchang Town, Anshun (26°35′ N, E 106°32′ E). As the ducks raised by surrounding residents in the mining area have similar feeding methods and feed, random sampling of purchased duck eggs was conducted. The eggs were from ducks raised by local households with free-range. Sampling locations, quantities, and times were shown in Table 1 (26). The collected duck eggs were brought to the laboratory within 24 h and stored at 4°C.
2.2 Analysis method
2.2.1 Materials and reagents
Process ultrapure grade HNO3, 30% H2O2 (superior purity), Milli-Q water (18.2 MΩ.cm, Millipore), ICP-MS (Thermos Scientific iCAP RQ).
2.2.2 Pretreatment of samples
The duck eggs were washed with 18.2 MΩ water, followed by separation of egg yolk and egg white, and then freeze-dried and mixed. Each sample (0.500 g) was digested with 5 mL of nitric acid (process ultrapure) and 1 mL of hydrogen peroxide at 160°C for 8 h. After cooling to room temperature, the inner chamber of the digestion tank was removed and the inner lid was rinsed with a small amount of ultrapure water, and inner chamber placed on a hot plate at 90°C, 2% nitric acid fixed volume to 10 mL and stored at 4°C before measurement.
2.2.3 Determination of metal concentrations and quality control
The heavy metals Cr, Zn, Sr, Ba, and Pb were determined by ICP-MS (Thermos Scientific iCAP RQ) at Guizhou University. Quality control was performed by standard reference materials (VAR-CAL-2 for trace elements; CLMS-1 for rare earth elements). The relative standard deviations of the metals were all below 10%, and the recoveries were 80% ~ 110%.
2.3 Evaluation of heavy metal pollution of duck eggs
According to the single factor pollution index, heavy metal contamination in duck eggs is evaluated as following:
Where Pi is the single-factor pollution index, Ci is the concentration of metals in duck eggs (μg/g, DW), and Si denotes the evaluation standard value of the five heavy metals in duck eggs (μg/g). Pb is the standard value according to the Chinese national standard for food safety (GB 2762–2022). Given the lack of standard limits for metals in duck eggs, the corresponding metal levels in the background area are used as the standard limit for other metals in this study. As shown in Supplementary Table S1 (27), are the grading standards.
2.4 Health risk assessment
The US Environmental Protection Agency (USEPA) health risk assessment model was used to assess non-carcinogenic and carcinogenic risks based on exposure parameters in the Chinese population (28). The noncarcinogenic risks for Cr, Zn, Sr, Ba, and Pb and the carcinogenic risks for Cr and Pb are calculated according to the International Agency for Research on Cancer (the International Agency for Cancer, 2020) carcinogen classification.
Heavy metal chronic daily intake can be calculated as following:
Where EDI is the estimated daily intake of heavy metals (mg/kg/day), is the concentration of metals in duck eggs (μg/g), IR is the dietary intake (kg/d), ED is the exposure time (a), EF is the exposure frequency (d/a), AT is the average exposure time (d), and BW is the body weight (kg). IR is 0.15 kg/d and 0.10 kg/d for adults and children, respectively; ED is 30 years and 10 years, respectively; EF are 365 d/a and 365 d/a, respectively; AT is 10950 d and 3,650 d, respectively; and BW are 70 kg and 16 kg, respectively (29–33).
The noncarcinogenic risk of consuming contaminated duck eggs is calculated and assessed by the health risk quotient (HQ) and the health risk index (HI, the sum of the HQ values of different metals, used to calculate the noncarcinogenic risk caused by multiple heavy metals). Heavy metal HQ and HI can be calculated as follows:
Where HQ is the one-factor noncarcinogenic risk index, EDI is the chronic daily intake of heavy metals (mg/kg/day), RfD is the reference consumption of heavy metals (0.003, 0.300, 0.600, 0.200, and 0.0035 mg/kg/day for Cr, Zn, Sr, Ba, and Pb, respectively), and HI is the total noncarcinogenic risk index for the five elements. HQ or HI > 1 indicates a potential non-carcinogenic risk, while HQ or HI < 1 indicates no potential risk (34–36).
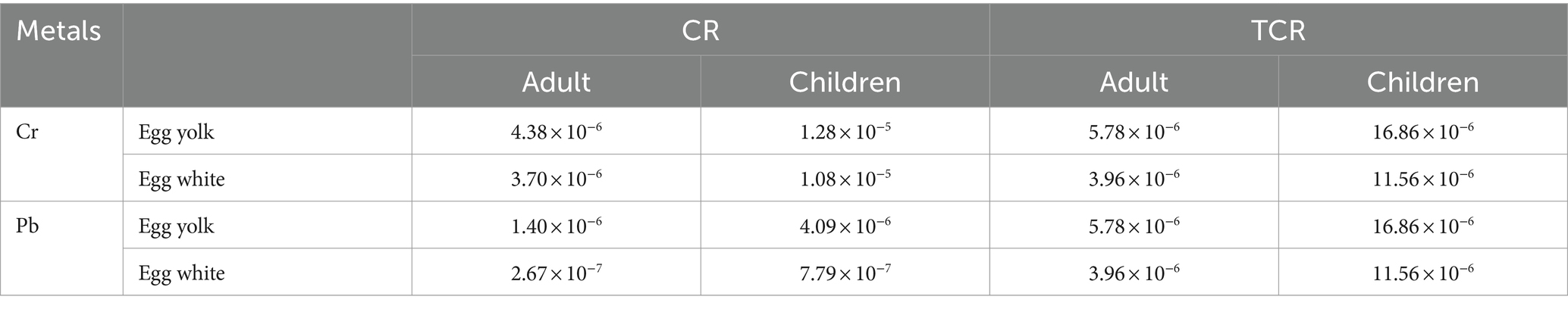
The total carcinogenic risk (TCR) is the sum of the carcinogenic risk (CR) values of different metals. Heavy metal CR and TCR can be calculated as follows:
Where CR is the heavy metal carcinogenic risk index, EDI is the chronic daily intake of heavy metals (mg/kg/day), SF is the slope factor of carcinogenic heavy metals (0.005 and 0.0085 for Cr and Pb, respectively), and TCR is the total heavy metal carcinogenic risk index. When CR or TCR ≤ 1 × 10−6, the carcinogenic risk is considered negligible, while CR or TCR < 1 × 10−4 indicates a low risk and is considered acceptable, and CR or TCR ≥ 1 × 10−4 indicates a potential carcinogenic risk (37, 38).
2.5 Data analysis
ArcGIS is used to plot the distribution of sampling points, and SPSS 26.0 is used to analyze the data. In this study, we applied the Wilcoxon rank sum test uniformly to analyze the significant differences in metal concentrations and pollution index. Moreover, the mean, median, minimum, and maximum values of metal concentration and pollution index were shown.
3 Results
3.1 Metal pollutions
The mean Cr, Zn, Sr, Ba, and Pb concentrations of duck eggs from the Hg mining area are 0.38, 63.06, 4.86, 10.08, and 0.05 μg/g, respectively, whereas those from the background area are 0.21, 24.65, 1.43, 1.05, and 0.01 μg/g, respectively. The concentrations of Cr, Zn, Sr, and Pb in duck eggs from the Hg mining area are significantly higher than those from the background area (p < 0.05; Figure 2A). Specifically, the mean concentrations of Cr, Zn, Sr, Ba, and Pb in egg yolk are 0.41, 79.90, 6.44, 17.98, and 0.08 μg/g, respectively, while the mean concentrations of Cr, Zn, Sr, Ba, and Pb in egg white are 0.35, 47.73, 7.62, 2.18, and 0.02 μg/g, respectively (Figure 2B; Table 2). In the Hg mining area, the mean concentrations of Sr, Ba, and Pb in the yolk are much higher than those in egg white (p < 0.05). In the background area, the mean concentrations of Cr, Zn, Sr, Ba, and Pb in egg yolk are 0.24, 48.94, 1.51, 2.01, and 0.01 μg/g, respectively. The mean concentrations of Cr, Zn, Sr, Ba, and Pb in egg white are 0.36, 3.75, 1.35, 0.10, and 0.01 μg/g, respectively. The mean concentration of Zn in egg yolk is also much higher than that in the duck egg whites from the background area (p < 0.05) (Figure 2C; Table 2). Overall, the concentrations of Cr, Zn, Sr, Ba, and Pb in duck eggs in the Hg mining area are higher than those in the background area, and the metal concentrations in yolks are higher than those in egg whites.
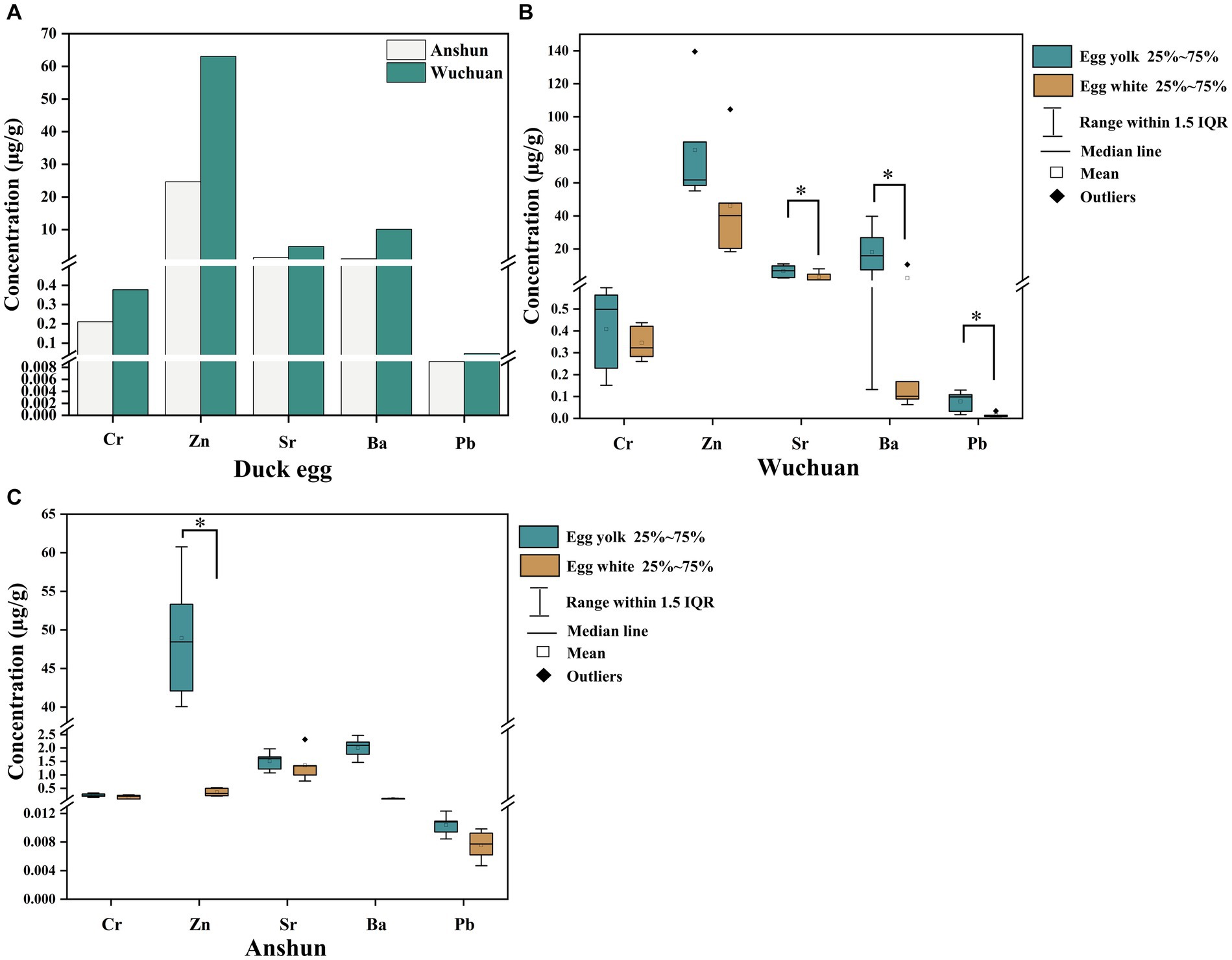
Figure 2. Heavy metal concentrations in duck egg yolk and egg white from the background area. (A) Cr, Zn, Sr, Ba, Pb concentrations in duck eggs from Wuchuan (Hg mining area) and Anshun (background area); (B) Cr, Zn, Sr, Ba, Pb concentrations in duck eggs from Wuchuan; (C) Cr, Zn, Sr, Ba, Pb concentrations in duck eggs from Anshun. “*” represents significantly different (p < 0.05).
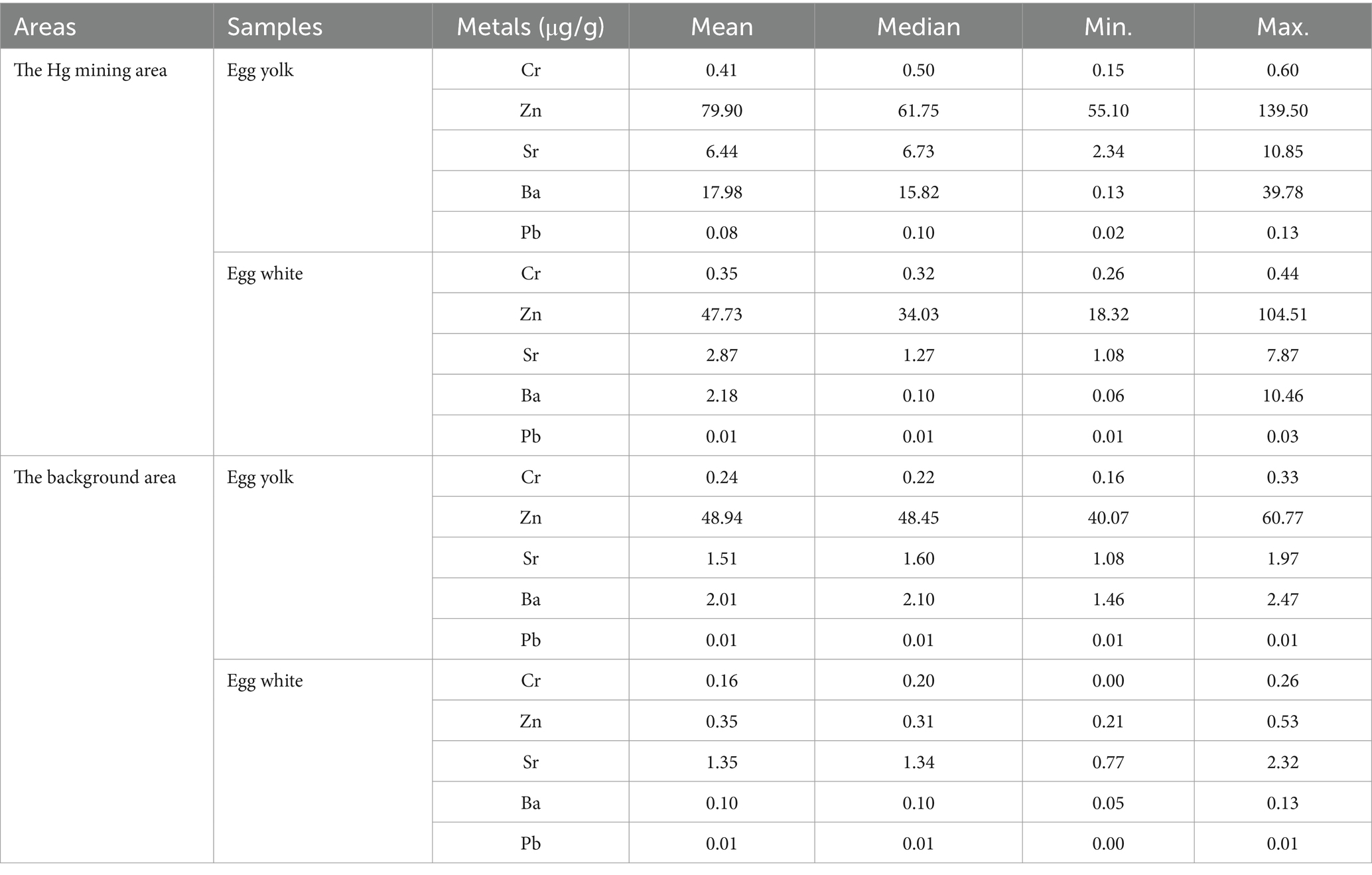
Table 2. The concentrations of Cr, Zn, Sr, Ba, and Pb in duck egg yolk and egg white at the Hg mining area and the background area.
3.2 The duck eggs of single factor pollution index of heavy metals
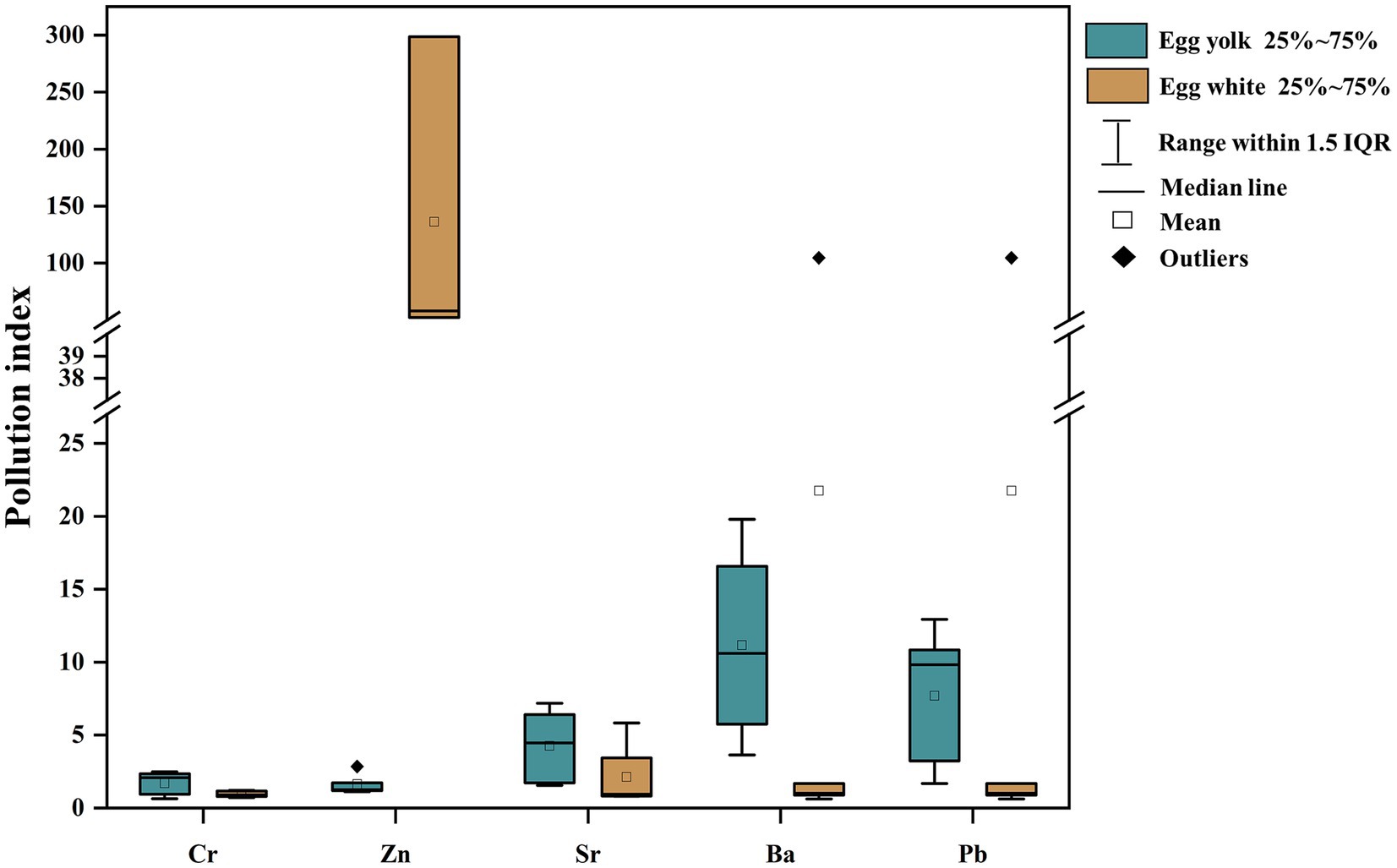
The single factor pollution index is an indicator to evaluate a single factor in heavy metal pollution, which has already been used in evaluations of heavy metal pollution in various environmental media and materials, including water, soil, crops, etc. Given the lack of standards for the five metals in this study, we take Anshun as the background area and assess the heavy metal pollution in duck eggs in the mercury mining area based on the related calculation (Figure 3). The single factor pollution index in egg yolk declines in the following order: Pb (9.83) > Ba (7.87) > Sr (4.46) > Cr (2.07) > Zn (1.26), and in egg white is characterized as Zn (58.12) > Pb (1.31) > Ba (1.01) > Cr (0.90) > Sr (0.83) (Table 3). Except for the Cr and Sr. single factor contamination index in egg white which is less than 1, other heavy metals are all >1 in egg yolk and egg white, indicating that duck eggs are contaminated with heavy metals at different levels.
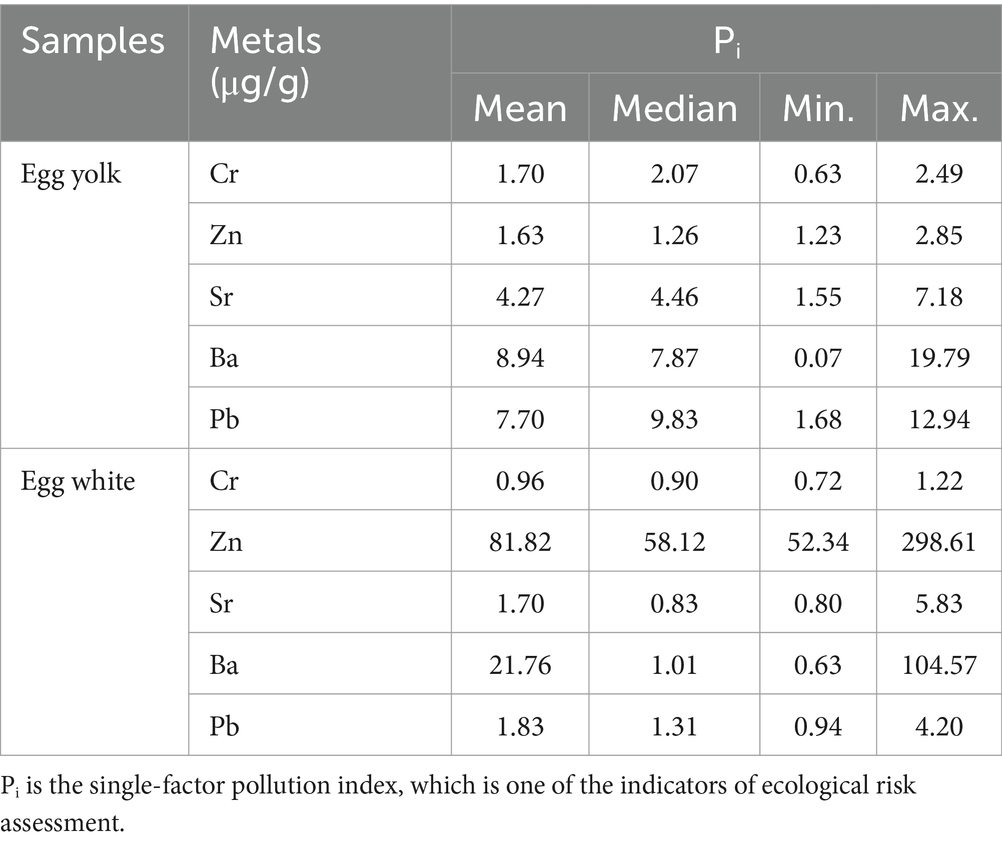
Table 3. The single factor pollution index of Cr, Zn, Sr, Ba, and Pb in duck egg yolk and egg white at the Hg mining area.
3.3 Health risk assessment of heavy metals
3.3.1 Noncarcinogenic risk assessment of heavy metals in duck eggs
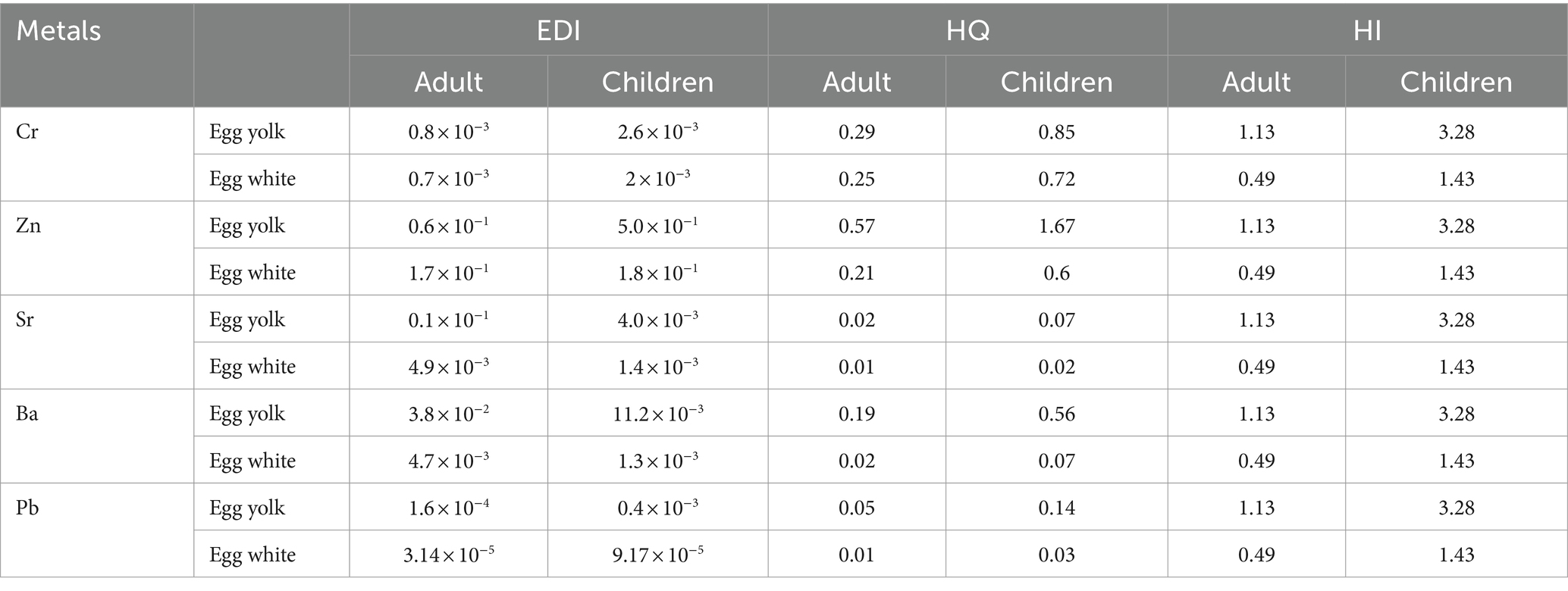
Carcinogenic and noncarcinogenic health risk estimates are widely recognized as important parameters for human health risk assessment. Table 4 shows the heavy metal intake and noncarcinogenic risk in duck eggs from the Hg mining area. The mean daily intake (EDI) of Cr, Zn, Sr, and Pb in egg yolk for adults and the Cr, Zn, and Pb in egg white are less than the reference exposure dose (RfD), while EDI for Ba in egg yolk and Sr, Ba, and Pb in egg white are greater than RfD. Children EDI is less than the RfD for Cr and Pb in egg yolk and Cr, Zn, Sr, Ba, and Pb in egg white, while EDI for Zn, Sr, and Ba in egg yolk and Pb in egg white are greater than RfD. Furthermore, the total health risk index of egg yolk consumption is >1 for adults, while the index of both egg yolk and egg white are >1 for children, higher than that for adults.
3.3.2 Carcinogenic risk assessment of heavy metals in duck eggs
Due to the lack of carcinogenic slope factors for Zn, Sr, and Ba, the carcinogenic risk assessment is only conducted for Cr and Pb. When the carcinogenic risk is greater than 1.00 × 10−6, it indicates a certain carcinogenic risk (39, 40). In this study, we observed that the carcinogenic risk of Cr and Pb in both yolk and white of duck eggs is >1.00 × 10−6. Cr has a greater carcinogenic risk than Pb (Table 5). It is noteworthy that the total carcinogenic risk of duck egg intake in children is greater than adults, and that of egg yolk is greater than egg white for both adults and children.
4 Discussion
4.1 Analysis of heavy metal pollution
In this study, preliminary results indicate that duck eggs in Hg mining areas are contaminated with Cr, Zn, Sr, Ba, and Pb. The higher metal concentration of duck eggs in the Hg mining area than in the background area is related to the high metal levels in the mining area environment. The tailings left after the cessation of mining impact the local soil and water, and the soil in the mining area is contaminated to varying degrees with Cd, Pb, Zn, Cr and As (7, 41, 42). Cr and As exceed the standard in the water of the mining area (8, 43). Heavy metal pollution causes elevated concentrations of heavy metals in crops (44). As and Ni levels in vegetables are higher than normal values, and the estimated mean daily intakes of As and Pb in vegetables are above the permissible limits (45). In addition, corn kernels of Zn, Pb, Cd, Cr, and Ni exceeded the limits of China’s food hygiene standards (30). Thus, ducks live in the mining area with prolonged metal exposure inducing contaminated duck eggs. Particularly, differences in metal concentrations in duck egg yolks and whites are observed. Differences in the metal levels in duck egg yolks and whites might be related to their formation mechanisms. Once the female ducks absorb higher heavy metal levels they subsequently transfer to the embryo. Duck eggs are formed in the reproductive system of the duck and minerals are deposited into the eggs by two pathways, including ovary to yolk and oviduct to egg white (46, 47). The yolk precursor molecule of egg yolk protein could transfer minerals to the yolk, and the yolk component is synthesized in the liver, which is the main organ enrich heavy metals in the body (39, 40, 48). Therefore, most heavy metal levels are higher in egg yolks than in egg whites.
We also measured Cr, Zn, Sr, Ba, and Pb concentrations in chicken eggs from the Wuchuan Hg mine, and the concentrations of Cr, Zn, Sr, Ba, and Pb in chicken eggs from the mine are not statistically different from those of the background area (p > 0.05; Figure 4A). Meanwhile, Cr, Zn, Sr, Ba, and Pb concentrations in duck eggs from the mining area are slightly higher than those in local chicken eggs (Figure 4B). Ducks belong to the waterfowl category of poultry, which eat not only crops, grasses earthworms but also fish and shrimps (49). Therefore, ducks are exposed to heavy metals from multiple sources with a possible higher heavy metal level compared to chickens and once ingested, heavy metals are enriched in the embryo then transferred to duck eggs, which may explain the higher heavy metal levels in duck eggs compared to chicken eggs (41, 49). Concentrations of Cr, Zn, Sr, Ba, and Pb in chicken eggs from the mine region do not differ from those in the background, indicating that chicken eggs are less contaminated than duck eggs in Hg mining region. However, the concentrations of Cr, Zn, Sr, Ba, and Pb in duck eggs in Hg mining area are higher than those in background area, suggesting that we should be more concerned about the potential risk of heavy metal contamination in duck eggs.
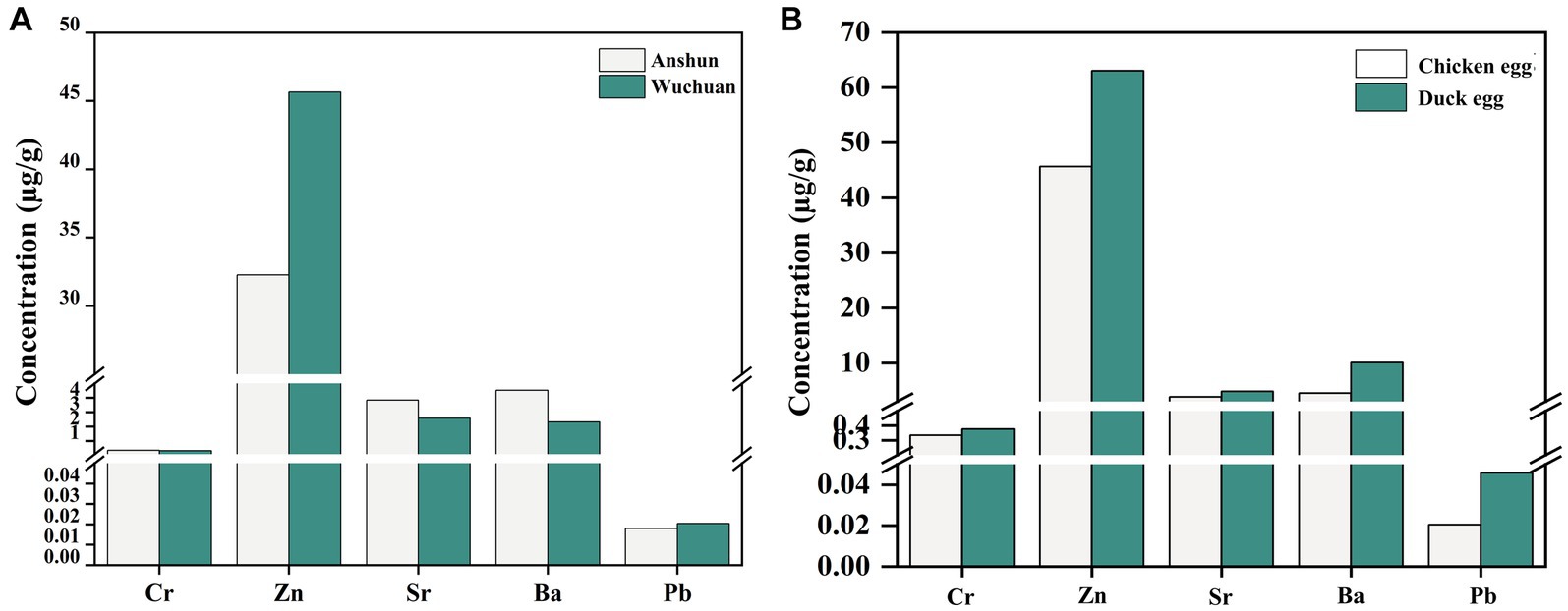
Figure 4. Heavy metal concentrations in duck eggs from mercury mining areas versus duck eggs from background areas (A), and metal concentrations in eggs from mercury mining areas and duck eggs (B).
In addition, the sampling was performed in July, the rainy season in Guizhou. The heavy metals exposure to ducks are different between the dry and rainy seasons (50). Heavy metals accumulate in the environment during the dry season as a result of evaporation. Conversely, during the rainy season, the heavy metal exposure could be decreased due to dilution effects (51). Thus, high heavy metal levels in duck eggs during the rainy season in this study suggest even higher levels during the dry season. Notably, there is an ecological risk of heavy metal exposure in duck eggs. The duck eggs have been heavily contaminated with Ba, Pb, and Zn, with extremely strong potential ecological risk. Consistent with previous results on single factor pollution indices for crops in mining areas, the rice collected in the vicinity of the mining area is more severely contaminated by As, Sb, Cd, Cu, and Zn (52). Potatoes are heavily contaminated by heavy metals while cabbage and radish are lightly polluted (53, 54). The single factor of Pb, Cd, Cr, and Ni in maize seeds are all greater than 1, suggesting that all heavy metal contamination in the edible part of the crops has reached heavy contamination levels (52, 54). This result illuminates that mining area duck eggs, like crops, are ecologically risky.
4.2 Health risk of heavy metals to local residents
The noncarcinogenic risk results suggest that non-age-specific, the total health risk index of egg yolk intake is >1. The contribution of the five heavy metals to the noncarcinogenic risk is illustrated in Figure 5A, Cr and Zn are the main noncarcinogenic risk metals for the inhabitants in the area, more significantly in children. The results indicate that noncarcinogenic health risks are associated with the consumption of duck eggs by both adults and children, and higher in children than in adults. Therefore, the noncarcinogenic risk of consuming duck eggs from Hg mining areas should not be ignored. Our results are consistent with crops in Hg mining areas, which indicate a health risk (55). Cr and Ni health risks are highest in maize from mining areas, and children are most sensitive to maize heavy metal exposure (55). The higher health risk of duck egg consumption in children than in adults suggests that children are more sensitive to environmental pollutions. Liver is the main organ that enriches and metabolism heavy metals (43, 53). However, children’s metabolic organs, such as the liver and kidney, are not yet well developed and have weaker detoxification functions for toxic and harmful substances (56). Whereas the health risks of egg yolks are greater than egg whites may be since the yolk protein precursor molecule in egg yolk can transfer minerals to the yolk, and the yolk component is synthesized in the liver (57). Therefore, concerns should be raised about the potential noncarcinogenic health risks to children from the consumption of mining area duck eggs, especially the yolks.
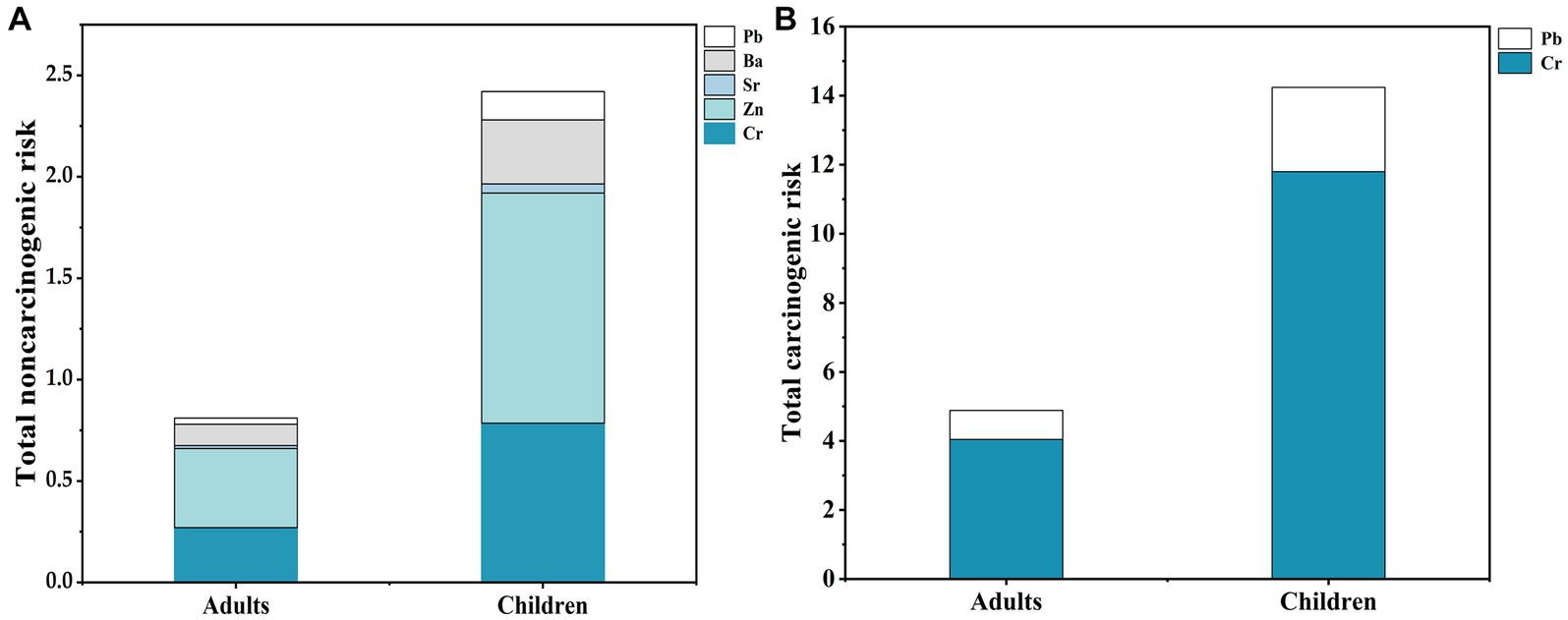
Figure 5. Total noncarcinogenic risk and total carcinogenic risk of heavy metals in adults and children. (A) Total noncarcinogenic risk of heavy metals in adults and children; (B) Total carcinogenic risk of heavy metals in adults and children.
We find that the TCRs of Cr and Pb in duck eggs are greater than 1.00 × 10−6 when consumed by adults and children, indicating both have a certain carcinogenic risk from the intake of egg yolk and white. CR combined with TCR shows that Cr is the main contributing factor, indicating that Cr is the most significant carcinogenic risk metal in Hg mining area (Figure 5B). Long-term consumption of brown rice poses potential noncarcinogenic and carcinogenic health risks to the local population (43, 58). The same goes for long-term consumption of duck eggs. Although the Hg mining area is dominated by Hg pollution, the carcinogenic risk of Cr in local duck eggs should not be ignored. To sum up, duck eggs from Hg mining areas are contaminated with heavy metals and may pose a potential health risk to local residents who consume them.
Previous studies have reported high levels of Hg in the hair, blood, and urine of people living near the Wuchuan Hg mines (59, 60). It is suggested to be related to Hg pollution in this region. Except for Hg, high levels of other heavy metals have been observed in the mining areas, such as soil and vegetables (41, 44). According to our results in this study, high heavy metal concentrations in duck eggs indicate high levels of heavy metals in the environment and crops and further illustrate that local residents could possibly be exposed to high levels of heavy metals via poultry products (e.g., eggs) and environmental materials. Thus, the risk of heavy metal pollution posing to the residents is non-negligible.
4.3 Analysis of heavy metal concentrations in free-range and caged eggs
Eggs as the paramount source of protein consumption for humans, which could be roughly categorized into free-range eggs and caged eggs (61). Investigations have revealed a gradual increase in the overall consumption of eggs, with a growing preference for free-range eggs among consumers (62). During the same period, sales of free-range eggs in the Australian egg industry surge by 21.7%, while caged eggs show a decline of 12.5% (61). Similar preferences for free-range eggs have also been observed among consumers in various countries, including Canada and China, who perceive them to possess higher nutritional value and safety (10, 63). Based on comprehensive avian egg research (Figure 6), we categorized poultry eggs (duck egg and chicken egg) into caged eggs, background free-range eggs, and mining area free-range eggs. Interestingly, for duck eggs and chicken eggs, the concentrations of Cr, Zn, Sr, Ba, and Pb in background free-range eggs are found to be lower than those in caged eggs, which also elucidates why consumers favor free-range eggs. However, for free-range eggs from mining areas, the concentrations of Cr, Zn, Sr, Ba, and Pb are notably higher than those in caged eggs and background free-range eggs. In addition, consistent with previous studies, the heavy metal concentration in mining area of duck eggs is higher than chicken eggs (64, 65).
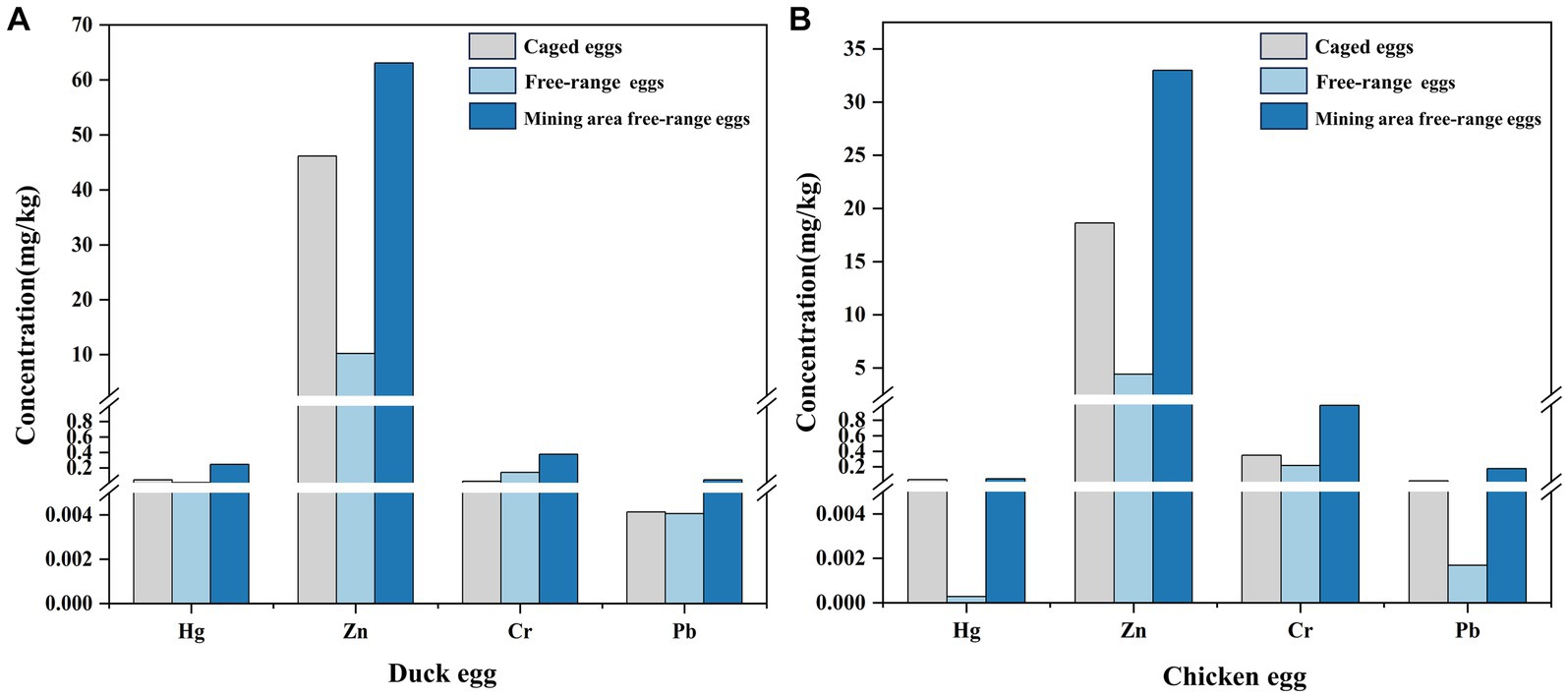
Figure 6. The concentrations of Hg, Zn, Cr, and Pb in free-range eggs and caged eggs (16, 17, 64–77). (A) Duck egg; (B) Chicken egg.
Disparities in heavy metal concentration among distinct egg types could be attributed to poultry rearing practices. Caged poultry are commonly provided with formulated feed, restricting their environmental exposure (78). On the other hand, free-range poultry predominantly feed on substances present in their surroundings, including insects and grains (63). Free-range poultry in mining areas feed on substances from their surrounding environment. It is widely recognized that mining areas face severe heavy metal pollution, with long half-lives and prolonged presence in the environment. Through the food chain, free-range poultry in mining areas are exposed to environmental heavy metal contamination, accumulating in their eggs. Therefore, the ingestion of mining area free-range eggs can pose a potential threat to human health. When choosing free range eggs, consumers should identify the producing areas.
5 Conclusion
In this study, we measured concentrations of five metals (Cr, Zn, Sr, Ba, and Pb) in duck eggs and chicken eggs from the Hg mining area and the background area, and found that duck eggs from the Hg mining area contained higher concentrations than those from the background area. Duck egg yolks contain higher concentrations than whites, which is related to the presence of yolk precursor proteins in the liver which is the main organ enrich heavy metals in the body. There is no difference in those metal concentrations between chicken eggs from Hg mining areas and background areas, which indicates that duck eggs are more susceptible to heavy metal contamination than chicken eggs. Duck eggs are contaminated by heavy metals to varying degrees, especially for Ba, Pb, and Zn, which have an extremely strong potential ecological risk. In view of different types of eggs from different areas, the concentration in free-range duck eggs and chicken eggs from mining areas are higher than that in farm and free-range duck eggs and chicken eggs. Therefore, when choosing free-range duck eggs as daily food, attention should be paid to identifying the producing regions, with a knowledge about the health risks of duck eggs from heavy metal contaminated areas, such as mining regions. Nevertheless, this is a preliminary study with limited number of duck egg samples. Further studies with increasing numbers of eggs and environmental (soil, water) and crop samples need to be performed to gain a better understanding of the sources of heavy metal pollution in duck eggs from Hg mining areas.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ZhuW: Conceptualization, Formal analysis, Investigation, Supervision, Writing – review & editing. XL: Data curation, Investigation, Visualization, Writing – review & editing. JL: Investigation, Writing – review & editing. XZ: Methodology, Visualization, Writing – review & editing. YR: Investigation, Writing – review & editing. QW: Formal analysis, Methodology, Software, Writing – review & editing. TZ: Software, Visualization, Writing – review & editing. ZhoW: Methodology, Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Chinese National Natural Science Foundation (no. 42273008).
Acknowledgments
We would like to thank all the authors and researchers who participated in this study. The useful and constructive comments from the editors and reviewers are also sincerely acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1352043/full#supplementary-material
Abbreviations
Hg, Mercury; Cr, Chromium; Zn, Zinc; Sr, Strontium; Ba, Barium; Pb, Lead.
References
1. Li, P, Feng, XB, Qiu, GL, Shang, LH, and Li, ZG. Mercury pollution in Asia: a review of the contaminated sites. J Hazard Mater. (2009) 168:591–601. doi: 10.1016/j.jhazmat.2009.03.031
2. Feng, XB, and Qiu, GL. Mercury pollution in Guizhou, southwestern China—an overview. Sci Total Environ. (2008) 400:227–37. doi: 10.1016/j.scitotenv.2008.05.040
3. Li, P, Feng, XB, Qiu, GL, Shang, LH, and Wang, SF. Mercury pollution in Wuchuan mercury mining area, Guizhou, southwestern China: the impacts from large scale and artisanal mercury mining. Environ Int. (2012) 42:59–66. doi: 10.1016/j.envint.2011.04.008
4. Li, P, Feng, XB, Qiu, GL, Li, ZG, Fu, XW, Sakamoto, M, et al. Mercury exposures and symptoms in smelting workers of artisanal mercury mines in Wuchuan, Guizhou, China. Environ Res. (2008) 107:108–14. doi: 10.1016/j.envres.2007.08.003
5. Huang, X, Wu, X, Tang, X, Zhang, Z, Ma, J, Zhang, J, et al. Distribution characteristics and risk of heavy metals and microbial community composition around the Wanshan mercury mine in Southwest China. Ecotox Environ Safe. (2021) 227:112897. doi: 10.1016/j.ecoenv.2021.112897
6. Wang, X, Li, YF, Li, B, Dong, Z, Qu, L, Gao, Y, et al. Multielemental contents of foodstuffs from the Wanshan (China) mercury mining area and the potential health risks. Appl Geochem. (2011) 26:182–7. doi: 10.1016/j.apgeochem.2010.11.017
7. Li, Z, Ma, Z, van der Kuijp, TJ, Yuan, Z, and Huang, L. A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ. (2014) 468-469:843–53. doi: 10.1016/j.scitotenv.2013.08.090
8. Khan, R, Israili, SH, Ahmad, H, and Mohan, A. Heavy metal pollution assessment in surface water bodies and its suitability for irrigation around the Neyevli lignite mines and associated industrial complex, Tamil Nadu, India. Mine Water Environ. (2005) 24:155–61. doi: 10.1007/s10230-005-0087-x
9. Zhang, H, Feng, X, Larssen, T, Qiu, G, and Vogt, RD. In inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ Health Persp. (2010) 118:1183–8. doi: 10.1289/ehp.1001915
10. Tchounwou, PB, Yedjou, CG, Patlolla, AK, and Sutton, D. Heavy metal toxicity and the environment In: A Luch, editor. Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology : Springer Science & Business Media (2012). 133–64.
11. Tassew Belete, AH, and Rao, VM. Determination of concentrations of selected heavy metals in cow’s milk: Borena zone, Ethiopia. J Health Sci. (2014) 4:105–12. doi: 10.5923/j.health.20140405.01
12. Tsipoura, N, Burger, J, Newhouse, M, Jeitner, C, Gochfeld, F, and Mizrahi, D. Lead, mercury, cadmium, chromium, and arsenic levels in eggs, feathers, and tissues of Canada geese of the New Jersey meadowlands. Environ Res. (2011) 111:775–84. doi: 10.1016/j.envres.2011.05.013
13. Kim, S, Arora, M, Fernandez, C, Landero, J, Caruso, J, and Aimin, C. Lead, mercury, and cadmium exposure and attention deficit hyperactivity disorder in children. Environ Res. (2013) 126:105–10. doi: 10.1016/j.envres.2013.08.008
14. Feng, SL, Wang, XM, Wei, GJ, Peng, PA, Yang, Y, and Cao, ZH. Leachates of municipal solid waste incineration bottom ash from Macao: heavy metal concentrations and genotoxicity. Chemosphere. (2007) 67:1133–7. doi: 10.1016/j.chemosphere.2006.11.030
15. Sajad, F, Mohsen, G, Haghbin, NH, and Azadeh, R. Lead exposure through eggs in Iran: health risk assessment. Food Raw Mater. (2021) 9:184–91. doi: 10.21603/2308-4057-2021-1-184-191
16. Hashemi, M, Sadeghi, A, Saghi, M, Aminzare, M, Raeisi, M, Rezayi, M, et al. Health risk assessment for human exposure to trace metals and arsenic via consumption of hen egg collected from largest poultry industry in Iran. Biol Trace Elem Res. (2019) 188:485–93. doi: 10.1007/s12011-018-1437-4
17. Zhao, X, Liang, K, Zhu, H, and Wang, J. Health risk assessment of heavy metals contamination in selenium-enriched eggs. Environ Sci Pollut R. (2021) 28:27047–55. doi: 10.1007/s11356-021-12547-z
18. He, S, Lin, J, Jin, Q, Ma, X, Liu, Z, Chen, H, et al. The relationship between animal welfare and farm profitability in cage and free-range housing systems for laying hens in China. Animals. (2022) 12:2090. doi: 10.3390/ani12162090
19. Wang, Y, Xiong, C, Luo, W, Li, J, Tu, YG, and Zhao, Y. Effects of packaging methods on the quality of heavy metals–free preserved duck eggs during storage. Poultry Sci. (2021) 100:101051. doi: 10.1016/j.psj.2021.101051
20. Gao, B, Hu, X, Xue, H, Li, R, Liu, H, Han, T, et al. The changes of umami substances and influencing factors in preserved egg yolk: pH, endogenous protease, and proteinaceous substance. Front Nutr. (2022) 9:998448. doi: 10.3389/fnut.2022.998448
21. Atamaleki, A, Sadani, M, Raoofi, A, Miri, A, Bajestani, SG, Fakhri, Y, et al. The concentration of potentially toxic elements (PTEs) in eggs: a global systematic review, meta-analysis and probabilistic health risk assessment. Trends Food Sci Tech. (2020) 95:1–9. doi: 10.1016/j.tifs.2019.11.003
22. Wang, Z, Liao, J, Guo, X, Li, X, and Kwon, SY. Total mercury in different egg tissues provides insights to mercury metabolisms in bird bodies. Ecotox Environ Safe. (2023) 249:114336. doi: 10.1016/j.ecoenv.2022.114336
23. Xu, X, Luo, P, Li, S, Zhang, Q, and Sun, D. Distributions of heavy metals in rice and corn and their health risk assessment in Guizhou province. Bull Environ Contam Toxicol. (2022) 108:926–35. doi: 10.1007/s00128-021-03407-0
24. Yang, H, Hu, JW, Huang, XF, Zhou, C, Li, LY, and Fan, MY. Risk assessment of heavy metals pollution for Rosa sterilis and soil from planting bases located in karst areas of Guizhou province. Appl Mech Mater. (2015) 700:47–52. doi: 10.4028/www.scientific.net/amm.700.47
25. Qiu, GL, Feng, XB, Wang, SF, and Shang, LH. Environmental contamination of mercury from hg-mining areas in Wuchuan, northeastern Guizhou, China. Environ Pollut. (2006) 142:549–58. doi: 10.1016/j.envpol.2005.10.015
26. Giannico, OV, Baldacci, S, Basile, FC, Pellegrino, A, Desiante, F, Franco, E, et al. PCDD/Fs and PCBs in hen eggs from a contaminated area in Italy: a 9 years spatio-temporal monitoring study[J]. Food Addit Contam Part A. (2023) 40:294–304. doi: 10.1080/19440049.2022.2157051
27. Xue, S, Korna, R, Fan, J, Ke, W, Lou, W, Wang, J, et al. Spatial distribution characteristics and source analysis of soil heavy metals at typical smelting industry sites. Environ Sci. (2021) 42:5930–7.
28. Guo, Z, Wang, M, Dai, H, and Pan, S. Contamination status and ecological security thresholds of fluoride in farmland around a phosphorus chemical plant in a karst area of southwestern China. Toxics. (2023) 11:587. doi: 10.3390/toxics11070587
29. Zhou, Y, Wan, JZ, Li, Q, Huang, JB, Zhang, ST, Long, T, et al. Heavy metal contamination and health risk assessment of corn grains from a Pb-Zn mining area. Environ Sci. (2020) 41:4733–9. doi: 10.13227/j.hjkx.202004139
30. Hu, Q, Nie, C, Shen, Q, Kong, C, and Zhang, S. Assessment of health risk of heavy metals in major crops in mining abandoned reclamation land. J Agro Environ Sci. (2019) 38:534–43. doi: 10.11654/jaes.2018-0630
31. USEPA. Superfund Public Health Evaluation Manual; Environmental Protection Agency. Washington, DC, USA: USEPA (1986).
32. USEPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part a); Environmental Protection Agency. Washington, DC, USA: USEPA (1989).
33. Wu, D, Yang, X, Li, C, Zhou, C, and Qin, F. Concentrations and health risk assessments of heavy metals in soil and rice in zinc-lead mining area in Guizhou Province. J Agro Environ Sci. (2013) 32:1992–8. doi: 10.16213/j.cnki.scjas.2012.03.005
34. USEPA. Exposure Factors Handbook, Final Edition; Environmental Protection Agency. Washington, DC, USA: USEPA (2011).
35. USEPA. Regional Screening Level (RSL) Summary Table; Environmental Protection Agency. Washington, DC, USA: USEPA (2018).
36. World Health Organization (WHO). Guidelines for Drinking Water Quality. Geneva, Switzerland: World Health Organization (2006).
37. USDOE. The Risk Assessment Information System (RAIS); Energy Information Agency. Oak Ridge, TN, USA: USDOE (2011).
38. Kaur, M, Kumar, A, Mehra, R, and Kaur, I. Quantitative assessment of exposure of heavy metals in groundwater and soil on human health in Reasi district, Jammu and Kashmir. Environ Geochem Health. (2020) 42:77–94. doi: 10.1007/s10653-019-00294-7
39. Gautron, J, Réhault-Godbert, S, Nys, Y, Mann, K, and Righetti, PG. Use of high-throughput technology to identify new egg components In: Improving the Safety and Quality of Eggs and Egg Products. Sawston, UK: Woodhead Publishing (2011). 133–50.
40. Van Immerseel, F, Nys, Y, and Bain, M. Improving the Safety and Quality of Eggs and Egg Products: Egg Safety and Nutritional Quality. Amsterdam, Netherlands: Elsevier (2011).
41. Wei, B, and Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J. (2010) 94:99–107. doi: 10.1016/j.microc.2009.09.014
42. Zhou, H. Soil heavy metal pollution evaluation around mine area with traditional and ecological assessment methods. J Geosci Environ Protect. (2015) 3:28–33. doi: 10.4236/gep.2015.310005
43. Reza, R, and Singh, G. Heavy metal contamination and its indexing approach for river water. Int J Environ Sci Tech. (2010) 7:785–92. doi: 10.1007/bf03326187
44. Zeng, F, Wei, W, Li, M, Huang, R, Yang, F, and Duan, Y. Heavy metal contamination in rice-producing soils of Hunan province, China and potential health risks. Int J Environ Res Pub Health. (2015) 12:15584–93. doi: 10.3390/ijerph121215005
45. Bempah, CK, and Ewusi, A. Heavy metals contamination and human health risk assessment around Obuasi gold mine in Ghana. Environ Monit Assess. (2016) 188:261–13. doi: 10.1007/s10661-016-5241-3
46. Anton, M, Nau, F, and Guérin-Dubiard, C. Bioactive Fractions of Eggs for Human and Animal Health. Improving the Safety and Quality of Eggs and Egg Products. Sawston, UK: Woodhead Publishing (2011).
47. Yair, R, and Uni, Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poultry Sci. (2011) 90:1523–31. doi: 10.3382/ps.2010-01283
48. Bargellini, A, Marchesi, I, Rizzi, L, Cauteruccio, L, Masironi, R, Simioli, M, et al. Selenium interactions with essential and toxic elements in egg yolk from commercial and fortified eggs. J Trace Elem Med Bio. (2008) 22:234–41. doi: 10.1016/j.jtemb.2008.03.004
49. Yin, R, Zhang, W, Sun, G, Feng, Z, Hurley, JP, Yang, L, et al. Mercury risk in poultry in the Wanshan mercury mine. China Environ Pollut. (2017) 230:810–6. doi: 10.1016/j.envpol.2017.07.027
50. Tyokumbur, E, and Daramola, T. Assessment of lead and cadmium in the eggs of Gallus gallus in Ibadan, Nigeria. Am J Food Sci Nutr. (2014) 1:47–54.
51. Nagabhushnam, R, Kodarkar, MS, and Sarojini, R. Textbook of Animal Physiology. New Delhi: Oxford and IBH (1983).
52. Tian, ML, Zhong, XM, Zhang, YX, Yu, YY, Pang, R, Zhou, L, et al. Concentrations and health risk assessments of heavy metal contents in soil and rice of mine contaminated areas. Environ Sci. (2018) 39:2919–26.
53. Yu, Z, Chen, F, Zhang, JF, Huang, DK, Yu, EJ, and Liu, HY. Contamination and risk of heavy metals in soils and vegetables from zinc smelting area. China Environ Sci. (2019) 39:2086–94.
54. Ravindran, B, Mupambwa, HA, Silwana, S, and Mnkeni, PNS. Assessment of nutrient quality, heavy metals and phytotoxic properties of chicken manure on selected commercial vegetable crops. Heliyon. (2017) 3:e00493. doi: 10.1016/j.heliyon.2017.e00493
55. Yang, X, Cheng, B, Gao, Y, Zhang, H, and Liu, L. Heavy metal contamination assessment and probabilistic health risks in soil and maize near coal mines. Front Public Health. (2022) 10:1004579. doi: 10.3389/fpubh.2022.1004579
56. Guo, Z, Dai, H, Wang, M, and Pan, S. Health risk assessment of heavy metal exposure through vegetable consumption around a phosphorus chemical plant in the Kaiyang karst area, southwestern China. Environ Sci Pollut R. (2023) 30:35617–34. doi: 10.1007/s11356-022-24662-6
57. Hincke, M, Gautron, J, Rodriguez-Navarro, AB, and McKee, MD. The eggshell: structure and protective function In: Improving the Safety and Quality of Eggs and Egg Products. Sawston, UK: Woodhead Publishing (2011). 151–82.
58. Sun, HF, Li, YH, Ji, YF, Yang, LS, Wang, WY, and Li, HR. Environmental contamination and health hazard of lead and cadmium around Chatian mercury mining deposit in western Hunan Province, China. Trans Nonferr Metal Soc China. (2010) 20:308–14. doi: 10.1016/s1003-6326(09)60139-4
59. Li, P, Feng, XB, Shang, LH, Qiu, GL, Meng, B, Zhang, H, et al. Human co-exposure to mercury vapor and methylmercury in artisanal mercury mining areas, Guizhou, China. Ecotox Environ Safe. (2011) 74:473–9. doi: 10.1016/j.ecoenv.2010.10.030
60. Feng, X, Li, P, Qiu, G, Wang, SF, Li, GH, Shang, LH, et al. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ Sci Technol. (2008) 42:326–32. doi: 10.1021/es071948x
61. Slack, NJ, Sharma, S, Cúg, J, and Singh, G. Factors forming consumer willingness to pay a premium for free-range eggs. Brit Food J. (2023) 125:2439–59. doi: 10.1108/BFJ-07-2022-0663
62. Cao, Y, Cranfield, J, Chen, C, and Widowski, T. Heterogeneous informational and attitudinal impacts on consumer preferences for eggs from welfare enhanced cage systems. Food Policy. (2021) 99:101979. doi: 10.1016/j.foodpol.2020.101979
63. English, MM. The chemical composition of free-range and conventionally-farmed eggs available to Canadians in rural Nova Scotia. PeerJ. (2021) 9:e11357. doi: 10.7717/peerj.11357
64. Aendo, P, De Garine-Wichatitsky, M, Mingkhwan, R, Senachai, K, Santativongchai, P, Krajanglikit, P, et al. Potential health effects of heavy metals and carcinogenic health risk estimation of pb and cd contaminated eggs from a closed gold mine area in northern Thailand. Foods. (2022) 11:2791. doi: 10.3390/foods11182791
65. Basha, AM, Yasovardhan, N, Satyanarayana, SV, Reddy, GVS, and Kumar, AV. Assessment of heavy metal content of hen eggs in the surroundings of uranium mining area, India. Ann Food Sci Technol. (2013) 344:349.
66. Kabeer, MS, Hameed, I, Kashif, SUR, Khan, M, Tahir, A, Anum, F, et al. Contamination of heavy metals in poultry eggs: a study presenting relation between heavy metals in feed intake and eggs. Arch Environ Occup Health. (2021) 76:220–32. doi: 10.1080/19338244.2020.1799182
67. Farahani, S, Eshghi, N, Abbasi, A, Karimi, F, Shiri Malekabad, E, and Rezaei, M. Determination of heavy metals in albumen of hen eggs from the Markazi Province (Iran) using ICP-OES technique. Toxin Rev. (2015) 34:96–100. doi: 10.3109/15569543.2015.1040166
68. Demirulus, H. The heavy metal content in chicken eggs consumed in Van Lake territory. Ekoloji. (2013) 22:19–25. doi: 10.5053/ekoloji.2013.863
69. Giri, S, and Singh, AK. Heavy metals in eggs and chicken and the associated human health risk assessment in the mining areas of Singhbhum copper belt, India. Arch Environ Occup Health. (2019) 74:161–70. doi: 10.1080/19338244.2017.1407284
70. Aliu, H, Dizman, SERDAR, Sinani, A, and Hodolli, G. Comparative study of heavy metal concentration in eggs originating from industrial poultry farms and free-range hens in Kosovo. J Food Quality. (2021) 2021:1–7. doi: 10.1155/2021/6615289
71. Şekeroğlu, A, Sarı, H, Sarıca, M, Yıldırım, A, and Duman, M. Effects of distance from the roadway on heavy metal content and egg quality of village laying hen’s egg along roadsides of Tokat-Turhal, Turkey. Pak J Agri Sci. (2013) 50:299–304.
72. Hu, Y, Zhou, J, Du, B, Liu, H, Zhang, W, Liang, J, et al. Health risks to local residents from the exposure of heavy metals around the largest copper smelter in China. Ecotox Environ Safe. (2019) 171:329–36. doi: 10.1016/j.ecoenv.2018.12.073
73. Samad, A, Roy, D, Hasan, MM, Ahmed, KS, Sarker, S, Hossain, MM, et al. Intake of toxic metals through dietary eggs consumption and its potential health risk assessment on the peoples of the capital city Dhaka, Bangladesh. Arab J Chem. (2023) 16:105104. doi: 10.1016/j.arabjc.2023.105104
74. Ullah, AA, Afrin, S, Hosen, MM, Musarrat, M, Ferdoushy, T, Nahar, Q, et al. Concentration, source identification, and potential human health risk assessment of heavy metals in chicken meat and egg in Bangladesh. Environ Sci Pollut R. (2022) 29:22031–42. doi: 10.1007/s11356-021-17342-4
75. Aendo, P, Thongyuan, S, Songserm, T, and Tulayakul, P. Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci Total Environ. (2019) 689:215–22. doi: 10.1016/j.scitotenv.2019.06.414
76. Tanhan, P, Apipongrattanasuk, N, Poapolathep, A, Poapolathep, S, Kruatrachue, M, and Imsilp, K. Heavy metal concentrations in duck eggs and potential human health risk via consumption. Jpn J Vet Res. (2020) 68:21–33. doi: 10.14943/jjvr.68.1.17
77. Aendo, P, Netvichian, R, Viriyarampa, S, Songserm, T, and Tulayakul, P. Comparison of zinc, lead, cadmium, cobalt, manganese, iron, chromium and copper in duck eggs from three duck farm systems in central and Western, Thailand. Ecotox Environ Safe. (2018) 161:691–8. doi: 10.1016/j.ecoenv.2018.06.052
Keywords: heavy metal, health risk, mercury mine area, free-range duck eggs, caged duck eggs
Citation: Guo X, Wang Z, Li X, Liao J, Zhang X, Ran Y, Wu Q, Zhang T and Wang Z (2024) Heavy metal contamination in duck eggs from a mercury mining area, southwestern China. Front. Public Health. 12:1352043. doi: 10.3389/fpubh.2024.1352043
Edited by:
Mahdi Ghorbanian, North Khorasan University of Medical Sciences, IranReviewed by:
Marcela Tamayo-Ortiz, Columbia University, United StatesOrazio Valerio Giannico, Local Health Authority of Taranto, Italy
Copyright © 2024 Guo, Wang, Li, Liao, Zhang, Ran, Wu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuhong Wang, d2FuZ3podWhvbmdAZ21jLmVkdS5jbg==
 Xiaoling Guo
Xiaoling Guo Zhuhong Wang1*
Zhuhong Wang1* Qixin Wu
Qixin Wu