
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 11 April 2024
Sec. Public Health and Nutrition
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1351510
This article is part of the Research Topic Dietary and Nutrigenetic Contributors to Metabolic Syndrome View all 8 articles
 Dorsa Hosseininasab1
Dorsa Hosseininasab1 Farideh Shiraseb2
Farideh Shiraseb2 Niki Bahrampour1
Niki Bahrampour1 Alessandra da Silva3
Alessandra da Silva3 Mohammad Mahdi Hajinasab4
Mohammad Mahdi Hajinasab4 Josefina Bressan3
Josefina Bressan3 Khadijeh Mirzaei2*
Khadijeh Mirzaei2*Background: Ultra-processed foods (UPFs) have been associated with a higher intake of added sugars, sodium, and unhealthy fats; however, the relationship between UPFs and quality of life (QoL) is not well understood.
Methods: The present cross-sectional study included 193 Iranian women aged 18–48 years with a body mass index (BMI) of ≥25 kg/m2. UPFs were identified using the NOVA classification. QoL was evaluated using the SF-36 questionnaire. Anthropometric measurements and body composition were assessed using an appropriate method.
Results: The mean BMI and fat-free mass (FFM) of the subjects were 30.90 kg/m2 and 46.69 kg, respectively. At first, a significant difference was observed in the height of women across tertiles of UPF consumption. The mean score of the total QoL scale was 66.90. Women who were in the tertile 3 of UPFs intake had 23.59 units lower the scale of limitation in physical capabilities and activity (score of role-physical) (β = −23.59, 95% CI: −37.77–9.40, p = 0.001). Among those with the highest adherence to UPF intake, there was an 8.76 unit reduction in addressing feelings of energy and fatigue (vitality domain) in model 2 (β = −8.76, 95% CI: −16.42–1.11, p = 0.02). Finally, a reduction of 15.78 units was observed in the mental health scale, specifically in the mental states of anxiety and depression, among participants in the third tertile of UPF intake (β = −15.78, 95% CI: −24.11–7.45, p < 0.001).
Conclusion: Increased UPF consumption was associated with lower QoL in Iranian women. Further studies are needed to confirm these findings and develop effective strategies to promote healthy food choices.
Finding ways to increase the quality of life (QoL) has always been one of the concerns of scientists. QoL is usually categorized into five dimensions: physical, material, social, and emotional wellbeing, and development and activity (1). Two individuals with the same health condition can have different QoLs based on their expectations and resilience toward health or illness, socioeconomic condition, age, and social support (2–4). Studies have considered QoL as a means to assess healthcare options, particularly for individuals with chronic/disabling diseases (5, 6). Rathnayake et al. indicated that women tend to express lower QoL (compared to men) (7). Additionally, differences in QoL scores have been identified between obese and non-obese women (8). Nutrition and eating habits may be related to individuals’ QoL and can significantly impact it (9); therefore, diverse eating indices have been known to evaluate the quality of a person’s nutrition.
Consumption of ultra-processed foods (UPFs) is increasing rapidly worldwide. According to the Nationwide Food Surveys, UPFs account for 25–60% of total daily energy consumption (10). Based on the NOVA classification system, UPFs are classified as foods made up entirely/predominantly from unhealthy components consisting of greater levels of total fat, saturated fat, added sugar, high-calorie content, salt, and lower fiber and vitamin content (11). Several studies displayed that consumption of UPFs is related to unfavorable health outcomes, including obesity (12–14). Our recent study reported an association between cardiometabolic risk factors and the consumption of UPFs (15).
Although several studies have assessed the relationship between eating habits and health outcomes, such as cardiovascular diseases, dementia, and mortality, studies evaluating the connection between eating habits and QoL are limited. Therefore, we intended to evaluate the relationship between UPF consumption and QoL among obese and overweight Iranian women.
This research was conducted in Tehran, Iran, using a multi-stage cluster random sampling procedure involving 193 overweight or obese women. The participants had a body mass index (BMI) ranging from 25 to 40 kg/m2 and were aged between 18 and 48 years. The sample size formula, N = (([(Z1 − α + Z1 − β) × √1−r2]/r)2 + 2), β = 95%, and α = 0.05, r = 0.25, was used. The exclusion criteria were as follows: total daily energy intake outside of 800–4,200 kcal (17,556–3,344 kJ) (16), presence of metabolic diseases, menopause, pregnancy, breastfeeding, taking lipid-lowering and blood glucose-lowering agents, alcohol consumption, and smoking. All the participants of our study signed an informed consent form, and all the methods of our study were performed in accordance with the relevant guidelines and regulations.
For evaluating the food consumption of participants throughout the previous year, we utilized a 147-item semi-quantitative food frequency questionnaire (FFQ) [its validity and reliability have already been authorized (17, 18)]. Participants evaluated their consumption frequency using four categories: daily, weekly, monthly, and infrequent. Using home measures, the portion sizes of the consumed foods were converted to grams (19). To evaluate energy intake and nutrients, version 7.0 of NUTRITIONIST IV was used. To evaluate NOVA components, we used the same method as our previous study (15).
To measure QoL, we utilized a self-administered SF-36 (short-form questionnaire), which comprises 36 questions. Thirty-five of these questions are compressed into eight multi-item scales, namely physical functioning (PF), role-physical (RP), role-emotional (RE), general health (GH), bodily pain (BP), vitality (VT), social functioning (SF), and mental health (MH) (20).
(1) The PF scale is a 10-question scale that assesses a person’s ability to deal with daily physical demands such as personal hygiene, flexibility, and walking. (2) The RP scale is a 4-item scale that assesses how much physical limitations hinder activity. (3) The BP scale is a 2-item measure that evaluates the discomfort felt in the last 4 weeks and how much that pain interfered with routine work duties. (4) The GH scale is a 5-item questionnaire that evaluates personal perceptions of general health. (5) The VT scale is a 4-item scale that evaluates a person’s sense of vigor, energy, and weariness. (6) The SF scale is a 2-item scale that assesses how long and how much physical health or emotional problems interfered with family, friends, and other social contacts in the previous 4 weeks. (7) The RE scale is a 3-item questionnaire that assesses how much emotional problems interfere with work or other activities. (8) The MH scale is a 5-item questionnaire to examine anxiety and depression symptoms (20, 21). The SF-36 also consists of a question about self-evaluating health changes over the last year, which is not part of the 8 categories or the total SF-36 score. The score of each of these 8 dimensions ranged from 0 to 100 (worst health to highest health).
The demographic and socioeconomic conditions were estimated using a questionnaire that has been used in recent studies (15). Anthropometric measures, including height (m), waist circumference (WC) (cm), and hip circumference (HC) (cm), were measured using standard protocols (22). The waist-to-hip ratio (WHR) was computed as WC (cm)/HC (cm). A body composition analyzer (BIA) (Inbody Co., Seoul, Korea) was used to assess the individuals’ weight, BMI, fat mass index (FMI), fat-free mass (FFM), and body fat percentage (%) according to a predetermined methodology (23). More than that, we used the validated International Physical Activity Questionnaire (IPAQ) to obtain the physical activity status of the participants. Metabolic equation hours per week (MET-hours/week) were calculated for each subject by trained professionals (16, 24).
The participants were categorized into tertiles of UPF consumption in grams according to the NOVA score. A one-way analysis of variance (ANOVA) and χ2 tests were performed to compare the mean difference of quantitative and frequency of categorical variables across UPF tertiles, respectively. An analysis of covariance (ANCOVA) adjusted for potential confounders (age, BMI, energy intake, and physical activity) was performed. We used the Bonferroni post-hoc test to find the statistically significant difference among UPF tertiles. Linear regression was performed to evaluate the association of UPF consumption (independent variable) with QoL factors (dependent variable). Model 1 was adjusted for age, BMI, physical activity, and total energy intake, while model 2 was additionally adjusted for education status. This analysis was presented as the β-value and a confidence interval (CI) of 95%. SPSS v.26 software was utilized for statistical analysis.
The analyzed sample was composed of 193 women with 36.37 (SD 8.05) years of age and a weight of 80.76 (SD 12.13) kg (Table 1). The mean BMI and FFM were 30.90 (SD 4.20) kg/m2 and 46.69 (SD 5.62) kg, respectively. Statistically significant differences were found in the height of women across tertiles of the NOVA score (p < 0.05). In addition, 45.6% of participants had a bachelor’s degree or higher. The percentages of low-income and high-income subjects were 20 and 33.3%, respectively. There were no other significant differences in socio-demographic and anthropometric variables among NOVA score tertiles.
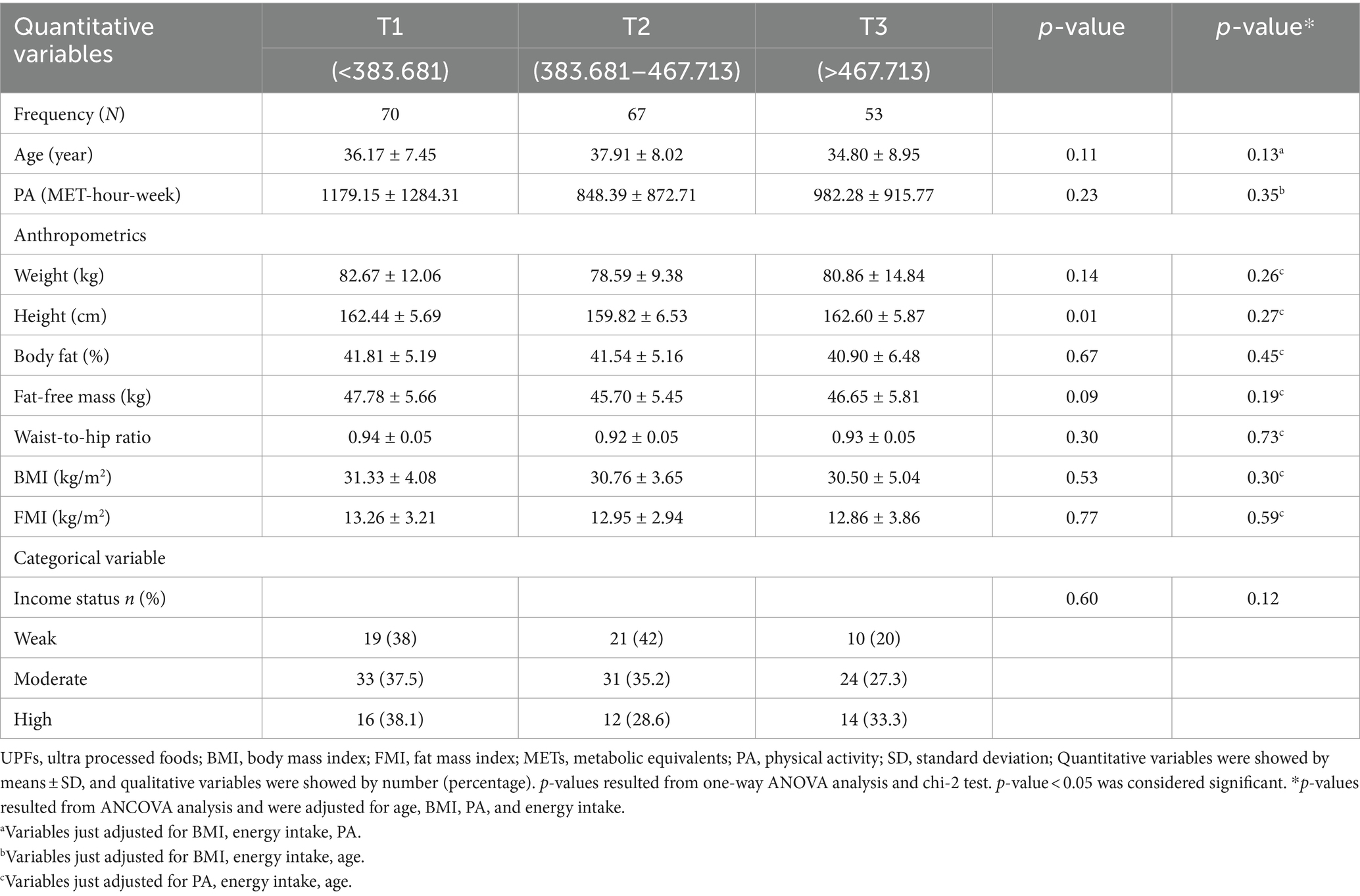
Table 1. Distribution of socio-demographic and lifestyle parameters of participants, according to tertiles of UPFs consumption (n = 193).
As shown in Table 2, all NOVA score subgroups increased significantly across tertiles of UPFs score (p < 0.05). In addition, vegetable consumption was lower according to higher adherence to the UPF intake tertiles (p < 0.05). On average, the total fiber intake among the women in the study was 40.89 (19.26) g. This amount decreased significantly across the tertiles (p < 0.01). Foods/beverages containing EPA and DHA were consumed in a small amount. Overall, vitamins and minerals were not statistically different between the tertiles, even after adjusting for energy intakes.
The mean score of the total QoL scale was 66.90 (SD 24.19) among Iranian women (Table 3). Regarding QoL subscales, role-physical and mental health domains were significantly different across tertiles of UPF intakes (p < 0.05). Women with lower adherence to UPF intake (first tertile) had a higher mean score in the role-physical domain, with a score of 86.03 (SD 34.39), compared to the third tertile. In addition, the first tertile of UPF consumption exhibited a higher mental health score of 79.38 (SD 20.77) compared to the third tertile of UPF consumption, which had a score of 70.82 (SD 21.29). These differences remained significant after adjusting confounders (p = 0.01 and p = 0.002). In addition, a marginal significance appeared in the vitality subgroup among tertiles of NOVA score in adjusted mode (p = 0.06).
Table 4 shows the relationship between NOVA score tertiles, QoL score, and its eight scales. Tertile 1 was chosen as the reference group. Being in the third tertile of consumption of UPFs decreased by 16,829 times in the role-physical domain units (β = −16.82, 95% CI: −30.94–2.71, p = 0.01), regardless of confounding variables in the crude model. This significance was improved after adjusting for confounders in model 1 (β = −21.90, 95% CI: −36.18–7.62, p = 0.003). Finally, women in the third tertile of UPF intake had a 23.59 unit lower score in the limitation in physical capabilities and activity (score of role-physical) (β = −23.59, 95% CI: −37.77–9.40, p = 0.001). In addition, another subgroup of the QoL scale called vitality had a negative association with the third tertile of UPF intake in model 1 (β = −8.48, 95% CI: −16.18–0.78, p = 0.03). The highest adherence to UPF intake (tertile 3) reduced by 8.76 units in addressing feelings of energy and fatigue (vitality domain) in model 2 (β = −8.76, 95% CI: −16.42–1.11, p = 0.02). Moreover, the mental health of the participants was inversely related to the second (β = −8.06, 95% CI: −15.66–0.47, p = 0.03) and third (β = 8.55, 95% CI: −16.74–0.36, p = 0.04) tertiles of UPF consumption in the crude model. This significance became even stronger in models 1 and 2. Finally, 15.78 decreasing units were found at mental states of anxiety and depression (mental health scale) between participants of the third tertile of UPF intake (β = −15.78, 95% CI: −24.11–7.45, p < 0.001).
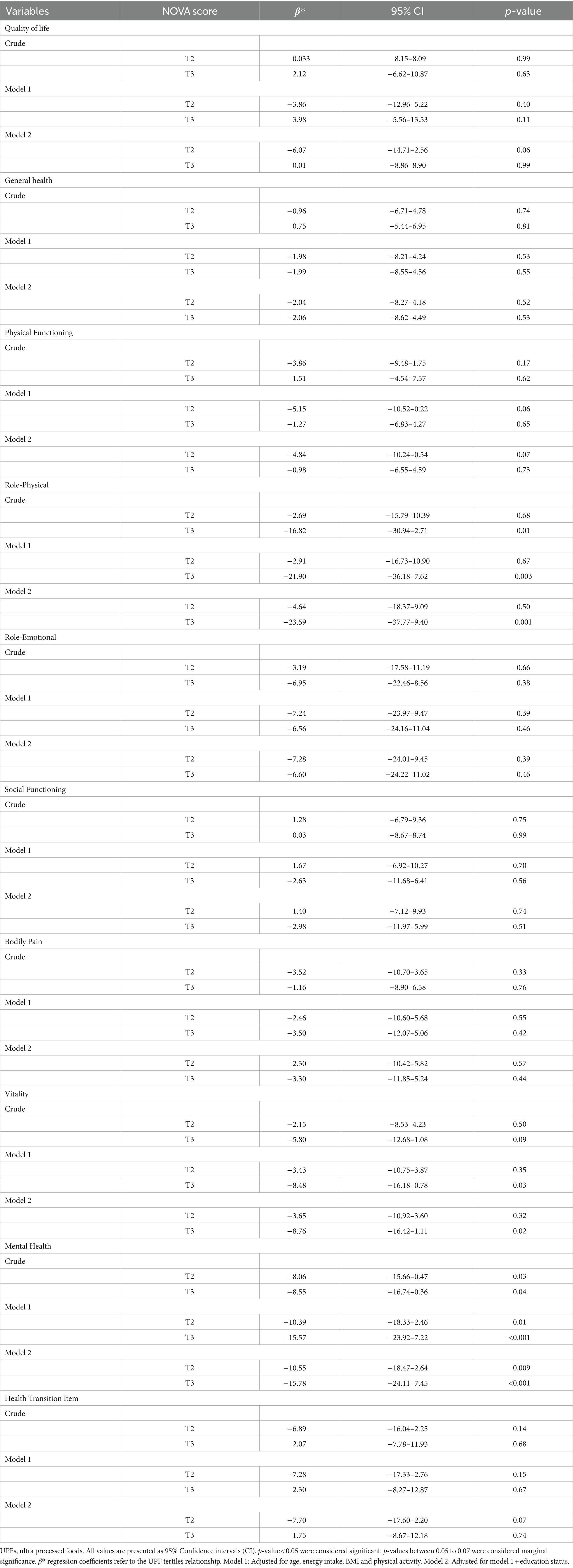
Table 4. Association between UPFs tertiles with quality of life and its subgroups in Iranian women (n = 193).
The current study investigated the associations between UPF intake and QoL in Iranian women for the first time. Participants with higher UPF consumption displayed a lower QoL (after adjustment for possible confounders). In other words, we observed a significant negative association between UPF intake and role-physical, mental health, and vitality in both models (crude and adjusted).
Similar to our study, another study in 2020 indicated that individuals with unhealthy dietary patterns, such as a Western diet characterized by high consumption of refined grains, red or processed meat, and sugary carbonated beverages, had lower QoL scores (25). Another study showed that adherence to healthy dietary patterns, such as the Mediterranean diet, could benefit at least one of the QoL domains (9). A study conducted in Paraguay in 2022 demonstrated that consumption of foods rich in sodium, free sugars, fat, and nitrites was associated with a lower QoL and insufficient sleep duration (26), and a study conducted in Brazil in 2020 revealed that a decrease in the consumption of UPFs led to a reduction in the range of body mass index and waist circumference and an improvement in QoL (27). Magaly Aceves et al. showed that often, due to the cheaper, more convenient, and lower nutritional quality of highly processed foods and their potential health risks, it can lead to increased medical costs and decreased productivity and quality of life (28). In addition, studies showed that increasing consumption of processed foods can affect self-confidence and social interactions by affecting physical appearance, such as weight gain (29–33).
Numerous situations in life, such as living arrangements, migration, loss of loved ones, being unemployed, aging, and a lack of social relationships, could affect food intake and nutritional status and, as a result, affect the QoL in individuals (34–36). On the other hand, nowadays, people are under tremendous financial pressure, especially in low-income countries such as Iran, and they have to work round the clock to satisfy their basic needs. Hence, they are too tired to spend time cooking healthy meals at home, and instead, they prefer consuming ready-to-eat foods or purchasing food from takeaway outlets. UPFs can have so many detrimental effects on various aspects of human health. Cheng et al. observed that higher intake of UPFs is associated with a greater risk of overall cancer, as well as an increased risk of overall mortality (37). A diet high in UPF content is nutritionally inferior, as it tends to be higher in energy, saturated fats, salt, and free sugars, while being lower in several micronutrients and fiber (38). Furthermore, evidence has shown the strong obesity-promoting potential of UPFs (39), which is a risk factor for several metabolic diseases, including some cancers in women (40–42).
Inflammation is a key mediator in chronic diseases such as diabetes and depression (43–47). Ultra-processed foods can induce metabolic endotoxemia, increase inflammatory cytokines, and impair endothelial function (48, 49). Furthermore, highly processed foods can alter neurotransmissions by altering the availability and activity of neurotransmitters such as dopamine, serotonin, and glutamate (50). These foods can stimulate the reward pathway, increase food cravings, and create addictive eating behaviors (51, 52). Studies have linked the consumption of highly processed foods with lower levels of dopamine and serotonin in the brain and higher cases of food addiction, overeating, and depression (53, 54). It also indicated that UPFs can affect the gut microbiome, potentially leading to chronic inflammation and diseases, such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and colorectal cancer (55).
Emerging evidence has shown additional properties of UPFs that could contribute to adverse human health outcomes. These include the use of controversial food additives, the formation of new contaminants during ultra-processing, and the migration of toxic contaminants from food packaging (37). Bisphenols and phthalates are endocrine-disrupting chemicals usually found in food storage, packaging, and contact materials, and higher urinary concentrations of phthalates and bisphenols have been found in people with higher UPF consumption (56). The available data on bisphenols have consistently shown many toxic effects, including DNA damage and impacts on the nervous and immune systems (57).
The present study has some strengths. To the best of our knowledge, this is the first study to investigate the associations between UPF consumption and QoL among obese and overweight Iranian women. Furthermore, dietary intake was assessed utilizing a validated questionnaire. Nevertheless, the study had several limitations. It was observational; hence, causal inference is limited. In addition, some errors may be present in the dietary assessment due to recall bias and misclassification errors. Moreover, our results are not generalizable to men and normal-weight women.
We observed an inverse association between UPF consumption and role-physical, mental health, and vitality in overweight and obese women. The causality may not be implied because of the observational nature of the study, but these findings highlight the importance of considering UPF intake in diets. Our findings suggest that limiting UPF intake could be beneficial in preventing/reducing some diseases and improving the QoL in Iranian women.
In conclusion, this study has highlighted the significant association between UPF consumption and QoL among overweight and obese women. The findings indicate that higher UPF intake is associated with poorer QoL. To gain a better understanding of the relationship between UPF and quality of life, we suggest conducting a series of clinical studies so that we can determine the cause-and-effect relationships and make practical recommendations for the future.
The raw data supporting the conclusions of this article will be made available by the authors, upon request to the corresponding author.
The studies involving humans were approved by Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences (TUMS), Tehran, Iran. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
DH: Writing – original draft, Writing – review & editing. FS: Formal analysis, Writing – review & editing. NB: Writing – review & editing. AS: Writing – review & editing. MH: Writing – review & editing. JB: Writing – review & editing. KM: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Tehran University of Medical Sciences (TUMS) grants (Grant ID: 97-03-161-41017).
The authors are grateful to all participants for their contribution to this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Felce, D, and Perry, J. Quality of life: its definition and measurement. Res Dev Disabil. (1995) 16:51–74. doi: 10.1016/0891-4222(94)00028-8
2. Rubin, RR, and Peyrot, M. Quality of life and diabetes. Diabetes Metab Res Rev. (1999) 15:205–18. doi: 10.1002/(SICI)1520-7560(199905/06)15:3<205::AID-DMRR29>3.0.CO;2-O
3. Campos, ACV, EF, FE, AMD, V, and Albala, C. Aging, gender and quality of life (AGEQOL) study: factors associated with good quality of life in older Brazilian community-dwelling adults. Health Qual Life Outcomes. (2014) 12:1–11. doi: 10.1186/s12955-014-0166-4
4. Kim, H-K, Hisata, M, Kai, I, and Lee, S-K. Social support exchange and quality of life among the Korean older adult. J Cross Cult Gerontol. (2000) 15:331–47. doi: 10.1023/A:1006765300028
5. Alexandre, TS, Cordeiro, RC, and Ramos, LR. Factors associated to quality of life in active older adult. Revista de saude Publica. (2009) 43:613–21. doi: 10.1590/S0034-89102009005000030
6. Pearlman, RA, and Uhlmann, RF. Quality of life in chronic diseases: perceptions of older adult patients. J Gerontol. (1988) 43:M25–30. doi: 10.1093/geronj/43.2.M25
7. Rathnayake, N, Lenora, J, Alwis, G, and Lekamwasam, S. Prevalence and severity of menopausal symptoms and the quality of life in middle-aged women: a study from Sri Lanka. Nurs Res Pract. (2019) 2019:1–9. doi: 10.1155/2019/2081507
8. Chu, D-T, Nguyet, NTM, Nga, VT, Lien, NVT, Vo, DD, Lien, N, et al. An update on obesity: mental consequences and psychological interventions. Diabetes Metab Syndr Clin Res Rev. (2019) 13:155–60. doi: 10.1016/j.dsx.2018.07.015
9. Govindaraju, T, Sahle, BW, McCaffrey, TA, McNeil, JJ, and Owen, AJ. Dietary patterns and quality of life in older adults: a systematic review. Nutrients. (2018) 10:971. doi: 10.3390/nu10080971
10. Baraldi, LG, Steele, EM, Canella, DS, and Monteiro, CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. (2018) 8:e020574. doi: 10.1136/bmjopen-2017-020574
11. Zinöcker, MK, and Lindseth, IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. (2018) 10:365. doi: 10.3390/nu10030365
12. Costa, CS, Del-Ponte, B, Assunção, MCF, and Santos, IS. Consumption of ultra-processed foods and body fat during childhood and adolescence: a systematic review. Public Health Nutr. (2018) 21:148–59. doi: 10.1017/S1368980017001331
13. Juul, F, Martinez-Steele, E, Parekh, N, Monteiro, CA, and Chang, VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. (2018) 120:90–100. doi: 10.1017/S0007114518001046
14. Marti, A . Ultra-processed foods are not “real food” but really affect your health. MDPI. (2019) 11:1902. doi: 10.3390/nu11081902
15. Hosseininasab, D, Shiraseb, F, Noori, S, Jamili, S, Mazaheri-Eftekhar, F, Dehghan, M, et al. The relationship between ultra-processed food intake and cardiometabolic risk factors in overweight and obese women: a cross-sectional study. Front Nutr. (2022) 9:945591. doi: 10.3389/fnut.2022.945591
16. Hagströmer, M, Oja, P, and Sjöström, M. The international physical activity questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. (2006) 9:755–62. doi: 10.1079/PHN2005898
17. Mirmiran, P, Esfahani, FH, Mehrabi, Y, Hedayati, M, and Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
18. Toorang, F, Sasanfar, B, Esmaillzadeh, A, Ebrahimpour-Koujan, S, and Zendehdel, K. Comparison of validity of the food frequency questionnaire and the diet history questionnaire for assessment of energy and nutrients intakes in an Iranian population. East Mediterr Health J. (2020) 26:1062–9. doi: 10.26719/emhj.19.099
19. Azizi, F, Ghanbarian, A, Madjid, M, and Rahmani, M. Distribution of blood pressure and prevalence of hypertension in Tehran adult population: Tehran lipid and glucose study (TLGS), 1999–2000. J Hum Hypertens. (2002) 16:305–12. doi: 10.1038/sj.jhh.1001399
20. McHorney, CA, Ware, JE, and Raczek, AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. (1993) 31:247–63. doi: 10.1097/00005650-199303000-00006
21. Kalantar-Zadeh, K, Kopple, JD, Block, G, and Humphreys, MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. (2001) 12:2797–806. doi: 10.1681/ASN.V12122797
22. Mahan, LK, and Raymond, JL. Krause’s food & the nutrition care process-e-book Elsevier Health Sciences (2016).
23. Yarizadeh, H, Setayesh, L, Roberts, C, Yekaninejad, MS, and Mirzaei, K. Nutrient pattern of unsaturated fatty acids and vitamin E increase resting metabolic rate of overweight and obese women. Int J Vitam Nutr Res. (2020) 92:214. doi: 10.1024/0300-9831/a000664
24. Ainsworth, BE, Haskell, WL, Herrmann, SD, Meckes, N, Bassett, DR Jr, Tudor-Locke, C, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. (2011) 43:1575–81. doi: 10.1249/MSS.0b013e31821ece12
25. Vajdi, M, and Farhangi, MA. A systematic review of the association between dietary patterns and health-related quality of life. Health Qual Life Outcomes. (2020) 18:337. doi: 10.1186/s12955-020-01581-z
26. Rodríguez, ALB, Amarilla, NJD, Rodríguez, MMT, Martínez, BEN, and Meza-Miranda, ER. Processed and ultra-processed foods consumption in adults and its relationship with quality of life and quality of sleep. Rev Nutr. (2022) 35:e173. doi: 10.1590/1678-9865202235e220173
27. Poll, FA, Miraglia, F, D’avila, HF, Reuter, CP, and Mello, ED. Impact of intervention on nutritional status, consumption of processed foods, and quality of life of adolescents with excess weight. J Pediatr. (2020) 96:621–9. doi: 10.1016/j.jped.2019.05.007
28. Aceves-Martins, M, Bates, RL, Craig, LCA, Chalmers, N, Horgan, G, Boskamp, B, et al. Nutritional quality, environmental impact and cost of ultra-processed foods: a UK food-based analysis. Int J Environ Res Public Health. (2022) 19:3191. doi: 10.3390/ijerph19063191
29. Martins, CA, Machado, PP, Louzada, MLC, Levy, RB, and Monteiro, CA. Parents’ cooking skills confidence reduce children’s consumption of ultra-processed foods. Appetite. (2020) 144:104452. doi: 10.1016/j.appet.2019.104452
30. Norman, J, Kelly, B, McMahon, A-T, Boyland, E, Baur, LA, Chapman, K, et al. Children’s self-regulation of eating provides no defense against television and online food marketing. Appetite. (2018) 125:438–44. doi: 10.1016/j.appet.2018.02.026
31. Crimarco, A, Landry, MJ, and Gardner, CD. Ultra-processed foods, weight gain, and co-morbidity risk. Curr Obes Rep. (2022) 11:80–92. doi: 10.1007/s13679-021-00460-y
32. Monteiro, CA, Moubarac, JC, Cannon, G, Ng, SW, and Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. (2013) 14:21–8. doi: 10.1111/obr.12107
33. Mekonnen, DA, Achterbosch, T, and Ruben, R. Social status and the consumption of highly processed foods in Nigeria. Q Open. (2023) 3:e36. doi: 10.1093/qopen/qoac036
34. Ryan, AS, Craig, LD, and Finn, SC. Nutrient intakes and dietary patterns of older Americans: a national study. J Gerontol. (1992) 47:M145–50. doi: 10.1093/geronj/47.5.M145
35. Watanabe, R, Hanamori, K, Kadoya, H, Nishimuta, M, and Miyazaki, H. Nutritional intakes in community-dwelling older Japanese adults: high intakes of energy and protein based on high consumption of fish, vegetables and fruits provide sufficient micronutrients. J Nutr Sci Vitaminol. (2004) 50:184–95. doi: 10.3177/jnsv.50.184
36. Nnakwe, N . Nutrient intakes of healthy independent living older adult persons consuming self-selected diets. FASEB J. (1997) 11:1095.
37. Chang, K, Gunter, MJ, Rauber, F, Levy, RB, Huybrechts, I, Kliemann, N, et al. Ultra-processed food consumption, cancer risk and cancer mortality: a large-scale prospective analysis within the UK biobank. EClinicalMedicine. (2023) 56:101840. doi: 10.1016/j.eclinm.2023.101840
38. Monteiro, CA, Cannon, G, Levy, RB, Moubarac, J-C, Louzada, ML, Rauber, F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. (2019) 22:936–41. doi: 10.1017/S1368980018003762
39. Srour, B, Kordahi, MC, Bonazzi, E, Deschasaux-Tanguy, M, Touvier, M, and Chassaing, B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. The lancet. Gastroenterol Hepatol. (2022) 7:1128–40. doi: 10.1016/S2468-1253(22)00169-8
40. Research W. Diet, nutrition, physical activity and cancer: A global perspective World Cancer Research Fund/American Institute for Cancer Research (2018).
41. Avgerinos, KI, Spyrou, N, Mantzoros, CS, and Dalamaga, M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
42. Pearson-Stuttard, J, Papadimitriou, N, Markozannes, G, Cividini, S, Kakourou, A, Gill, D, et al. Type 2 diabetes and cancer: an umbrella review of observational and mendelian randomization studies. Cancer Epidemiol Biomarkers Prev. (2021) 30:1218–28. doi: 10.1158/1055-9965.EPI-20-1245
43. Tsalamandris, S, Antonopoulos, AS, Oikonomou, E, Papamikroulis, GA, Vogiatzi, G, Papaioannou, S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. (2019) 14:50–9. doi: 10.15420/ecr.2018.33.1
44. Dandona, P, Aljada, A, and Bandyopadhyay, A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. (2004) 25:4–7. doi: 10.1016/j.it.2003.10.013
45. Forrester, JV, Kuffova, L, and Delibegovic, M. The role of inflammation in diabetic retinopathy. Front Immunol. (2020) 11:583687. doi: 10.3389/fimmu.2020.583687
46. Miller, AH . Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry. (2020) 19:108–9. doi: 10.1002/wps.20723
47. Milaneschi, Y, Kappelmann, N, Ye, Z, Lamers, F, Moser, S, Jones, PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK biobank and NESDA cohorts. Mol Psychiatry. (2021) 26:7393–402. doi: 10.1038/s41380-021-01188-w
48. Lopes, AESC, Araújo, LF, Levy, RB, Barreto, SM, and Giatti, L. Association between consumption of ultra-processed foods and serum C-reactive protein levels: cross-sectional results from the ELSA-Brasil study. São Paulo Med J. (2019) 137:169–76. doi: 10.1590/1516-3180.2018.0363070219
49. Martins, GMS, França, AKTC, Viola, PCAF, Carvalho, CA, Marques, KDS, Santos, AM, et al. Intake of ultra-processed foods is associated with inflammatory markers in Brazilian adolescents. Public Health Nutr. (2022) 25:591–9. doi: 10.1017/S1368980021004523
50. Dhailappan, A, and Samiappan, S. Impact of diet on neurotransmitters. S Rajagopal, S Ramachandran, G Sundararaman, and S Gadde Venkata, (Ed). Role of nutrients in neurological disorders. Singapore: Springer Singapore; (2022). 363–383
51. Gearhardt, AN, Bueno, NB, DiFeliceantonio, AG, Roberto, CA, Jiménez-Murcia, S, and Fernandez-Aranda, F. Social, clinical, and policy implications of ultra-processed food addiction. BMJ. (2023) 383:e075354. doi: 10.1136/bmj-2023-075354
52. Schulte, EM, Chao, AM, and Allison, KC. Advances in the neurobiology of food addiction. Curr Behav Neurosci Rep. (2021) 8:103–12. doi: 10.1007/s40473-021-00234-9
53. Lane, MM, Gamage, E, Travica, N, Dissanayaka, T, Ashtree, DN, Gauci, S, et al. Ultra-processed food consumption and mental health: a systematic review and meta-analysis of observational studies. Nutrients. (2022) 14:2568. doi: 10.3390/nu14132568
54. MD ES. Nutritional psychiatry: your brain on food 2022, Available at: https://www.health.harvard.edu/blog/nutritional-psychiatry-your-brain-on-food-201511168626
55. Whelan, K, Bancil, AS, Lindsay, JO, and Chassaing, B. Ultra-processed foods and food additives in gut health and disease. Nature reviews. Gastroenterology & Hepatology. (2024) 24:e5. doi: 10.1038/s41575-024-00893-5
56. Martínez Steele, E, Khandpur, N, da Costa Louzada, ML, and Monteiro, CA. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS One. (2020) 15:e0236738. doi: 10.1371/journal.pone.0236738
57. Thoene, M, Dzika, E, Gonkowski, S, and Wojtkiewicz, J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol a: a literature review. Nutrients. (2020) 12:532. doi: 10.3390/nu12020532
Keywords: ultra-processed food, quality of life, obesity, overweight, women
Citation: Hosseininasab D, Shiraseb F, Bahrampour N, da Silva A, Hajinasab MM, Bressan J and Mirzaei K (2024) Ultra-processed food consumption and quality of life: a cross-sectional study in Iranian women. Front. Public Health. 12:1351510. doi: 10.3389/fpubh.2024.1351510
Received: 06 December 2023; Accepted: 13 March 2024;
Published: 11 April 2024.
Edited by:
Maroof Alam, University of Michigan, United StatesReviewed by:
Asim Rizvi, Aligarh Muslim University, IndiaCopyright © 2024 Hosseininasab, Shiraseb, Bahrampour, da Silva, Hajinasab, Bressan and Mirzaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khadijeh Mirzaei, bWluYV9taXJ6YWVpMTAxQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.