- 1Institute for Mental Health Policy and Research at CAMH, Toronto, ON, Canada
- 2Department of Psychiatry, Institute of Medical Sciences, University of Toronto, Toronto, ON, Canada
- 3Department of Psychology, McGill University, Montreal, QC, Canada
Background: Problematic cannabis use is highly prevalent among people with mood disorders. This underscores the need to understand the effects of cannabis and cannabinoids in this population, especially considering legalization of recreational cannabis use.
Objectives: We aimed to (1) systematically evaluate cross-sectional and longitudinal studies investigating the interplay between cannabis use, cannabis use disorder (CUD), and the occurrence of mood disorders and symptoms, with a focus on major depressive disorder (MDD) and bipolar disorder (BD) and; (2) examine the effects of cannabis on the prognosis and treatment outcomes of MDD and BD.
Methods: Following PRISMA guidelines, we conducted an extensive search for English-language studies investigating the potential impact of cannabis on the development and prognosis of mood disorders published from inception through November 2023, using EMBASE, PsycINFO, PubMed, and MEDLINE databases.
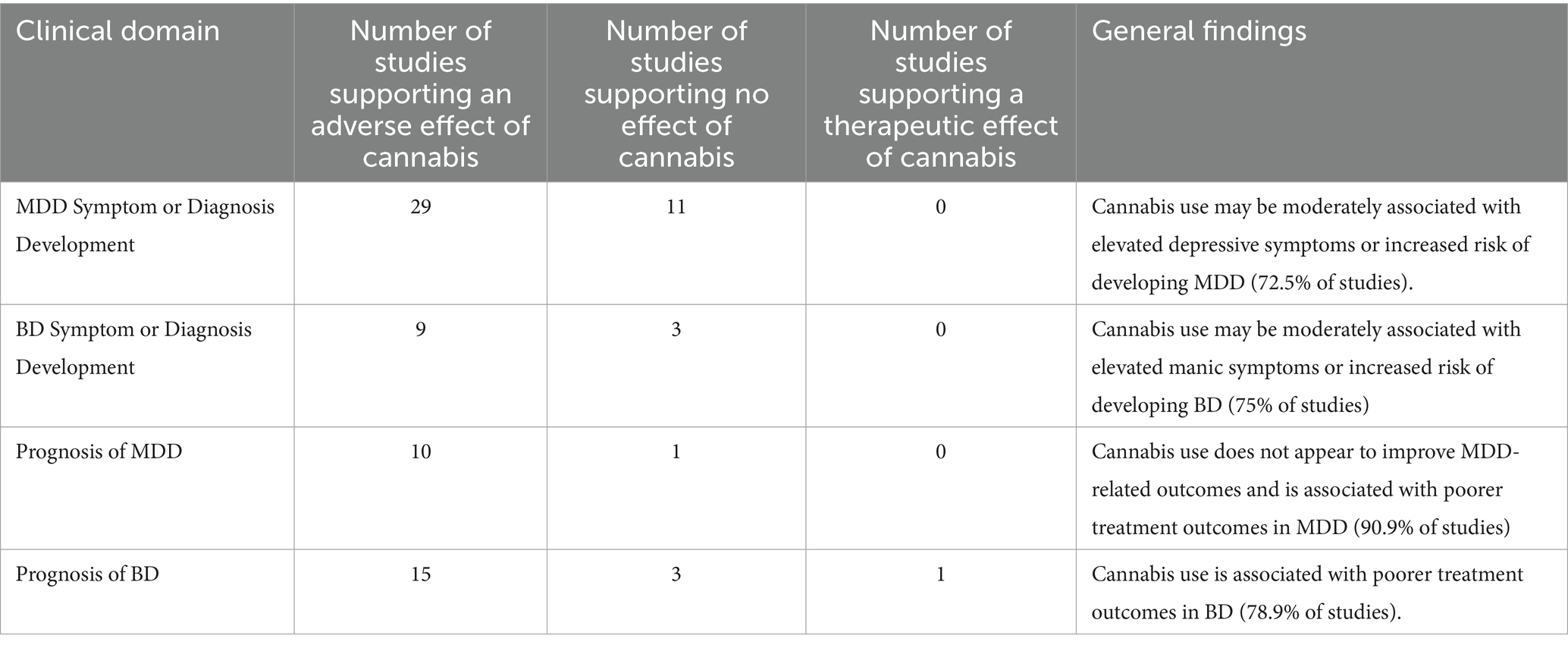
Results: Our literature search identified 3,262 studies, with 78 meeting inclusion criteria. We found that cannabis use is associated with increased depressive and manic symptoms in the general population in addition to an elevated likelihood of developing MDD and BD. Furthermore, we observed that cannabis use is linked to an unfavorable prognosis in both MDD or BD.
Discussion: Our findings suggest that cannabis use may negatively influence the development, course, and prognosis of MDD and BD. Future well-designed studies, considering type, amount, and frequency of cannabis use while addressing confounding factors, are imperative for a comprehensive understanding of this relationship.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023481634.
Introduction
Mood disorders represent a substantial global mental health challenge, with major depressive disorder (MDD) affecting up to 15% of the population, or approximately 300 million individuals worldwide (1). Moreover, bipolar disorder (BD) exhibits a lifetime prevalence of 1.9%, with an additional 4.6% of individuals experiencing subclinical presentations (e.g., cyclothymia) (2). These disorders typically start in adolescence and follow a chronic course, commonly leading to substantial functional impairment (3, 4). Moreover, both MDD and BD contribute significantly to years marked by disability, underscoring their profound impact on quality of life and well-being.
Concurrently, the landscape of cannabis use is evolving globally, with 192 million people reporting its use in 2018 (5), and rates of cannabis use disorder (CUD) estimated at ~3% (6). Notably, the rates of cannabis use among people with mood disorders are increasing at a faster rate compared to those without such conditions (7, 8). For instance, between 2005 and 2006, people with MDD showed a 30% higher likelihood of daily cannabis use than their non-depressed counterparts. However, this proportion surged to 216% between 2015 and 2016 (9).
This evolving trend in cannabis use among people with mood disorders is particularly relevant because it raises pivotal questions on how the compounds in cannabis may influence the development, course, and treatment of mood disorders. Cannabis contains ~400 compounds, with over 100 identified cannabinoids (10). Two extensively studied compounds in cannabis are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is known for producing psychotomimetic and anxiogenic effects (11, 12), whereas CBD is believed to possess antipsychotic, pro-cognitive, and anti-anxiety properties (13, 14). These compounds target the endocannabinoid system, which is involved in brain development, cognition, and emotion regulation (14, 15). Manipulating this system through cannabis or exogenous cannabinoids may affect the pathophysiology and progression of mental health disorders.
Concerning the effects of cannabis use on mood disorders, a complex relationship exists, characterized by proposals of both harmful and therapeutic effects in animal and human populations. Evidence indicates that a significant number of people report relief of depressive symptoms during acute cannabis intoxication. A recent meta-analysis found that approximately 34% of people using medical cannabis reported the alleviation of mood symptoms, potentially including attenuation of cannabis withdrawal, as their primary reason for using cannabis (16). However, long-term, or heavy cannabis use is also associated with the exacerbation of mood symptoms (17, 18). This paradox raises questions about how cannabis might impact the development and progression of mood disorders.
Evidence on cannabis use and mood disorders is limited and, at times, inconsistent. While some studies have not established an association between self-reported cannabis use and MDD or BD following confounder adjustments (19), other observational studies have identified a positive relationship between cannabis use and subsequent MDD, BD, and manic symptoms (20, 21). Therefore, the objectives of this systematic review are to: (1) comprehensively evaluate cross-sectional and longitudinal studies investigating the interplay between cannabis use, cannabis use disorder (CUD), and the occurrence of mood disorders and symptoms, with a focus on MDD and BD and; (2) examine the effects of cannabis, including, tetrahydrocannabinol (THC) and cannabidiol (CBD), on prognosis, treatment outcomes, and non-clinical aspects such as cognition and neural functioning in MDD and BD.
Methods
Search strategy
This systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22). Using EMBASE, PsycINFO, PubMed, and MEDLINE, we identified English-language studies published from inception through November 2023. A combination of free-text terms and medical subject heading terms were used for the subject search. Search terms (found in the title or abstract) included the following: ‘cannabis’, ‘tetrahydrocannabinol’, ‘cannabidiol’, ‘cannabinoid’, ‘bipolar disorder’, ‘major depressive disorder’, ‘mania’, and ‘suicide’. Furthermore, we manually searched through the reference lists of all eligible articles and relevant systematic reviews to retrieve additional studies.
Title and abstract screening in addition to full-text review were conducted by two of the reviewers (MS and ED). Any uncertainties were reviewed and reconciled by the senior author (TPG). This systematic review was registered on PROSPERO (ID: CRD42023481634).
Inclusion and exclusion criteria
We considered observational studies focusing on the influence of cannabis on the initiation of mood disorders, giving priority to both longitudinal and cross-sectional studies. Included studies were required to assess major depressive disorder (MDD), bipolar disorder (BD), mania, hypomania, suicidality, dysthymia, or depressive symptoms using validated clinical tools. For studies investigating the development of mood disorders or symptoms, we additionally limited our scope to studies conducted in the general population. In the context of studies evaluating the effects of cannabinoids on the treatment outcomes and prognosis of MDD and BD, we considered both experimental and observational studies. Despite the anticipated limited availability of large, well-controlled randomized controlled trials (RCTs) in this domain, our search maintained inclusivity to comprehensively assess the available literature.
We excluded the following from our review: (a) literature reviews, meta-analyses, dissertations, commentaries, conference presentations, abstracts, and case studies; (b) studies examining the effect of mood disorders on subsequent cannabis use; (c) studies lacking a baseline measure of cannabis use; (d) animal models of cannabis use; (e) studies involving a special population (e.g., persons living with human immunodeficiency virus) and; (f) non-English publications.
Data extraction and quality assessment
Data was extracted by one reviewer (MS). The following variables from each article were recorded: author, publication year, study design, number of participants, age range of participants, follow-up time, cannabis use measure, mood disorder/symptom measure, and relevant findings.
The Newcastle-Ottawa Scale (NOS) evaluated the quality of included observational studies (23). The NOS assesses non-randomized studies on a scale from 0–9 based on three main criteria: (1) selection of cases and controls (e.g., adequate definition, representativeness, and selection of cases); (2) comparability of cases and controls (e.g., adequate controlling or adjustment for confounding variables); and (3) ascertainment of exposure (e.g., adequate assurance that the cases were exposed to the variable of interest). Studies receiving a score ≥ 7 on the NOS are categorized as high quality, while studies receiving a score ≤ 4 are considered to exhibit low methodological quality (23).
We evaluated the quality of randomized controlled trials (RCTs) by assessing their risk of bias, through the Cochrane Collaboration Risk of Bias tool 2.0 (RoB) (24). For non-randomized interventions, the ROBINS-I tool was implemented (25). Each study was categorized as having high, low, or unclear risk in domains such as randomization, intervention adherence (deviation from intended interventions), missing outcome data, outcome measurement, and selective reporting. The worst grading on individual items defines the overall risk of bias for each single study.
Results
Study characteristics
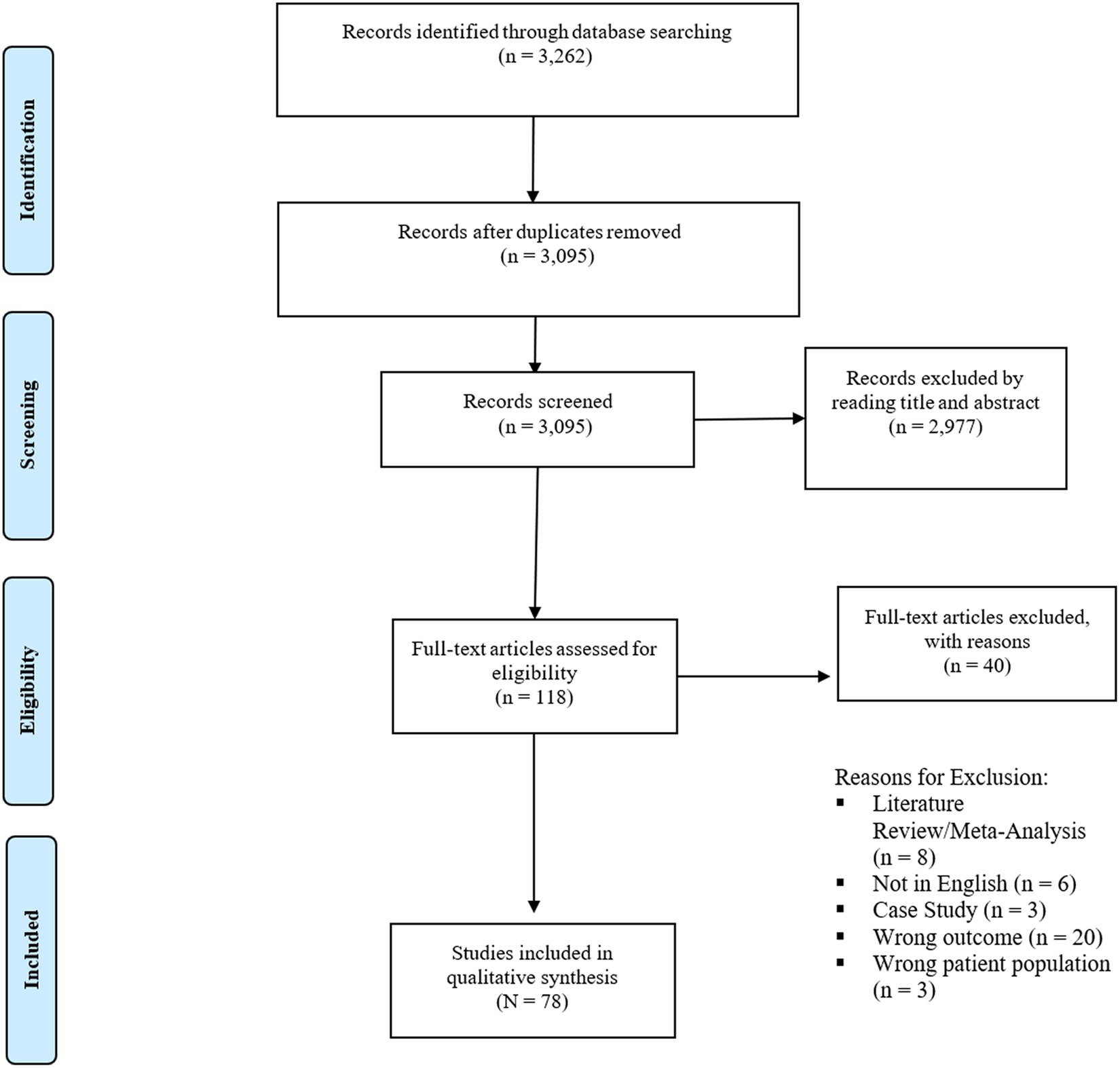
Our initial literature search yielded a total of 3,262 studies. After eliminating 167 duplicates, we retained 3,095 records for screening based on their titles and abstracts. Following this screening process, 118 articles underwent full-text review. Of these, 78 papers met our inclusion criteria. Specific reasons for exclusion are listed in the PRISMA flowchart (Figure 1). The included studies spanned from 2001 to 2023 and were conducted in a wide range of countries. Among the 78 studies, there was one RCT, specifically examining the therapeutic potential of cannabidiol in treating BD (26). The remaining were observational or non-randomized in nature, with 52 adopting longitudinal designs and 25 utilizing cross-sectional designs. All but three publications (26–28) relied on self-report measures to assess cannabis use. Forty-eight studies examined the connection between cannabis use and MDD or depressive symptoms, 27 studies examined the relationship between cannabis use and BD or mania, and 3 studies examined the relationship between cannabis use and both depressive and manic symptoms. Table 1A outlines cross-sectional and longitudinal studies examining the association between cannabis use and MDD symptoms and diagnoses, while Table 1B addresses the corresponding relationship with manic symptoms and BD diagnoses. Table 2A provides a detailed account of cross-sectional and longitudinal studies that explore the impact of cannabis use on MDD while Table 2B examines the corresponding relationship within BD.
Table 1A. Summary of observational studies exploring the relationship between cannabis use, depressive symptoms, and MDD diagnoses (40 Studies, N = 1,015,726).
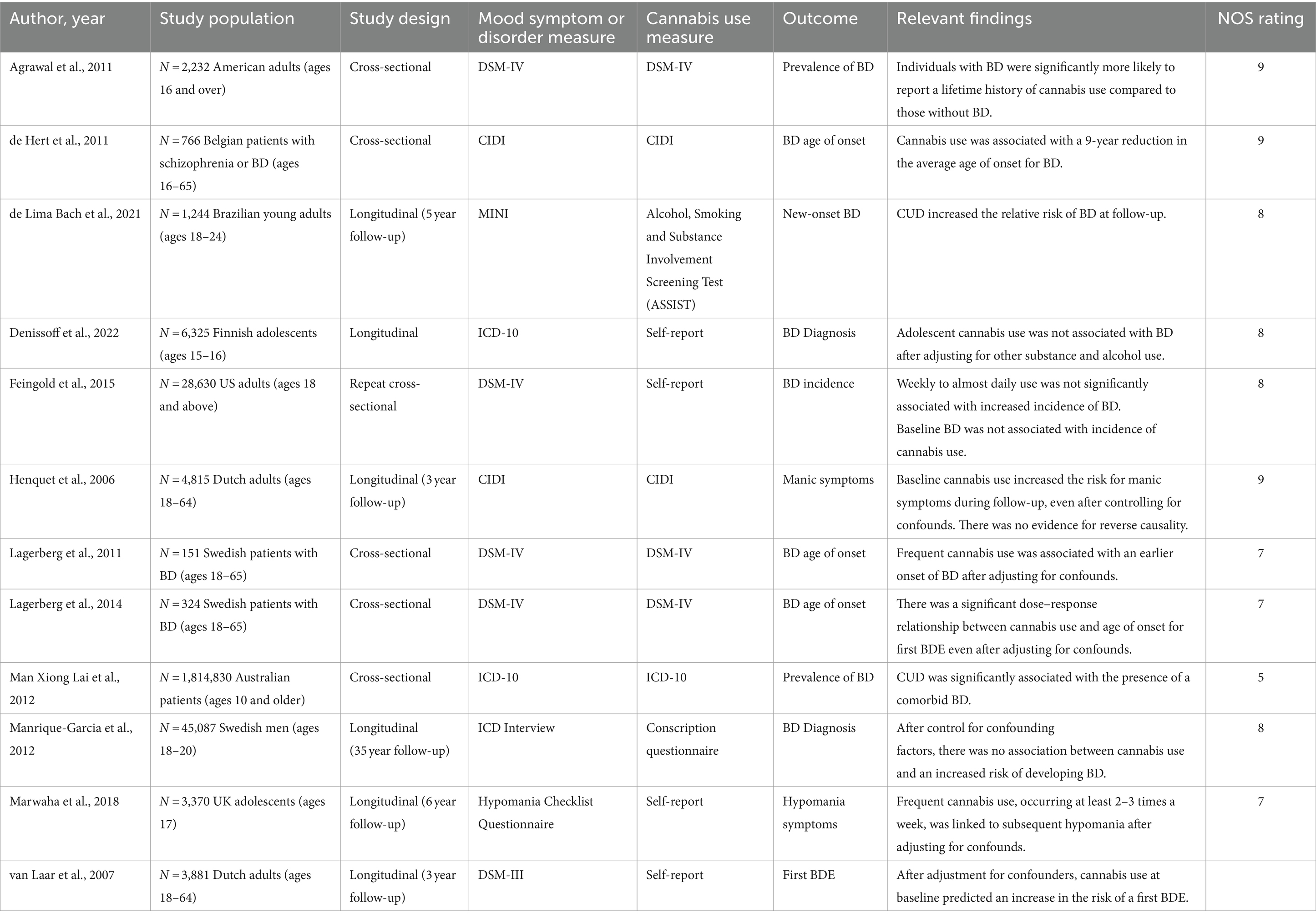
Table 1B. Summary of observational studies exploring the relationship between Cannabis use, manic symptoms, and BD diagnoses (12 Studies, N = 1,907,623).
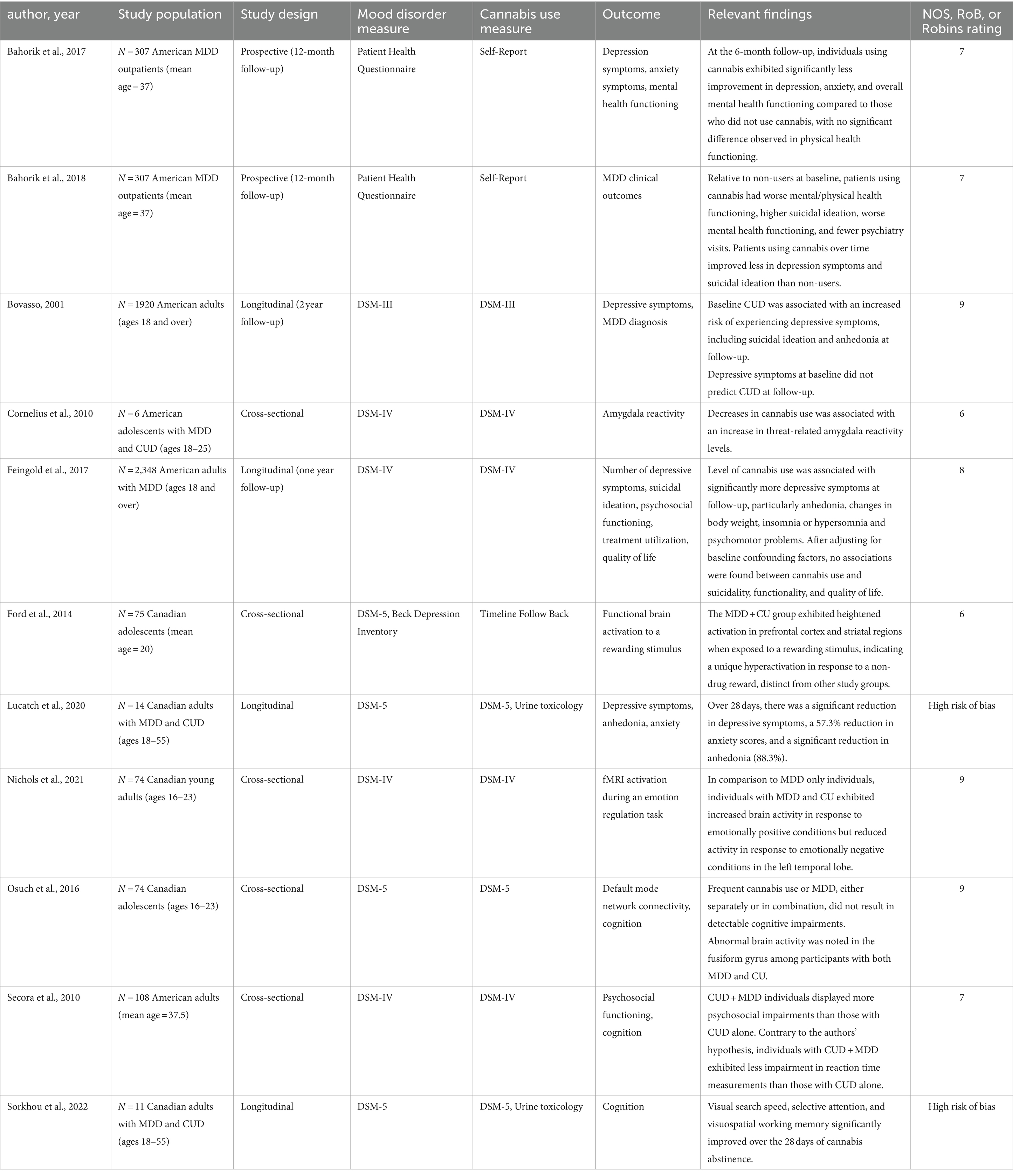
Table 2A. Summary of studies exploring the impact of cannabis on course of illness in MDD (11 Studies, N = 4,778).
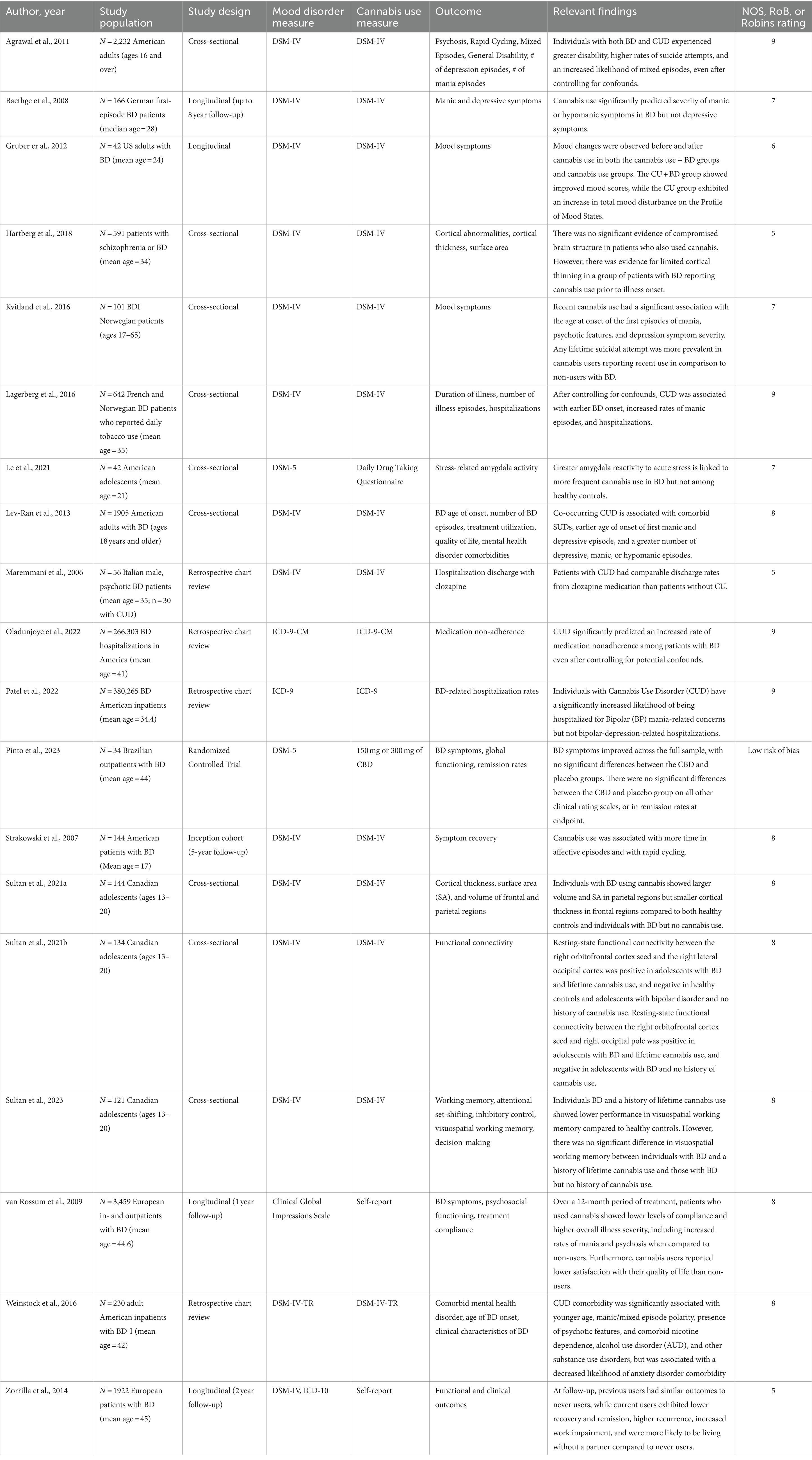
Table 2B. Summary of studies exploring the impact of cannabis on course of illness in BD (19 Studies, N = 658,278).
The complete risk of bias assessment for each study is reported in Tables 3, 4. Concerning the NOS ratings, 78.7% (59 out of 75) of the studies exhibited high methodological quality, with the remaining studies indicating moderate methodological quality (21.3%; comprising 16 out of 75 studies). The ROBINS-I tool was applied to two studies conducted by the same research group (27, 28), where both studies displayed a high risk of bias. The only randomized controlled trial included in the review demonstrated a low risk of bias.
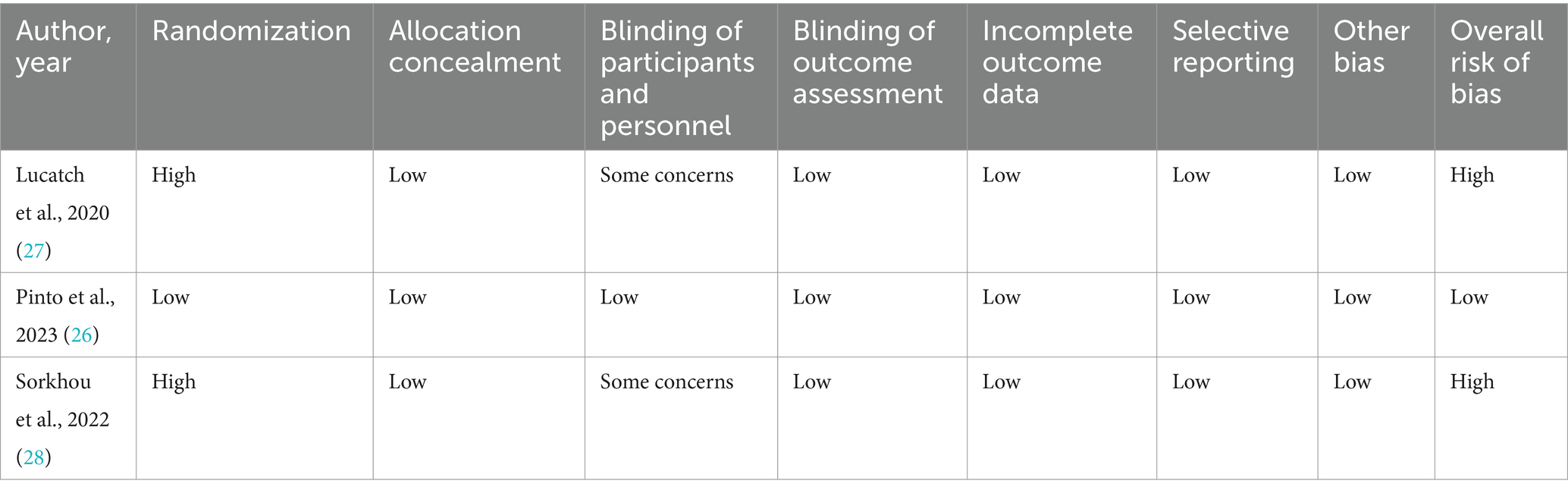
Table 4. Risk of bias using Cochrane’s risk of bias in non-randomized studies of interventions tool (ROBINS-I) and Cochrane’s risk of bias tool (RoB-2).
Relationship between cannabis use, depressive symptoms and MDD diagnoses
Nine studies examined the cross-sectional relationship between cannabis use and depressive symptoms or MDD diagnoses, with seven identifying significant associations (30, 46–48, 69, 86, 101). In a retrospective analysis of data from the Australian Twin Registry, frequent cannabis use (≥ 100 times across their lifetime) was associated with MDD and suicidal ideation in both monozygotic and dizygotic twins (30). In a similar study using nationally representative data to investigate the relationship between cannabis use and a past-year experience of a major depressive episode (MDE), Fairman et al. (48) found that, compared to individuals with no cannabis use, those reporting both early-onset and adult-onset cannabis use had an increased likelihood of experiencing a MDE, even after adjusting for other substance use (with odds ratios of 1.7 and 1.8, respectively). These findings contrast with an epidemiological study conducted in the UK among young adults, where self-reported use of high- or low-potency cannabis did not correlate with a diagnosis of MDD after controlling for relevant factors, including other substance use (61). Similarly, Chabrol et al. found no significant association between cannabis use disorder (CUD) with suicidal ideation or depressive symptoms in a sample of 1,034 French postsecondary students (38).
In two smaller cross-sectional studies that examined the role of cannabis use in depressive symptoms among youth, cannabis use emerged as a significant predictor of this outcome (47, 101). Medina and colleagues (101) demonstrated that adolescent cannabis users, in comparison to never users, had exhibited neural abnormalities, including reduced hippocampal volumes and white matter alterations, along with higher levels of depressive symptoms. In a separate study examining depressive symptoms among 707 Spanish youth, Espada and colleagues (47) found that, after controlling for alcohol and tobacco use, adolescents who reported past 30-day cannabis use reported significantly higher levels of depressive symptoms than non-users.
Thirty-one studies prospectively explored the relationship between cannabis use and subsequent depression, including depressive symptoms, MDD diagnoses, and suicidality. Among these studies, 22 identified a significant temporal association between baseline cannabis use and subsequent depression. Cannabis use was a significant predictor of adulthood depression across 12 sample sets following adolescents (20, 34, 42, 59, 60, 66, 70, 74, 78, 82, 84, 89). In a sample of LGB and heterosexual adolescents, London-Nadeau and colleagues observed that cannabis use at age 13 predicted depression symptoms at ages 15 and 17 among LGB participants only (70). However, a bidirectional relationship was also identified among LGB adolescents, where baseline depressive scores predicted subsequent cannabis use. In one study that tracked a cohort of Australian young adults from birth, and after controlling for covariates, individuals who used cannabis at least once a week were more likely to report depressive symptoms than those who never used cannabis or those who used cannabis less than weekly (42). Furthermore, among young adults who used cannabis at least weekly, the risk of reporting depressive symptoms was greater among those who initiated cannabis use before the age of 15. Additionally, one study prospectively explored the relationship between cannabis use, depressive symptoms, and the serotonin transporter gene (5-HTTLPR) in adolescents (82). The serotonin transporter gene is highly regarded as a key candidate gene due to its involvement in development of depression and other mental health disorders [(see 102)]. Specifically, this gene encodes the serotonin transporter protein, which plays a crucial role in reuptake of serotonin into presynaptic neurons. The authors found that cannabis use elevated the risk of experiencing heightened depressive symptoms over a 5-year period, but only among people with the short allele of the 5-HTTLPR genotype.
In prospective studies that identified gender-specific effects between cannabis use and subsequent depression, findings were mixed. In a nationally representative study of American youth and adults by Carra and colleagues (37), it was found that women who reported cannabis use at baseline were more likely than men to experience a major depressive episode (MDE) 1 year later. Moreover, among adults only, higher potency cannabis use predicted a subsequent MDE. Similarly, in a study that tracked cannabis use trends among Australian adolescents over a seven-year period, daily cannabis use was associated with a more than five-fold increase in depressive symptoms among girls, but not among boys (84). In contrast, three studies detected stronger associations between cannabis use and subsequent depressive symptoms in men (39, 50, 66, 90). A longitudinal study spanning 4 years and involving 951 adolescents observed that increases in cannabis use were associated with increases in the severity of depressive symptoms in boys only (50).
Three prospective studies examined the link between cannabis use and suicidality, with all reporting significant associations (55, 85, 90). In a study that followed adolescents for 13 years, the authors found that while frequent or any cannabis use did not predict later depression, any cannabis use was linked to later suicidal ideation, and frequent use was associated with suicide attempts (85).
However, nine longitudinal studies did not establish a temporal relationship between cannabis use and subsequent depressive symptoms or diagnoses when controlling for relevant confounding variables, including other alcohol or substance use (35, 41, 49, 56, 58, 71, 87, 99, 103). In a recent study examining the relationship between various substance use disorders (SUDs) and the incidence of a first MDE, Hassan et al. (58) found that CUD alone did not increase the likelihood of experiencing a MDE 1 year later. However, the comorbidity of CUD and opioid use disorder (OUD) significantly increased this association. Moreover, two prospective studies found that while baseline cannabis use did not predict a diagnosis of MDD at follow-up, baseline severity of depressive symptoms was predictive of subsequent cannabis use (49, 87).
Relationship between cannabis use, mania, and BD diagnoses
Five studies examined the cross-sectional association between cannabis use and the presence of (hypo) manic symptoms or the diagnosis of BD, all of which identified a significant relationship (29, 43, 63, 64, 72). Lagerberg and colleagues (64) found a significant dose-dependent relationship between cannabis use and age of onset for BD. This effect of cannabis was not confounded by multiple variables, including gender, family history of BD, lifetime psychotic symptoms, and other substance or alcohol use. Similarly, in a retrospective chart review of more than one million Australian patients, which focused on the relationship between SUDs and mental health disorders, Lai and colleagues found a significant association between CUD and BD diagnoses (72).
Moreover, seven studies prospectively examined the relationship between cannabis use and BD diagnoses and symptoms, with four establishing a significant association (21, 44, 76, 95). In a nationally representative sample of Dutch adults, cannabis use at baseline nearly tripled the risk of reporting manic symptoms at a 3-year follow-up, even after controlling for confounds (21). No evidence of reverse causality was found. Similarly, Marwaha et al. (76) found that among 3,370 UK adolescents, cannabis use at least 2–3 times per week at baseline doubled the risk of reporting manic symptoms at a 6-year follow-up.
However, three studies did not detect a temporal relationship between cannabis use and BD diagnoses or symptoms (45, 49, 71). Using Waves 1 and 2 of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), Feingold et al. (49) found that baseline cannabis use was not associated with an increased incidence of BD or MDD when controlling for other substance use. In a separate epidemiological study that examined lifetime cannabis exposure and its association with BD within a Finnish birth cohort, no association was found between adolescent cannabis use and BD diagnosis in adulthood after adjusting for alcohol use, smoking, and lifetime drug use (45).
Impact of cannabis on the course of MDD
Five cross-sectional studies evaluated the effects of cannabis use in patients with MDD (40, 51, 79, 81, 88), with three identifying neural abnormalities compared to controls (40, 51, 79). Ford et al. (51) employed a passive music listening paradigm to investigate variations in reward processing in brain activity across four groups of youth: healthy controls, frequent cannabis users, individuals with MDD, and individuals with MDD who use cannabis. They observed significant hyperactivation in prefrontal and limbic regions during the task only within the MDD cannabis-using group. In a study assessing cognition and psychosocial functioning, Secora and colleagues found that adults with comorbid CUD and MDD reported more psychosocial impairments than those with CUD alone (88). However, when assessing memory, verbal skills, and attention, there were no significant differences between the two groups. Similar findings were noted in a more recent study that examined cognitive functioning and neural activity across four groups of adolescents: healthy controls, youth with current MDD without frequent cannabis use, youth with frequent cannabis use, and youth with frequent cannabis use and MDD (81). The authors found no evidence of cognitive impairments in any of the cannabis-using groups when compared to non-users. Furthermore, there was no interaction observed between MDD and cannabis use on resting state fMRI connectivity.
When considering the longitudinal effects in MDD, six prospective studies demonstrated an adverse effect of cannabis use (19, 27, 28, 32, 33, 36). In a recent open-label, single-arm investigation utilizing monetary contingency management (CM), the cognitive and clinical effects of 28 days of cannabis abstinence were examined in people with co-occurring MDD and CUD (27, 28). With 8/11 participants achieving cannabis abstinence as confirmed by urine toxicology, significant improvements were observed in depressive symptoms and anhedonia (27), and in specific cognitive areas including visual search speed, visual sustained attention, response inhibition, and visuospatial working memory (28). Moreover, in a secondary analysis of a randomized controlled trial involving 307 outpatients with MDD receiving substance/alcohol use treatment, patients using cannabis for medical purposes experienced worse mental and physical health functioning at study endpoint, in comparison to non-users (32, 33). Furthermore, non-medical use of cannabis was linked to higher suicidal ideation, poorer global functioning, and fewer psychiatry visits. Over time, patients using non-medical cannabis exhibited less improvement in depression symptoms and suicidal ideation compared to non-users. Utilizing data from Waves 1 and 2 of NESARC, Feingold et al. (19) explored the effect of cannabis use and CUD on the course and outcome of MDD over a three-year period. Cannabis use at baseline was associated with a significant increase in depressive symptoms at follow-up, but did not correlate with suicidality, quality of life, or psychosocial functioning. Similar findings were obtained by Bovasso (36) where baseline CUD was predictive of worsened depressive symptoms, suicidal ideation, and anhedonia in a community sample of adults with MDD over a 2-year period.
Impact of cannabis on the course of BD
Of 13 cross-sectional studies that investigated cannabis use in BD, 11 identified a negative impact associated with this substance (29, 57, 62, 64, 67, 68, 80, 83, 92, 93, 97). In one study examining clinical characteristics associated with comorbid CUDs in BD inpatients, Weinstock et al. (97) found that CUD comorbidity was significantly associated with a greater presence of psychotic features, diagnosis at a younger age and a greater likelihood of meeting diagnostic criteria for an alcohol use disorder (AUD) or other SUD. Other evidence indicates that cannabis use in BD is associated with greater severity of depressive and manic symptoms (29, 62, 64, 68), an increased number of hospitalizations (64, 83), reduced medication adherence (80), greater disability (29) and a higher number of suicide attempts (29, 62). Moreover, two studies found that compared to BD-only patients, those with comorbid CUD exhibited a reduction in cortical thickness in frontal brain regions (57, 93). Similarly, Sultan and colleagues (92) observed that patients with BD and comorbid CUD demonstrated reduced resting-state functional connectivity across multiple frontal-temporal regions, in contrast to those with BD only.
However, two studies did not find a significant relationship between cannabis use and BD-related outcomes (75, 94). In a retrospective chart review, Maremmani and colleagues discovered that, contrary to their initial hypothesis, hospital discharge rates were comparable between BD patients with CUD and BD patients without cannabis use (75). In a separate study that compared various cognitive outcomes among three distinct groups, including adolescents with BD and no history of cannabis use, those with BD and cannabis use, and healthy controls without cannabis use, the authors found that the BD and cannabis use group and the BD group exhibited similar cognitive performance, which was inferior to controls (94). This finding contradicted the researchers’ initial hypothesis that cannabis use exacerbates cognitive impairments in adolescents with BD.
Five of the studies included in the review prospectively explored the effects of cannabis in BD. All of these studies (31, 91, 96, 100), except one (104), revealed negative outcomes linked to cannabis use. In a 2-year prospective observational study, individuals with BD who continued using cannabis demonstrated lower recovery rates and remission, in addition to poorer psychosocial functioning than never users and those who reduced their cannabis use (100). In a study examining clinical outcomes among a sample of 3,459 European patients diagnosed with BD, those who self-reported cannabis use exhibited reduced treatment compliance, increased illness severity, and lower satisfaction with their quality of life at a one-year follow-up (96). Other evidence suggests that cannabis use in BD is temporally associated with severity of mania (31), greater number of manic and depressive episodes (91), and rapid cycling (91).
However, in a prospective observational study that examined the immediate effects of smoking cannabis on mood in BD, results showed that the BD group experienced significant mood improvement compared to the group who used cannabis without BD (54). This improvement was evident in reductions in total mood disturbance as measured by the Profile of Mood States. These findings provide empirical support for anecdotal reports indicating that cannabis is sometimes used to alleviate mood-related symptoms (e.g., alleviation of cannabis withdrawal) in at least a subset of people with BD.
In the only randomized controlled trial (RCT) included in our review, Pinto and colleagues (26) investigated the potential of adjunctive cannabidiol (CBD; 0, 150 and 300 mg/day) for BD treatment. BD. Both the CBD and placebo groups exhibited a significant reduction in BD symptoms at study endpoint (Week 8), with no significant between group differences. Adverse events were comparable between groups, indicating that CBD could be a safe and well-tolerated treatment option.
Discussion
This systematic review provides a comprehensive assessment of the impact of cannabis use on the development of mood disorders and associated symptoms, specifically emphasizing MDD and BD. Furthermore, we examined how cannabis use impacts prognosis and treatment outcomes in people with MDD or BD. Our findings suggest that cannabis use is associated with elevated depressive and manic symptoms in the general population, as well as an increased risk of developing MDD and BD. Furthermore, there is minimal evidence to support the notion that cannabis improves clinical or treatment outcomes for MDD or BD; instead, its use is associated with a poorer prognosis for both disorders (see Table 5).
Our review serves as an extension of prior reviews and meta-analyses by identifying the associations between cannabis use and depressive and manic symptoms across both clinical and non-clinical populations. While previous reviews have focused particularly on specified clinical populations (105), or solely examined the prospective risk associated with cannabis use and mood disorders (106), our review uniquely bridges these perspectives. Furthermore, our review conducts a thorough investigation into the multifaceted impact of cannabis on a range of outcomes within MDD and BD. This exploration extends beyond the traditional focus on mood symptoms, encompassing critical domains such as cognition and neural functioning. We also examine the potential therapeutic effects of cannabinoids in mood disorders, noting that only one trial met the specified criteria (26). Overall, this expanded analysis enriches our comprehension of the intricate relationship between cannabis use and mood-related outcomes, offering a more holistic and comprehensive perspective.
Cannabis use, mood symptoms, and mood disorder diagnoses
We found that cannabis use is associated with elevated depressive symptoms, mania, and suicidality. Consistent with other reviews and meta-analyses (107–109), a dose-dependent relationship has been observed between the duration of cannabis use, particularly when initiated in adolescence, and the heightened occurrence of symptoms related to depression and mania [(e.g., 37, 64)]. These findings underscore the critical role of age of onset in cannabis use. Adolescence is a period of dynamic brain development, marked by significant structural and functional changes, including maturation of key brain regions implicated in mood regulation and emotional processing (110, 111). This period represents a heightened vulnerability to the potential long-term consequences of environmental stressors, including THC exposure. Indeed, a recent review of over 30 human magnetic resonance imaging studies comparing former cannabis users with control groups has highlighted the emergence of neuroanatomical abnormalities among cannabis users in regions with a high density of CB1 receptors (112).
However, our review obtained some evidence that the relationship between cannabis and mood disorders may be influenced by additional factors, such as concurrent substance use (41, 45, 49). People who engage in cannabis use often co-use other substances (113, 114). Importantly, these co-occurring substances also have the potential to impact brain development and influence mood. This underscores the critical need to account for other substance use when investigating the complex relationship between cannabis use and mood disorders.
Cannabis and the course of mood disorders
In MDD, we found compelling evidence indicating that cannabis use and CUD are linked to more pronounced symptomatology and a less favorable prognosis compared to patients who do not use cannabis. This includes greater levels of anhedonia (27, 36), suicidality (33), reduced adherence (33) and poorer cognition (28). Notably, a study assessing the effects of 28 days of cannabis abstinence demonstrated substantial enhancements in specific cognitive domains and depressive symptoms (27, 28). These findings imply that the adverse effects of cannabis use may be reversible. Nonetheless, it is imperative to acknowledge a significant limitation in this study—the absence of a non-abstinent control group. This limitation underscores the need for further controlled, long-term research to elucidate the impact of cannabis on clinical outcomes and cognition in MDD. Interestingly, other data suggests that cannabis use in MDD have better cognitive functioning than those who use cannabis without MDD (88). These findings underscore the need for more controlled research on cannabis and cognition in MDD.
Our systematic review also revealed a multitude of adverse consequences of cannabis use in people with BD. This includes more severe symptom profiles (29, 31, 96), a greater number of manic and depressive episodes (62, 64), more rapid cycling (91) and higher levels of suicidality (29). Intriguingly, one study suggested that people with BD who use cannabis experienced greater immediate mood improvements than cannabis-using controls (104). This observation is in line with other research indicating that people with psychiatric disorders may use cannabis for short-term symptom relief (e.g., withdrawal alleviation) (115, 116), despite adverse long-term consequences.
Moreover, we found evidence that baseline manic or depressive symptoms were predictive of subsequent cannabis initiation [(e.g., 37, 50)]. These findings have significant implications for treatment, suggesting that following the onset of a mood disorder, people may be more prone to initiate cannabis use. Given the available evidence indicating that cannabis use may be detrimental to people with MDD and BD, this association warrants further study. Thus, it is crucial for clinicians and researchers to explore alternative, evidence-based therapeutic approaches for people with mood disorders who may use cannabis for symptom management.
We found no compelling evidence supporting the therapeutic potential of cannabinoids in mood disorders. The only RCT included in our review that examined CBD’s efficacy and safety for BD yielded inconclusive clinical results. Nonetheless, the findings indicate CBD’s potential as a safe option, and further extensive clinical trials are warranted. Other research has evaluated cannabinoids such as dronabinol, nabilone, and nabiximols on depressive symptoms (117, 118). These studies have been conducted within the context of treatment studies focused on other primary conditions, notably, chronic pain disorders. While there is some evidence that cannabinoids may offer benefits for mood and anxiety symptoms in specific patient populations (119), it is important to note that these people may not necessarily meet the clinical criteria for mood disorders. Overall, there is a need for further investigation, particularly in clinical cohorts with established mental health diagnoses.
Strengths and limitations
This systematic review has several notable strengths. First, the review encompassed a comprehensive range of studies, including both cross-sectional and prospective designs. This ensured a thorough examination of relationships between cannabis use and mood disorders, providing a holistic view of available evidence. Furthermore, we investigated a diverse set of outcomes. By considering various dimensions, such as depressive and manic symptoms, diagnostic outcomes, cognition, neuroimaging and treatment outcomes, this review provides multifaceted assessment of the impact of cannabis use on mood disorders.
However, there were several limitations. The inherent heterogeneity across the included studies, including variations in methodology, outcome measures, and participant characteristics is a significant limitation. For example, measurements of cannabis use exhibited substantial variability across studies, with many relying on single-item self-reports and few employing objective measures. This lack of standardized assessments of cannabis use introduces variability that may impact our findings. Future research would benefit from more comprehensive methods for quantifying cannabis use (e.g., urine toxicology). Second, few studies examined variables associated with cannabis use, including potency and route of administration, in relation to our outcomes. Two studies (36, 50) examined the relationship between low and high potency cannabis use and depressive symptoms and diagnoses, yielding mixed findings. In contrast, only one study (68) delved into the association between cannabis potency and the initial onset of a bipolar disorder episode, revealing a positive relationship. Relatedly, while only one of the included studies captured data on the route of cannabis administration, no analyses were reported on its relationship with mood symptoms (98). The route of cannabis administration may influence mood-related symptoms through its impact on the speed of onset, bioavailability, peak concentrations, metabolism, and the context of use (120–122). Thus, studying these factors may be crucial for developing a nuanced understanding of how cannabis use may affect mental health. Furthermore, the review predominantly relies on observational studies, which inherently limited our ability to establish causality or determine the direction of the relationship between cannabis use and mood disorders. Moreover, several studies did not control for important confounding factors such as other substance misuse or psychosocial variables. Finally, the majority of included studies did not conduct gender-specific analyses, despite existing evidence indicating gender differences in the effects of cannabis use in the general population (123, 124). This adds to the heterogeneity across studies and calls for more gender-inclusive research to better understand the nuances of this relationship.
Conclusion
We found that cannabis use is linked to increased depressive and manic symptoms in the general population, and an elevated risk of developing MDD and BD. Moreover, there was evidence of cannabis-related harms in mood disorders. While these findings should be interpreted with caution when considering the observational nature of most eligible studies, the potential implications of these findings are substantial for practitioners and policymakers. Mental health practitioners should incorporate cannabis use screening into their standard assessments, ensuring that people with mood disorders are aware of the potential risks associated with cannabis use. Patients with mood disorders may use cannabis to manage mood symptoms, and clinicians should consider evidence-based alternatives for symptom management. Policymakers and public health officials may consider the need for targeted interventions and education programs focusing on the risks of cannabis use, particularly among people at risk for, or diagnosed with mood disorders.
Finally, a deeper exploration of the neurobiological underpinnings and causal relationships between cannabis use and mood disorders, both in human studies and animal models, would provide a more comprehensive understanding of the mechanisms involved. Moreover, well-controlled prospective studies with comprehensive assessments of cannabis use are needed to further elucidate the nuances of this relationship, while also considering sex- and gender-specific analyses. These studies will advance our knowledge and ultimately improve the mental health and well-being of people impacted by mood disorders.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the data that support the findings of this study are available on request from the corresponding author. Requests to access these datasets should be directed to dG9ueS5nZW9yZ2VAY2FtaC5jYQ==.
Author contributions
MS: Data curation, Methodology, Writing – original draft, Writing – review & editing. ED: Data curation, Writing – review & editing. TG: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by an operating grant from the Canadian Institutes of Health Research (CIHR, PJT-190053) to TG.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lim, GY, Tam, WW, Lu, Y, Ho, CS, Zhang, MW, and Ho, RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. (2018) 8:2861. doi: 10.1038/s41598-018-21243-x
2. Stubbs, B, Vancampfort, D, Solmi, M, Veronese, N, and Fornaro, M. How common is bipolar disorder in general primary care attendees? A systematic review and meta-analysis investigating prevalence determined according to structured clinical assessments. Australian & New Zealand J Psychiatry. (2016) 50:631–9. doi: 10.1177/0004867415623857
3. McIntyre, RS, Cha, DS, Soczynska, JK, Woldeyohannes, HO, Gallaugher, LA, Kudlow, P, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. (2013) 30:515–27. doi: 10.1002/da.22063
4. Miller, S, Dell'Osso, B, and Ketter, TA. The prevalence and burden of bipolar depression. J Affect Disord. (2014) 169:S3–S11. doi: 10.1016/S0165-0327(14)70003-5
5. United Nations Office on Drugs and Crime . World drug report 2019: 35 million people worldwide suffer from drug use disorders while only 1 in 7 people receive treatment, United Nations Office on Drugs and Crime. (2019).
6. Hasin, DS . US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. (2018) 43:195–212. doi: 10.1038/npp.2017.198
7. Hasin, D, and Walsh, C. Cannabis use, cannabis use disorder, and comorbid psychiatric illness: a narrative review. J Clin Med. (2020) 10:15. doi: 10.3390/jcm10010015
8. Marti, CN, Arora, S, and Loukas, A. Depressive symptoms predict trajectories of electronic delivery nicotine systems, cigarette, and cannabis use across 4.5 years among college students. Addict Behav. (2023) 146:107809. doi: 10.1016/j.addbeh.2023.107809
9. Gorfinkel, LR, Stohl, M, and Hasin, D. Association of depression with past-month cannabis use among US adults aged 20 to 59 years, 2005 to 2016. JAMA Netw Open. (2020) 3:e2013802-e. doi: 10.1001/jamanetworkopen.2020.13802
10. Ashton, CH . Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. (2001) 178:101–6. doi: 10.1192/bjp.178.2.101
11. D’Souza, DC, Abi-Saab, WM, Madonick, S, Forselius-Bielen, K, Doersch, A, Braley, G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. (2005) 57:594–608. doi: 10.1016/j.biopsych.2004.12.006
12. Lowe, DJE, Sasiadek, JD, Coles, AS, and George, TP. Cannabis and mental illness: a review. Eur Arch Psychiatry Clin Neurosci. (2019) 269:107–20. doi: 10.1007/s00406-018-0970-7
13. Iseger, TA, and Bossong, MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. (2015) 162:153–61. doi: 10.1016/j.schres.2015.01.033
14. Chadwick, VL, Rohleder, C, Koethe, D, and Leweke, FM. Cannabinoids and the endocannabinoid system in anxiety, depression, and dysregulation of emotion in humans. Curr Opin Psychiatry. (2020) 33:20–42. doi: 10.1097/YCO.0000000000000562
15. Fride, E . Endocannabinoids in the central nervous system-an overview. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA). (2002) 66:221–33. doi: 10.1054/plef.2001.0360
16. Kosiba, JD, Maisto, SA, and Ditre, JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. (2019) 233:181–92. doi: 10.1016/j.socscimed.2019.06.005
17. Lev-Ran, S, Roerecke, M, Le Foll, B, George, TP, McKenzie, K, and Rehm, J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. (2014) 44:797–810. doi: 10.1017/S0033291713001438
18. Lucatch, AM, Coles, AS, Hill, KP, and George, TP. Cannabis and mood disorders. Curr Addict Rep. (2018) 5:336–45. doi: 10.1007/s40429-018-0214-y
19. Feingold, D, Rehm, J, and Lev-Ran, S. Cannabis use and the course and outcome of major depressive disorder: a population based longitudinal study. Psychiatry Res. (2017) 251:225–34. doi: 10.1016/j.psychres.2017.02.027
20. Meier, MH, Beardslee, J, and Pardini, D. Associations between recent and cumulative cannabis use and internalizing problems in boys from adolescence to young adulthood. J Abnorm Child Psychol. (2020) 48:771–82. doi: 10.1007/s10802-020-00641-8
21. Henquet, C, Krabbendam, L, de Graaf, R, ten Have, M, and van Os, J. Cannabis use and expression of mania in the general population. J Affect Disord. (2006) 95:103–10. doi: 10.1016/j.jad.2006.05.002
22. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
23. Peterson, J, Welch, V, Losos, M, and Tugwell, P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute (2011).
24. Sterne, JA, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019):l4898. doi: 10.1136/bmj.l4898
25. Sterne, JA, Hernán, MA, Reeves, BC, Savović, J, Berkman, ND, Viswanathan, M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016):355. doi: 10.1136/bmj.i4919
26. Pinto, JV, Crippa, JAS, Ceresér, KM, Vianna-Sulzbach, MF, Silveira Júnior, ÉM, Santana da Rosa, G, et al. Cannabidiol as an adjunctive treatment for acute bipolar depression: a pilot study. Can J Psychiatr. (2023) 69:07067437231209650. doi: 10.1177/07067437231209650
27. Lucatch, AM, Kloiber, SM, Meyer, JH, Rizvi, SJ, and George, TP. Effects of extended Cannabis abstinence in major depressive disorder. Canadian J Addiction. (2020) 11:33–41. doi: 10.1097/CXA.0000000000000090
28. Sorkhou, M, Rabin, RA, Rabin, J, Kloiber, S, McIntyre, RS, and George, TP. Effects of Cananbis abstinence on cognition in major depressive disorder: a pilot study. Am J Addict. (2022) 31:454–62. doi: 10.1111/ajad.13305
29. Agrawal, A, Nurnberger JI Jr,, and Lynskey, MTBipolar Genome Study. Cannabis involvement in individuals with bipolar disorder. Psychiatry Res. (2011) 185:459–61. doi: 10.1016/j.psychres.2010.07.007
30. Agrawal, A, Nelson, EC, Bucholz, KK, Tillman, R, Grucza, RA, Statham, DJ, et al. Major depressive disorder, suicidal thoughts and behaviours, and cannabis involvement in discordant twins: a retrospective cohort study. Lancet Psychiatry. (2017) 4:706–14. doi: 10.1016/S2215-0366(17)30280-8
31. Baethge, C, Hennen, J, Khalsa, HMK, Salvatore, P, Tohen, M, and Baldessarini, RJ. Sequencing of substance use and affective morbidity in 166 first-episode bipolar I disorder patients. Bipolar Disord. (2008) 10:738–41. doi: 10.1111/j.1399-5618.2007.00575.x
32. Bahorik, AL, Leibowitz, A, Sterling, SA, Travis, A, Weisner, C, and Satre, DD. Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J Affect Disord. (2017) 213:168–71. doi: 10.1016/j.jad.2017.02.016
33. Bahorik, AL, Sterling, SA, Campbell, CI, Weisner, C, Ramo, D, and Satre, DD. Medical and non-medical marijuana use in depression: longitudinal associations with suicidal ideation, everyday functioning, and psychiatry service utilization. J Affect Disord. (2018) 241:8–14. doi: 10.1016/j.jad.2018.05.065
34. Bataineh, BS, Wilkinson, AV, Sumbe, A, Clendennen, SL, Chen, B, Messiah, SE, et al. The association between tobacco and Cannabis use and the age of onset of depression and anxiety symptoms: among adolescents and young adults. Nicotine Tob Res. (2023) 25:1455–64. doi: 10.1093/ntr/ntad058
35. Bolanis, D, Orri, M, Castellanos-Ryan, N, Renaud, J, Montreuil, T, Boivin, M, et al. Cannabis use, depression and suicidal ideation in adolescence: direction of associations in a population based cohort. J Affect Disord. (2020) 274:1076–83. doi: 10.1016/j.jad.2020.05.136
36. Bovasso, GB . Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiatry. (2001) 158:2033–7. doi: 10.1176/appi.ajp.158.12.2033
37. Carrà, G, Bartoli, F, and Crocamo, C. Trends of major depressive episode among people with cannabis use: findings from the National Survey on drug use and health 2006-2015. Subst Abus. (2019) 40:178–84. doi: 10.1080/08897077.2018.1550464
38. Chabrol, H, Chassagne, J, Henry, L, and Raynal, P. Influence of cannabis use disorder symptoms on suicidal ideation in college students. Int J Ment Heal Addict. (2021) 19:865–71. doi: 10.1007/s11469-019-00201-2
39. Crane, NA, Langenecker, SA, and Mermelstein, RJ. Gender differences in the associations among marijuana use, cigarette use, and symptoms of depression during adolescence and young adulthood. Addict Behav. (2015) 49:33–9. doi: 10.1016/j.addbeh.2015.05.014
40. Cornelius, JR, Aizenstein, HJ, and Hariri, AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. (2010) 35:644–6. doi: 10.1016/j.addbeh.2010.02.004
41. Danielsson, A-K, Lundin, A, Agardh, E, Allebeck, P, and Forsell, Y. Cannabis use, depression and anxiety: a 3-year prospective population-based study. J Affect Disord. (2016) 193:103–8. doi: 10.1016/j.jad.2015.12.045
42. Davis, JP, Pedersen, ER, Tucker, JS, Prindle, J, Dunbar, MS, Rodriguez, A, et al. Directional associations between cannabis use and depression from late adolescence to young adulthood: the role of adverse childhood experiences. Addiction. (2023) 118:1083–92. doi: 10.1111/add.16130
43. De Hert, M, Wampers, M, Jendricko, T, Franic, T, Vidovic, D, De Vriendt, N, et al. Effects of cannabis use on age at onset in schizophrenia and bipolar disorder. Schizophr Res. (2011) 126:270–6. doi: 10.1016/j.schres.2010.07.003
44. de Lima, BS, de Azevedo, CT, Moreira, FP, Mondin, TC, Simjanoski, M, Kapczinski, FP, et al. Risk factors for new-onset bipolar disorder in a community cohort: a five-year follow up study. Psychiatry Res. (2021) 303:114109. doi: 10.1016/j.psychres.2021.114109
45. Denissoff, A, Mustonen, A, Alakokkare, AE, Scott, JG, Sami, MB, Miettunen, J, et al. Is early exposure to cannabis associated with bipolar disorder? Results from a Finnish birth cohort study. Addiction. (2022) 117:2264–72. doi: 10.1111/add.15881
46. Durdle, H, Lundahl, LH, Johanson, CE, and Tancer, M. Major depression: the relative contribution of gender, MDMA, and cannabis use. Depress Anxiety. (2008) 25:241–7. doi: 10.1002/da.20297
47. Espada, JP, Sussman, S, Medina, TBH, and Alfonso, JP. Relation between substance use and depression among Spanish adolescents. Int J Psychol Psychol Ther. (2011) 11:79–90.
48. Fairman, BJ, and Anthony, JC. Are early-onset cannabis smokers at an increased risk of depression spells? J Affect Disord. (2012) 138:54–62. doi: 10.1016/j.jad.2011.12.031
49. Feingold, D, Weiser, M, Rehm, J, and Lev-Ran, S. The association between cannabis use and mood disorders: a longitudinal study. J Affect Disord. (2015) 172:211–8. doi: 10.1016/j.jad.2014.10.006
50. Fleming, CB, Mason, WA, Mazza, JJ, Abbott, RD, and Catalano, RF. Latent growth modeling of the relationship between depressive symptoms and substance use during adolescence. Psychol Addict Behav. (2008) 22:186–97. doi: 10.1037/0893-164X.22.2.186
51. Ford, KA, Wammes, M, Neufeld, RW, Mitchell, D, Théberge, J, Williamson, P, et al. Unique functional abnormalities in youth with combined marijuana use and depression: an FMRI study. Front Psychol. (2014) 5:130. doi: 10.3389/fpsyt.2014.00130
52. Frohe, T, Beseler, CL, Mendoza, AM, Cottler, LB, and Leeman, RF. Perceived health, medical, and psychiatric conditions in individual and dual-use of marijuana and nonprescription opioids. J Consult Clin Psychol. (2019) 87:859–71. doi: 10.1037/ccp0000431
53. Gage, S, Hickman, M, Heron, J, Munafò, M, Lewis, G, Macleod, J, et al. Associations of cannabis and cigarette use with psychotic experiences at age 18: findings from the Avon longitudinal study of parents and children. Psychol Med. (2014) 44:3435–44. doi: 10.1017/S0033291714000531
54. Gruber, SA, Dahlgren, MK, Sagar, KA, Gönenc, A, and Killgore, WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. (2012) 511:89–94. doi: 10.1016/j.neulet.2012.01.039
55. Halladay, JE, Munn, C, Boyle, M, Jack, SM, and Georgiades, K. Temporal changes in the cross-sectional associations between Cannabis use, suicidal ideation, and depression in a nationally representative sample of Canadian adults in 2012 compared to 2002. Can J Psychiatr. (2020) 65:115–23. doi: 10.1177/0706743719854071
56. Harder, VS, Morral, AR, and Arkes, J. Marijuana use and depression among adults: testing for causal associations. Addiction. (2006) 101:1463–72. doi: 10.1111/j.1360-0443.2006.01545.x
57. Hartberg, CB, Lange, EH, Lagerberg, TV, Haukvik, UK, Andreassen, OA, Melle, I, et al. Cortical thickness, cortical surface area and subcortical volumes in schizophrenia and bipolar disorder patients with cannabis use. Eur Neuropsychopharmacol. (2018) 28:37–47. doi: 10.1016/j.euroneuro.2017.11.019
58. Hassan, AN, and Le Foll, B. Survival probabilities and predictors of major depressive episode incidence among individuals with various types of substance use disorders. J Clin Psychiatry. (2021) 82:35506. 10.4088/JCP.20m13637
59. Hayatbakhsh, MR, Najman, JM, Jamrozik, K, Mamun, AA, Alati, R, and Bor, W. Cannabis and anxiety and depression in young adults: a large prospective study. J Am Acad Child Adolesc Psychiatry. (2007) 46:408–17. doi: 10.1097/chi.0b013e31802dc54d
60. Hengartner, MP, Angst, J, Ajdacic-Gross, V, and Rössler, W. Cannabis use during adolescence and the occurrence of depression, suicidality and anxiety disorder across adulthood: findings from a longitudinal cohort study over 30 years. J Affect Disord. (2020) 272:98–103. doi: 10.1016/j.jad.2020.03.126
61. Hines, LA, Freeman, TP, Gage, SH, Zammit, S, Hickman, M, Cannon, M, et al. Association of high-potency cannabis use with mental health and substance use in adolescence. JAMA Psychiatry. (2020) 77:1044–51. doi: 10.1001/jamapsychiatry.2020.1035
62. Kvitland, LR, Melle, I, Aminoff, SR, Lagerberg, TV, Andreassen, OA, and Ringen, PA. Cannabis use in first-treatment bipolar I disorder: relations to clinical characteristics. Early Interv Psychiatry. (2016) 10:36–44. doi: 10.1111/eip.12138
63. Lagerberg, TV, Sundet, K, Aminoff, SR, Berg, AO, Ringen, PA, Andreassen, OA, et al. Excessive cannabis use is associated with earlier age at onset in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. (2011) 261:397–405. doi: 10.1007/s00406-011-0188-4
64. Lagerberg, TV, Kvitland, LR, Aminoff, SR, Aas, M, Ringen, PA, Andreassen, OA, et al. Indications of a dose–response relationship between cannabis use and age at onset in bipolar disorder. Psychiatry Res. (2014) 215:101–4. doi: 10.1016/j.psychres.2013.10.029
65. Lagerberg, T, Icick, R, Andreassen, O, Ringen, P, Etain, B, Aas, M, et al. Cannabis use disorder is associated with greater illness severity in tobacco smoking patients with bipolar disorder. J Affect Disord. (2016) 190:286–93. doi: 10.1016/j.jad.2015.10.023
66. Leadbeater, BJ, Ames, ME, and Linden-Carmichael, AN. Age-varying effects of cannabis use frequency and disorder on symptoms of psychosis, depression and anxiety in adolescents and adults. Addiction. (2019) 114:278–93. doi: 10.1111/add.14459
67. Le, V, Kirsch, DE, Tretyak, V, Weber, W, Strakowski, SM, and Lippard, ET. Recent perceived stress, amygdala reactivity to acute psychosocial stress, and alcohol and Cannabis use in adolescents and young adults with bipolar disorder. Front Psychol. (2021) 12:767309. doi: 10.3389/fpsyt.2021.767309
68. Lev-Ran, S, Le Foll, B, McKenzie, K, George, TP, and Rehm, J. Bipolar disorder and co-occurring cannabis use disorders: characteristics, co-morbidities and clinical correlates. Psychiatry Res. (2013) 209:459–65. doi: 10.1016/j.psychres.2012.12.014
69. Livne, O, Razon, L, Rehm, J, Hasin, DS, and Lev-Ran, S. The association between lifetime cannabis use and dysthymia across six birth decades. J Affect Disord. (2018) 234:327–34. doi: 10.1016/j.jad.2018.03.005
70. London-Nadeau, K, Rioux, C, Parent, S, Vitaro, F, Côté, SM, Boivin, M, et al. Longitudinal associations of cannabis, depression, and anxiety in heterosexual and LGB adolescents. J Abnorm Psychol. (2021) 130:333–45. doi: 10.1037/abn0000542
71. Manrique-Garcia, E, Zammit, S, Dalman, C, Hemmingsson, T, and Allebeck, P. Cannabis use and depression: a longitudinal study of a national cohort of Swedish conscripts. BMC Psychiatry. (2012) 12:112. doi: 10.1186/1471-244X-12-112
72. Lai, HMX, and Sitharthan, T. Exploration of the comorbidity of cannabis use disorders and mental health disorders among inpatients presenting to all hospitals in New South Wales, Australia. Am J Drug Alcohol Abuse. (2012) 38:567–74. doi: 10.3109/00952990.2012.694523
73. Marmorstein, NR . Longitudinal associations between alcohol problems and depressive symptoms: early adolescence through early adulthood. Alcohol Clin Exp Res. (2009) 33:49–59. doi: 10.1111/j.1530-0277.2008.00810.x
74. Marmorstein, NR, and Iacono, WG. Explaining associations between cannabis use disorders in adolescence and later major depression: a test of the psychosocial failure model. Addict Behav. (2011) 36:773–6. doi: 10.1016/j.addbeh.2011.02.006
75. Maremmani, I, Pacini, M, Lazzeri, A, Perugi, G, and Deltito, J. Concurrent abuse of cannabis is associated with a shorter duration of hospitalization in treatment-resistant psychotic bipolar inpatients treated with clozapine. Addict Disord Treat. (2006) 5:1–7. doi: 10.1097/01.adt.0000210703.70788.65
76. Marwaha, S, Winsper, C, Bebbington, P, and Smith, D. Cannabis use and hypomania in young people: a prospective analysis. Schizophr Bull. (2018) 44:1267–74. doi: 10.1093/schbul/sbx158
77. Medina, KL, Hanson, KL, Schweinsburg, AD, Cohen-Zion, M, Nagel, BJ, and Tapert, SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Society: JINS. (2007) 13:807–20. doi: 10.1017/S1355617707071032
78. Mustonen, A, Hielscher, E, Miettunen, J, Denissoff, A, Alakokkare, A-E, Scott, JG, et al. Adolescent cannabis use, depression and anxiety disorders in the northern Finland birth cohort 1986. BJPsych open. (2021) 7:e137. doi: 10.1192/bjo.2021.967
79. Nichols, ES, Penner, J, Ford, KA, Wammes, M, Neufeld, RW, Mitchell, DG, et al. Emotion regulation in emerging adults with major depressive disorder and frequent cannabis use. Neuroimage: clinical. (2021) 30:102575. doi: 10.1016/j.nicl.2021.102575
80. Oladunjoye, AF, Kaleem, SZ, Suresh, A, Sahni, V, Thoonkuzhy, MJ, Anugwom, G, et al. Cannabis use and medication nonadherence in bipolar disorder: a nationwide inpatient sample database analysis. J Affect Disord. (2022) 299:174–9. doi: 10.1016/j.jad.2021.11.067
81. Osuch, E, Manning, K, Hegele, R, Théberge, J, Neufeld, R, Mitchell, D, et al. Depression, marijuana use and early-onset marijuana use conferred unique effects on neural connectivity and cognition. Acta Psychiatr Scand. (2016) 134:399–409. doi: 10.1111/acps.12629
82. Otten, R, and Engels, RC. Testing bidirectional effects between cannabis use and depressive symptoms: moderation by the serotonin transporter gene. Addict Biol. (2013) 18:826–35. doi: 10.1111/j.1369-1600.2011.00380.x
83. Patel, RS, Cheema, Z, Singla, A, Cornejo, M, and Verma, G. Cannabis use is an independent risk factor for manic episode: a report from 380, 265 bipolar inpatients. Subst Use Misuse. (2022) 57:344–9. doi: 10.1080/10826084.2021.2012690
84. Patton, GC, Coffey, C, Carlin, JB, Degenhardt, L, Lynskey, M, and Hall, W. Cannabis use and mental health in young people: cohort study. BMJ. (2002) 325:1195–8. doi: 10.1136/bmj.325.7374.1195
85. Pedersen, W . Does cannabis use lead to depression and suicidal behaviours? A population-based longitudinal study. Acta Psychiatr Scand. (2008) 118:395–403. doi: 10.1111/j.1600-0447.2008.01259.x
86. Rabiee, R, Lundin, A, Agardh, E, Hensing, G, Allebeck, P, and Danielsson, A-K. Cannabis use and the risk of anxiety and depression in women: a comparison of three Swedish cohorts. Drug Alcohol Depend. (2020) 216:108332. doi: 10.1016/j.drugalcdep.2020.108332
87. Repetto, PB, Zimmerman, MA, and Caldwell, CH. A longitudinal study of depressive symptoms and marijuana use in a sample of inner-city African Americans. J Res Adolesc. (2008) 18:421–47. doi: 10.1111/j.1532-7795.2008.00566.x
88. Secora, AM, Eddie, D, Wyman, BJ, Brooks, DJ, Mariani, JJ, and Levin, FR. A comparison of psychosocial and cognitive functioning between depressed and non-depressed patients with cannabis dependence. J Addict Dis. (2010) 29:325–37. doi: 10.1080/10550887.2010.489444
89. Schoeler, T, Theobald, D, Pingault, J-B, Farrington, D, Coid, J, and Bhattacharyya, S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. Psychol Med. (2018) 48:2169–76. doi: 10.1017/S0033291717003658
90. Shalit, N, Shoval, G, Shlosberg, D, Feingold, D, and Lev-Ran, S. The association between cannabis use and suicidality among men and women: a population-based longitudinal study. J Affect Disord. (2016) 205:216–24. doi: 10.1016/j.jad.2016.07.010
91. Strakowski, SM, Del Bello, MP, Fleck, DE, Adler, CM, Anthenelli, RM, Keck, PE, et al. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry. (2007) 64:57–64. doi: 10.1001/archpsyc.64.1.57
92. Sultan, AA, Hird, MA, Dimick, MK, Mac Intosh, BJ, and Goldstein, BI. Cannabis use and resting state functional connectivity in adolescent bipolar disorder. J Psychiatry Neurosci. (2021) 46:E559–67. doi: 10.1503/jpn.200228
93. Sultan, AA, Kennedy, KG, Fiksenbaum, L, Mac Intosh, BJ, and Goldstein, BI. Neurostructural correlates of cannabis use in adolescent bipolar disorder. Int J Neuropsychopharmacol. (2021) 24:181–90. doi: 10.1093/ijnp/pyaa077
94. Sultan, AA, Mio, M, Dimick, MK, Zou, Y, Karthikeyan, S, Kolla, N, et al. Association of cannabis use with neurocognition in adolescents with bipolar disorder. J Psychopharmacol. (2023) 37:920–7. doi: 10.1177/02698811231187128
95. Van Laar, M, Van Dorsselaer, S, Monshouwer, K, and De Graaf, R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. (2007) 102:1251–60. doi: 10.1111/j.1360-0443.2007.01875.x
96. van Rossum, I, Boomsma, M, Tenback, D, Reed, C, and van Os, J. Does cannabis use affect treatment outcome in bipolar disorder?: a longitudinal analysis. J Nerv Ment Dis. (2009) 197:35–40. doi: 10.1097/NMD.0b013e31819292a6
97. Weinstock, LM, Gaudiano, BA, Wenze, SJ, Epstein-Lubow, G, and Miller, IW. Demographic and clinical characteristics associated with comorbid cannabis use disorders (CUDs) in hospitalized patients with bipolar I disorder. Compr Psychiatry. (2016) 65:57–62. doi: 10.1016/j.comppsych.2015.10.003
98. Wittchen, H-U, Fröhlich, C, Behrendt, S, Günther, A, Rehm, J, Zimmermann, P, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. (2007) 88:S60–70. doi: 10.1016/j.drugalcdep.2006.12.013
99. Womack, SR, Shaw, DS, Weaver, CM, and Forbes, EE. Bidirectional associations between cannabis use and depressive symptoms from adolescence through early adulthood among at-risk young men. J Stud Alcohol Drugs. (2016) 77:287–97. doi: 10.15288/jsad.2016.77.287
100. Zorrilla, I, Aguado, J, Haro, J, Barbeito, S, Lopez Zurbano, S, Ortiz, A, et al. Cannabis and bipolar disorder: does quitting cannabis use during manic/mixed episode improve clinical/functional outcomes? Acta Psychiatr Scand. (2015) 131:100–10. doi: 10.1111/acps.12366
101. Medina, KL, Nagel, BJ, Park, A, McQueeny, T, and Tapert, SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. (2007) 48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x
102. Nordquist, N, and Oreland, L. Serotonin, genetic variability, behaviour, and psychiatric disorders-a review. Ups J Med Sci. (2010) 115:2–10. doi: 10.3109/03009730903573246
103. Gage, SH, Hickman, M, Heron, J, Munafò, MR, Lewis, G, Macleod, J, et al. Associations of cannabis and cigarette use with depression and anxiety at age 18: findings from the Avon longitudinal study of parents and children. PloS one. (2015) 10:e0122896. doi: 10.1371/journal.pone.0122896
104. Gruber, SA, Sagar, KA, Dahlgren, MK, Olson, DP, Centorrino, F, and Lukas, SE. Marijuana impacts mood in bipolar disorder: a pilot study. Ment Health Subst Use. (2012) 5:228–39. doi: 10.1080/17523281.2012.659751
105. Tourjman, SV, Buck, G, Jutras-Aswad, D, Khullar, A, McInerney, S, Saraf, G, et al. Canadian network for mood and anxiety treatments (CANMAT) task force report: a systematic review and recommendations of Cannabis use in bipolar disorder and major depressive disorder. Can J Psychiatr. (2023) 68:299–311. doi: 10.1177/07067437221099769
106. Mammen, G, Rueda, S, Roerecke, M, Bonato, S, Lev-Ran, S, and Rehm, J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. (2018) 79:1–12. doi: 10.4088/JCP.17r11839
107. Sorkhou, M, Bedder, RH, and George, TP. The behavioral sequelae of cannabis use in healthy people: a systematic review. Front Psychol. (2021) 12:122. doi: 10.3389/fpsyt.2021.630247
108. Onaemo, VN, Fawehinmi, TO, and D'Arcy, C. Comorbid cannabis use disorder with major depression and generalized anxiety disorder: a systematic review with meta-analysis of nationally representative epidemiological surveys. J Affect Disord. (2021) 281:467–75. doi: 10.1016/j.jad.2020.12.043
109. Gobbi, G, Atkin, T, Zytynski, T, Wang, S, Askari, S, Boruff, J, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry. (2019) 76:426–34. doi: 10.1001/jamapsychiatry.2018.4500
110. Lubman, DI, Cheetham, A, and Yücel, M. Cannabis and adolescent brain development. Pharmacol Ther. (2015) 148:1–16. doi: 10.1016/j.pharmthera.2014.11.009
111. Tirado-Muñoz, J, Lopez-Rodriguez, AB, Fonseca, F, Farré, M, Torrens, M, and Viveros, M-P. Effects of cannabis exposure in the prenatal and adolescent periods: preclinical and clinical studies in both sexes. Front Neuroendocrinol. (2020) 57:100841. doi: 10.1016/j.yfrne.2020.100841
112. Lorenzetti, V, Solowij, N, and Yücel, M. The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry. (2016) 79:e17–31. doi: 10.1016/j.biopsych.2015.11.013
113. White, J, Walton, D, and Walker, N. Exploring comorbid use of marijuana, tobacco, and alcohol among 14 to 15-year-olds: findings from a national survey on adolescent substance use. BMC Public Health. (2015) 15:233–9. doi: 10.1186/s12889-015-1585-9
114. Rabin, RA, and George, TP. A review of co-morbid tobacco and cannabis use disorders: possible mechanisms to explain high rates of co-use. Am J Addict. (2015) 24:105–16. doi: 10.1111/ajad.12186
115. Li, X, Diviant, JP, Stith, SS, Brockelman, F, Keeling, K, Hall, B, et al. Focus: plant-based medicine and pharmacology: the effectiveness of cannabis flower for immediate relief from symptoms of depression. Yale J Biol Med. (2020) 93:251–64.
116. Dekker, N, Linszen, D, and De Haan, L. Reasons for cannabis use and effects of cannabis use as reported by patients with psychotic disorders. Psychopathology. (2009) 42:350–60. doi: 10.1159/000236906
117. Narang, S, Gibson, D, Wasan, AD, Ross, EL, Michna, E, Nedeljkovic, SS, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. (2008) 9:254–64. doi: 10.1016/j.jpain.2007.10.018
118. Novotna, A, Mares, J, Ratcliffe, S, Novakova, I, Vachova, M, Zapletalova, O, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols*(Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. (2011) 18:1122–31. doi: 10.1111/j.1468-1331.2010.03328.x
119. Müller-Vahl, KR, Pisarenko, A, Szejko, N, Haas, M, Fremer, C, Jakubovski, E, et al. CANNA-TICS: efficacy and safety of oral treatment with nabiximols in adults with chronic tic disorders–results of a prospective, multicenter, randomized, double-blind, placebo controlled, phase IIIb superiority study. Psychiatry Res. (2023) 323:115135. doi: 10.1016/j.psychres.2023.115135
120. Mills, L, Lintzeris, N, O'Malley, M, Arnold, JC, and McGregor, IS. Prevalence and correlates of cannabis use disorder among Australians using cannabis products to treat a medical condition. Drug Alcohol Rev. (2022) 41:1095–108. doi: 10.1111/dar.13444
121. Baggio, S, Deline, S, Studer, J, Mohler-Kuo, M, Daeppen, J-B, and Gmel, G. Routes of administration of cannabis used for nonmedical purposes and associations with patterns of drug use. J Adolesc Health. (2014) 54:235–40. doi: 10.1016/j.jadohealth.2013.08.013
122. Russell, C, Rueda, S, Room, R, Tyndall, M, and Fischer, B. Routes of administration for cannabis use–basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. (2018) 52:87–96. doi: 10.1016/j.drugpo.2017.11.008
123. Greaves, L, and Hemsing, N. Sex and gender interactions on the use and impact of recreational cannabis. Int J Environ Res Public Health. (2020) 17:509. doi: 10.3390/ijerph17020509
Keywords: cannabis, major depressive disorder, bipolar disorder, depression, mania, suicidality
Citation: Sorkhou M, Dent EL and George TP (2024) Cannabis use and mood disorders: a systematic review. Front. Public Health. 12:1346207. doi: 10.3389/fpubh.2024.1346207
Edited by:
Kevin Hill, Harvard Medical School, United StatesReviewed by:
Michael Hsu, United States Department of Veterans Affairs, United StatesMirjana Ratko Jovanovic, University of Kragujevac, Serbia
Copyright © 2024 Sorkhou, Dent and George. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tony P. George, dG9ueS5nZW9yZ2VAY2FtaC5jYQ==
 Maryam Sorkhou
Maryam Sorkhou Eliza L. Dent3
Eliza L. Dent3
 Tony P. George
Tony P. George