- 1Department of Geriatrics, The Second Clinical Medical College, Jinan University (Shenzhen People’s Hospital), Shenzhen, China
- 2The First Affiliated Hospital, Jinan University, Guangzhou, China
- 3Department of Neurology, The Second Clinical Medical College, Jinan University (Shenzhen People’s Hospital), Shenzhen, China
- 4Shenzhen Clinical Research Center for Geriatrics, Shenzhen People’s Hospital, Shenzhen, China
Strong epidemiological evidence has shown that early life adversity (ELA) has a profound negative impact on health in adulthood, including an increased risk of cardiovascular disease, the leading cause of death worldwide. Here, we review cohort studies on the effects of ELA on cardiovascular outcomes and the possible underlying mechanisms. In addition, we summarize relevant studies in rodent models of ELA. This review reveals that the prevalence of ELA varies between regions, time periods, and sexes. ELA increases cardiovascular health risk behaviors, susceptibility to mental illnesses, and neuroendocrine and immune system dysfunction in humans. Rodent models of ELA have been developed and show similar cardiovascular outcomes to those in humans but cannot fully replicate all ELA subtypes. Therefore, combining cohort and rodent studies to further investigate the mechanisms underlying the association between ELA and cardiovascular diseases may be a feasible future research strategy.
1 Introduction
Since Felitti et al. conducted the Adverse Childhood Experiences Study in 1998, the relationship between Early Life Adversity (ELA) and negative long-term health outcomes has attracted much research attention (1). Also called adverse childhood experiences in human studies, ELA is defined as potentially traumatic events that occur before adulthood, including threats to the child’s bodily, familial, or social safety and security, and ranges from broad categories of abuse, neglect, and household dysfunction to more specific experiences of bullying, exposure to crime, victimization, and economic hardship (1–4). ELA is highly prevalent, with regional variations occur. According to data from the Behavioral Risk Factor Surveillance System, 61.55% of adults in the United States have experienced ELA (2); in European countries, this figure ranges from 20.4 to 69.4% (5). In Chinese middle-aged or older adults it can be up to 80.9%, according to data from the China Health and Retirement Longitudinal Study (6). Societal changes over time affect the prevalence of specific adverse experiences. For example, during the first 15–18 years of the 21st century, parental illness, sibling death, exposure to domestic violence, childhood poverty, parental divorce, serious childhood illness, physical abuse, sexual abuse, physical and emotional bullying, and exposure to community violence declined; however, parental alcohol and drug abuse increased (7).
ELA exposure has been strongly associated with the development of various health conditions in later life, including cardiovascular disease, which is the leading cause of death worldwide. Cohort studies indicate that ELA increases the odds of cardiovascular health risk behaviors, such as smoking and alcoholism, as well as having long-term effects on the stress response system, leading to autonomic, vascular, and inflammatory dysfunction, and contributing to the onset and development of cardiovascular disease (4). Rodent models such as maternal separation have been developed to study the underlying mechanisms of cardiovascular effects of ELA. Consistent with human cohort studies, negative cardiovascular outcomes were observed (8).
This review provides an overview of the epidemiological evidence linking ELA to the development of cardiovascular disease and the mechanisms underlying the effects of ELA on the cardiovascular system. In addition, we summarize the rodent models that have been developed to mimic ELA and mechanistic studies performed using these models. Finally, we discuss the direction of future research.
2 ELA and cardiovascular outcomes in humans
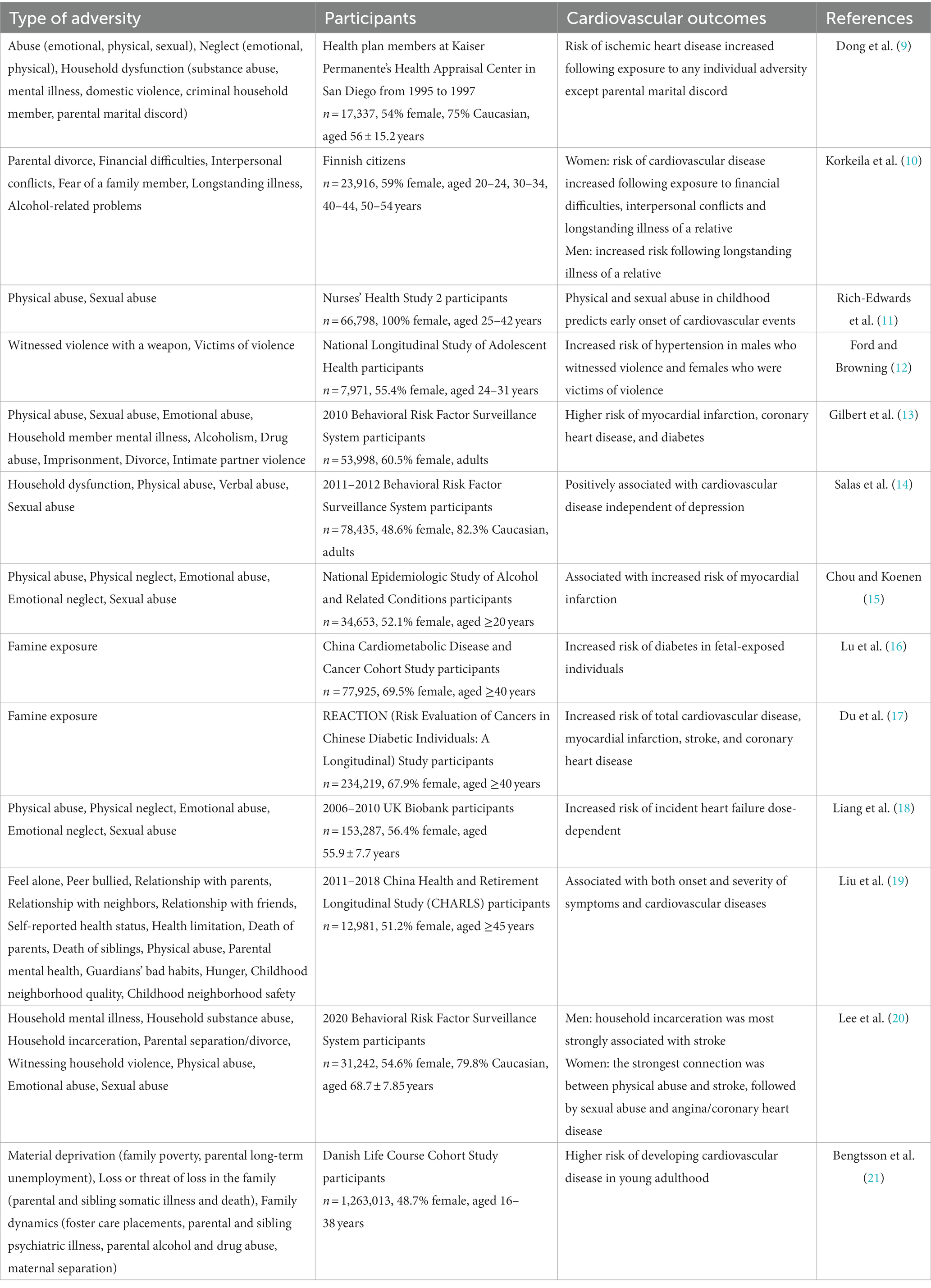
An increasing number of cohort studies have shown ELA to be strongly associated with negative cardiovascular outcomes (Table 1). Different types of adversities have been carefully classified, and their effects studied. Abuse, neglect, and household dysfunction are the three most common types of adversity; all of these have been shown to increase the risk of cardiovascular events in adulthood, including ischemic heart disease, myocardial infarction, coronary heart disease, stroke, and heart failure (9, 11, 13–15, 18, 19, 21). These correlations were dose-dependent, the more types of adversities experienced, the higher the risk (9, 13, 18). Exposure to a single adversity type has been associated with a 1.3- to 1.7-fold increase in the risk of ischemic heart disease compared with that of individuals not exposed to adversity (9). The risk of developing myocardial infarction was increased in individuals who experienced ELA compared with those who did not, while the risk of developing coronary heart disease and stroke was increased in individuals exposed to more than four types of ELA compared with those who experienced fewer types (13). The risk of developing heart failure doubled following exposure to three to five types of ELA (18).
Certain specific types of ELA have also been associated with cardiovascular events. The risk of hypertension was increased in males who have witnessed violence with a weapon and female victims of violence (12). Fetal exposure to famine increased the risk of diabetes (16), while famine experienced in early life increased the risk of total cardiovascular disease, myocardial infarction, stroke, and coronary heart disease (17). In addition, early exposure to air pollution increased the lifetime burden on the cardiovascular system, contributing to the development of cardiovascular disease (22).
Sex differences are observed in the incidence and effects of ELA. Within the same racial and ethnic population, females are more likely than males to experience ELA (23). The risk of cardiovascular disease increased in women who were exposed to financial difficulties, interpersonal conflicts, and chronic illness in a relative. For men, an increased risk was observed only following chronic illness in a relative (10). Stoke was most strongly associated with physical abuse in women and household incarceration in men (20).
3 Possible mechanisms linking ELA and cardiovascular disease in humans
3.1 Behavioral factors
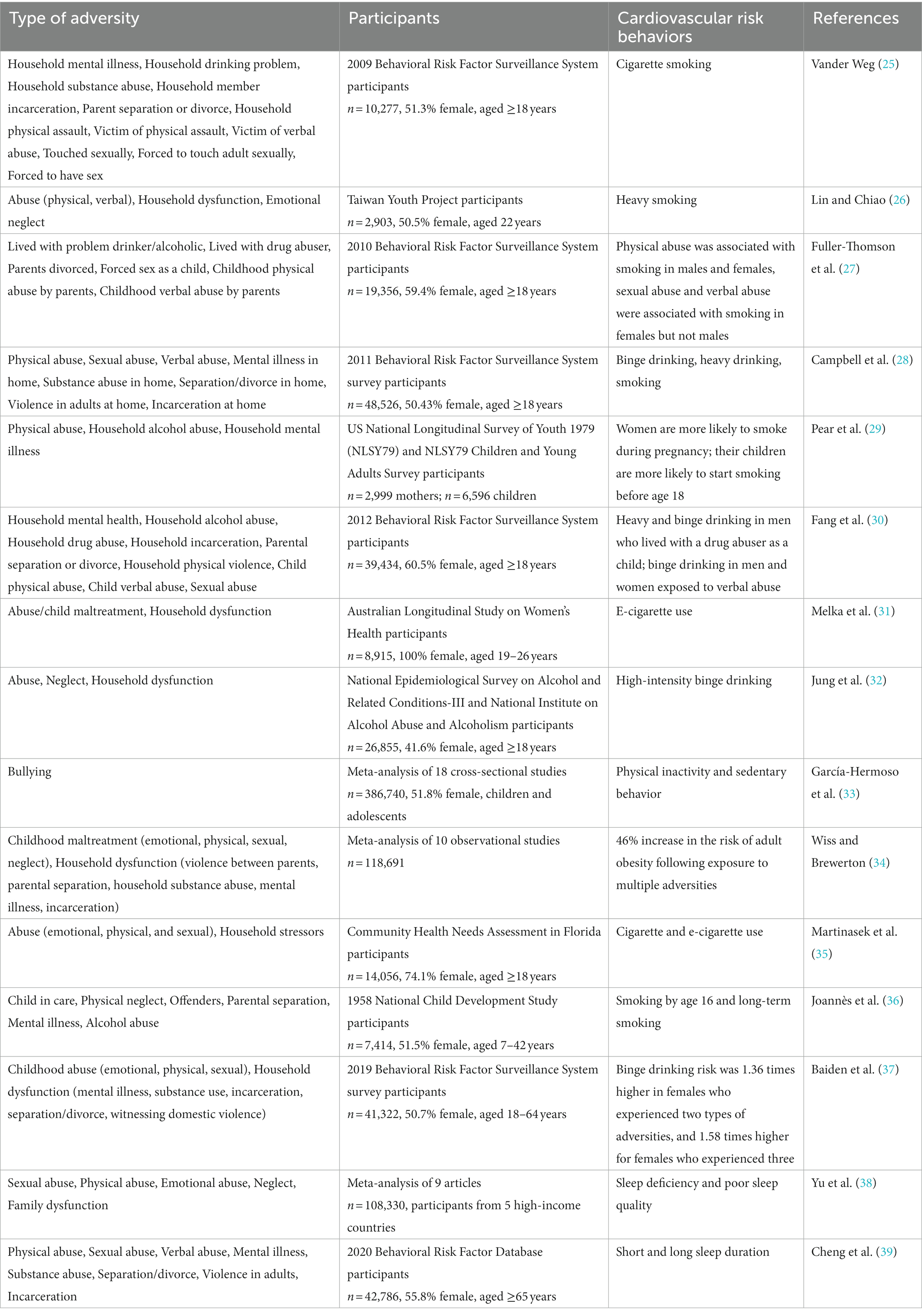
Diet, physical activity, nicotine exposure, sleep health, body mass index, blood lipids, blood glucose, and blood pressure are recognized as Life’s Essential 8, the eight most important contributors to cardiovascular health (24). ELA exposure significantly increases the incidence of smoking, alcoholism, physical inactivity, obesity, and poor sleep (Table 2) (1, 38, 40–44).
ELA increases the risk of both cigarette and e-cigarette smoking (25, 26, 31, 35), with exposed individuals more likely to start smoking by 16 years of age and smoke for a lifetime (36). Physical abuse was associated with smoking in both males and females; sexual and verbal abuse were associated with smoking in females but not males (27). This correlation has intergenerational effects, maternal exposure to childhood physical abuse was associated with an increased risk of both smoking during pregnancy and the child smoking (29).
ELA is also associated with high-intensity binge drinking (32). Heavy and binge drinking was more common in men who lived with a drug abuser as a child than in those who did not, and verbal abuse was correlated with binge drinking in both men and women (30). The greater the total number of adversities experienced, the higher the probability of binge drinking (28, 37). Although some studies suggest that moderate drinking may be beneficial for cardiovascular health, large cohort studies have shown that all levels of alcohol consumption are associated with an increased cardiovascular risk (45–47).
A meta-analysis showed that physical inactivity increased among children and adolescents exposed to bullying (33). A separate meta-analysis of 10 observational studies showed that the risk of obesity in adulthood increased 46% following ELA (34). Physical inactivity and obesity have been closely associated with high blood lipid, glucose, and blood pressure levels, which are major contributors to cardiovascular disease (48, 49).
A systematic review and meta-analysis indicated that ELA has a considerable impact on long-term sleep health: the greater the exposure, the higher the risk of sleep disorders (38). ELA exposure was associated with both short and long sleep duration: victims of childhood sexual abuse were 146% more likely to short sleep and 99% more likely to long sleep; reporting more than four types of adversities raised these risks to 3.10 and 2.13 times, respectively (39). Both short and long sleep duration and poor sleep quality are associated with an increased risk of all-cause mortality and cardiovascular events (50, 51).
3.2 Mental factors
ELA is associated with the development of mental illnesses in adulthood, including anxiety, depression, post-traumatic stress disorder (PTSD), and schizophrenia. Cohort studies have shown that ELA increases the incidence of anxiety, depression, and PTSD (52–54). ELA exposure has been associated with both the onset and severity of depression and cardiovascular disease (19). The number of adversity types experienced was a nonlinear predictor of depression (55). A meta-analysis including over 200,000 participants showed that individuals maltreated in childhood had an increased risk of depression and cardiometabolic disease, and were three times more likely to have comorbid depression and cardiometabolic disease (56). A systematic review and meta-analysis associated childhood trauma with the severity of hallucinations and delusions in individuals with psychotic disorders (57). In addition, patients with schizophrenia report more exposure to ELA than healthy controls (58).
Mental illness increases the burden on the cardiovascular system through biological pathways such as elevated cortisol, oxidative stress, inflammation, and autonomic nervous system dysfunction, as reviewed in detail by Treur et al. (59). Patients with severe mental illness have twice the cardiovascular mortality rate of the general population (60, 61). Both anxiety and depression are associated with an increased risk of mortality owing to cardiovascular events (62, 63). Schizophrenia reduces life expectancy by 15–20 years, with 40–50% of deaths due to cardiovascular disease (64). Thus, ELA may predispose individuals to cardiovascular diseases later in life through their susceptibility to mental illnesses.
3.3 Biological pathways
ELA disturbs the neuroendocrine and immune systems in adulthood; this dysregulation has been associated with cardiovascular diseases. Hypothalamic–pituitary–adrenal (HPA) axis dysfunction has been observed in individuals exposed to ELA (65–68). A meta-analysis indicated that cortisol levels affected by ELA (67). Bullying, emotional abuse, and cumulative exposure to adversity have been associated with low daytime salivary cortisol levels in adolescents (66). Increased levels of hair cortisone were detected among middle-aged and older individuals exposed to ELA (65, 68). Meta analyses have indicated that ELA increases levels of inflammatory mediators such as C-reactive protein, interleukin-6, and tumor necrosis factor-α (69, 70). These effects persist into late adulthood (71). Dysregulated inflammation and cortisol levels may contribute to the development of mental illnesses such as depression (72–74), increasing the risk of cardiovascular disease.
ELA has been reported to directly increase circulating endothelin-1 levels in young adulthood, with greater numbers of adversity types faced further increasing endothelin-1 levels (75). Endothelin-1 is a peptide produced by the vascular endothelium in response to stress (76, 77) and is implicated in the pathogenesis of cardiovascular diseases (78). Physical exercise decreases endothelin-1 levels among women exposed to ELA and improves cardiovascular psychophysiological outcomes; endothelin-1 may therefore be a potential therapeutic target for the treatment of cardiovascular diseases following ELA (79).
4 Impact of ELA on the cardiovascular system in rodents
4.1 Rodent models of ELA
In humans, ELA primarily involves abuse, neglect, and household dysfunction. To mimic these adversities, several types of rodent models have been developed: (1) Maternal separation, in which pups are removed from their mother for several hours per day (at different times each day) during the postnatal period (2). Social deprivation, in which pups are separated from both their mother and littermates for several hours per day (at different times each day) during the postnatal period (3). Limited bedding and nesting, in which the mother and pups have limited access to nesting material during the postnatal period (4). Early weaning, in which pups are weaned before postnatal day 21, usually on postnatal day 17. Of these, maternal separation is the most widely used. To simulate multiple ELA exposures, these models are used in combination, such as maternal separation combined with limited bedding and nesting and maternal separation combined with early weaning (80, 81). These rodent models effectively simulate the negative outcomes of ELA, behavioral experiments have shown that rodents exposed to ELA elicit negative psychopathological outcomes in adulthood. Mice exposed to maternal separation and limited nesting were more susceptible to depression-like behaviors after social defeat (82). Social deprivation during postnatal days 1–14 increased the risk of social dysfunction later in life (83).
4.2 Mechanisms underlying the effect of ELA on the cardiovascular system in rodents
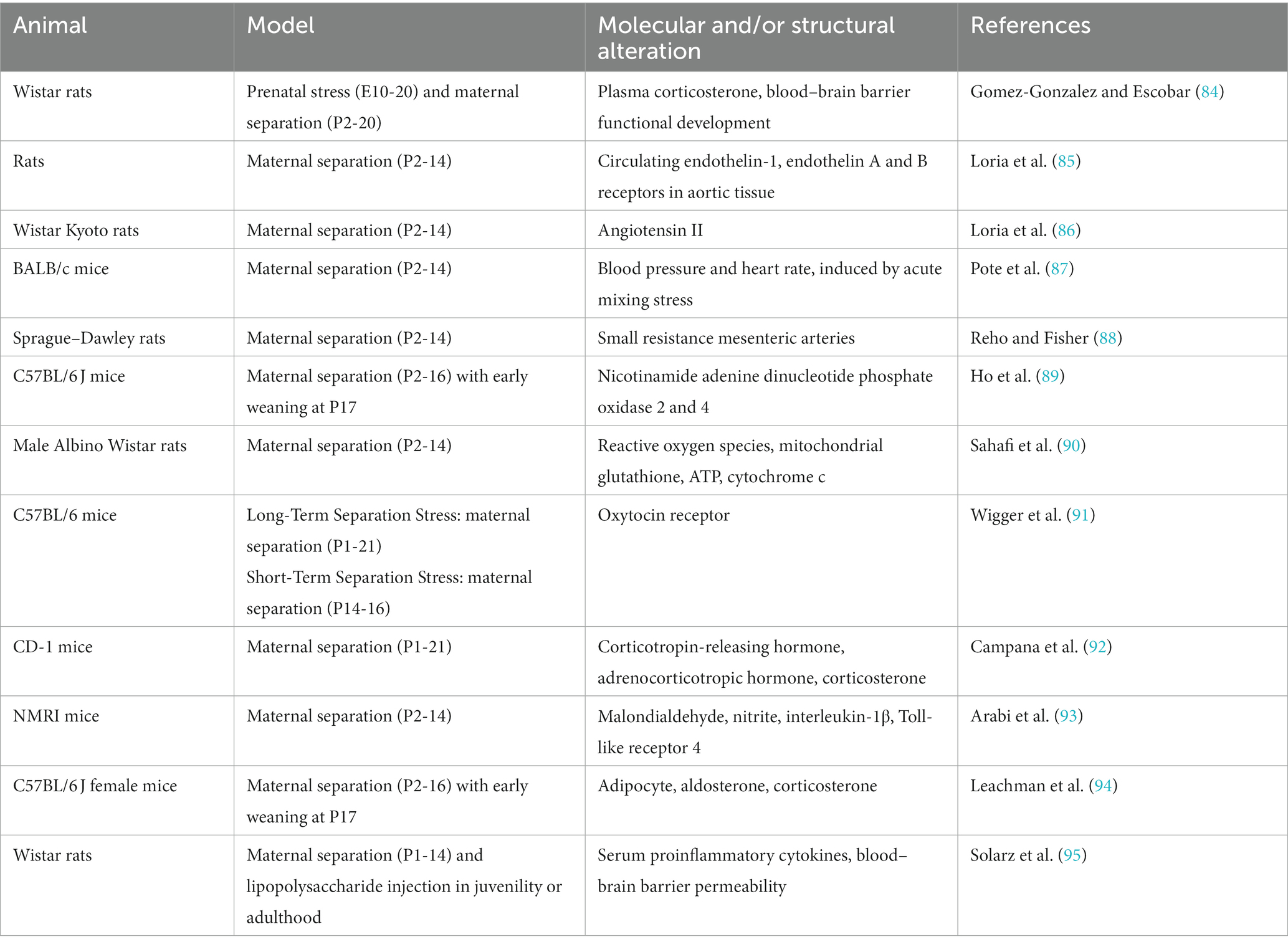
Consistent with results from human studies, ELA in rodents negatively affects the cardiovascular system (Table 3). Neuroendocrine, immune system and vascular dysfunction have been identified following ELA in rodents. Dysfunction in the HPA axis and renin-angiotensin-aldosterone system were detected in rats and mice following maternal separation, as evidenced by increased levels of corticotropin-releasing hormone, adrenocorticotropic hormone, corticosterone, and angiotensin II (84, 86, 92). Circulating endothelin-1 levels were significantly increased in rats following maternal separation (85). In a mouse model of maternal separation, acute mixing stress induced lower blood pressure and heart rate responses (87). Maternal separation in rats resulted in misprogramming of resistance artery smooth muscle, increased vasoconstriction and blood pressure (88), and blood–brain barrier dysfunction (84, 95). ELA also increased oxidative stress and inflammation. Levels of the reactive oxygen species nicotinamide adenine dinucleotide phosphate oxidases 2 and 4 (89, 90) and the inflammatory protein interleukin-1β and Toll-link receptor 4 (93, 95) were increased in rodents following ELA. ELA may be linked to oxytocin signaling, cardiac oxytocin receptors decreased following maternal separation on postnatal days 1–21, but increased following maternal separation on postnatal days 12–16 (91). Maternal separation and early weaning increased adiposity in female mice, which may be the mechanism underlying the development of obesity and diabetes following ELA (94).
5 Discussion and future directions
Despite the progress in community and family support, ELA remains highly prevalent and contributes to the morbidity and mortality of cardiovascular diseases worldwide. The prevalence of ELA varies between countries and regions, and between sexes. Over time, the prevalence of different subtypes of ELA changes. This means that the adoption of rigorous sampling strategies is required to dynamically surveil the prevalence of subtypes of ELA and minimize data bias (7). It also means that policymakers need to be flexible in the use of prevention strategies to reduce the incidence of ELA. Numerous cohort studies have revealed the association between ELA and cardiovascular disease, with the risk increasing with greater ELA exposure. However, the frequency, intensity, exposure duration, and exposure timing of each subtype of ELA, which may also have an impact on cardiovascular outcomes, have not been fully assessed (96). Each individual’s childhood experience is unique and may experience some specific subtypes of ELA. Therefore, how to accurately evaluate the effect of ELA on an individual’s cardiovascular system requires further investigation.
Mechanistic studies in humans have shown that ELA increases cardiovascular health risk behaviors, susceptibility to mental illnesses, and neuroendocrine and immune system dysfunction. But whether ELA directly affects the development of cardiovascular disease or whether the association between ELA and cardiovascular disease is fully mediated through risk behaviors remains to be determined. Rodent models have been developed to study the mechanisms underlying the effects of ELA on cardiovascular outcomes and result in cardiovascular dysfunction similar to that observed in humans. Rodents exposed to ELA showed similar behavioral tendencies to humans exposed to ELA, such as alcohol consumption (97), drug addiction (98), and depression-like behavior (82). However, whether these behavioral effects contribute to cardiovascular outcomes remains largely unknown. Rodent models provide an important research tool as they allow the association between ELA and cardiovascular disease to be analyzed in a homogenous population under controlled conditions. However, these models cannot fully replicate all of ELA subtypes. Therefore, combining cohort and rodent studies to analyze the association between ELA and cardiovascular diseases may be a feasible future research strategy.
Author contributions
HT: Conceptualization, Resources, Visualization, Writing – original draft, Writing – review & editing. HZ: Writing – review & editing. JC: Writing – review & editing. HR: Writing – review & editing. YG: Funding acquisition, Project administration, Supervision, Writing – review & editing. XJ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Sustainable Development Science and Technology Project of the Shenzhen Science and Technology Innovation Commission (KCXFZ20201221173411032 and KCXFZ20201221173400001) and the “Five Threes” Clinical Research Program of Shenzhen People’s Hospital (SYWGSLCYJ202204).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Felitti, VJ, Anda, RF, Nordenberg, D, Williamson, DF, Spitz, AM, Edwards, V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. (1998) 14:245–58. doi: 10.1016/S0749-3797(98)00017-8
2. Suglia, SF, Koenen, KC, Boynton-Jarrett, R, Chan, PS, Clark, CJ, Danese, A, et al. Childhood and adolescent adversity and Cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation. (2018) 137:e15–28. doi: 10.1161/CIR.0000000000000536
3. Cronholm, PF, Forke, CM, Wade, R, Bair-Merritt, MH, Davis, M, Harkins-Schwarz, M, et al. Adverse childhood experiences: expanding the concept of adversity. Am J Prev Med. (2015) 49:354–61. doi: 10.1016/j.amepre.2015.02.001
4. Godoy, LC, Frankfurter, C, Cooper, M, Lay, C, Maunder, R, and Farkouh, ME. Association of Adverse Childhood Experiences with Cardiovascular Disease Later in life: a review. JAMA Cardiol. (2021) 6:228–35. doi: 10.1001/jamacardio.2020.6050
5. Hughes, K, Ford, K, Bellis, MA, Glendinning, F, Harrison, E, and Passmore, J. Health and financial costs of adverse childhood experiences in 28 European countries: a systematic review and meta-analysis. Lancet Public Health. (2021) 6:e848–57. doi: 10.1016/S2468-2667(21)00232-2
6. Lin, L, Wang, HH, Lu, C, Chen, W, and Guo, VY. Adverse childhood experiences and subsequent chronic diseases among middle-aged or older adults in China and associations with demographic and socioeconomic characteristics. JAMA Netw Open. (2021) 4:e2130143. doi: 10.1001/jamanetworkopen.2021.30143
7. Finkelhor, D. Trends in adverse childhood experiences (ACEs) in the United States. Child Abuse Negl. (2020) 108:104641. doi: 10.1016/j.chiabu.2020.104641
8. Murphy, MO, Cohn, DM, and Loria, AS. Developmental origins of cardiovascular disease: impact of early life stress in humans and rodents. Neurosci Biobehav Rev. (2017) 74:453–65. doi: 10.1016/j.neubiorev.2016.07.018
9. Dong, M, Giles, WH, Felitti, VJ, Dube, SR, Williams, JE, Chapman, DP, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. (2004) 110:1761–6. doi: 10.1161/01.CIR.0000143074.54995.7F
10. Korkeila, J, Vahtera, J, Korkeila, K, Kivimaki, M, Sumanen, M, Koskenvuo, K, et al. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. (2010) 96:298–303. doi: 10.1136/hrt.2009.188250
11. Rich-Edwards, JW, Mason, S, Rexrode, K, Spiegelman, D, Hibert, E, Kawachi, I, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. (2012) 126:920–7. doi: 10.1161/CIRCULATIONAHA.111.076877
12. Ford, JL, and Browning, CR. Effects of exposure to violence with a weapon during adolescence on adult hypertension. Ann Epidemiol. (2014) 24:193–8. doi: 10.1016/j.annepidem.2013.12.004
13. Gilbert, LK, Breiding, MJ, Merrick, MT, Thompson, WW, Ford, DC, Dhingra, SS, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. (2015) 48:345–9. doi: 10.1016/j.amepre.2014.09.006
14. Salas, J, van den Berk-Clark, C, Skiold-Hanlin, S, Schneider, FD, and Scherrer, JF. Adverse childhood experiences, depression, and cardiometabolic disease in a nationally representative sample. J Psychosom Res. (2019) 127:109842. doi: 10.1016/j.jpsychores.2019.109842
15. Chou, PH, and Koenen, KC. Associations between childhood maltreatment and risk of myocardial infarction in adulthood: results from the National Epidemiologic Survey on alcohol and related conditions. J Psychiatr Res. (2019) 116:172–7. doi: 10.1016/j.jpsychires.2018.12.001
16. Lu, J, Li, M, Xu, Y, Bi, Y, Qin, Y, Li, Q, et al. Early life famine exposure, ideal cardiovascular health metrics, and risk of incident diabetes: findings from the 4C study. Diabetes Care. (2020) 43:1902–9. doi: 10.2337/dc19-2325
17. Du, R, Zheng, R, Xu, Y, Zhu, Y, Yu, X, Li, M, et al. Early-life famine exposure and risk of cardiovascular diseases in later life: findings from the REACTION study. J Am Heart Assoc. (2020) 9:e014175. doi: 10.1161/JAHA.119.014175
18. Liang Yy, MP, Ai, S, Weng, F, Feng, H, Yang, L, He, Z, et al. Associations of childhood maltreatment and genetic risks with incident heart failure in later life. J Am Heart Assoc. (2022) 11:e026536. doi: 10.1161/JAHA.122.026536
19. Liu, Y, Wang, C, and Liu, Y. Association between adverse childhood experiences and later-life cardiovascular diseases among middle-aged and older Chinese adults: the mediation effect of depressive symptoms. J Affect Disord. (2022) 319:277–85. doi: 10.1016/j.jad.2022.09.080
20. Lee, C, Cao, J, Eagen-Torkko, M, and Mohammed, SA. Network analysis of adverse childhood experiences and cardiovascular diseases. SSM Popul Health. (2023) 22:101358. doi: 10.1016/j.ssmph.2023.101405
21. Bengtsson, J, Elsenburg, LK, Andersen, GS, Larsen, ML, Rieckmann, A, and Rod, NH. Childhood adversity and cardiovascular disease in early adulthood: a Danish cohort study. Eur Heart J. (2023) 44:586–93. doi: 10.1093/eurheartj/ehac607
22. Kim, JB, Prunicki, M, Haddad, F, Dant, C, Sampath, V, Patel, R, et al. Cumulative lifetime burden of cardiovascular disease from early exposure to air pollution. J Am Heart Assoc. (2020) 9:e014944. doi: 10.1161/JAHA.119.014944
23. Cole, AB, Armstrong, CM, Giano, ZD, and Hubach, RD. An update on ACEs domain frequencies across race/ethnicity and sex in a nationally representative sample. Child Abuse Negl. (2022) 129:105686. doi: 10.1016/j.chiabu.2022.105686
24. Lloyd-Jones, DM, Allen, NB, Anderson, CAM, Black, T, Brewer, LC, Foraker, RE, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. (2022) 146:e18–43. doi: 10.1161/CIR.0000000000001078
25. Vander Weg, MW. Adverse childhood experiences and cigarette smoking: the 2009 Arkansas and Louisiana behavioral risk factor surveillance systems. Nicotine Tob Res. (2011) 13:616–22. doi: 10.1093/ntr/ntr023
26. Lin, WH, and Chiao, C. The relationship between adverse childhood experience and heavy smoking in emerging adulthood: the role of not in education, employment, or training status. J Adolesc Health. (2022) 70:155–62. doi: 10.1016/j.jadohealth.2021.07.022
27. Fuller-Thomson, E, Filippelli, J, and Lue-Crisostomo, CA. Gender-specific association between childhood adversities and smoking in adulthood: findings from a population-based study. Public Health. (2013) 127:449–60. doi: 10.1016/j.puhe.2013.01.006
28. Campbell, JA, Walker, RJ, and Egede, LE. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. Am J Prev Med. (2016) 50:344–52. doi: 10.1016/j.amepre.2015.07.022
29. Pear, VA, Petito, LC, and Abrams, B. The role of maternal adverse childhood experiences and race in intergenerational high-risk smoking behaviors. Nicotine Tob Res. (2017) 19:623–30. doi: 10.1093/ntr/ntw295
30. Fang, L, and McNeil, S. Is there a relationship between adverse childhood experiences and problem drinking behaviors? Findings from a population-based sample. Public Health. (2017) 150:34–42. doi: 10.1016/j.puhe.2017.05.005
31. Melka, A, Chojenta, C, Holliday, E, and Loxton, D. Adverse childhood experiences and electronic cigarette use among young Australian women. Prev Med. (2019) 126:105759. doi: 10.1016/j.ypmed.2019.105759
32. Jung, J, Rosoff, DB, Muench, C, Luo, A, Longley, M, Lee, J, et al. Adverse childhood experiences are associated with high-intensity binge drinking behavior in adulthood and mediated by psychiatric disorders. Alcohol Alcohol. (2020) 55:204–14. doi: 10.1093/alcalc/agz098
33. Garcia-Hermoso, A, Hormazabal-Aguayo, I, Oriol-Granado, X, Fernandez-Vergara, O, and Del Pozo, CB. Bullying victimization, physical inactivity and sedentary behavior among children and adolescents: a meta-analysis. Int J Behav Nutr Phys Act. (2020) 17:114. doi: 10.1186/s12966-020-01016-4
34. Wiss, DA, and Brewerton, TD. Adverse childhood experiences and adult obesity: a systematic review of plausible mechanisms and Meta-analysis of cross-sectional studies. Physiol Behav. (2020) 223:112964. doi: 10.1016/j.physbeh.2020.112964
35. Martinasek, MP, Wheldon, CW, Parsons, CA, Bell, LA, and Lipski, BK. Understanding adverse childhood experiences as predictors of cigarette and E-cigarette use. Am J Prev Med. (2021) 60:737–46. doi: 10.1016/j.amepre.2021.01.004
36. Joannes, C, Castagne, R, and Kelly-Irving, M. Associations of adverse childhood experiences with smoking initiation in adolescence and persistence in adulthood, and the role of the childhood environment: findings from the 1958 British birth cohort. Prev Med. (2022) 156:106995. doi: 10.1016/j.ypmed.2022.106995
37. Baiden, P, Onyeaka, HK, Kyeremeh, E, Panisch, LS, LaBrenz, CA, Kim, Y, et al. An Association of Adverse Childhood Experiences with binge drinking in adulthood: findings from a population-based study. Subst Use Misuse. (2022) 57:360–72. doi: 10.1080/10826084.2021.2012692
38. Yu, HJ, Liu, X, Yang, HG, Chen, R, and He, QQ. The association of adverse childhood experiences and its subtypes with adulthood sleep problems: a systematic review and meta-analysis of cohort studies. Sleep Med. (2022) 98:26–33. doi: 10.1016/j.sleep.2022.06.006
39. Cheng, X, Dong, X, Liu, J, Qu, S, Xu, H, Yao, Y, et al. Adverse childhood experiences and sleep duration among U.S. 65 years and older: results from the 2020 BRFSS. J Affect Disord. (2023) 336:35–41. doi: 10.1016/j.jad.2023.05.061
40. Allen, H, Wright, BJ, Vartanian, K, Dulacki, K, and Li, HF. Examining the prevalence of adverse childhood experiences and associated cardiovascular disease risk factors among low-income uninsured adults. Circ Cardiovasc Qual Outcomes. (2019) 12:e004391. doi: 10.1161/CIRCOUTCOMES.117.004391
41. Flores-Torres, MH, Comerford, E, Signorello, L, Grodstein, F, Lopez-Ridaura, R, de Castro, F, et al. Impact of adverse childhood experiences on cardiovascular disease risk factors in adulthood among Mexican women. Child Abuse Negl. (2020) 99:104175. doi: 10.1016/j.chiabu.2019.104175
42. Miller, NE, and Lacey, RE. Childhood adversity and cardiometabolic biomarkers in mid-adulthood in the 1958 British birth cohort. SSM Popul Health. (2022) 19:101260. doi: 10.1016/j.ssmph.2022.101260
43. Wooldridge, JS, Tynan, M, Rossi, FS, Gasperi, M, McLean, CL, Bosch, J, et al. Patterns of adverse childhood experiences and cardiovascular risk factors in U.S. adults. Stress Health. (2023) 39:48–58. doi: 10.1002/smi.3167
44. Kajeepeta, S, Gelaye, B, Jackson, CL, and Williams, MA. Adverse childhood experiences are associated with adult sleep disorders: a systematic review. Sleep Med. (2015) 16:320–30. doi: 10.1016/j.sleep.2014.12.013
45. Biddinger, KJ, Emdin, CA, Haas, ME, Wang, M, Hindy, G, Ellinor, PT, et al. Association of Habitual Alcohol Intake with Risk of cardiovascular disease. JAMA Netw Open. (2022) 5:e223849. doi: 10.1001/jamanetworkopen.2022.3849
46. Im, PK, Wright, N, Yang, L, Chan, KH, Chen, Y, Guo, Y, et al. Alcohol consumption and risks of more than 200 diseases in Chinese men. Nat Med. (2023) 29:1476–86. doi: 10.1038/s41591-023-02383-8
47. Krittanawong, C, Isath, A, Rosenson, RS, Khawaja, M, Wang, Z, Fogg, SE, et al. Alcohol consumption and cardiovascular health. Am J Med. (2022) 135:1213–30 e3. doi: 10.1016/j.amjmed.2022.04.021
48. Elagizi, A, Kachur, S, Carbone, S, Lavie, CJ, and Blair, SN. A review of obesity, physical activity, and cardiovascular disease. Curr Obes Rep. (2020) 9:571–81. doi: 10.1007/s13679-020-00403-z
49. Valenzuela, PL, Carrera-Bastos, P, Castillo-Garcia, A, Lieberman, DE, Santos-Lozano, A, and Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol. (2023) 20:475–94. doi: 10.1038/s41569-023-00847-5
50. Yin, J, Jin, X, Shan, Z, Li, S, Huang, H, Li, P, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response Meta-analysis of prospective cohort studies. J Am Heart Assoc. (2017) 6:e005947. doi: 10.1161/JAHA.117.005947
51. Saz-Lara, A, Luceron-Lucas-Torres, M, Mesas, AE, Notario-Pacheco, B, Lopez-Gil, JF, and Cavero-Redondo, I. Association between sleep duration and sleep quality with arterial stiffness: a systematic review and meta-analysis. Sleep Health. (2022) 8:663–70. doi: 10.1016/j.sleh.2022.07.001
52. King, AR. Childhood adversity links to self-reported mood, anxiety, and stress-related disorders. J Affect Disord. (2021) 292:623–32. doi: 10.1016/j.jad.2021.05.112
53. Whitaker, RC, Dearth-Wesley, T, Herman, AN, Block, AE, Holderness, MH, Waring, NA, et al. The interaction of adverse childhood experiences and gender as risk factors for depression and anxiety disorders in US adults: a cross-sectional study. BMC Public Health. (2021) 21:2078. doi: 10.1186/s12889-021-12058-z
54. Kuzminskaite, E, Vinkers, CH, Milaneschi, Y, Giltay, EJ, and Penninx, B. Childhood trauma and its impact on depressive and anxiety symptomatology in adulthood: a 6-year longitudinal study. J Affect Disord. (2022) 312:322–30. doi: 10.1016/j.jad.2022.06.057
55. Tan, M, and Mao, P. Type and dose-response effect of adverse childhood experiences in predicting depression: a systematic review and meta-analysis. Child Abuse Negl. (2023) 139:106091. doi: 10.1016/j.chiabu.2023.106091
56. Souama, C, Lamers, F, Milaneschi, Y, Vinkers, CH, Defina, S, Garvert, L, et al. Depression, cardiometabolic disease, and their co-occurrence after childhood maltreatment: an individual participant data meta-analysis including over 200,000 participants. BMC Med. (2023) 21:93. doi: 10.1186/s12916-023-02769-y
57. Bailey, T, Alvarez-Jimenez, M, Garcia-Sanchez, AM, Hulbert, C, Barlow, E, and Bendall, S. Childhood trauma is associated with severity of hallucinations and delusions in psychotic disorders: a systematic review and Meta-analysis. Schizophr Bull. (2018) 44:1111–22. doi: 10.1093/schbul/sbx161
58. Wells, R, Jacomb, I, Swaminathan, V, Sundram, S, Weinberg, D, Bruggemann, J, et al. The impact of childhood adversity on cognitive development in schizophrenia. Schizophr Bull. (2020) 46:140–53. doi: 10.1093/schbul/sbz033
59. Treur, JL, Veeneman, RR, Vermeulen, JM, and Verweij, KJH. Unravelling the relation between mental illness and cardiovascular disease by triangulating evidence from different methods. Eur Heart J. (2023) 44:1851–4. doi: 10.1093/eurheartj/ehad049
60. Goldfarb, M, De Hert, M, Detraux, J, Di Palo, K, Munir, H, Music, S, et al. Severe mental illness and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 80:918–33. doi: 10.1016/j.jacc.2022.06.017
61. Lambert, AM, Parretti, HM, Pearce, E, Price, MJ, Riley, M, Ryan, R, et al. Temporal trends in associations between severe mental illness and risk of cardiovascular disease: a systematic review and meta-analysis. PLoS Med. (2022) 19:e1003960. doi: 10.1371/journal.pmed.1003960
62. Flygare, O, Boberg, J, Ruck, C, Hofmann, R, Leosdottir, M, Mataix-Cols, D, et al. Association of anxiety or depression with risk of recurrent cardiovascular events and death after myocardial infarction: a nationwide registry study. Int J Cardiol. (2023) 381:120–7. doi: 10.1016/j.ijcard.2023.04.023
63. Nakada, S, Ho, FK, Celis-Morales, C, Jackson, CA, and Pell, JP. Individual and joint associations of anxiety disorder and depression with cardiovascular disease: a UK biobank prospective cohort study. Eur Psychiatry. (2023) 66:e54. doi: 10.1192/j.eurpsy.2023.2425
64. Ringen, PA, Engh, JA, Birkenaes, AB, Dieset, I, and Andreassen, OA. Increased mortality in schizophrenia due to cardiovascular disease - a non-systematic review of epidemiology, possible causes, and interventions. Front Psych. (2014) 5:137. doi: 10.3389/fpsyt.2014.00137
65. Green, C, Stolicyn, A, Harris, MA, Shen, X, Romaniuk, L, Barbu, MC, et al. Hair glucocorticoids are associated with childhood adversity, depressive symptoms and reduced global and lobar grey matter in generation Scotland. Transl Psychiatry. (2021) 11:523. doi: 10.1038/s41398-021-01644-9
66. Iob, E, Baldwin, JR, Plomin, R, and Steptoe, A. Adverse childhood experiences, daytime salivary cortisol, and depressive symptoms in early adulthood: a longitudinal genetically informed twin study. Transl Psychiatry. (2021) 11:420. doi: 10.1038/s41398-021-01538-w
67. Khoury, JE, Bosquet Enlow, M, Plamondon, A, and Lyons-Ruth, K. The association between adversity and hair cortisol levels in humans: a meta-analysis. Psychoneuroendocrinology. (2019) 103:104–17. doi: 10.1016/j.psyneuen.2019.01.009
68. Bevan, K, and Kumari, M. Maternal separation in childhood and hair cortisol concentrations in late adulthood. Psychoneuroendocrinology. (2021) 130:105253. doi: 10.1016/j.psyneuen.2021.105253
69. Baumeister, D, Akhtar, R, Ciufolini, S, Pariante, CM, and Mondelli, V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry. (2016) 21:642–9. doi: 10.1038/mp.2015.67
70. Brown, M, Worrell, C, and Pariante, CM. Inflammation and early life stress: an updated review of childhood trauma and inflammatory markers in adulthood. Pharmacol Biochem Behav. (2021) 211:173291. doi: 10.1016/j.pbb.2021.173291
71. Iob, E, Lacey, R, and Steptoe, A. The long-term association of adverse childhood experiences with C-reactive protein and hair cortisol: cumulative risk versus dimensions of adversity. Brain Behav Immun. (2020) 87:318–28. doi: 10.1016/j.bbi.2019.12.019
72. Taylor, KS, Steptoe, A, and Iob, E. The relationship of adverse childhood experiences, hair cortisol, C-reactive protein, and polygenic susceptibility with older adults’ psychological distress during the COVID-19 pandemic. Mol Psychiatry. (2022) 27:5038–48. doi: 10.1038/s41380-022-01805-2
73. Yuan, JP, Ho, TC, Coury, SM, Chahal, R, Colich, NL, and Gotlib, IH. Early life stress, systemic inflammation, and neural correlates of implicit emotion regulation in adolescents. Brain Behav Immun. (2022) 105:169–79. doi: 10.1016/j.bbi.2022.07.007
74. Li, C, and Xiang, S. Adverse childhood experiences, inflammation, and depressive symptoms in late life: a population-based study. J Gerontol B Psychol Sci Soc Sci. (2023) 78:220–9. doi: 10.1093/geronb/gbac179
75. Su, S, Wang, X, Kapuku, GK, Treiber, FA, Pollock, DM, Harshfield, GA, et al. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension. (2014) 64:201–7. doi: 10.1161/HYPERTENSIONAHA.113.02755
76. Yammine, L, Kang, DH, Baun, MM, and Meininger, JC. Endothelin-1 and psychosocial risk factors for cardiovascular disease: a systematic review. Psychosom Med. (2014) 76:109–21. doi: 10.1097/PSY.0000000000000026
77. Fox, BM, Becker, BK, Loria, AS, Hyndman, KA, Jin, C, Clark, H, et al. Acute pressor response to psychosocial stress is dependent on endothelium-derived Endothelin-1. J Am Heart Assoc. (2018) 7:e007863. doi: 10.1161/JAHA.117.007863
78. Ford, TJ, Corcoran, D, Padmanabhan, S, Aman, A, Rocchiccioli, P, Good, R, et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J. (2020) 41:3239–52. doi: 10.1093/eurheartj/ehz915
79. Rogers, EM, Banks, NF, Tomko, PM, Sciarrillo, CM, Emerson, SR, Thomas, EBK, et al. Progressive exercise training improves cardiovascular psychophysiological outcomes in young adult women with a history of adverse childhood experiences. J Appl Physiol. (2023) 134:742–52. doi: 10.1152/japplphysiol.00524.2022
80. Murthy, S, and Gould, E. Early life stress in rodents: animal models of illness or resilience? Front Behav Neurosci. (2018) 12:157. doi: 10.3389/fnbeh.2018.00157
81. Pena, CJ, Nestler, EJ, and Bagot, RC. Environmental programming of susceptibility and resilience to stress in adulthood in male mice. Front Behav Neurosci. (2019) 13:40. doi: 10.3389/fnbeh.2019.00040
82. Pena, CJ, Kronman, HG, Walker, DM, Cates, HM, Bagot, RC, Purushothaman, I, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. (2017) 356:1185–8. doi: 10.1126/science.aan4491
83. Shin, S, Pribiag, H, Lilascharoen, V, Knowland, D, Wang, XY, and Lim, BK. Drd3 signaling in the lateral septum mediates early life stress-induced social dysfunction. Neuron. (2018) 97:195–208 e6. doi: 10.1016/j.neuron.2017.11.040
84. Gomez-Gonzalez, B, and Escobar, A. Altered functional development of the blood-brain barrier after early life stress in the rat. Brain Res Bull. (2009) 79:376–87. doi: 10.1016/j.brainresbull.2009.05.012
85. Loria, AS, D’Angelo, G, Pollock, DM, and Pollock, JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol. (2010) 299:R185–91. doi: 10.1152/ajpregu.00333.2009
86. Loria, AS, Kang, KT, Pollock, DM, and Pollock, JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension. (2011) 58:619–26. doi: 10.1161/HYPERTENSIONAHA.110.168674
87. Pote, W, Tagwireyi, D, Chinyanga, HM, Musara, C, Nyandoro, G, Chifamba, J, et al. Cardiovascular effects of Boophone disticha aqueous ethanolic extract on early maternally separated BALB/C mice. J Ethnopharmacol. (2013) 148:379–85. doi: 10.1016/j.jep.2013.03.001
88. Reho, JJ, and Fisher, SA. The stress of maternal separation causes misprogramming in the postnatal maturation of rat resistance arteries. Am J Physiol Heart Circ Physiol. (2015) 309:H1468–78. doi: 10.1152/ajpheart.00567.2015
89. Ho, DH, Burch, ML, Musall, B, Musall, JB, Hyndman, KA, and Pollock, JS. Early life stress in male mice induces superoxide production and endothelial dysfunction in adulthood. Am J Physiol Heart Circ Physiol. (2016) 310:H1267–74. doi: 10.1152/ajpheart.00016.2016
90. Sahafi, E, Peeri, M, Hosseini, MJ, and Azarbayjani, MA. Cardiac oxidative stress following maternal separation stress was mitigated following adolescent voluntary exercise in adult male rat. Physiol Behav. (2018) 183:39–45. doi: 10.1016/j.physbeh.2017.10.022
91. Wigger, DC, Groger, N, Lesse, A, Krause, S, Merz, T, Gundel, H, et al. Maternal separation induces long-term alterations in the cardiac oxytocin receptor and cystathionine gamma-Lyase expression in mice. Oxidative Med Cell Longev. (2020) 2020:4309605. doi: 10.1155/2020/4309605
92. Campana, G, Loizzo, S, Fortuna, A, Rimondini, R, Maroccia, Z, Scillitani, A, et al. Early post-natal life stress induces permanent adrenocorticotropin-dependent hypercortisolism in male mice. Endocrine. (2021) 73:186–95. doi: 10.1007/s12020-021-02659-4
93. Arabi, M, Nasab, SH, Lorigooini, Z, Boroujeni, SN, Mortazavi, SM, Anjomshoa, M, et al. Auraptene exerts protective effects on maternal separation stress-induced changes in behavior, hippocampus, heart and serum of mice. Int Immunopharmacol. (2021) 93:107436. doi: 10.1016/j.intimp.2021.107436
94. Leachman, JR, Cincinelli, C, Ahmed, N, Dalmasso, C, Xu, M, Gatineau, E, et al. Early life stress exacerbates obesity in adult female mice via mineralocorticoid receptor-dependent increases in adipocyte triglyceride and glycerol content. Life Sci. (2022) 304:120718. doi: 10.1016/j.lfs.2022.120718
95. Solarz, A, Majcher-Maslanka, I, Kryst, J, and Chocyk, A. Early-life stress affects peripheral, blood-brain barrier, and brain responses to immune challenge in juvenile and adult rats. Brain Behav Immun. (2023) 108:1–15. doi: 10.1016/j.bbi.2022.11.005
96. Anda, RF, Porter, LE, and Brown, DW. Inside the adverse childhood experience score: strengths, limitations, and misapplications. Am J Prev Med. (2020) 59:293–5. doi: 10.1016/j.amepre.2020.01.009
97. Bendre, M, Granholm, L, Drennan, R, Meyer, A, Yan, L, Nilsson, KW, et al. Early life stress and voluntary alcohol consumption in relation to Maoa methylation in male rats. Alcohol (Fayetteville, NY). (2019) 79:7–16. doi: 10.1016/j.alcohol.2018.11.001
Keywords: early life adversity, adverse childhood experiences, cardiovascular disease, risk behavior, mental health
Citation: Tan H, Zhou H, Chen J, Ren H, Guo Y and Jiang X (2024) Association of early life adversity with cardiovascular disease and its potential mechanisms: a narrative review. Front. Public Health. 12:1341266. doi: 10.3389/fpubh.2024.1341266
Edited by:
Michelle Plusquin, University of Hasselt, BelgiumReviewed by:
Yuna Koyama, Tokyo Medical and Dental University, JapanAmanda Sonnega, University of Michigan, United States
Copyright © 2024 Tan, Zhou, Chen, Ren, Guo and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Guo, eHVhbnlpX2d1b0AxNjMuY29t; Xin Jiang, amlhbmd4aW5zekAxNjMuY29t
 Huiying Tan1,2
Huiying Tan1,2 Huixia Ren
Huixia Ren Yi Guo
Yi Guo Xin Jiang
Xin Jiang