- 1Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 2Schools of Public Health, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
- 3Department of Biomedical Sciences, School of Medicine, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
- 4School of Public Health, College of Medicine and Health Sciences, Wachamo University, Hosaena, Ethiopia
Background: Adolescent girls and young women (AGYW) are expected to be healthy in life. However, the unique health challenges faced by AGYW include unsafe sex practices and substance abuse. Only 46.3% of AGYW in Africa are aware of their HIV status, and difficulties are underlined in HIV testing among adolescents and young people. To demarcate the areas with low and high HIV testing, this study aimed to map predictors of ever-tested for HIV among adolescent girls and young women in Ethiopia.
Methods: Secondary data analysis was conducted using the dataset from the 2016 Ethiopia Demographic and Health Survey (EHDS). We conducted spatial autocorrelation and Moran's I statistics to investigate the regional variance of HIV being ever-tested in AGYW. In addition, spatial regression analyses such as ordinary least squares (OLS) regression and geographically weighted regression (GWR) were carried out to determine the predictors of being ever-tested for HIV among AGYW.
Results: Addis Ababa, some parts of Amhara, Dire Dawa, Gambela, and Tigray were the primary regions and city administrations for being ever-tested for HIV among AGYW. A lesser proportion of AGYW being ever-tested for HIV was found in Somalia, Afar, Benshangul Gumuz, and southern nations. Spatial regression analyses identified an age range of 15–19 years, being Muslim, having no formal education, having no knowledge about HIV, and experiencing severe stigma as predictors of being ever-tested for HIV among AGYW.
Conclusion: The proportion of AGYW being ever-tested for HIV was high in Addis Ababa, some parts of Amhara, Dire Dawa, Gambela, and Tigray. Spatial regression analyses identified that AGYW aged 15–19 years, having no formal education, having no knowledge about HIV, and experiencing severe community stigma as predictors negatively affecting the proportion of being ever-tested for HIV, while being Muslim was a predictor that positively affected the proportion of being ever-tested for HIV. The governments and other stakeholders should focus on increasing HIV testing among these special groups of the population.
Introduction
The HIV epidemic continues to spread globally, though at varying rates across regions and countries. In Sub-Saharan Africa (SSA), adolescent girls and young women (AGYW) are at a notable risk of acquiring HIV, with nearly 63% of all newly reported HIV infections in 2021 occurring within this demographic population (1). Youth constitute an increasing proportion of people with HIV/AIDS, specifically AGYW, with a concentration of over 2.8 million people under the age of 19 with HIV in 2020 in eastern and southern Africa (2, 3). Globally, more than two-thirds of new HIV infections are found in African nations, which are home to 60% of youth under the age of 25 years (2). In Sub-Saharan Africa, the proportion of HIV infection is high and widespread, with South Africa accounting for the majority (3). Over two-thirds of the world's youth living with HIV are found in this region (2).
Research conducted across seven African nations revealed that 3.6% of individuals in this demographic were living with HIV (4). A systematic review and meta-analysis across 10 high-prevalence African countries found variations in HIV incidence rates among different geographical locations and age groups (5). In 2019, there was an expected decrease in the population of adolescents and youth living with HIV globally, particularly in Eastern and Southern Africa (6). However, the reduction in new infections may not be rapid enough to eliminate AIDS as a public health threat among individuals of this age group. This demographic population tends to seroconvert earlier than their male counterparts and constitutes ~30% of all new cases (7). Risk factors such as a lack of family support and inadequate education are associated with a high HIV incidence among adolescent girls and young women (AGYW) (8). Furthermore, insufficient awareness of HIV prevention measures, including HIV testing, contributes to the high HIV incidence among AGYW (4). For example, only 46.3% of AGYW are aware of their HIV status (4). A study also highlighted the challenges faced in HIV testing among adolescents and youth (9).
Studies conducted in Ethiopia reveal a higher prevalence of HIV among AGYW compared to men of the same age group and older women, especially among the highly vulnerable groups (10). Despite this high proportion, the uptake of HIV testing among AGYW remains low, with only 21.8% reporting testing in the past year. However, people living with HIV and those receiving antiretroviral therapy tend to engage in safer sexual behaviors (11). In Sub-Saharan African countries, including Ethiopia, HIV testing uptake among AGYW is low but is showing an increasing trend (6, 12, 13). Factors associated with a higher uptake of HIV testing include older age (14), better education, comprehensive HIV knowledge (15), discussions about HIV with mothers or female guardians (14), having multiple sexual partners, having nondiscriminatory attitudes, and pregnancy (12), whereas factors such as being unmarried, living in rural areas, having lower financial status, having limited media exposure, experiencing early sexual initiation, having perceived low risk, and having only one sexual partner are associated with lower HIV testing uptake (13, 15).
In Ethiopia, HIV prevalence is higher among urban and young populations, with an estimated 1.52% prevalence among women (16). A smaller proportion of AGYW (27%) tested for HIV (17). These figures also vary from region to region (11, 16–18). For an AIDS-free generation and the pandemic to be under control, prevention of HIV infection in this age group is essential (7). To manage the pandemic among AGYW, the government must make consistent efforts to detect HIV infection in such individuals (4).
GPS technology helps integrate spatial data, including demographics, location of healthcare facilities, HIV prevalence, and critical areas requiring targeted testing strategies (19). However, the spatial distribution of HIV being ever-tested AGYW has not been examined in Ethiopia. Therefore, this study aimed to identify hot spots and cold spots for HIV being ever-tested in AGYW to enhance testing strategies and improve the response to HIV/AIDS (11, 16–18).
Methods
Study area and population
The current study was conducted in Ethiopia, the second-most populated nation in Africa located in the Horn of Africa between 33 and 48°E longitude and 3° and 15°N latitude. The Ethiopian Public Health Institute (EPHI) conducted a comprehensive study on the demographic and health characteristics of the nation, as commissioned by the Ethiopia Federal Ministry of Health. Ethiopia is geographically partitioned into nine distinct regions. The 2016 Ethiopia Demographic and Health Survey (EDHS) was stratified, and the sample was drawn using a two-stage process. In the first phase, a sample of 645 enumeration areas (EAs) was chosen, consisting of 202 EAs in urban areas and 443 EAs in rural areas. The selection procedure was implemented by using a probability proportional to the size of each EA taken from the sample frame of the Population and Housing Census (PHC) in 2007. In the second stage, 28 households were selected in each cluster. The EDHS conducted in 2016 encompasses a comprehensive representation of all geographical areas and municipal governments (20). The specifics of sampling techniques for EDHS populations are covered elsewhere (20).
As part of the EDHS 2016, all women aged between 15 and 59 years were assessed for ever having an HIV test (Figure 1). AGYW aged 15–24 years were included in the analysis, while women aged >24 years were excluded from this study.
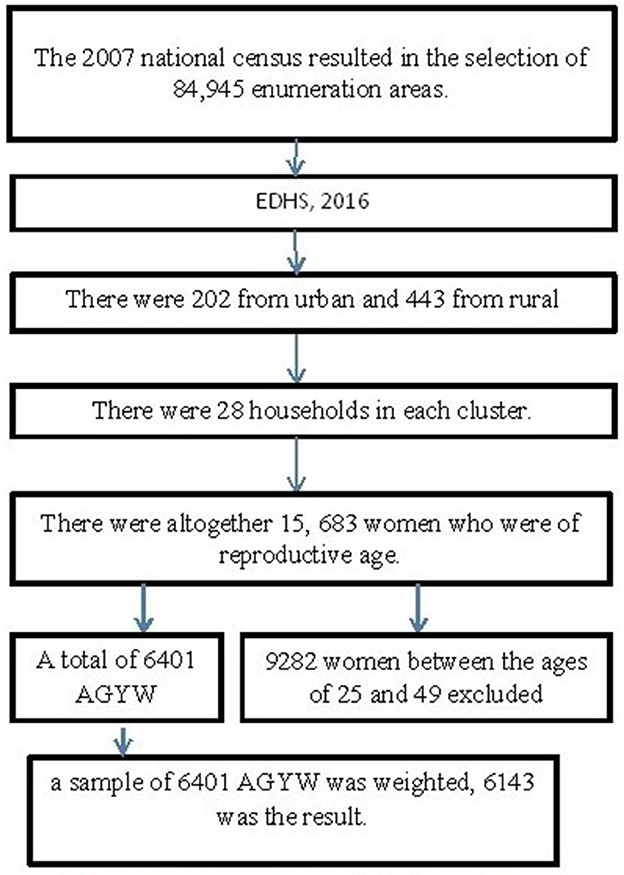
Figure 1. Sampling frame, sampling procedure, and data extraction process. AGYW, adolescent girls and young women; EHDs, Ethiopia Demographic and Health Survey.
Outcome and explanatory variables
Dependent variable
The dependent variable was being ever-tested for HIV and coded as “no = 0” or “yes = 1”. The proportion of individuals who have ever been tested for HIV was calculated. The number of respondents who answered “yes” was divided by the total number of both “yes” and “no” responses for being ever-tested for HIV.
Explanatory variables
The variables related to HIV testing status were selected based on an in-depth analysis of the existing literature (21, 22). The variables measured in this research include age, education attainment, employment status, marital status, place of residence, wealth index, religious affiliation, knowledge of HIV, awareness of sexually transmitted infections (STIs), number of sexual partners, exposure to media, stigma, geographical region, and proximity to healthcare facilities. In this study, all of these variables were taken into account for spatial regression analysis.
Operational definitions and measurement
Knowledge about HIV
This variable evaluated the participant's knowledge about HIV based on the combination of eleven knowledge indicator questions: (1) physically healthy-looking individuals can have HIV; (2) the risk of acquiring HIV can be minimized by having one sexual partner; (3) HIV transmitted through mosquito bites; (4) sharing food with an AIDS patient can transmit HIV; (5) HIV can be transmitted through supernatural means or witchcraft; (6) the risk of getting HIV can be increased by never skipping the condom during sex; (7) during pregnancy, HIV can be transmitted; (8) during delivery, HIV can be transmitted; (9) breastfeeding can transmit HIV; (10) if a husband or partner has sexually transmitted infection, the spouse reasonably asking the husband to use a condom; and (11) during pregnancy, there are drugs to be used to avoid HIV transmission to the baby. Participants' knowledge of HIV was categorized into not knowledgeable (scored ≤ 5) and knowledgeable (score 6–11) based on the right response to all of the indicators (23).
Access to health facility
Participants were assessed for accessibility to the health facility. If the facility was not far from the residence, the participant responded by citing it as no big problem, while if it was far from the residence, the participant responded by citing it as a big problem.
Exposure to media
Based on their prior history, the respondents were assessed for exposure to reading magazines or newspapers, listening radio, watching television, or using the internet to generate a variable (exposure to media). The response was coded as “0 = no” if an individual had no access to any of the media listed above and “1 = yes” if an individual had access to at least one of these media. The proportion of exposure to media was generated using “yes” as the numerator and “yes” plus “no” as the denominator.
Risky sexual activities
AGYW were assessed for at what age sex was started and multisexual partners (23). The age when first sex was started and the proportion of multisexual partners were also generated.
Community stigma
Seven measures that show unfavorable views toward people living with HIV were used to generate community stigma as follows: (1) children who acquired HIV should not be allowed schooling with children who did not acquire HIV; (2) people would not purchase fresh vegetables from vendors who are living with HIV; (3) people lose respect for those who have HIV or who are suspected of having it; (4) people speak negatively about such individuals; (5) because of how others might respond, people are reluctant to get an HIV test; (6) if a member of the family has HIV, it would cause them to be embarrassed; and (7) people fear of contracting HIV from touch with a contaminated person's saliva. This index was then classified as having no stigma if the subject scored 7, low stigma if the participant scored 6, moderate stigma if the participant scored (4, or 3), and high stigma if the individual scored 2 or 1 (23). Then, the proportion was generated for each category.
Wealth index
The proportions of AGYW in the wealth quintile, namely, poorest, poor, middle, rich, and richest, were generated from data on the combined wealth index. The household wealth index scores were calculated by summing the weighted scores of the indicators of households such as televisions and bicycles, materials used for housing construction, and types of water access and sanitation facilities.
Data extraction and management
The EDHS 2016 data were obtained from the DHS website after a formal online request for data. The request included a comprehensive explanation of the objectives of the research. The data are accessible for downloading on the official website of the DHS:http://www.dhsprogram.com. We downloaded the data from the DHS website and used the ETIR71DT folder to identify and extract important information for the research investigation. We also formally requested and downloaded the geographical coordinates such as latitude and longitude to visualize the hotspot areas. To maintain the confidentiality of the participants, the geographic coordinates have been deliberately displaced by a distance of 5 km in a random manner. We used Stata SE 18 (STATA Corporation. IC., TX, USA) (24) to extract variables from the original study dataset, clean, and conduct descriptive analysis.
Secondary data analysis
The global Moran's I statistic was analyzed to investigate the presence of spatial autocorrelations. A geographical autocorrelation was assessed using the global Moran's I statistic to determine the spatial pattern of HIV testing across different regions in Ethiopia. The spatial autocorrelation can show patterns of dispersion, clustering, or random distribution of outcome measures throughout the study areas (25). Global Moran's I is a widely used geographic statistical measure that evaluates the presence of spatial autocorrelation. It has a single output value that ranges from −1 to 1. A Moran's I value of −1 implies that the distribution of individuals who have been tested for HIV is low. In contrast, a Moran's I value of +1 implies a spatial clustering of being ever-tested for HIV. A Moran's I value of 0 indicates that the distribution of individuals who have ever been tested for HIV is random. The spatial autocorrelation patterns within the study area were analyzed using the Getis-Ord Gi* statistics. The realization of this outcome was accomplished by the computation of the GI* statistic for every region. The statistical significance cluster is determined using a p-value of >0.05. The statistically significant findings imply that a high geographic information (GI) value is suggestive of “hotspots” defined by an increased likelihood of HIV testing, whereas places with a low GI value are related to “cold spots,” which imply a reduced probability of HIV testing (26).
Spatial regression is a statistical methodology used to examine the association between a dependent variable and independent variables. The spatial regression analysis method could involve both local and global analysis techniques (27). To ensure the coefficient variability within each cluster or region, we first examined the global geographical regression models and then performed local geographical analysis (25).
Following that analysis, we conducted OLS regression with the appropriate tests to verify the assumptions. The normality assumption of the residuals was evaluated using the Jarque–Bera test. To assess the presence of spatial autocorrelation in the geographically weighted regression model, the Koenker BP test was used. The use of this test was justified due to the observation of non-spatial autocorrelation in the residuals. To reduce redundancy among the independent variables, the presence of multicollinearity was evaluated using the variance inflation factor (VIF) to confirm its value was below a certain threshold of 10 (28–30).
The geographically weighted regression (GWR) analysis was conducted using ArcGIS 10.8 software. The criteria were used to develop a spatial regression model including the coefficients that satisfy the previously specified criteria: they demonstrate the anticipated directionality, there is no presence of multicollinearity among the explanatory variables, the coefficients are statistically significant, and they display high adjusted R2 values. Variable coefficients showed that a p-value of <0.05 was selected. The most appropriate model for the data was selected by considering the lowest AICc score and a higher adjusted R2 value (28–30).
Ethical statement
This secondary data analysis did not involve participants' contacts. Thus, informed consent is not required in this study. The uses of the secondary data were formally requested for the current study, with permission being granted to identify the research questions and objectives, conduct analysis, and develop a manuscript. The original study also collected data according to the Declaration of Helsinki. The DHS dataset does not include personal identifiers such as names of people. Furthermore, the data were handled with confidentiality, and it is imperative to refrain from any endeavor to ascertain the identity of any household or individual respondent who participated in the survey. The information obtained was only used for statistical reporting and analysis of our registered study.
Results
Background, knowledge, and behavior characteristics of AGYW in the study
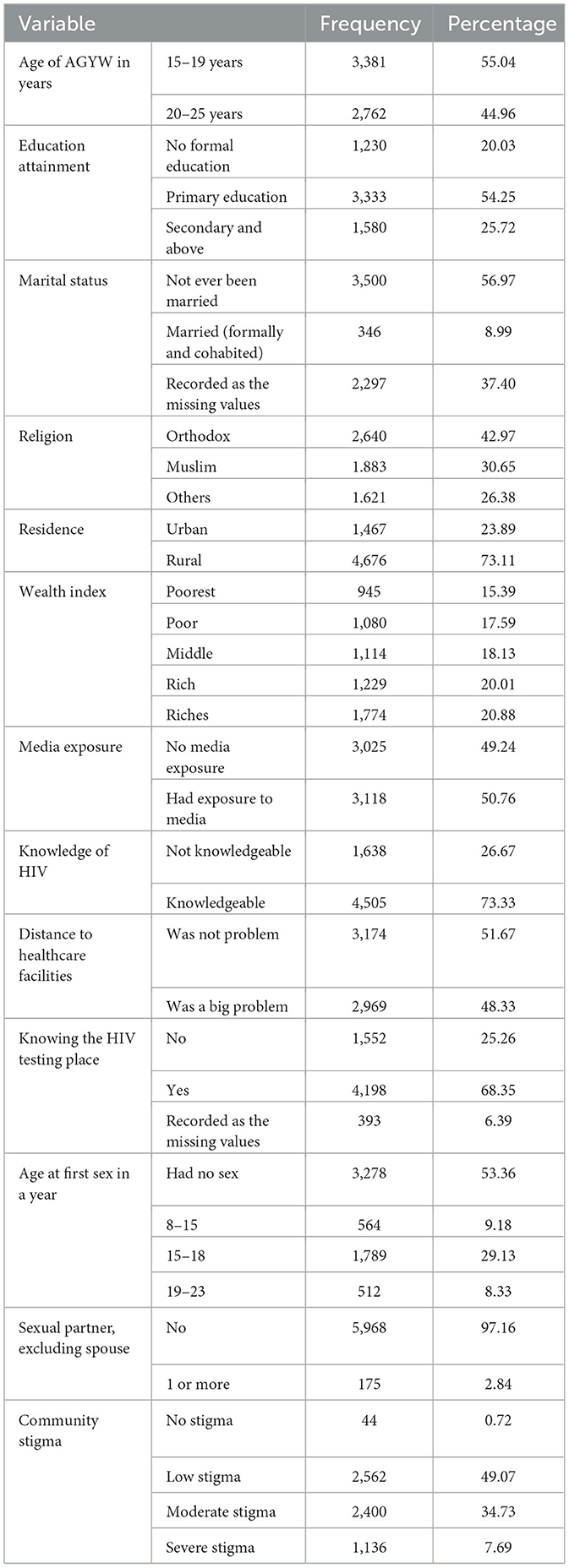
A total of 6,143 AGYW aged 15–24 years were included in this study. More than half of the responders (55%) were aged 15–19 years. Of the total AGYW, only 25.7% attained secondary or higher education. A majority of the respondents (57%) were never married, and 37.4% of the responses from respondents were coded as missing values. In terms of religion, 43% of the respondents identified as orthodox. Rural inhabitants made up more than two-thirds (73.1%) of the respondents, and 33% of AGYW were from low-income families. Regarding media exposure, approximately half of them (50.8%) were not exposed to any newspapers, radio, television, or internet, while 26.3% of AGYW had no knowledge about HIV. Regarding the testing place, 25.3% of AGYW did not know the place of HIV testing, and 48.3% of them mentioned that distance to health facilities was a big problem (Table 1).
In terms of sexual exposure, 53.4% of adolescent girls and young women (AGYW) reported no sexual activity, while 9.2 and 29.1% had initiated sex between the ages of 8–14 and 15–19 years, respectively. Regarding sexual partners, the majority of AGYW (97.2%) reported no sexual activity, excluding spouses. Among all participants, 41.7% and 18.5% reported low and severe community stigma related to HIV, respectively (Table 1).
Spatial autocorrelation and HIV testing clusters
Regarding the autocorrelation analysis, findings showed that significant spatial variance on being ever-tested for HIV with Global Moran's I was 0.46 and p-value was 0.000 (Figure 2). Addis Ababa, the northern part of Amahar, Dire Dawa, Gambela, and Tigray regions were shown to be the statistically significant hotspot areas for being ever-tested for HIV. In contrast, Afar, most parts of Amhara, Benshangul Gumuz, SNNPR, and Somalia were significant cold spots (Figures 3, 4).
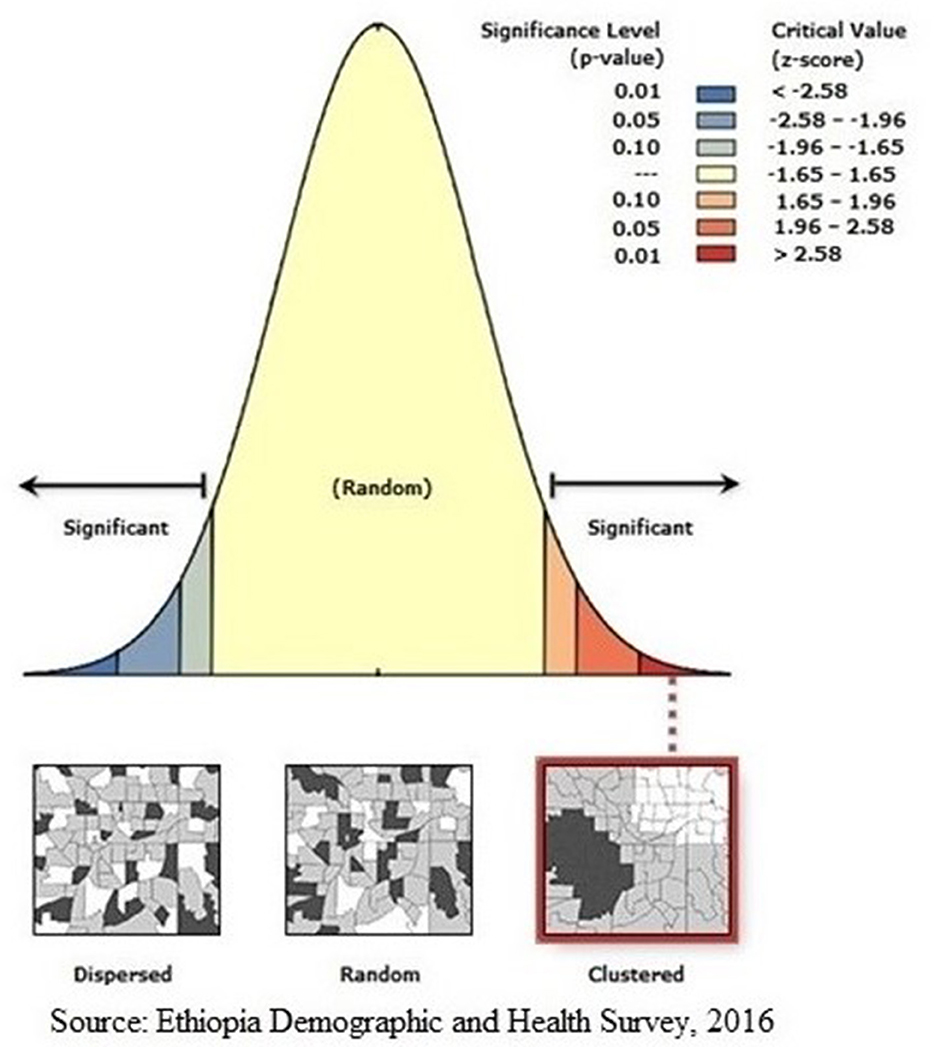
Figure 2. Global Moran's I analysis of AGYW being ever-tested for HIV. The Moran's I index value indicated that ever-tested. The Moran's I index value indicated that ever-tested pattern is not random. The significant P-value and positive z-score showed the spatial distribution of high values and low values in this study was more spatially clustered, while significant P-value and negative z-score showed the spatial distribution of high values and low values in this study was more spatially dispersed.
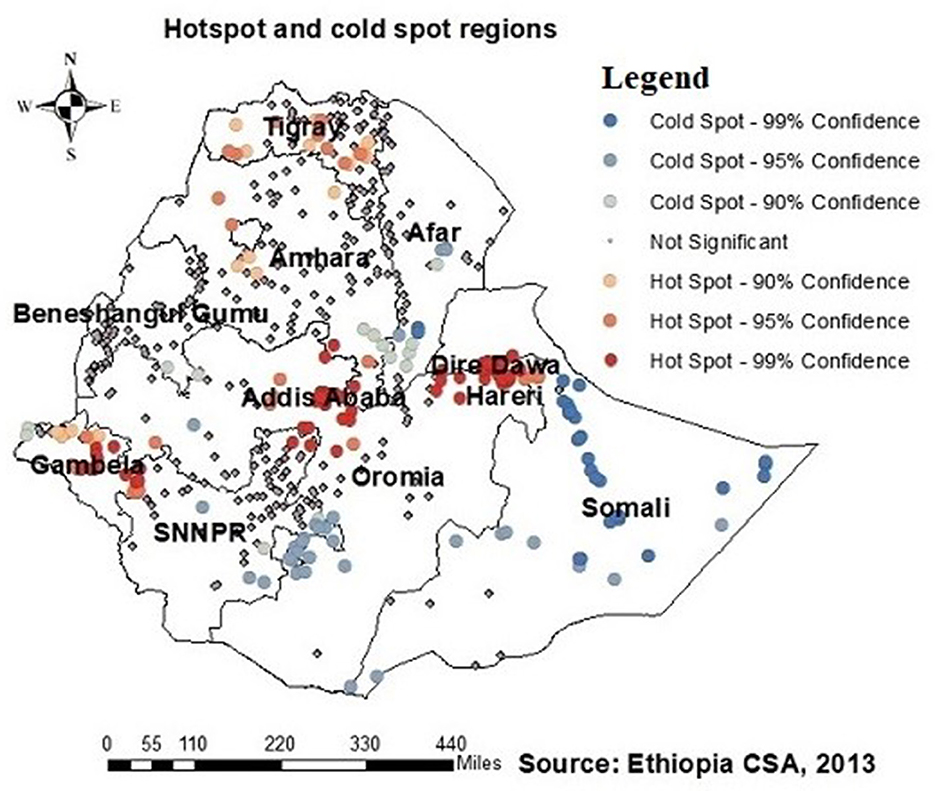
Figure 3. Hotspots and cold spots of AGYW being ever-tested for HIV in Ethiopia. Hotsport regions (red colors) were Addis Ababa, Northern Amhara, Dire Dawa, Tigray, and Gambella, while cold spot regions (blue colors) were Afar, most of Mahara, Somalia, Benishangul-Gumuz, and Southern Nation, Nationality and People Region (SNNPR).
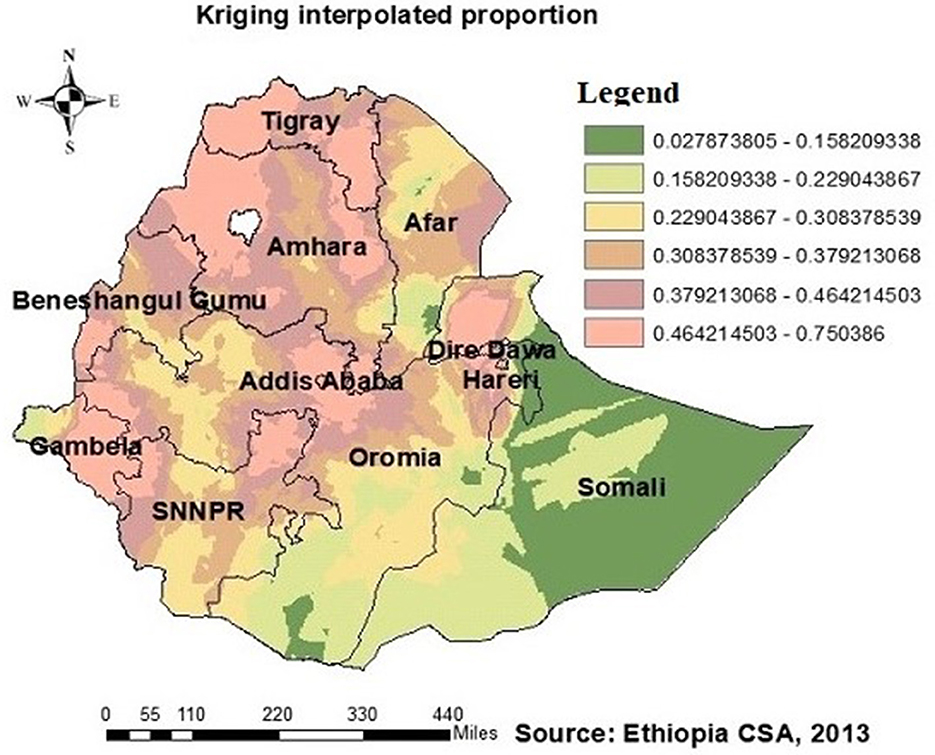
Figure 4. HIV ever-tested AGYW in Ethiopia using kriging interpolation. The interpolated areas (orange) were with high proportion of AGYW ever-tested for HIV while interpolated areas (olive green) were with low proportion of AGYW ever-tested for HIV.
Predictors of ever tested for HIV
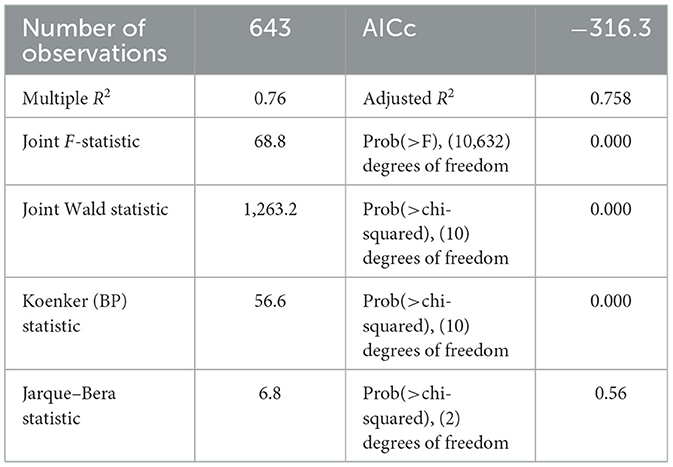
The OLS model identifies the relationships between independent and dependent variables based on geographic locations and affects HIV ever-tested in AGYW in Ethiopia with an adjusted R2 of 0.758; this model accounted for 75.8% of the variation in being ever-tested for HIV. If the Koenker BP test in this study showed a significant association, the geographically weighted regression is advised. The Jarque–Bera statistic was not significant (P = 0.060); hence, it was believed that the model residuals would be normally distributed. In addition, the Joint Wald statistic demonstrated that the entire model was significant (P < 0.001) (Table 2). Since each explanatory variable had a local coefficient, we used a geographically weighted regression model and retrieved those values. The study identified various factors that predict the locations of hotspots for HIV testing, including the percentage of adolescent girls and young women (AGYW) aged 15–19 years, the proportion of Muslim individuals, the proportion of those with no formal education, the proportion of those not knowledgeable about HIV, and the proportion of community stigma (Table 2). The proportion of being ever-tested for HIV differed among AGYW in various demographic groups. For AGYW aged 15–19 years, with no formal education, not knowledgeable about HIV, and experiencing community stigma, the likelihood of being ever-tested for HIV decreased by −0.30, −0.21, 0.22, and −0.22 times, respectively. Conversely, the proportion of Muslim AGYW who reported being ever-tested for HIV increased by 0.16 times with an increase in their proportion.
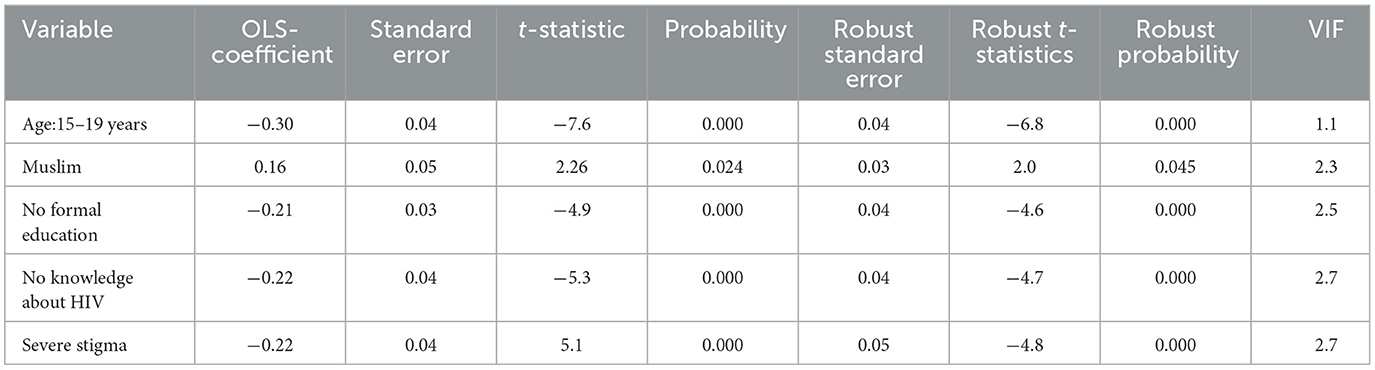
Table 2. Predictors of a high proportion of ever been tested for HIV: result from secondary data analysis of EDHS 2016.
Geographically weighted regression analysis
The GWR analysis showed an improvement in the model. The findings indicated a notable difference in the “AICc” values, with a decrease from −316.3 in the OLS regression to −329.13 in the GWR analysis (Table 3). Moreover, the adjusted R2 value of 0.758 obtained using ordinary least squares (OLS) regression increased to 0.78 by use of GWR. This finding indicates that GWR improved the model's ability to predict the likelihood of individuals having been tested for HIV. Therefore, the results of this research indicate that the GWR analysis showed superior performance compared to the OLS model (Table 3). The model identified both positive and negative associations with the proportion of being ever-tested for HIV. The proportion of being ever-tested for HIV increases with an increase in the proportion of AGYW aged 15–19 years in Tigray, central Afar, Amhara, Addis Ababa, Dire Dawa, Gambela, Harari, and a central part of the Oromia region (Figure 5). However, the proportion of being ever-tested for HIV decreased in Gambela (south and southwest) Oromia, SNNPR, and (south and western) Somalia.
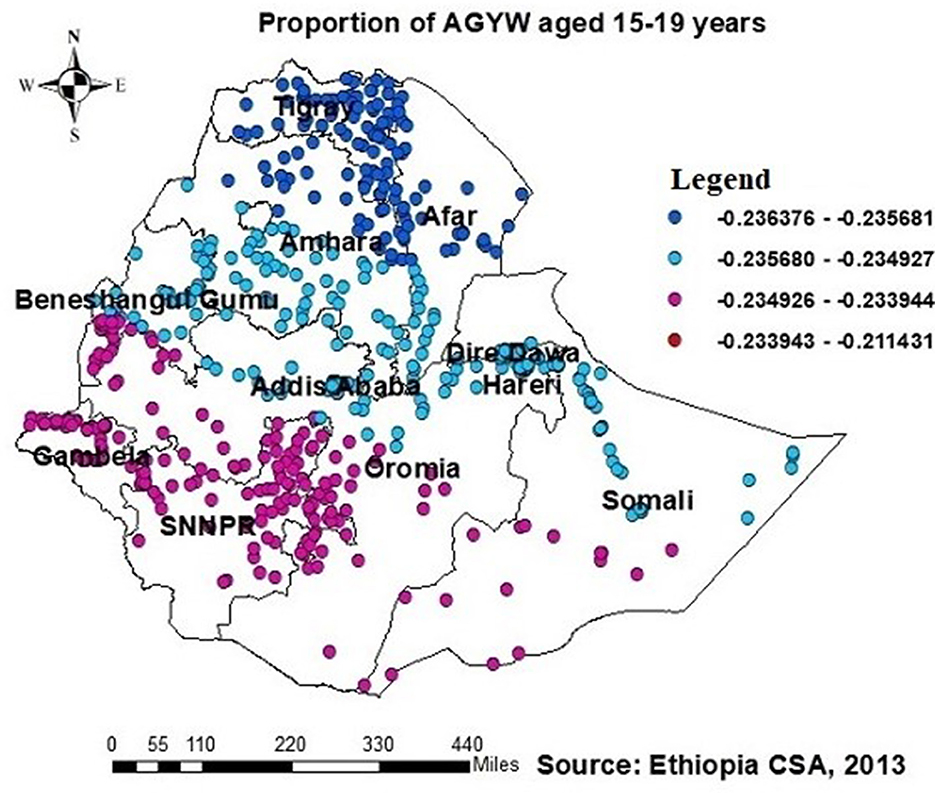
Figure 5. GWR coefficients of the proportion of AGYW aged 15–19 years as a predictor of those being ever-tested for HIV. The proportion of ever-tested for HIV (b1ue legend areas) increases when the proportion of adolescent girls and young women (AGYW) aged 15–19 years increases, while the proportion of ever-tested for HIV (red legend areas) decreases when the proportion of AGYW increases.
The proportion of individuals with no formal education showed a negative relationship with the proportion of those being ever-tested for HIV. The proportion of individuals being ever-tested for HIV decreased in Afar, Addis Ababa, southern Amhara, Dire Daw, Harari, most of the Oromia region, Somalia, and SNNPR with an increase in the proportion of no formal education (Figure 6). Regarding knowledge of HIV, the proportion of those not knowledgeable about HIV showed a negative association with the proportion of those being ever-tested for HIV. The proportion of individuals being ever-tested for HIV decreased in the northern areas of Amhara, Gambela, Benshangul Gumezu, and Tigra with an increase in the proportion of those not knowledgeable about HIV (Figure 7). The research finding showed a negative correlation between the proportion of community stigma and the proportion of those being ever-tested for HIV. The areas of Afar, Addis Ababa, Harari, Amhara, and the northern part of SNNPR, Oromiya, and Tigray had a decreased proportion of those being ever-tested for HIV when community stigma was identified as a prominent contributing factor (Figure 8).
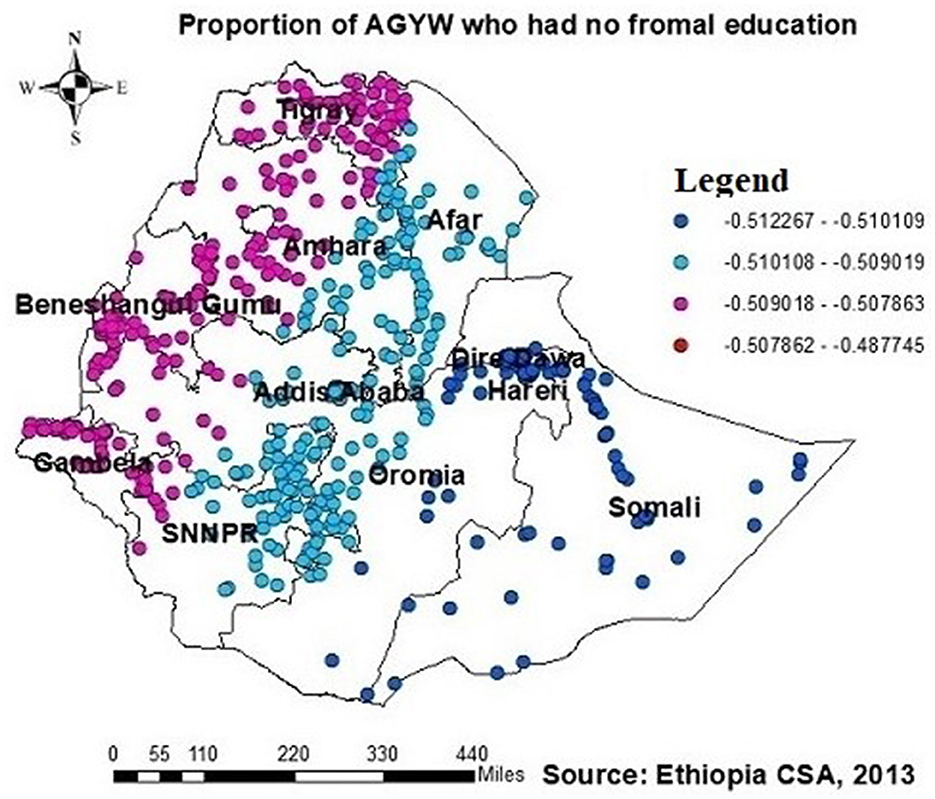
Figure 6. GWR coefficients of the proportion of those with no formal education as a predictor of those being ever-tested for HIV. The proportion of ever-tested for HIV (b1ue legend areas) decreases when the proportion of no formal education increases, while the proportion of ever-tested for HIV (red legend areas) increases when the proportion of no formal education decreases.
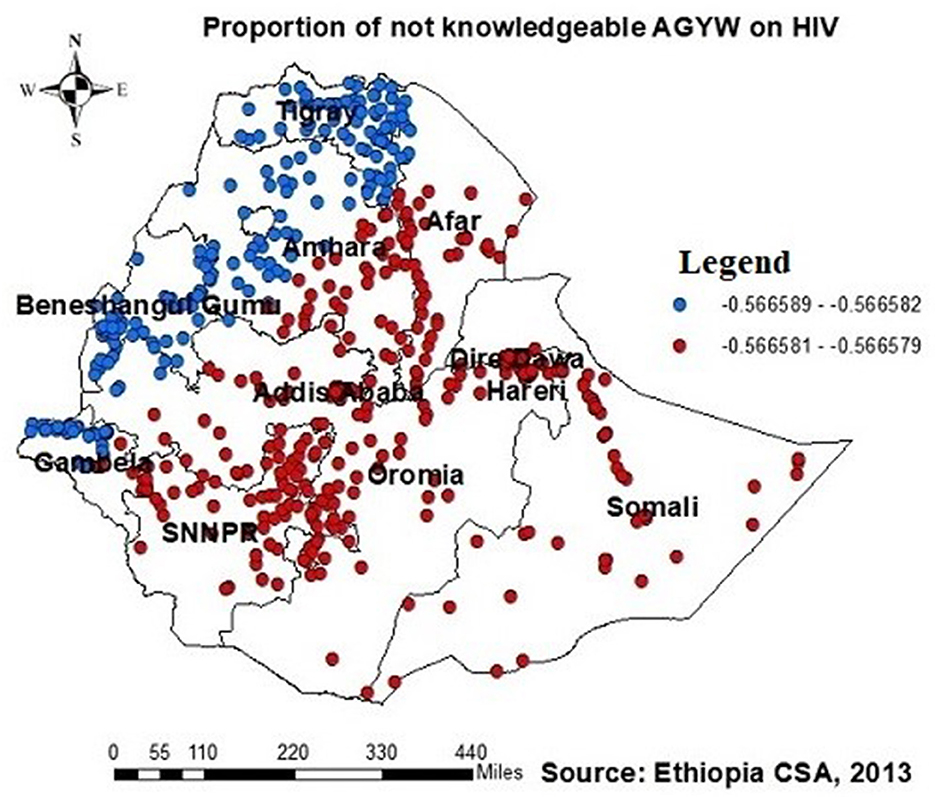
Figure 7. GWR coefficients of the proportion of those not knowledgeable about HIV as a predictor of those being ever-tested for HIV. The proportion of ever-tested for HIV decreases (b1ue legend areas) decreases when the proportion of those not knowledgeable about HIV increases, while the proportion of ever-tested for HIV (red legend areas) increases when the proportion of those not knowledgeable about HIV decreases.
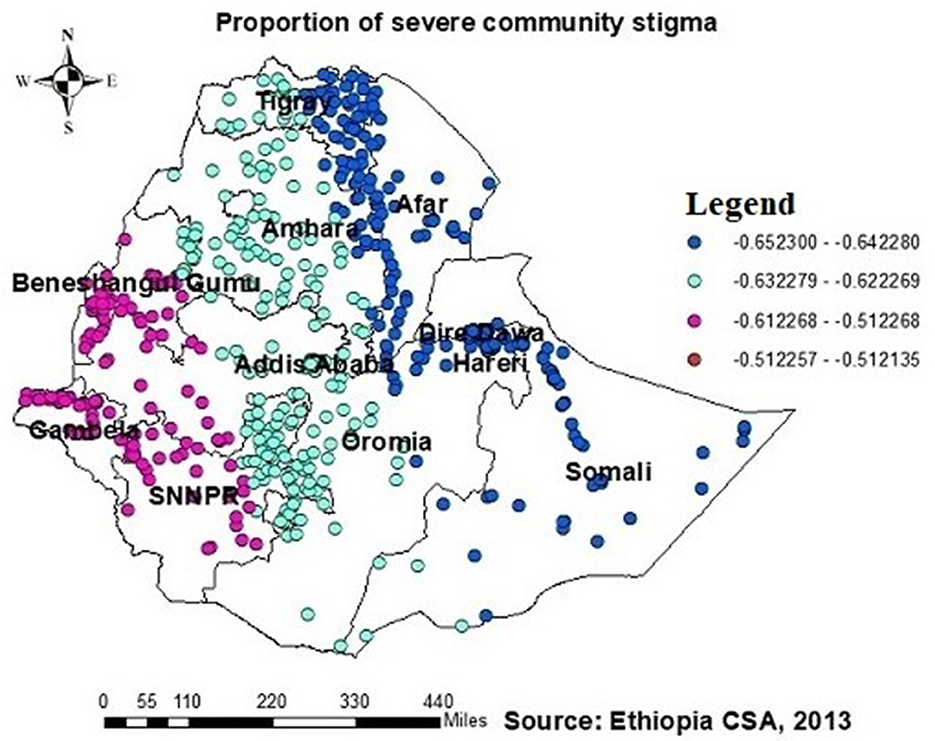
Figure 8. GWR coefficients of the proportion of those experiencing severe community stigma as a predictor of those being ever-tested for HIV. The proportion of ever-tested for HIV (b1ue legend areas) decreased when community stigma was identified as a prominent contributing factor.
In Addis Ababa, Afar, the southern part of Amhara, Dire Dawa, the northern part of SNNPR, and (south and southeast) part of the Oromia region, Harari, and Somalia, the proportion of muslims had a significant and positive association with the proportion of those being ever-tested for HIV (Figure 9).
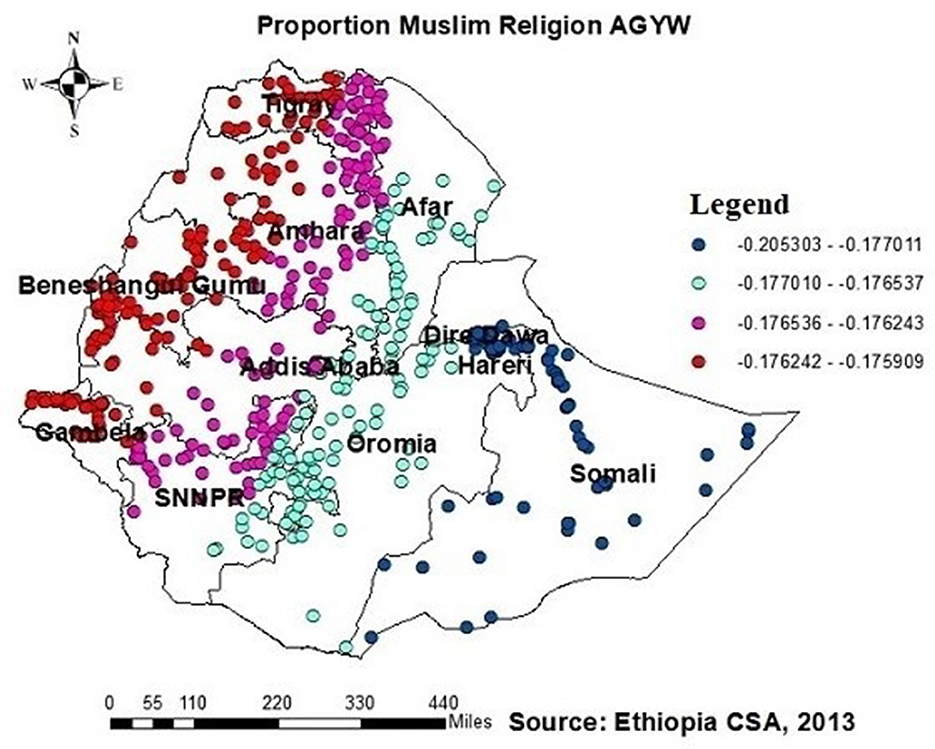
Figure 9. GWR coefficients of the proportion of AGYW being Muslim as a predictor of those being ever-tested for HIV. The proportion of ever-tested for HIV (b1ue legend areas) increases when the proportion of Muslim AGYW increases, while the proportion of ever-tested for HIV (red legend areas) less increases when the proportion of Muslim AGYW increases.
Discussion
The study identified hotspots of being ever-tested for HIV among AGYW in Addis Ababa, northern Amhara, Dire Dawa, Tigray, and Gambela regions. Cold spot regions, such as Afar, most of Amhara, Somalia, Benishangul-Gumuz, and SNNPR, were also identified. These findings align with those of previous studies conducted on the spatial distribution of HIV testing and counseling in Ethiopia (18, 21), as well as a similar study in Nigeria. This finding is supported by the geographic concentration of HIV infections within the nation and regional disparities in prioritizing HIV testing (22, 31–33). The presence of cold spots in HIV testing could be linked to the limited availability of medical services in specific areas. Conversely, hotspot regions, which are often characterized by higher levels of economic development and infrastructure, tend to have better access to healthcare facilities and HIV testing services, leading to higher testing rates. On the contrary, cold spot regions may face economic challenges or political instability, making it difficult to provide sufficient healthcare services, thereby resulting in lower testing rates.
Furthermore, the differences in education access across geographical regions, media exposure, cultural factors, and living conditions may contribute to these variations (21). For example, our study revealed that 54.97% of AGYW in Somalia lacked formal education, whereas only 3.9% of AGYW in Addis Ababa had no formal education. Although the proportion of media exposure did not exhibit a direct relationship with the high prevalence of HIV testing in certain areas, our findings indicate that only 2.4% of AGYW in Addis Ababa lacked media exposure, while a significant 74.69% of AGYW in Somalia were not exposed to media. This lack of media exposure in Somalia likely contributed to lower rates of HIV testing than in other regional states in Ethiopia, consistent with findings from other studies (34, 35). While the use of media platforms such as radio, television, and social media can effectively increase HIV awareness and encourage HIV testing, depending solely on these media for disseminating health information may marginalize individuals with restricted access to electronic media, especially in rural or isolated areas where such platforms are scarce.
This study employed GWR models to analyze the spatial variations and predictors of the proportion of HIV testing among AGYW in Ethiopia. Positive and negative associations with the proportion of those being ever-tested for HIV were identified, including the proportion of participants aged 15–19 years, who lack of formal education, being Muslim, who lack of knowledge about HIV, and who are experiencing community stigma.
The current study found a negative association between the proportion of AGYW aged 15–19 and those prediciting being ever-tested for HIV. The GWR coefficients ranged from 0.23 to 0.21 across districts, indicating a negative association with HIV testing among AGYW. This finding suggests a lower uptake of HIV testing among AGYW aged 15–19. Similar findings are reported in other studies (21, 23, 34). This can be attributed to the decreased self-perceived risk of HIV among AGYW aged 15–19 (21, 36, 37). Additionally, factors such as lower engagement in sexual activity, delayed marriage, and limited economic power among AGYW aged 15–19 contributed to this trend (38).
The study revealed a significant negative association between the proportion of AGYW with no formal education and those being ever-tested for HIV. The GWR coefficients for the proportion of AGYW with no formal education ranged from 0.51 to 0.48. This factor strongly influenced the likelihood of them being ever-tested for HIV, with notable occurrences in regions such as Afar, Addis Ababa, southern Amhara, Dire Dawa, Harari, most of the Oromia region, Somalia, and SNNPR.
Educated AGYW are more likely to understand the benefits of HIV testing and the importance of protection against HIV infection through various media sources such as newspapers, television, and social media, as evidenced in studies from Burkina Faso, Ethiopia, Malawi, Nigeria, and Zambia (21, 34, 35, 39–42). Conversely, lack of formal education constrains access to information and awareness about the importance of HIV testing. Additionally, it diminishes one's autonomy in making decisions to seek healthcare services and utilize healthcare facilities (11, 21, 43, 44).
Similarly, the proportion of individuals lacking knowledge about HIV showed a negative relationship with the proportion of those being ever-tested for HIV, with GWR coefficients ranging between 0.57 and 0.56. This finding is supported by disparities across geographical regions in awareness of HIV transmission and the importance of HIV testing in Ethiopia (45), leading to a varied distribution of being ever-tested AGYW across the country. Moreover, communities with limited knowledge about HIV may experience increased HIV-related stigma, resulting in reduced rates of HIV testing due to decreased awareness (11, 21, 46, 47).
Additionally, this study revealed a negative relationship between the proportion of individuals being ever-tested for HIV among AGYW and severe community stigma. The GWR coefficients for the proportion of severe community stigma ranged between 0.65 and 0.51 (Figure 8). This finding is consistent with those of studies conducted in Ethiopia, China, Malawi, and Nigeria (21, 23, 34, 37, 39, 42, 48). However, in regions such as Tigray, Amhara, Dire Dawa, the northern part of southern Ethiopia, Gambela, and southwest and southern Shewa Oromia, the proportion of muslim AGYW exhibited a positive association with those being ever-tested for HIV, with GWR coefficients ranging from 0.21 to 0.17. This finding may be attributed to muslim participants potentially having better access to education than orthodox and other religious groups; while it is possible for muslim communities to arrange health-related initiatives during religious gatherings or prayer days, these endeavors must prioritize HIV testing and ensure accessibility to the broader community to have a notable effect. Nonetheless, there is limited evidence indicating that such endeavors are prevalent or unique to muslim communities.
Several studies conducted in various Sub-Saharan African countries have highlighted the significance of access to healthcare facilities for HIV testing (19, 49–52). However, in this study, the proportion of distance to healthcare facilities did not show a significant relationship with the proportion of those being ever-tested for HIV among AGYW. Similar findings have been reported in other studies conducted in Ethiopia (21, 53). This could be due to AGYW traveling long distances to access healthcare services to avoid community stigma. Studies conducted in South Africa have also shown that individuals often travel significant distances to access healthcare facilities, indicating that individuals living near regional borders might access services outside their residential region (54, 55). This finding suggests that healthcare access patterns might not strictly adhere to regional boundaries.
The current analysis comprised a representative population of AGYW in Ethiopia. In addition, most of the previous studies identified factors using the logistic regression analysis. However, this study used OLS and GWR to account for geographical or regional heterogeneity variation in determining the factors of high HIV ever-tested areas. Despite the strengths mentioned above, the study has its limitations. The original study was a cross-sectional survey, causing difficulty in establishing a cause and relationship. In addition, self-response indicated that there could be potential memory loss among AGYW, and the original study might cause socially desirable biases. Finally, due to the secondary data used in this study, crucial variables such as service delivery, including quality, cost, distance, and accessibility to HIV testing services, were not assessed in this study, which could cause missing important predictors.
Conclusion
The proportion of hotspot regions for HIV ever-tested for HIV hotspot regions found include Amhara, Addis Ababa, Dire Dawa, Tigray, and Gambela regions. Somali, Afar, Benshangul Gumuz, and SNNPR regions were demarcated as cold spot regions for HIV ever-tested. AGYW aged 15–19 years, Muslim religion, had no formal schooling, no knowledge on HIV, and with severe community stigma were found to be predictors of HIV ever-tested in hotspot areas using GWR. To prevent HIV infection and control the disease, governments and other stakeholders should give due attention to increasing HIV testing among these special groups of the population.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: it is EDHS data that will be available to the corresponding author and will be given up on request. Requests to access these datasets should be directed to DHS; email address: YXJjaGl2ZUBkaHNwcm9ncmFtLmNvbQ==; web: https://dhsprogram.com/.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
MS: Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Conceptualization, Writing – review & editing, Writing – original draft. AT: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing, Investigation. BB: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Formal analysis, Data curation. AG: Conceptualization, Data curation, Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis. SH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Writing – review & editing. LF: Writing – review & editing, Visualization, Software, Methodology, Investigation, Conceptualization. WM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express gratitude to DHS for allowing access to data for the secondary analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AGYW, adolescent girls and young women; AIDS, acquired immunodeficiency syndrome; AICc's, Akaike's Information Criteria; DHS, Demographic and Health Survey; EHDS, Ethiopia Demographic and Health Survey; GWR, geographically weighted regression; HIV, human immunodeficiency virus; OLS, ordinary least square; SNNPR, Southern Nation Nationality and People's Region; VIF, variance inflation factor.
References
1. Murewanhema G, Musuka G, Moyo P, Moyo E, Dzinamarira T, HIV. and adolescent girls and young women in sub-Saharan Africa: A call for expedited action to reduce new infections. IJID Regions. (2022) 5:30–2. doi: 10.1016/j.ijregi.2022.08.009
2. Global H. AIDS Statistics—Fact Sheet. UNAIDS (2021). Available online at: https://www.unaidsorg/en/resources/fact-sheet (accessed November 10, 2023).
3. WHO. Epidemiological Fact Sheet: HIV Statistics, Globally and by WHO Region. (2023) Available online at: https://cdnwhoint/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/j0294-who-hiv-epi-factsheet-v7pdf (accessed November 10, 2023).
4. Brown K, Williams DB, Kinchen S, Saito S, Radin E, Patel H, et al. Status of HIV epidemic control among adolescent girls and young women aged 15–24 years—seven African countries, 2015–2017. Morbid Mortal Wkly Rep. (2018) 67:29. doi: 10.15585/mmwr.mm6701a6
5. Birdthistle I, Tanton C, Tomita A, de Graaf K, Schaffnit SB, Tanser F, et al. Recent levels and trends in HIV incidence rates among adolescent girls and young women in ten high-prevalence African countries: a systematic review and meta-analysis. Lancet Global Health. (2019) 7:e1521–e40. doi: 10.1016/S2214-109X(19)30410-3
6. Khalifa A, Stover J, Mahy M, Idele P, Porth T, Lwamba C. Demographic change and HIV epidemic projections to 2050 for adolescents and young people aged 15-24. Glob Health Action. (2019) 12:1662685. doi: 10.1080/16549716.2019.1662685
7. Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc. (2015) 18:19408. doi: 10.7448/IAS.18.2.19408
8. Lewis L, Kharsany AB, Humphries H, Maughan-Brown B, Beckett S, Govender K, et al. HIV incidence and associated risk factors in adolescent girls and young women in South Africa: a population-based cohort study. PLoS ONE. (2022) 17:e0279289. doi: 10.1371/journal.pone.0279289
9. Wong VJ, Murray KR, Phelps BR, Vermund SH, McCarraher DR. Adolescents, young people, and the 90–90–90 goals: a call to improve HIV testing and linkage to treatment. AIDS. (2017) 31:S191. doi: 10.1097/QAD.0000000000001539
10. Comins CA, Rucinski KB, Baral S, Abebe SA, Mulu A, Schwartz SR. Vulnerability profiles and prevalence of HIV and other sexually transmitted infections among adolescent girls and young women in Ethiopia: a latent class analysis. PLoS ONE. (2020) 15:e0232598. doi: 10.1371/journal.pone.0232598
11. Ajiboye AS, Eshetu F, Lulseged S, Getaneh Y, Tademe N, Kifle T, et al. Predictors of HIV testing among youth 15–24 years in urban Ethiopia, 2017–2018 Ethiopia population-based HIV impact assessment. PLoS ONE. (2023) 18:e0265710. doi: 10.1371/journal.pone.0265710
12. Pachena A, Musekiwa A. Trends in HIV testing and associated factors among adolescent girls and young women in Zimbabwe: cross-sectional analysis of demographic and health survey data from 2005 to 2015. Int J Environ Res Public Health. (2022) 19:5165. doi: 10.3390/ijerph19095165
13. Maviso MK, Kalembo FW. Determinants of not testing for HIV among young adult women in Papua New Guinea: findings from the 2016–2018 Demographic and Health Survey. medRxiv. doi: 10.1101/2023.05.07.23289638
14. Peltzer K, Matseke G. Determinants of HIV testing among young people aged 18–24 years in South Africa. Afr Health Sci. (2013) 13:1012–20. doi: 10.4314/ahs.v13i4.22
15. Stangl AL, Sebany M, Kapungu C, Jessee C, Ricker CL, Chard E. Is HIV index testing and partner notification safe for adolescent girls and young women in low-and middle-income countries? J Int AIDS Soc. (2020) 23:e25562. doi: 10.1002/jia2.25562
16. Kebede A, Desale A, Mulugeta A, Yaregal Z, Gebreegziabxier A, Ayana G, et al. Quality assurance for point-of-care testing: Ethiopia's experience: country profile. Afr J Lab Med. (2016) 5:1–5. doi: 10.4102/ajlm.v5i2.452
17. Bekele YA, Fekadu GA. Factors associated with HIV testing among young females; further analysis of the 2016 Ethiopian demographic and health survey data. PLoS ONE. (2020) 15:e0228783. doi: 10.1371/journal.pone.0228783
18. Diress G, Ahmed M, Adane S, Linger M, Alemnew B. Barriers and facilitators for HIV testing practice among Ethiopian women aged 15-24 years: analysis of the. Ethiopian demographic and health survey. HIV/AIDS Res Palliat Care. (2016) 2021:963–70. doi: 10.2147/HIV.S280590
19. Simms I, Gibin M, Petersen J. Location, Location, Location: What Can Geographic Information Science (GIS) Offer Sexual Health Research? London: BMJ Publishing Group Ltd. (2014). p. 442−3.
20. ECSA aI. Ethiopia Demographic and Health Survey 2016. Addis Ababa: CSA and ICF (2016). Available online at: https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf (accessed November 10, 2023).
21. Alem AZ, Liyew AM, Guadie HA. Spatial pattern and associated factors of HIV testing and counselling among youths (15–24 years) in Ethiopia. BMC Public Health. (2021) 21:1–13. doi: 10.1186/s12889-021-10677-0
22. Nwachukwu CE, Odimegwu C. Regional patterns and correlates of HIV voluntary counselling and testing among youths in Nigeria. Afr J Reprod Health. (2011) 15:131–46.
23. Erena AN, Shen G, Lei P. Factors affecting HIV counselling and testing among Ethiopian women aged 15–49. BMC Infect Dis. (2019) 19:1–12. doi: 10.1186/s12879-019-4701-0
25. Chen Y. New approaches for calculating Moran's index of spatial autocorrelation. PLoS ONE. (2013) 8:e68336. doi: 10.1371/journal.pone.0068336
26. Tsai P-J, Lin M-L, Chu C-M, Perng C-H. Spatial autocorrelation analysis of health care hotspots in Taiwan in 2006. BMC Public Health. (2009) 9:1–13. doi: 10.1186/1471-2458-9-464
27. Shrestha PM. Comparison of ordinary least square regression, spatial autoregression, and geographically weighted regression for modeling forest structural attributes using a Geographical Information System (GIS). Remote Sensing (RS) Approach Thesis Canada, University of Calgary (2006). Available online at: http://peopleucalgaryca/~mcdermid/Docs/Theses/Shrestha_2006pdf (accessed October 30, 2012).
28. Nazeer M, Bilal M. Evaluation of ordinary least square (OLS) and geographically weighted regression (GWR) for water quality monitoring: a case study for the estimation of salinity. J. Ocean Univ China. (2018) 17:305–10. doi: 10.1007/s11802-018-3380-6
29. Belay DG, Adane SM, Ferede OL, Lakew AM. Geographically weighted regression analysis of anemia and its associated factors among reproductive age women in Ethiopia using 2016 demographic and health survey. PLoS ONE. (2022) 17:e0274995. doi: 10.1371/journal.pone.0274995
30. Tesema GA, Tessema ZT, Angaw DA, Tamirat KS, Teshale AB. Geographic weighted regression analysis of hot spots of anemia and its associated factors among children aged 6–59 months in Ethiopia: a geographic weighted regression analysis and multilevel robust Poisson regression analysis. PLoS ONE. (2021) 16:e0259147. doi: 10.1371/journal.pone.0259147
31. Ying R, Fekadu L, Schackman BR, Verguet S. Spatial distribution and characteristics of HIV clusters in Ethiopia. Trop Med Int Health. (2020) 25:301–7. doi: 10.1111/tmi.13356
32. Gelibo T, Lulseged S, Eshetu F, Abdella S, Melaku Z, Ajiboye S, et al. Spatial distribution and determinants of HIV prevalence among adults in urban Ethiopia: findings from the Ethiopia population-based HIV Impact Assessment Survey (2017–2018). PLoS ONE. (2022) 17:e0271221. doi: 10.1371/journal.pone.0271221
33. Lakew Y, Benedict S, Haile D. Social determinants of HIV infection, hotspot areas and subpopulation groups in Ethiopia: evidence from the National Demographic and Health Survey in 2011. BMJ Open. (2015) 5:e008669. doi: 10.1136/bmjopen-2015-008669
34. Teklehaimanot HD, Teklehaimanot A, Yohannes M, Biratu D. Factors influencing the uptake of voluntary HIV counseling and testing in rural Ethiopia: a cross sectional study. BMC Public Health. (2016) 16:1–13. doi: 10.1186/s12889-016-2918-z
35. Qiao S, Zhang Y, Li X, Menon JA. Facilitators and barriers for HIV-testing in Zambia: a systematic review of multi-level factors. PLoS ONE. (2018) 13:e0192327. doi: 10.1371/journal.pone.0192327
36. Lemma T, Silesh M, Taye BT, Desta K, Kitaw TM, Tekalign T, et al. Serostatus disclosure and its predictors among children living with HIV in Ethiopia: a systematic review and meta-analysis. Front Public Health. (2022) 10:859469. doi: 10.3389/fpubh.2022.859469
37. Leta TH, Sandøy IF, Fylkesnes K. Factors affecting voluntary HIV counselling and testing among men in Ethiopia: a cross-sectional survey. BMC Public Health. (2012) 12:1–12. doi: 10.1186/1471-2458-12-438
38. Jones C, Ritchwood TD, Taggart T. Barriers and facilitators to the successful transition of adolescents living with HIV from pediatric to adult care in low and middle-income countries: a systematic review and policy analysis. AIDS Behav. (2019) 23:2498–513. doi: 10.1007/s10461-019-02621-6
39. Lépine A, Terris-Prestholt F, Vickerman P. Determinants of HIV testing among Nigerian couples: a multilevel modelling approach. Health Policy Plan. (2015) 30:579–92. doi: 10.1093/heapol/czu036
40. Bibiana N, Emmanuel P, Amos D, Ramsey Y, Idris A. Knowledge, attitude and factors affecting voluntary HIV counseling and testing services among women of reproductive age group in an Abuja Suburb community, Nigeria. Med J Zambia. (2018) 45:13–22. doi: 10.55320/mjz.45.1.155
41. Kirakoya-Samadoulougou F, Jean K, Maheu-Giroux M. Uptake of HIV testing in Burkina Faso: an assessment of individual and community-level determinants. BMC Public Health. (2017) 17:1–11. doi: 10.1186/s12889-017-4417-2
42. Berendes S, Rimal RN. Addressing the slow uptake of HIV testing in Malawi: the role of stigma, self-efficacy, and knowledge in the Malawi BRIDGE Project. J Assoc Nurses AIDS Care. (2011) 22:215–28. doi: 10.1016/j.jana.2010.08.005
43. Latunji O, Akinyemi O. Factors influencing health-seeking behaviour among civil servants in Ibadan, Nigeria. Ann Ibadan Postgrad Med. (2018) 16:52–60.
44. Kifle D, Azale T, Gelaw YA, Melsew YA. Maternal health care service seeking behaviors and associated factors among women in rural Haramaya District, Eastern Ethiopia: a triangulated community-based cross-sectional study. Reprod Health. (2017) 14:1–11. doi: 10.1186/s12978-016-0270-5
45. Gurmu E, Etana D. HIV/AIDS knowledge and stigma among women of reproductive age in Ethiopia. Afr J AIDS Res. (2015) 14:191–9. doi: 10.2989/16085906.2015.1051066
46. Letshwenyo-Maruatona SB, Madisa M, Boitshwarelo T, George-Kefilwe B, Kingori C, Ice G, et al. Association between HIV/AIDS knowledge and stigma towards people living with HIV/AIDS in Botswana. Afr J AIDS Res. (2019) 18:58–64. doi: 10.2989/16085906.2018.1552879
47. Alemi Q, Stempel C. Association between HIV knowledge and stigmatizing attitudes towards people living with HIV in Afghanistan: findings from the 2015 Afghanistan Demographic and Health Survey. Int Health. (2019) 11:440–6. doi: 10.1093/inthealth/ihz013
48. Zhang Q, Fu Y-S, Liu X-M, Ding Z-Q, Li M-Q, Fan Y-G. HIV prevalence and factors influencing the uptake of voluntary HIV counseling and testing among older clients of female sex workers in Liuzhou and Fuyang Cities, China, 2016-2017: a cross-sectional study. BioMed Res Int. (2020) 2020:9634328. doi: 10.1155/2020/9634328
49. Fundisi E, Dlamini S, Mokhele T, Weir-Smith G, Motolwana E, editors. Exploring determinants of HIV/AIDS self-testing uptake in South Africa using generalised linear poisson and geographically weighted poisson regression. Healthcare. (2023) 11:881. doi: 10.3390/healthcare11060881
50. Jooste S, Mabaso M, Taylor M, North A, Shean Y, Simbayi LC, et al. Geographical variation in HIV testing in South Africa: evidence from the 2017 national household HIV survey. South Afr J HIV Med. (2021) 22:1–9. doi: 10.4102/sajhivmed.v22i1.1273
51. Hansoti B, Mishra A, Rao A, Chimoyi L, Redd AD, Reynolds SJ, et al. The geography of emergency department-based HIV testing in South Africa: can patients link to care? EClinicalMedicine. (2021) 40:101091. doi: 10.1016/j.eclinm.2021.101091
52. Chimoyi L, Matsena-Zingoni Z, Charalambous S, Marinda E, Manda S, Musenge E. Assessing spatial patterns of HIV prevalence and interventions in semi-urban settings in South Africa. Implications for spatially targeted interventions. Geospatial Health. (2022) 17:1084. doi: 10.4081/gh.2022.1084
53. Tsega NT, Belay DG, Asratie MH, Gashaw M, Endalew M, Aragaw FM. Individual and community-level determinants and spatial distribution of prenatal HIV test uptake in Ethiopia: Spatial and multilevel analysis. Front Public Health. (2023) 11:962539. doi: 10.3389/fpubh.2023.962539
54. Bassett IV, Regan S, Mbonambi H, Blossom J, Bogan S, Bearnot B, et al. Finding HIV in hard to reach populations: mobile HIV testing and geospatial mapping in Umlazi township, Durban, South Africa. AIDS Behav. (2015) 19:1888–95. doi: 10.1007/s10461-015-1012-3
Keywords: mapping, ever tested for HIV, adolescent girls, young women, Ethiopia
Citation: Shimbre MS, Tunja A, Bodicha BB, Belete AG, Hailgebereal S, Fornah L and Ma W (2024) Spatial mapping and predictors of ever-tested for HIV in adolescent girls and young women in Ethiopia. Front. Public Health 12:1337354. doi: 10.3389/fpubh.2024.1337354
Received: 17 November 2023; Accepted: 29 February 2024;
Published: 03 April 2024.
Edited by:
Daniel Diaz, National Autonomous University of Mexico, MexicoReviewed by:
Machiko Otani, World Health Organization, DenmarkYunia Mayanja, Medical Research Council, Uganda
Copyright © 2024 Shimbre, Tunja, Bodicha, Belete, Hailgebereal, Fornah and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mulugeta Shegaze Shimbre, bXVsc2hlZ0B5YWhvby5jb20=; Wei Ma, d2VpbWFAc2R1LmVkdS5jbg==
 Mulugeta Shegaze Shimbre
Mulugeta Shegaze Shimbre Abayneh Tunja2
Abayneh Tunja2 Belay Boda Bodicha
Belay Boda Bodicha Abebe Gedefaw Belete
Abebe Gedefaw Belete Samuel Hailgebereal
Samuel Hailgebereal Lovel Fornah
Lovel Fornah Wei Ma
Wei Ma