- 1Laboratorio Microbiología Molecular – Laboratorios de Investigación y Desarrollo, Facultad de Ciencias e Ingeniería, Universidad Peruana Cayetano Heredia, Lima, Peru
- 2Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
- 3Asociación Benéfica Prisma, Lima, Peru
- 4Department of Virology, Tohoku University Graduate School of Medicine, Sendai, Japan
Background: Enterotoxigenic E. coli (ETEC) is a leading cause of diarrheal morbidity and mortality in children, although the data on disease burden, epidemiology, and impact on health at the community level are limited.
Methods: In a longitudinal birth cohort study of 345 children followed until 24 months of age in Lima, Peru, we measured ETEC burden in diarrheal and non-diarrheal samples using quantitative PCR (LT, STh, and STp toxin genes), studied epidemiology and measured anthropometry in children.
Results: About 70% of children suffered from one or more ETEC diarrhea episodes. Overall, the ETEC incidence rate (IR) was 73 per 100 child-years. ETEC infections began early after birth causing 10% (8.9–11.1) ETEC-attributable diarrheal burden at the population level (PAF) in neonates and most of the infections (58%) were attributed to ST-ETEC [PAF 7.9% (1.9–13.5)] and LT + ST-ETEC (29%) of which all the episodes were associated with diarrhea. ETEC infections increased with age, peaking at 17% PAF (4.6–27.7%; p = 0.026) at 21 to 24 months. ST-ETEC was the most prevalent type (IR 32.1) with frequent serial infections in a child. The common colonization factors in ETEC diarrhea cases were CFA/I, CS12, CS21, CS3, and CS6, while in asymptomatic ETEC cases were CS12, CS6 and CS21. Only few (5.7%) children had repeated infections with the same combination of ETEC toxin(s) and CFs, suggested genotype-specific immunity from each infection. For an average ETEC diarrhea episode of 5 days, reductions of 0.060 weight-for-length z-score (0.007 to 0.114; p = 0.027) and 0.061 weight-for-age z-score (0.015 to 0.108; p = 0.009) were noted in the following 30 days.
Conclusion: This study showed that ETEC is a significant pathogen in Peruvian children who experience serial infections with multiple age-specific pathotypes, resulting in transitory growth impairment.
Introduction
Enterotoxigenic E. coli (ETEC) causes significant global morbidity and mortality in low-and middle-income countries (1–3). Despite being an important pathogen, the paucity of data on ETEC disease burden, particularly at the national and sub-national levels, has created uncertainties in the reported ETEC-associated morbidity and mortality estimates (4, 5). This lack of data called for a widespread improvement in the quality and quantity of data, including improved surveillance systems, and using appropriate diagnostic tools to reveal the disease burden (6, 7). The region-specific estimates for the acute and long-term burden of ETEC would guide funders and public health officials to make evidence-based decisions to design effective vaccines and age-appropriate vaccination schedules for the regions with high pathogen burden (8).
ETEC toxin, heat-labile (LT) and colonization factors (CFs) are the primary target antigens in ETEC vaccine development (9–12). After adherence to the intestinal mucosa, ETEC produce one or both of two enterotoxins, heat-labile enterotoxin (LT) and heat-stable enterotoxin (ST). There are two types of ST, STa and STb. ETEC strains isolated from humans produce STa, and STb predominates in ETEC from animals. There are two subtypes of STa: STh and STp (1). STh (human) is a short (19-amino-acid) peptide and poorly immunogenic; thus, it itself cannot be used as a vaccine component (13, 14) and is not included in the most advanced ETEC vaccine candidates (9–12); although, there are current approaches to include STh in the vaccine candidates (15, 16). Evidence about the role of ETEC strains producing STp (porcine) as the only enterotoxin in causing moderate to severe diarrhea is contradictory (17, 18). While the prevalence of the ETEC toxin types, CFs and O serogroups varies substantially by region (19, 20), conflicting data are available on the relative importance of these virulence factors in protection from ETEC (1, 21). Data on the sequelae of the infections associated with types of ETEC by age are needed to elucidate the role of these virulence factors. Understanding the shedding duration of the pathotypes of ETEC in stool following infection could bring insight to our planning for a reduction in transmission of this enteric disease. The effect of ETEC diarrhea on immediate and long-term growth faltering is an important aspect that needs to be further studied (22, 23).
We conducted a prospective birth cohort study of Peruvian children in a censused population in Lima, Peru, to determine the natural history of ETEC infections, age specific ETEC disease burden, the impact of ETEC infections on morbidity and nutritional status during the first two years of life and identify the ETEC vaccine antigens and natural protection relevant to this region.
Materials and methods
Participants and procedures
A total of 345 newborns within 35 days and their mothers were enrolled in a staggered fashion to control for seasonality in a peri-urban community in Lima from 2016 to 2019. The children were followed until they were 24 months old. The children who presented with severe disease or a birth weight < 1,500 g were excluded.
The diarrheal episodes, and morbidity data were recorded by the fieldworkers through daily home visits. The diarrhea severity scoring criteria were based on the CODA index (24). CODA uses the number of days with fever, anorexia, vomiting, the number of liquid stools, and the maximum number of stools in a 24-h period during the episode. The anthropometry (length and weight) of the children was measured every month. A stool sample was collected every week and during the diarrhea episode and transported to the laboratory in insulated cooler box. All stools from diarrhea episodes and one routine stool every three months were tested for ETEC from DNA extracted from the stool followed by qPCR for the LT, STh, and STp genes (7, 25). Samples with a cycle threshold (Cq) value of <40 for LT, STh, or STp were considered positive for ETEC. We also calculated the incidence rates with a more stringent cutoff, Cq35, and presented the difference in the Supplementary Table S1. Conventional culture for E. coli was performed on MacConkey agar from the collected stool, followed by PCR for LT, STh, and STp genes of five selected E. coli colonies per sample (7). Thirteen colonization factors (CFA/I, CS1-8, CS12, CS21, CS17, CS17/19) were tested from the ETEC isolates or from DNA isolated from stool (when isolates were not available) using multiplex PCR (26) and confirmation with simplex PCR. To further characterize the ETEC strains, we detected the O serogroups. Randomly selected 44 ETEC isolates from ETEC diarrhea stool were tested using slide agglutination with poly and monoclonal O-antigen antisera (Denka Seiken, Japan) (27). Stools from randomly selected 147 ETEC positive cases (98 asymptomatic and 50 diarrheal) were tested for co-pathogens norovirus (GI and GII) (28), rotavirus (29), sapovirus (30), adenovirus (31), Shigella spp. (7), and Campylobacter spp. (32) using qPCR. For blood grouping, Hematest A1 (Diagast) kit was used. The secretory status was performed on saliva samples using a direct ELISA assay to identify the presence of H antigen. The sample was considered positive when the absorbance was at least 4 times greater than the absorbance of the negative control.
Statistical analysis
The cumulative incidence and 95% CI of ETEC diarrhea and infections were estimated using Kaplan Meier. A non-diarrhea ETEC episode was defined as a positive sample collected more than 30 days after a diarrhea episode ended and at least 15 days before a diarrhea episode started. The ETEC-attributable diarrheal burden at the population level (PAF) (33) over two years was estimated by age group at three-month intervals. PAF is defined as the fraction of all cases of a particular disease or other adverse condition in a population that is attributable to a specific exposure. ETEC shedding duration by toxin types was compared using the Kruskal Wallis test and post hoc Dunn’s test. Toxin and CFs-specific protection from repeated infections was estimated using time-dependent variable Cox proportional hazards regression for multiple event data using the age of children for the model timeline, accounting for intra-child correlation using standard robust variance estimates. Diarrhea and non-diarrhea samples were compared using GEE logistic regressions with exchangeable correlation to account for intra child correlation. We evaluated the association between the total number of ETEC episodes over the follow-up and anthropometric measurements at the end of the follow up by means of linear regression models. The velocity model was used to assess the more immediate association between the proportion of ETEC diarrhea days during any anthropometric measurement interval and the average change in anthropometric measurements over the following measurement interval. Study definitions and analysis details are provided in the Supplementary material.
Results
The burden of ETEC infection and diarrhea
Out of 345 enrolled children, 259 (75.1%) completed follow-up until 24 months of age, with a total follow-up period of 201,520 days (Table 1). ETEC was detected in 393 (25.6%) of the diarrhea episodes tested. The average number of ETEC diarrheal days was 5.3 days/episode. The number of ETEC diarrhea episodes was not different between girls and boys, accounting for intra-child correlation and follow-up time (p = 0.941). Most ETEC episodes were moderate to severe diarrhea (86.7%); 6.4% were persistent diarrhea, of which 44% episodes lasted for ≥14 days (maximum 26 days); ~21% of cases were with fever and vomiting each. No significant differences were noted between the clinical outcomes of the ETEC positive and negative diarrhea episodes (Table 2). Among the routine non-diarrheal specimens, ETEC was detected in 373 (22.1%) [highest 30% at 21–24 months and lowest 7% at 0–3 months]; 18.5% in the first and 30% in the second year of life.
Of the children with blood group and secretor status detected, the majority were blood type O [80% (221 of 277)] and secretors [98.5% (329 of 334)] with no significant association with the number or severity of ETEC diarrhea (see Supplementary Figure S1 and Supplementary Table S2).
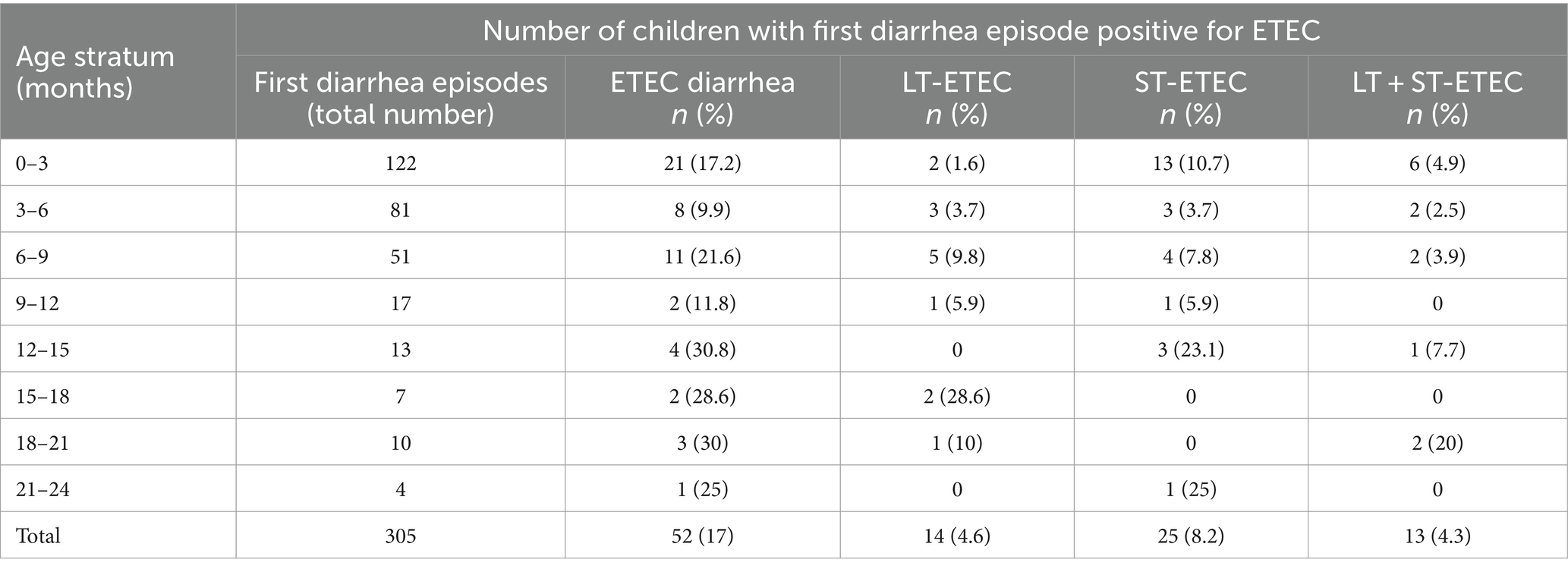
ETEC diarrhea incidence started early after birth and markedly increased with age, while ETEC negative diarrhea incidence decreased after 200 days of life (Figure 1). The overall incidence rate per 100 child years (IR) of ETEC positive diarrhea was 72.9 episodes. By the 1st year of life, 33.8% and by the 2nd year of life, 68% of the children suffered from one or more (up to 7) ETEC diarrhea episodes (Figure 2A). The frequency of two or more ST-ETEC diarrhea episodes (46.4%) per child was higher than LT-ETEC (29.8%) and LT + ST-ETEC (23.8%) (Figures 2B–D; Table 3). The ETEC diarrhea IR was significantly higher in the second year of life than in the first year (91.2 vs. 56.1; p = 0.023, 95% CI 6.7–62.9%) (Figure 3). The highest IR 106.4 was at 18–21 months of age. The overall IR of ST-ETEC (32.1) was highest, followed by LT + ST-ETEC (20.6) and LT-ETEC (20.2) (Figure 3). The IR of STp-ETEC was 9.3.
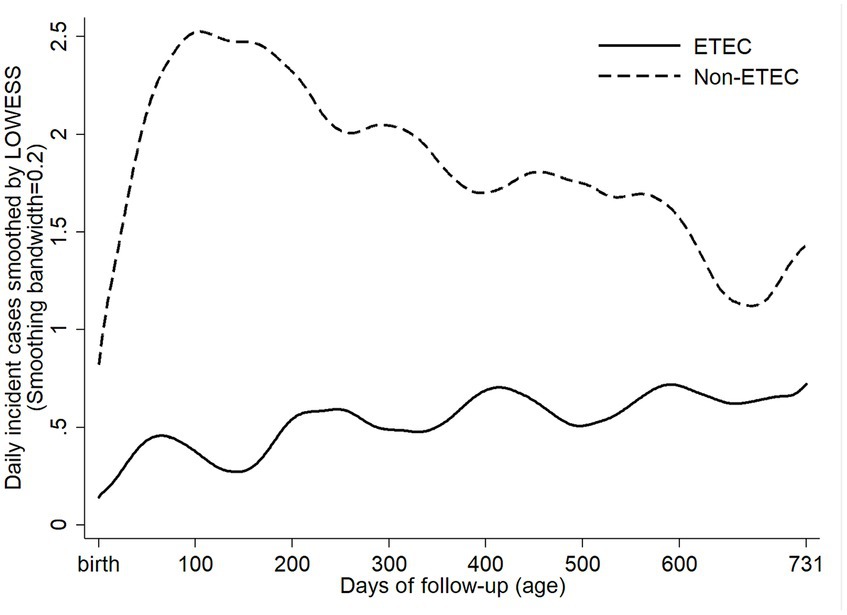
Figure 1. Daily incident cases of ETEC positive and ETEC negative diarrhea episodes. Daily incident cases of ETEC positive and non-ETEC diarrhea from birth to the end of the follow-up, smoothed by LOWESS (locally weighted scatterplot smoothing).
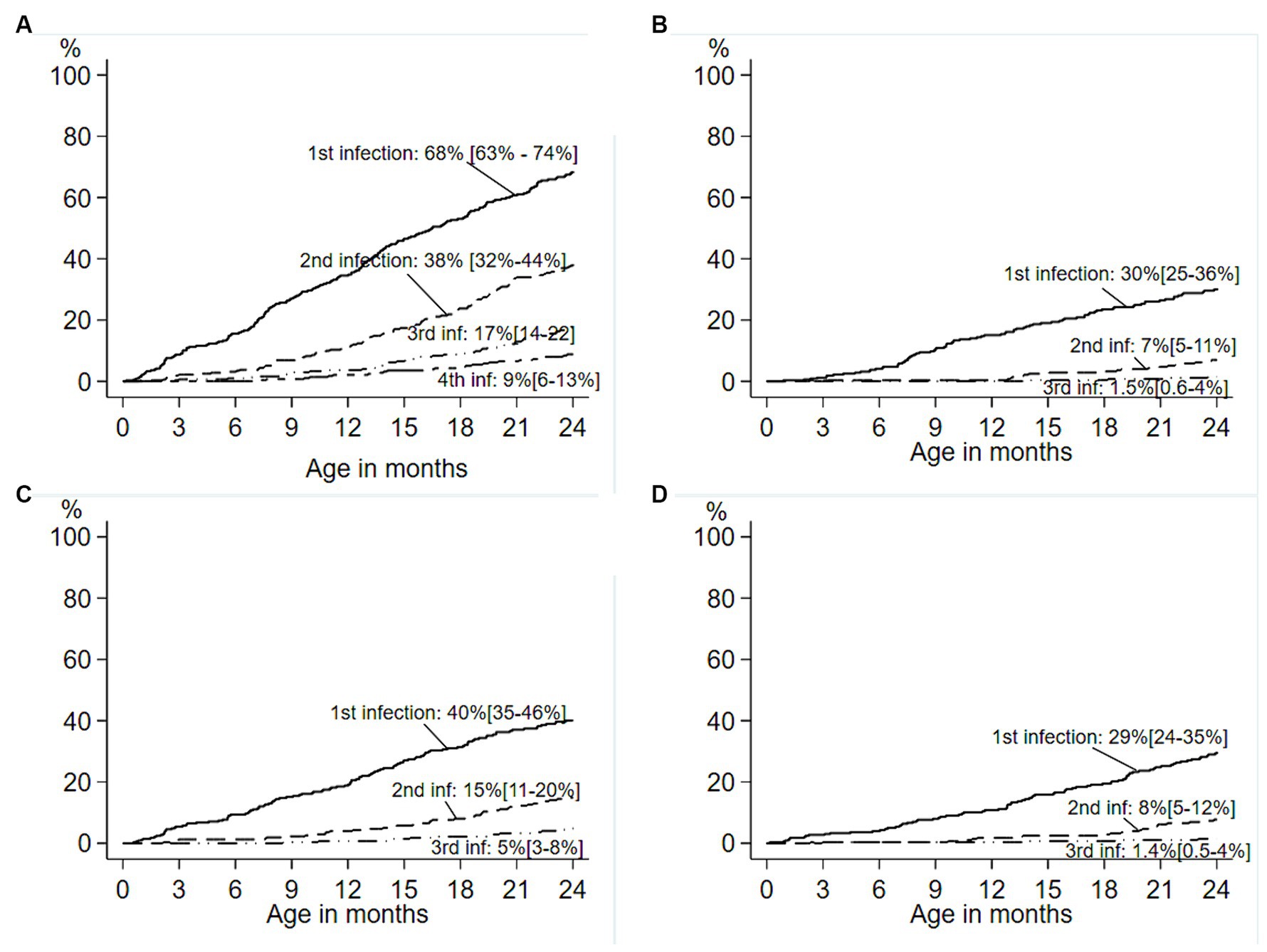
Figure 2. Cumulative incidence of first and subsequent ETEC diarrhea in the birth cohort. Cumulative incidence of first and subsequent ETEC diarrhea episodes in the birth cohort of 345 children. (A) Survival curves showing cumulative incidence of the first through fourth ETEC diarrhea episodes during the first two years of life. (B–D) Toxin-specific survival curves showing the cumulative incidence of the first through third episodes of ETEC diarrhea in the children 0–2 years age. (B) LT-ETEC; (C) ST-ETEC; (D) LT + ST-ETEC. Percentage is showing the cumulative incidence, and 95% confidence intervals are based on Kaplan–Meier survival analysis. (—— first infection; − - - - second infection; — • • — third infection; — - - — fourth infection).
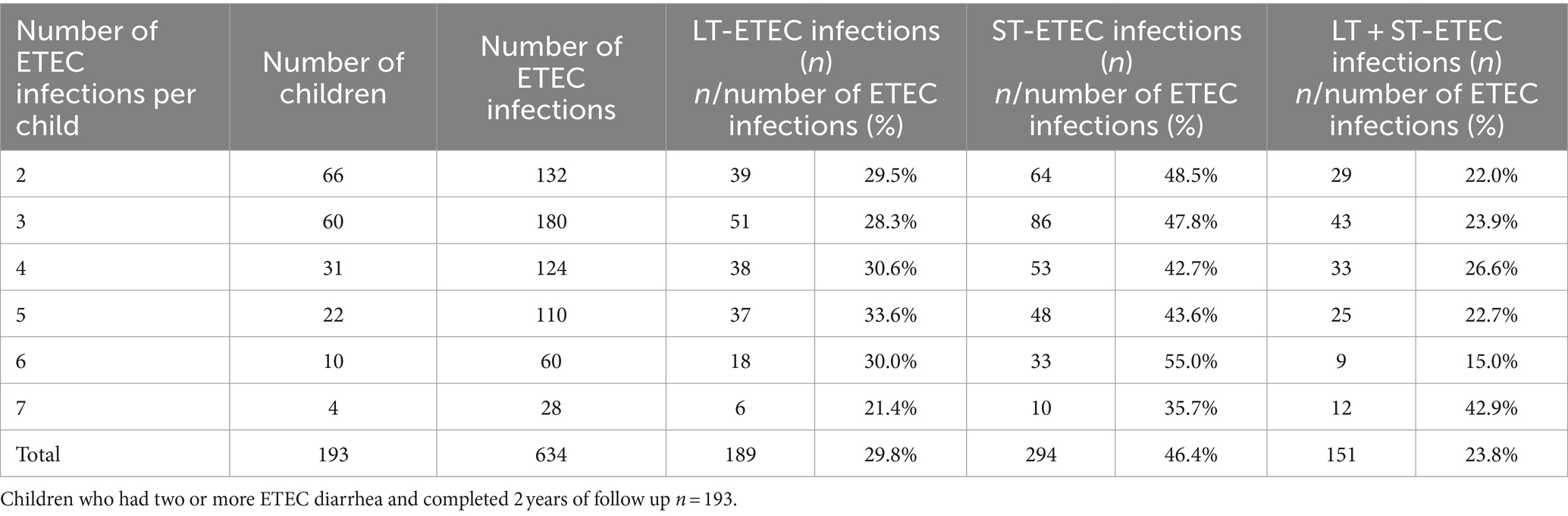
Table 3. Frequency of repeated ETEC diarrhea episodes by the toxin types in children in the 2 years of follow up.
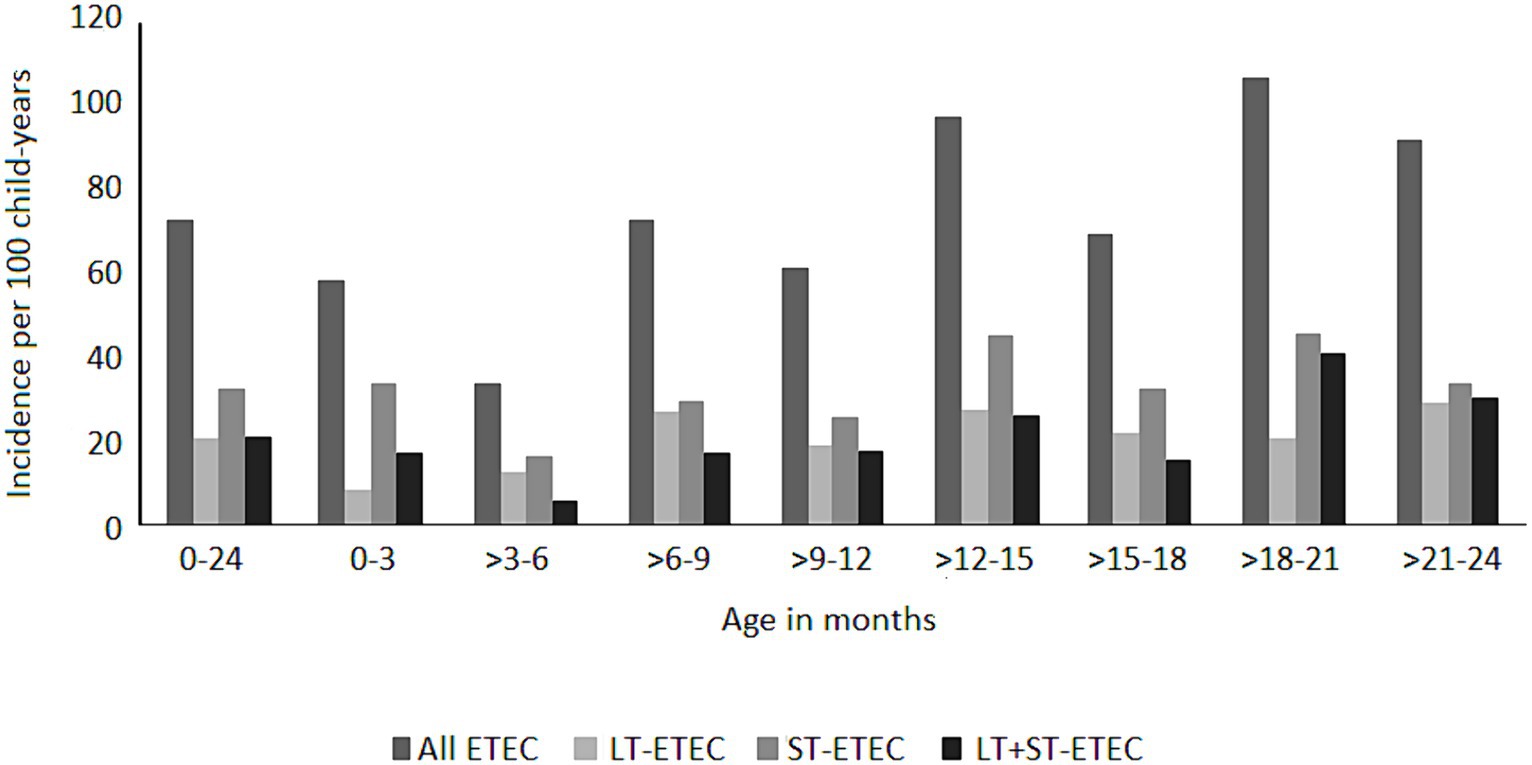
Figure 3. Incidence of ETEC diarrhea episodes by age stratum. Incidence of ETEC diarrhea per 100 child-years by the type of toxins at different age groups at three months intervals. “0–24” shows the two years follow up period.
The ETEC attributable diarrheal burden (PAF) over 2 years was 5.2% (2–8.3) (Figure 4, see Supplementary Table S3), which widely varied by age. Notably, the ETEC PAF was 10% (8.9–11.1) in the neonates (0–3 months), and most of the infections (58%) were attributed to ST-ETEC [PAF 7.9% (1.9–13.5)] and LT + ST-ETEC (29%) of which all the episodes were associated with diarrhea. Of the ST-ETEC diarrhea, 77% was contributed by the STh and STh + STp-ETEC. ETEC was increasingly associated with asymptomatic infections in the subsequent age strata in the first year of life. At 3–6 months, the prevalence of ETEC infections was the lowest of all age stratum and was primarily contributed by ST-ETEC (48%) and LT-ETEC (36%). The LT-ETEC PAF [2.8% (1.9–3.7)] was significant only in the 3–6 months of age. The prevalence of the ETEC toxin types was equally distributed in 6–12 months of age. The ETEC PAF significantly increased at the beginning of the second year of life, with the highest being 17% (4.6–27.7) at the age of 21–24 months. In fact, at 18–24 months of age, 40% of the total diarrhea episodes was positive for ETEC, mostly attributed to LT + ST-ETEC [PAF 11.6% (4.8–12.3) at 18–21 months and 11.9% (1.1–11.9), at 21–24 months]. The overall STp-ETEC PAF was 1.4% (0.7–2.2) and was significant at the 6–9 months [2.7% (1.4–4.0)] and 12–15 months [4.1% (2.0–6.2)] age strata.
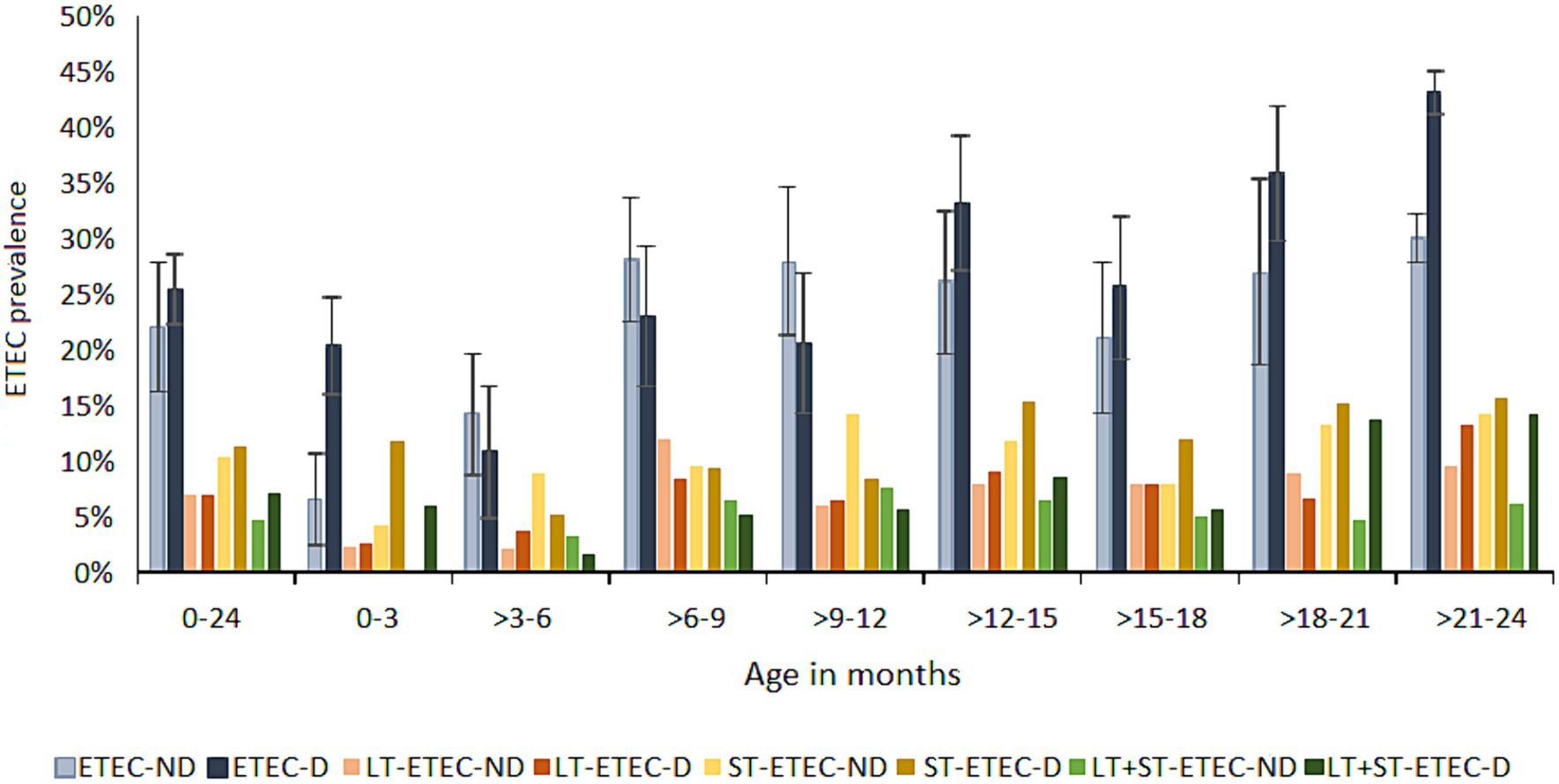
Figure 4. Prevalence of ETEC in diarrhea and asymptomatic routine surveillance samples. ETEC prevalence in diarrhea and non-diarrhea samples by toxin types at three months of age intervals. 0–24: the prevalence of ETEC in the total follow up period of 24 months in each child. Error bars showing 95% confidence intervals. ND: no diarrhea; D: diarrhea.
Among the first diarrhea episodes in children, 52 (17%) were positive for ETEC, with the youngest child being five days old. The mean age of the first ETEC diarrhea episode was 9.4 months (min 0.2, max 23.9). Of the 122 neonates who had their first diarrhea episode within three months of age, 21 (17.2%) were positive for ETEC, of which 13 (61.9%) were ST-ETEC (Table 4).
The most frequent co-pathogens among the ETEC diarrhea cases were Campylobacter spp. (25%), sapovirus (22%) and Shigella spp. (13.2%) and among the ETEC asymptomatic cases, Campylobacter spp. (13.7%) and norovirus GII (8.2%) (see Supplementary Table S4).
Duration of shedding of ETEC following diarrhea and asymptomatic ETEC cases
Ninety ETEC episodes [diarrhea (n = 70) and asymptomatic (n = 20)] were randomly selected, and weekly stool samples (total n = 189) were tested for ETEC following each of those episodes until two consecutive stools were negative for ETEC with the same toxin types. The mean shedding duration of ETEC was 10.6 days (SD 7.4, range 1 to 37 days), which was similar between diarrhea and asymptomatic cases (median 10.7 and 10.6 days; SD 8.5 and 7.1; p = 0.601). Among the diarrhea episodes, the shedding was significantly longer for LT + ST-ETEC episodes (mean 15.4 days, SD 7.3) compared to LT-ETEC (mean 10.1, SD 5.9, p = 0.026) and ST-ETEC (mean 8.3 days; SD 7.2; p < 0.001) (Figure 5).
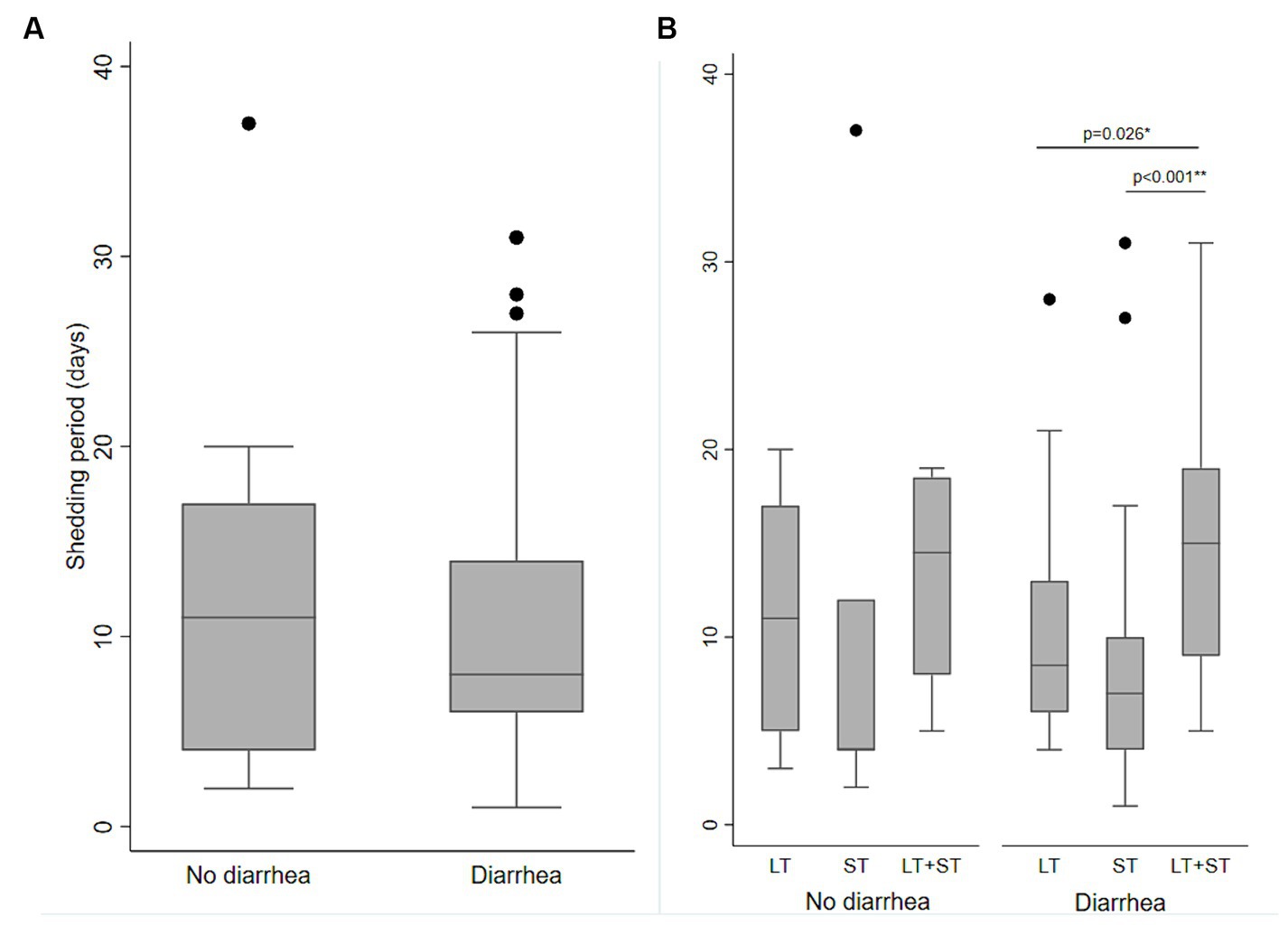
Figure 5. Shedding of ETEC following ETEC diarrhea and asymptomatic infections. (A) Length of shedding of ETEC following ETEC infections in the diarrhea and no diarrhea episodes. (B) Length of shedding of ETEC by the ETEC toxin types following ETEC infections in diarrhea and no diarrhea episodes. Significant differences are shown by Kruskal Wallis test and then Dunn test for pairwise comparison, (A) no diarrhea vs. diarrhea; (B) LT-ETEC vs. ST-ETEC and LT-ETEC vs. LT + ST-ETEC.
ETEC colonization factors and serogroups
CFs were detectable in 212 (69.7%) of the 304 ETEC diarrhea episodes tested. Overall, CFA/I (16.4%) was the most commonly occurring CF, followed by CS12 (13.2%), CS21 (10.5%), CS3 (7.2%) and CS6 (6.6%). Among 101 asymptomatic ETEC cases tested, CFs could not be detected in 30 (29.7%). The most commonly occurring CFs were CS12 (10.9%) followed by CS6 (5%) and CS21 (4%). The prevalence of CFs and their association with toxins by age and clinical outcome (diarrhea or non-diarrhea) are shown in Figure 6.
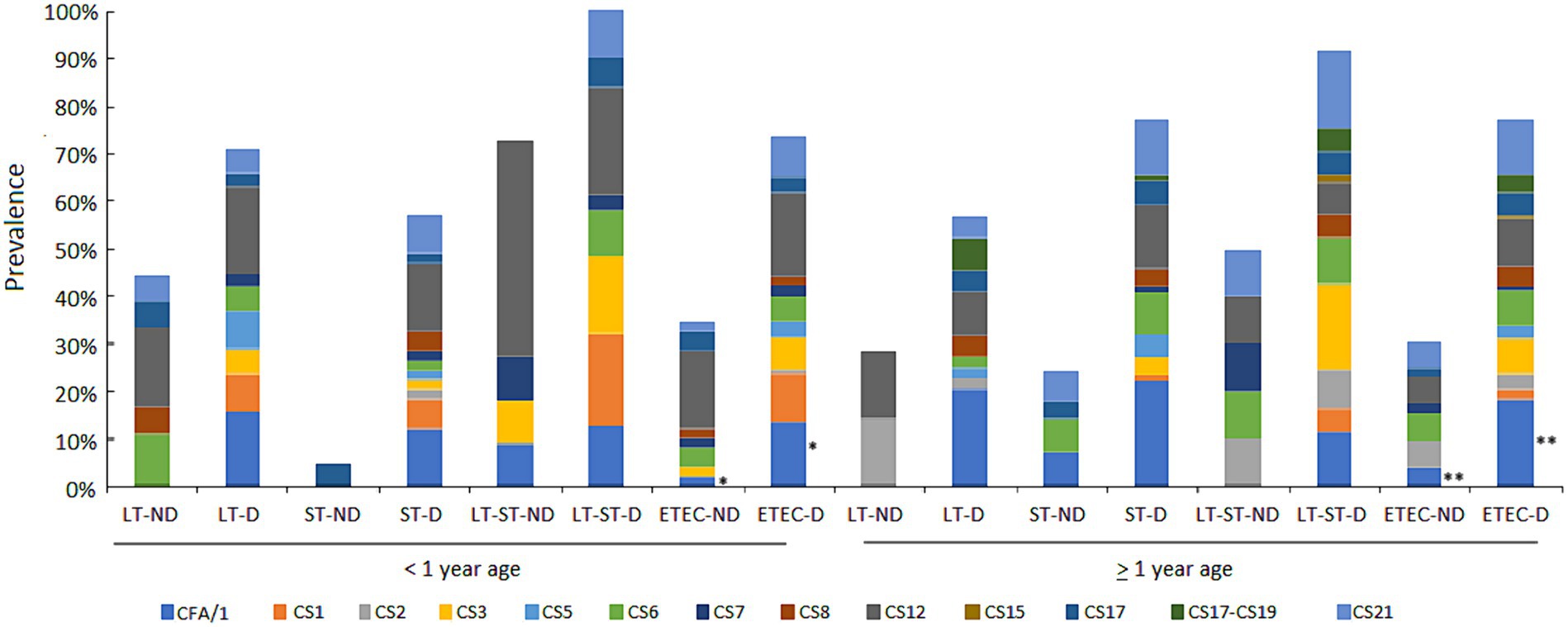
Figure 6. Proportion of ETEC CFs by toxin types in diarrhea and asymptomatic cases in the first and second year of life. The prevalence of CFs and associations with the ETEC toxin types by ETEC diarrhea and ETEC no diarrhea episodes among the children less than 1 year and above 1 year of age. Comparisons of CFs among diarrhea and asymptomatic cases was performed using GEE population averaged model to adjust for repeated measures. *A marginal difference was found in children less than 1 year p = 0.058 and ** a significant difference was found in children above 1 year of age p = 0.023 for CFA/I.
The ETEC infection was significantly associated with diarrhea when CFs were detected (OR:3.37, p < 0.001). Only CFA/I ± CS21 was significantly associated with ETEC diarrhea episodes [OR = 3.97 (1.76–9.12, p = 0.001)] and was more prevalent in children >12 months old [OR = 1.96 (1.14–3.35, p = 0.029)], all adjusted for intra-child correlation.
Among the 44 ETEC isolates tested, 20 different O serogroups were detected, of which O148 was detected most frequently (18.2%), followed by O164 and O158 (9.1% each), and O128 and O125 (6.8% each).
Repeat infections with ETEC
Although 193 children had serial ETEC infections, only 11 (5.7%) had repeat infections with the same combination of ETEC toxin(s) and CFs. We analyzed if a primary ETEC infection prevented reinfection with ETEC of a homologous toxin or CF type within three months of the first infection. Because of the small number, although no significant homologous protection was noted for any toxin and CFs, a longer intervals of CFA/I ETEC infections, regardless of toxins was noted when previous ETEC infection was with CFA/I. The median time between the repeat infections with CFA/I was 269 days, while that for CS21, CS12, CS3, and CS17 were 36, 29.5, 14 and 6 days. There was no repeat infection with CS6. There were more repeat infections of ST-ETEC, regardless of CFs in the same child [49.35%; (39.0–59.7)] compared to LT-ETEC [24.68%; (16.4–35.4)] or LT + ST-ETEC infections [25.97%; (17.6–36.5)].
The impact of ETEC on growth
Out of 332 children whose birth weight could be collected, 10 (3%) were born underweight (< 2.5 kg; range 1.7 to 2.49) and of 314 children who completed 3 m of follow up, 15 (4.7%) were stunted (z-score height for age < −2.0) at 3 months of age.
We evaluated the impact of ETEC diarrhea on the immediate and long-term growth of the children. Using a velocity model, for an average ETEC diarrhea episode of 5.3 days in the previous measurement interval (approximately one month), there was a reduction of 0.060 (0.007 to 0.114; p = 0.027) in the weight for length Z-score (WLZ) and 0.061 (0.015 to 0.108; p = 0.009) in the weight for age z-score (WAZ) in the following 30 day interval, compared to no ETEC infection (see Supplementary material for details). The total number of ETEC diarrhea episodes over two years of follow up was not significantly associated with WAZ (coefficient = 0.032 (−0.074 to 0.139, p = 0.547) or length for age z-score (LAZ) (coefficient = 0.045 (−0.053 to 0.144, p = 0.366) measured at the end of the follow-up, adjusted by sex, age at the end of follow up and birth weight (Supplementary Tables S5–S8).
Discussion
In this peri-urban shantytown, ETEC diarrhea began as early as five days after birth, and the burden was substantial, infecting about 70% of the children by the time they were two years old and having up to seven ETEC diarrhea episodes, impacting the growth of the children. Most ETEC diarrhea was moderate to severe, and a considerable proportion was associated with fever and vomiting.
The incidence rates and diarrheal episodes attributed to ETEC among the neonates were significantly high, and notably, most infections were attributed to ST-ETEC. ST is poorly immunogenic, and therefore neonates may have suboptimal protection against ST-ETEC infections through maternal immunity. The immune responses to the majority of the CFs from natural ETEC infections are generally low and may need repeated exposures to achieve optimum immunity (21, 34). To protect the vulnerable neonatal period, an ETEC vaccine that includes ST antigen and overexpressed CF antigens to achieve increased immunity, as in the ETVAX ETEC vaccine (11, 12), could be given at birth or as a maternal vaccine. The later immunization route could avoid the challenge of low immunity in infants from oral vaccines.
The decrease in total ETEC infections at 3–6 months and increase in asymptomatic ETEC infections in the first year of life, with equal distributions of the toxin types, likely reflect the protection from transferred maternal antibodies through breastfeeding as well as immunity from past infections in children. In the second year of life, following weaning to complementary foods, ETEC infections steadily increased, accounting for about half of the total diarrhea cases, mostly attributable to LT + ST-ETEC, and were more strongly associated with diarrhea than in the first year.
Overall, ST-ETEC was the most frequent in this cohort, followed by LT + ST-ETEC, which had the longest shedding. LT-ETEC, although highly prevalent in all age groups, was associated on average equally with diarrhea and asymptomatic infections, which could be a result of the higher immunogenicity of LT (35) and the protection from prior LT-ETEC infections, which resulted in fewer repeat infections with LT-ETEC in the same child. As reported before, STp-ETEC was detected at higher frequencies than in Asian and some African countries (36, 37). Notably, STp-ETEC contributed 15% of the ETEC diarrhea episodes and was significantly attributed to diarrhea in at least two age strata, which signifies the importance of this ETEC pathotype in causing diarrhea. Consistent with prior studies from Latin American countries (38, 39), minor CF, CS21, was frequently detected in the ETEC diarrhea cases. CS21 pilus had shown to contribute to adhesion to intestinal cells and to pathogenesis under in vivo conditions (40). Another minor CF, CS12, was detected in both diarrheal and asymptomatic cases. Notably, CS21 and CS12 are not included in the advanced vaccine candidates (10, 11). Although repeated ETEC infections were frequent in children, repeat infections by the same toxin (other than ST) and CF types of ETEC were rare.
Our study population appears to have had less acute malnutrition at birth than those in South Asia (37, 41). However, ETEC diarrhea had a significant negative impact on the short-term growth of the children.
This study has several strengths. This is the first birth cohort study of ETEC in Lima, which is geographically distinct from the Peruvian Amazon, one of the sites in the MAL-ED study (42). We conducted frequent home visits to ensure all episodes of diarrhea were captured, and we tested samples frequently for asymptomatic infections. Study limitations should be acknowledged. We included stringent requirements to define the end of infection episodes and their association with diarrhea; our incidence estimates are therefore conservative. We tested 14 CFs which although includes all the major CFs except CS14 (43), we may have missed some minor CFs circulating in this area. Limited testing for other enteric pathogens in this study means that some study children with ETEC likely had undetected co-pathogens contributing to the synergistic effect on pathogenicity, although the impact of individual co-pathogen in a diarrhea episode is difficult to determine and depends on multiple factors. In addition, as reported before, the incidence of mixed infections seems to increase with age and fewer co-pathogens were seen in infants than in older children and adults with ETEC diarrhea (1).
Conclusion
This study highlights the significance of ETEC for Peruvian children in Lima and underscores the importance of the development and implementation of ETEC vaccine. The high neonatal ETEC burden noted in our study suggests that an ETEC vaccine should be given at birth or as a maternal vaccine. To achieve considerable protection from ETEC in this area, the current vaccine candidates need to include CS21 and CS12, along with ST. The serial ETEC infections by multiple genotypes in a child suggest genotype-specific immunity from each infection, which should be considered when developing vaccines. The data from this study will strengthen modeled disease burden estimates and facilitate the design of improved vaccines to prevent ETEC diarrhea and infections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional review boards of Asociación Benéfica PRISMA, Universidad Peruana Cayetano Heredia, and Johns Hopkins University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MP: Writing – original draft, Writing – review & editing. SN: Writing – review & editing. LC: Writing – review & editing. AT: Writing – review & editing. MV: Writing – review & editing. LA: Writing – review & editing. MO: Writing – review & editing. LM: Writing – review & editing. MS: Writing – review & editing. RG: Writing – review & editing. SC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number R01AI108695 (SC and RG).
Acknowledgments
We thank the families who participated in this study. We also thank the project field and lab staff for their hard work and dedication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer DS declared a shared affiliation with the author MS to the handling editor at the time of review.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIAID.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1332319/full#supplementary-material
References
1. Qadri, F, Svennerholm, AM, Faruque, AS, and Sack, RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. (2005) 18:465–83. doi: 10.1128/CMR.18.3.465-483.2005
2. Anderson, JD, Bagamian, KH, Muhib, F, Amaya, MP, Laytner, LA, Wierzba, T, et al. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. (2019) 7:e321–30. doi: 10.1016/S2214-109X(18)30483-2
3. Kotloff, KL, Nataro, JP, Blackwelder, WC, Nasrin, D, Farag, TH, Panchalingam, S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet. (2013) 382:209–22. doi: 10.1016/S0140-6736(13)60844-2
4. Bourgeois, AL, Wierzba, TF, and Walker, RI. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. (2016) 34:2880–6. doi: 10.1016/j.vaccine.2016.02.076
5. Khalil, I, Troeger, CE, Blacker, BF, and Reiner, RC Jr. Capturing the true burden of Shigella and ETEC: the way forward. Vaccine. (2019) 37:4784–6. doi: 10.1016/j.vaccine.2019.01.031
6. Khalil, I, Walker, R, Porter, CK, Muhib, F, Chilengi, R, Cravioto, A, et al. Enterotoxigenic Escherichia coli (ETEC) vaccines: priority activities to enable product development, licensure, and global access. Vaccine. (2021) 39:4266–77. doi: 10.1016/j.vaccine.2021.04.018
7. Connor, S, Velagic, M, Zhang, X, Johura, FT, Chowdhury, G, Mukhopadhyay, AK, et al. Evaluation of a simple, rapid and field-adapted diagnostic assay for enterotoxigenic E. Coli and Shigella. PLoS Negl Trop Dis. (2022) 16:e0010192. doi: 10.1371/journal.pntd.0010192
8. Hosangadi, D, Smith, PG, and Giersing, BK. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine. (2019) 37:7372–80. doi: 10.1016/j.vaccine.2017.09.083
9. Darsley, MJ, Chakraborty, S, DeNearing, B, Sack, DA, Feller, A, Buchwaldt, C, et al. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol. (2012) 19:1921–31. doi: 10.1128/CVI.00364-12
10. Harro, C, Louis Bourgeois, A, Sack, D, Walker, R, DeNearing, B, Brubaker, J, et al. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine. (2019) 37:1978–86. doi: 10.1016/j.vaccine.2019.02.025
11. Akhtar, M, Chowdhury, MI, Bhuiyan, TR, Kaim, J, Ahmed, T, Rafique, TA, et al. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine. (2019) 37:5645–56. doi: 10.1016/j.vaccine.2018.11.040
12. Qadri, F, Akhtar, M, Bhuiyan, TR, Chowdhury, MI, Ahmed, T, Rafique, TA, et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. (2020) 20:208–19. doi: 10.1016/S1473-3099(19)30571-7
13. Zhang, W, and Sack, DA. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines. (2012) 11:677–94. doi: 10.1586/erv.12.37
14. Seo, H, Lu, T, Nandre, RM, Duan, Q, and Zhang, W. Immunogenicity characterization of genetically fused or chemically conjugated heat-stable toxin toxoids of enterotoxigenic Escherichia coli in mice and pigs. FEMS Microbiol Lett. (2019) 366. doi: 10.1093/femsle/fnz037
15. Seo, H, Garcia, C, Ruan, X, Duan, Q, Sack, DA, and Zhang, W. Preclinical characterization of immunogenicity and efficacy against diarrhea from MecVax, a multivalent enterotoxigenic E. coli vaccine candidate. Infect Immun. (2021) 89:e0010621. doi: 10.1128/IAI.00106-21
16. Govasli, ML, Diaz, Y, Zegeye, ED, Darbakk, C, Taxt, AM, and Puntervoll, P. Purification and characterization of native and vaccine candidate mutant enterotoxigenic Escherichia coli heat-stable toxins. Toxins (Basel). (2018) 10:274. doi: 10.3390/toxins10070274
17. Steinsland, H, Valentiner-Branth, P, Perch, M, Dias, F, Fischer, TK, Aaby, P, et al. Enterotoxigenic Escherichia coli infections and diarrhea in a cohort of young children in Guinea-Bissau. J Infect Dis. (2002) 186:1740–7. doi: 10.1086/345817
18. Bölin, I, Wiklund, G, Qadri, F, Torres, O, Bourgeois, AL, Savarino, S, et al. Enterotoxigenic Escherichia coli with STh and STp genotypes is associated with diarrhea both in children in areas of endemicity and in travelers. J Clin Microbiol. (2006) 44:3872–7. doi: 10.1128/JCM.00790-06
19. Isidean, SD, Riddle, MS, Savarino, SJ, and Porter, CK. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine. (2011) 29:6167–78. doi: 10.1016/j.vaccine.2011.06.084
20. Vidal, RM, Muhsen, K, Tennant, SM, Svennerholm, AM, Sow, SO, Sur, D, et al. Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the global enteric multicenter study (GEMS). PLoS Negl Trop Dis. (2019) 13:e0007037. doi: 10.1371/journal.pntd.0007037
21. Chakraborty, S, Harro, C, DeNearing, B, Ram, M, Feller, A, Cage, A, et al. Characterization of mucosal immune responses to enterotoxigenic Escherichia coli vaccine antigens in a human challenge model: response profiles after primary infection and homologous rechallenge with strain H10407. Clin Vaccine Immunol. (2015) 23:55–64. doi: 10.1128/CVI.00617-15
22. Gazi, MA, Alam, MA, Fahim, SM, Wahid, BZ, Khan, SS, Islam, MO, et al. Infection with Escherichia Coli pathotypes is associated with biomarkers of gut enteropathy and nutritional status among malnourished children in Bangladesh. Front Cell Infect Microbiol. (2022) 12:901324. doi: 10.3389/fcimb.2022.901324
23. Kosek, MNMAL-ED Network Investigators. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED Birth Cohort Study. EBioMedicine. (2017) 18:109–17. doi: 10.1016/j.ebiom.2017.02.024
24. Lee, G, Peñataro Yori, P, Paredes Olortegui, M, Caulfield, LE, Sack, DA, Fischer-Walker, C, et al. An instrument for the assessment of diarrhoeal severity based on a longitudinal community-based study. BMJ Open. (2014) 4:e004816. doi: 10.1136/bmjopen-2014-004816
25. Lothigius, A, Janzon, A, Begum, Y, Sjöling, Å, Qadri, F, Svennerholm, AM, et al. Enterotoxigenic Escherichia coli is detectable in water samples from an endemic area by real-time PCR. J Appl Microbiol. (2008) 104:1128–36. doi: 10.1111/j.1365-2672.2007.03628.x
26. Rodas, C, Iniguez, V, Qadri, F, Wiklund, G, Svennerholm, AM, and Sjöling, A. Development of multiplex PCR assays for detection of enterotoxigenic Escherichia coli colonization factors and toxins. J Clin Microbiol. (2009) 47:1218–20. doi: 10.1128/JCM.00316-09
27. Chakraborty, S, Deokule, JS, Garg, P, Bhattacharya, SK, Nandy, RK, Nair, GB, et al. Concomitant infection of enterotoxigenic Escherichia coli in an outbreak of cholera caused by Vibrio cholerae O1 and O139 in Ahmedabad, India. J Clin Microbiol. (2001) 39:3241–6. doi: 10.1128/JCM.39.9.3241-3246.2001
28. Saito, M, Goel-Apaza, S, Espetia, S, Velasquez, D, Cabrera, L, Loli, S, et al. Multiple norovirus infections in a birth cohort in a Peruvian Periurban community. Clin Infect Dis. (2014) 58:483–91. doi: 10.1093/cid/cit763
29. Freeman, MM, Kerin, T, Hull, J, McCaustland, K, and Gentsch, J. Enhancement of detection and quantification of rotavirus in stool using a modified real-time RT-PCR assay. J Med Virol. (2008) 80:1489–96. doi: 10.1002/jmv.21228
30. Sánchez, GJ, Mayta, H, Pajuelo, MJ, Neira, K, Xiaofang, L, Cabrera, L, et al. Epidemiology of Sapovirus infections in a birth cohort in Peru. Clin Infect Dis. (2018) 66:1858–63. doi: 10.1093/cid/cix1103
31. Chhabra, P, Payne, DC, Szilagyi, PG, Edwards, KM, Staat, MA, Shirley, SH, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008-2009. J Infect Dis. (2013) 208:790–800. doi: 10.1093/infdis/jit254
32. Lappan, R, Henry, R, Chown, SL, Luby, SP, Higginson, EE, Bata, L, et al. Monitoring of diverse enteric pathogens across environmental and host reservoirs with TaqMan array cards and standard qPCR: a methodological comparison study. Lancet Planet Health. (2021) 5:e297–308. doi: 10.1016/S2542-5196(21)00051-6
33. Blackwelder, WC, Biswas, K, Wu, Y, Kotloff, KL, Farag, TH, Nasrin, D, et al. Statistical methods in the global enteric multicenter study (GEMS). Clin Infect Dis. (2012) 55 Suppl 4:S246–53. doi: 10.1093/cid/cis788
34. Tobias, J, and Svennerholm, AM. Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole-cell inactivated ETEC vaccine candidates. Appl Microbiol Biotechnol. (2012) 93:2291–300. doi: 10.1007/s00253-012-3930-6
35. Norton, EB, Branco, LM, and Clements, JD. Evaluating the A-subunit of the heat-labile toxin (LT) as an immunogen and a protective antigen against enterotoxigenic Escherichia coli (ETEC). PLoS One. (2015) 10:e0136302. doi: 10.1371/journal.pone.0136302
36. Qadri, F, Saha, A, Ahmed, T, Al Tarique, A, Begum, YA, and Svennerholm, AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun. (2007) 75:3961–8. doi: 10.1128/IAI.00459-07
37. Mansour, A, Shaheen, HI, Amine, M, Hassan, K, Sanders, JW, Riddle, MS, et al. Pathogenicity and phenotypic characterization of enterotoxigenic Escherichia coli isolates from a birth cohort of children in rural Egypt. J Clin Microbiol. (2014) 52:587–91. doi: 10.1128/JCM.01639-13
38. Guerra, JA, Romero-Herazo, YC, Arzuza, O, and Gómez-Duarte, OG. Phenotypic and genotypic characterization of enterotoxigenic Escherichia coli clinical isolates from northern Colombia, South America. Biomed Res Int. (2014) 2014:236260:1–11. doi: 10.1155/2014/236260
39. Rasko, DA, Del Canto, F, Luo, Q, Fleckenstein, JM, Vidal, R, and Hazen, TH. Comparative genomic analysis and molecular examination of the diversity of enterotoxigenic Escherichia coli isolates from Chile. PLoS Negl Trop Dis. (2019) 13:e0007828. doi: 10.1371/journal.pntd.0007828
40. Guevara, CP, Luiz, WB, Sierra, A, Cruz, C, Qadri, F, Kaushik, RS, et al. Enterotoxigenic Escherichia coli CS21 pilus contributes to adhesion to intestinal cells and to pathogenesis under in vivo conditions. Microbiology (Reading). (2013) 159:1725–35. doi: 10.1099/mic.0.065532-0
41. Black, RE, Brown, KH, and Becker, S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. (1984) 73:799–805. doi: 10.1542/peds.73.6.799
42. Platts-Mills, JA, Babji, S, Bodhidatta, L, Gratz, J, Haque, R, Havt, A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health. (2015) 3:e564–75. doi: 10.1016/S2214-109X(15)00151-5
Keywords: diarrhea, enterotoxigenic E. coli, disease burden, growth in children, Peru, birth cohort, epidemiology, colonization factors
Citation: Pajuelo MJ, Noazin S, Cabrera L, Toledo A, Velagic M, Arias L, Ochoa M, Moulton LH, Saito M, Gilman RH and Chakraborty S (2024) Epidemiology of enterotoxigenic Escherichia coli and impact on the growth of children in the first two years of life in Lima, Peru. Front. Public Health. 12:1332319. doi: 10.3389/fpubh.2024.1332319
Edited by:
Adrian Canizalez-Roman, Autonomous University of Sinaloa, MexicoReviewed by:
Sergio George, University of Chile, ChileDaisuke Sano, Tohoku University, Japan
Gabriela Tapia-Pastrana, Hospital Regional de Alta Especialidad de Oaxaca, Mexico
Hector Manuel Flores-Villaseñor, Autonomous University of Sinaloa, Mexico
Copyright © 2024 Pajuelo, Noazin, Cabrera, Toledo, Velagic, Arias, Ochoa, Moulton, Saito, Gilman and Chakraborty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subhra Chakraborty, c2NoYWtyMTFAamh1LmVkdQ==
 Monica J. Pajuelo
Monica J. Pajuelo Sassan Noazin2
Sassan Noazin2 Angie Toledo
Angie Toledo Subhra Chakraborty
Subhra Chakraborty