- 1Gastroenterology and Hepatology Unit, San Giovanni Battista Hospital, Turin, Italy
- 2Real World Solutions, IQVIA Solutions Italy S.R.L., Milan, Italy
- 3Department of General Surgery and Transplantation, Niguarda Hospital, Milan, Italy
- 4Hepatobiliary Surgery and Liver Transplantation Unit, University of Pisa Medical School Hospital, Pisa, Italy
- 5Gastroenterology Department of Emergency and Organ Transplantation, University Hospital Policlinico di Bari, Bari, Italy
- 6Department of Medicine, University of Milan Bicocca and Gastroenterology Hepatology and Transplantation Unit, Papa Giovanni XXIII Hospital, Bergamo, Italy
Introduction: In Italy, post-liver transplant (LT) hepatitis B virus (HBV) reinfection prophylaxis is frequently based on a combined regimen of anti-HBV immunoglobulin (HBIG) and oral antivirals. However, little information is available at the national level on the cost of LT and the contribution of HBV prophylaxis. This study aimed to quantify the direct healthcare cost for adult patients undergoing LT for HBV-related disease over a lifetime horizon and from the perspective of a National Healthcare Service.
Methods: A pharmaco-economic model was implemented with a 4-tiered approach consisting of 1) preliminary literature research to define the research question; 2) pragmatic literature review to retrieve existing information and inform the model; 3) micro-simulated patient cycles; and 4) validation from a panel of national experts.
Results: The average lifetime healthcare cost of LT for HBV-related disease was €395,986. The greatest cost drivers were post-transplant end-stage renal failure (31.9% of the total), immunosuppression (20.6%), and acute transplant phase (15.8%). HBV reinfection prophylaxis with HBIG and antivirals accounted for 12.4% and 6.4% of the total cost, respectively; however, lifetime HBIG prophylaxis was only associated with a 6.6% increase (~€422 k). Various sensitivity analyses have shown that discount rates have the greatest impact on total costs.
Conclusion: This analysis showed that the burden of LT due to HBV is not only clinical but also economic.
Introduction
Hepatitis B virus (HBV) infection is the most common chronic viral disease worldwide, with an estimated prevalence of 4.1% in 2019 (1). According to the most recent estimates (1, 2), approximately 425,000 people were chronically infected in Italy in 2014. Over the last 30 years, the epidemiological and clinical scenarios of both the general population and liver transplant patients have radically changed in most Western countries owing to the introduction of vaccination and nucleos(t)ide analogs (NAs) in the former and the use of anti-hepatitis B immunoglobulin (HBIG) in the latter. Vaccination (1) has resulted in a significant reduction in the incidence of HBV infection [from approximately 10 cases/100,000 inhabitants in the 1990s to 1 case/100,000 in 2011 in Italy (3)], while HBIG and antiviral drugs have improved the outcome of both chronic HBV patients and liver transplant recipients, resulting in a 5-year survival rate above 80% versus 45% prior to HBIG introduction (4).
Despite these advancements, HBV infection remains a major public health burden (5) and a major risk factor for liver cirrhosis, hepatocellular carcinoma (3), and liver failure (6). Liver transplantation (LT) is the best therapeutic option for HBV-related end-stage liver disease but is associated with early and long-term complications, immunosuppression-related comorbidities, and high socio-economic costs (7).
Post-transplant HBV prophylaxis with a combination of HBIG and NA tailored to patient-, transplant-, and virus-related risk factors is crucial to favorable long-term results, but concerns about the cost of HBIG and the availability of high-barrier NA have gradually reduced the use, dose, and duration of immunoglobulin in the last few years (5). However, information on the overall economic burden of LT in general, and post-transplant HBV prophylaxis in particular, is still scarce in Europe (8). To fill this gap, we performed the present study to quantify the direct healthcare costs for patients undergoing LT for HBV infection over a lifetime horizon and from the perspective of the Italian National Healthcare System (NHS).
Materials and methods
To estimate the direct lifetime healthcare cost of LT for HBV-related liver disease, we conducted an analysis using a multi-step approach, in line with the methodology reported by the National Institute for Health and Care Excellence (NICE) (9). Initially, a targeted literature review was performed to conceptualize the research question and identify the key components needed to implement a lifetime model for HBV patients undergoing LT. A pragmatic literature review of the available evidence on LT in Europe was then performed to retrieve the necessary information to inform the model. Finally, the model was conceptualized and implemented using the statistical program Stata/MP, version 14.0. The model structure and inputs were validated by two Italian key opinion leaders (KOLs), while resource consumption and costs were calibrated with the support of three additional KOLs. The model was then adjusted and finalized following the discussions with the five clinical experts.
Problem conceptualization and pragmatic literature review
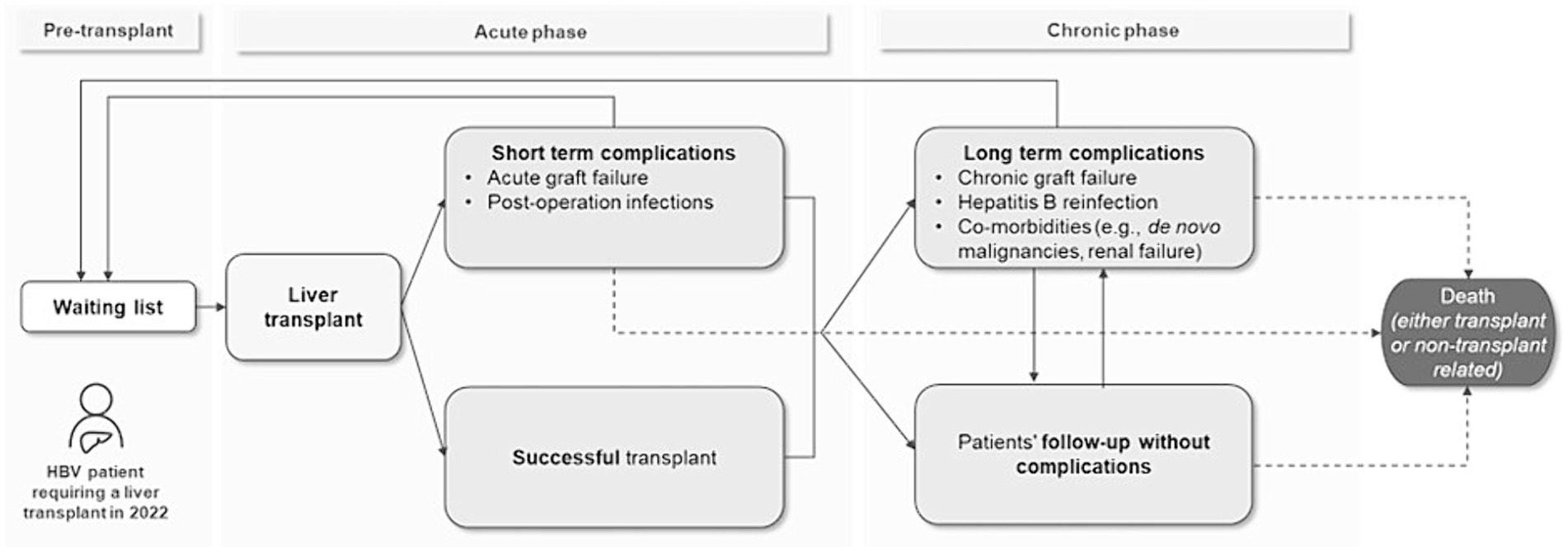
To assess model feasibility, the research question was focused on through preliminary literature research, and the patient flow was outlined based on published evidence (4, 10). The initial flow is divided into three phases: pre-transplant, post-transplant acute phase and post-transplant chronic phase (Figure 1). The patients begin the flow entering the waiting list for liver transplantation and after an average waiting time they undergo transplantation, successfully or with acute complications. Finally, they enter the post-transplant chronic phases, where they might have a post-LT follow-up without any event or incur in chronic complications. At any point, patients might undergo re-transplantation (re-LT) or die, due to transplant- or non-transplant-related causes. The initial flow’s structure was discussed and validated with two KOLs.
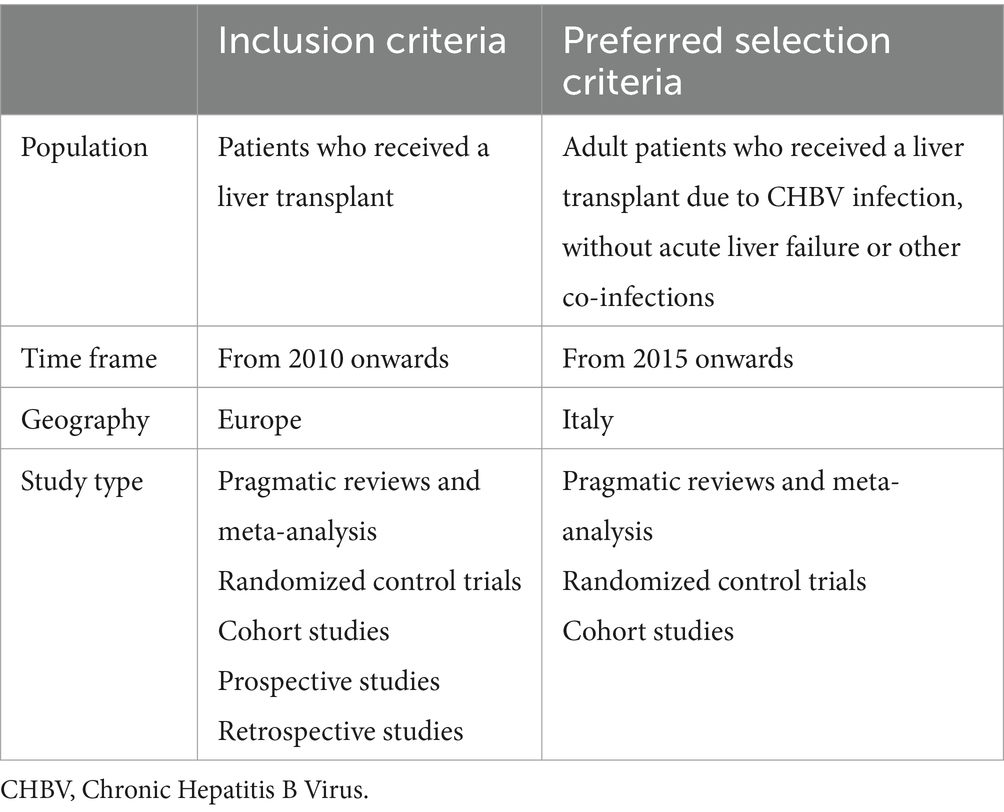
After conceptualizing the problem and validating the initial flow, a pragmatic review of the scientific literature was conducted according to the PRISMA guidelines to retrieve the available information and define the final model structure. Information retrieved from literature included probabilities of events’ occurrence, time to transplant, time to complications, time to death, and resource consumption for each phase. The search was conducted in January 2022 by querying the online databases Medline (Pubmed), Cochrane Library, and Embase with a combination of eight different research strings, and it was focused on papers published for the European context from January 1st, 2010, onwards (Table 1). Inclusion, selection criteria, and search terms were discussed and validated with two KOLs. Search terms referred to the patient flow phases identified during the problem conceptualization phase in terms of events, patient management, and cost analyses. Detailed information on the PRISMA flow diagram and the resulting sources is provided in the Supplementary material.
Model conceptualization
Based on the information retrieved from the pragmatic literature review, the final model structure was conceptualized (Figure 2). The patient enters the model in the waiting list and undergoes transplantation after an average waiting time, retrieved from published data reports (11–13). In the post-transplant period, patients are either followed up regularly with routine examinations and HBV prophylactic treatment, or they experience complications (postoperative infections, graft rejection, HBV reinfection) and comorbidities [de novo malignancies, renal failure, diabetes, major adverse cardiovascular events (MACEs)].1 At any time, patients may undergo re-transplantation (re-LT)2 or die due to either transplant- or non-transplant-related causes.
Due to the complexity of patient follow-up, a microsimulation approach was chosen to build the model, since it is considered more appropriate for complex chronic diseases where patient-level information is relevant (14). Microsimulations allow the replication of the healthcare trajectory of individual patients (15), which can be affected by more than one complication/comorbidity at a time, and to keep track of each patient’s individual history. Patients enter the model in the initial state and proceed individually through various transition states based on the probabilities of transition and are subject to events with a time-varying probability of occurrence.
The model simulated 10,000 adult patients who underwent LT for HBV-related disease and were followed up until death. It consisted of 12 one-month cycles in the early (i.e.,1 year) post-transplant period followed by 44 one-year cycles, for a maximum time horizon of 45 years [which corresponds to a projected 100-year life span assuming a mean age of 55 years at wait listing (11)]. Costs were discounted at the present value using an annual discount rate of 3%, according to the Italian guidelines for economic evaluations (16).
The model starts in the waiting list and assumes that all patients are transplanted, since the study aims to estimate the costs associated with LT from the pre-transplant through the post-transplant phase. After LT, patients may experience complications and comorbidities, according to the estimated probabilities of occurrence. The model assumes that complications are independent of each other and that patients may only undergo re-LT once during their lifetime. If a patient develops any long-term complication in cycle t, the complication will persist over time (i.e., from cycle t + 1 onwards), and the cost associated with the events is quantified and assigned to the patient.
At the end of each cycle, patients may die according to general LT population survival curves or comorbidities-specific curves, consistently with literature data (17). Finally, if, at any time point, the death probability was lower than that of the sex- and age-adjusted Italian population, the latter was applied.
The direct healthcare lifetime cost of an average patient undergoing LT for HBV-related disease was calculated by running 1,000 simulations of the model for 10,000 patients, to allow for the convergence of in-sample standard errors toward zero (see the Supplementary material for more details). Results were extracted from each simulation in terms of average total lifetime cost, average lifetime cost by cost component, average cost by transplantation phase, and probability of occurrence of each complication and comorbidity at different model cycles. The average total lifetime cost for one patient was then multiplied by the estimated eligible population in Italy of 233 patients (18, 19).3
Model input parameters
Incidence curves
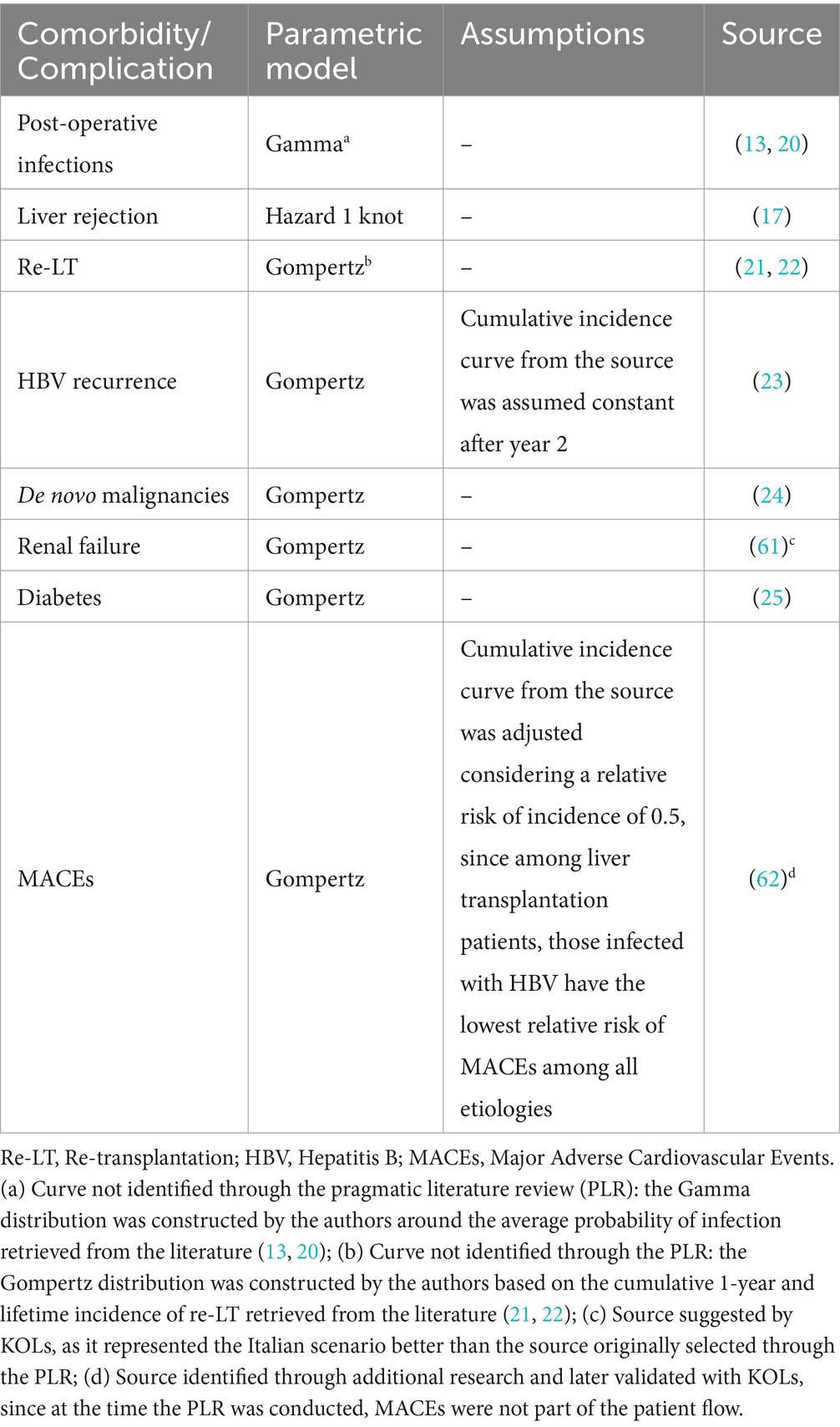
To assess probability of occurrence of comorbidities and complications in each cycle of the model, incidence curves related to model events were retrieved from the literature, except for postoperative infection and re-transplantation, which were modeled by the authors using different probability distributions based on literature data (13, 20–22). The curves retrieved from the literature were extrapolated beyond their original follow-up period to cover the entire patient’s life, using the fittest parametric model. The choice of the parametric model was made on a case-by-case basis on both visual inspection and statistical criteria (see Supplementary material for more details) and was later validated by two KOLs. In the final model, some of the original curves were adjusted to better mirror Italian clinical practice, according to inputs from the KOLs. Table 2 provides an overview of the sources and the models used for each incidence curve, as well as the assumptions made in terms of curve adjustment following KOLs’ validation.
Survival curves
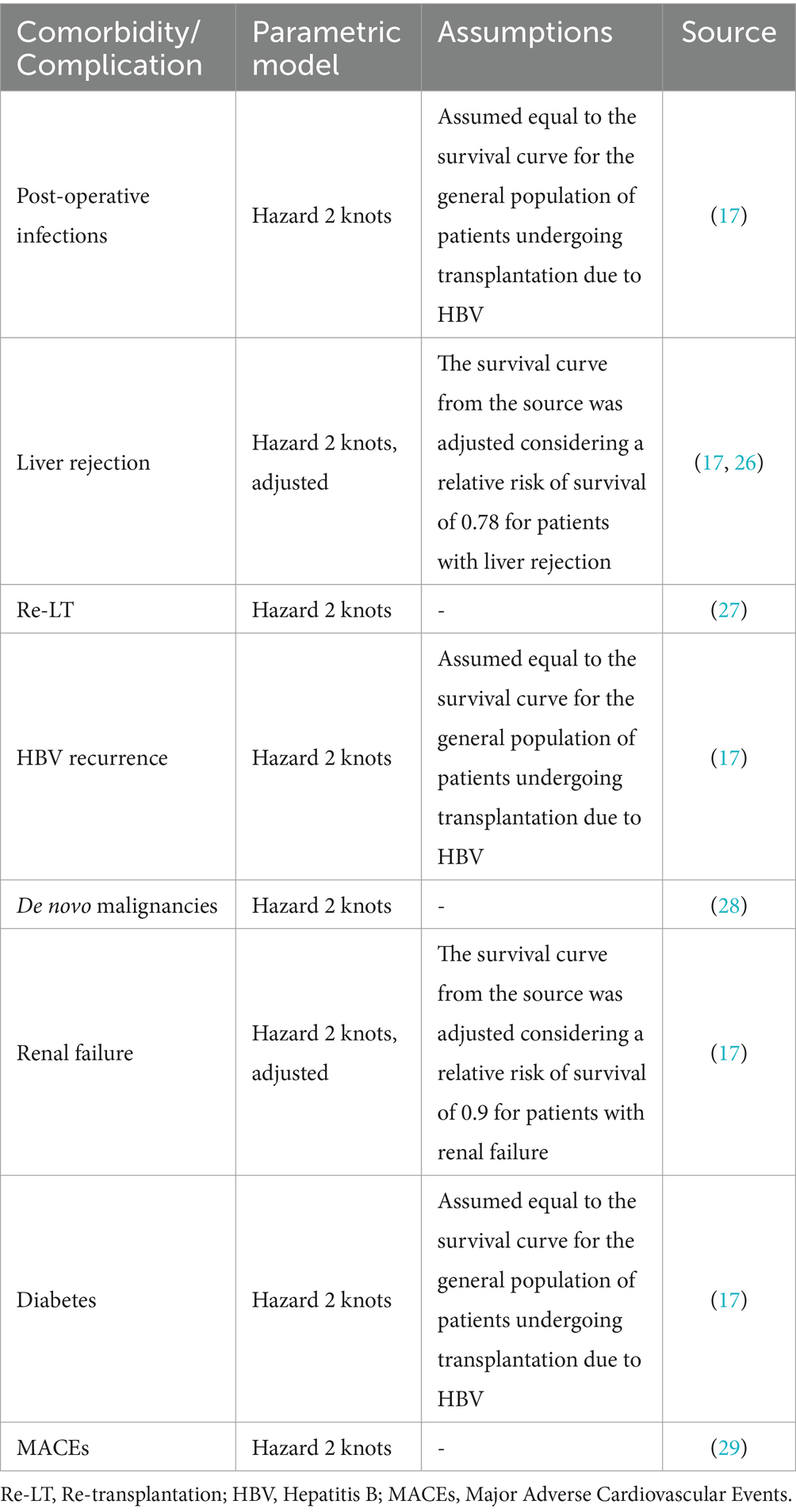
Mortality in each cycle was modeled by extrapolating complication- and comorbidity-specific survival curves from the literature. Similarly to incidence curves, the choice of the fittest parametric model for survival curve extrapolations was assessed through visual inspection and statistical criteria (see Supplementary material for more details) and was later validated and adjusted with two KOLs (Table 3). In particular, event-specific mortality for patients who developed post-operative infections, diabetes, and HBV recurrence was assumed equal to the general mortality for patients undergoing transplantation due to HBV retrieved from the literature (17), while mortality for patients who develop liver rejection and renal failure was computed by adjusting the same general mortality curve using relative risk factors. Patients who develop de novo tumors or MACEs and patients who are subject to re-transplantation follow their respective event-specific mortality. Finally, patients who persist in post-LT follow-up without any comorbidities or complications are subject to their own mortality curve. This curve was computed at each cycle by considering the weighted sum of all other curves applied in the same cycle, aiming to achieve a general mortality for patients who undergo LT due to HBV that aligns with the literature (17).
Resource consumption and costs
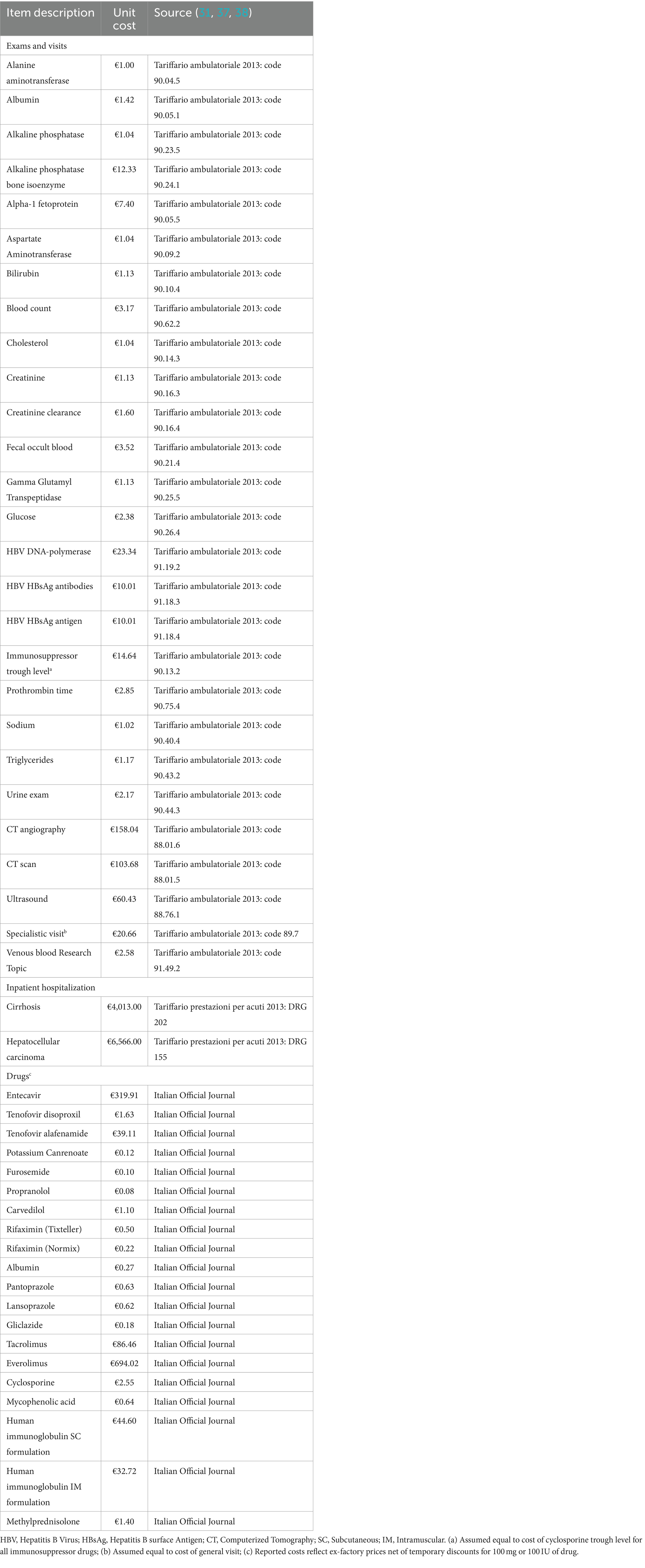
Unit costs were either per event or annual. The formers were assigned only once to the corresponding cycle of occurrence; the latter were assigned to the patient annually, from the date of complication/comorbidity until death, except for de novo malignancies and HBIG prophylaxis. Consistent with other modeling approaches (30), the annual costs of de novo malignancies started in the incident year throughout the five subsequent years.
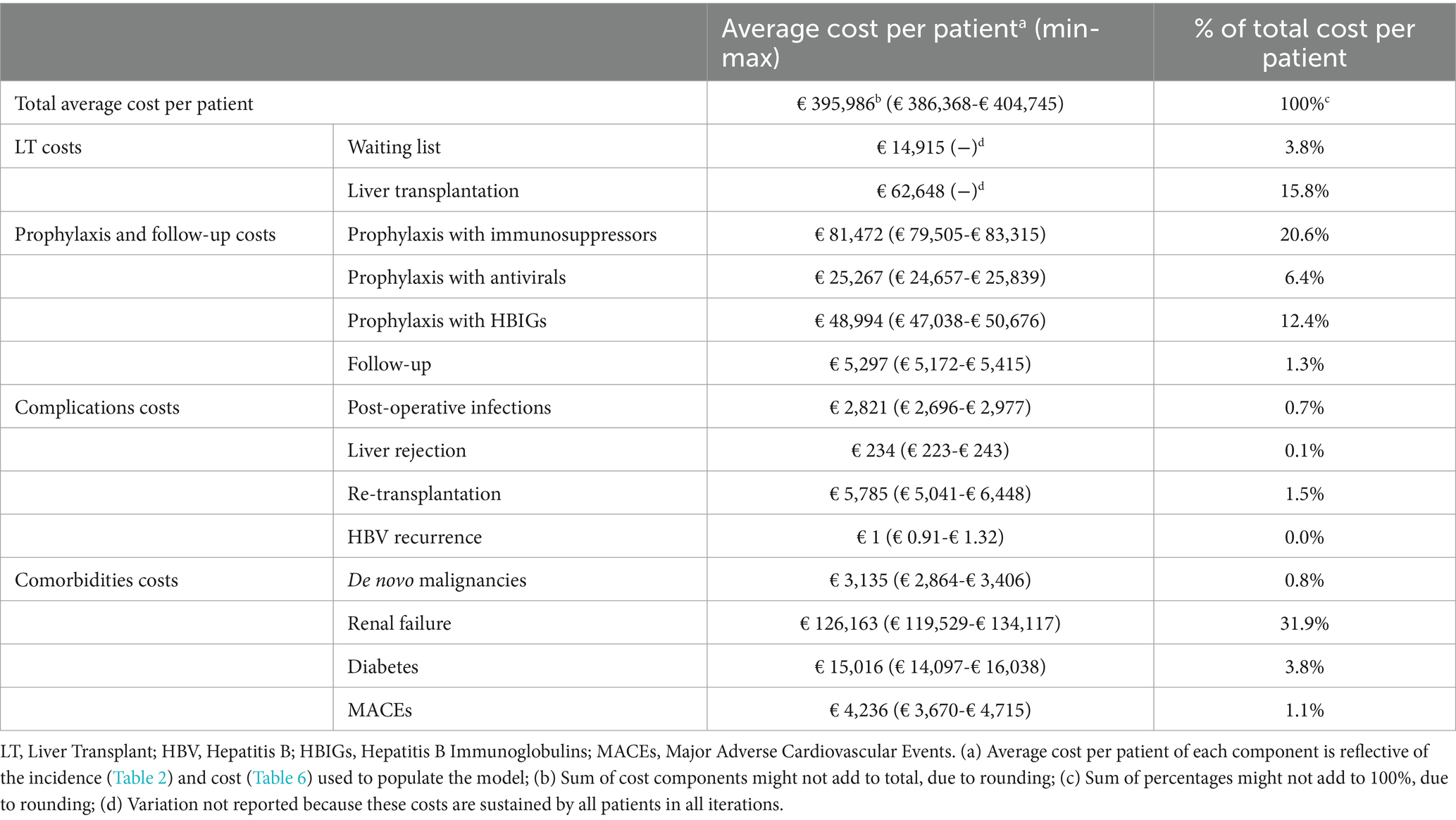
The unit cost of LT and postoperative infections was estimated based on 2022 national tariffs (31). The unit costs of the waiting list, complications, follow-up, and prophylactic treatment were calculated using a micro-costing approach, considering the relevant resource consumption of visits and exams, hospitalization episodes, and pharmacological treatment for each component. Resource consumption was retrieved from national guidelines (32), summary of product characteristics of relevant drugs (33), and published literature (34–36) and was later validated with the five KOLs. The unit costs of visits, exams, and hospitalizations were retrieved from national tariffs (31, 37), while the cost of drugs was calculated from the ex-factory prices net of mandatory discounts, as reported in the Italian Official Journal (38). Finally, the costs of comorbidities were retrieved from Italian literature (31, 39–55) and inflated to 2022.
Annual costs of HBIG were imputed considering their variable duration and were interrupted in the event of HBV recurrence (56). The duration of HBIG prophylaxis was modeled considering the consensus gathered from the Italian IMMUNOHBs expert meeting in 2020, during which 24 experts from the Italian liver transplantation community agreed on post-LT prophylaxis protocols based on the available evidence and clinical practice (36). The resource consumption and unit cost details are outlined in Tables 4, 5, respectively. Table 6 presents the cost values used to inform the model, expressed in euros (€) for the year 2022.
Deterministic sensitivity analyses
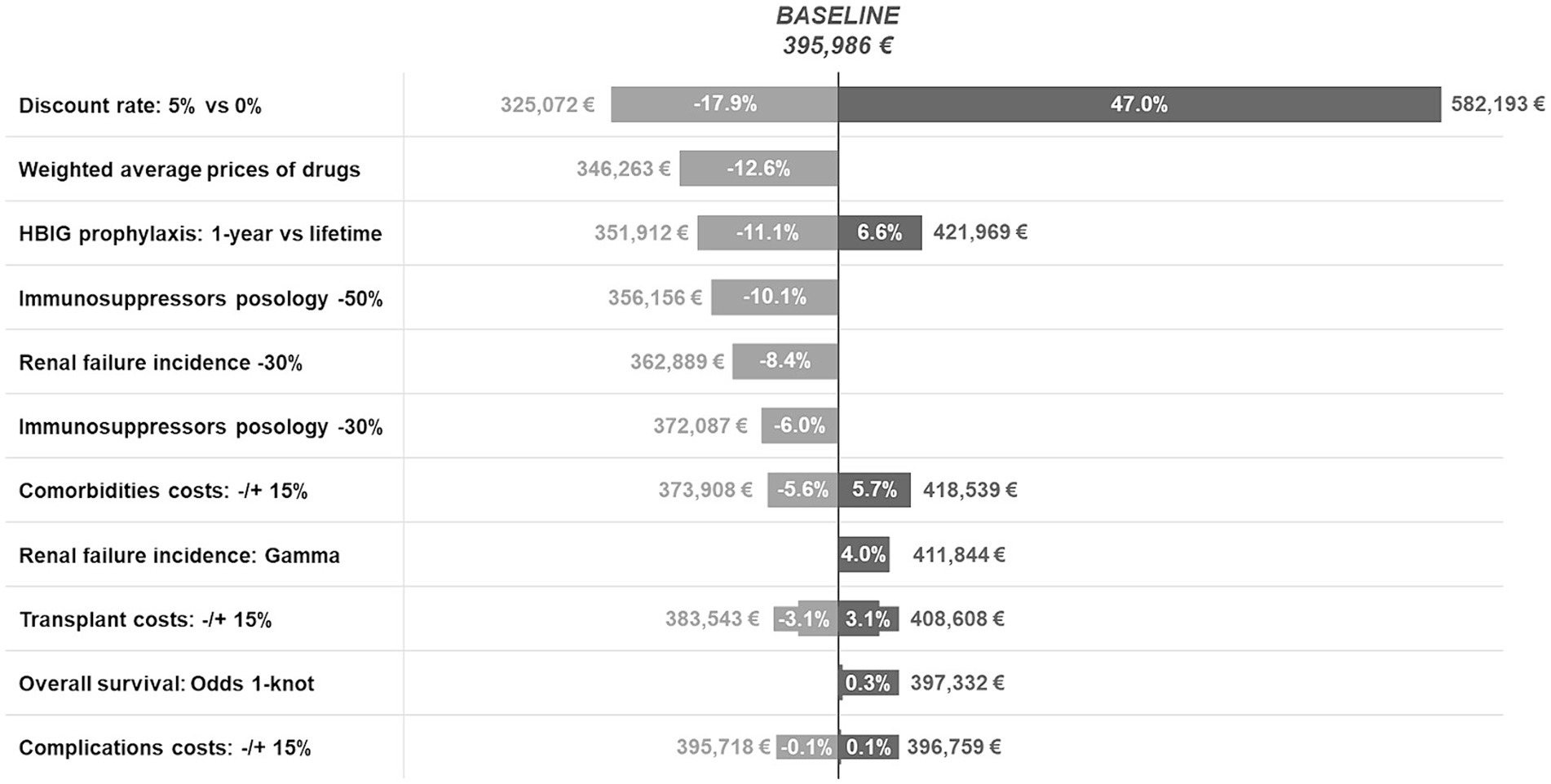
A set of deterministic sensitivity analyses was conducted based on the uncertainties of some of the model parameters. Regarding resource consumption, different HBIG and immunosuppressive regimens were considered. For HBIG treatment, based on previous discussions about the optimal duration of treatment in clinical practice (36, 56), two alternative scenarios were explored: 1-year prophylaxis versus lifetime administration in order to assess variation of costs resulting from the two extreme cases. These scenarios were modeled by assigning related costs for the respective durations, without varying the effect of prophylaxis on health outcomes since no published evidence relating prophylaxis duration and risk health outcomes was found (6). For immunosuppression, posology variations of −30% and −50% were explored owing to drug dose modifications in the early post-transplant period (35). Regarding complications/comorbidities, the incidence of end-stage renal failure derived from Ojo et al. (61) was decreased by 30% due to changes observed in clinical practice over the last two decades. Furthermore, to address possible uncertainties which might arise from the choice of the parametric models used to extrapolate incident and survival curves, two sensitivity analyses were conducted on the curve informing the cost items with the highest impact on the model results (i.e., overall survival and renal failure incidence). In particular, overall survival was modeled using the odds 1-knots spline model, while renal failure incidence was modeled after incidence using the gamma distribution. The first analysis allows to assess results in the best-case scenario, i.e., the scenario with the lowest mortality, while the second in the worst-case scenario, i.e., the scenario with the highest renal failure incidence.
Two sensitivity analyses were conducted to assess the impact of different unit costs. First, the weighted average price4 of all drugs included in the model was used instead of the ex-factory price net of mandatory discounts (38). Second, variations of ±15% were considered for cost components of LT (cost of waiting list and cost of surgery), complications (cost of postoperative infections, liver rejection, and HBV recurrence), and comorbidities (cost of de novo malignancies, renal failure, diabetes, and MACEs), to address concerns of possible underestimation or overestimation of cost components. Finally, two scenarios were explored by considering alternative discount rates (0% versus 5%) in line with the Italian guidelines for economic evaluations (16).
Results
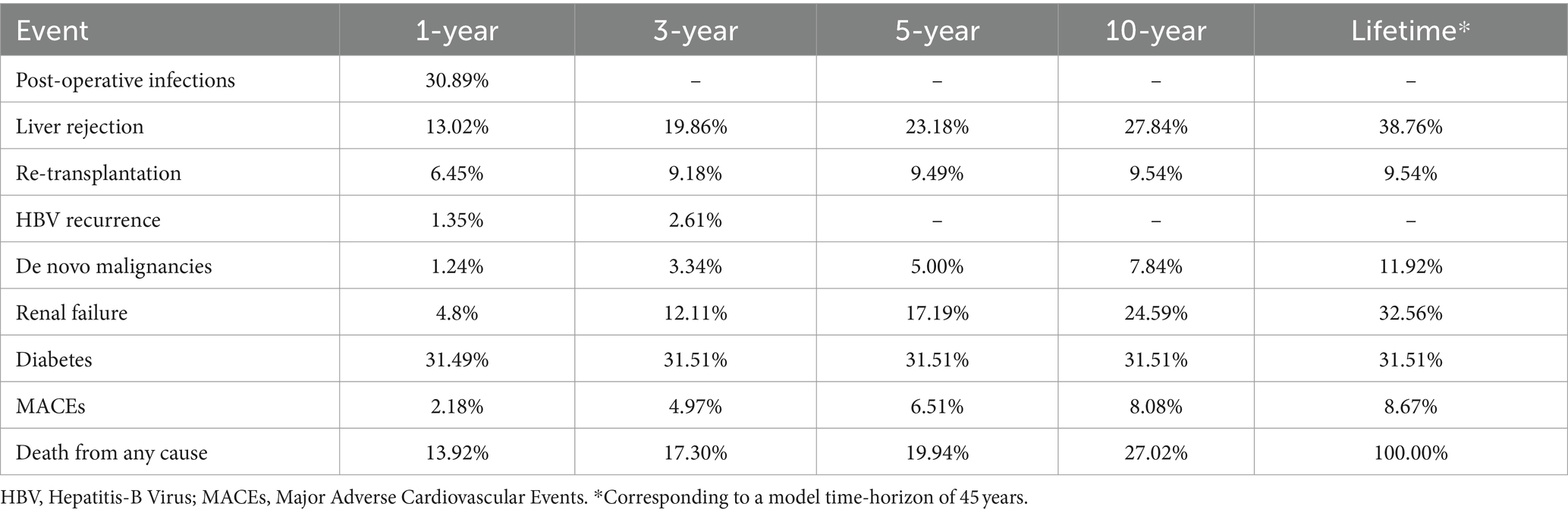
The main results of the analysis in terms of costs are shown in Table 7 and Figure 3. The average lifetime direct healthcare cost for an adult recipient undergoing LT for HBV-related disease in Italy was estimated to be €395,986, varying between € 386,368 and € 404,745 among the different iterations (Table 7). Considering the incidence estimates derived from the literature (Table 2) and the values of cost components used to populate the model (Table 6), the greatest cost driver was post-transplant end-stage renal failure, accounting for 31.9% (~€126 k) of the total cost, followed by the cost of immunosuppression (20.6%, ~€81 k) and cost of liver transplantation (15.8%, ~€63 k). HBV prophylaxis with HBIG and antivirals accounted for 12.4% (~€49 k) and 6.4% (~€25 k) of the total cost, respectively. Finally, the cost of the waiting list accounted for 3.8% (~€15 k) of the total cost, while follow-up, complications, and comorbidities other than renal failure were residual.
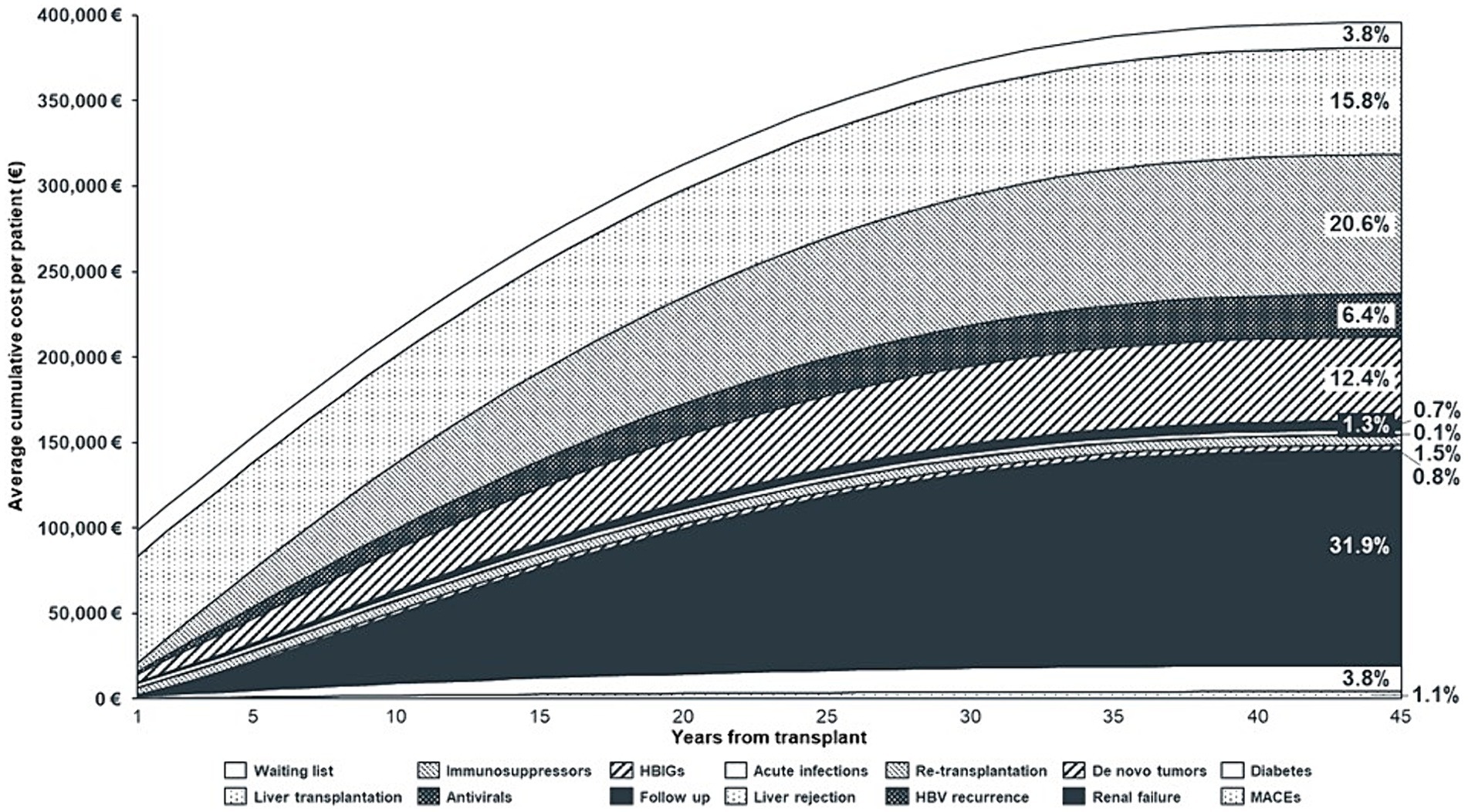
Figure 3. Average cumulative cost per patient, by year and cost component (Discounted Values). HBIGs, Hepatitis B Immunoglobulins; HBV, Hepatitis B Virus; MACEs, Major Adverse Cardiovascular Events.
Regarding the liver transplantation phases, the cost of the waiting list and transplantation was €77,563. In the first-year post-transplantation, when follow-up visits are more frequent, HBV prophylaxis doses are higher (32, 35, 36) and complications and comorbidities – such as infections, re-LT, HBV recurrence, and diabetes – are more likely to occur (13, 20–23, 25); the average annual cost per patient was €20,818. From the second year onwards, this cost decreased to an average of €6,764/patient per year.
Figure 3 illustrates the cumulative cost of LT and shows that the annual increase in costs declines over time. This was due to: (1) some costs being on–off at the beginning of the model (waiting list and transplant), (2) higher incidence of complications/comorbidities in the early post-transplant period, (3) higher resource consumption in the early years following transplantation (follow-up and prophylaxis), (4) patients exiting the model due to death, and (5) application of the discount rate.
Considering the eligible population, that is, patients who underwent liver transplantation in Italy due to HBV in 2022 (233 patients) (see foot note 3) (18, 19), the discounted lifetime economic burden for the Italian NHS related to HBV-patients was €92.3 million.
Table 8 reports the probability of occurrence of complications predicted by the model. The most frequent complications on a lifetime horizon were liver rejection, renal failure, and diabetes, all reaching estimates of cumulative incidence above 30%. Focusing on short-term comorbidities and complication, re-transplantation emerges as one of the most frequent complications (6.5%). Overall, patients in the model had an average life expectancy of 22 years, with a 1-year, 3-year, and 5-year probability of survival of 86.1, 82.7, and 80.1%, respectively.
These estimates are aligned with published data from Italian reports and observational studies. For example, Angelico et al. (58) report a re-transplantation rate of 6.1% at 18-month follow-up, while the Italian national guidelines on LT (32) report an incidence rate between 3 and 15% for de novo tumors and between 19 and 30% for renal failure. Similarly, an official report from the Italian National Transplant Center evaluating activities related to LT in Italy provides patients’ and grafts’ survival estimates comparable to our study (63).
Deterministic sensitivity analyses
The results of the deterministic sensitivity analyses are shown in Figure 4. According to the different scenarios, the cost of LT for HBV-related disease ranged from €325,072 to €582,193 per patient compared to the baseline scenario (€395,986/patient). Discount rates had the greatest impact on cost, increasing by 47.0% (~€582 k) for a 0% discount rate.
As for drugs, when the weighted average price was considered instead of the official ex-factory price, the average total lifetime cost of LT was €346,263 per patient, namely 12.6% lower than baseline, due to a 24.9% reduction in the annual cost of immunosuppression and a 26.3% and 61.8% decrease in the annual cost of HIBG and antivirals, respectively.
One-year HBIG prophylaxis resulted in an 11.1% decrease (~€352 k) in the total cost, while lifetime prophylaxis was associated with a 6.6% increase (~€422 k). Accordingly, a 30 and 50% reduction in immunosuppressant posology 3 months post-transplantation resulted in a 6.0% (~€372 k) and 10.1% (~€356 k) decrease in total cost, respectively.
Variations of −/+ 15% for cost components related to comorbidities, liver transplantation, and complications led to variations from the baseline results of −/+ 5.7% (~€374 k; ~€419 k), −/+ 3.1% (~€384 k; ~€409 k), and −/+ 0.1% (~€396 k; €397 k), respectively, whereas a reduction in the incidence of renal failure of 30% resulted in an 8.4% decrease in overall cost (~€363 K).
Finally, the analyses on the parametric extrapolation of the overall survival curve and the renal failure incidence curve led to an increase of 0.3% (~€397 K) and 4.0% (~€411 K), respectively. This limited variation is due to the fact that the new extrapolations mostly impact the tails of the curves, where patients incur in low costs as they progressively decease and exit the model.
Discussion
HBV is a major risk factor for liver cirrhosis and hepatocellular carcinoma in Italy (3), and liver transplantation (LT) is the best therapeutic option for patients with end-stage liver disease caused by HBV infection (64). Even though other studies have estimated the economic burden associated to LT due to different etiologies (65) and in different geographies (66), to the best of our knowledge, no previous analysis has investigated the lifetime cost for adult patients receiving a liver graft for HBV recipients in Italy or Europe. The modeling methodology we adopted followed a robust multi-step approach (9) from problem conceptualization to pragmatic literature review, model implementation, and model validation with a panel of national experts working in different Italian regions. Additionally, the model adopts a microsimulation approach to simulate the complex clinical pathway of this category of patients and provides statistically stable (15, 67) estimates of the average cost for patients undergoing LT in Italy in 2022.
The current analysis shows that the economic burden of LT for HBV-related diseases is high, with a discounted average lifetime cost per transplant patient of €395,986 and an overall impact for the Italian NHS of €92.3 million for the entire population of HBV patients transplanted in 2022. The greatest cost driver is end-stage renal failure, which accounts for 31.9% (~€126 k) of the total cost. Although this comorbidity has a 10-year cumulative incidence of less than 25% (61), as it also emerges from model results (Table 8), its management cost is high (~€33 k per year on average) (49–51) when considering dialysis and referral to renal transplantation (61). This evidence highlights the need for reinforcing “renal sparing” policies in the management of post liver transplant recipients in order to reduce both the incidence and the severity of renal complications, thus greatly impacting on total costs. The major cost drivers of liver transplantation are immunosuppression (20.6% of the total cost, ~€81 k) and liver transplant procedures (15.8% of the total cost, ~€63 k). HBV recurrence prophylaxis with HBIG and antiviral drugs accounts for 12.4 and 6.4% of the total cost, respectively, whereas all other cost components are residual. Additionally, sensitivity analyses have shown that considering the weighted average price of drugs instead of official drug prices leads to a reduction in total costs of 12.6% (~€346 k), with the relative contribution of anti-HBV recurrence prophylaxis of 13.2% instead of 18.8%. Furthermore, the lifetime duration of HBIG prophylaxis results in a limited cost increase (6.6%) versus −11.1% for the 1-year prophylaxis regimen, highlighting the maximum variation of costs resulting from the two limit durations adopted for patients with detectable HBV at time of transplantation in Italian clinical practice (36).
The reliability of the model was confirmed by sensitivity analyses, with variations ranging from €346,263 to €421,969 compared to the baseline value of €395,986/patient. Discount rate variations resulted in fluctuations between −17.9% (~€325 k) and + 47.0% (~€582 k) at 5 and 0% discount rates, respectively. The significant impact of discount rate variations is due to the long-term economic burden of LT, whereby consequences are spread over time and are consequently affected by higher discount rates.
While there are no published studies quantifying the overall lifetime cost for HBV patients undergoing LT in Italy, our analysis is consistent with previous Italian (68) and international (34, 69, 70) estimates of the cost components. To allow comparisons and cross-validation with these studies, costs reported in the literature were inflated to 2022 and adjusted for purchase power parity (PPP). Filipponi et al. (68) estimated the cost of transplantation and post-transplantation hospital stay during the acute phase. Considering a one-month acute-phase period, the corresponding cost from our model was aligned with this estimate (~€67 k vs. ~€77 k). Van der Hilst et al. and Longworth et al. (69, 70) estimated the LT-associated cost for a two-year follow-up at ~€122 k and ~ €95 k, respectively (versus ~€113 k in our analysis). Harries et al. (34) reported on the cost of waiting lists versus LT plus a three-year follow-up. Both costs were consistent with the results of our model, with estimates of ~€8 k and ~ €138 k, respectively, versus ~€15 k and ~ €126 k in the present study. Finally, Bjørnelv and co-authors (65) estimate the cost of liver transplantation for patients with colorectal metastases in Norway. When considering inflation and PPP, their lifetime cost estimation amounts to ~€180 k for the entire cohort of patients and ~ €200 k for a selected cohort, being largely lower than this study estimates. The difference may be explained by the shorter time horizon considered (25 years instead of lifetime), higher a discount rate (4.0% instead of 3.0%), the inclusion in their analysis of a lower number of cost components (transplantation and re-transplantation, post-operative complications, follow-up, immunosuppressive drugs, and anti-tumoral drugs in case of cancer recurrence), and clinical differences associated to the health system (Norway vs. Italian) and the etiology (colorectal metastases instead of HBV).
In addition to the validation by Italian KOLs in various phases of the study and the comparison with results from published analyses on costs related to liver transplantation, the model has been subject to other validations by the authors. Published data from observational studies and reports on LT in Italy allow for verification of model results in terms of incidence of comorbidities and complications and patient survival. Furthermore, the probabilities of occurrence reported in Table 8 are reflective of the incidence curves and survival curves extrapolated for model calibration (Tables 2, 3), highlighting the internal validity of the model ad well as the solidity of the microsimulation approach.
Despite its novelty and these validations, the model presents some limitations. First, some costs may have been underestimated. The model was constructed to estimate the average lifetime direct healthcare costs for HBV patients without any comorbidity or co-infection at the time of transplantation, thus considering a conservative resource-consumption scenario (32, 35). In addition, we assumed a maximum of one re-LT, as further re-transplantation is extremely rare (27). Finally, some estimates were based on the national tariffs in force in 2022, which have not been updated since 2013. This may lead to the underestimation of real costs (45, 71); however, it is the standard methodology for cost studies (72). To address this limitation, three sensitivity analyses were conducted varying the values of different cost components, which resulted in limited variations in total cost with respect to baseline.
In terms of model conceptualization and structure, there are some limitations due to the complexity of the LT clinical pathway of LT (15). Some post-transplant complications such as biliary complications and chronic rejection were not included in the model. Biliary complications encompass a wide spectrum of clinically variable scenarios (i.e., casts, stones, stenosis, leaks, and ischemic cholangiopathy) and cannot be easily encapsulated in a general model structure, while the incidence of chronic rejection is considered negligible in the modern era of immunosuppression regimens (4, 7, 21). Furthermore, the probabilities of complications were assumed to be independent of patient and donor characteristics, drug dosage regimen, and institutional framework and were independent of each other. This limitation in the model structure is due to the lack of highly detailed inputs and might lead to an overestimation of the cost of complications, as they are added on top of each other without considering that resource consumption increases less than proportionally with the increase in the number of complications (73).
Finally, the model was based on data from national guidelines and the literature, and although it had been validated with a panel of Italian experts, real-world evidence for further validation and verification of results is scarce.
In conclusion, this is the first study to estimate the lifetime direct healthcare cost of HBV patients undergoing LT in Italy and to provide data on the economic burden of liver transplantation for the Italian NHS. We firmly believe that the current results may pave the way for further research on this topic at a national level. The current model may be integrated with health technology assessment of new technologies introduced in transplantation, which may impact the cost trajectory of transplant patients. Furthermore, it may be adapted and adjusted to estimate costs related to LT for other indications (e.g., alcohol-associated liver disease and fatty liver disease). Finally, the analysis may be enriched by considering broader perspectives (including costs incurred by society and patients), with the involvement of patients and stakeholders.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
AM: Writing – review & editing, Validation, Supervision, Conceptualization. BC: Methodology, Formal analysis, Writing – review & editing, Writing – original draft, Data curation, Conceptualization. LDC: Writing – review & editing, Validation, Supervision. PDS: Writing – review & editing, Validation, Supervision. FF: Methodology, Formal analysis, Writing – review & editing, Writing – original draft, Data curation, Conceptualization. MR: Writing – review & editing, Validation, Supervision. CV: Methodology, Formal analysis, Writing – review & editing, Writing – original draft, Data curation, Conceptualization. SF: Writing – review & editing, Validation, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported with an unconditional grant by Kedrion Biopharma Inc.
Acknowledgments
The authors wish to acknowledge Veronica Andrea Fittipaldo for her contribution in the pragmatic literature review phase and her work in defining the research strings, creating the online databases, and conducting the first screening of the identified records.
Conflict of interest
BC, FF, and CV were employees of IQVIA Solutions S.r.l., which received a grant from Kedrion Biopharma Inc. to conduct the study. LC, PS, SF, AM, and MR received a fee from IQVIA Solutions S.r.l. as Advisors.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1328782/full#supplementary-material
Footnotes
1. ^Since at the time of the pragmatic literature review, MACEs had yet to be included in the patient flow, incidence curve for this comorbidity was retrieved in a second place through research of published literature and later validated with two KOLs.
2. ^The model assumes that re-transplantation can only occur once during the patients’ lifetime.
3. ^Calculated as the number of registered LT in Italy in 2022 [1,474 from the National Transplant Network report 2023 (19)] multiplied by the percentage of CHBV-related transplant from Italian literature [15.8% from Brancaccio et al., 2020 (18)].
4. ^IQVIA statistical elaboration on consumptions and expenditure data from a panel of hospital pharmacies across the Italian territory.
References
1. Trépo, C, Chan, HLY, and Lok, A. Hepatitis B virus infection. Lancet. (2014) 384:2053–63. doi: 10.1016/S0140-6736(14)60220-8
2. European Centre for Disease Prevention and Control. Systematic review on hepatitis B and C prevalence in the EU/EEA. Publications Office. Stockholm: European Centre for Disease Prevention and Control (2016).
3. Iannazzo, S, De Francesco, M, Coco, B, Brunetto, MR, Tomic, R, Paolini, D, et al. Impatto sul budget di un trattamento personalizzato dell’epatite B cronica HBeAg-negativa in Italia mediante peg-interferone alfa-2a associato alla stopping-rule alla 12a settimana. PharmacoEcon Ital Res Artic. (2013) 15:123–30. doi: 10.1007/s40276-013-0015-1
5. Orfanidou, A, Papatheodoridis, G, and Cholongitas, E. Antiviral prophylaxis against hepatitis B recurrence after liver transplantation: current concepts. Liver Int. (2021) 41:1448–61. doi: 10.1111/liv.14860
6. Maiwall, R, and Kumar, M. Prevention and treatment of recurrent hepatitis B after liver transplantation. J Clin Transl Hepatol. (2016) 4:54–65. doi: 10.14218/JCTH.2015.00041
7. Ammori, JB, Pelletier, SJ, Lynch, R, Cohn, J, Ads, Y, Campbell, DA, et al. Incremental costs of post–liver transplantation complications. J Am Coll Surg. (2008) 206:89–95. doi: 10.1016/j.jamcollsurg.2007.06.292
8. Harbarth, S, Szucs, T, Berger, K, and Jilg, W. The economic burden of hepatitis B in Germany. Eur J Epidemiol. (2000) 16:173–7. doi: 10.1023/a:1007624415699
9. Kaltenthaler, E, Tappenden, P, Paisley, S, and Squires, H. NICE DSU technical support document 13: Identifying and reviewing evidence to inform the conceptualization and population of cost-effectiveness models. (2011).
10. Millson, C, Considine, A, Cramp, ME, Holt, A, Hubscher, S, Hutchinson, J, et al. Adult liver transplantation: UK clinical guideline - part 2: surgery and post-operation. Front Gastroenterol. (2020) 11:385–96. doi: 10.1136/flgastro-2019-101216
11. Cillo, U, Saracino, L, Vitale, A, Bertacco, A, Salizzoni, M, Lupo, F, et al. Very early introduction of Everolimus in De novo liver transplantation: results of a multicenter, prospective. Randomized Trial Liver Transpl. (2019) 25:242–51. doi: 10.1002/lt.25400
12. Ettorre, GM, Piselli, P, Galatioto, L, Rendina, M, Nudo, F, Sforza, D, et al. De novo malignancies following liver transplantation: results from a multicentric study in central and southern Italy, 1990–2008. Transplant Proc. (2013) 45:2729–32. doi: 10.1016/j.transproceed.2013.07.050
13. Gagliotti, C, Morsillo, F, Moro, ML, Masiero, L, Procaccio, F, Vespasiano, F, et al. Infections in liver and lung transplant recipients: a national prospective cohort. Eur J Clin Microbiol Infect Dis. (2018) 37:399–407. doi: 10.1007/s10096-018-3183-0
14. Graves, J, Garbett, S, Zhou, Z, Schildcrout, J, and Peterson, J. Comparison of decision modeling approaches for health technology and policy evaluation. Value Health. (2020) 23:S17. doi: 10.1016/j.jval.2020.04.087
15. Qu, Z, Krauth, C, Amelung, VE, Kaltenborn, A, Gwiasda, J, Harries, L, et al. Decision modelling for economic evaluation of liver transplantation. World J Hepatol. (2018) 10:837–48. doi: 10.4254/wjh.v10.i11.837
16. AIFA. Linee guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale. (2021).
17. Haldar, D, Kern, B, Hodson, J, Armstrong, MJ, Adam, R, Berlakovich, G, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European liver transplant registry study. J Hepatol. (2019) 71:313–22. doi: 10.1016/j.jhep.2019.04.011
18. Brancaccio, G, Vitale, A, Signoriello, G, Gaeta, G, and Cillo, U. Changing indications for liver transplant: Slow decline of hepatitis viruses in Italy. (2020).
20. Freire, MP, Song, ATW, Oshiro, ICV, Andraus, W, D’Albuquerque, LAC, and Abdala, E. Surgical site infection after liver transplantation in the era of multidrug-resistant bacteria: what new risks should be considered? Diagn Microbiol Infect Dis. (2021) 99:115220. doi: 10.1016/j.diagmicrobio.2020.115220
21. Adam, R, Karam, V, and Cailliez, V. O Grady JG, Mirza D, Cherqui D, et al. 2018 annual report of the European liver transplant registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. (2018) 31:1293–317. doi: 10.1111/tri.13358
22. Fosby, B, Melum, E, Bjøro, K, Bennet, W, Rasmussen, A, Andersen, IM, et al. Liver transplantation in the Nordic countries – an intention to treat and post-transplant analysis from the Nordic liver transplant registry 1982–2013. Scand J Gastroenterol. (2015) 50:797–808. doi: 10.3109/00365521.2015.1036359
23. Pauwelyn, K, Cassiman, D, Laleman, W, Verslype, C, Monbaliu, D, Aerts, R, et al. Outcomes of long-term Administration of Intravenous Hepatitis B Immunoglobulins for the prevention of recurrent hepatitis B after liver transplantation. Transplant Proc. (2010) 42:4399–402. doi: 10.1016/j.transproceed.2010.07.011
24. Taborelli, M, Piselli, P, Ettorre, GM, Lauro, A, Galatioto, L, Baccarani, U, et al. Risk of virus and non-virus related malignancies following immunosuppression in a cohort of liver transplant recipients. Italy, 1985–2014. Intl J Cancer. (2018) 143:1588–94. doi: 10.1002/ijc.31552
25. Boudjema, K, Camus, C, Saliba, F, Calmus, Y, Salamé, E, Pageaux, G, et al. Reduced-dose tacrolimus with mycophenolate Mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant. (2011) 11:965–76. doi: 10.1111/j.1600-6143.2011.03486.x
26. Rostved, AA, Lundgren, JD, Hillingsø, J, Peters, L, Mocroft, A, and Rasmussen, A. MELD score measured day 10 after orthotopic liver transplantation predicts death and re-transplantation within the first year. Scand J Gastroenterol. (2016) 51:1360–6. doi: 10.1080/00365521.2016.1196497
27. Takagi, K, Domagala, P, Porte, RJ, Alwayn, I, Metselaar, HJ, Van Den Berg, AP, et al. Liver retransplantation in adult recipients: analysis of a 38-year experience in the Netherlands. J Hepato Biliary Pancreat. (2020) 27:26–33. doi: 10.1002/jhbp.701
28. Baccarani, U, Piselli, P, Serraino, D, Adani, GL, Lorenzin, D, Gambato, M, et al. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Dig Liver Dis. (2010) 42:55–60. doi: 10.1016/j.dld.2009.04.017
29. Tovikkai, C, Charman, SC, Praseedom, RK, Gimson, AE, and Van Der Meulen, J. Time-varying impact of comorbidities on mortality after liver transplantation: a national cohort study using linked clinical and administrative data. BMJ Open. (2015) 5:e006971–1. doi: 10.1136/bmjopen-2014-006971
30. Brain, D, and Jadambaa, A. Economic evaluation of long-term survivorship Care for Cancer Patients in OECD countries: a systematic review for decision-makers. Int J Environ Res Public Health. (2021) 18:11558. doi: 10.3390/ijerph182111558
31. Gazzetta Ufficiale della Repubblica Italiana. Osp GU Serie Generale n.23 del 28-01-2013 - Suppl. Ordinario n. 8 -Allegato I. (2013).
32. Associazione Italiana per lo Studio del Fegato. Raccomandazioni per il trapianto di fegato. (2008).
33. Agenzia Italiana del Farmaco. (2023). Available at: https://www.aifa.gov.it/.
34. Harries, L, Schrem, H, Stahmeyer, JT, Krauth, C, and Amelung, VE. High resource utilization in liver transplantation-how strongly differ costs between the care sectors and what are the main cost drivers?: a retrospective study. Transpl Int. (2017) 30:621–37. doi: 10.1111/tri.12950
35. Cillo, U, De Carlis, L, Del Gaudio, M, De Simone, P, Fagiuoli, S, Lupo, F, et al. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian working group. Hepatol Int. (2020) 14:930–43. doi: 10.1007/s12072-020-10091-5
36. Cecchini, I, Monti, T, and Panza, A. Verso una modalità condivisa di profilassi post-OLT con immunoglobuline antiepatite B. BIFE (2021).
37. Gazzetta Ufficiale della Repubblica Italiana. GU Serie Generale n.23 del 28-01-2013 - Suppl. Ordinario n. 8 -Allegato III. (2013).
38. Gazzetta Ufficiale della Repubblica Italiana. (2024). Available at: www.gazzettaufficiale.it.
39. Marcellusi, A, Bini, C, Peris, K, Ascierto, PA, and Mennini, FS. Cost of illness of cutaneous squamous cell carcinoma (CSCC). Grhta. (2020) 7:148–53. doi: 10.33393/grhta.2020.2171
40. Altini, M, Solinas, L, Bucchi, L, Gentili, N, Gallegati, D, Balzi, W, et al. Assessment of Cancer care costs in disease-specific Cancer care pathways. Int J Environ Res Public Health. (2020) 17:4765. doi: 10.3390/ijerph17134765
41. Polesel, J, Lupato, V, Collarile, P, Vaccher, E, Fanetti, G, Giacomarra, V, et al. Direct health-care cost of head and neck cancers: a population-based study in North-Eastern Italy. Med Oncol. (2019) 36:31. doi: 10.1007/s12032-019-1256-2
42. Leal, J, Luengo-Fernandez, R, Sullivan, R, and Witjes, JA. Economic burden of bladder Cancer across the European Union. Eur Urol. (2016) 69:438–47. doi: 10.1016/j.eururo.2015.10.024
43. Gerace, C, Montorsi, F, Tambaro, R, Cartenì, G, De Luca, S, Tucci, M, et al. Cost of illness of urothelial bladder cancer in Italy. Clinicoecon Outcomes Res. (2017) 9:433–42. doi: 10.2147/CEOR.S135065
44. Maio, M, Ascierto, P, Testori, A, Ridolfi, R, Bajetta, E, Queirolo, P, et al. The cost of unresectable stage III or stage IV melanoma in Italy. J Exp Clin Cancer Res. (2012) 31:91. doi: 10.1186/1756-9966-31-91
45. Filetti, S, Ladenson, PW, Biffoni, M, D’Ambrosio, MG, Giacomelli, L, and Lopatriello, S. The true cost of thyroid surgery determined by a micro-costing approach. Endocrine. (2017) 55:519–29. doi: 10.1007/s12020-016-0980-z
46. Mantovani, LG, Morsanutto, A, Tosolini, F, Mustacchi, G, Esti, R, Belisari, A, et al. The burden of renal cell cancer: a retrospective longitudinal study on occurrence, outcomes and cost using an administrative claims database. Eur J Cancer Suppl. (2008) 6:46–51. doi: 10.1016/j.ejcsup.2008.06.013
47. Francisci, S, Guzzinati, S, Capodaglio, G, Pierannunzio, D, Mallone, S, Tavilla, A, et al. Patterns of care and cost profiles of women with breast cancer in Italy: EPICOST study based on real world data. Eur J Health Econ. (2020) 21:1003–13. doi: 10.1007/s10198-020-01190-z
48. Capri, S, and Russo, A. Cost of breast cancer based on real-world data: a cancer registry study in Italy. BMC Health Serv Res. (2017) 17:84. doi: 10.1186/s12913-017-2006-9
49. Turchetti, G, Bellelli, S, Amato, M, Bianchi, S, Conti, P, Cupisti, A, et al. The social cost of chronic kidney disease in Italy. Eur J Health Econ. (2017) 18:847–58. doi: 10.1007/s10198-016-0830-1
50. Jommi, C, Armeni, P, Battista, M, Di Procolo, P, Conte, G, Ronco, C, et al. The cost of patients with chronic kidney failure before Dialysis: results from the IRIDE observational study. PharmacoEconomics Open. (2018) 2:459–67. doi: 10.1007/s41669-017-0062-z
51. Società Italiana Nefrologia. Il valore del trapianto: un’analisi empirica dei consumi sanitari e dei costi dei trapienti di rene in Italia 2013. (2013).
52. Pucciarelli, G, Rebora, P, Arisido, MW, Ausili, D, Simeone, S, Vellone, E, et al. Direct cost related to stroke: a longitudinal analysis of survivors after discharge from a rehabilitation hospital. J Cardiovasc Nurs. (2020) 35:86–94. doi: 10.1097/JCN.0000000000000620
53. Grimaccia, F, and Kanavos, P. Cost, outcomes, treatment pathways and challenges for diabetes care in Italy. Glob Health. (2014) 10:58. doi: 10.1186/1744-8603-10-58
54. Lucioni, C, Mazzi, S, Rossi, E, Rielli, R, Calabria, S, Maggioni, AP, et al. Therapeutic strategies and health costs of patients admitted for a cardiovascular event in Italy. Glob Reg Health Technol Assess. (2016) 3:5000221. doi: 10.33393/grhta.2016.413
55. Roggeri, A, Gnavi, R, Dalmasso, M, Rusciani, R, Giammaria, M, Anselmino, M, et al. Resource consumption and healthcare costs of acute coronary syndrome: a retrospective observational administrative database analysis. Crit Pathways Cardiol. (2013) 12:204–9. doi: 10.1097/HPC.0b013e3182a78c06
56. Duvoux, C, Belli, LS, Fung, J, Angelico, M, Buti, M, Coilly, A, et al. 2020 position statement and recommendations of the European liver and intestine transplantation association (ELITA): management of hepatitis B virus-related infection before and after liver transplantation. Aliment Pharmacol Ther. (2021) 54:583–605. doi: 10.1111/apt.16374
57. De Simone, P, Nevens, F, De Carlis, L, Metselaar, HJ, Beckebaum, S, Saliba, F, et al. Everolimus with reduced tacrolimus improves renal function in De novo liver transplant recipients: a randomized controlled trial. Am J Transplant. (2012) 12:3008–20. doi: 10.1111/j.1600-6143.2012.04212.x
58. Angelico, R, Trapani, S, Spada, M, Colledan, M, De Ville De Goyet, J, Salizzoni, M, et al. A national mandatory-split liver policy: a report from the Italian experience. Am J Transplant. (2019) 19:2029–43. doi: 10.1111/ajt.15300
59. Harries, L, Gwiasda, J, Qu, Z, Schrem, H, Krauth, C, and Amelung, VE. Potential savings in the treatment pathway of liver transplantation: an inter-sectorial analysis of cost-rising factors. Eur J Health Econ. (2019) 20:281–301. doi: 10.1007/s10198-018-0994-y
60. Maffei, P, Wiramus, S, Bensoussan, L, Bienvenu, L, Haddad, E, Morange, S, et al. Intensive early rehabilitation in the intensive care unit for liver transplant recipients: a randomized controlled trial. Arch Phys Med Rehabil. (2017) 98:1518–25. doi: 10.1016/j.apmr.2017.01.028
61. Ojo, AO, Leichtman, AB, and Merion, RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. (2003) 349:931–40. doi: 10.1056/NEJMoa021744
62. Albeldawi, M, Aggarwal, A, Madhwal, S, Cywinski, J, Lopez, R, Eghtesad, B, et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. (2012) 18:370–5. doi: 10.1002/lt.22468
64. Vida Perez, L, Montero Alvarez, JL, Poyato Gonzalez, A, Briceño Delgado, J, Costan Rodero, G, Fraga Rivas, E, et al. Prevalence and predictors of metabolic syndrome after liver transplantation. Transplant Proc. (2016) 48:2519–24. doi: 10.1016/j.transproceed.2016.08.029
65. Bjørnelv, GM, Dueland, S, Sørbye, H, and Aas, E. Cost-effectiveness of liver transplantation in patients with colorectal metastases confined to the liver. Br J Surg. (2018) 106:132–41. doi: 10.1002/bjs.10962
66. Neff, GW, Duncan, CW, and Schiff, ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (NY). 7:661–71.
67. Skedgel, C, Bulut, M, and Steuten, L. After the transplant: Potential benefits for the NHS and UK kidney transplant patients. (2021).
68. Filipponi, F, Pisati, R, Cavicchini, G, Ulivieri, MI, Ferrara, R, and Mosca, F. Cost and outcome analysis and cost determinants of liver transplantation in a European National Health Service Hospital. Transplantation. (2003) 75:1731–6. doi: 10.1097/01.TP.0000063828.20960.35
69. Van Der Hilst, CS, Ijtsma, AJC, Slooff, MJH, and TenVergert, EM. Cost of liver transplantation: a systematic review and Meta-analysis comparing the United States with other OECD countries. Med Care Res Rev. (2009) 66:3–22. doi: 10.1177/1077558708324299
70. Longworth, L, Young, T, Buxton, MJ, Ratcliffe, J, Neuberger, J, Burroughs, A, et al. Midterm cost-effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transpl. (2003) 9:1295–307. doi: 10.1016/j.lts.2003.09.012
71. Palmieri, V, Baldi, C, Di Blasi, PE, Citro, R, Di Lorenzo, E, Bellino, E, et al. Impact of DRG billing system on health budget consumption in percutaneous treatment of mitral valve regurgitation in heart failure. J Med Econ. (2015) 18:89–95. doi: 10.3111/13696998.2014.980502
72. Tarricone, R . Cost-of-illness analysis. Health Policy. (2006) 77:51–63. doi: 10.1016/j.healthpol.2005.07.016
73. Lock, JF, Reinhold, T, Bloch, A, Malinowski, M, Schmidt, SC, Neuhaus, P, et al. The cost of graft failure and other severe complications after liver transplantation – experience from a German transplant center. Ann Transplant. 15:11–8.
Keywords: hepatitis B, immunoglobulin, liver transplant, cost analysis, cost of illness, Italy
Citation: Marzano A, Canali B, De Carlis L, De Simone P, Fiorentino F, Rendina M, Vassallo C and Fagiuoli S (2024) Estimation of lifetime costs for patients receiving a transplant: the case of liver transplantation related to hepatitis B in Italy. Front. Public Health. 12:1328782. doi: 10.3389/fpubh.2024.1328782
Edited by:
Liliana Chemello, University of Padua, ItalyCopyright © 2024 Marzano, Canali, De Carlis, De Simone, Fiorentino, Rendina, Vassallo and Fagiuoli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beatrice Canali, YmVhdHJpY2UuY2FuYWxpQGlxdmlhLmNvbQ==
 Alfredo Marzano1
Alfredo Marzano1 Beatrice Canali
Beatrice Canali Paolo De Simone
Paolo De Simone Francesca Fiorentino
Francesca Fiorentino Maria Rendina
Maria Rendina Chiara Vassallo
Chiara Vassallo Stefano Fagiuoli
Stefano Fagiuoli