
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 07 June 2024
Sec. Environmental Health and Exposome
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1325856
 Xi Chen1,2†
Xi Chen1,2† Tengkun Wang1,3†
Tengkun Wang1,3† Yushan Tian4†
Yushan Tian4† Yinchao Ma1,4
Yinchao Ma1,4 Yuan Liu1
Yuan Liu1 Huan Chen4*
Huan Chen4* Hongwei Hou4*
Hongwei Hou4* Qingyuan Hu4*
Qingyuan Hu4* Ming Chu1,4*
Ming Chu1,4*Background: Smoking is a risk factor for a wide range of diseases. Previous research has confirmed over 30 Smoking-Associated Diseases in diverse systems. There is limited research exploring the correlation among multiple diseases, with an absence of comprehensive investigations. Few studies concentrate on diseases exhibiting a negative correlation with smoking, wherein smokers demonstrate a lower prevalence.
Objective: This study aimed to detect the correlation between smoking and other diseases using data from National Health and Nutrition Examination Surveys (NHANES) and construct a Smoking-Diseases Correlation Database (SDCD). The second aim is to obtain an extensive screening test for diseases that may be linked to smoking.
Methods: 39,126 subjects’ data from the NHANES 2013–2018 dataset were extracted. The baseline information, difference in blood routine and blood chemistry indicators between smokers and non-smokers, and diseases’ correlation with smoking in four different models were analyzed by R. The data and statistics were aggregated into an online SDCD.
Results: Our study reported 46 Smoking-Associated Diseases (SAD), including 29 Smoking Positively Associated Diseases (SPAD) and 17 Smoking Negatively Associated Diseases (SNAD). The SDCD of 422 diseases was constructed and can be accessed at https://chatgptmodel.shinyapps.io/sdcd/.
Conclusion: Our findings revealed 46 SADs including 29 SPADs and 17 SNADs. We aggregated the statistics and developed online SDCD, advancing our understanding of the correlation between smoking and diseases.
Smoking is the main cause of preventable early death around the world. Approximately one person dies every 6 s due to smoking, accounting for one in five deaths in the age group between 35 and 69 (1, 2). According to the WHO, the total number of tobacco users is 1.30 billion in 2020, and the prevalence rate of tobacco use is 22.3% of the global population. Over 8 million people died from tobacco-related diseases in 2019 (3). In the 1950s, Ernst Wynder et al. put forward a hypothesis that lung cancer is associated with smoking. As the evidence accumulated, in 1964, a report from the United States Department of Health confirmed the causal relationship between smoking and lung cancer (4). From then on, a quantity of evidence was regularly updated and more Smoking-Associated Diseases (SAD) were found. Studies have established that smoking can affect different organs and systems such as cardiovascular (5), respiratory (6), brain (7), reproductive (8), immune (9) systems, etc. The number of SADs increases gradually and has reached at least 30, but there are still SADs that are not being detected or lack enough evidence (10).
Tremendous research focuses on Smoking Positively Associated Diseases (SPAD). However, studies have also found that some diseases were Smoking Negatively Associated Diseases (SNAD). For example, smokers have a lower prevalence of Parkinson’s disease than nonsmokers, which is consistently shown by previous epidemiology research. Because combustible tobacco smoke has been seen as a cause of adverse health outcomes, this inverse correlation was counterintuitive and fascinating (11). Furthermore, studies suggest that non-smokers have a relatively higher risk of ulcerative colitis and osteoarthritis than smokers (12). If we were to document favorable effects of smoking on different diseases, this might provide an opportunity to identify constituents of combustible tobacco smoke that have favorable effects on disease and to test these as potential treatments (12).
The majority of present studies are dispersed and specialize in a certain disease, and lack comprehensive research including multiple diseases in one study. The comprehensive study is important for the understanding of tobacco’s effect on health. Therefore, we evaluated the correlation between smoking and 422 different diseases using data of 39,126 people from the National Health and Nutrition Examination Survey dataset of the United States (NHANES) and paid attention to both SPAD and SNAD. By extracting and analyzing a giant collection of data, knowledge of this database could provide a new landscape on smoking and disease, laying a foundation for further research on SAD.
For this investigation, we utilized the data extracted from the National Health and Nutrition Examination Survey dataset of the United States (NHANES) spanning from 2013 to 2018. All the data we used could be accessed at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. 12,226 individuals were excluded from the study due to missing or incomplete data on body mass index (BMI), education level, smoking records, and uncertain or incomplete information. Additionally, 24 individuals who lacked ICD-10 diagnosis information were also ruled out in this study. A total of 39,126 subjects remained for the final analysis. The study of NHANES 2013–2018 was performed according to the guidelines approved by the Research Ethics Review Board of the National Center for Health Statistics (Protocol #2011-17, #2018-01). Moreover, written informed consent was duly obtained from all participants involved in the NHANES project.
Variables data from NHANES is collected through a combination of interviews, physical examinations, and laboratory tests. Participants are interviewed to gather information on their medical history, lifestyle factors, and dietary habits. Additionally, a comprehensive physical examination is conducted, which includes measuring vital signs and collecting blood samples. The blood samples are then analyzed in a laboratory, where tests are performed to measure the various parameters of interest. These data contribute to the rich dataset available in the NHANES database, which allows researchers and public health professionals to study and monitor the health and nutritional status of the United States population.
National Health and Nutrition Examination Surveys employs a weighted sampling approach to ensure its findings are representative of the United States population. Participants are assigned base weights reflecting their probability of selection. Nonresponse adjustments account for those who choose not to participate, and post-stratification aligns the sample with known population demographics.
The population variables involved in this study include age, BMI, sex, race, and education level. Ethnicity and race are determined by a series of questions in interviews. Hispanic or Latino refers to anyone who says they were born in or had ancestors from Spain or one of the western hemisphere territories or countries where Spanish is the primary language. The blood routine and blood chemistry indicators involved in this study include white blood cell count (WBC), neutrophils (NEU), lymphocytes (LYM), monocytes (MO), platelets (PLT), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), systemic immune inflammation index (SII), systemic immune response index (SIRI), alkaline phosphatase (ALP), total bilirubin (TBIL), albumin (ALB), gamma-glutamyl transferase (GGT), iron (Fe), and alpha-klotho (a-klotho).
The dependent variables of this research were 422 diseases with ICD-10 coding reported by each individual’s response to the questionnaire using the following question: “Has a doctor ever diagnosed you with….” 422 diseases involved multiple organs and systems cover the smoking’s influence on health as comprehensive as possible.
In this study, the smoking population is delineated as individuals who have consumed a minimum of 100 cigarettes throughout their lifetime. This definition is consistent with the report methods of other diseases in the questionnaire in order to get a better correlation analysis.
We have performed all statistical analysis with the R software (Version 4.2.2) according to the CDC guidelines.1 Sample weights were taken into account in all of the estimates to produce representative data of the civilian noninstitutionalized United States population. The correlation between smoking and each disease was assessed through four multivariable logistic regression models: one unadjusted and three adjusted models. Model 1 did not include any covariate adjustments. In Model 2, adjustments were made for age, gender, and race. Model 3 accounted for age, gender, race, education level, and poverty-to-income ratio. Finally, Model 4 incorporated adjustments for age, gender, race, education level, poverty-to-income ratio, smoking status, body mass index, and bone mineral density.
We constructed a Smoking-Diseases Correlation Database (SDCD), an online repository of smoking-disease correlations, using the R software package Shiny and the statistics. The database can be accessed at the following web address: https://chatgptmodel.shinyapps.io/sdcd/. There are the characteristics of the participants, the association between smoking and selected diseases, and subgroup analysis data on the website. Subgroup analysis refers to an analysis of OR values of diseases among smokers relative to non-smokers in different gender and ethnic groups separately.
A total of 39,126 participants (mean age 53.90 ± 0.35, 46% males) were included in this study. 48.18% of the participants have a history of smoking. The weighted distribution of the characteristics according to smoking is shown in Table 1. The average age and BMI in these two groups did not have a significant difference. Regarding sex and race, Males and non-Hispanic white people were more likely to have a higher rate of smoking. There are more smokers than non-smokers in the College or above and the High School or equivalent education level participants groups and fewer smokers than non-smokers in the College graduate or above and the Less than high school groups.
We conducted a study examining the disparities in blood routine and blood biochemistry variables between smokers and non-smokers (Table 2). To assess the difference between smokers and non-smokers, a t-test was computed. Compared to the non-smoking population, the majority of immunological inflammatory markers were significantly elevated in the smoking population, including white blood cell count (WBC), neutrophil count (NEU), lymphocyte count (LYM), monocyte count (MO), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), and systemic immune response index (SIRI). Additionally, there were notable increases in certain oxidative stress indicators such as Alkaline Phosphatase (ALP), albumin (ALB), Gamma-Glutamyl Transferase (GGT), and decreases of α-Klotho and total bilirubin (TBIL), in the smoking population.
Among non-smokers, for example, the WBC was 7.26, while this rose 9% to 7.91 in former smokers. NEU indicator rises 11% in smokers. MO rises 9%. Smokers’ GGT indicator (34.50) is 27% larger than non-smokers (27.21) and it has the biggest increase percentage in all risen blood indicators. Interestingly, TBIL and a-klotho, respectively, decreased by 5 and 4%, and they are both the only decreased indicators with statistical significance.
Within this investigation, we delved into the correlation between smoking and various diseases. We examined a comprehensive collection of 422 prevalent illnesses categorized by the ICD-10 classification system and calculated the disparities in disease incidence between smokers and non-smokers. Ultimately, we identified 29 diseases that exhibited a significant positive correlation with smoking (Table 3). SPADs listed in Table 3 primarily focus on COPD, mental health disorders, pain, and gastrointestinal diseases. It also includes muscle spasm.
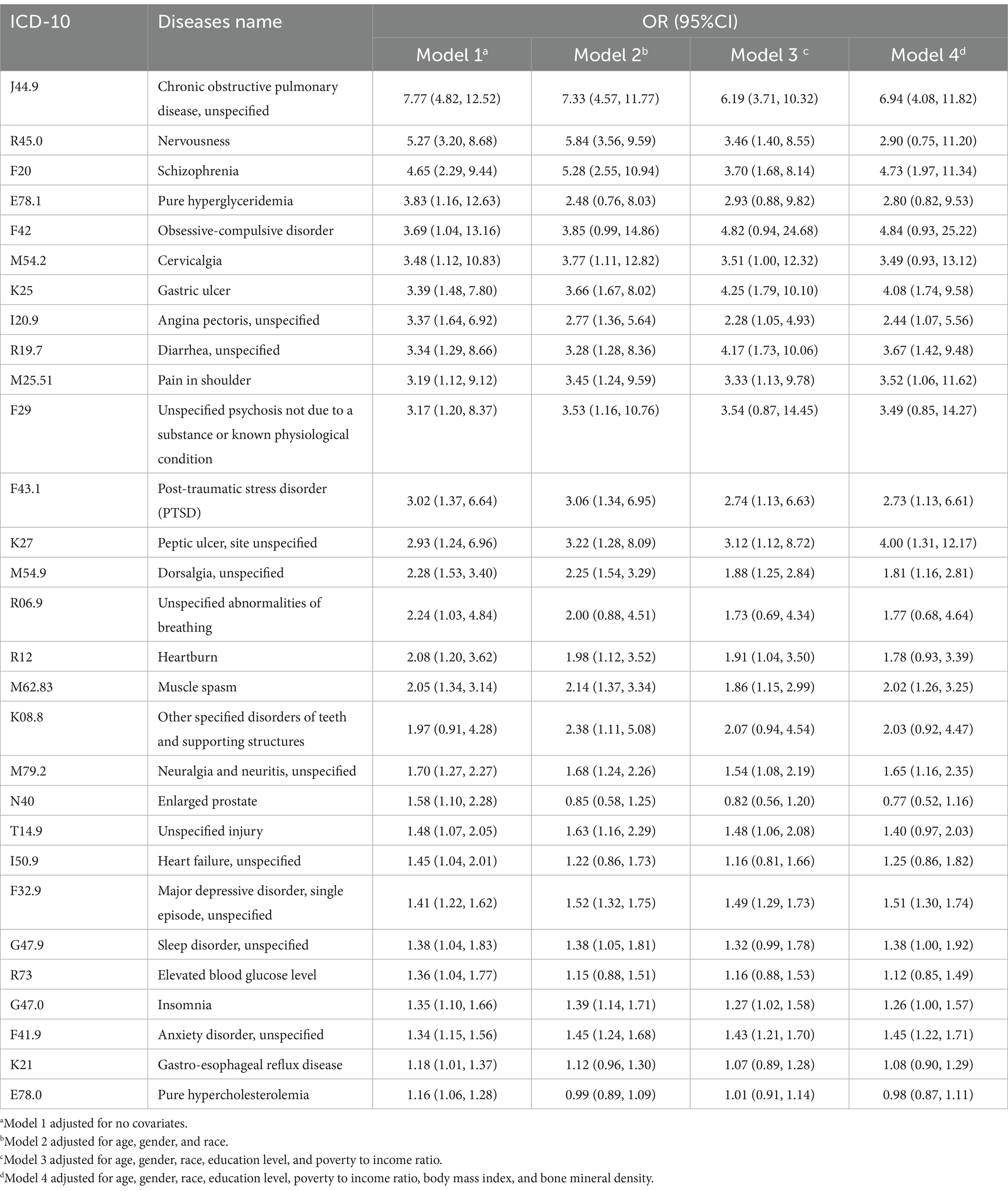
Table 3. SPADs’ names, ICD-10 numbers, and OR values among smokers relative to non-smokers in four models after adjusting for variables of interest.
Notably, we also identified 17 diseases that displayed a significant negative correlation with smoking (Table 4). SNADs listed in Table 4 cover a broader range of diseases across various medical domains, including infectious diseases, endocrine disorders (diabetes), neurological conditions (Parkinson’s disease), eye disorders, respiratory conditions (pulmonary embolism, otitis media), musculoskeletal conditions (psoriasis, osteoporosis), and genitourinary disorders (kidney failure, menstrual irregularities).
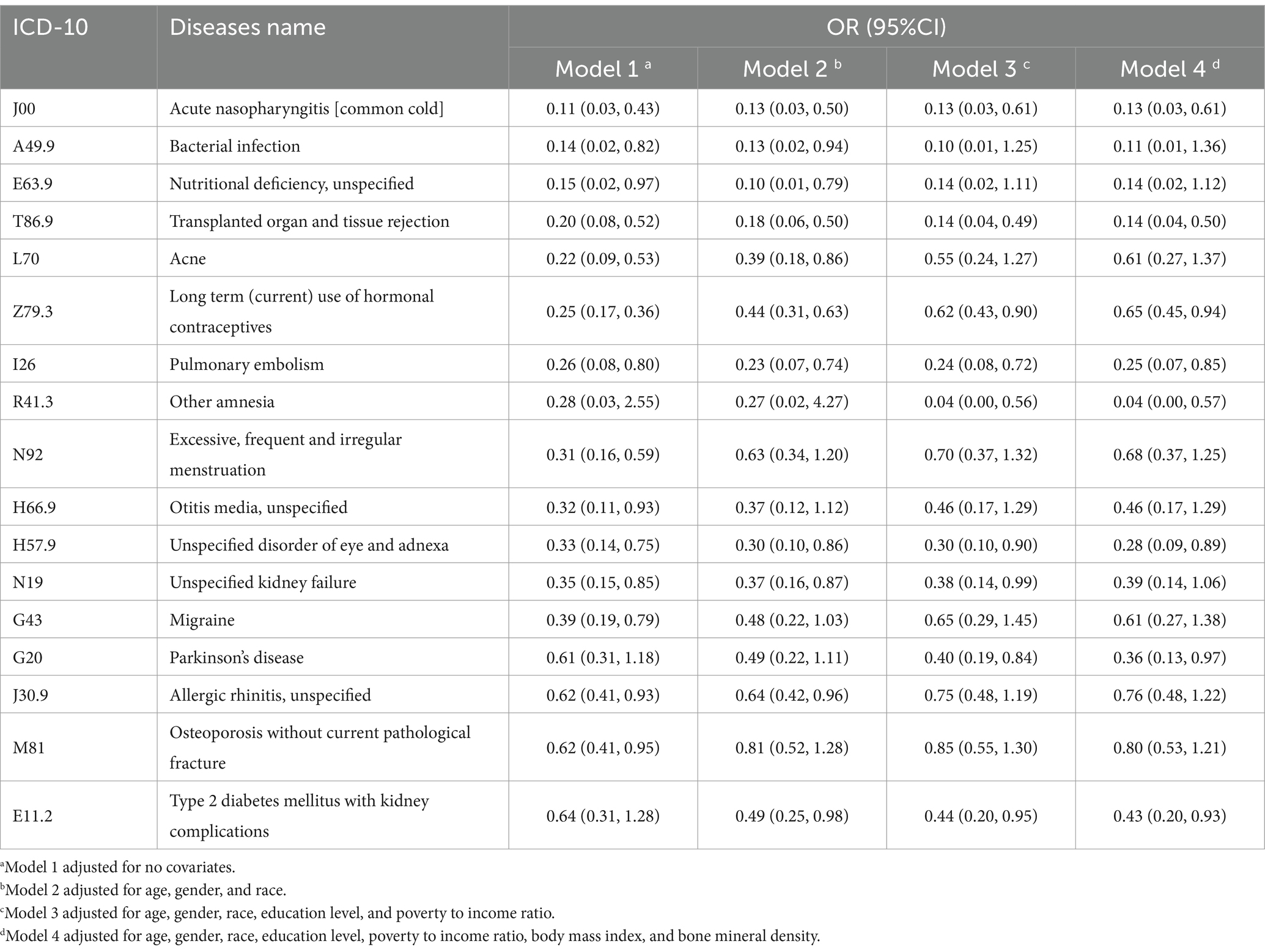
Table 4. SNADs’ names, ICD-10 numbers, and OR values among smokers relative to non-smokers in four models after adjusting for variables of interest.
Blood routine and blood biochemistry indicators had 12 differences between smokers and non-smokers, with the majority of indicators showing higher levels in smokers. Elevated indicators suggest an increased inflammatory load and oxidative stress in smokers. One protein α-klotho caught our attention. Data showed that the α-klotho level in smokers is 4% lower than in non-smokers. Klotho gene is one of the most important genes related to the aging process and controls many metabolic pathways. Klotho protein has anti-aging, anti-inflammatory, anti-oxidant, and cardioprotective effects. A study suggested that oxidative stress from smoking can disrupt the anti-oxidant defense and decrease α-klotho protein synthesis (13).
Notably, our study identified 29 diseases possibly with a positive correlation with smoking. The most prominent one of these 29 SPADs is COPD, while many SPADs can be categorized into groups by their features, the mechanism of which requires further research, such as mental disorders, pain, and gastrointestinal disorders.
Chronic obstructive pulmonary disease has the highest OR value, respectively 7.77, 7.33, 6.19, and 6.94 among both four models and 29 SPADs. COPD is commonly associated with smoking; in addition to that, second-hand smoke, occupational exposures, air pollutants, and a history of previous lung infections are risk factors (14). One possible mechanism is that cigarette smoke can reprogram the epithelium of trachea and cause mitochondrial dysfunction (14, 15). A perspective suggested that COPD is a polygenetic disease, with complex environment risk factors accumulating through lifetime and interacting with susceptible genes (16).
Many mental disorders are SPADs. The rate of smoking among schizophrenia patients is 4.65 times higher compared to the general population, and other research indicates rates ranging from 2 to 5 times higher (17, 18). Several hypotheses have been proposed to explain this correlation, including (a) the causal relationship hypothesis, (b) the self-medication hypothesis, and (c) the shared genetic background hypothesis (17–20). In addition to schizophrenia, patients with nervousness, anxiety, and depression have respective OR values of 5.27, 1.34, and 1.41 in model 1.
Pain constitutes another category of SPADs that we have reported. Individuals experiencing chronic pain are approximately twice as likely to be current smokers compared to those without chronic pain (21). Firstly, nicotine exhibits an acute analgesic effect, intensifying pain in nicotine-deprived smokers and thereby making smoking cessation efforts more difficult (22). Secondly, smokers may be driven to use tobacco as a response to pain through intricate psychological mechanisms. An interactive reciprocal model of pain and smoking suggests that pain and smoking form a positive feedback loop, resulting in exacerbated pain and increased smoking (23).
More interestingly, we identified 18 different SNADs in this study, in which Parkinson’s disease (PD), allergic rhinitis, and long-term use of hormonal contraceptives (OCs) aroused our interest.
The OR value for Parkinson’s disease is 0.36 in model 4. Numerous epidemiological studies have consistently reported the same negative result. This correlation exhibits dose-dependent and time-dependent characteristics: RRs of PD were 0.8, 0.6, 0.5, and 0.4, for 1–9, 10–24, 25–44, and 45+ pack-years, respectively, relative to never-smokers (11). Notably, there have been compelling evidence for the relationship between smoking and the declining prevalence of PD. Arguments against this correlation, such as confounding by unknown factors, shared genes, and personality characteristics of PD patients, remain unlikely (24). The neuroprotective role shown by smoking might be related to the biological effect of nicotine, which has demonstrated neuroprotective effects in animal models of PD (25).
Allergic rhinitis showed a significant negative correlation in models 1 and 2 but did not show significance in models 3 and 4. The OR values were comparable between model 1 (0.62) and 2 (0.64), as well as between 3 (0.75) and 4 (0.76). A two-sample Mendelian randomization study also revealed that smoking can decrease the risk of allergic rhinitis and increase the risk of vasomotor rhinitis. One hypothesis suggested that individuals with allergic rhinitis may have a preference for smoking. Another hypothesis suggested that smoking might inhibit the immune system, thereby reducing the risk of allergic rhinitis (26).
Long-term use of hormonal contraceptives has a low prevalence (OR = 0.44) in smokers after gender was adjusted in model 2. Currently, there is limited understanding of the correlation between OCs’ use and smoking, with conflicting data reported in various studies. The most common OCs, which contain estrogen and progesterone components, can alter body progesterone and estradiol levels. Former studies have explored the potential impact of OCs on nicotine metabolism, cardiovascular reactivity, mood, and withdrawal (27). Some studies have investigated the clinical trial of exogenous progesterone’s effect on smoking cessation. One study discovered that exogenous progesterone increased the odds of quitting in females nearly 3-fold and averaged 6 more days before relapse (28). The proposition of exogenous progesterone, either as an adjunctive or stand-alone therapy for smoking cessation has been put forward (29). Further epidemiological studies are required to better understand the correlation between OCs and tobacco use.
Based on NHANES 2013–2018, we made a comprehensive analysis of the correlation between smoking and 422 diseases and found 46 SADs including 29 SPADs. Interestingly, we revealed 17 SNADs such as Parkinson’s disease, allergic rhinitis, and long-term use of hormonal contraceptives. It is noted that we put forward some previously not well-known Smoking-Associated Diseases, which require further research. Therefore, we constructed the online SDCD and summed up the correlation data between 422 diseases and smoking, making it easy to find. Our SDCD proves the importance of a large database in smoking research. These results facilitated our understanding of the correlation between smoking and diseases, providing the basis for studying the correlation between smoking and diseases.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://chatgptmodel.shinyapps.io/sdcd/.
The studies involving humans were approved by the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
XC: Formal analysis, Methodology, Software, Writing – original draft. TW: Formal analysis, Visualization, Writing – original draft. YT: Data curation, Resources, Writing – review & editing. YM: Writing – review & editing. YL: Writing – review & editing. HC: Funding acquisition, Writing – review & editing. HH: Funding acquisition, Writing – review & editing. QH: Writing – review & editing, Funding acquisition. MC: Writing – review & editing, Conceptualization, Project administration.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Significant Science and Technology Project of Beijing Life Science Academy (grant number 2023000CA0040); the National Natural Science Foundation of China (grant number 81603119); the Natural Science Foundation of Beijing Municipality (grant number 7174316); the Peking University Medicine Seed Fund for Interdisciplinary Research supported by “the Fundamental Research Funds for the Central Universities” (grant number Nos. BMU2022MX017 and BMU2022MX003).
We would like to thank Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database, which makes it easier for us to explore. Thanks to those who contributed to NHANES data, including all participants in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
SDCD, Smoking-diseases correlation database; SAD, Smoking-associated diseases; SPAD, Smoking positively associated diseases; SNAD, Smoking negatively associated diseases; NHANES, National Health and Nutrition Examination Surveys.
1. Goldenberg, M, Danovitch, I, and Ishak, WW. Quality of life and smoking. Am J Addict. (2014) 23:540–62. doi: 10.1111/j.1521-0391.2014.12148.x
2. Jha, P, Ramasundarahettige, C, Landsman, V, Rostron, B, Thun, M, Anderson, RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. (2013) 368:341–50. doi: 10.1056/nejmsa1211128
3. World Health Organisation WHO global report on trends in prevalence of tobacco use 2000–2025. 4th Edn. World Health Organization, Geneva. (2021).
4. Aj, A, Dr, S, and Km, C. The 2014 surgeon General’s report: commemorating the 50th anniversary of the 1964 report of the advisory committee to the US surgeon general and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. (2014) 179:403–12. doi: 10.1093/aje/kwt335
5. Benowitz, NL, and Liakoni, E. Tobacco use disorder and cardiovascular health. Addiction. (2022) 117:1128–38. doi: 10.1111/add.15703
6. Saracen, A . Cigarette smoking and respiratory system diseases in adolescents. Adv Exp Med Biol. (2017) 944:81–5. doi: 10.1007/5584_2016_60
7. Campos, MW, Serebrisky, D, and Castaldelli-Maia, JM. Smoking and cognition. Curr Drug Abuse Rev. (2016) 9:76–9. doi: 10.2174/1874473709666160803101633
8. Penzias, A, Bendikson, K, Butts, S, Coutifaris, C, Falcone, T, Gitlin, S, et al. Smoking and infertility: a committee opinion. Fertil Steril. (2018) 110:611–8. doi: 10.1016/j.fertnstert.2018.06.016
9. Qiu, F, Liang, C-L, Liu, H, Zeng, Y-Q, Hou, S, Huang, S, et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. (2017) 8:268–84. doi: 10.18632/oncotarget.13613
10. Mackenbach, JP, Damhuis, RA, and Been, JV. The effects of smoking on health: growth of knowledge reveals even grimmer picture. Ned Tijdschr Geneeskd. (2017) 160:D869.
11. Ma, C, Liu, Y, Neumann, S, and Gao, X. Nicotine from cigarette smoking and diet and Parkinson disease: a review. Transl Neurodegener. (2017) 6:18. doi: 10.1186/s40035-017-0090-8
12. Felson, DT, and Zhang, Y. Smoking and osteoarthritis: a review of the evidence and its implications. Osteoarthr Cartil. (2015) 23:331–3. doi: 10.1016/j.joca.2014.11.022
13. Onmaz, M, Demirbas, N, Eryavuz Onmaz, D, Kutlu, R, and Unlu, A. Effect of cigarette smoking on serum methylarginine and α-klotho levels. Nutr Metab Cardiovasc Dis. (2023) 33:602–9. doi: 10.1016/j.numecd.2022.12.020
14. Christenson, SA, Smith, BM, Bafadhel, M, and Putcha, N. Chronic obstructive pulmonary disease. Lancet. (2022) 399:2227–42. doi: 10.1016/S0140-6736(22)00470-6
15. Maremanda, KP, Sundar, IK, and Rahman, I. Role of inner mitochondrial protein OPA1 in mitochondrial dysfunction by tobacco smoking and in the pathogenesis of COPD. Redox Biol. (2021) 45:102055. doi: 10.1016/j.redox.2021.102055
16. Agustí, A, Vogelmeier, C, and Faner, R. COPD 2020: changes and challenges. Am J Phys Lung Cell Mol Phys. (2020) 319:L879–83. doi: 10.1152/ajplung.00429.2020
17. Chen, J, Chen, R, Xiang, S, Li, N, Gao, C, Wu, C, et al. Cigarette smoking and schizophrenia: Mendelian randomisation study. Br J Psychiatry. (2021) 218:98–103. doi: 10.1192/bjp.2020.116
18. Coustals, N, Martelli, C, Brunet-Lecomte, M, Petillion, A, Romeo, B, and Benyamina, A. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. (2020) 222:113–21. doi: 10.1016/j.schres.2020.03.071
19. Sagud, M, Mihaljevic Peles, A, and Pivac, N. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. (2019) 32:402–8. doi: 10.1097/YCO.0000000000000529
20. Goodwin, RD . Next steps toward understanding the relationship between cigarette smoking and depression/anxiety disorders: a Lifecourse perspective. Nicotine Tob Res. (2017) 19:1–2. doi: 10.1093/ntr/ntw296
21. Zale, EL, Maisto, SA, and Ditre, JW. Anxiety and depression in bidirectional relations between pain and smoking: implications for smoking cessation. Behav Modif. (2016) 40:7–28. doi: 10.1177/0145445515610744
22. LaRowe, LR, and Ditre, JW. Pain, nicotine, and tobacco smoking: current state of the science. Pain. (2020) 161:1688–93. doi: 10.1097/j.pain.0000000000001874
23. Ditre, JW, Brandon, TH, Zale, EL, and Meagher, MM. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. (2011) 137:1065–93. doi: 10.1037/a0025544
24. Ascherio, A, and Schwarzschild, MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. (2016) 15:1257–72. doi: 10.1016/S1474-4422(16)30230-7
25. Belvisi, D, Pellicciari, R, Fabbrini, G, Tinazzi, M, Berardelli, A, and Defazio, G. Modifiable risk and protective factors in disease development, progression and clinical subtypes of Parkinson’s disease: what do prospective studies suggest? Neurobiol Dis. (2020) 134:104671. doi: 10.1016/j.nbd.2019.104671
26. Wang, S, Qi, L, Wei, H, Jiang, F, and Yan, A. Smoking behavior might affect allergic rhinitis and vasomotor rhinitis differently: a mendelian randomization appraisal. World Allergy Organ J. (2022) 15:100630. doi: 10.1016/j.waojou.2022.100630
27. Allen, AM, Weinberger, AH, Wetherill, RR, Howe, CL, and McKee, SA. Oral contraceptives and cigarette smoking: a review of the literature and future directions. Nicotine Tob Res. (2017) 21:592–601. doi: 10.1093/ntr/ntx258
28. Tosun, NL, Fieberg, AM, Eberly, LE, Harrison, KA, Tipp, AR, Allen, AM, et al. Exogenous progesterone for smoking cessation in men and women: a pilot double-blind, placebo-controlled randomized clinical trial. Addiction. (2019) 114:1800–13. doi: 10.1111/add.14645
Keywords: smoking, smoking-diseases correlation database, smoking-associated diseases, smoking positively associated diseases, smoking negatively associated diseases
Citation: Chen X, Wang T, Tian Y, Ma Y, Liu Y, Chen H, Hou H, Hu Q and Chu M (2024) Smoking-diseases correlation database: comprehensive analysis of the correlation between smoking and 422 diseases based on NHANES 2013–2018. Front. Public Health. 12:1325856. doi: 10.3389/fpubh.2024.1325856
Received: 22 October 2023; Accepted: 24 May 2024;
Published: 07 June 2024.
Edited by:
Mohammad Javad Mohammadi, Ahvaz Jundishapur University of Medical Sciences, IranReviewed by:
Shirley Ann James, University of Oklahoma Health Sciences Center, United StatesCopyright © 2024 Chen, Wang, Tian, Ma, Liu, Chen, Hou, Hu and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Chen, aHVubnlfY2hAMTYzLmNvbQ==; Hongwei Hou, cXNmY3RjQDE2My5jb20=; Qingyuan Hu, aHVxeTE5NjVAMTYzLmNvbQ==; Ming Chu, ZmFtb3VzQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.