- 1Department of Tuberculosis, Shandong Public Health Clinical Center, Shandong University, Jinan, Shandong, China
- 2Department of Tuberculosis, Qingdao Chest Hospital, Qingdao, Shandong, China
- 3Department of Tuberculosis, Weifang Second People's Hospital, Weifang, Shandong, China
- 4Department of Tuberculosis, Dezhou Second People's Hospital, Dezhou, Shandong, China
- 5Department of Tuberculosis, Yantai Pulmonary Hospital, Yantai, Shandong, China
- 6Department of Internal Medicine, Zaozhuang Tumor Hospital, Zaozhuang, Shandong, China
- 7Department of Tuberculosis, Liaocheng Infectious Disease Hospital, Liaocheng, Shandong, China
- 8Department of Respiratory Medicine, Tai'an Tumor Prevention and Treatment Hospital, Tai'an, Shandong, China
- 9Department of Tuberculosis, Linyi People's Hospital, Linyi, Shandong, China
- 10Department of Tuberculosis, Binzhou Central Hospital, Binzhou, Shandong, China
- 11Department of Internal Medicine, Zibo First Hospital, Zibo, Shandong, China
- 12Third Department of Respiratory Medicine, Yantai Beihai Hospital, Yantai, Shandong, China
- 13Respiratory Center, Shandong Public Health Clinical Center, Shandong University, Jinan, Shandong, China
Objective: To investigate the positivity rates and drug resistance characteristics of Mycobacterium tuberculosis (MTB) among suspected tuberculosis (TB) patients in Shandong Province, the second-largest population province in China.
Methods: A prospective, multi-center study was conducted from April 2022 to June 2023. Pathogen and drug resistance were identified using nucleotide matrix-assisted laser desorption ionization time-of-flight mass spectrometry (nucleotide MALDI-TOF MS).
Results: Of 940 suspected TB patients included in this study, 552 cases were found to be infected with MTB giving an overall positivity rate of 58.72%. Total of 346 cases were resistant to arbitrary anti-TB drug (62.68%), with Zibo (76.47%), Liaocheng and Weihai (both 69.23%) ranking top three and TB treatment history might be a related factor. Monoresistance was the most common pattern (33.53%), with isoniazid the highest at 12.43%, followed by rifampicin at 9.54%. Further analysis of gene mutations conferring resistance revealed diverse types with high heteroresistance rate found in multiple anti-TB drugs.
Conclusion: A relatively high rate of MTB positivity and drug resistance was found in Shandong Province during and after the COVID-19 pandemic, indicating the need for strengthening rapid identification of species and drug resistance among suspected TB patients to guide better medication and minimize the occurrence of drug resistance.
1 Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), remains a global public health event affecting public health worldwide. Approximately 10 million people are still afflicted with TB each year, resulting in around 1.5 million deaths. China continues to be a high-burden country for TB. According to the 2022 World Health Organization (WHO) Tuberculosis Report, over one-third of the TB cases in 2021 were concentrated in India and China, accounting for 28 and 7.4%, respectively (1). The situation for TB prevention and control has become more severe with the emergence and spread of drug-resistant strains. Treatment of drug-resistant TB (DR-TB) is prolonged, comes with significant side effects, and poses a heavy economic burden (2). Moreover, with extension of treatment time, the risk of infection with non-tuberculous mycobacteria (NTM) also increases (3). Rapid and accurate diagnosis of MTB infection and drug resistance will contribute to the timely implementation of precise treatment and control of TB transmission.
Shandong Province is located in the eastern coastal region of China, with a population of over 100 million, ranked second-most populous province. In 1992, Shandong Province began to implement the WHO-recommended directly observed treatment, short-course (DOTS) strategy for TB control. By 1997, the DOTS strategy had achieved full coverage at the county level in Shandong Province. The incidence rate of TB decreased from 40.8 per 100,000 in 2005 to 26.25 per 100,000 in 2017 (4). The reported incidence of MTB per 100,000 people in Shandong was 20.60 in 2022. According to a study by Song et al. in 2018, the prevalence rate of mono-resistant TB (MR-TB), multidrug-resistant TB (MDR-TB), and extensively drug-resistant TB (XDR-TB) among newly diagnosed TB patients in Shandong Province were 13.35, 3.73, and 4.30%, respectively (5).
Although several published studies have described the epidemiology of TB in Shandong Province (4, 5), there is still limited prospective research on the prevalence and characteristics of TB and DR-TB in recent years, particularly during and after the COVID-19 pandemic. As the second-largest populous province in China, it is necessary to comprehensively characterize the recent trends and features of TB and DR-TB in Shandong Province, providing a theoretical basis for better prevention and control of TB and DR-TB in this region.
The matrix-assisted laser desorption ionization time-of-flight mass spectrometry based on nucleic acid extracted from clinical samples (nucleotide MALDI-TOF MS) is a novel molecular diagnostic technology (6). By performing multiple PCR-specific amplifications of target gene segments and undergoing single nucleotide extension reactions, the reaction product is combined with matrix, and a mass spectrum is obtained finally, providing information on the species and drug resistance. Several studies have confirmed the high accuracy of nucleotide MALDI-TOF MS in identifying MTB and its drug resistance (6, 7). In recent years, this technique has been highly favored by clinicians due to its high accuracy, high throughput, rapidity, high resolution and low cost. Recently, China has also issued an expert consensus on the application of nucleotide MALDI-TOF MS in TB and NTM infection diseases (8), further recognizing the diagnostic capability of this technology.
In this study, we prospectively enrolled suspected TB patients from 12 designated TB hospitals in Shandong Province. Nucleotide MALDI-TOF MS on clinical samples from participants was applied for identification of MTB and its resistance to anti-TB drugs and basic information of them were simultaneously collected. Based on the results of nucleotide MALDI-TOF MS, we analyzed and characterized the positivity rates of TB and its drug resistance patterns among suspected TB patients in Shandong Province, providing theoretical support for the prevention and control of TB in Shandong Province.
2 Methods
2.1 Study design and study population
This is a prospective, cross-sectional, multi-center study conducted in Shandong Province, which is located in the eastern coastal region of China, bordered by the Bohai Sea and the Yellow Sea and composed of 16 prefecture-level cities, with an area of 157,100 square kilometers. Shandong Province has the second-largest population in China with over 100 million residents. Twelve designated TB hospitals participated in this study, including Public Health Clinical Center Affiliated to Shandong University, Yantai Pulmonary Hospital, Zibo First Hospital, Dezhou Second People's Hospital, Liaocheng Infectious Disease Hospital, Linyi People's Hospital, Qingdao Chest Hospital, Yantai Beihai Hospital, Zaozhuang Tumor Hospital, Binzhou Central Hospital, Tai'an Tumor Prevention and treatment Hospital, and Weifang Second People's Hospital. Supplementary Figure 1 displays the cities where the 12 participated hospitals are located. Considering Publich Health Clinical Center Affiliated to Shandong University is the largest TB designated hospital in Shandong Province, it nearly carries more than half of the TB patients' diagnosis and treatment, with many patients from other cities referring to the hospital themselves or being referred to the hospital. The other 11 hospitals are also responsible for most of the TB patients in their own city and the neighboring cities. Therefore, the population enrolled in this study could well represent the whole Shandong Province. Prior to the study, we provided standardized training to the principles of the 12 participating hospitals, including the purpose of this study, the procedures, sample handling, etc., to ensure, as far as possible, uniformity of operation across hospitals. All patients included in the study were seen at TB department specialized for TB care.
During the study period, trained and experienced clinicians conducted screening based on symptoms and signs of suspected TB and obtained informed consent from eligible individuals who met the inclusion criteria. Each participant was required to provide at least one sample, which was sent to Shanghai Conlight Medical Co., Ltd. for nucleotide MALDI-TOF MS assay. Basic information of each participant, including demographic and clinical characteristics, was collected simultaneously. Inclusion criteria: (1) individuals with suspected symptoms and signs of TB; (2) no severe illnesses; (3) no antibiotic use within the past 6 months; (4) willingness to participate in the study and sign the informed consent. Exclusion criteria: (1) presence of severe illnesses; (2) inability to provide informed consent. The severe illnesses in our study were defined by the presence of severe shortness of breath, significant impairment of daily activities and the high risk of death associated with a particular disease or condition.
2.2 Ethical approval
The study has obtained approval from the Ethics Committee of Public Health Clinical Center Affiliated to Shandong University (No. GWLCZXEC2022-74). All participants were provided informed consent, and all data were kept confidential. Research involving human subjects compiled with all relevant national regulations and is in accordance with the tenets of the Helsinki Declaration.
2.3 Sample size
Sample size was estimated according to the cross-sectional study type by taking MTB positivity rate among suspected TB patients in Shandong Province at 50%, with DR-TB accounting for about 50% of TB patients, 5% margin of error (d = 0.05) and 95% confidence interval (z = 1.96). The sample size was 922 by considering a 20% non-response rate. The final sample size we included was 940 based on our actual situation.
2.4 Sample collection
Enrolled patients were asked to provide eligible samples at any time within 7 days after their visit at hospital. The sample types included but were not limited to sputum, bronchoalveolar lavage fluid (BALF), pus, tissue, cerebrospinal fluid (CSF), and puncture fluid. Experienced clinicians collected these samples according to the requirements and performed appropriate processing procedures based on the sample type, preserving them in sample preservation solution. All of the samples were then sent to Shanghai Conlight Medical Co., Ltd. according to the sample transportation requirements for subsequent processing and testing.
2.5 Nucleotide MALDI-TOF MS detection
Nucleotide MALDI-TOF MS detection to assess the mycobacterial species and MTB drug sensitivity was based on Conlight TB&DR assay (7). After receiving the clinical specimens, the following experimental steps were conducted: (1) Sample DNA extraction, strictly following the manufacturer's instructions of the kit used; (2) PCR amplification, preparing PCR mix and adding it to the 96-well plate, followed by adding to the DNA template and running the PCR program accordingly; (3) Alkaline phosphatase (SAP) reaction, preparing the SAP mix and adding it to the 96-well plate to remove residual deoxynucleotide triphosphates; (4) Extension reaction, preparing extension primers and iPLEX extension mix, adding to the 96-well plate, and performing the iPLEX extension reaction; (5) Sample desalting and loading it into the chip plate for mass spectrometry detection in the instrument; (6) Analyzing the results of mass spectrometry. For each specific steps, the concrete information can be referred to the citation (7). The information on drug resistance associated gene loci designed by Shanghai Conlight Medical Co., Ltd. is provided in Supplementary Table 1.
2.6 Data collection and statistical analysis
Date analysis was performed using R software (R 4.2.0 version). Demographic and clinical information of the included patients were recorded in EXCEL spreadsheets and assessed with descriptive statistics. Continuous data were expressed as average with standard errors, whereas categorical data were expressed as numbers and percentages. For intergroup comparisons of categorical variables, chi-square test was carried out; for continuous variables, the Mann-Whitney U-test was employed. A two-tailed p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Characteristics of enrolled participants
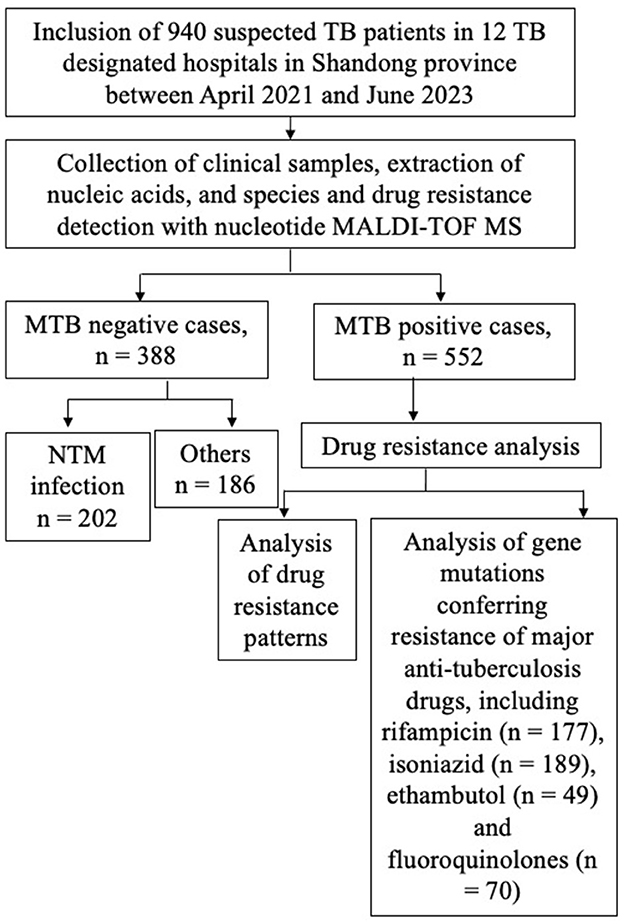
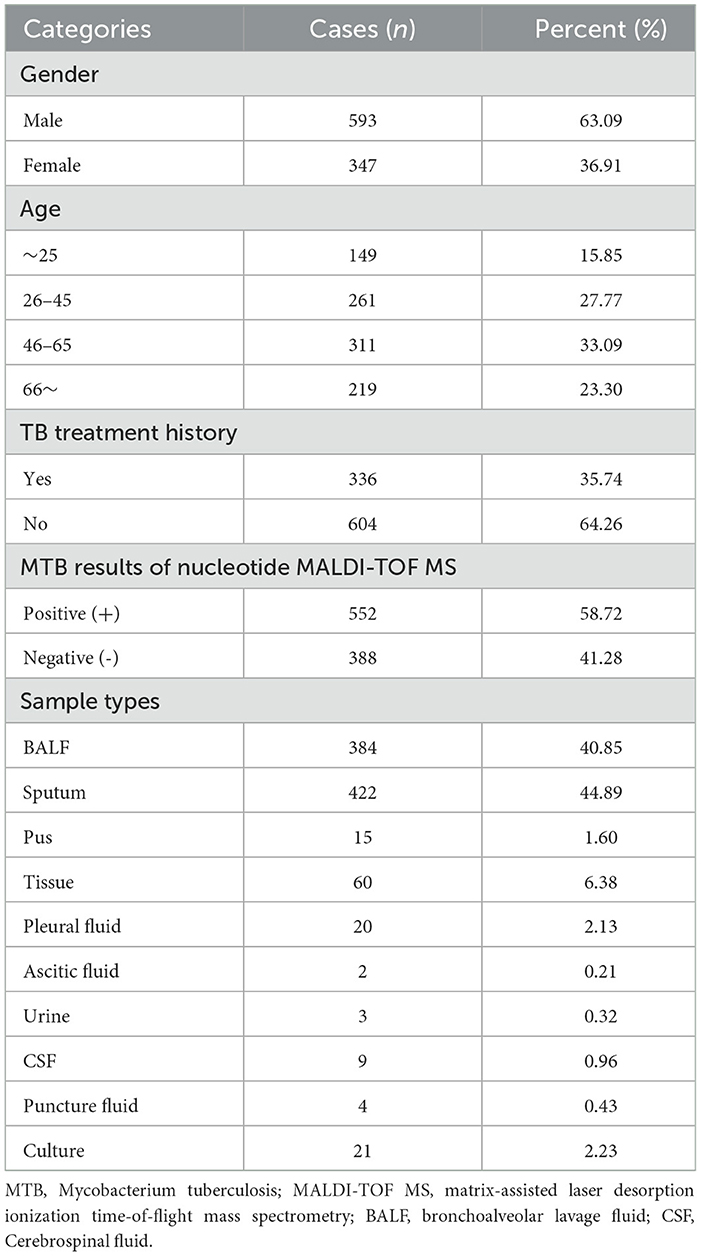
The flowchart of the study design is shown in Figure 1. In order to study the MTB positivity rates and characteristics of TB and DR-TB in Shandong Province, we prospectively included 940 suspected TB patients from 12 designated TB hospitals between April 2021 and June 2023, based on the inclusion criteria. Table 1 presents the basic information of the study population. Nearly two-thirds were male (63.09%), with an average age of 48.59 years (95% CI: 47.35–49.83 years), and an overall distribution was uniform across all ages, with the largest number of people in the 46–65 years (33.09%). A previous TB treatment history was found in 35.74% (336/940) of enrolled patients. The samples collected were diverse, with sputum being the most common (44.89%), followed by BALF samples at 40.85%, and tissue samples with 60 cases (6.38%). Other samples included pleural fluid (2.13%), pus (1.60%), nine cases of CSF, four cases of puncture fluid, and only three and two cases of urine and ascites, respectively. Based on the identification results by nucleotide MALDI-TOF MS assay, 552 cases (58.72%) were infected with MTB, classified as TB patients, with the remaining as non-TB patients (41.28%, 388/940).
3.2 Characteristics of TB patients in Shandong Province
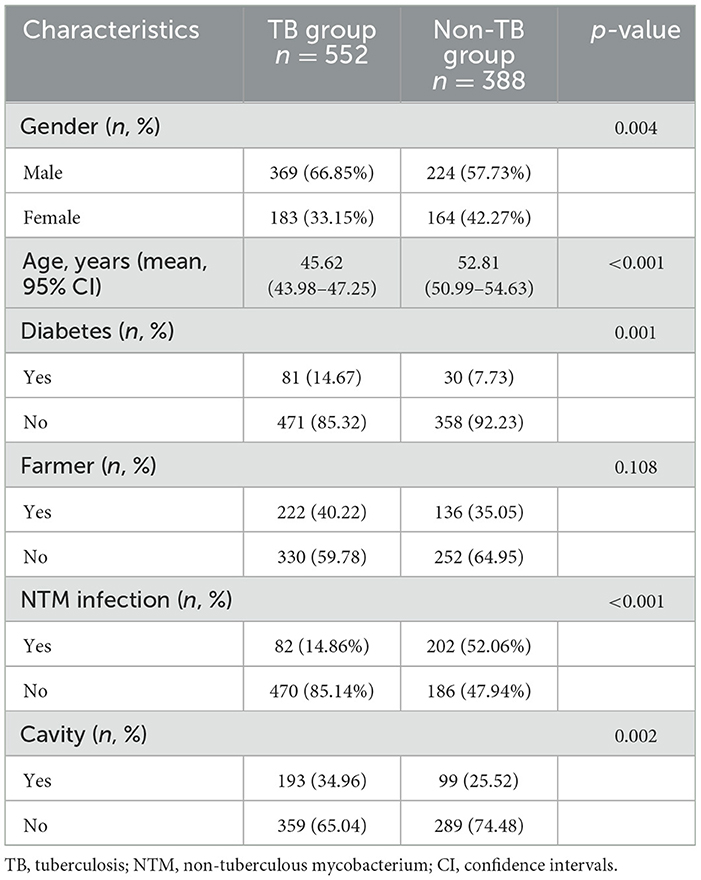
In order to characterize the basic features of TB patients in Shandong Province, we further compared the demographic and relevant clinical characteristics between TB patient and non-tuberculosis (non-TB) patients in the study population. There were significant differences between the TB group and non-TB group in terms of age, gender, comorbid diabetes, and radiological findings (Table 2). The TB group had a higher proportion of male patients and individuals in the younger to middle-aged group. Among TB group, 14.67% of patients had diabetes, while only 7.73% of cases were found in non-TB group. In terms of chest radiograph features, 34.96% of patients in TB group had cavities, where 25.52% in non-TB group. Additionally, 14.86% of patients were simultaneously infected with NTM in TB group, while 52.06% of cases had NTM infection in non-TB group, suggesting NTM infection is an important confounding factor in suspected TB patients where MTB is not detected.
3.3 Description of DR-TB characteristics and patterns in Shandong Province
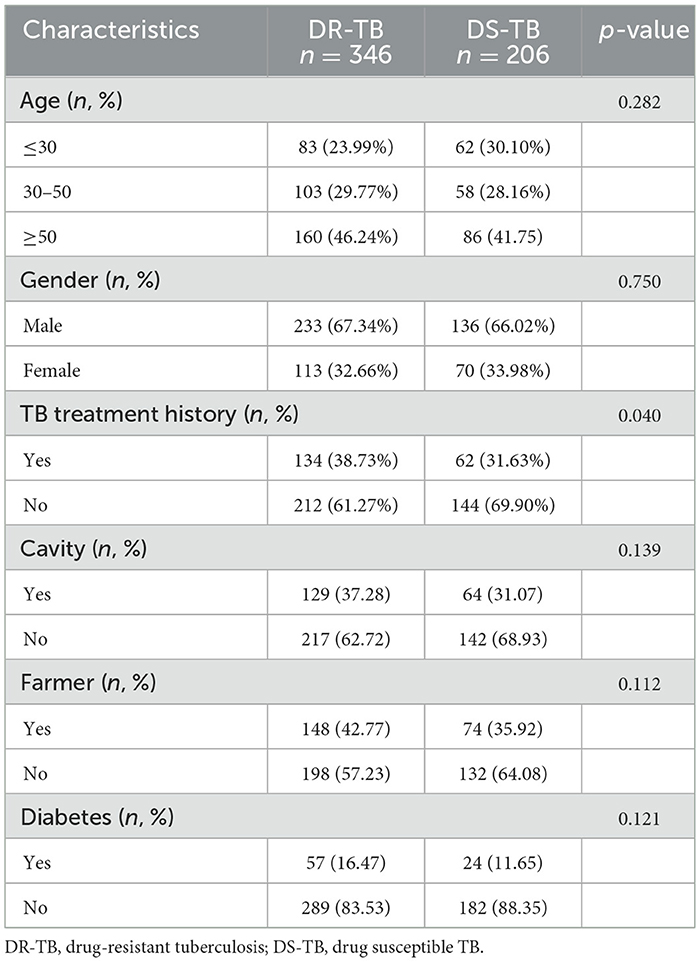
DR-TB has always been a challenge in the prevention and treatment of TB. Therefore, we further conducted a detailed description of the drug resistance status in the identified group of TB patients in this study. According to the results of nucleotide MALDI-TOF MS assay, MTB strains from 206 cases (37.32%) didn't show any mutations in the detected drug resistance associated gene loci, indicating drug susceptible TB (DS-TB) and the remaining 346 (62.68%) TB patients were resistant to at least one anti-TB drug, classified as DR-TB. Here, we compared the clinical characteristics of DR-TB and DS-TB patients (Table 3). Both groups showed no significant differences in terms of age, gender, cavities, diabetes, or occupation as farmers (Table 3). Only the previous TB treatment history was associated with drug resistance (p = 0.040; Table 3).
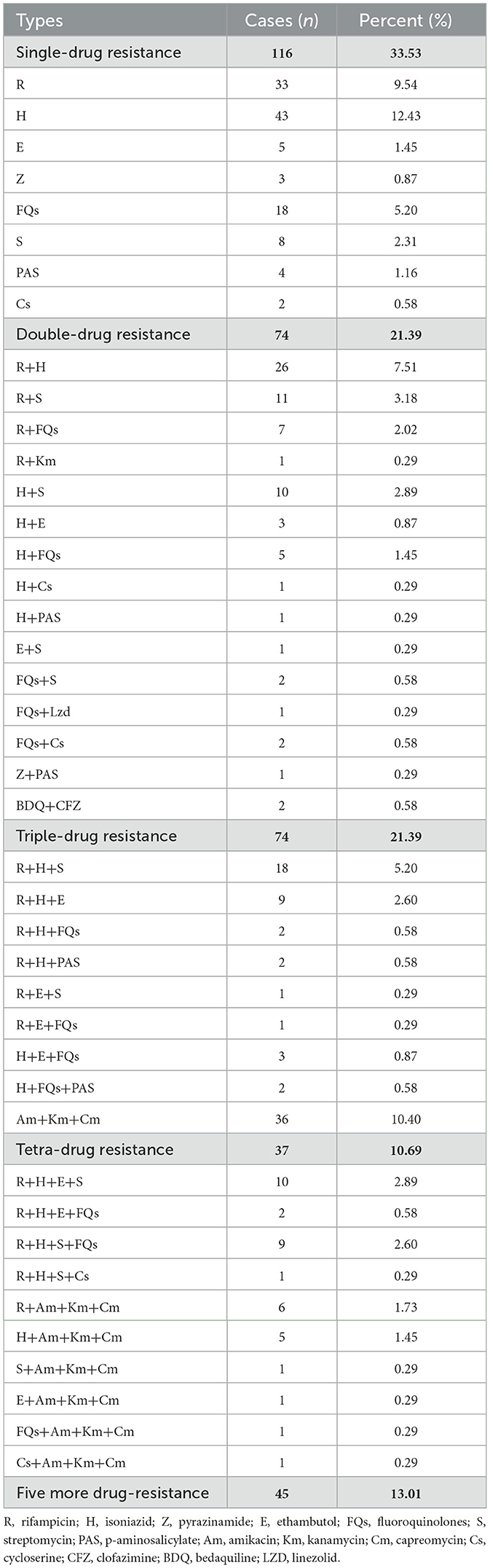
DR-TB in Shandong Province displayed diverse patterns of drug resistance, ranging from resistance to a single drug to over five drugs (Table 4). Mono-resistant TB was the most common, with a total of 116 cases, accounting for 33.53% (116/346) of all DR-TB cases. Among them, isoniazid mono-resistant TB was the most prevalent, accounting for 12.43% (43/346), followed by rifampicin mono-resistant TB (9.54%, 33/346), and fluoroquinolones mono-resistant TB at 5.20% (18/346). There were 74 cases (21.39%) of dual-drug resistance, the same as triple-drug resistance. In the dual-drug resistant cases, resistance to rifampicin combined with isoniazid was the most common, with 26 cases, followed by resistance to rifampicin combined with streptomycin, with 11 cases. In the triple-drug resistant cases, resistance to injectable drugs (amikacin + kanamycin + capreomycin) was the most common, with 36 cases. There were 37 cases (10.69%) of four-drug resistant TB and 45 cases (13.01%) of resistance to five or more drugs.
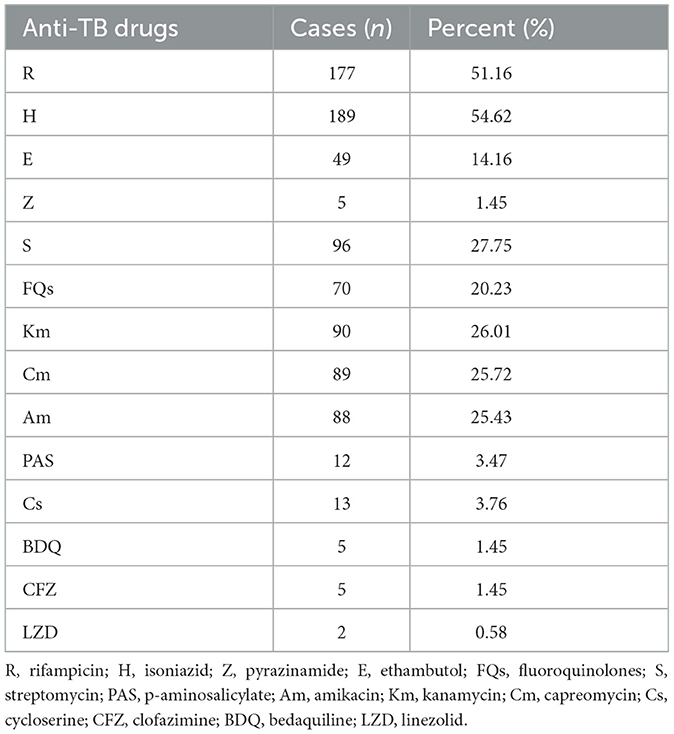
Analyzing the resistance to any single anti-TB drug among DR-TB in Shandong Province (Table 5), we found that isoniazid resistance was the highest (54.62%, 189/346), followed by rifampicin (51.16%, 177/346), and resistance to infectable drugs, including streptomycin, kanamycin, capreomycin and amikacin, was also relatively high, all above 25%. Of note, the resistance rate to fluoroquinolones, a core group of drugs for DR-TB, reached 20.23% (40/346). Five cases of resistance to the new drug, bedaquiline, were also observed.
3.4 Geographical distribution of MTB arbitrary drug resistance rates in Shandong Province
We further analyzed the MTB arbitrary drug resistance rates in the 16 prefecture-level cities of Shandong Province. The results showed that more than half of the cities had MTB arbitrary drug resistance rates higher than the average rate of Shandong Province (Figures 2A, B), with Zibo the highest (76.47%), followed by Liaocheng and Weihai (both 69.23%). Interestingly, these three cities are geographically located in the center, west and east respectively, with no concentration in one region (Figure 2B). Conversely, cities with lower MTB arbitrary drug resistance rates than the provincial average were concentrated in the southwestern of Shandong Province, with Binzhou lowest (45.45%), followed by Jining (50.00%) and Jinan (52.50%) (Figures 2A, B).
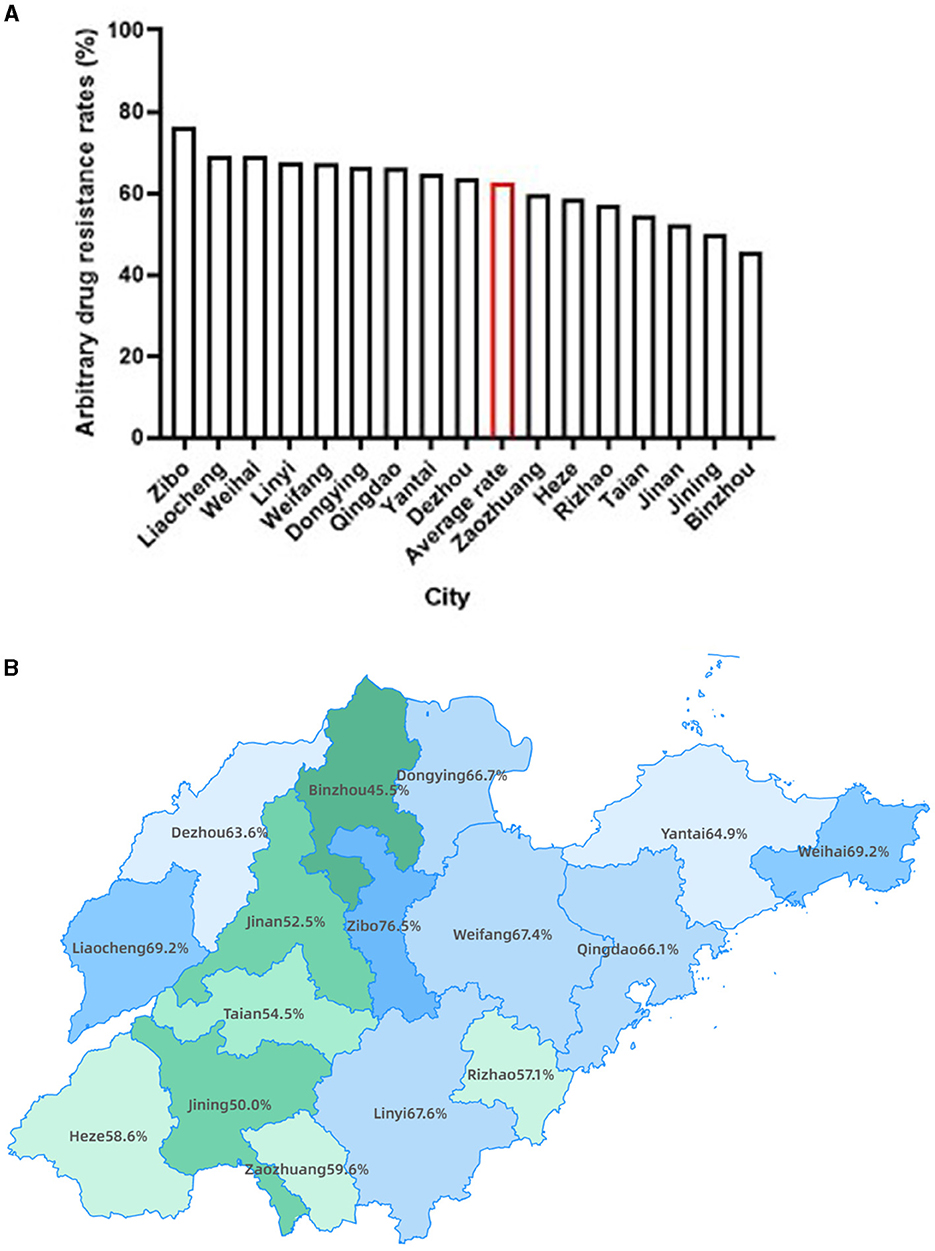
Figure 2. The distribution of MTB arbitrary drug resistance rates in Shandong province. (A) Bars presenting the MTB arbitrary drug resistance rate for each city of Shandong province, as well as the average rate of Shandong province highlighted with red. (B) Geographic distribution of MTB arbitrary drug resistance rate by city.
3.5 Molecular characteristics of drug-resistant MTB in Shandong Province
Finally, we compiled the molecular characteristics of all DR-TB isolates (refer to Supplementary Table 2 for details). Below, we provide a detailed description of the molecular characteristics of resistance to rifampicin, isoniazid, ethambutol, and fluoroquinolones.
Rifampicin: Rifampicin resistance is mainly concentrated in the rifampicin resistance-determining region (RRDR) of rpoB gene. Among the 177 rifampicin-resistant strains, rpoB codon 531 had the highest percentage of mutations (67.23%, 119/177), with five types of mutations detected and TCG > TTG (Ser > Leu) being the most common. rpoB codon 526 was found in 24 rifampicin- resistant strains, with a total of 11 different mutation types (Figure 3A). Heteroresistance (co-existence of wild-type and mutant strains) was detected at codons 511, 516, 526, 531, and 533, among which, the most common was codon 531 TCG, with 34 cases. The proportion of rifampicin heteroresistance was 37.85% (67/177) (Supplementary Table 2). We further conducted a more extensive examination of the geographical distribution of rifampicin-resistant cases. Notably, Yantai exhibited the highest incidence with a total of 28 cases, followed closely by Weifang and Qingdao, with 22 and 20 cases, respectively. It is worth mentioning that these three cities are situated in close proximity to one another within the eastern region of Shandong Province. However, Liaocheng city, located in the western part of the province, also reported 20 cases displaying resistance to rifampicin (Supplementary Figure 2A).
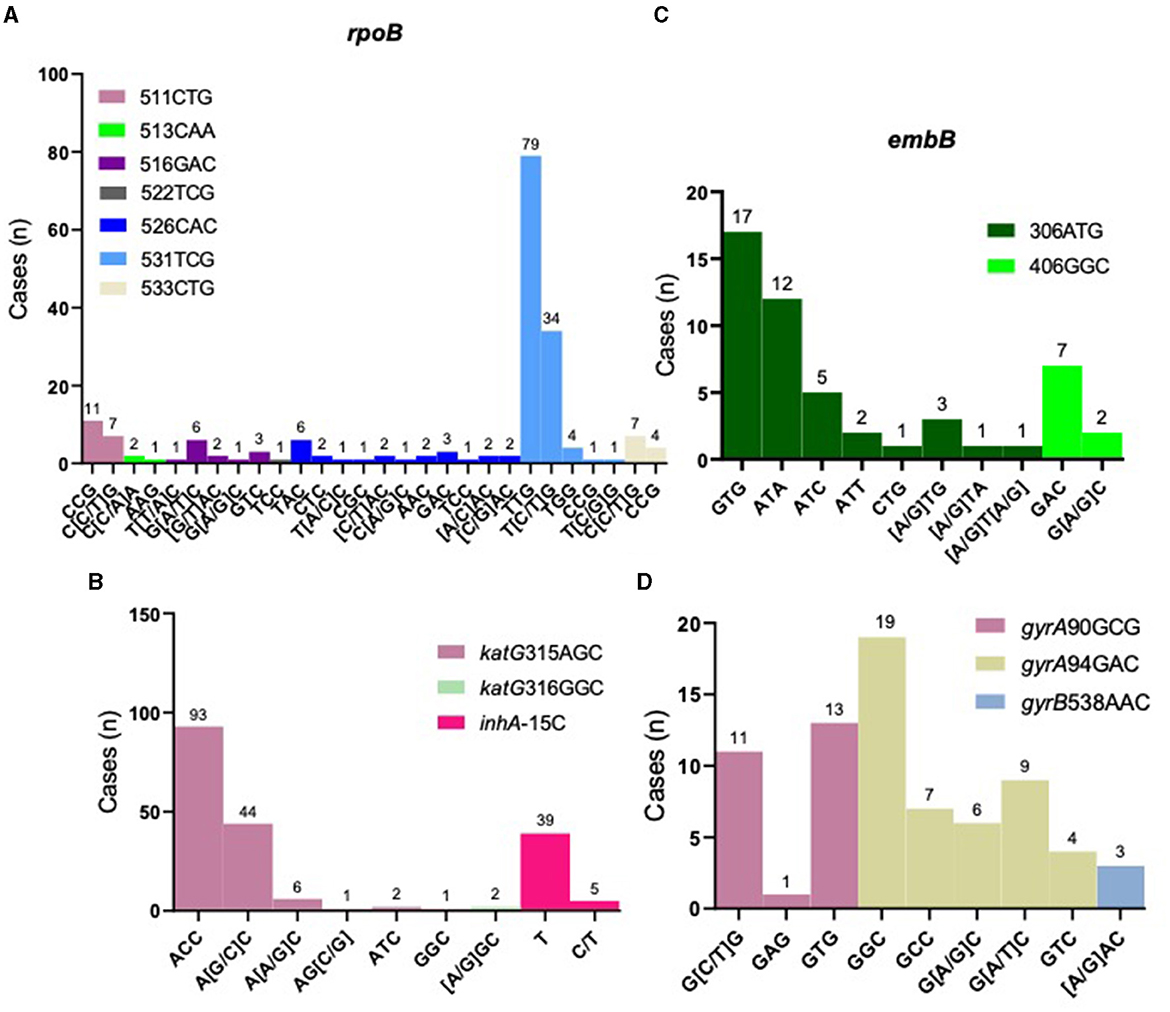
Figure 3. Distribution of resistance-related gene loci. Distribution of resistance loci mutations for rifampicin resistance-related gene, rpoB (A); isoniazid resistance-related genes, katG and inhA (B); ethambutol resistance-related gene, embB (C) and fluoroquinolones resistance-related genes, gyrA and gyrB (D).
Isoniazid: Isoniazid resistance is mainly concentrated in katG gene. Our results showed that katG codon 315 was the most common mutation conferring resistance, accounting for 77.78% (147/189), of which six types of mutations were found with AGC > ACC (Ser > Thr) being the most frequent. inhA C-15 mutation was found in 44 cases, representing 23.28% (44/189), two of which also harbored katG codon 315 mutation (Figure 3B and Supplementary Table 2). Isoniazid heteroresistance was present in 52 strains (27.51%, 52/189), with codon 315 AGC > AGC/ACC being the most prevalent (Supplementary Table 2).
Ethambutol: Ethambutol resistance is mainly associated with mutations in embB gene. Our results showed that embB codon 306 was the most frequent mutation, accounting for 85.71% (42/49), of which eight types of mutations were found, with ATG > GTG (Met > Val) being the most common (Figure 3C and Supplementary Table 2). Heteroresistance was detected in six cases (12.24%, 6/49) (Supplementary Table 2).
Fluoroquinolones: Resistance to fluoroquinolones is primarily associated with gyrA gene. Our results showed that gyrA codon 94 was the most frequent, accounting for 64.29% (45/70), of which five types of mutations were observed, with GCG > GGC (Ser > Leu) the most common (Figure 3D and Supplementary Table 2). Two strains with gyrB codon 538 mutation also harbored mutants in gyrA gene. Twenty-seven cases were found to have heteroresistance, possessing 38.57% (27/70) (Supplementary Table 2).
4 Discussion
Although there have been several studies reporting the prevalence and drug resistance of TB from various aspects in Shandong Province (5, 9, 10), most of them are retrospective studies, and the data were collected before the outbreak of the COVID-19 pandemic. Currently, there is still a lack of multi-center prospective studies characterizing the detection and drug resistance patterns of MTB in suspected TB patients in Shandong Province during and after the COVID-19 pandemic. In this study, we conducted a multi-center prospective study in Shandong Province to assess the infection rate and drug resistance of MTB in suspected TB patients based on nucleotide MALDI-TOF MS assay. Our results revealed an overall MTB positivity rate was 58.72% among suspected TB patients and relatively high proportion of DR-TB cases (62.68%) in Shandong Province during and after the COVID-19 pandemic, with Zibo, Liaocheng, and Weihai ranking top three.
The results of our study showed much higher MTB arbitrary drug resistance rate as compared with the previous report (5). There were several contributing factors. The period in which this study was conducted was during and at the end of the COVID-19 pandemic, where many patients were more difficult with access to a doctor, and most of them came to the hospital when their symptoms had progressed to the point where they were very obvious and difficult to control, with more complex. Secondly, many of the patients in our study had TB treatment history. To some extent, this study suggests that the COVID-19 pandemic might have some impact on TB drug resistance. Our findings of high MTB arbitrary drug resistance rates of TB patients in Shandong Province might partially attributed to the previous TB treatment history. It was further found by geographical distribution that more patients with TB treatment history existed in areas with high arbitrary drug resistance rates. We found cities with lower MTB arbitrary drug resistance rates than the provincial average were concentrated in the southwestern of Shandong Province, which might be related to the high MTB positivity rates in these regions. Here, we also analyzed the MTB positivity rates in the 16 prefecture-level cities of Shandong Province and found the cities with high levels of MTB positivity rates were concentrated in the southwestern of Shandong Province (Supplementary Figures 3A, B). Furthermore, we also analyzed the geographic distribution of TB patients resistant to three or more anti-TB drugs. The findings revealed that Yantai and Qingdao, located in the eastern part of Shandong Province, exhibited the highest incidence, followed by Dezhou in the west and Linyi, also in the east (Supplementary Figure 4). Based on the integrated data analysis of this study, a high TB positivity and drug resistance rate during and after the COVID-19 pandemic in Shandong Province can be noticed, which also give us some insights: (1) The timely diagnosis of TB is of utmost important, necessitating increased emphasis on the prompt detection of suspected TB cases to facilitate early diagnosis and treatment, thereby impeding its further transmission; (2) The assessment of TB patients' resistance to anti-TB drugs assumes significant important, particularly for key first-line drugs such as rifampicin and isoniazid, to ensure their precise administration and enhance the efficacy of treatment; (3) Cities exhibiting elevated levels of drug resistance warrant considerable attention, with a specific focus on promoting the rational and accurate utilization of antibiotics.
In recent years, nucleotide MALDI-TOF MS assay has been widely applied in various fields such as tumor, genetic diseases, bacterial and viral typing (11–13), including identification of MTB and its drug resistance (7, 14). Multiple studies have demonstrated that nucleotide MALDI-TOF MS assay performs well in TB diagnosis and drug resistance identification, with excellent sensitivity, specificity, and accuracy (15). Due to its high sensitivity, precision, high throughput, low cost, user-friendliness, and ease of analysis, more and more clinicians have recognized the clinical value of this technology. Moreover, China has released an expert consensus on the clinical application of nucleotide MALDI-TOF MS assay in TB and NTM diseases, recently (8). In this study, we utilized this technique to determine the etiology and drug resistance of clinically suspected TB patients in Shandong Province. The nucleotide MALDI-TOF MS assay was designed with multiple target genes for MTB identification, including commonly used genes such as IS6110, IS1081, and gyrB (16, 17), which to some extent increased the sensitivity of MTB detection. The results of this study showed a prevalence rate of MTB of 58.72% among suspected TB patients in Shandong Province, which is close to the reported MTB detection rate (59.3%) based on clinical diagnosis (18).
According to existing research reports, nucleotide MALDI-TOF MS assay exhibits high sensitivity, specificity, and concordance with phenotypic drug susceptibility testing results for various anti-TB drugs, especially for first-line drugs rifampicin and isoniazid (7). Our study shows that drug resistance to these two drugs among TB patients are both over 50%, possibly due to patients' non-compliant use of drugs or direct infection with drug-resistant MTB (19). In addition, a substantial number of patients have more complicated conditions and may also have a history of other infections for which similar antibiotics have been used. Currently, isoniazid mono-resistant TB is emerging as a growing global public health concern due to its association with poor treatment outcomes (20). The incidence of isoniazid mono-resistant TB is increasing progressively more frequently than rifampicin mono-resistant TB, and an estimated 11% of TB patients are isoniazid-resistant and rifampicin-sensitive, globally (21). Our study yielded similar results, showing that the incidence of isoniazid mono-resistant TB was 12.43%, slightly higher than the global estimate but also higher than that of rifampicin mono-resistant TB, suggesting that we should pay attention to the detection of isoniazid resistance. Additionally, it is noteworthy that the resistance rate to fluoroquinolones is also relatively high, ranking third after rifampicin and isoniazid, although the proportion of patients with monoreistance to it is not very high. Fluoroquinolones are widely used not only for treating TB but also for other types of infectious diseases (22), which might contribute to the high fluoroquinolone resistance rate. Considering that fluoroquinolones are core drugs for treating DR-TB patients, we need to pay attention to and strengthen monitoring of its drug resistance.
In this study, we also analyzed the geographical distribution of rifampicin-resistant cases, revealing that the three neighboring cities in the east had the highest number of cases, while Liaocheng in the west also contained 20 cases. We attempted to analyze rpoB mutations in the top six cities with the highest number of rifampicin-resistant cases. Among the four cities located in the east of Shandong Province, Weifang displayed the highest occurrence of mutated loci in rpoB, with mutations detected in all codons except for codon 513, which was exclusively found in Dezhou in the western region (Supplementary Figure 2B). Our results show that rpoB mutations that result in rifampicin resistance differed in different cities of Shandong Province.
Heteroresistance in TB has always been a matter of clinical concern (23), and there are differing opinions on the treatment of patients with heterogeneous DR-TB. Some believe that drugs corresponding to the resistant strains can still be used to kill the drug-resistant strains through increasing drug dosage. On the other hand, some argue that drugs corresponding to heterogeneous drug-resistant strains should not be continued, as doing so may exacerbate drug resistance, and under drug pressure, MTB could evolve into dominant drug-resistant strains. A consensus on how to treat patients with heterogeneous DR-TB has not been reached in clinical practice. Nucleotide MALDI-TOF MS assay has advantages in detecting heterogeneous drug resistance. Zhang et al. have reported this technology can detect 1% heteroresistance (24), reaching the level of phenotypic test, much higher than other common rapid molecular diagnostic methods, such as Xpert MTB/RIF, melting curve analysis and conventional sequencing (25). In addition, it is possible to achieve a detection limit of 0.1% through improvements and optimizations of nucleotide MALDI-TOF MS assay (26). Our results showed that MTB exhibited a certain proportion of heteroresistance to various anti-TB drugs, with rifampicin and fluoroquinolones the most significant rates of heteroresistance at 37.85 and 38.57%, respectively. Faye et al., have found the phenomenon of heteroresistance rate in the Rural Eastern Cape Province increased over time, from 9.6% in 2018 to 32.2% in 2020 (27). Recent study has characterized the trend of MTB drug resistance changes in a few TB patients during the treatment process and the proportion of drug-resistant strains indeed increases in some cases as treatment progresses, leading to complete drug resistance in later stages (22). If very low proportions of drug-resistant strains can be detected early and appropriate measures are taken to eliminate these strains, it may improve the success rate of subsequent treatment. Further related research is needed to confirm this.
Through nucleotide MALDI-TOF MS assay, we can also detect NTM infections. Our study showed that 14.86% of TB patients were infected with NTM, with Mycobacterium intracellulare being the leading species (data not shown), which is consistent with previous research findings (28). We also detected NTM infections in 52.06% of cases among non-TB group, indicating a relatively high NTM infection rate among suspected TB patients, which requires our attention. Indeed, with changes in people's lifestyles, and increase in sub-healthy and older-adult populations, and continuous progress in socio-economic and medical systems, the incidence of NTM infections has been on the rise, and NTM infected pulmonary diseases are gradually attracting clinical attention (29). Our results of this study suggest the risk of NTM infection among TB patients in Shandong Province, as well as the possibility of NTM infection among suspected TB patients, which should be given much attention, especially in cases with poor TB treatment outcomes.
The biggest strength of this study is its large sample size and multi-center prospective design, which provides a certain level of representativeness. However, we also acknowledge that the detection technology has some limitations. First, nucleotide MALDI-TOF MS assay is designed based on the current understanding of drug-resistant genes (30), and it is restricted to the detection of known drug-resistant loci. As a result, this study may have underestimated the resistance rates of certain drugs to some extent. Second, the assay does not systematically assess the accuracy of sample types other than respiratory specimens. However, the vast majority of samples included in this study were respiratory samples, making the impact more limited. Third, the assay was used for one sample per patient. However, the lab conducted three replicates to ensure accuracy for each sample.
In summary, this study utilized nucleotide MALDI-TOF MS assay to assess the MTB positivity rate and drug resistance among suspected TB patients in Shandong Province. The MTB positivity rate was found to be 58.72%, and the overall drug resistance rate was 62.68%, among which, the southwestern regions had lower MTB arbitrary drug resistance rates. The resistance rate for isoniazid and rifampicin were at high level, and fluoroquinolone drug resistance rate was also relatively high. Heteroresistance was common, with rifampicin and fluoroquinolones the most frequent. These results suggest that relevant authorities in Shandong Province should strengthen monitoring and management in cities with high MTB positivity rates and enhance drug resistance test for key drugs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Public Health Clinical Center Affiliated to Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
XG: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. TL: Methodology, Writing – original draft. WH: Data curation, Writing – review & editing. YX: Data curation, Writing – review & editing. SX: Investigation, Methodology, Writing – review & editing. HM: Investigation, Methodology, Writing – review & editing. QW: Formal analysis, Writing – review & editing. QZ: Investigation, Methodology, Writing – review & editing. GY: Investigation, Methodology, Writing – review & editing. DX: Formal analysis, Writing – review & editing. PJ: Investigation, Methodology, Writing – review & editing. HW: Investigation, Methodology, Writing – review & editing. MLin: Investigation, Methodology, Writing – review & editing. MLiu: Investigation, Methodology, Writing – review & editing. MN: Investigation, Methodology, Writing – review & editing. DW: Investigation, Methodology, Writing – review & editing. YL: Investigation, Methodology, Writing – review & editing. LJ: Conceptualization, Data curation, Writing – review & editing. CD: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ZZ: Conceptualization, Data curation, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Key Technology Research and Development Program of Shandong under Grant (No. 2021SFGC0504). The Foundation had no influence on this study other than provision of funding.
Acknowledgments
We thank all of the staff at 12 hospitals that participated in this study. Chengchen Huang and Jingfeng Tong from Shanghai Conlight Medical Co., Ltd. are acknowledged for their help of nucleotide MALDI-TOF MS results collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1322426/full#supplementary-material
References
1. World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization (2022). Available online at: https://www.who.int/publications/i/item/9789240061729
2. Lelisa Duga A, Salvo F, Kay A, Figueras A. Safety profile of medicines used for the treatment of drug-resistant tuberculosis: a descriptive study based on the WHO database (VigiBase®). Antibiotics. (2023) 12:50811. doi: 10.3390/antibiotics12050811
3. Huang M, Tan Y, Zhang X, Wang Y, Su B, Xue Z, et al. Effect of mixed infections with Mycobacterium tuberculosis and nontuberculous mycobacteria on diagnosis of multidrug-resistant tuberculosis: a retrospective multicentre study in China. Infect Drug Resist. (2022) 15:157–66. doi: 10.2147/IDR.S341817
4. Tao N-N, Li Y-F, Wang S-S, Liu Y-X, Liu J-Y, Song W-M, et al. Epidemiological characteristics of pulmonary tuberculosis in Shandong, China, 2005–2017. Medicine. (2019) 98:e15778. doi: 10.1097/MD.0000000000015778
5. Song WM, Li YF, Ma XB, Liu JY, Tao NN, Liu Y, et al. Primary drug resistance of mycobacterium tuberculosis in Shandong, China, 2004-2018. Respir Res. (2019) 20:3. doi: 10.1186/s12931-019-1199-3
6. Su KY, Yan BS, Chiu HC, Yu CJ, Chang SY, Jou R, et al. Rapid sputum multiplex detection of the M. tuberculosis complex (MTBC) and resistance mutations for eight antibiotics by nucleotide MALDI-TOF MS. Sci Rep. (2017) 7:1–10. doi: 10.1038/srep41486
7. Wu X, Tan G, Yang J, Guo Y, Huang C, Sha W, et al. Prediction of Mycobacterium tuberculosis drug resistance by nucleotide MALDI-TOF-MS. Int J Infect Dis. (2022) 121:47–54. doi: 10.1016/j.ijid.2022.04.061
8. Liang J, Wu X, An H. Expert consensus on the clinical application of nucleic acid MALDI-TOF MS technique in the diagnosis of tuberculosis and non-tuberculosis mycobacteriosis. Chin J Antituberc. (2023) 45:545–58. doi: 10.19982/j.issn.1000-6621.20230113
9. An Q, Song W, Liu J, Tao N, Liu Y, Zhang Q, et al. Primary drug-resistance pattern and trend in elderly tuberculosis patients in Shandong, China, from 2004 to 2019. Infect Drug Resist. (2020) 13:4133–45. doi: 10.2147/IDR.S277203
10. Tao NN, Li YF, Song WM, Liu JY, Zhang QY, Xu TT, et al. Risk factors for drug-resistant tuberculosis, the association between comorbidity status and drug-resistant patterns: a retrospective study of previously treated pulmonary tuberculosis in Shandong, China, during 2004–2019. Br Med J Open. (2021) 11:44349. doi: 10.1136/bmjopen-2020-044349
11. Cheung K-W, Peng Q, He L, Cai K, Jiang Q, Zhou B, et al. Rapid and simultaneous detection of major drug resistance mutations in reverse transcriptase gene for HIV-1 CRF01_AE, CRF07_BC and Subtype B in China using Sequenom MassARRAY® system. PLoS ONE. (2016) 11:e0153641. doi: 10.1371/journal.pone.0153641
12. Wu S, Wang M, Wang Y, Zhang M, He JQ. Polymorphisms in the STAT4 gene and tuberculosis susceptibility in a Chinese Han population. Microb Pathog. (2019) 128:288–93. doi: 10.1016/j.micpath.2019.01.024
13. Shah R, Sharma V, Bhat A, Singh H, Sharma I, Verma S, et al. MassARRAY analysis of twelve cancer related SNPs in esophageal squamous cell carcinoma in J&K, India. BMC Cancer. (2020) 20:1–6. doi: 10.1186/s12885-020-06991-2
14. Shi J, He G, Ning H, Wu L, Wu Z, Ye X, et al. Application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) in the detection of drug resistance of Mycobacterium tuberculosis in re-treated patients. Tuberculosis. (2022) 135:102209. doi: 10.1016/j.tube.2022.102209
15. Yang H, Li A, Dang L, Kang T, Ren F, Ma J, et al. A rapid, accurate, and low-cost method for detecting Mycobacterium tuberculosis and its drug-resistant genes in pulmonary tuberculosis: applications of MassARRAY DNA mass spectrometry. Front Microbiol. (2023) 14:1093745. doi: 10.3389/fmicb.2023.1093745
16. Tram TTB, Ha VTN, Trieu LPT, Ashton PM, Crawford ED, Thu DDA, et al. FLASH-TB: an application of next-generation CRISPR to detect drug resistant tuberculosis from direct sputum. J Clin Microbiol. (2023) 22:e0163422. doi: 10.1128/jcm.01634-22
17. Liu Z, Yang Y, Wang Q, Wang L, Nie W, Chu N. Diagnostic value of a nanopore sequencing assay of bronchoalveolar lavage fluid in pulmonary tuberculosis. BMC Pulm Med. (2023) 23:77. doi: 10.1186/s12890-023-02337-3
18. Bai W, Liu L, Wu L, Chen S, Wu S, Wang Z, et al. Assessing the utility of the Xpert Mycobacterium tuberculosis/rifampin assay for analysis of bronchoalveolar lavage fluid in patients with suspected pulmonary tuberculosis. J Clin Lab Anal. (2022) 36:24154. doi: 10.1002/jcla.24154
19. Shi T, Li T, Li J, Wang J, Zhang Z. Genetic diversity of drug resistant Mycobacterium tuberculosis in local area of Southwest China: a retrospective study. BMC Infect Dis. (2018) 18:3503. doi: 10.1186/s12879-018-3503-0
20. Alemu A, Bitew ZW, Diriba G, Seid G, Moga S, Abdella S, et al. Poor treatment outcome and associated risk factors among patients with isoniazid mono-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. (2023) 18:e0286194. doi: 10.1371/journal.pone.0286194
21. World Health Organization. Global Tuberculosis Report 2020. Geneva: World Health Organization (2020).
22. Perumal R, Khan A, Naidoo K, Ngema SL, Nandlal L, Padayatchi N, et al. Mycobacterium tuberculosis intra-host evolution among drug-resistant tuberculosis patients failing treatment. Infect Drug Resist. Volume. (2023) 16:2849–59. doi: 10.2147/IDR.S408976
23. Koch A, Cox H, Mizrahi V. Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment. Curr Opin Pharmacol. (2018) 42:7–15. doi: 10.1016/j.coph.2018.05.013
24. Zhang X, Zhao B, Huang H, Zhu Y, Peng J, Dai G, et al. Co-occurrence of amikacin-resistant and -susceptible Mycobacterium tuberculosis isolates in clinical samples from Beijing, China. J Antimicrob Chemother. (2013) 68:1537–42. doi: 10.1093/jac/dkt082
25. Hu Y, Chi Y, Feng X, Yu F, Li H, Shang Y, et al. Comparison of the diagnostic performance of MeltPro and next-generation sequencing in determining fluoroquinolone resistance in multidrug-resistant tuberculosis isolates. J Mol Diagnost. (2023) 25:342–51. doi: 10.1016/j.jmoldx.2023.02.003
26. Mosko MJ, Nakorchevsky AA, Flores E, Metzler H, Ehrich M, Van Den Boom DJ, et al. Ultrasensitive detection of multiplexed somatic mutations using MALDI-TOF mass spectrometry. J Mol Diagnost. (2016) 18:23–31. doi: 10.1016/j.jmoldx.2015.08.001
27. Faye LM, Hosu MC, Oostvogels S, Dippenaar A, Warren RM, Sineke N, et al. The detection of mutations and genotyping of drug-resistant Mycobacterium tuberculosis strains isolated from patients in the Rural Eastern Cape Province. Infect Dis Rep. (2023) 15:403–16. doi: 10.3390/idr15040041
28. Chen S, Wang F, Xue Y, Huo F, Jia J, Dong L, et al. Doubled nontuberculous mycobacteria isolation as a consequence of changes in the diagnosis algorithm. Infect Drug Resist. (2022) 15:3347–55. doi: 10.2147/IDR.S368671
29. Tan Y, Deng Y, Yan X, Liu F, Tan Y, Wang Q, et al. Nontuberculous mycobacterial pulmonary disease and associated risk factors in China: a prospective surveillance study. J Infect. (2021) 83:46–53. doi: 10.1016/j.jinf.2021.05.019
Keywords: tuberculosis, Shandong Province, nucleotide MALDI-TOF MS, drug resistance, mutation
Citation: Gao X, Li T, Han W, Xiong Y, Xu S, Ma H, Wang Q, Zhang Q, Yang G, Xie D, Jiang P, Wu H, Lin M, Liu M, Ni M, Wang D, Li Y, Jiao L, Ding C and Zhang Z (2024) The positivity rates and drug resistance patterns of Mycobacterium tuberculosis using nucleotide MALDI-TOF MS assay among suspected tuberculosis patients in Shandong, China: a multi-center prospective study. Front. Public Health 12:1322426. doi: 10.3389/fpubh.2024.1322426
Received: 16 October 2023; Accepted: 08 January 2024;
Published: 18 January 2024.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Jie Lu, Beijing Children's Hospital, ChinaQiong Meng, University of Pennsylvania, United States
Copyright © 2024 Gao, Li, Han, Xiong, Xu, Ma, Wang, Zhang, Yang, Xie, Jiang, Wu, Lin, Liu, Ni, Wang, Li, Jiao, Ding and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongfa Zhang, MTU4NjY2NDc2NzdAMTYzLmNvbQ==; Caihong Ding, ZGluZ2RkaW5nXzY0QDE2My5jb20=; Lunxian Jiao, amlhb2x1bnhpYW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xusheng Gao1†
Xusheng Gao1† Zhongfa Zhang
Zhongfa Zhang