
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 23 January 2024
Sec. Injury Prevention and Control
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1284594
This article is part of the Research TopicExploring the Interaction between Health-promoting and Health Risk Behaviours in HealthView all 16 articles
 Youjia Qiu1†
Youjia Qiu1† Xingzhou Wei2†
Xingzhou Wei2† Yuchen Tao2†
Yuchen Tao2† Bingyi Song1
Bingyi Song1 Menghan Wang2
Menghan Wang2 Ziqian Yin1
Ziqian Yin1 Minjia Xie1
Minjia Xie1 Aojie Duan1
Aojie Duan1 Zhouqing Chen1*
Zhouqing Chen1* Zhong Wang1*
Zhong Wang1*Background: Some studies suggest sedentary behavior is a risk factor for musculoskeletal disorders. This study aimed to investigate the potential causal association between leisure sedentary behavior (LSB) (including television (TV) viewing, computer use, and driving) and the incidence of sciatica, intervertebral disk degeneration (IVDD), low back pain (LBP), and cervical spondylosis (CS).
Methods: We obtained the data of LSB, CS, IVDD, LBP, sciatica and proposed mediators from the gene-wide association studies (GWAS). The causal effects were examined by Inverse Variance Weighted (IVW) test, MR-Egger, weighted median, weighted mode and simple mode. And sensitivity analysis was performed using MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) and MR-Egger intercept test. Multivariable MR (MVMR) was conducted to investigate the independent factor of other LSB; while two-step MR analysis was used to explore the potential mediators including Body mass index (BMI), smoking initiation, type 2 diabetes mellitus (T2DM), major depressive disorder (MDD), schizophrenia, bipolar disorder between the causal association of LSB and these diseases based on previous studies.
Results: Genetically associated TV viewing was positively associated with the risk of CS (OR = 1.61, 95%CI = 1.25 to 2.07, p = 0.002), IVDD (OR = 2.10, 95%CI = 1.77 to 2.48, p = 3.79 × 10−18), LBP (OR = 1.84, 95%CI = 1.53 to 2.21, p = 1.04 × 10−10) and sciatica (OR = 1.82, 95% CI = 1.45 to 2.27, p = 1.42 × 10−7). While computer use was associated with a reduced risk of IVDD (OR = 0.66, 95%CI = 0.55 to 0.79, p = 8.06 × 10−6), LBP (OR = 0.49, 95%CI = 0.40 to 0.59, p = 2.68 × 10−13) and sciatica (OR = 0.58, 95%CI = 0.46 to 0.75, p = 1.98 × 10−5). Sensitivity analysis validated the robustness of MR outcomes. MVMR analysis showed that the causal effect of TV viewing on IVDD (OR = 1.59, 95%CI = 1.13 to 2.25, p = 0.008), LBP (OR = 2.15, 95%CI = 1.50 to 3.08, p = 3.38 × 10−5), and sciatica (OR = 1.61, 95%CI = 1.03 to 2.52, p = 0.037) was independent of other LSB. Furthermore, two-step MR analysis indicated that BMI, smoking initiation, T2DM may mediate the causal effect of TV viewing on these diseases.
Conclusion: This study provides empirical evidence supporting a positive causal association between TV viewing and sciatica, IVDD and LBP, which were potentially mediated by BMI, smoking initiation and T2DM.
Cervical spondylosis (CS) is a degenerative condition characterized by the compression of the cervical spinal cord and/or surrounding blood vessels and has been shown to be associated with musculoskeletal neck disorders (1, 2). More than one third of the global population experiences mechanical neck pain for a duration of at least 3 months (3). In addition, prolonged neck flexion is a significant contributing factor in the development of myofascial neck pain (4). Intervertebral disk disorders (IVDD) is a common musculoskeletal condition and age-related degenerative disorder in which the amounts of proteoglycans and water in the nucleus pulposus within the disk gradually decreases (5–7). With increasing age, intervertebral disks gradually lose flexibility, elasticity and shock absorbency due to the degeneration, and the fibrosis surrounding the disks can become fragile and prone to rupture (8). The primary clinical manifestation of IVDD is usually low back pain (LBP) and can lead to radiculopathy and myelopathy (9, 10). Sciatica is considered a symptom, rather than a specific disease diagnosis, resulting from the inflammation or compression of the lumbosacral nerve roots L4-S1 by IVDD (11, 12). Research has shown a substantial range in the occurrence of sciatica symptoms, with prevalence rates ranging from 1.6 to 43% (13). In addition, several systematic reviews have suggested that smoking, obesity, and engaging in physically demanding work are potential risk factors for the initial onset of sciatica (14). LBP is not a distinct disease but rather a symptom characterized by pain in the dorsal region between the lower ribs and the gluteal fold (15). A systematic review found that the prevalence ranged from 1.4 to 20.0% in North America, Northern Europe, and Israel (16).
Leisure sedentary behavior (LSB) encompasses activities that involve maintaining a reclined or seated position, leading to limited physical exertion and low metabolic activity (energy expenditure ≤1.5 metabolic equivalents) (17). Such activities include watching television (TV), using a computer, and driving (18). Studies have demonstrated a link between LSB and an increased risk of cardiovascular disease, all-cause mortality, metabolic syndrome, and obesity (19, 20). Additionally, a large cohort study suggests an association between prolonged sedentary leisure time exceeding 6 h and an increased likelihood of neurological, sensory, and musculoskeletal disorders (21). Furthermore, a meta-analysis showed that there may be association between full-day sedentary or sitting time and the risk of cervical, and shoulder pain and LBP (22). However, due to the deficits in potential confounding factors and reverse causality, the precise understanding of the relationship between LSB and sciatica, CS, IVDD, and LBP remains incomplete (21, 23).
Mendelian Randomization (MR) analysis is an analytical method to evaluate the causal effect of specific exposures on outcomes using genetic variants available on genome-wide association study (GWAS) (24). GWAS is a systematic analysis of genes which examines and identify DNA sequence variations regulate a complex trait or affect the risk of the disease (25, 26). Previous observational studies of disk disease are subject to unavoidable potential confounding factors such as heterogeneity of the included studies, individual factors in the study population, investigator subjectivity and measurement error, as well as reverse causation due to the effect of disease phenotypes on exposures during disease progression (27–30). As single nucleotide polymorphisms (SNPs) are randomly assigned at conception, they are unlikely to be influenced by lifestyle and environmental factors (31). This feature of MR reduces the risk of confounding factors and reverse causal association, which is common in observational studies (32). Therefore, using a two-sample MR design to analyze summary statistics from GWAS could increase the statistical efficacy of causal association (33). Multivariable Mendelian Randomization (MVMR) analysis is a further developed exploration of traditional MR, which could evaluate more than two exposures simultaneously, and assess the causal association after adjusting other exposures (34). This study aimed to assess the causal associations between LSB (TV viewing, computer use, and driving) and CS, IVDD, LBP, and sciatica with MR approach. Then we further investigated the potential factor independent of other LSB. Several previous studies suggested there may exist an association between LSB and body mass index (BMI), type 2 diabetes mellitus (T2DM) and smoking (35, 36). In addition, LSB may be risk factors for neuropsychiatric disorders (37) and a recent MR study confirmed that LSB (TV viewing) is a high risk factor for major depressive disorder (MDD) (38). In addition, previous epidemiological studies on risk factors of these musculoskeletal disease indicated that several lifestyle related factors may also associated with these diseases, such as depression, education, smoking, obesity, and physical activities (39–41). These modifiable risk factors may also play a role between the LSB and CS, IVDD, LBP, and sciatica. Mediation analyses using two-step MR method could identify the causal pathways through which exposure affects outcomes and their relative importance, which can help identify which factors mediate the relationship between exposure and outcome, which in turn can be intervened or prevented to reduce the impact of exposure on outcomes (42). Therefore, we also evaluated the potential mediator between the causal association of LSB and these skeletomuscular diseases, which will help to optimize disease prevention at both clinical and health levels.
Our study is a re-analysis of data already included in GWAS; all ethical approvals have been obtained by the original GWAS authors. Thus, no additional ethical approval is required.
In this study, we used MR analysis to detect the association between LSB (TV viewing, computer use and driving) and CS, IVDD, LBP and sciatica using publicly available datasets from large GWAS. We used strict selection criteria to identify SNPs associated with specific LSB (including prolonged TV viewing, computer use, and driving), which were subsequently used as instrumental variables (IVs). The MR design was based on the following assumptions: (1) the genetic variants were directly and robustly associated with LSB and met the GWAS significance threshold; (2) the genetic variants used were not linked to any confounders; (3) the selected genetic variants influenced the development of CS and sciatica only through LSB. We used MR Steiger analysis to determine the precision of the direction. In addition, we also investigated the independent causal role of an exposure after adjusting for other exposures using MVMR. Furthermore, we sought to further explore the potential mechanisms by which genetic proxies for LSB influence susceptibility to CS, IVDD, sciatica and LBP through assessing the effects of potential mediating risk factors (including BMI, T2DM smoking initiation, MDD, schizophrenia, bipolar disorder) using two-step MR analysis (Figure 1). Confounding factors including alcohol use, smoking, low density lipoprotein, triglyceride, etc. are unrelated to genetic variation (43).
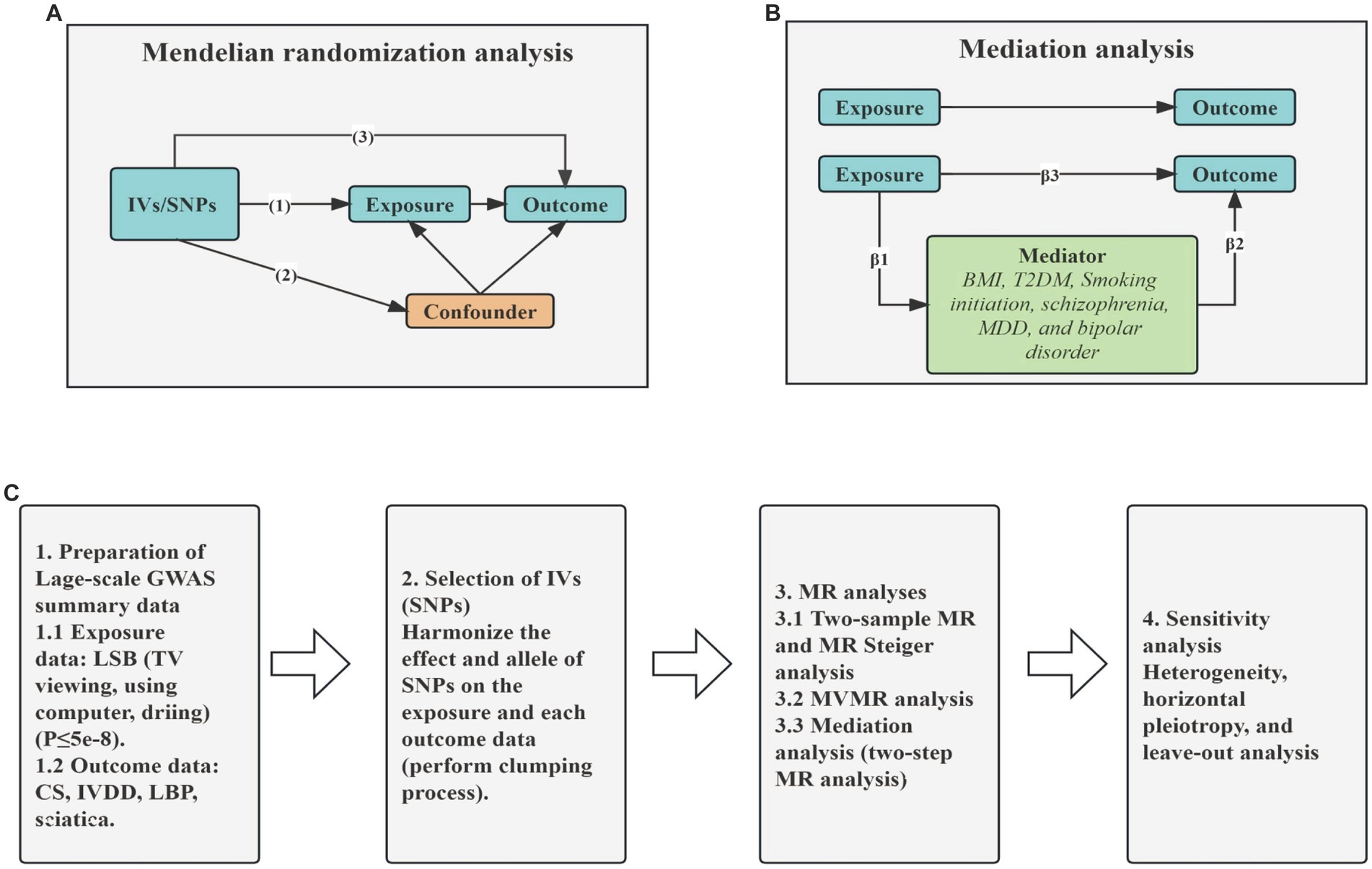
Figure 1. Graphical overview of the MR process. (A) The principles for two-sample MR analysis. (B) The principles for two-step MR analysis. (C) The whole workflow of MR analysis. IVs, instrumental variables; SNPs, single nucleotide polymorphisms; BMI, Body mass index; T2DM, type 2 diabetes mellitus; MDD, major depressive disorder; TV, television; CS, cervical spondylosis; IVDD, intervertebral disk disorders; LBP, low back pain; MR, Mendelian Randomization; MVMR, Multivariable Mendelian Randomization.
The GWAS summary data of LSB was obtained from a previous publication of the UK Biobank Repository (N = 422,218; European ancestry) (43). The study conducted a GWAS of sedentary behavior in 422,218 individuals of European origin. We selected IVs significantly associated (p < 5 × 10−8) with LSB on the UK Biobank website,1 including TV viewing, computer use, and driving. The amount of time respondents spent on these three behaviors was measured by their responses to the following questions: “In a typical day, how many hours do you spend watching television?,” “In a typical day, how many hours do you spend using a computer (excluding using a computer at work)?” and “In a typical day, how many hours do you spend driving?.” The mean age of the cohort at first assessment was 57.4 years (SD 8.0), and 45.7% of the study population was male. The average daily TV viewing was 2.8 h, computer use was 1.0 h and driving was 0.9 h, with standard deviations (SD) of 1.5 h, 1.2 h and 1.0 h, respectively. A strict threshold (R2 < 0.001, kb = 10,000) clustering procedure was used to ensure the independence of the selected SNPs. SNPs with a significant association with outcomes (p < 5 × 10−8) were also diskarded, and the mean F-statistic of all included exposures was greater than 10 (24).
Summary level GWAS results for CS (N = 284,358), IVDD (N = 308,600), sciatica (N = 377,277), and LBP (N = 300,293) were obtained from the Finn-Gen, and participant details, statistical protocols, and genetic information are available on the website.2 The trait of CS was labeled as “Cervical disk disorders” (44).
We also obtained genetic association for potential mediator (such as BMI, smoking initiation and T2DM) from different database in the IEU open GWAS,3 and the detailed information of the source of mediators is shown in Table 1. We selected phenotypes of potential mediators with a non-overlapped population to minimize the bias of weak instruments caused by sample overlap.
Inverse variance method (IVW) was used as the primary method for MR analysis. Random-effects IVW was used when significant heterogeneity (I2 > 50%) was detected, otherwise fixed-effects IVW was used. MR-Egger, weighted median, MR Pleiotropy Residuals and Outliers (MR-PRESSO) were also applied for additional statistical analysis. MR-Egger can detect and correct for potential horizontal pleiotropy, but results may be affected by the presence of outlying genetic variables (45–47). Moreover, MR-Egger slopes are relatively effective as estimates of MR in the presence of horizontal pleiotropy. The weighted median method ensures the stability of causality estimates by eliminating errors in the presence of 50% invalid IVs, and may provide better causality detection than the MR-egger under certain conditions (48, 49). MR Steiger directionality test was performed to rule out possibilities of reverse causal association. The estimates are provided for each increase of one standard deviation (SD), and the impact magnitude was reported as the odds ratio (OR) along with a 95% confidence interval (95%CI). Finally, various diagnostic plots were used to detect the robustness of MR estimates. The scatter plots showcase the association of SNPs with exposure and outcome, while the forest plots illustrate the influence of individual instrumental variable on the overall estimation of causality (50). Leave-one-out plots were utilized to visually present the findings of leave-one-out analysis, which involved recalculating the causal estimates obtained from IVW by excluding one SNP at a time. This approach was carried out to assess whether the estimates were affected by biases or driven by outliers (45).
The MR-PRESSO method identifies and corrects outliers by detecting the presence of horizontal multi-effects through global tests, outlier tests and bias tests (51). It is identified horizontal pleiotropy that genetic variants associated with the exposure (LSB) of interest have a direct effect on the outcome (CS, IVDD, sciatica and LBP) through multiple pathways other than the hypothesized exposure, and if horizontal pleiotropy occurs in MR analyses, the results of MR analyses will become unreliable (52). Cochran’s Q test and I2 statistics were carried out to evaluate the heterogeneity of the instrumental genetic variable, and a p-value <0.05 indicated significant heterogeneity. MR-Egger intercept, ME-PRESSO global test were performed to assess pleiotropy between IVs. Directional pleiotropy was assessed using the intercept term in MR Egger regressions, while in the MR-PRESSO method, heterogeneity is minimized by finding and removing outliers, then reassessing causal estimates. When horizontal pleiotropy still existed, we used the Radial MR method to filter variants identified as outliers (53). In addition, Bonferroni test was used for multiple comparisons, and a p-value of 0.016 (0.05/3 exposures) was considered significant, p-value ranged from 0.016 to 0.05 was considered suggested significant. In addition, p-value <0.05 was considered statistically significant in MVMR, which did not involve errors in multiple comparison.
We used MVMR to further assess the independent effects of these three LSB on these outcomes. MVMR can be used to assess which characteristics maintain causal relationships with outcomes, reflecting the direct effects of exposure on the outcome (54, 55). MR responds to the total effect of exposure and outcome and is composed of both direct (MVMR) and indirect effects (mediation effects) (42, 56). In addition, we further explored the effect of potential mediators that may mediate the causality between LSB and these outcomes using two-step MR analysis. The effect of LSB on these outcomes after adjusting for potential mediators is referred to as the direct effect, whereas the effect mediated by potential mediators is referred to as the indirect effect. In two-step MR, the first step is to test the influence of LSB on potential mediators; the second step is to test the influence of potential mediators on CS, IVDD, LBP, and sciatica. All statistical analyses were two-sided. The following packages were all used in R software (version 4.3.0) for analyses: MendelR (version 7.6.2), RadialMR, MR-PRESSO (1.0), and Forestploter (1.1.0) packages.
All instrumental variables used to genetically proxy LSB are shown in Supplementary Table S1, with a total of 113 SNPs for TV viewing, 83 SNPs for computer use, and 7 SNPs for driving. Meanwhile, Steiger filter test showed no reverse causality between the exposure and outcome (Supplementary Table S2). The results of MR estimates are shown in Supplementary Table S3. However, MR-PRESSO and radial MR test detected some of outliers, we therefore preformed MR analysis after remove these outliers (Supplementary Tables S4, S5). The number of eventually enrolled SNPs are shown in Supplementary Table S6.
In the IVW test, no significant causal relationship was found between computer use and CS (OR = 0.80, 95%CI = 0.61 to 1.05, p = 0.11). There were also no significant causal associations between driving and CS (OR = 0.40, 95%CI = 0.11 to 1.55, p = 0.19), IVDD (OR = 1.67, 95%CI = 0.66 to 4.22, p = 0.28), sciatica (OR = 1.73, 95%CI = 0.44 to 6.71, p = 0.43) and LBP (OR = 1.38, 95%CI = 0.67 to 2.83, p = 0.38) (Figure 2). However, genetically predicted computer use was associated with a reduced risk of IVDD (OR = 0.66, 95%CI = 0.55 to 0.79, p = 8.06 × 10−6), sciatica (OR = 0.58, 95%CI = 0.46 to 0.75, p = 1.98 × 10−5) and LBP (OR = 0.49, 95%CI = 0.40 to 0.59, p = 2.68 × 10−13) (Figure 3); while genetically predicted TV viewing was positively associated with the risk of CS (OR = 1.61, 95%CI = 1.25 to 2.07, p = 0.002), IVDD (OR = 2.10, 95%CI = 1.77 to 2.48, p = 3.79 × 10−18), sciatica (OR = 1.82, 95%CI = 1.45 to 2.27, p = 1.42 × 10−7) and LBP (OR = 1.84, 95%CI = 1.53 to 2.21, p = 1.04 × 10−10) (Figure 4). In addition, weight median and other methods (weight mode and MR-Egger) also provided consistent results with IVW, showing the same directions, indicating the robustness of the identified SNP.
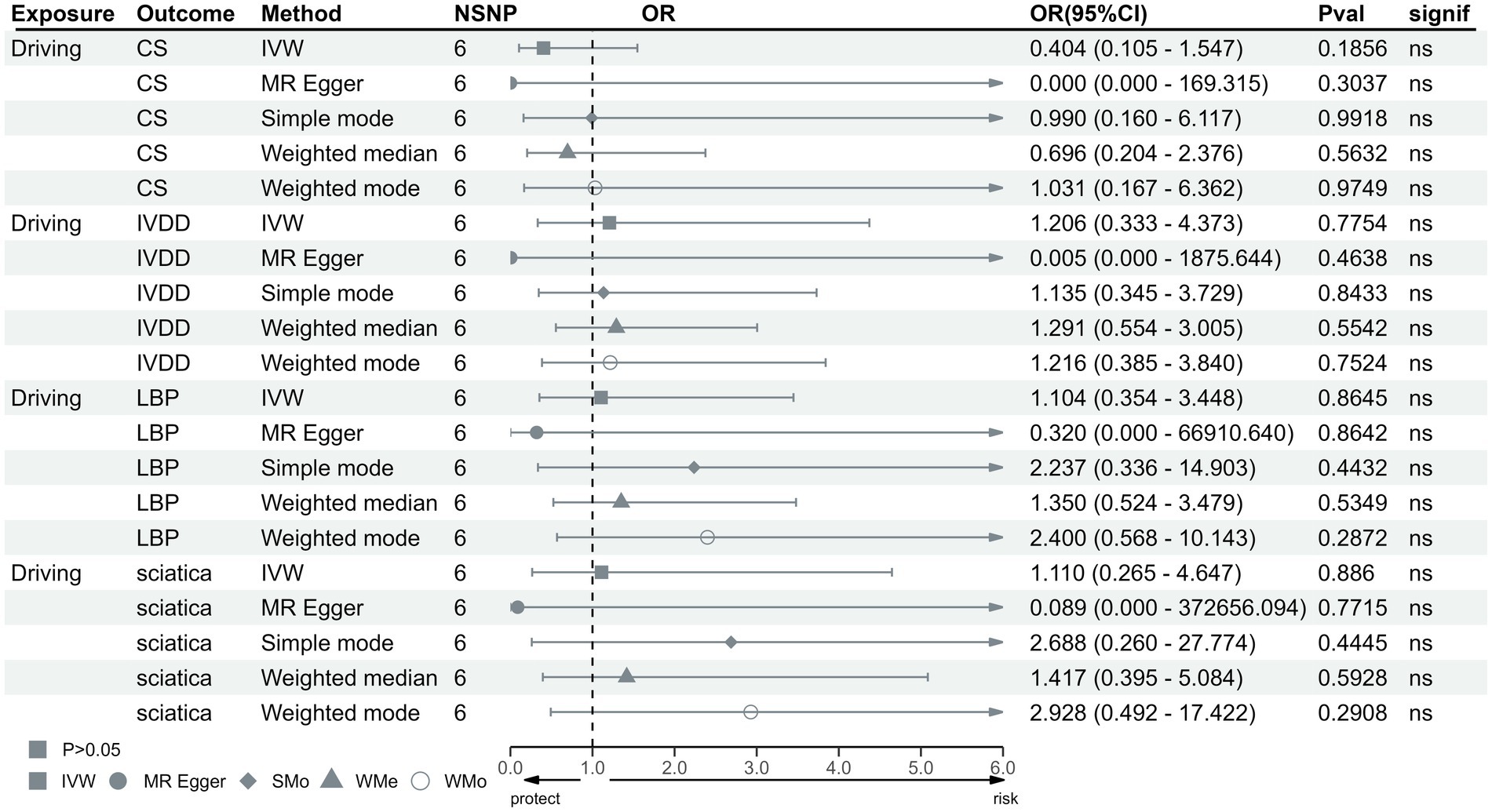
Figure 2. MR analysis for time spent driving on outcomes. MR, Mendelian Randomization; IVW, invers variance weighted; CS, cervical spondylosis; IVDD, intervertebral disk disorders; LBP, low back pain; OR, odds ratio; CI, confidence interval.
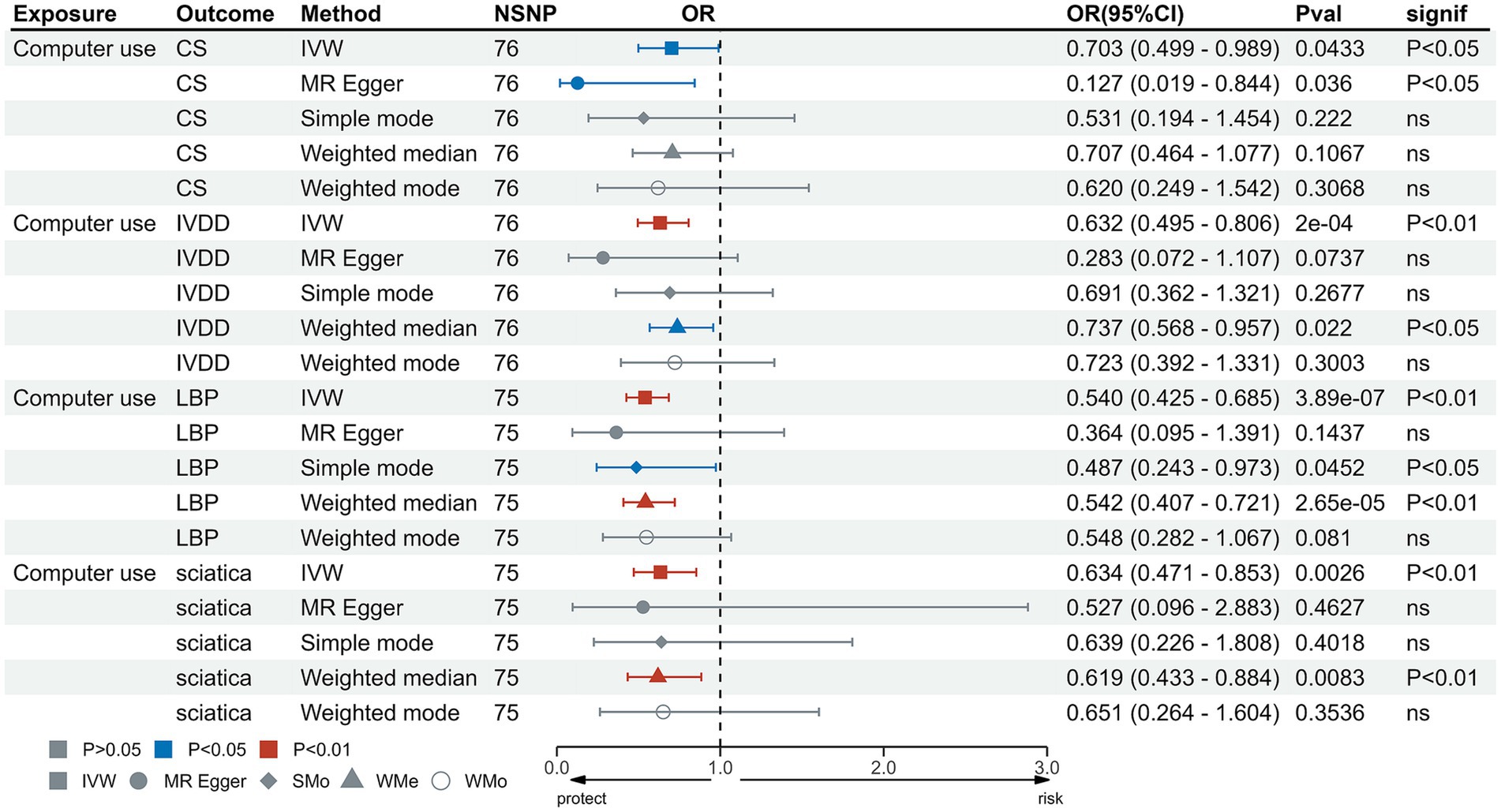
Figure 3. MR analysis for time spent using computer on outcomes. MR, Mendelian Randomization; IVW, inverse variance weighted; CS, cervical spondylosis; IVDD, intervertebral disk disorders; LBP, low back pain; OR, odds ratio; CI, confidence interval.
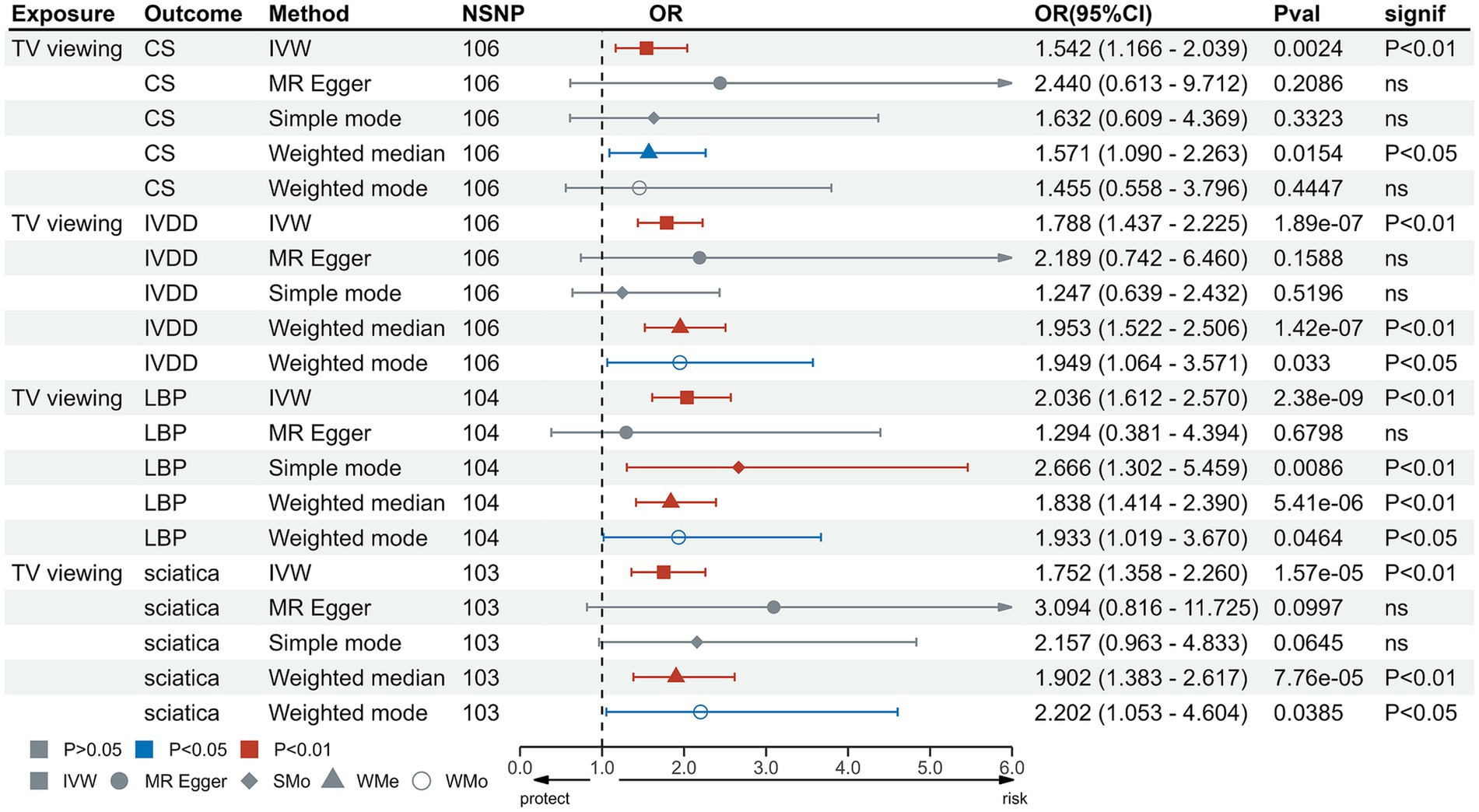
Figure 4. MR analysis for TV viewing on outcomes. MR, Mendelian Randomization; IVW, inverse variance weighted; TV, television; CS, cervical spondylosis; IVDD, intervertebral disk disorders; LBP, low back pain; OR, odds ratio; CI, confidence interval.
Heterogeneity test results were consistent with a value of p >0.05 except for driving and CS (p < 0.05) (Supplementary Table S7). The leave-one-out test suggested no SNP derived the causal association between exposure and outcome (Supplementary Figures S1–S12). Furthermore, scatter plot showed the same direction of different methods (Supplementary Figures S13–S24), and the funnel plot displayed a mostly symmetrical distribution (Supplementary Figures S25–S36), indicating the robustness of MR results. Funnel plot showed no specific outlier among SNPs. The results of the MR-PRESSO test after removing outliers showed no potential pleiotropy in MR analysis (Supplementary Table S8). The results of forest plot are shown in Supplementary Figures S37–S48.
MVMR was used to evaluate the independent effects of these three LSB on these outcomes. The results of the MVMR analysis showed that TV viewing remained independently causally associated with IVDD (OR = 1.59, 95%CI = 1.13 to 2.25, p = 0.008), LBP (OR = 2.15, 95%CI = 1.50 to 3.08, p = 3.38 × 10−5) and sciatica (OR = 1.61, 95%CI = 1.03 to 2.52, p = 0.037). However, MVMR analysis demonstrated that TV viewing was not significantly causally related to CS (OR = 1.38, 95%CI = 0.88 to 2.18, p = 0.16) and computer use was not significantly causally related to CS (OR = 0.97, 95%CI = 0.60 to 1.57, p = 0.91), IVDD (OR = 0.89, 95%CI = 0.62 to 1.29, p = 0.55), LBP (OR = 0.85, 95%CI = 0.58 to 1.24, p = 0.39) and sciatica (OR = 0.73, 95%CI = 0.46 to 1.18, p = 0.20) (Table 2 and Figure 5).
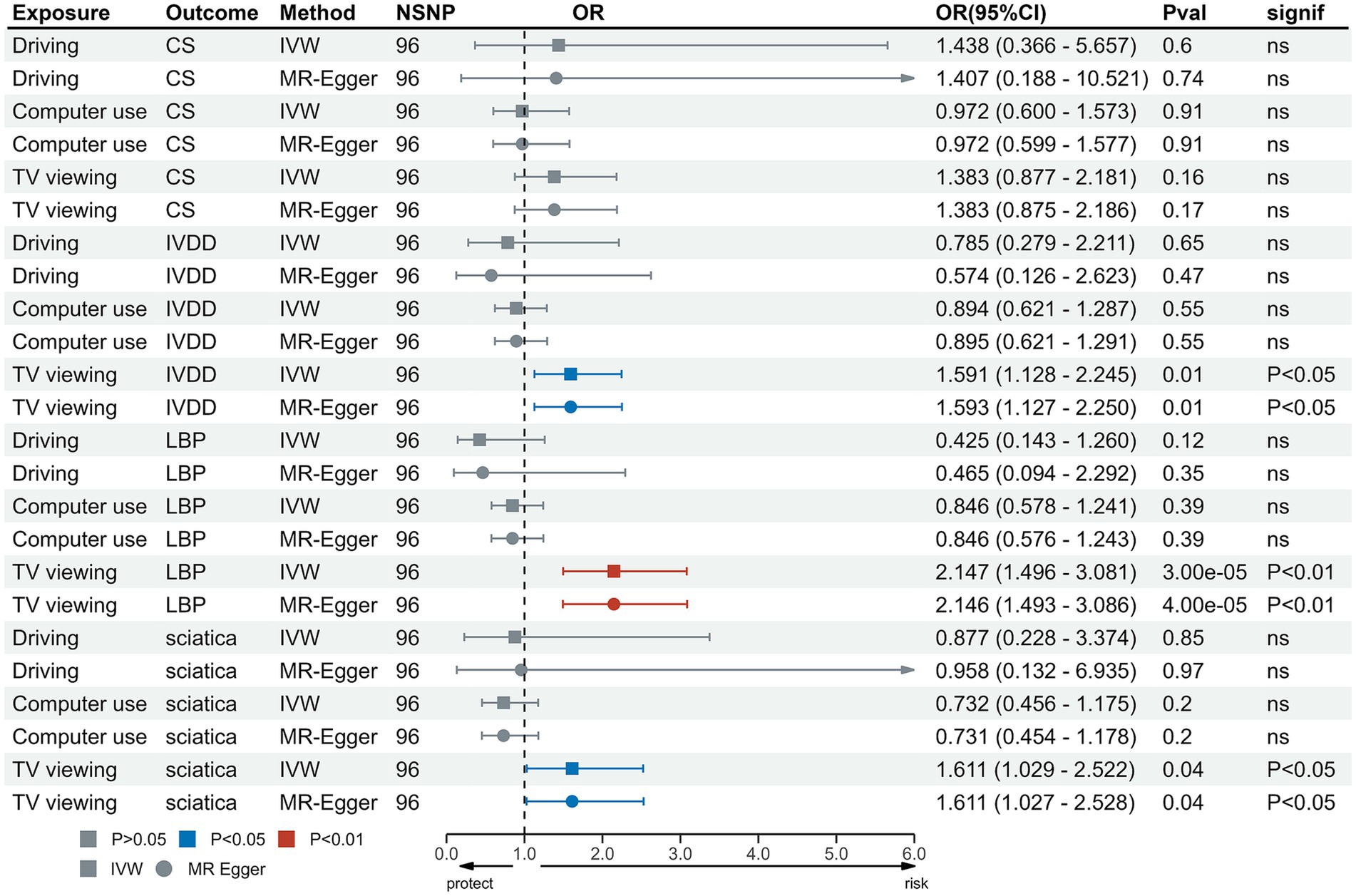
Figure 5. MVMR analysis for TV viewing on outcomes. MVMR, Multivariable Mendelian Randomization; IVW, inverse variance weighted; TV, television; CS, cervical spondylosis; IVDD, intervertebral disk disorders; LBP, low back pain; OR, odds ratio; CI, confidence interval.
The proportion of mediation is shown in Table 3 and Figure 6. MDD (p > 0.05), schizophrenia (p > 0.05), bipolar disorder (p > 0.05) did not exhibit mediating effects between the relationship of LSB and these outcomes. In the relationship between TV viewing and CS, BMI (0.174), smoking initiation (0.118) and T2DM (0.088) were identified as potential intermediary factors. And in the relationship between TV viewing and IVDD, BMI (0.176), smoking initiation (0.107) and T2DM (0.062) were identified as factors. Meanwhile, BMI (0.14), smoking initiation (0.104) and T2DM (0.01) were found to mediate the effect of TV viewing on sciatica. In particular, BMI (0.187), smoking initiation (0.126) and T2DM (0.034) were also found to mediate the effect of TV viewing on LBP (Supplementary Table S9).
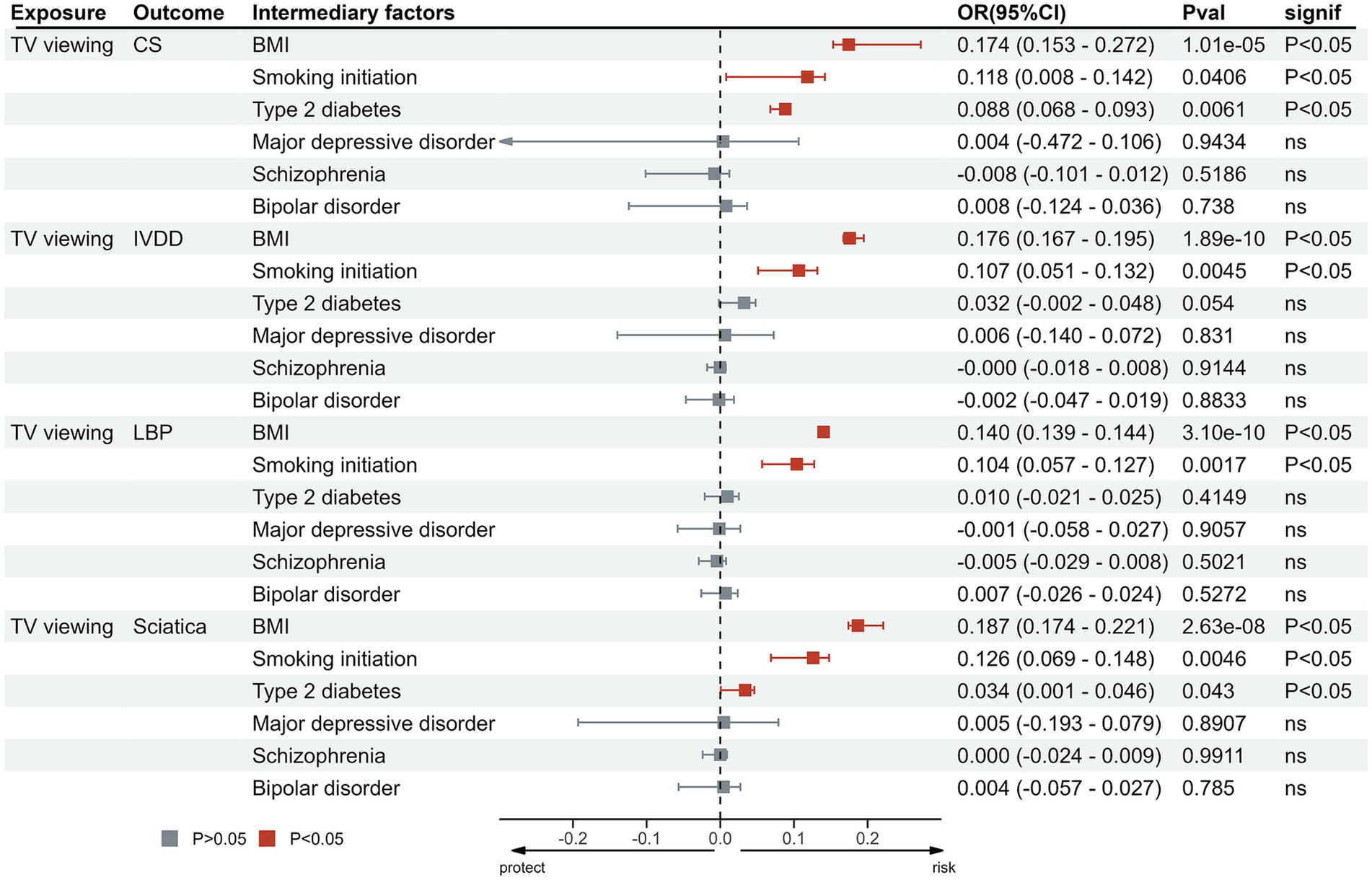
Figure 6. Proportion of the effect of TV viewing on outcomes mediated by potential mediators. MR, Mendelian Randomization; IVW, inverse variance weighted; TV, television; CS, cervical spondylosis; IVDD, intervertebral disk disorders; LBP, low back pain.
Our MR analysis suggested that LSB (TV viewing) may serve as a significant risk factor that is causally related to the development of IVDD, sciatica, and LBP. Additionally, factors such as BMI, T2DM, and smoking initiation may act as potential mediators in the relationship between TV viewing and IVDD, sciatica, and LBP.
IVDD is a common musculoskeletal disease caused by degenerative changes in the nucleus pulposus of the intervertebral disk (7, 11, 57). And the main cause of LBP is IVDD and results from a variety of known or unknown pathologies or diseases (58, 59). While the etiology of sciatica is attributed to the involvement of the L4-S1 nerve roots by the IVDD (11). Additionally, patients with IVDD may experience LBP and sciatica as a result of inflammation caused by IVDD (7). One study showed that LSB was associated with an increased risk of IVDD and LBP (60) and Euro et al. found a significant association between sitting and the incidence of sciatica (61), which are consistent with our findings.
LSB (TV viewing) may promote the onset and progression of IVDD, sciatica, and LBP by altering disk biomechanical relationships and causing chronic disk inflammation, due to body weight gain. One of the main causes of disk degeneration is damage to the intervertebral disks caused by disturbed biomechanical relationships between the vertebrae (62), and vertebral endplate defects have been shown to be the primary cause of disk degeneration (63, 64). Several studies have demonstrated that LSB plays a role in the development of high BMI and obesity, as evidenced by research conducted by various authors (65–71). TV viewing is considered a ‘mentally passive’ behavior, whereas using a computer is considered a ‘mentally active’ behavior (38). Furthermore, as a ‘mentally passive’ behavior, TV viewing is often perceived as an immersive and less reflective form of leisure and entertainment (52) and unhealthy eating, alcohol consumption and snacking are also associated with TV viewing (72), which may lead to obesity by having more involuntary intake and less consumption compared to non-sedentary population. And obesity can lead to severe postural changes that affect joint loading, while prolonged TV viewing can lead to prolonged periods of poor posture due to ‘mentally passivity’, which increases spinal strain and muscle fatigue leading disturbed biomechanical relationships and vertebral endplate defects (73–75). In addition, an increase in BMI increases the lumbosacral angle, causing greater flexion of the sacroiliac joints, increased lumbar disk and joint torque, which in turn increases joint loading and causes LBP (76, 77). At the same time, high BMI and obesity lead to metabolic dysregulation and chronic low-grade inflammatory response causing abnormal cytokine production, increased acute phase reactants and activation of inflammatory signaling pathways (78, 79). Moreover, fatty tissue has been shown to promote an inflammatory response through the release of leptin and resistin (80–82). Vertebral endplate defects may allow pro-inflammatory mediators which may be caused by obesity to be transported from the disk to the vertebral body, which in turn cause degenerative disk changes (63).
It has been shown that smoking is associated with LSB using MR analysis (52), and several studies have shown that smoking is an adverse risk factor for LBP and sciatica (83–85), which are consistent with the results of our mediation analysis. By reducing the blood supply to the intervertebral disks, smoking can cause intervertebral disk dystrophy (84), and tobacco smoke has been shown to contribute to degenerative changes in intervertebral disks in animal models (86). In addition, tobacco smoke inhalation promotes the production and release of cytokines from inflammatory cells in the intervertebral disk, which causes disk fibrosis and interferes with disk healing and repair (87, 88). Smoking may cause elevated serum levels of advanced glycation end-products (AGEs) and elevated AGEs promote degenerative disk changes by promoting nucleus pulposus apoptosis, facilitating collagen degradation of the annulus fibrosus, and inducing endplate sclerosis (89). In addition, T2DM has also been shown to be causally associated with an increased risk of IVDD (90, 91). In patients with T2DM mellitus, hyperglycemia causes the irreversible formation and accumulation of glycosylation end products, leading to pathophysiological changes in the cartilaginous endplates of the intervertebral disks, while at the same time hyperglycemia affects disk nutrition, cell viability and matrix homeostasis, resulting in changes in disk biomechanics and ultimately leading to IVDD (90). Diabetes accelerates hyperglycemia-induced accumulation of AGEs (92) and the continued accumulation of AGEs associated with hyperglycemia in T2DM was responsible for disk stiffening and the subsequent destructive chain of events (93). Moreover, LSB is considered a potential risk factor for neuropsychiatric disorders (37), and TV viewing was regard as a ‘mentally passive’ behavior which was considered that associated with an increased MDD risk (38). However, through mediation analyses, we did not find a mediating role for these psychiatric disorders (schizophrenia, MDD and bipolar disorder) between TV viewing and CS, IVDD, LBP, and sciatica. This may be due to the fact that we used mediation analyses (two-step MR analysis) to examine the potential relationship and role of neuropsychiatric disorders in the relationship between LSB (TV viewing) and those outcomes.
In addition, the mechanisms underlying neck pain and CS have been identified as increased intramuscular pressure in the neck, abnormal fascial tension on peripheral nerves, and altered muscle tissue mechanics (2, 4, 94). This condition is primarily caused by increased stress on the intervertebral disks in the neck and a decrease in flexion strength, resulting in the splitting of the annulus and subsequent herniation of the nucleus pulposus (cervical disk herniation), which in turn compresses the spinal cord and blood vessels (95). And studies have shown that inactivity and occasional sitting are associated with more perceived neck pain (96, 97). Although the results of two-sample MR analysis showed a significant association between TV viewing and CS, the results of our MVMR analysis showed no significant association between LSB and CS, which is inconsistent with the expected result. This suggests that the LSB of TV viewing does not have an effect on CS independently of other sedentary behaviors.
As mentioned above, although several observational studies have suggested an association between LSB and CS, IVDD, LBP, and sciatica, they have not provided clear evidence of a causal relationship. In addition, strong evidence of an association may not be available due to confounding variables, reverse causality and survival bias. We therefore performed MR analysis of sedentary behavior and CS, IVDD, LBP, and sciatica to resolve this uncertainty. Our study demonstrated that using computer and TV viewing are causally related to the aforementioned musculoskeletal disease, which should be emphasized in the preventive strategies. At the same time, avoiding smoking, maintaining a healthy BMI and preventing the onset of T2DM are also associated with avoiding these musculoskeletal diseases.
Our study has several strengths. First, the causal effect of LSB on IVDD, sciatica, and LBP was investigated using a large, publicly available GWAS database, the results were less likely to be influenced by confounding factors and reverse causality. Second, the population we selected was restricted to European origin to reduce the bias introduced by population stratification. Third, we further assessed the existence possible mediators in the relationship between LSB (TV viewing) and these outcomes, which may play a role in the prevention of these diseases. Fourth, the data sources for the mediators we analyzed were different from the exposures and outcomes, which effectively avoided the problem of overlapping sample sizes. However, there are some limitations to this study. Firstly, we only enrolled European ancestry because there is no GWAS of other origins with large sample size. Therefore, this result may not apply to other ancestries. Second, we did not conduct subgroup analysis on different sex due to the application of summary statistics instead of individual-level data. Additionally, although mediation analysis was conducted and mediators were identified, some other potential mediators, such as poor regions and chances of medical care, need to be heritable and available in GWAS.
In a summary, our two-sample MR study provides evidence that LSB is associated with the risk of CS, IVDD, sciatica, and LBP, and the causal effect of TV viewing on these diseases was independent of other LSB factors. In addition, mediation analysis indicated that BMI, smoking initiation, and T2DM may mediate the causal associations of TV viewing with IVDD, sciatica and LBP. These modifiable risk factors were the promising interventions for reducing the risk of these diseases.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by original GWAS authors. Thus, no additional ethical approval is required. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YQ: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing. XW: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing. YT: Conceptualization, Investigation, Validation, Writing – original draft. BS: Formal analysis, Methodology, Resources, Software, Writing – original draft. MW: Formal analysis, Methodology, Resources, Software, Writing – original draft. ZY: Data curation, Formal analysis, Methodology, Software, Writing – original draft. MX: Data curation, Methodology, Software, Writing – review & editing. AD: Data curation, Methodology, Supervision, Writing – original draft. ZC: Funding acquisition, Supervision, Writing – review & editing. ZW: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Suzhou Health Talents Training Project (Grants No GSWS2019002) and the National Natural Science Foundation of China (No 82201445).
The authors acknowledge the investigators of the original studies for sharing the GWAS data used in this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1284594/full#supplementary-material
1. McCormick, JR, Sama, AJ, Schiller, NC, Butler, AJ, and Donnally, CJ III. Cervical Spondylotic myelopathy: a guide to diagnosis and management. J Am Board Fam Med. (2020) 33:303–13. doi: 10.3122/jabfm.2020.02.190195
2. Money, S. Pathophysiology of trigger points in myofascial pain syndrome. J Pain Palliat Care Pharmacother. (2017) 31:158–9. doi: 10.1080/15360288.2017.1298688
3. Hurwitz, EL, Randhawa, K, Yu, H, Côté, P, and Haldeman, S. The global spine care initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J. (2018) 27:796–801. doi: 10.1007/s00586-017-5432-9
4. Lluch, E, Nijs, J, de Kooning, M, van Dyck, D, Vanderstraeten, R, Struyf, F, et al. Prevalence, incidence, localization, and pathophysiology of myofascial trigger points in patients with spinal pain: a systematic literature review. J Manip Physiol Ther. (2015) 38:587–600. doi: 10.1016/j.jmpt.2015.08.004
5. Hadjipavlou, AG, Tzermiadianos, MN, Bogduk, N, and Zindrick, MR. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. (2008) 90:1261–70. doi: 10.1302/0301-620X.90B10.20910
6. Silagi, ES, Shapiro, IM, and Risbud, MV. Glycosaminoglycan synthesis in the nucleus pulposus: dysregulation and the pathogenesis of disc degeneration. Matrix Biol. (2018) 71-72:368–79. doi: 10.1016/j.matbio.2018.02.025
7. Ravichandran, D, Pillai, J, and Krishnamurthy, K. Genetics of intervertebral disc disease: a review. Clin Anat. (2022) 35:116–20. doi: 10.1002/ca.23803
8. Chuah, YJ, Wu, Y, Cheong, MLS, Chia, YQ, Tee, CA, Hee, HT, et al. Development of annulus fibrosus tissue construct with hydrogel coils containing pre-conditioned mesenchymal stem cell. J Mater Sci Technol. (2021) 63:27–34. doi: 10.1016/j.jmst.2020.03.051
9. Vadalà, G, Russo, F, di Martino, A, and Denaro, V. Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med. (2015) 9:679–90. doi: 10.1002/term.1719
10. Cannata, F, Vadalà, G, Ambrosio, L, Fallucca, S, Napoli, N, Papalia, R, et al. Intervertebral disc degeneration: a focus on obesity and type 2 diabetes. Diabetes Metab Res Rev. (2020) 36:e3224. doi: 10.1002/dmrr.3224
11. Valat, JP, Genevay, S, Marty, M, Rozenberg, S, and Koes, B. Sciatica. Best Pract Res Clin Rheumatol. (2010) 24:241–52. doi: 10.1016/j.berh.2009.11.005
12. Jensen, RK, Kongsted, A, Kjaer, P, and Koes, B. Diagnosis and treatment of sciatica. BMJ. (2019) 367:l6273. doi: 10.1136/bmj.l6273
13. Konstantinou, K, and Dunn, KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine. (2008) 33:2464–72. doi: 10.1097/BRS.0b013e318183a4a2
14. Cook, CE, Taylor, J, Wright, A, Milosavljevic, S, Goode, A, and Whitford, M. Risk factors for first time incidence sciatica: a systematic review. Physiother Res Int. (2014) 19:65–78. doi: 10.1002/pri.1572
15. Knezevic, NN, Candido, KD, Vlaeyen, JWS, van Zundert, J, and Cohen, SP. Low back pain. Lancet. (2021) 398:78–92. doi: 10.1016/S0140-6736(21)00733-9
16. Fatoye, F, Gebrye, T, and Odeyemi, I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol Int. (2019) 39:619–26. doi: 10.1007/s00296-019-04273-0
17. on behalf of SBRN Terminology Consensus Project Participants Tremblay, MS, Aubert, S, Barnes, JD, Saunders, TJ, Carson, V, et al. Sedentary behavior research network (SBRN) -terminology consensus project process and outcome. Int J Behav Nutr Phys Act. (2017) 14:75. doi: 10.1186/s12966-017-0525-8
18. Pettee Gabriel, KK, Morrow, JR Jr, and Woolsey, AL. Framework for physical activity as a complex and multidimensional behavior. J Phys Act Health. (2012) 9:S11–8. doi: 10.1123/jpah.9.s1.s11
19. Grøntved, A, and Hu, FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. (2011) 305:2448–55. doi: 10.1001/jama.2011.812
20. Patel, AV, Maliniak, ML, Rees-Punia, E, Matthews, CE, and Gapstur, SM. Prolonged leisure time spent sitting in relation to cause-specific mortality in a large US cohort. Am J Epidemiol. (2018) 187:2151–8. doi: 10.1093/aje/kwy125
21. Dzakpasu, FQS, Carver, A, Brakenridge, CJ, Cicuttini, F, Urquhart, DM, Owen, N, et al. Musculoskeletal pain and sedentary behaviour in occupational and non-occupational settings: a systematic review with meta-analysis. Int J Behav Nutr Phys Act. (2021) 18:159. doi: 10.1186/s12966-021-01191-y
22. Zhou, J, Mi, J, Peng, Y, Han, H, and Liu, Z. Causal associations of obesity with the intervertebral degeneration, low Back pain, and sciatica: a two-sample Mendelian randomization study. Front Endocrinol. (2021) 12:740200. doi: 10.3389/fendo.2021.740200
23. de Rezende, LF, Rodrigues Lopes, M, Rey-López, JP, Matsudo, VKR, and Luiz, OC. Sedentary behavior and health outcomes: an overview of systematic reviews. PLoS One. (2014) 9:e105620. doi: 10.1371/journal.pone.0105620
24. Palmer, TM, Lawlor, DA, Harbord, RM, Sheehan, NA, Tobias, JH, Timpson, NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. (2012) 21:223–42. doi: 10.1177/0962280210394459
25. Smith, JG, and Newton-Cheh, C. Genome-wide association study in humans. Methods Mol Biol. (2009) 573:231–58. doi: 10.1007/978-1-60761-247-6_14
26. Dehghan, A. Genome-wide association studies. Methods Mol Biol. (2018) 1793:37–49. doi: 10.1007/978-1-4939-7868-7_4
27. Chun, SW, Lim, CY, Kim, K, Hwang, J, and Chung, SG. The relationships between low back pain and lumbar lordosis: a systematic review and meta-analysis. Spine J. (2017) 17:1180–91. doi: 10.1016/j.spinee.2017.04.034
28. Mertimo, T, Karppinen, J, Niinimäki, J, Blanco, R, Määttä, J, Kankaanpää, M, et al. Association of lumbar disc degeneration with low back pain in middle age in the northern Finland birth cohort 1966. BMC Musculoskelet Disord. (2022) 23:359. doi: 10.1186/s12891-022-05302-z
29. Ruffilli, A, Viroli, G, Neri, S, Traversari, M, Barile, F, Manzetti, M, et al. Mechanobiology of the human intervertebral disc: systematic review of the literature and future perspectives. Int J Mol Sci. (2023) 24:2728. doi: 10.3390/ijms24032728
30. Suo, M, Zhang, J, Sun, T, Wang, J, Liu, X, Huang, H, et al. The association between morphological characteristics of paraspinal muscle and spinal disorders. Ann Med. (2023) 55:2258922. doi: 10.1080/07853890.2023.2258922
31. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
32. Smith, GD, and Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
33. Pierce, BL, and Burgess, S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
34. Sanderson, E, Smith, GD, Windmeijer, F, and Bowden, J. Corrigendum to: an examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. (2020) 49:1057. doi: 10.1093/ije/dyaa101
35. Kang, JB, Shah, MA, Park, DJ, and Koh, PO. Retinoic acid regulates the ubiquitin-proteasome system in a middle cerebral artery occlusion animal model. Lab Anim Res. (2022) 38:13. doi: 10.1186/s42826-022-00123-6
36. Biddle, SJH, Bengoechea García, E, Pedisic, Z, Bennie, J, Vergeer, I, and Wiesner, G. Screen time, other sedentary Behaviours, and obesity risk in adults: a review of reviews. Curr Obes Rep. (2017) 6:134–47. doi: 10.1007/s13679-017-0256-9
37. Hoare, E, Milton, K, Foster, C, and Allender, S. The associations between sedentary behaviour and mental health among adolescents: a systematic review. Int J Behav Nutr Phys Act. (2016) 13:108. doi: 10.1186/s12966-016-0432-4
38. He, Q, Bennett, AN, Fan, B, Han, X, Liu, J, Wu, KCH, et al. Assessment of bidirectional relationships between leisure sedentary behaviors and neuropsychiatric disorders: a two-sample Mendelian randomization study. Genes. (2022) 13:962. doi: 10.3390/genes13060962
39. Yang, H, and Haldeman, S. Behavior-related factors associated with low Back pain in the US adult population. Spine. (2018) 43:28–34. doi: 10.1097/BRS.0000000000001665
40. Shiri, R, Solovieva, S, Husgafvel-Pursiainen, K, Telama, R, Yang, X, Viikari, J, et al. The role of obesity and physical activity in non-specific and radiating low back pain: the young Finns study. Semin Arthritis Rheum. (2013) 42:640–50. doi: 10.1016/j.semarthrit.2012.09.002
41. Shiri, R, and Falah-Hassani, K. Does leisure time physical activity protect against low back pain? Systematic review and meta-analysis of 36 prospective cohort studies. Br J Sports Med. (2017) 51:1410–8. doi: 10.1136/bjsports-2016-097352
42. Sanderson, E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. (2021) 11:a038984. doi: 10.1101/cshperspect.a038984
43. van de Vegte, YJ, Said, MA, Rienstra, M, van der Harst, P, and Verweij, N. Genome-wide association studies and Mendelian randomization analyses for leisure sedentary behaviours. Nat Commun. (2020) 11:1770. doi: 10.1038/s41467-020-15553-w
44. Sun, Y, Jin, M, Yu, T, and Zhang, J. Cardiovascular risk factors mediating the protective effect of education on cervical spondylosis risk. Sci Rep. (2023) 13:936. doi: 10.1038/s41598-023-28153-7
45. Burgess, S, and Thompson, SG. Interpreting findings from Mendelian randomization using the MR-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
46. Xiang, K, Wang, P, Xu, Z, Hu, YQ, He, YS, Chen, Y, et al. Causal effects of gut microbiome on systemic lupus erythematosus: a two-sample Mendelian randomization study. Front Immunol. (2021) 12:667097. doi: 10.3389/fimmu.2021.667097
47. Morris, DR, Jones, GT, Holmes, MV, Bown, MJ, Bulbulia, R, Singh, TP, et al. Genetic predisposition to diabetes and abdominal aortic aneurysm: a two stage Mendelian randomisation study. Eur J Vasc Endovasc Surg. (2022) 63:512–9. doi: 10.1016/j.ejvs.2021.10.038
48. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
49. Hartwig, FP, Davey Smith, G, and Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
50. Gao, Y, Mi, J, Liu, Z, and Song, Q. Leisure sedentary behavior and risk of lung Cancer: a two-sample Mendelian randomization study and mediation analysis. Front Genet. (2021) 12:763626. doi: 10.3389/fgene.2021.763626
51. Verbanck, M, Chen, CY, Neale, B, and do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
52. Chen, X, Hong, X, Gao, W, Luo, S, Cai, J, Liu, G, et al. Causal relationship between physical activity, leisure sedentary behaviors and COVID-19 risk: a Mendelian randomization study. J Transl Med. (2022) 20:216. doi: 10.1186/s12967-022-03407-6
53. Bowden, J, Spiller, W, del Greco M, F, Sheehan, N, Thompson, J, Minelli, C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol. (2018) 47:2100. doi: 10.1093/ije/dyy265
54. Sanderson, E, Davey Smith, G, Windmeijer, F, and Bowden, J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. (2019) 48:713–27. doi: 10.1093/ije/dyy262
55. Wang, M, Zhang, Z, Liu, D, Xie, W, Ma, Y, Yao, J, et al. Educational attainment protects against epilepsy independent of cognitive function: a Mendelian randomization study. Epilepsia. (2021) 62:1362–8. doi: 10.1111/epi.16894
56. Burgess, S, and Thompson, SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251–60. doi: 10.1093/aje/kwu283
57. Ropper, AH, and Zafonte, RD. Sciatica. N Engl J Med. (2015) 372:1240–8. doi: 10.1056/NEJMra1410151
58. Hartvigsen, J, Hancock, MJ, Kongsted, A, Louw, Q, Ferreira, ML, Genevay, S, et al. What low back pain is and why we need to pay attention. Lancet. (2018) 391:2356–67. doi: 10.1016/S0140-6736(18)30480-X
59. Brinjikji, W, Diehn, FE, Jarvik, JG, Carr, CM, Kallmes, DF, Murad, MH, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and Meta-analysis. AJNR Am J Neuroradiol. (2015) 36:2394–9. doi: 10.3174/ajnr.A4498
60. Zhao, X, Yang, Y, Yue, R, and Su, C. Potential causal association between leisure sedentary behaviors, physical activity and musculoskeletal health: a Mendelian randomization study. PLoS One. (2023) 18:e0283014. doi: 10.1371/journal.pone.0283014
61. Euro, U, Heliövaara, M, Shiri, R, Knekt, P, Rissanen, H, Aromaa, A, et al. Work-related risk factors for sciatica leading to hospitalization. Sci Rep. (2019) 9:6562. doi: 10.1038/s41598-019-42597-w
62. Adams, MA, and Dolan, P. Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat. (2012) 221:497–506. doi: 10.1111/j.1469-7580.2012.01551.x
63. Määttä, JH, Rade, M, Freidin, MB, Airaksinen, O, Karppinen, J, and Williams, FMK. Strong association between vertebral endplate defect and Modic change in the general population. Sci Rep. (2018) 8:16630. doi: 10.1038/s41598-018-34933-3
64. Munir, S, Freidin, MB, Rade, M, Määttä, J, Livshits, G, and Williams, FMK. Endplate defect is heritable, associated with low Back pain and triggers intervertebral disc degeneration: a longitudinal study from TwinsUK. Spine. (2018) 43:1496–501. doi: 10.1097/BRS.0000000000002721
65. Chinapaw, MJ, Proper, KI, Brug, J, van Mechelen, W, and Singh, AS. Relationship between young peoples’ sedentary behaviour and biomedical health indicators: a systematic review of prospective studies. Obes Rev. (2011) 12:e621–32. doi: 10.1111/j.1467-789X.2011.00865.x
66. Costigan, SA, Barnett, L, Plotnikoff, RC, and Lubans, DR. The health indicators associated with screen-based sedentary behavior among adolescent girls: a systematic review. J Adolesc Health. (2013) 52:382–92. doi: 10.1016/j.jadohealth.2012.07.018
67. Hoare, E, Skouteris, H, Fuller-Tyszkiewicz, M, Millar, L, and Allender, S. Associations between obesogenic risk factors and depression among adolescents: a systematic review. Obes Rev. (2014) 15:40–51. doi: 10.1111/obr.12069
68. Prentice-Dunn, H, and Prentice-Dunn, S. Physical activity, sedentary behavior, and childhood obesity: a review of cross-sectional studies. Psychol Health Med. (2012) 17:255–73. doi: 10.1080/13548506.2011.608806
69. Proper, KI, Singh, AS, van Mechelen, W, and Chinapaw, MJM. Sedentary behaviors and health outcomes among adults: a systematic review of prospective studies. Am J Prev Med. (2011) 40:174–82. doi: 10.1016/j.amepre.2010.10.015
70. Thorp, AA, Owen, N, Neuhaus, M, and Dunstan, DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011. Am J Prev Med. (2011) 41:207–15. doi: 10.1016/j.amepre.2011.05.004
71. van Uffelen, JG, Wong, J, Chau, JY, van der Ploeg, HP, Riphagen, I, Gilson, ND, et al. Occupational sitting and health risks: a systematic review. Am J Prev Med. (2010) 39:379–88. doi: 10.1016/j.amepre.2010.05.024
72. Frydenlund, G, Jørgensen, T, Toft, U, Pisinger, C, and Aadahl, M. Sedentary leisure time behavior, snacking habits and cardiovascular biomarkers: the Inter99 study. Eur J Prev Cardiol. (2012) 19:1111–9. doi: 10.1177/1741826711419999
73. Davis, KG, and Kotowski, SE. Postural variability: an effective way to reduce musculoskeletal discomfort in office work. Hum Factors. (2014) 56:1249–61. doi: 10.1177/0018720814528003
74. van Dieën, JH, de Looze, MP, and Hermans, V. Effects of dynamic office chairs on trunk kinematics, trunk extensor EMG and spinal shrinkage. Ergonomics. (2001) 44:739–50. doi: 10.1080/00140130120297
75. Fabris de Souza, SA, Faintuch, J, Valezi, AC, Sant’Anna, AF, Gama-Rodrigues, JJ, de Batista Fonseca, IC, et al. Postural changes in morbidly obese patients. Obes Surg. (2005) 15:1013–6. doi: 10.1381/0960892054621224
76. Onyemaechi, NO, Anyanwu, GE, Obikili, EN, Onwuasoigwe, O, and Nwankwo, OE. Impact of overweight and obesity on the musculoskeletal system using lumbosacral angles. Patient Prefer Adherence. (2016) 10:291–6. doi: 10.2147/PPA.S90967
77. Rodríguez-Martínez, E, Nava-Ruiz, C, Escamilla-Chimal, E, Borgonio-Perez, G, and Rivas-Arancibia, S. The effect of chronic ozone exposure on the activation of endoplasmic reticulum stress and apoptosis in rat Hippocampus. Front Aging Neurosci. (2016) 8:245. doi: 10.3389/fnagi.2016.00245
78. Miscio, G, Guastamacchia, G, Brunani, A, Priano, L, Baudo, S, and Mauro, A. Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst. (2005) 10:354–8. doi: 10.1111/j.1085-9489.2005.00047.x
79. Wang, Y, and Huang, F. N-3 polyunsaturated fatty acids and inflammation in obesity: local effect and systemic benefit. Biomed Res Int. (2015) 2015:581469. doi: 10.1155/2015/581469
80. Tilg, H, and Moschen, AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. (2006) 6:772–83. doi: 10.1038/nri1937
81. Lord, GM, Matarese, G, Howard, JK, Baker, RJ, Bloom, SR, and Lechler, RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. (1998) 394:897–901. doi: 10.1038/29795
82. Sheng, B, Feng, C, Zhang, D, Spitler, H, and Shi, L. Associations between obesity and spinal diseases: a medical expenditure panel study analysis. Int J Environ Res Public Health. (2017) 14:183. doi: 10.3390/ijerph14020183
83. Parreira, P, Maher, CG, Steffens, D, Hancock, MJ, and Ferreira, ML. Risk factors for low back pain and sciatica: an umbrella review. Spine J. (2018) 18:1715–21. doi: 10.1016/j.spinee.2018.05.018
84. Shiri, R, and Falah-Hassani, K. The effect of smoking on the risk of sciatica: a Meta-analysis. Am J Med. (2016) 129:64–73.e20. doi: 10.1016/j.amjmed.2015.07.041
85. Lv, Z, Cui, J, and Zhang, J. Smoking, alcohol and coffee consumption and risk of low back pain: a Mendelian randomization study. Eur Spine J. (2022) 31:2913–9. doi: 10.1007/s00586-022-07389-3
86. Wang, D, Nasto, LA, Roughley, P, Leme, AS, Houghton, AM, Usas, A, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthr Cartil. (2012) 20:896–905. doi: 10.1016/j.joca.2012.04.010
87. Oda, H, Matsuzaki, H, Tokuhashi, Y, Wakabayashi, K, Uematsu, Y, and Iwahashi, M. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. J Orthop Sci. (2004) 9:135–41. doi: 10.1007/s00776-003-0759-y
88. Nemoto, Y, Matsuzaki, H, Tokuhasi, Y, Okawa, A, Uematu, Y, Nishimura, T, et al. Histological changes in intervertebral discs after smoking and cessation: experimental study using a rat passive smoking model. J Orthop Sci. (2006) 11:191–7. doi: 10.1007/s00776-005-0987-4
89. Yang, F, Zhu, D, Wang, Z, Ma, Y, Huang, L, and Kang, X. Role of advanced glycation end products in intervertebral disc degeneration: mechanism and therapeutic potential. Oxidative Med Cell Longev. (2022) 2022:7299005. doi: 10.1155/2022/7299005
90. Jin, P, Xing, Y, Xiao, B, Wei, Y, Yan, K, Zhao, J, et al. Diabetes and intervertebral disc degeneration: a Mendelian randomization study. Front Endocrinol. (2023) 14:1100874. doi: 10.3389/fendo.2023.1100874
91. Alpantaki, K, Kampouroglou, A, Koutserimpas, C, Effraimidis, G, and Hadjipavlou, A. Diabetes mellitus as a risk factor for intervertebral disc degeneration: a critical review. Eur Spine J. (2019) 28:2129–44. doi: 10.1007/s00586-019-06029-7
92. Illien-Junger, S, Grosjean, F, Laudier, DM, Vlassara, H, Striker, GE, and Iatridis, JC. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS One. (2013) 8:e64302. doi: 10.1371/journal.pone.0064302
93. Krishnamoorthy, D, Hoy, RC, Natelson, DM, Torre, OM, Laudier, DM, and Iatridis, JC. Dietary advanced glycation end-product consumption leads to mechanical stiffening of murine intervertebral discs. Dis Model Mech. (2018) 11:dmm036012. doi: 10.1242/dmm.036012
94. Shah, JP, Thaker, N, Heimur, J, Aredo, JV, Sikdar, S, and Gerber, L. Myofascial trigger points then and now: a historical and scientific perspective. PM R. (2015) 7:746–61. doi: 10.1016/j.pmrj.2015.01.024
95. Theodore, N. Degenerative cervical spondylosis. N Engl J Med. (2020) 383:159–68. doi: 10.1056/NEJMra2003558
96. Ekblom-Bak, E, Stenling, A, Salier Eriksson, J, Hemmingsson, E, Kallings, LV, Andersson, G, et al. Latent profile analysis patterns of exercise, sitting and fitness in adults -associations with metabolic risk factors, perceived health, and perceived symptoms. PLoS One. (2020) 15:e0232210. doi: 10.1371/journal.pone.0232210
Keywords: leisure sedentary behavior, sciatica, cervical spondylosis, intervertebral disk disorders, low back pain, Mendelian randomization
Citation: Qiu Y, Wei X, Tao Y, Song B, Wang M, Yin Z, Xie M, Duan A, Chen Z and Wang Z (2024) Causal association of leisure sedentary behavior and cervical spondylosis, sciatica, intervertebral disk disorders, and low back pain: a Mendelian randomization study. Front. Public Health. 12:1284594. doi: 10.3389/fpubh.2024.1284594
Received: 28 August 2023; Accepted: 08 January 2024;
Published: 23 January 2024.
Edited by:
Huixuan Zhou, Beijing Sport University, ChinaReviewed by:
Isabel Rada, Universidad del Desarrollo, ChileCopyright © 2024 Qiu, Wei, Tao, Song, Wang, Yin, Xie, Duan, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Wang, d2FuZ3pob25nNzYxQDE2My5jb20=; Zhouqing Chen, enFjaGVuNkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.