- 1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China
- 2Breast Tumor Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 3Department of Gastroenterology, Dongguan Songshan Lake Tungwah Hospital, Dongguan, China
Background: The relationships between heavy metals and fatty liver, especially the threshold values, have not been fully elucidated. The objective of this research was to further investigate the correlation between blood heavy metal exposures and the risk of Metabolic dysfunction Associated Fatty Liver Disease (MAFLD) in adults.
Methods: Laboratory data on blood metal exposure levels were obtained from National Health and Nutrition Examination Survey (NHANES) data for the period 2015 to 2020 for a cross-sectional study in adults. Associations between blood levels of common heavy metals and the risk of MAFLD in adults were analyzed using multifactorial logistic regression and ranked for heavy metal importance using a random forest model. Finally, thresholds for important heavy metals were calculated using piecewise linear regression model.
Results: In a multifactorial logistic regression model, we found that elevated levels of selenium (Se) and manganese (Mn) blood exposure were strongly associated with the risk of MAFLD in adults. The random forest model importance ranking also found that Se and Mn blood exposure levels were in the top two positions of importance for the risk of disease in adults. The restricted cubic spline suggested a non-linear relationship between Se and Mn blood exposure and adult risk of disease. The OR (95% CI) for MAFLD prevalence was 3.936 (2.631–5.887) for every 1 unit increase in Log Mn until serum Mn levels rose to the turning point (Log Mn = 1.10, Mn = 12.61 μg/L). This correlation was not significant (p > 0.05) after serum Mn levels rose to the turning point. A similar phenomenon was observed for serum Se levels, with a turning point of (Log Se = 2.30, Se = 199.55 μg/L).
Conclusion: Blood heavy metals, especially Se and Mn, are significantly associated with MAFLD in adults. They have a non-linear relationship with a clear threshold.
Introduction
MAFLD, is the most common cause of chronic liver disease worldwide (1). Originally referred to as non-alcoholic fatty liver disease (NAFLD), but proposed to be redefined by an international expert group in 2020 (2). MAFLD is a condition of liver fat accumulation with metabolic dysfunction in the form of overweight or obesity and insulin resistance (3). MAFLD is closely related to diabetes, chronic kidney disease, cardiovascular disease (4) and is also prevalent in patients with hepatocellular carcinoma (5, 6), posing a significant potential economic burden to society.
The relationship between heavy metals and liver diseases has been confirmed in previous studies. One study showed that liver function was significantly correlated with blood heavy metals, and different heavy metals had different effects on various indicators of liver function (7). Study has also shown a positive association between blood Se and NAFLD (8). In addition, blood Mn and Se are associated with hepatic steatosis and fibrosis (9). However, previous studies have mostly focused on the effects of heavy metals on the liver, lacking of description of the thresholds for the relationship between heavy metal exposure levels and the risk of developing MAFLD in adults. Given the importance of thresholds in describing relationship, the relationship between heavy metal exposures and the risk of MAFLD in adults must be further explored.
This study retrieved the data of the NHANES from 2015 to 2020, used multi-factor logistics regression to analyze the association between blood levels of common heavy metals and the risk of MAFLD in adults, and ranked the importance of heavy metals by random forest model. Furthermore, the piecewise linear regression model was used to calculate the threshold values of the two most significantly correlated heavy metals, which is important for understanding the relationship between exposure to heavy metals and MAFLD in adults.
Methods
Study population
The NHANES is a cross-sectional survey administered by the National Center for Health Statistics (NCHS) and the Centers for Disease Control and Prevention with data from the civilian population of the United States and is nationally representative. The data collection protocol was approved by the NCHS Ethics Review Committee and all survey participants provided informed consent prior to being interviewed and examined. Because laboratory data on blood metal exposure levels were complete for the period 2015 to 2020 compared to other years, the NHANES public data file for that cycle was used to construct the dataset for this study, and the study population consisted of all NHANES respondents.
Exposure
The exposure variable in this study was blood heavy metal levels. Blood levels of 10 different metals [plumbum (Pb), hydrargyrum (Hg), cadmium (Cd), Mn, Se, chromium (Cr), cobalt (Co), inorganic hydrargyrum (InHg), methyl hydrargyrum (MeHg) and ethyl hydrargyrum (EtHg)] were obtained by direct extraction of participant laboratory data. The test method is described in detail in the NHANES database1 and focuses on the direct measurement of heavy metals in whole blood samples using mass spectrometry after a simple dilution sample preparation procedure.
Outcome
The primary outcome of this study was the presence or absence of MAFLD, which was defined as the combination of steatosis, irrespective of the gradation, and metabolic dysfunction. This was characterized by either overweight (Body mass index (BMI) ≥25 kg/m2), type 2 diabetes mellitus defined as antidiabetic drug use, fasting plasma glucose ≥7.0 mmol/L, HbA1c > 6.4% or based on oral glucose tolerance test (OGTT), or a combination of at least two of the following metabolic abnormalities: (1) waist circumference > 102 cm for male and > 88 cm for female; (2) blood pressure ≥ 130/85 mmHg or antihypertensive drug use; (3) plasma triglycerides ≥1.70 mmol/L or lipid-lowering drug treatment; (4) high-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L for men and < 1.3 mmol/L for women or lipid-lowering drug treatment; (5) prediabetes defined as fasting plasma glucose 5.6–6.9 mmol/L, HbA1c 5.7–6.4% or matching OGTT; (6) homeostatic model assessment of insulin resistance (HOMA-IR) of ≥2.5; (7) or C-reactive protein (CRP) level > 2 mg/L (10). Those who met the diagnostic criteria were identified as MAFLD patients, those who did not were identified as controls, and those with missing diagnosis-related data were removed.
Baseline data on the study population
Demographic characteristics, such as age, gender and ethnicity were included. Social factors, including education level, marital status, ratio of household income to poverty level and health insurance coverage were included. Data on everyday health-related behaviors were collected for smoking and alcohol consumption. Variables related to medical comorbidities, such as liver and kidney function, BMI, diabetes, hypertension and cancer, are collected.
Data analysis
The quantitative data met a normal distribution by selecting a t-test or analysis of variance (ANOVA), or a rank sum test if they did not meet a normal distribution. Data on categorical variables were analyzed for differences in baseline characteristics and blood heavy metal levels between outcome groups using the χ2 test. We used Spearman correlation matrices to identify correlations for the 10 heavy metals. Subsequently, multifactorial logistic regression models were used to identify exposure factors associated with the screening outcome variables for the preliminary analysis. A random forest (RF) model was used to determine the importance of the variables. Feature importance was assessed based on the out-of-bag (OOB) error rate, reflecting the level of contribution of each variable when classifying the metabolic dysfunction-related fatty liver with the control population.
To explore the non-linear relationship between blood heavy metal concentrations and the risk of MAFLD, as well as the threshold effect, serum heavy metal concentrations were transformed on a Log10 scale. We used the Restrictive Cubic Spline (RCS) function to analyze the odds ratio (OR) relationship between blood heavy metal concentrations and the risk of MAFLD, as serum heavy metal concentrations showed a skewed distribution. Additionally, a piecewise linear regression model was applied to examine the threshold effect of serum heavy metal concentrations on the risk of MAFLD using a smoothing function. Threshold levels (i.e., turning points) were determined by iterative trials involving the selection of turning points along predefined intervals, followed by the selection of turning points that gave the maximum model likelihood.
All data analyzes were conducted using R.3.5.2/R4.2.2.2 Sample sizes were based on available data and no ex ante sample size calculations were performed. Significance was tested for all descriptive analyzes by two-sided tests at the p < 0.05 level of significance.
Results
Baseline characteristics of the study population
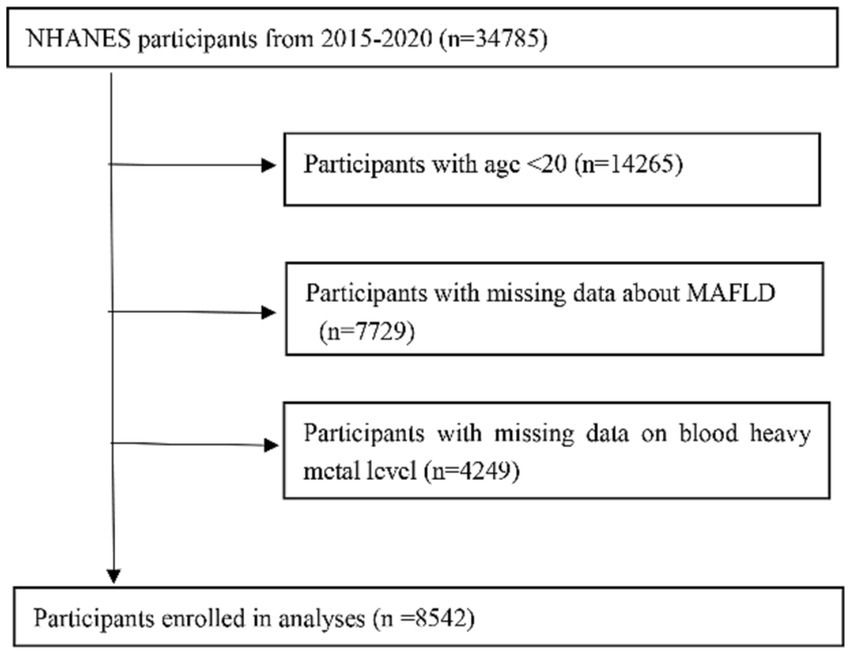
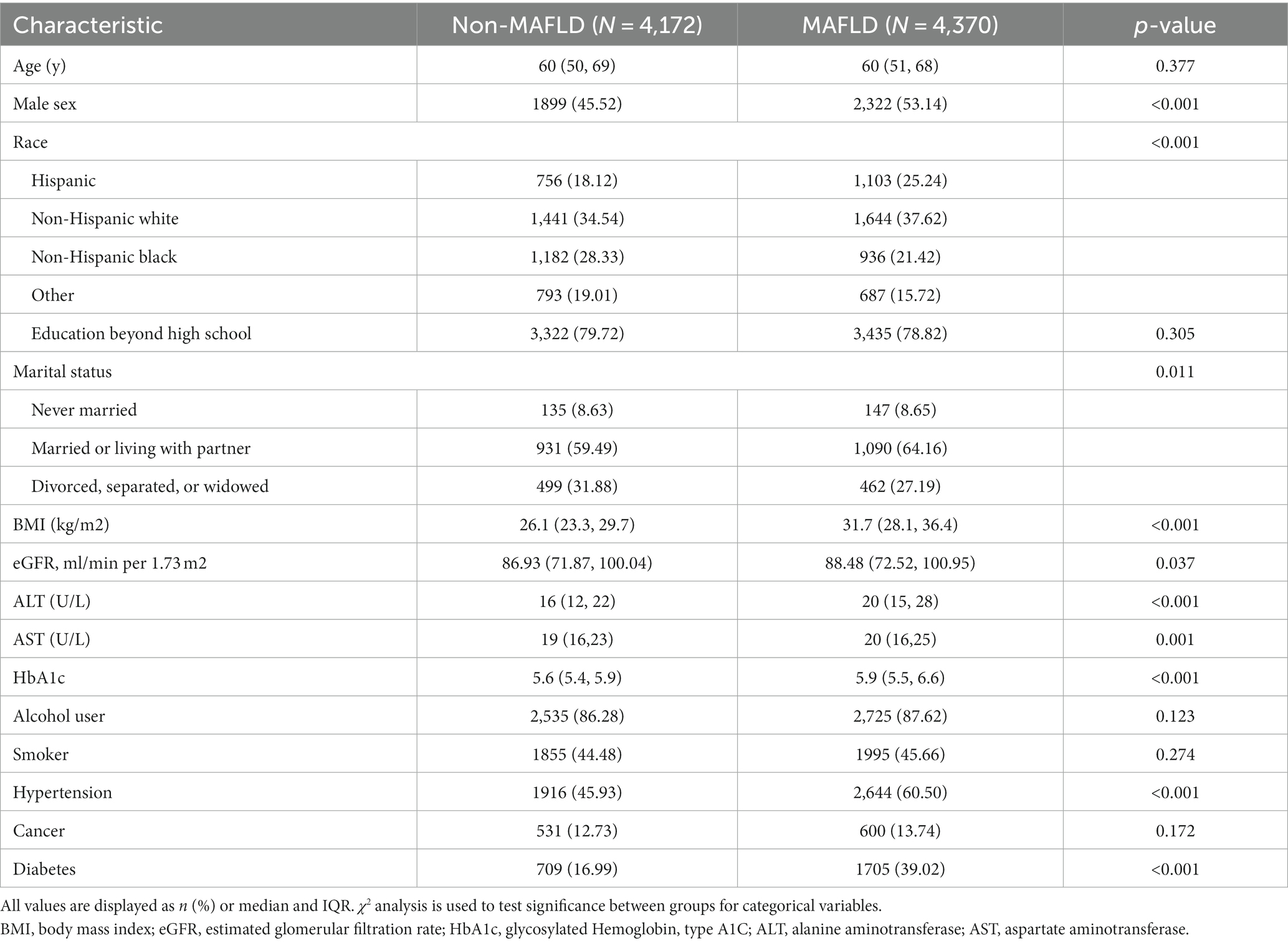
Survey data were collected from 34,785 participants. After excluding those lacking MAFLD, heavy metal blood level data (with the exceptions mentioned above), 8,542 adult participants (age ≥ 20 years) were included in the analysis (Figure 1). The distribution of baseline characteristics stratified by outcome is shown in Table 1. In the preliminary analysis, the MAFLD group participants, 53.14% male, were significantly higher than the Non-MAFLD group, in addition the age distribution of participants in the MAFLD group was not significantly different from the Non-MAFLD group. In terms of social factors, the proportion of Married or living with partner was higher in the MAFLD group than in the Non-MAFLD group, and there was no significant difference in the education level of the two groups. For comorbidities, BMI was significantly higher in the MAFLD group than in the Non-MAFLD group (33.05 ± 6.84 vs. 26.97 ± 5.56, p < 0.001). estimated glomerular filtration rate (eGFR), alanine transaminase(ALT) and aspartate transaminase (AST) were in the normal range in both groups, and HbA1c was in the normal range in the Non-MAFLD group, while HbA1c was above the normal range in the MAFLD group. The MAFLD group had a higher proportion of combined hypertension and diabetes than Non-MAFLD, and the difference was statistically significant (p < 0.05).
Blood levels of heavy metal exposure in the study population
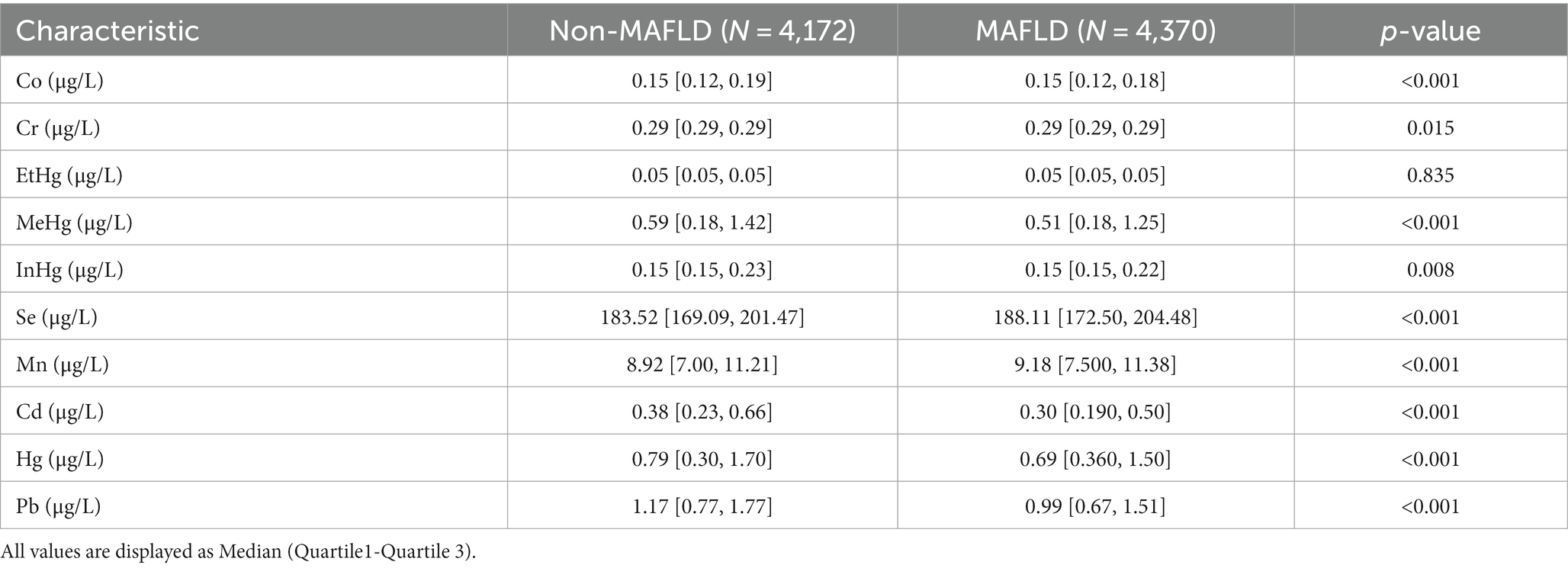
In the preliminary analysis, the 10 heavy metal blood exposure levels were skewed data. Comparing the participants in the Non-MAFLD group, there were differences in the distribution of heavy metal blood levels in Pb, Hg, Mn, Se, MeHg, InHg, Cd and Co among the participants in the MAFLD group, with Mn and Se levels being high and the differences being statistically significant (p < 0.05) (Table 2).
Influence of blood heavy metal exposure levels on the risk of MAFLD
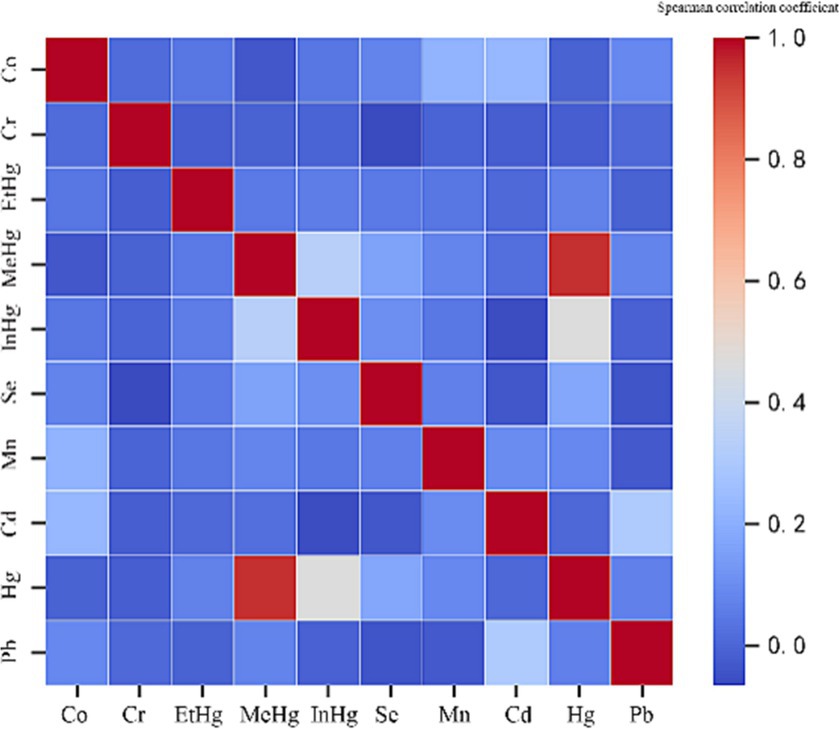
Firstly, Spearman’s correlation matrix analysis (Figure 2) was performed for the correlation of 10 environmental heavy metals. There was a significant correlation between MeHg and Hg (correlation value of 0.951, p﹤0.001). Therefore, MeHg should be eliminated in the next Poisson review. Exploratory factor analysis confirmed highly correlated covariates, with the other nine metals having limited correlation with each other.
Analysis using a multifactorial logistic regression model found that high blood Mn and Se exposure levels were associated with an increased risk of disease in patients in the MAFLD group compared to the Non-MAFLD group [OR (95% CI): Mn 1.028 (1.015–1.04); Se 1.006 (1.004–1.008)] (Figure 3A). The random forest model determined the importance of the variables, with the top 2 rankings of characteristic importance Se and Mn, which matched the multi-factor logistic regression model (Figure 3B).
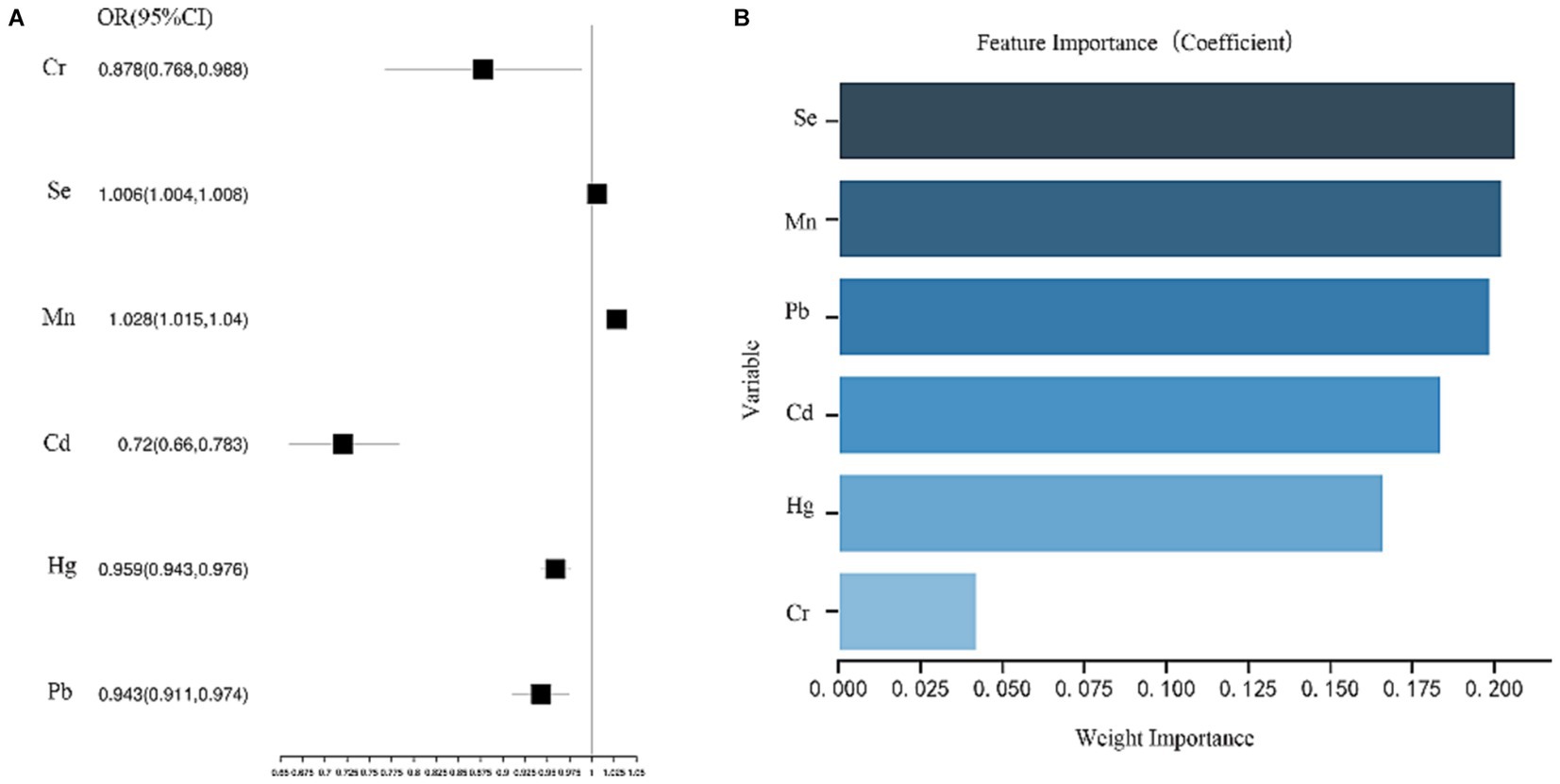
Figure 3. (A) Analysis results of multi-factor logistics regression model. (B) The order of importance of variables.
Threshold effect of blood Mn and Se exposure levels on the risk of MAFLD prevalence
The RCS plot (Figure 4) shows a non-linear relationship between serum Mn and Se exposure levels and the risk of MAFLD. The OR (95% CI) for MAFLD prevalence (Table 3) was 3.936 (2.631–5.887) for every 1 unit increase in Log Mn until serum Mn levels rose to the turning point (Log Mn = 1.10, Mn = 12.61 μg/L). This correlation was not significant (p > 0.05) after serum Mn levels rose to the turning point. A similar phenomenon was observed for serum Se levels, with a turning point of (Log Se = 2.30, Se = 199.55 μg/L).
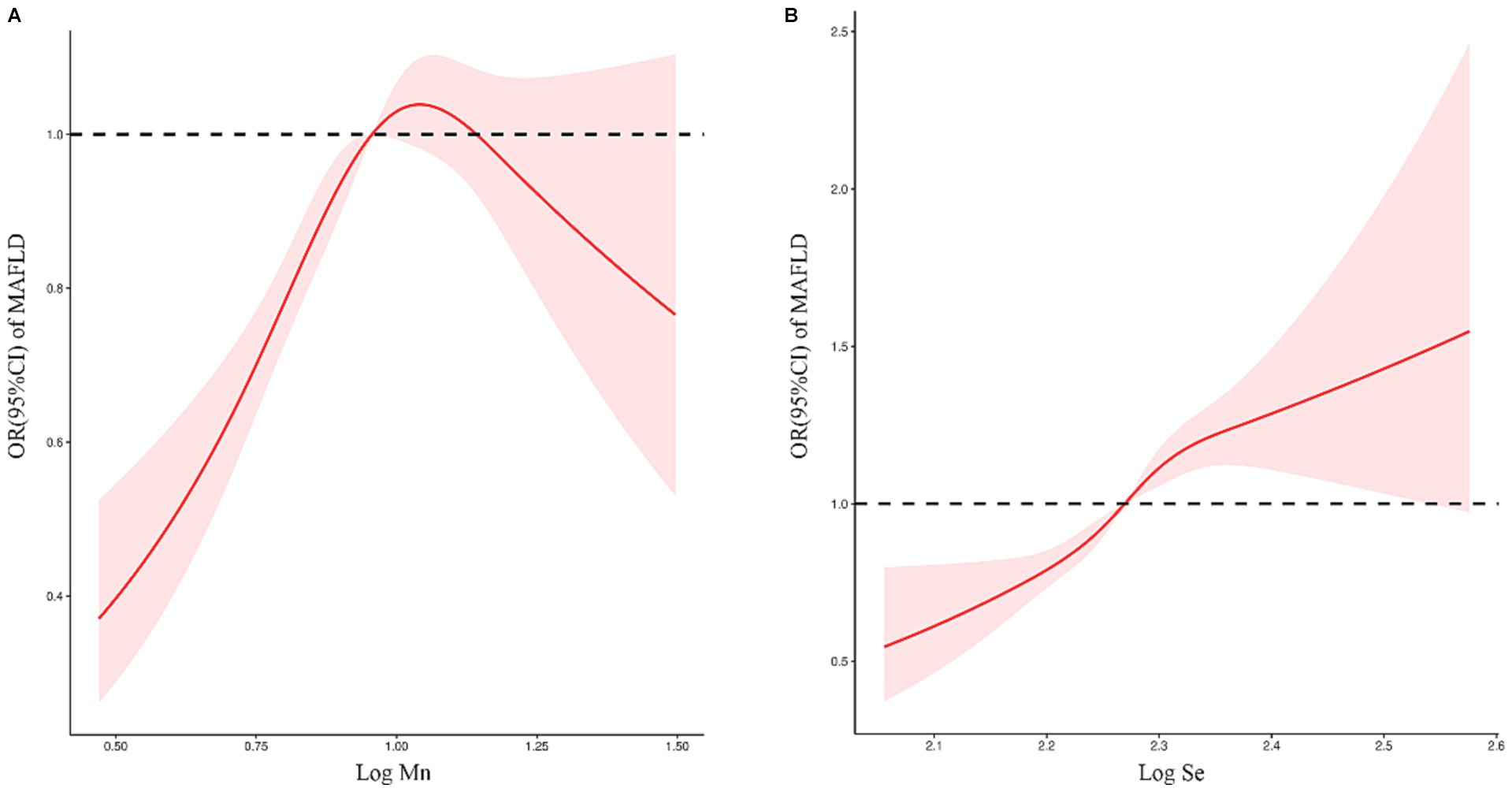
Figure 4. (A) Relationship between serum Mn exposure level and risk of MAFLD. (B) Relationship between serum Se exposure level and risk of MAFLD.
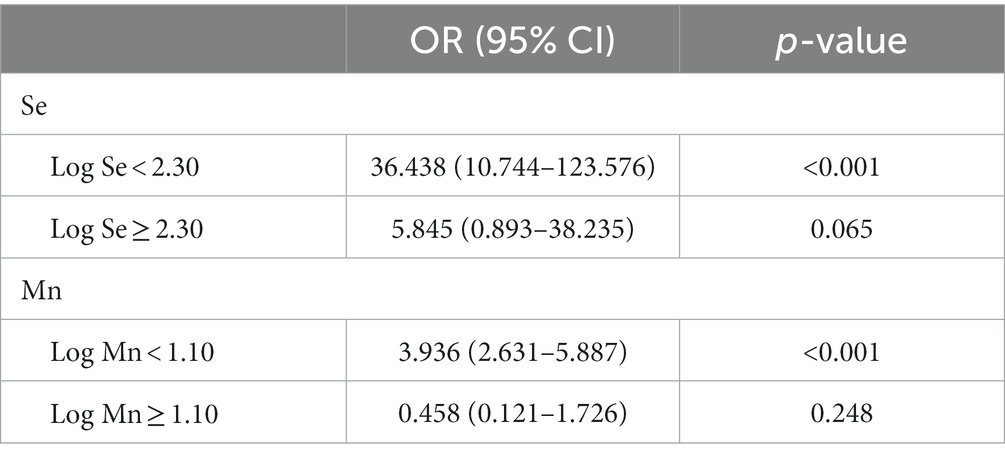
Table 3. Threshold effect analysis of blood heavy metals on MAFLD using piecewise linear regression.
Discussion
MAFLD, the most common cause of chronic liver disease (1), poses a huge potential economic burden on society. The etiology of MAFLD has not been fully elucidated, with oxidative stress and metabolic imbalance of fatty acids playing a crucial role (11).
Due to the continuous development of human industry, mineral resources have been extensively exploited, leading to severe heavy metal pollution of water and soil, which in turn poses a threat to human health. With the increase in heavy metal pollution, the relationship between heavy metal exposure and metabolic diseases in adults has attracted widespread attention. Studies have shown that the liver is one of the major accumulation organs of heavy metals, especially plumbum, cadmium, nickel and chromium, which accumulate in large quantities in the liver (12). The toxic effects of heavy metals occur mainly through oxidative stress induced by the production of reactive oxygen species (ROS) in cells, which may cause liver damage and lead to the development of fatty liver (13, 14). Previous studies have focused on the effects of heavy metals on the liver, and there is a lack of description of thresholds for the relationship between heavy metal exposures and the risk of developing MAFLD in adults, so more research is needed.
We obtained blood level data for 10 different metals at NHANES from 2015 to 2020 and obtained data on demographic characteristics, social factors, daily health-related behaviors and variables related to medical comorbidities. First, we compared the blood levels of heavy metals between participants in the Non-MAFLD and MAFLD groups and found differences in the distribution of heavy metal blood levels in the MAFLD group for Pb, Hg, Mn, Se, MeHg, InHg, Cd, and Co, with statistically significant differences (p < 0.05) for Mn and Se levels. We then analyzed the effect of heavy metal exposure levels on the risk of MAFLD prevalence using a multifactorial logistic regression model and found that high blood Mn and Se exposure levels were associated with an increased risk of prevalence in patients in the MAFLD group. The importance of the variables in the random forest model also indicated that Se and Mn ranked in the top 2, which was consistent with the multi-factor logistic regression model. We then used the RCS model to find a non-linear relationship between serum Mn and Se exposure levels and the risk of MAFLD. Finally, based on this non-linear relationship, we used a piecewise linear regression model to derive a turning point of Log Mn = 1.10 for serum Mn = 12.61 μg/L and Log Se = 2.30 for serum Se = 199.55 μg/L.
Both Mn and Se are controversial and contradictory in influencing the risk of MAFLD. Mn is an essential element for humans and Mn-containing superoxide dismutase (MnSOD) is highly expressed in differentiated organs containing large numbers of mitochondria, such as the heart, liver and kidney, and is the main antioxidant enzyme in mitochondria, playing a key role in the detoxification of superoxide radicals and protecting cells from oxidative stress (15). A clinical study found that there may be sex differences in the association between blood Mn and MAFLD, higher blood Mn may be a potential protective factor for MAFLD in males, but there is no significant association in females (16). The sex difference may be related to the fact that Mn can act as an endocrine disrupter. Study have shown that Mn can increase the level of serum testosterone (17). The association between testosterone and MAFLD differs by sex, with higher testosterone levels decreasing the odds of MAFLD in men and increasing the odds of NAFLD in women (18, 19). In addition, some clinical studies have shown that higher levels of Mn exposure are positively associated with MAFLD and hepatic steatosis degeneration and liver fibrosis (8, 9, 20). However, hepatic steatosis, fibrosis, and hepatocellular carcinoma all occurred more frequently in men than in women (21, 22). This suggests that there is a sex difference in liver disease itself. Perhaps, Mn may have magnified this difference, which requires more study. Mn also has an effect on liver function. In a cross-sectional study of Chinese manganese miners, ALT, AST and direct bilirubin (DBIL) were found to be significantly elevated in workers exposed to high levels of Mn (23). Additional studies have also shown an association between Mn and elevated liver enzyme levels and mortality associated with chronic liver disease (24), with the most significant association between Mn and ALT (7). Animal studies have also shown that Mn exposure can cause liver damage through oxidative stress, mitochondrial damage and thus elevated liver enzymes (25, 26).
We generally believe that the antioxidant effect of Se may attenuate the development of metabolic diseases including MAFLD (27–29). In an animal study, dietary selenium was shown to promote selenoprotein P1 (SEPP1) synthesis and modulate the Kelch-like ECH-associated protein 1 (KEAP1)/NF-E2-related factor 2 (NRF2) pathway to protect against hepatocyte oxidative stress (30). However, several previous clinical studies, including ours, have shown that high levels of selenium are associated with the risk of developing MAFLD, positively correlated with hepatic steatosis (8, 9, 31–33) and positively correlated with changes in liver function, with Se being most significantly associated with changes in ALT (7). We suggest that this may be related to the fact that high dietary selenium intake increases MAFLD risk by modulating dysregulation of insulin biosynthesis and secretion as well as stimulating glucagon secretion, insulin resistance and dyslipidemia (33, 34). It has also been proposed that MAFLD and liver fibrosis are caused by an imbalance in selenium homeostasis rather than by dietary selenium intake (8).
Although our study confirmed the association of Mn and Se with the risk of MAFLD prevalence and also calculated their thresholds precisely, there are still some unavoidable limitations and shortcomings. On the one hand, our study is a retrospective study, based on published data for analysis, and has inherent shortcomings compared to prospective studies. On the other hand, the mechanisms underlying the effects of Mn and Se on MAFLD are not fully understood, and previous studies have had conflicting findings. Therefore, more studies, especially prospective studies and laboratory studies, are needed to further clarify the mechanisms of the effects of Mn and Se on MAFLD.
Conclusion
Our study further clarified the relationship between blood heavy metal exposure levels and MAFLD in adults. Notably, Se and Mn exhibited the most significant influence on MAFLD. Although higher exposure levels of Se and Mn were linked to an elevated risk of MAFLD, this association demonstrated a non-linear pattern with clear thresholds. Furthermore, the exact mechanism behind the impact of heavy metals on MAFLD remains incompletely understood based on current research, necessitating future prospective and laboratory investigations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because Institutional Review Board approval was not required as the NHANES represents an adequately de-identifed and publicly available dataset. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements because the NHANES represents an adequately de-identifed and publicly available dataset.
Author contributions
ST: Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – review & editing. SL: Writing – original draft. ZW: Writing – review & editing. JS: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Dongguan Songshan Lake Tungwah Hospital.
Acknowledgments
We thank those people and staff who contributed data to NHANES.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1280163/full#supplementary-material
Footnotes
References
1. Huang, DQ, El-Serag, HB, and Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
2. Eslam, M, Newsome, PN, Sarin, SK, Anstee, QM, Targher, G, Romero-Gomez, M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
3. Badmus, OO, Hillhouse, SA, Anderson, CD, Hinds, TD, and Stec, DE. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci (Lond). (2022) 136:1347–66. doi: 10.1042/CS20220572
4. Liang, Y, Chen, H, Liu, Y, Hou, X, Wei, L, Bao, Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab. (2022) 107:88–97. doi: 10.1210/clinem/dgab641
5. Bae, SDW, George, J, and Qiao, L. From MAFLD to hepatocellular carcinoma and everything in between. Chin Med J. (2022) 135:547–56. doi: 10.1097/CM9.0000000000002089
6. Vitale, A, Svegliati-Baroni, G, Ortolani, A, Cucco, M, Dalla Riva, GV, Giannini, EG, et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002-2033: the ITA.LI.CA database. Gut. (2023) 72:141–52. doi: 10.1136/gutjnl-2021-324915
7. Li, W, Li, X, Su, J, Chen, H, Zhao, P, Qian, H, et al. Associations of blood metals with liver function: analysis of NHANES from 2011 to 2018. Chemosphere. (2023) 317:137854. doi: 10.1016/j.chemosphere.2023.137854
8. Liu, J, Tan, L, Liu, Z, and Shi, R. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with blood selenium level based on the NHANES 2017-2018. Ann Med. (2022) 54:2259–68. doi: 10.1080/07853890.2022.2110277
9. Spaur, M, Nigra, AE, Sanchez, TR, Navas-Acien, A, Lazo, M, and Wu, HC. Association of blood manganese, selenium with steatosis, fibrosis in the National Health and nutrition examination survey, 2017-18. Environ Res. (2022) 213:113647. doi: 10.1016/j.envres.2022.113647
10. Eslam, M, Newsome, P, Sarin, S, Anstee, Q, Targher, G, Romero-Gomez, M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:1575–9. doi: 10.1016/j.jhep.2020.07.045
11. Mota, M, Banini, BA, Cazanave, SC, and Sanyal, AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metab Clin Exp. (2016) 65:1049–61. doi: 10.1016/j.metabol.2016.02.014
12. Bawuro, AA, Voegborlo, RB, and Adimado, AA. Bioaccumulation of heavy metals in some tissues of fish in Lake Geriyo, Adamawa state, Nigeria. J Environ Public Health. (2018) 2018:1–7. doi: 10.1155/2018/1854892
13. Renu, K, Chakraborty, R, Myakala, H, Koti, R, Famurewa, AC, Madhyastha, H, et al. Molecular mechanism of heavy metals (Lead, chromium, arsenic, mercury, nickel and cadmium) - induced hepatotoxicity - a review. Chemosphere. (2021) 271:129735. doi: 10.1016/j.chemosphere.2021.129735
14. Saïdi, SA, Azaza, MS, Windmolders, P, van Pelt, J, and El-Feki, A. Cytotoxicity evaluation and antioxidant enzyme expression related to heavy metals found in tuna by-products meal: an in vitro study in human and rat liver cell lines. Exp Toxicol Pathol. (2013) 65:1025–33. doi: 10.1016/j.etp.2013.03.001
15. Li, S, Lu, L, Hao, S, Wang, Y, Zhang, L, Liu, S, et al. Dietary manganese modulates expression of the manganese-containing superoxide dismutase gene in chickens. J Nutr. (2011) 141:189–94. doi: 10.3945/jn.110.126680
16. Zhang, D, Wu, S, Lan, Y, Chen, S, Wang, Y, Sun, Y, et al. Blood manganese and nonalcoholic fatty liver disease: a cohort-based case-control study. Chemosphere. (2022) 287:132316. doi: 10.1016/j.chemosphere.2021.132316
17. Iavicoli, I, Fontana, L, and Bergamaschi, A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. (2009) 12:206–23. doi: 10.1080/10937400902902062
18. Jaruvongvanich, V, Sanguankeo, A, Riangwiwat, T, and Upala, S. Testosterone, sex hormone-binding globulin and nonalcoholic fatty liver disease: a systematic review and Meta-analysis. Ann Hepatol. (2017) 16:382–94. doi: 10.5604/01.3001.0009.8593
19. Sarkar, M. Testosterone levels in women: implications for fatty liver and beyond. J Women's Health. (2002) 28:1015–6. doi: 10.1089/jwh.2019.7661
20. Liu, J, Tan, L, Liu, Z, and Shi, R. Blood and urine manganese exposure in non-alcoholic fatty liver disease and advanced liver fibrosis: an observational study. Environ Sci Pollut Res Int. (2023) 30:22222–31. doi: 10.1007/s11356-022-23630-4
21. Bissell, DM. Sex and hepatic fibrosis. Hepatology (Baltimore, Md). (1999) 29:988–9. doi: 10.1002/hep.510290351
22. Goulart, AC, Bianchi, LLT, Bismarchi, D, Miname, MH, Lourenção, ACM, Henares, BB, et al. Sex differences in the relationship between hepatic steatosis, mood and anxiety disorders. J Psychosom Res. (2023) 168:111216. doi: 10.1016/j.jpsychores.2023.111216
23. Deng, Q, Liu, J, Li, Q, Chen, K, Liu, Z, Shen, Y, et al. Interaction of occupational manganese exposure and alcohol drinking aggravates the increase of liver enzyme concentrations from a cross-sectional study in China. Environ Health. (2013) 12:30. doi: 10.1186/1476-069X-12-30
24. Spangler, JG. Air manganese levels and chronic liver disease mortality in North Carolina counties: an ecological study. Int J Environ Res Public Health. (2012) 9:3258–63. doi: 10.3390/ijerph9093258
25. Chtourou, Y, Garoui, E, Boudawara, T, and Zeghal, N. Therapeutic efficacy of silymarin from milk thistle in reducing manganese-induced hepatic damage and apoptosis in rats. Hum Exp Toxicol. (2013) 32:70–81. doi: 10.1177/0960327112455674
26. Jiang, J, Wang, F, Wang, L, Xiao, J, and Guo, D. Manganese chloride exposure causes disorder of energy metabolism and induces oxidative stress and autophagy in chicken liver. Biol Trace Elem Res. (2020) 197:254–61. doi: 10.1007/s12011-019-01960-8
27. Huang, J, Xie, L, Song, A, and Zhang, C. Selenium status and its antioxidant role in metabolic diseases. Oxidative Med Cell Longev. (2022) 2022:1–15. doi: 10.1155/2022/7009863
28. Wang, N, Tan, HY, Li, S, Xu, Y, Guo, W, and Feng, Y. Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxidative Med Cell Longev. (2017) 2017:1–13. doi: 10.1155/2017/7478523
29. Xu, L, Lu, Y, Wang, N, and Feng, Y. The role and mechanisms of selenium supplementation on fatty liver-associated disorder. Antioxidants Basel, Switzerland. (2022) 11:922. doi: 10.3390/antiox11050922
30. Wang, Y, Liu, B, Wu, P, Chu, Y, Gui, S, Zheng, Y, et al. Dietary selenium alleviated mouse liver oxidative stress and NAFLD induced by obesity by regulating the KEAP1/NRF2 pathway. Antioxidants (Basel, Switzerland). (2022) 11:349. doi: 10.3390/antiox11020349
31. Wang, X, Seo, YA, and Park, SK. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and nutrition examination survey (NHANES) 2011-2016. Environ Res. (2021) 197:111190. doi: 10.1016/j.envres.2021.111190
32. Wu, J, Zeng, C, Yang, Z, Li, X, Lei, G, Xie, D, et al. Association between dietary selenium intake and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study. J Am Coll Nutr. (2020) 39:103–11. doi: 10.1080/07315724.2019.1613271
33. Yang, Z, Yan, C, Liu, G, Niu, Y, Zhang, W, Lu, S, et al. Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: a cross-sectional analysis. Sci Rep. (2016) 6:37288. doi: 10.1038/srep37288
Keywords: MAFLD, heavy metal, NHANES, manganese, selenium
Citation: Tang S, Luo S, Wu Z and Su J (2024) Association between blood heavy metal exposure levels and risk of metabolic dysfunction associated fatty liver disease in adults: 2015–2020 NHANES large cross-sectional study. Front. Public Health. 12:1280163. doi: 10.3389/fpubh.2024.1280163
Edited by:
Mohammad Imran Ansari, University of Maryland, Baltimore, United StatesReviewed by:
Aziz-Ur-Rahim Bacha, Harbin Institute of Technology, Shenzhen, ChinaBrigitte Le Magueresse-Battistoni, INSERM U1060 Laboratoire de Recherche en Cardiovasculaire, Métabolisme, diabétologie et Nutrition, France
Copyright © 2024 Tang, Luo, Wu and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiandong Su, c3R1bXNqZEAxNjMuY29t
†These authors have contributed equally to this work
 Song Tang
Song Tang Simin Luo
Simin Luo Zhendong Wu3†
Zhendong Wu3†