
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 20 February 2024
Sec. Substance Use Disorders and Behavioral Addictions
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1278834
This article is part of the Research TopicThe Impact of Prenatal Cannabinoid Exposure on Offspring DevelopmentView all 6 articles
Background: Marijuana potency and utilization both continue to increase across the United States. While the overall prevalence of cannabinoid utilization during pregnancy has been surveyed in various studies, the direct impact of changing governmental policies on pregnancy use is less characterized. Thus, we aimed to investigate how the legalization of recreational cannabinoid products impacted use during pregnancy in the state of New Mexico.
Methods: Participants who had a live birth during two study epochs were included: pre-legalization (Epoch 1: 1 January 2019–31 March 2021) and post-legalization (Epoch 2: 1 November 2021–30 November 2022). Participants were further divided into case group [prenatal cannabinoid exposure (PCE)] vs. control (no PCE), with cases being identified by documented self-report or a positive laboratory toxicology test for cannabinoid use during pregnancy.
Results: A total of 1,191 maternal/infant dyads were included in Epoch 1, and 378 maternal/infant dyads were included in Epoch 2. In Epoch 1, 788 dyads were controls with 403 cases, while Epoch 2 had 292 controls and 86 cases. Interestingly there was a significant decrease in self-report or positive laboratory toxicology tests in Epoch 2 compared to Epoch 1. Infants born following PCE in both Epoch groups were more commonly born via Cesarean section, had significantly smaller birth weight, length, and head circumference as well as significantly lower Apgar scores at 1 and 5 min.
Conclusion: The finding of decreased reported cannabinoid use in the post-legalization group is contradictory to previous studies which have shown increased rates of cannabinoid use after legalization. This could be due to multiple factors including changes in screening practices, the COVID-19 pandemic, and lack of commercialization of THC products. Additional studies are needed to further characterize how changing governmental policies impacts utilization during pregnancy.
Marijuana (Cannabis sativa) is a plant native to Eastern Asia and contains over 100 unique cannabinoids responsible for its psychoactive and medicinal properties (1). Of the more than 100 cannabinoids, trans-delta-9-tetrahydrocannabinol (THC) is the most psychoactive (2, 3). As a lipophilic molecule, THC can readily cross the placenta and penetrate the fetal central nervous system (CNS) (4–9). The concentration of THC in cannabinoid products used in the present day is up to 300% greater than the 1990s, with nearly triple the potency (10, 11). Compounding these changes is the increasing availability of cannabinoid products as states across the country continue to legalize them for recreational use.
In April 2021, the state of New Mexico passed the Cannabis Regulation Act, legalizing the consumption, purchase, possession, and cultivation of marijuana or other products containing THC (12). This came shortly after the state decriminalized possession of these products in 2019 (see Figure 1 for a timeline of key regulatory events in New Mexico and the United States). To date, 22 other states have legalized cannabinoid products for recreational use (13). Many of these states have commercial markets for cannabinoid products, and in New Mexico, licensed retail sales began in April 2022 (14). However, marijuana is currently classified as a schedule I drug by the United States Drug Enforcement Administration (DEA) and is an illegal drug federally. Despite the conflicting legalization status at the state and national level, cannabinoids remain the most widely used illicit drug in the United States. This also translates to pregnancy, where cannabinoid products remain the most used illicit substance in pregnancy (10). The impacts of legalizing cannabinoid products have been a controversial and pressing public health concern, and many studies show an increase in utilization after legalization (15–17). One study in particular analyzed data from six states that legalized cannabinoid products and the analysis revealed an increase in utilization in pregnant individuals throughout the preconception, prenatal, and postpartum stages (16). Legalization has also been shown to normalize use and minimize perceived harms. Dispensaries in Colorado recommend cannabinoid products for the treatment of morning sickness in pregnancy (18). Approximately 70% of pregnant and non-pregnant individuals believe there is only slight or no risk of harm from using cannabinoids during pregnancy (10, 19).
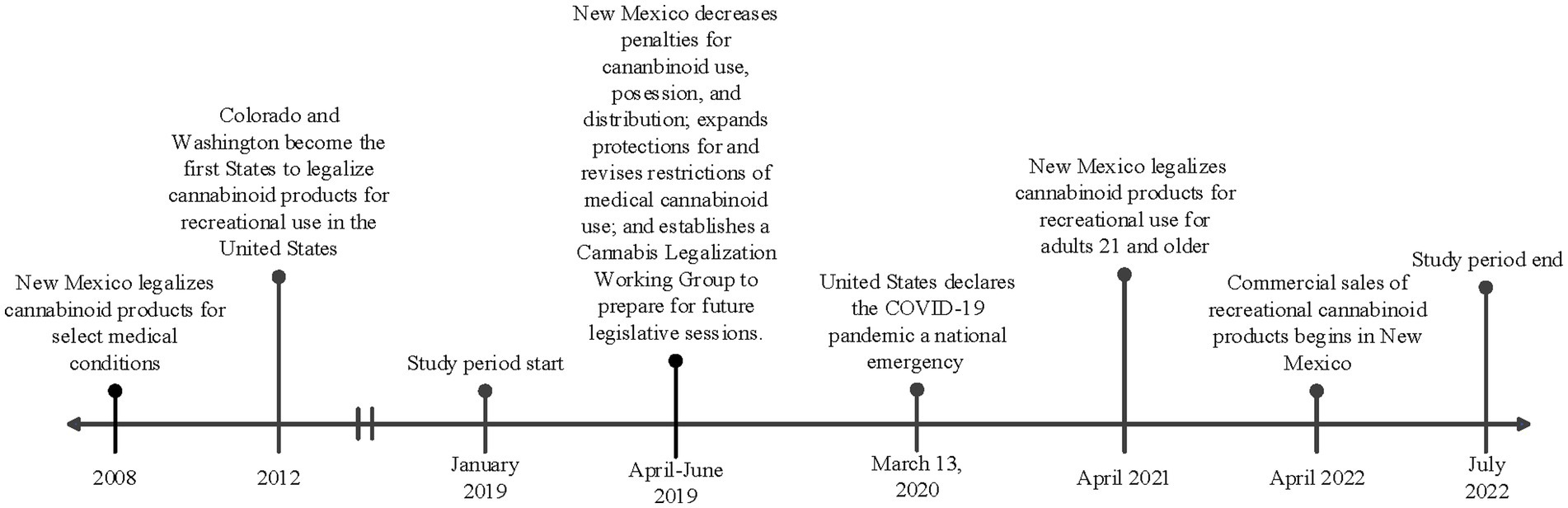
Figure 1. Timeline of key regulatory and contextual events in New Mexico and United States in relation to the study period.
Confounding the notion that cannabinoids are safe is the difficulty obtaining high quality studies to determine the impact on fetal growth and development. The concomitant use of other substances such as tobacco or alcohol, in the setting of changing cannabinoid potency, creates difficulty ascertaining if changes in fetal growth and development are directly related to the consumption of cannabinoids during pregnancy (20–22). Increased stress from social and environmental stressors, including socioeconomic status and access to prenatal care also directly impact neonatal outcomes (23–26). Additionally, THC itself may have adverse impacts on maternal stress and anxiety throughout pregnancy (27, 28). THC is a partial agonist of the cannabinoid receptor 1 (CB1) (27–29). While CB1 receptor activation can have an anxiolytic effect at low doses, it has a biphasic effect with higher doses of THC having an anxiogenic effect (27). Through this biphasic effect, the increased potency and concentration of THC products may lead to increased levels of maternal stress and anxiety, which may also impact fetal well-being (23).
Despite these challenges, it is known that cannabinoid receptors are present throughout the fetus during development, including in the CNS, cardiovascular, respiratory, immune, reproductive, hepatic, muscular, gastrointestinal, and skeletal systems, implicating the potential for vast alterations in development (20, 30–33). The expression of the primary brain cannabinoid receptor (CB1) is highest during gestation and is related to the development of other neurotransmitters such as the opioid and dopaminergic systems (1, 30, 34). It is also intensely expressed in the mesocorticolimbic system (1, 34–37). Cannabinoids and their receptors play a prominent role in synaptogenesis, neurite formation, neural migration, proliferation, and maturation (35, 36, 38–40). Thus, it is not surprising that several studies have observed alterations in dopaminergic activity in the amygdala, alterations in memory, verbal reasoning, visual–spatial processing, attention, sleep efficiency, increased impulsivity, and hyperactivity later in childhood following prenatal cannabinoid exposure (1, 32–34, 41–45). A review of the overall impact of cannabinoid exposure on an individual can be found in Lin et al. (46). Our understanding of population and developmental outcomes following in-utero cannabinoid exposure is evolving. Additional studies are needed to better characterize the consequences of prenatal cannabinoid exposure.
Due to the clinical implications of use following legalization, we sought to determine how legalization of cannabinoids would impact utilization in a pregnant population in New Mexico. We hypothesized that following legalization, a rise in the observed cannabinoid use rates would occur in the pregnant population.
Following approval by the University of New Mexico Health Sciences Center Institutional Review Board (IRB), study participants were identified through use of an honest broker. Individuals were included if they delivered a liveborn infant during the two epochs (pre-legalization and post-legalization) at the University of New Mexico Hospital (UNMH). UNMH serves the immediate urban and surrounding rural areas of New Mexico and accepts transports from across the state and surrounding states. The first epoch (pre-legalization) included individuals that delivered between 1 January 2019 and 31 March 2021. The second epoch (post-legalization) included individuals that delivered between 1 November 2021 and 30 November 2022. Individuals who delivered in between the two epochs were excluded from the study, as this was a transition period around the legalization of recreational cannabinoid use. The timeline of key regulatory and contextual events in New Mexico are shown in Figure 1, including the study periods.
The two epoch groups were further divided into prenatal cannabinoid exposure (case group, hereafter referred to as PCE) and no known prenatal cannabinoid exposure (control group). Participants were included in the case group if there was self-reported cannabinoid use documented during the pregnancy or if at least one laboratory test was positive for metabolites of marijuana during the pregnancy. Individuals with no positive laboratory and no self-reported use were included in the control group. No other laboratory tests were analyzed as part of the study protocol.
Information related to substance use in pregnancy, demographic information, and birth outcomes were obtained for all participants from the medical record. Specifically, maternal demographic information included maternal age, marital status (categorized as single/separated/divorced, married/civil union, partnered, minor/other), ethnicity (categorized as Hispanic/Latino, Non-Hispanic/Latino, other), race (categorized as White, Black/African American, American Indian/Alaska Native, Asian/Pacific Islander, other), and medical insurance type (categorized as Medicaid, commercial/self-pay, other/other government). Maternal information during pregnancy was obtained including gravida, parity, trimester of initiation of prenatal care, number of prenatal care visits, illicit substance use other than cannabinoids, chronic and perinatal maternal medical conditions, and prenatal medications. Infant information obtained included the sex, gestational age at birth, mode of delivery (vaginal or cesarean section), Apgar scores at 1 and 5 min, birth weight, birth length, head circumference, birth growth assessment (small, appropriate, or large for gestational age), congenital abnormalities if present, and need for oxygen therapy at birth.
Demographic and clinical characteristics were summarized using percentages for categorical variables and means and standard deviations for continuous variables. Statistical analysis was completed along two primary lines of comparison. First, analysis was done comparing demographic and clinical characteristics in maternal/infant dyads in total controls vs. total cases from all epochs combined. Second, analysis was completed comparing these same characteristics in maternal/infant dyad groups (control vs. case) in Epoch 1 vs. Epoch 2. Statistical analysis was completed primarily through two-sample t-test assuming equal variances for continuous variables and chi-square tests for categorical variables, with expected values for cannabinoid utilization calculated within the chi-square test. Statistical significance was defined with an alpha level of 0.05.
Following these analyses, we performed three multiple logistic regression models to ascertain the independent effects of COVID-19 shutdown, insurance type, other substance use, and legalization on the likelihood of prenatal cannabinoid exposure. In all models, the period of COVID-19 shutdown was determined by the New Mexico Department of Health guidelines; the shutdown officially began on 11 March 2020, and ended on 31 March 2022. In Model 1, the COVID-19 shutdown was coded as a binary variable. All participants in Epoch 1 with infant birthdates prior to 11 March 2020 were classified into the non-COVID-19 shutdown group, and those with infant birthdates after 11 March 2020 were classified into the COVID-19-shutdown group. All Epoch 2 participants with infant birthdates prior to 31 March 2022 were classified into the COVID-19 shutdown group, and all participants with infants born after that date were classified into the non-COVID-19-shutdown group.
Model 2 separated the data into three COVID-19 periods: (1) pre-COVID-19 shutdown (infant birthdates prior to 11 March 2020), (2) during COVID-19 shutdown (infant birthdates between 11 March 2020 and 31 March 2022), and (3) post-COVID-19 shutdown (infant birthdates after 31 March 2022). Model 3 separated the data into three time periods according to cannabinoid accessibility: (1) pre-legalization (infant birthdates prior to 1 April 2021), (2) post-legalization/pre-legal retail sales (infant birthdates between 1 April 2021 and 1 April 2022), and (3) post-legal retail sales (infant birthdates after 1 April 2022).
We used odds ratios to quantify the strength and direction of associations and assessed the overall fit of all models using McFadden’s pseudo-R (2). A significance threshold of 0.05 was used to determine the statistical significance of the model and individual predictors.
A total of 1,871 maternal/infant dyads were admitted to UNMH during the entire study duration, as shown in Figure 2. However, 302 of the infants were born during the transition period between pre- and post-legalization and were excluded from further analysis. The pre-legalization Epoch (Epoch 1) included 1,191 maternal/infant dyad, of which 788 were controls (no known prenatal cannabinoid use) and 403 were cases (confirmed prenatal cannabinoid use). The post-legalization Epoch (Epoch 2) included 378 maternal/infant dyads, with 292 controls and 86 cases.
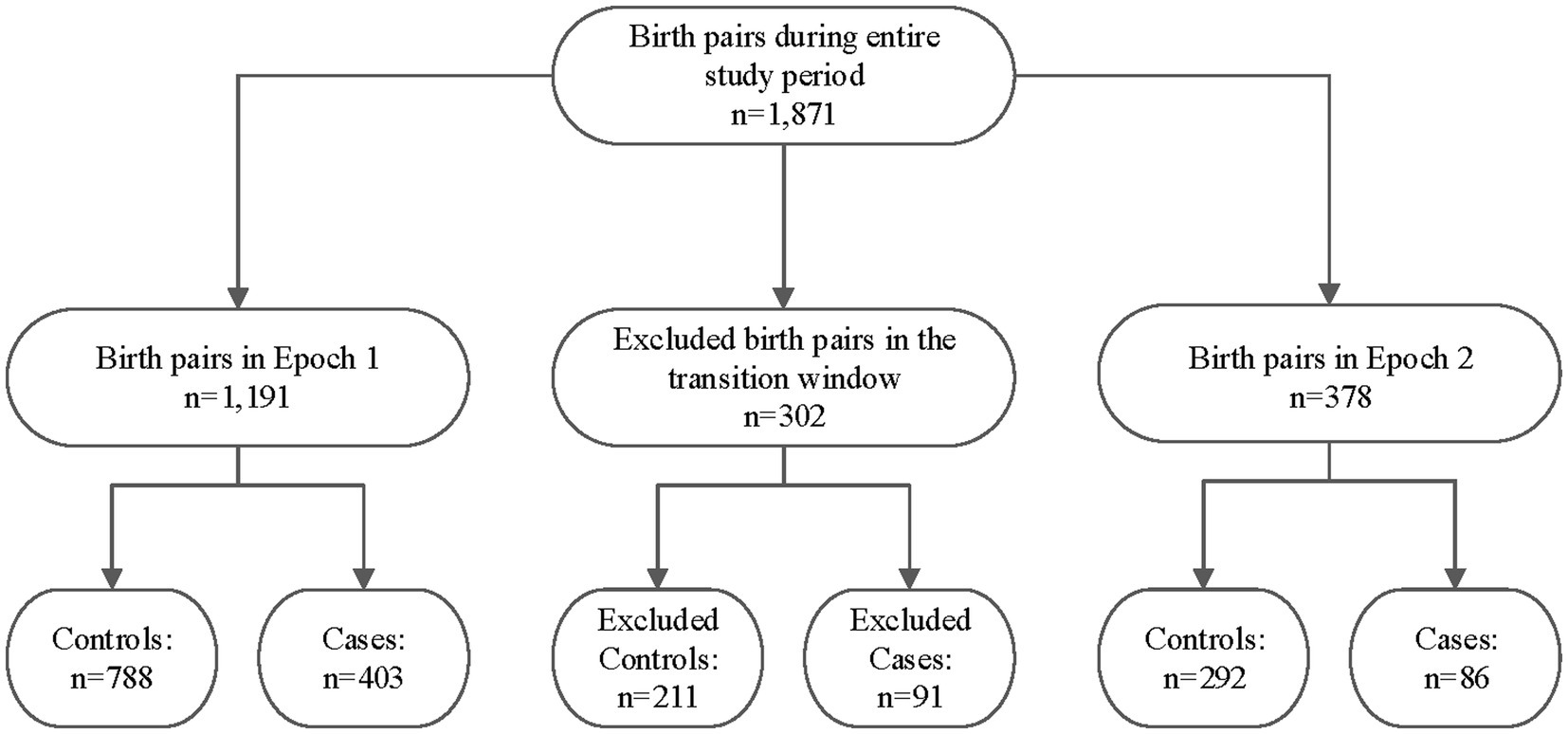
Figure 2. Study sample breakdown by epoch, transition window, and case/control status. Epoch 1: The pre-legalization window of the study period (1 January 2019–31 March 2021) Transition window: The period between when the New Mexico Cannabis Regulation Act was signed and several months after it went into effect (1 April–31 October 2021) Epoch 2: The post-legalization window of the study period (1 November 2021–November 2022).
There was no difference in maternal age between Epoch 1 and Epoch 2, as well as between all controls compared to all PCE (p = 0.07, see Table 1). Interestingly, there was a difference in maternal marital status, with more individuals reporting single status in Epoch 1 compared to Epoch 2 (p < 0.05), as well as all controls compared to all PCE (p < 0.001). No statistical difference was noted in maternal ethnicity between Epoch 1 and Epoch 2, but fewer individuals identified as Asian/Pacific Islander in PCE compared to the control group (p < 0.001, see Table 1). More individuals had Medicaid insurance in the PCE group than expected (p < 0.001, see Table 1). Fewer individuals had adequate prenatal care (defined as <3 prenatal visits during the pregnancy) in Epoch 2 compared to Epoch 1 (p < 0.01), with a higher number of individuals in the PCE group having no or inadequate prenatal care (p < 0.001, see Table 1).
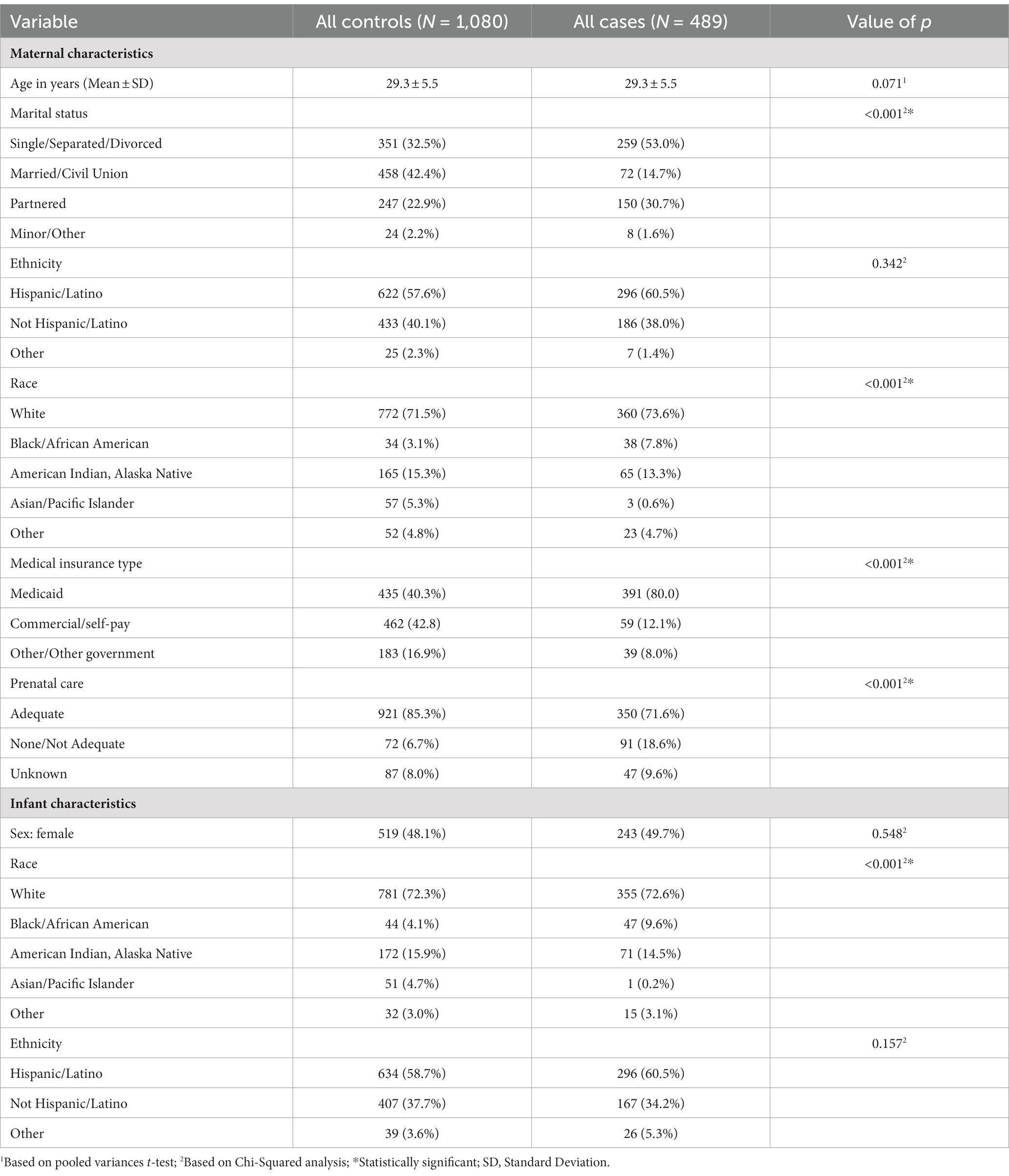
Table 1. Characteristics of the maternal and infant study participants stratified by case and control status.
Utilization of substances other than cannabinoids was different between groups as well (see Table 2). Tobacco use was significantly different between Epoch 1 and Epoch 2 (p < 0.05), and more individuals in all cases used tobacco compared to individuals with no prenatal cannabinoid exposure (p < 0.001, see Table 2). Similarly, alcohol use was higher in the PCE group compared to those in the control group (p < 0.001, see Table 2), although no difference was noted between Epoch 1 and Epoch 2. Opioid use was also significantly higher in the PCE group compared to the controls (p < 0.001, see Table 2).
There was no difference observed in the infant sex at birth between groups (p = 0.55, see Table 1), although the gestational age was significantly decreased in the PCE group compared to the controls (p < 0.01, see Table 3). Similar to maternal results, there were fewer individuals identifying as Asian/Pacific Islander in the PCE group compared to the controls (p < 0.001, see Table 1). In the control group, more deliveries occurred via vaginal delivery than expected, while more of the PCE group was delivered via Cesarean section (p < 0.05, see Table 3). Infants born following prenatal cannabinoid exposure were significantly lower weight (p < 0.001, see Figure 3), shorter in length (p < 0.001, see Table 3), and had smaller head circumferences (p < 0.001, see Table 3) at birth compared to infants in the control group. Additionally, the Apgar scores at 1 and 5 min were significantly lower in the PCE group compared to the control group (p < 0.001 and p < 0.01, respectively, see Table 3).
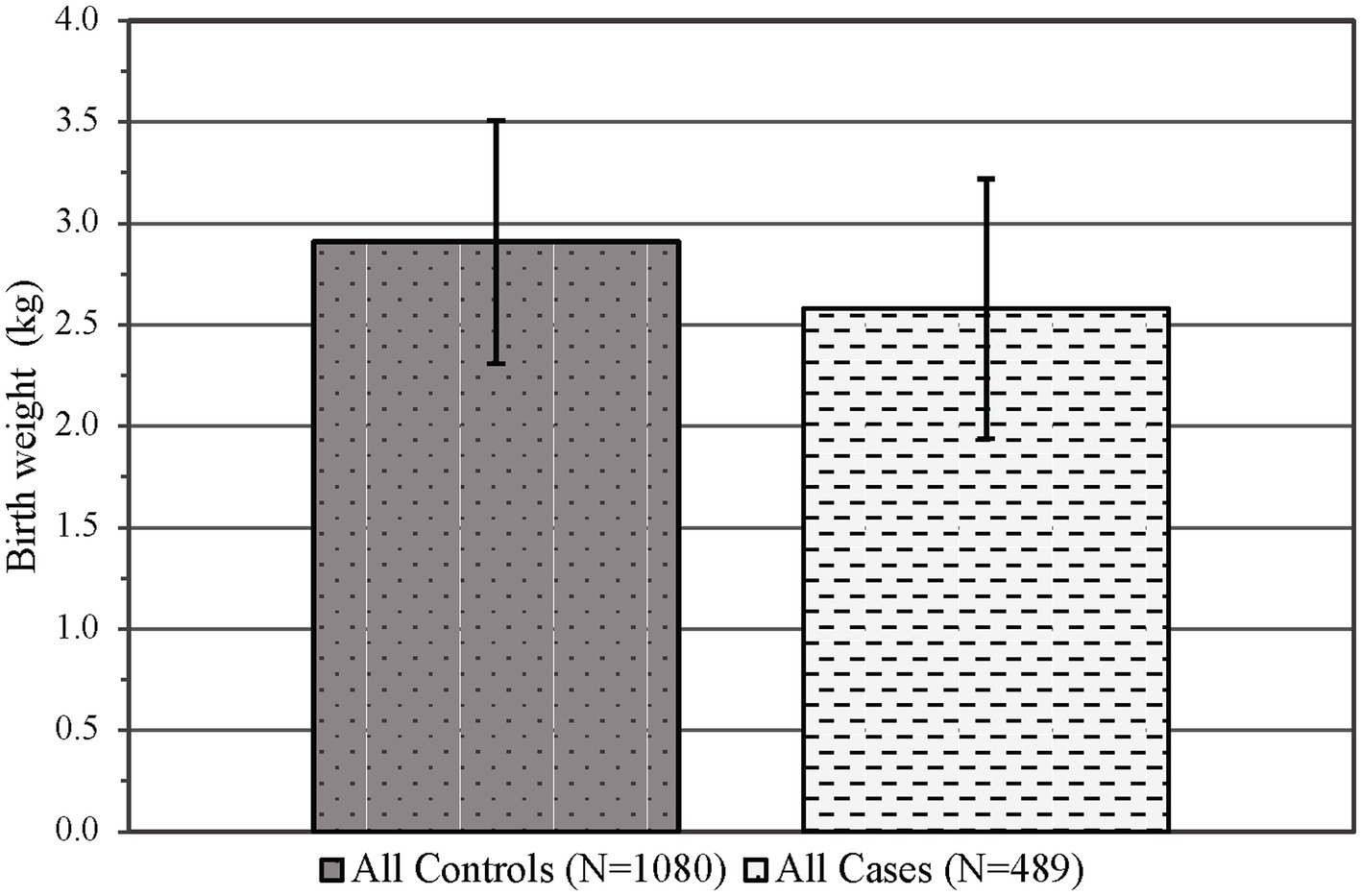
Figure 3. Infant mean birth weight in kilograms (±SD) by control and case status across both epochs. p < 0.001, SD, Standard deviation.
The utilization of cannabinoids during pregnancy was significantly different between Epoch 1 and Epoch 2. Interestingly, the utilization prior to legalization (Epoch 1) was higher than expected and was lower than expected following legalization (Epoch 2, p < 0.001, see Figure 4). In Epoch 2, the number of individuals identified with cannabinoid use during pregnancy through verbal screen alone was significantly higher than expected, with the number of individuals identified through toxicology screen alone or toxicology screen and verbal report both significantly lower than expected (p < 0.001). The number of individuals with no cannabinoid use was lower than expected prior to legalization (Epoch 1) and was higher than expected following legalization (Epoch 2, p < 0.001, see Figure 4).
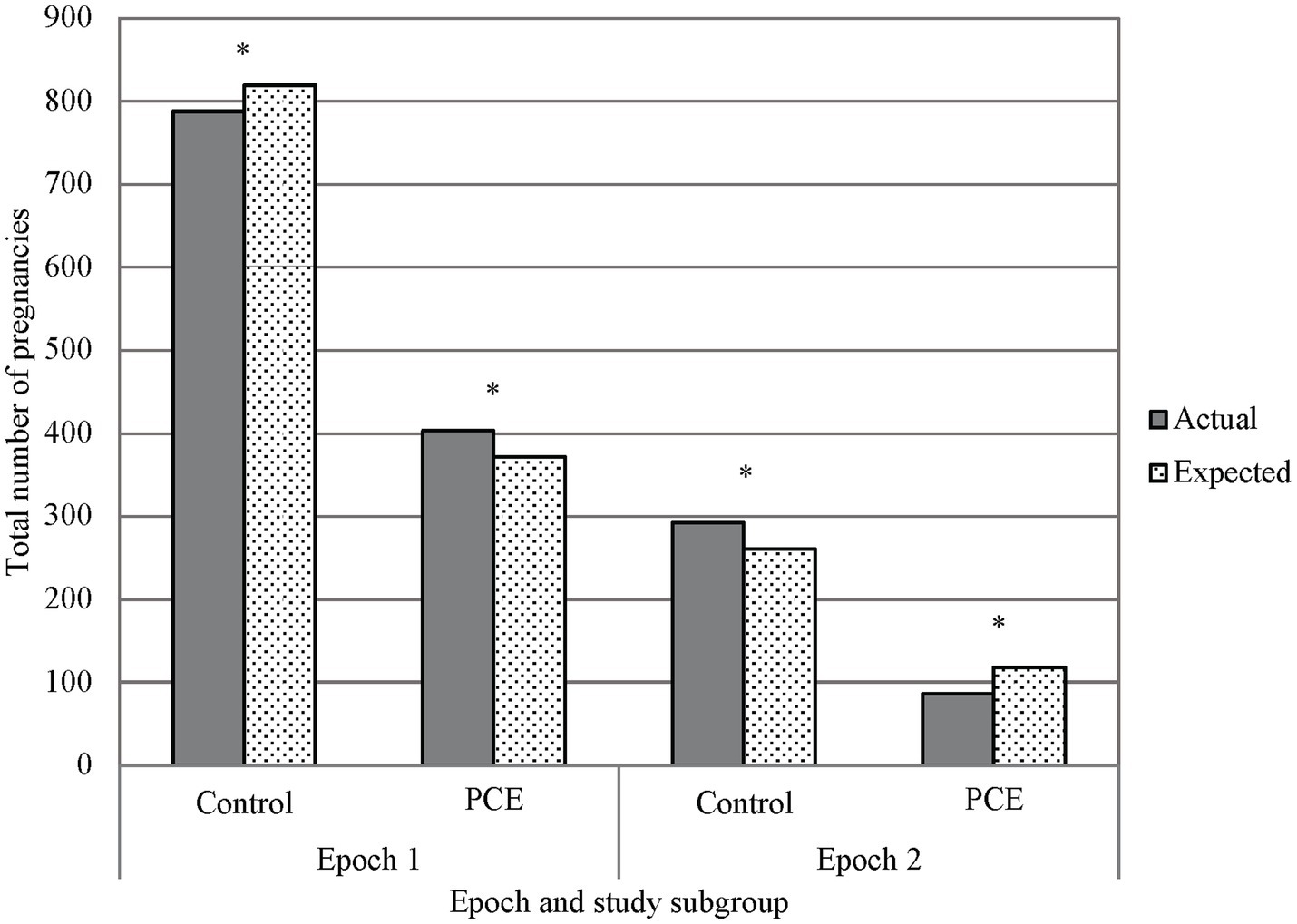
Figure 4. Primary outcome, marijuana exposure vs. control in epochs 1 and 2. A chi-squared analysis comparing PCE and no PCE in pregnancy before and after THC was legalized (Epochs 1 and 2 respectively) showed less PCE in Epoch 2 than what would be expected, and PCE in Epoch 1 was greater than what would be expected. Additionally, there was a noted decrease in reported PCE in Epoch 2 when compared to Epoch 1. *p < 0.001; PCE, Prenatal cannabis exposure.
The logistic regression model was statistically significant, χ2(7) = 594.02, p < 0.001. The model explained 31.0% (McFadden pseudo-R2) of the variance in PCE use and correctly classified 81.5% of patients in the PCE group (see Table 4). Most independent variables included in the model significantly contributed to variation in cannabinoid use during pregnancy, however the difference between participants in the commercial/self-pay and other/other government insurance groups was not significant (p = 0.068), and the COVID-19 shutdown did not contribute significantly to the variation in cannabinoid use among participants (p = 0.072).
The results of the logistic regression are shown in Table 5. Participants in the pre-legalization epoch were nearly twice as likely (OR = 1.95) to use cannabinoids than post-legalization epoch participants, which is consistent with the results of the chi-square tests. Utilization of substances other than cannabinoids contributed significantly to the variation in cannabinoid use among participants. Participants who used tobacco were approximately 10.8 times more likely to be in the PCE group compared to those who did not use tobacco. Similarly, participants who used alcohol were 5.4 times more likely to be in the PCE group than those who did not use alcohol, and participants who used opiates were 3.9 times more likely to be in the PCE group than those who did not use opiates. Insurance type and epoch also had significant independent effects on PCE status. Participants with Medicaid were 4.6 more likely to be in the PCE group relative to participants in the commercial/self-pay insurance group.
To further examine the decrease in utilization in Epoch 2, we tested two additional models. Model 2 separated the dataset into three time periods relating to the COVID-19 shutdown. We hypothesized that the reduction in utilization identified in the chi-square analyses stemmed from variation in utilization rates before, during, and after the shutdown. Additionally, any potential increase after the shutdown might not have been detectable in the initial analyses. However, the results confirm COVID-19 shutdown did not have a significant effect on cannabinoid utilization in our sample. Therefore, this model did not further explain the decrease in utilization following legalization (see Table 6).
Model 3 divided the dataset into three time periods relating to the availability of cannabinoids: (1) pre-retail availability, and (2) post-retail availability. Our hypothesis was that cannabinoid utilization was not more readily accessible until licensed retail stores opened. Since most participants in Epoch 2 predated the opening of licensed retail stores this may have prevented us from detecting an increase associated with legalization. However, we did not find support for this hypothesis; participants in the pre-retail group were 2.44 times more likely to utilize cannabinoids than those in the post-retail group (Table 7).
The results of this study did not support our hypothesis that cannabinoid use would increase during the post-legalization period; rather, there was a statistically significant decrease in observed utilization. This is inconsistent with other studies which have found increased rates of cannabinoid use in both the general population (15, 47, 48) and specifically during the pre-conception, prenatal, and post-partum time periods (16, 17) following the legalization period in other states. This trend of increased use of cannabinoid products during pregnancy has also been shown in other countries including Canada (17).
Several different theories may explain this significant decrease in cannabinoid use in pregnancy during the post-legalization period. Changes in substance use reporting, decreased verbal and toxicology screening for substance use by medical providers, impacts of the COVID-19 pandemic on screening priorities and substance use reporting, and ease of access to cannabinoid products may all have impacted the results observed in this study (see Figure 5).
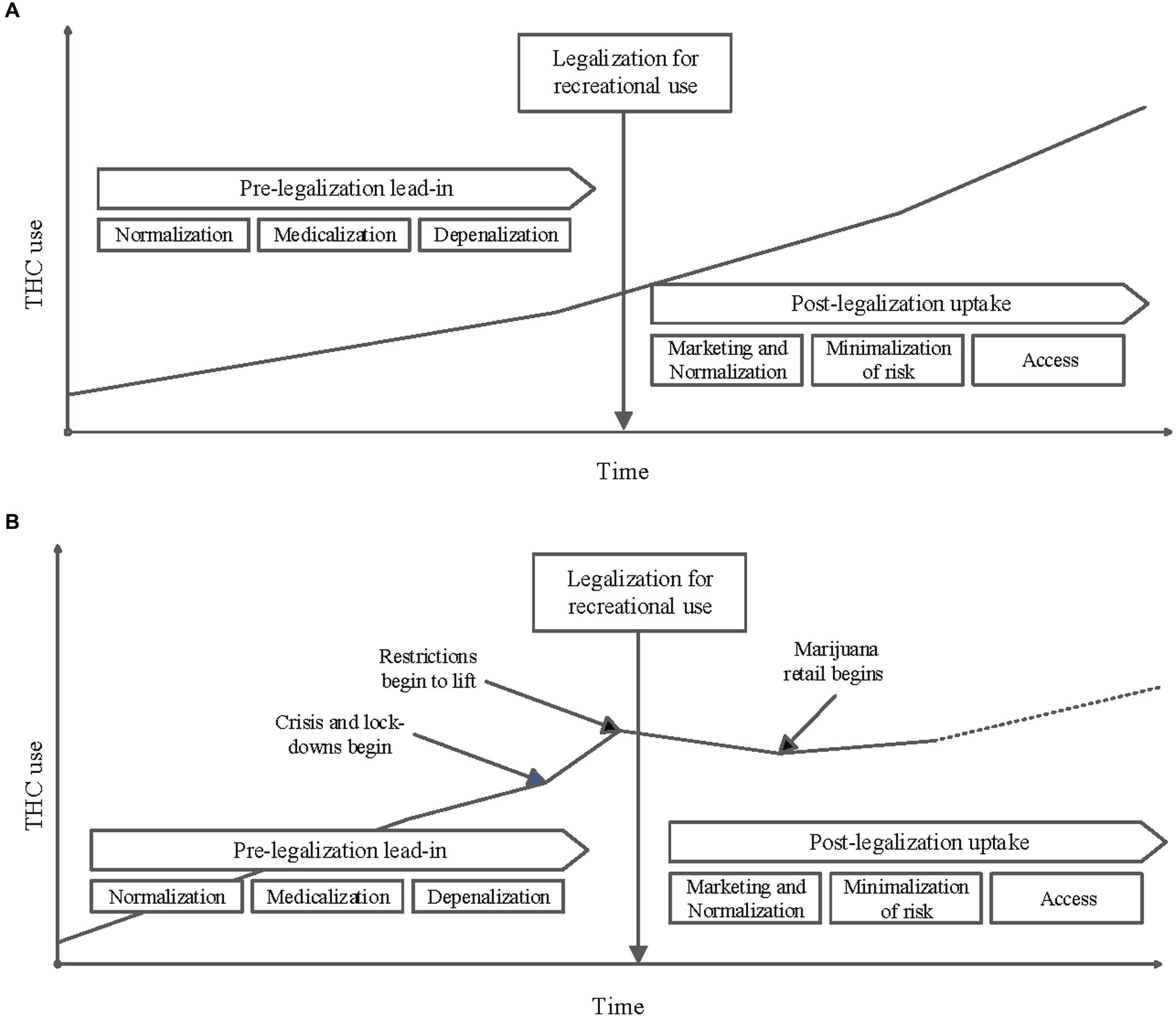
Figure 5. (A) Conceptual diagram of THC use in the population over time before and after it is legalized for recreational use: An increase in marijuana consumption is often observed in the time leading up to its legalization for recreational use. This is often attributed to changing social norms, decriminalization and depenalization, and legalizing marijuana for medicinal use. A further increase in use in the population is frequently seen post-legalization. This increase if often attributed to increased access that comes with commercial dispensaries, marketing, minimalization of risks associated with use, continued normalization and further medicalization. (B) Conceptual diagram of THC use in the study population over time as influenced by key events: The results of this study do not follow the trends depicted in A. While a pre-legalization lead-in period is visualized above, it is possible that the stresses of the Pandemic caused a sharp increase in use before legalization. As restrictions lifted in the Pandemic response, it is possible that this relative increase in THC use have started to decrease. The observed decrease in THC use after legalization also could be a reflection of decreased reporting secondary to Pandemic stressors. Additionally, it is not unreasonable to consider a slight increase of THC use after commercial distribution began with a potential upward trend supersceeding previously observed use rates.
Changes in substance use reporting, including verbal and toxicology screening for cannabinoids during the post-legalization period may be one of the contributing factors as to why there was a decrease in cannabinoid use during this period. Previous studies have shown providers discomfort counseling patients regarding cannabinoid use (49). Additionally, providers are also more likely to use punitive counseling, focusing on the negative social impacts of cannabinoid use (50). This may be due to a lack of general medical knowledge of the effects of cannabinoid use on the developing fetus, which may lead providers to look to other negative impacts of cannabinoids to dissuade patients from use. When cannabinoid products were legalized, many providers may have lost their main punitive counseling point. They could no longer dissuade patients from use solely on the grounds of legality of the drug. This may have discouraged providers from further screening and/or reporting cannabinoid use, leading to the observed results of this study. Additionally, hospital screening policies may change as a result of the legalization status, which could also directly impact the provider having knowledge of cannabinoid use during pregnancy. A recent study in Massachusetts analyzing cannabis use and documentation before and after legalization in the state revealed that despite there being an overall increase in cannabis use related documentation since legalization, only a small portion of medical notes documented actual cannabis use despite the uptake in usage in the state. This suggests a discrepancy between patient reports of cannabis use and electronic medical record documentation of cannabis use (51). Indeed, as the number of individuals identified in Epoch 2 through verbal screen alone was significantly lower than expected, it is very feasible that self-report was not provided and/or utilization of cannabinoids during pregnancy not asked by providers. Thus, the actual use may be overall be higher in the population than what we identified in this review, as the toxicology screening for cannabinoid use during pregnancy decreased following legalization.
Legalization of recreational cannabinoid use in New Mexico took place during the COVID-19 pandemic. The pre-legalization period began January 2019, just before the start of the pandemic, and continued through March 2021. While our logistic regression analysis did not show that COVID-19 alone was a significant contributor to the results of this study, it may have contributed to a complex interaction between multiple factors. A study in California reported increased rates of toxicology confirmed prenatal cannabinoid use during the early phases of the COVID-19 pandemic (from March 2020 to December 2020) (52). This was attributed to COVID-19 related stressors. The initial pre-legalization period included in our study were the earliest stages of the COVID-19 pandemic, at which time lockdown orders were more restrictive, and stressors were potentially higher. Therefore, it is possible the rates of pre-legalization cannabinoid use were higher than normal rates of use during a non-pandemic period, as more individuals used cannabinoid products as a means of stress relief. Our post-legalization period includes the later stages of the COVID-19 pandemic. The decreased rate of cannabinoid use observed in this study may be reflective of a return to a pre-pandemic baseline. In addition, the COVID-19 pandemic led to staffing shortages, and increased stress on healthcare workers (53). These stressors have continued past the COVID-19 pandemic into the present, shifting priorities to screening for viral exposure. Furthermore, an overburdened work force may be less thorough in substance use screening.
As shown in Figure 1, cannabinoid use was decriminalized in April of 2021 and legalized in June of 2021, but retail sales of cannabinoid products as commercial products did not begin until April of 2022. This could explain why we did not observe an increase in cannabinoid use in pregnancy during this period, as individuals may not have had the ease of access to cannabinoid products. It would be interesting to follow the population forward to determine if the utilization increases as the commercial product availability increases and as the world recovers from a pandemic.
As described in other studies, the pregnant population in the PCE group were significantly more likely to use other substances during pregnancy such as tobacco, alcohol, and opioids compared to the control group (see Table 2) (20–22). In evaluating social determinates of health, the pregnant population in the PCE group were more likely to have Medicaid insurance and less likely to have adequate prenatal care (see Table 1) compared to the control group. These findings may have been exacerbated by the COVID-19 pandemic as Epoch 2 showed a significant decrease in prenatal care compared to Epoch 1. It is likely that birthing individuals had higher stress levels due to the stressors of the COVID-19 pandemic which was exacerbated by the higher levels of THC in cannabis products (23, 27–29). No specific markers of maternal stress such as serum cortisol were obtained for this project, however this would be an interesting future direction for additional studies. It is, therefore, not unexpected that the infants in the PCE group had significantly lower birth weights, lengths, head circumferences, 1- and 5-min Apgar scores, and more increased likelihood of birth via Cesarean delivery compared to their counterparts. However, it is difficult to ascertain the extent to which these findings can be attributed to PCE verses the other factors shown to impact infant physical characteristics and health outcomes mentioned above (20–26).
There are several strengths for this study. The data collected by the honest broker through independent chart review of PCE and controls was verified, which decreased errors in data collection and ensured the veracity of the data. As the hospital serves a large geographic region, there is diversity in the participant population. Limitations of the study primarily stem from the nature of a retrospective chart review. The dosage or frequency of cannabinoid use was not typically documented and could not be reliably included in the analysis. The limited window for detecting marijuana metabolites in a urine sample may also have impacted the results, leading to an increase in false negatives. There may be a lead-in period observed during the pre-legalization phase of our study that showed increased THC utilization during this period. As cannabinoid products were decriminalized during the pre-legalization period, individuals may have increased use without the fear of criminal penalties. Additionally, the limitation of the sample size could affect these results. Future analyses completed over time may with a larger sample size may provide additional insights.
While this study looked at the pre-and post-legalization time periods of cannabinoid products in the state of New Mexico, future studies could investigate prenatal cannabinoid use moving forward, as increased commercialization could lead to increased ease of access and higher rates of usage, as would be consistent with other literature. Other studies could look specifically at rates of cannabinoid use in the pre-pandemic pre-legalization period, and the post-pandemic post legalization period to ascertain rates of cannabinoid use without the possible confounding factor of the COVID-19 pandemic.
Cannabinoid use in pregnancy had a statistically significant decrease during the post-legalization period compared to the pre-legalization period in this study. These findings are contradictory to previous studies which have shown increased rates of cannabinoid use following legalization in both the general and the pregnant population (15–17, 47, 48). Healthcare providers should be aware of these results to underline the importance of continued screening and harm reduction counseling in individuals using cannabinoid products during pregnancy, as the correlation between prenatal cannabinoid exposure and child development continues to be fully characterized. It is critically important to determine the impact legalization of cannabinoid products has on the pregnant population, as this may have lasting impacts for generations to come.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of New Mexico Health Sciences Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it was a retrospective chart review, thus not requiring consent.
JT: Data curation, Investigation, Writing – original draft, Writing – review & editing. CM: Data curation, Investigation, Writing – original draft, Writing – review & editing. MA: Data curation, Investigation, Writing – original draft, Writing – review & editing. JG: Formal analysis, Methodology, Writing – review & editing. JM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by an award from the National Center for Advancing Translational Sciences, National Institutes of Health under grant number UL1TR001449.
The authors would like to thank the support provided by the University of New Mexico Department of Pediatrics and the Division of Neonatology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Richardson, KA, Hester, AK, and McLemore, GL. Prenatal cannabis exposure—the "first hit" to the endocannabinoid system. Neurotoxicol Teratol. (2016) 58:5–14. doi: 10.1016/j.ntt.2016.08.003
2. Cooper, ZD, and Haney, M. Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatry. (2009) 21:104–12. doi: 10.1080/09540260902782752
3. Elsohly, MA, and Slade, D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. (2005) 78:539–48. doi: 10.1016/j.lfs.2005.09.011
4. Rokeby, ACE, Natale, BV, and Natale, DRC. Cannabinoids and the placenta: receptors, signaling and outcomes. Placenta. (2023) 135:51–61. doi: 10.1016/j.placenta.2023.03.002
5. Thompson, R, DeJong, K, and Lo, J. Marijuana use in pregnancy: a review. Obstet Gynecol Surv. (2019) 74:415–28. doi: 10.1097/OGX.0000000000000685
6. Kumar, AR, Sheikh, ED, Monson, JW, Ligon, SE, Talley, RL, Dornisch, EM, et al. Understanding the mechanism and extent of Transplacental transfer of (−)-∆(9) -tetrahydrocannabinol (THC) in the perfused human placenta to predict in vivo fetal THC exposure. Clin Pharmacol Ther. (2023) 114:446–58. doi: 10.1002/cpt.2964
7. Nyoni, EC, Sitaram, BR, and Taylor, DA. Determination of delta 9-tetrahydrocannabinol levels in brain tissue using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. (1996) 679:79–84. doi: 10.1016/0378-4347(96)00027-8
8. Grant, KS, Petroff, R, Isoherranen, N, Stella, N, and Burbacher, TM. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol Ther. (2018) 182:133–51. doi: 10.1016/j.pharmthera.2017.08.014
9. Sundram, S . Cannabis and neurodevelopment: implications for psychiatric disorders. Hum Psychopharmacol. (2006) 21:245–54. doi: 10.1002/hup.762
10. Committee on Obstetric Practice . Committee opinion no. 722: marijuana use during pregnancy and lactation. Obstet Gynecol. (2017) 130:e205–9. doi: 10.1097/AOG.0000000000002354
11. Skelton, KR, and Benjamin-Neelon, SE. Reexamining risks of prenatal Cannabis use-mounting evidence and a call to action. JAMA Netw Open. (2022) 5:e2145666. doi: 10.1001/jamanetworkopen.2021.45666
12. Lozier, B NV, and Thomas, D. State Approaches to Marijuana Policy. The Council of State Governments. (2023). Available at: https://www.csg.org/2023/02/13/state-approaches-to-marijuana-policy/.
13. Hartman, M. Cannabis Overview. National Conference of State Legislatures. (2022). Available at: https://www.ncsl.org/civil-and-criminal-justice/cannabis-overview.
14. McKay, D. Cannabis in NM: how will it work? Albuquerque Journal (2021). Available at: https://www.abqjournal.com/news/local/cannabis-in-nm-how-will-it-work/article_cffbfa2f-82c6-5d3e-81c5-7e7037214b27.html.
15. Zellers, SM, Ross, JM, Saunders, GRB, Ellingson, JM, Anderson, JE, Corley, RP, et al. Impacts of recreational cannabis legalization on cannabis use: a longitudinal discordant twin study. Addiction. (2023) 118:110–8. doi: 10.1111/add.16016
16. Skelton, KR, Hecht, AA, and Benjamin-Neelon, SE. Recreational Cannabis legalization in the US and maternal use during the preconception, prenatal, and postpartum periods. Int J Environ Res Public Health. (2020) 17:909. doi: 10.3390/ijerph17030909
17. Myran, DT, Roberts, R, Pugliese, M, Corsi, D, Walker, M, el-Chaâr, D, et al. Acute care related to cannabis use during pregnancy after the legalization of nonmedical cannabis in Ontario. CMAJ. (2023) 195:E699–708. doi: 10.1503/cmaj.230045
18. Dickson, B, Mansfield, C, Guiahi, M, Allshouse, AA, Borgelt, LM, Sheeder, J, et al. Recommendations from Cannabis dispensaries about first-trimester Cannabis use. Obstet Gynecol. (2018) 131:1031–8. doi: 10.1097/AOG.0000000000002619
19. Ko, JY, Farr, SL, Tong, VT, Creanga, AA, and Callaghan, WM. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol. (2015) 213:201.e1. doi: 10.1016/j.ajog.2015.03.021
20. Calvigioni, D, Hurd, YL, Harkany, T, and Keimpema, E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur Child Adolesc Psychiatry. (2014) 23:931–41. doi: 10.1007/s00787-014-0550-y
21. Varner, MW, Silver, RM, Rowland Hogue, CJ, Willinger, M, Parker, CB, Thorsten, VR, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol. (2014) 123:113–25. doi: 10.1097/AOG.0000000000000052
22. Metz, TD, and Stickrath, EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. (2015) 213:761–78. doi: 10.1016/j.ajog.2015.05.025
23. Muglia, LJ, Benhalima, K, Tong, S, and Ozanne, S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. (2022) 20:418. doi: 10.1186/s12916-022-02632-6
24. Wallace, HM, Micik, S, and Wise, P. Community study of infant mortality in San Diego County. J Trop Pediatr. (1994) 40:172–8. doi: 10.1093/tropej/40.3.172
25. Singh, GK, and Kogan, MD. Persistent socioeconomic disparities in infant, neonatal, and postneonatal mortality rates in the United States, 1969-2001. Pediatrics. (2007) 119:e928–39. doi: 10.1542/peds.2005-2181
26. Blumenshine, P, Egerter, S, Barclay, CJ, Cubbin, C, and Braveman, PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. (2010) 39:263–72. doi: 10.1016/j.amepre.2010.05.012
27. Rey, AA, Purrio, M, Viveros, MP, and Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. (2012) 37:2624–34. doi: 10.1038/npp.2012.123
28. De Aquino, JP, Sherif, M, Radhakrishnan, R, Cahill, JD, Ranganathan, M, and D'Souza, DC. The psychiatric consequences of cannabinoids. Clin Ther. (2018) 40:1448–56. doi: 10.1016/j.clinthera.2018.03.013
29. Lutz, B, Marsicano, G, Maldonado, R, and Hillard, CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. (2015) 16:705–18. doi: 10.1038/nrn4036
30. Roncero, C, Valriberas-Herrero, I, Mezzatesta-Gava, M, Villegas, JL, Aguilar, L, and Grau-Lopez, L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod Health. (2020) 17:25. doi: 10.1186/s12978-020-0880-9
31. Dong, C, Chen, J, Harrington, A, Vinod, KY, Hegde, ML, and Hegde, VL. Cannabinoid exposure during pregnancy and its impact on immune function. Cell Mol Life Sci. (2019) 76:729–43. doi: 10.1007/s00018-018-2955-0
32. Sujan, AC, Pal, A, Avalos, LA, and Young-Wolff, KC. A systematic review of in utero cannabis exposure and risk for structural birth defects. Front Pediatr. (2023) 11:1149401. doi: 10.3389/fped.2023.1149401
33. Paul, SE, Hatoum, AS, Fine, JD, Johnson, EC, Hansen, I, Karcher, NR, et al. Associations between prenatal Cannabis exposure and childhood outcomes: results from the ABCD study. JAMA Psychiatry. (2021) 78:64–76. doi: 10.1001/jamapsychiatry.2020.2902
34. Navarrete, F, Garcia-Gutierrez, MS, Gasparyan, A, Austrich-Olivares, A, Femenia, T, and Manzanares, J. Cannabis use in pregnant and breastfeeding women: behavioral and neurobiological consequences. Front Psych. (2020) 11:586447. doi: 10.3389/fpsyt.2020.586447
35. Jutras-Aswad, D, DiNieri, JA, Harkany, T, and Hurd, YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci. (2009) 259:395–412. doi: 10.1007/s00406-009-0027-z
36. Scheyer, AF, Melis, M, Trezza, V, and Manzoni, OJJ. Consequences of perinatal Cannabis exposure. Trends Neurosci. (2019) 42:871–84. doi: 10.1016/j.tins.2019.08.010
37. Wang, X, Dow-Edwards, D, Keller, E, and Hurd, YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. (2003) 118:681–94. doi: 10.1016/s0306-4522(03)00020-4
38. Watson, S, Chambers, D, Hobbs, C, Doherty, P, and Graham, A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol Cell Neurosci. (2008) 38:89–97. doi: 10.1016/j.mcn.2008.02.001
39. Mato, S, Del Olmo, E, and Pazos, A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. (2003) 17:1747–54. doi: 10.1046/j.1460-9568.2003.02599.x
40. Wu, CS, Jew, CP, and Lu, HC. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. (2011) 6:459–80. doi: 10.2217/fnl.11.27
41. Winiger, EA, and Hewitt, JK. Prenatal cannabis exposure and sleep outcomes in children 9-10 years of age in the adolescent brain cognitive development (SM) study. Sleep Health. (2020) 6:787–9. doi: 10.1016/j.sleh.2020.05.006
42. Stickrath, E . Marijuana use in pregnancy: an updated look at marijuana use and its impact on pregnancy. Clin Obstet Gynecol. (2019) 62:185–90. doi: 10.1097/GRF.0000000000000415
43. Marchand, G, Masoud, AT, Govindan, M, Ware, K, King, A, Ruther, S, et al. Birth outcomes of neonates exposed to marijuana in utero: a systematic review and Meta-analysis. JAMA Netw Open. (2022) 5:e2145653. doi: 10.1001/jamanetworkopen.2021.45653
44. Bailey, BA, and Osborne, JB. Prenatal marijuana exposure and visual perception in toddlers: evidence of a sensory processing deficit. Front Pediatr. (2023) 11:1113047. doi: 10.3389/fped.2023.1113047
45. Wang, X, Dow-Edwards, D, Anderson, V, Minkoff, H, and Hurd, YL. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. (2004) 56:909–15. doi: 10.1016/j.biopsych.2004.10.015
46. Lin, A, Dent, GL, Davies, S, Dominguez, ZM, Cioffredi, LA, McLemore, GL, et al. Prenatal cannabinoid exposure: why expecting individuals should take a pregnancy pause from using cannabinoid products. Front Pediatr. (2023) 11:1278227. doi: 10.3389/fped.2023.1278227
47. Hinckley, JD, and Hopfer, C. Marijuana legalization in Colorado: increasing potency, changing risk perceptions, and emerging public health concerns for youth. Adolesc Psychiatry. (2021) 11:95–116. doi: 10.2174/2210676611666210616163340
48. Kilmer, JR, Rhew, IC, Guttmannova, K, Fleming, CB, Hultgren, BA, Gilson, MS, et al. Cannabis use among Young adults in Washington state after legalization of nonmedical Cannabis. Am J Public Health. (2022) 112:638–45. doi: 10.2105/AJPH.2021.306641
49. Panday, J, Taneja, S, Popoola, A, Pack, R, Greyson, D, McDonald, SD, et al. Clinician responses to cannabis use during pregnancy and lactation: a systematic review and integrative mixed-methods research synthesis. Fam Pract. (2022) 39:504–14. doi: 10.1093/fampra/cmab146
50. Holland, CL, Rubio, D, Rodriguez, KL, Kraemer, KL, Day, N, Arnold, RM, et al. Obstetric health care Providers' counseling responses to pregnant patient disclosures of marijuana use. Obstet Gynecol. (2016) 127:681–7. doi: 10.1097/AOG.0000000000001343
51. Tavabi, N, Raza, M, Singh, M, Golchin, S, Singh, H, Hogue, GD, et al. Disparities in cannabis use and documentation in electronic health records among children and young adults. NPJ Digit Med. (2023) 6:138. doi: 10.1038/s41746-023-00885-w
52. Young-Wolff, KC, Ray, GT, Alexeeff, SE, Adams, SR, Does, MB, Ansley, D, et al. Rates of prenatal Cannabis use among pregnant women before and during the COVID-19 pandemic. JAMA. (2021) 326:1745–7. doi: 10.1001/jama.2021.16328
Keywords: prenatal cannabinoid exposure, prenatal cannabis, pregnancy, pregnant substance users, tetrahydrocannabinol
Citation: Torres J, Miller C, Apostol M, Gross J and Maxwell JR (2024) The impact of recreational cannabinoid legalization on utilization in a pregnant population. Front. Public Health. 12:1278834. doi: 10.3389/fpubh.2024.1278834
Received: 18 August 2023; Accepted: 02 February 2024;
Published: 20 February 2024.
Edited by:
Marc N. Potenza, Yale University, United StatesReviewed by:
Sraboni Chaudhury, University of Michigan, United StatesCopyright © 2024 Torres, Miller, Apostol, Gross and Maxwell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessie R. Maxwell, anJtYXh3ZWxsQHNhbHVkLnVubS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.