
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 30 January 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1194844
 Philippe C. G. Adam1,2,3
Philippe C. G. Adam1,2,3 Eline L. M. Op de Coul4
Eline L. M. Op de Coul4 Paul Zantkuijl5
Paul Zantkuijl5 Maria Xiridou4
Maria Xiridou4 Hanna Bos5
Hanna Bos5 Cor Blom5
Cor Blom5 Itsada Ketsuwan3
Itsada Ketsuwan3 Margreet J. M. te Wierik4
Margreet J. M. te Wierik4 Silke David4
Silke David4 John B. F. de Wit6*
John B. F. de Wit6*Background: The 2022 multicountry mpox outbreaks predominantly affected gay, bisexual and other men who have sex with men (GBMSM) in non-endemic countries, including in the Netherlands. We conducted a survey-based assessment of the alignment between the risk factors associated with mpox diagnosis among GBMSM in the Netherlands and the eligibility criteria used in 2022 for vaccinating this group, with the aim to refine these criteria.
Methods: An online self-report survey was conducted among adult GBMSM in the Netherlands between 29 July and 30 August 2022, corresponding to the first month of the Dutch mpox vaccination campaign. GBMSM were recruited via advertisements on social media and gay dating apps. Participants reported on their sexual behaviour, mpox diagnosis, and/or (initial) mpox vaccination since the start of the outbreak. Covariables of mpox diagnosis and vaccination were assessed using logistic regression analyses.
Results: Of the 2,460 participants, 73 (3.0%, 95% CI 2.3–3.6%) were diagnosed with mpox and 485 (19.7%, 95% CI 18.1–21.3%) had received (initial) mpox vaccination. Using sample weighting, we estimated that, of the GBMSM population aged 18–80 years in the Netherlands, 1.1% (95% CI 0.7–1.6%) had been diagnosed with mpox and 7.8% (95% CI 6.8–8.9%) had received (initial) vaccination. HIV-PrEP use, living with HIV, reporting ≥20 sex partners in the past 12 months, and sex in sex venues/parties in the past 2 months were independent risk factors for mpox diagnosis. Except for sex in sex venues/parties, these variables were also independently associated with mpox vaccination.
Conclusion: This study provides novel evidence regarding the degree to which the 2022 eligibility criteria for mpox vaccination align with the risk factors for mpox among GBMSM in the Netherlands. The findings contribute to a refinement of the eligibility criteria for mpox vaccination, to which sex in sex venues/parties should be added.
Mpox [formerly named monkeypox (1)], is a zoonotic infection caused by the monkeypox virus (MPXV) (2), that is endemic in parts of West and Central Africa (3). With an estimated global total of about 30,000 cases in humans until 2019, its occurrence was considered rare until early 2022 (4). Following initial reports of unusual cases in the United Kingdom in early May 2022 (5), multicountry outbreaks of human-to-human transmission of mpox have been identified in other non-endemic regions, including in Europe (6, 7). Between 1 January 2022 and 18 February 2023, 86,019 laboratory confirmed cases of mpox from 110 countries were reported to the World Health Organization (WHO) (8). The 2022 mpox outbreaks predominantly occurred in gay, bisexual and other men who have sex with men (GBMSM) and likely resulted from transmission during sexual contact, making individuals with a higher number of sex partners and/or participating in specific sexual networks more susceptible to mpox (9–11).
As observational studies suggested that smallpox vaccination attenuates mpox disease severity and acquisition risk (2, 3, 12), WHO recommended the primary prevention vaccination of individuals at high risk, notably GBMSM with multiple sexual partners (13). However, as in other countries (14), the (smallpox) vaccine initially available in the Netherlands was part of the strategic stockpile. The limited supply of vaccine necessitated the prioritization of vaccination to population groups at highest risk (15). The optimal allocation of vaccine required an alignment of eligibility criteria for vaccination with risk factors for mpox, knowledge of which was limited when mpox vaccination programs were initiated. Based on data from the Netherlands until the end of August 2022, this study provides new evidence on risk factors related to mpox diagnosis among GBMSM and assesses to what extent the 2022 eligibility criteria for mpox vaccination aligned with risk factors for mpox diagnosis in the Netherlands.
The Netherlands is one of the European countries most affected by the mpox outbreak (7). As of 16 February 2023, there were 1,261 confirmed cases of mpox in the Netherlands (16), a country with nearly 18 million inhabitants (17). The first mpox case in the Netherlands was reported on 20 May 2022, and the number of new diagnoses peaked at the beginning of July 2022. After that time, the number of new diagnoses decreased and became sporadic as of mid-September 2022 (16). In the second half of 2022, the number of mpox cases declined in the Netherlands (16), as well as in Europe (18) and globally (8). Future trends in mpox infections remain uncertain, although new outbreaks are likely to be of smaller magnitude than the 2022 outbreak (19, 20). In this context, mpox remains a public health concern, and achieving and sustaining elimination are priorities (21).
The early mpox response in the Netherlands aimed at halting transmission and consisted of identifying infected people and implementing public health protection measures, including isolation, contact tracing and post-exposure prophylaxis (PEP) vaccination (11). Awareness campaigns were also conducted among GBMSM. The stepwise roll-out of a centralized pre-exposure prophylaxis mpox vaccination program, using 32,000 doses of Imvanex® third generation smallpox vaccine, was initiated on 25 July 2022 (22). The vaccination program made use of initial information on the characteristics of individuals diagnosed with mpox (11) to prioritize which GBMSM would (first) receive mpox vaccination.
General eligibility criteria for mpox vaccination in 2022 were defined during meetings of the mpox expert council (23) and further specified for practical program implementation from available data on risk indicators routinely recorded in HIV-PrEP, HIV and STI programs. This resulted in practical eligibility criteria for mpox vaccination for GBMSM and trangender persons encompassing: (1) prescribed HIV-PrEP at a sexual health centre (SHC) or by a general practitioner (GP), or registered on a waiting list for HIV-PrEP at a SHC; (2) living with HIV and receiving regular HCV screening as a proxy for high risk behaviour; or (3) having attended a SHC in the past 6 months because of (3a) partner notification related to HIV or STI, (3b) prior diagnosis of syphilis, gonorrhoea or chlamydia, or (3c) having had more than three partners in the past 6 months (22).
High-risk individuals eligible for vaccination were identified by the National Institute for Public Health and the Environment (RIVM) from the national surveillance database (SOAP) that includes information pertaining to all visits to SHCs, encompassing anonymised data on age, sex, (sexual) behaviour, STI diagnoses, HIV status, and use of HIV-PrEP (M. Haverkate, personal communication, 6 July 2023). From these data, lists of individuals eligible for vaccination were provided to each SHC by region. For persons receiving HIV-PrEP from their GP, GPs were contacted by the Public Health Services (PHS) in their region to invite eligible patients for vaccination. Similarly, the HIV healthcare providers of people living with HIV were asked by the PHS to invite eligible patients for vaccination. All eligible individuals identified received an invitation to vaccinate against mpox.
To gather comprehensive information on vaccination among individuals most at risk for mpox, we conducted an online survey among adult GBMSM in the Netherlands. Drawing on the survey data, we examined the proportions of GBMSM reporting mpox diagnoses and mpox vaccination, and the associations of mpox diagnosis and mpox vaccination with a range of potential risk factors. These factors encompassed the use of HIV-PrEP, living with HIV, prior STI diagnosis, number of sex partners, and engagement in group sex, chemsex, and sex in gay saunas, sex clubs or at sex parties (for brevity referred to as sex in sex venues or at sex parties in the remainder of the text). The inclusion of these specific risk factors reflects the aim of encompassing, at a higher conceptual level, both the eligibility criteria employed by the program in the Netherlands, as well as additional potential behavioural risk factors, including those highlighted in guidance from the European Centre for Disease Prevention and Control (ECDC) (24). Potential behavioural risk factors related to sexual activity that need to be considered extend beyond the number of sex partners to allow assessing the potential contribution of participating in the specific sexual networks, including those of GBMSM practicing chemsex, group sex and/or attending sex venues or sex parties.
We compared the factors associated with mpox diagnosis and mpox vaccination to evaluate the degree of alignment between the risk factors for mpox, the 2022 eligibility criteria for mpox vaccination, and the factors associated with mpox vaccination in various groups of GBMSM. Furthermore, we estimated the proportion of GBMSM reporting one or more risk factors for mpox and estimated the presumed level of immunity towards mpox achieved within this group due to mpox diagnosis, mpox vaccination or prior smallpox vaccination. In addition to providing novel evidence on the risk factors for mpox among GBMSM, the study findings contribute to guiding the targeting of the mpox vaccination program in the Netherlands to GBMSM most at risk.
A new, purposive cross-sectional self-report survey entitled Monkeypox: a new challenge for your sex life was conducted online among GBMSM in the Netherlands between 29 July and 30 August 2022. Participants were recruited via ads appropriate for the GBMSM population of interest on social media (i.e., Facebook and Instagram), gay dating sites and apps (i.e., Grindr and Recon), and Man tot Man the principal sexual health promotion platform for GBMSM in the Netherlands.1 The ads provided a link to a web page with information about the study. People were eligible to participate if they: lived in the Netherlands, were 18 years or older, identified as male (or non-binary or transgender) and ever had sex with a male partner. All participants provided informed consent and received no compensation. Ethics approval for this study was granted on 25 July 2022 by the Research Ethics Board of the Faculty of Social and Behavioural Sciences, Utrecht University, the Netherlands (reference number: 22-0358).
Participant characteristics: age (continuous in years and in five age ranges: 18–29 years, 30–39 years, 40–49 years, 50–59 years, and 60 or more years), education (tertiary education completed or ongoing, no/yes), province of residence (recoded as the Randstad metropolitan area, no/yes, that is, the main urban area of the Netherlands encompassing the four largest cities, their suburbs and towns in between), sexual orientation (gay, bisexual, heterosexual, unsure).
Sexual behaviours: number of male sex partners in the past 12 months (0, 1–9, 10–19, 20–49, 50–99, 100 or more; recoded as 0–9, 10–19, 20 or more), group sex (sex with two or more partners at the same time) in the past 2 months (no/yes), chemsex (the intentional use of drugs to enhance sex) in the past 2 months (no/yes), sex in a gay sauna, sex club or at a sex party (i.e., sex in sex venues or at sex parties) in the past 2 months (no/yes).
HIV/STI-related indicators: current HIV-PrEP use (no/yes), living with HIV (no/yes), STI diagnosis in the past 12 months (no/yes).
Mpox-related indicators: mpox diagnosis (no/yes), invitation received to vaccinate against mpox (no/yes), and having received (initial) mpox vaccination since the start of the 2022 outbreak (no/yes). Participants were not asked about the specific number of vaccine doses they had received. However, it is likely that the participants reporting mpox vaccination in this survey had only received one dose of the mpox vaccine. This is because the survey was conducted during the first month of the mpox vaccination campaign and the recommended interval between the first and second doses is at least 4 weeks (16).
Only eligible participants who fully completed the questionnaire were retained in the analyses presented in this paper. All analyses were conducted using SPSS (version 28).
Descriptive statistics were computed to describe the characteristics of the sample. Due to a lack of data regarding the characteristics of the GBMSM population in the Netherlands, it was not possible to evaluate the sample’s representativeness with confidence. Nonetheless, it was clear from a comparison with national records (25) that the sample overrepresented GBMSM who use HIV-PrEP or live with HIV. A proportional weighting procedure was therefore applied to the data to redress the overrepresentation of GBMSM using HIV-PrEP or living with HIV. Sample weights were calculated based on data from national records showing that 11,576 GBMSM nationally used HIV-PrEP, and 13,289 GBMSM were in HIV care in 2022 (25). The size of the sexually active GBMSM population aged 18–80 years living in the Netherlands was estimated at 310,000, based on an update of a previous estimate (26). Our update was guided by social science research data regarding same-sex attraction and behaviour as well as gay and bisexual identity in the Netherlands (27). We also accounted for the growth of the general population of the Netherlands (28). Details of the study population size estimation are described in the Supplementary materials.
The proportional weighting procedure kept the sample size constant compared to the unweighted sample. Per convention, numbers are only reported for the unweighted data. To assess the impact of the weighting procedure on the sample characteristics, we calculated differences in percentages between unweighted and weighted estimates and computed the ratio of the two estimates.
Descriptive statistics were also calculated to estimate the proportions of participants (and 95% confidence intervals [CI]) reporting mpox diagnosis or mpox vaccination using both unweighted and weighted data. Univariable and multivariable logistic regression analyses were used to identify the covariables associated with mpox diagnosis or mpox vaccination. The selected set of covariables included in the models encompassed both the general and specific eligibility criteria for vaccination used in the Netherlands (see Table 1). Additional sexual behaviours were also included that may be related to the transmission of MPXV but were not listed as eligibility criteria for mpox vaccination in the Netherlands in 2022. In total, seven potential covariables were assessed: current use of HIV-PrEP, living with HIV, STI diagnosis in the past 12 months, number of sex partners in the past 12 months, group sex in the past 2 months, chemsex in the past 2 months, and sex in sex venues or at sex parties in the past 2 months. Except for living with HIV, all these potential covariables were also suggested as possible risk factors for mpox by ECDC (24). A backward elimination procedure was employed in the multivariable regression models to retain only the significantly associated covariables. Covariable analyses were conducted on unweighted data, as potential covariables were used for data weighting. Odds ratios and adjusted odds ratios, along with their corresponding 95% CIs and p-values, were calculated for univariable and multivariable analyses. Additionally, Nagelkerke R-squared values were calculated as an approximation of the proportion of variance in the dependent variable that can be explained by the independent variables in a logistic regression model.
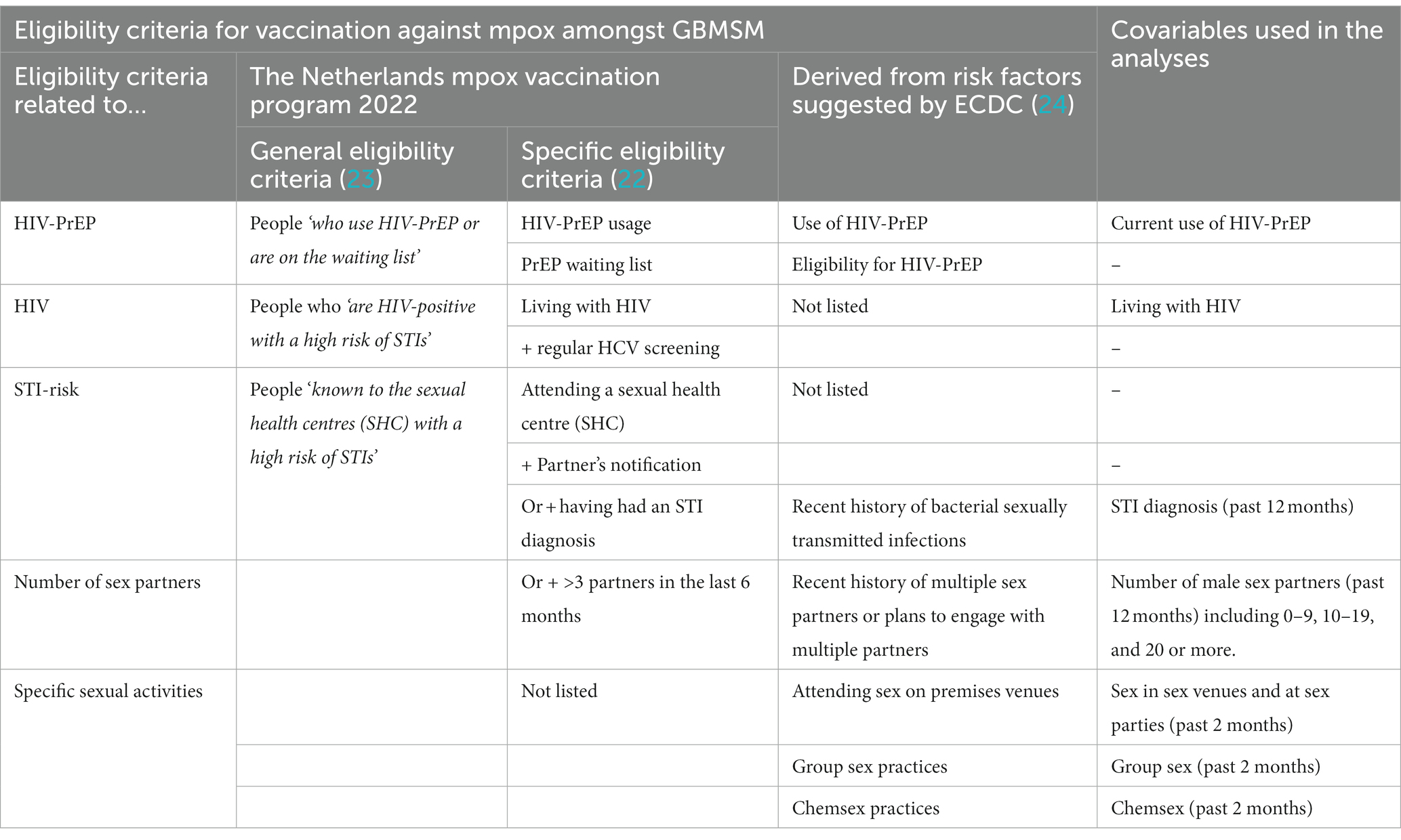
Table 1. Eligibility criteria for mpox (monkeypox) vaccination and related covariables included in the analyses.
The proportion of participants reporting one or more independent risk factors (IRFs) for mpox diagnosis, along with their 95% CIs, was calculated in the unweighted and weighted samples. Additional analyses were conducted to estimate the proportions of participants reporting specific numbers of IRFs, including one, two or three. The cumulative number of IRFs ranged from zero to three instead of four, as two of the four IRFs, namely current HIV-PrEP use and living with HIV, are mutually exclusive.
We estimated the proportion of individuals with presumed adequate immunity towards mpox due to previous mpox diagnosis, smallpox and mpox vaccination among participants with any independent risk factors for mpox. All participants diagnosed with mpox were considered to have presumably achieved adequate immunity. Immunity through vaccination was also presumed to be adequate if participants received two recent doses of the mpox vaccine. Note, however, that this was unlikely amongst participants as the survey was undertaken in the first month of the mpox vaccination program in the Netherlands. Immunity was also presumed to be adequate if participants received one dose of the mpox vaccine and had presumably been previously vaccinated through the historic smallpox vaccination program. Smallpox vaccination officially ended in November 1975 in the Netherlands (Manon Haverkate, Personal communication, 5 July 2023), and all participants born before 1976 were presumed to have received smallpox vaccination in the past. Analyses related to presumed immunity among participants were conducted on unweighted data only as the variables used for data weighting consisted of IRFs for mpox.
Overall, 2,899 individuals accessed the survey, of whom 2,744 (94.7%) were adult GBMSM 18 years or over living in the Netherlands who provided informed consent to participate in the survey. Of the 2,744 eligible participants, 2,460 (89.7%) completed all questions and were included in the analyses. A comparison of sample characteristics before and after weighting is presented in Table 2. Sample age was slightly higher in the unweighted sample (Median = 42.0 years, IQR 23) than in the weighted sample (Median = 41.0, IQR 24). The weighting reduced the proportional representation of participants living in the Randstad metropolitan area (unweighted sample: 67.4%, weighted sample: 64.3%), and of the proportion of participants who self-identified as gay (unweighted sample: 91.5%, weighted sample: 88.9%). Reductions were also observed in the proportions of participants currently using HIV-PrEP (unweighted sample: 32.5%, weighted sample: 3.8%), living with HIV (unweighted sample: 11.0%, weighted sample: 4.3%), and diagnosed with an STI in the past 12 months (unweighted sample: 23.1%, weighted sample: 12.5%). The proportion of participants with 10–19 sex partners in the past 12 months was also reduced (unweighted sample: 18.4%, weighted sample: 15.0%), as was the proportion of participants with ≥20 sex partners in the past 12 months (unweighted sample: 23.9%, weighted sample: 14.3%). Reductions were also observed in the proportions of participants who, in the past 2 months, engaged in group sex (unweighted sample: 22.3%, weighted sample: 14.6%), chemsex (unweighted sample: 15.3%, weighted sample: 8.5%), or sex in sex venues or at parties (unweighted sample: 21.5%, weighted sample: 14.5%).
Of the 2,460 participants in the unweighted sample, 73 (3.0%, 95% CI 2.3–3.6%) reported that they had been diagnosed with mpox (see Table 3). The estimated proportion of mpox diagnoses in the weighted sample was 1.1% (95% CI 0.7–1.6%).
Results from regression analyses aimed at identifying the covariables of mpox diagnosis are presented in Table 4. In univariable analyses, mpox diagnosis was significantly associated with six potential covariables but not with living with HIV. All seven variables were included in the multivariate model, of which three were removed in the backward estimation (STI diagnosis in the past 12 months, group sex in the past 2 months and chemsex in the past 2 months) due to their lack of independent association with mpox diagnosis. In the final multivariable model, mpox diagnosis was significantly independently associated with current use of HIV-PrEP (aOR = 3.92, 95% CI 2.01–7.66, p < 0.001) and living with HIV (aOR = 2.67, 95% CI 1.15–6.22, p = 0.023). An independent association was also found with having had ≥20 sex partners in the past 12 months (aOR = 5.16, 95% CI 2.24–10.68, p < 0.001) but not with having had 10–19 sex partners. Lastly, mpox diagnosis was independently associated with sex in sex venues or at sex parties in the past 2 months (aOR = 2.11, 95% CI 1.26–3.52, p = 0.004). The multivariate model, however, only explained about a fifth of the variance in the risk of mpox diagnosis (Nagelkerke R-squared = 0.18).
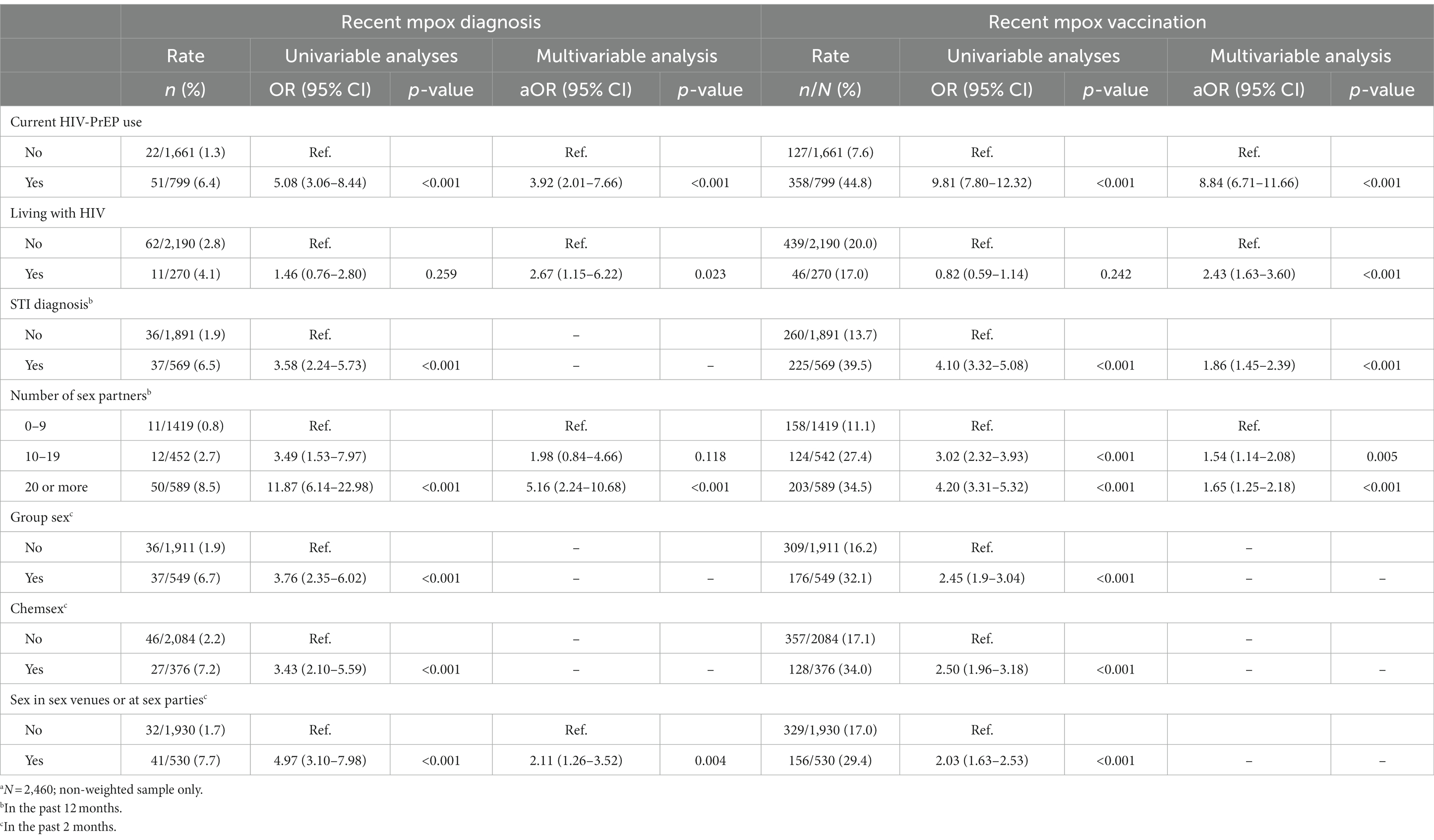
Table 4. Covariates of mpox diagnosis and mpox vaccinationa.
Of the 2,460 participants in the unweighted sample, 708 (28.8%, 95% CI 27.0–30.6%) had received an invitation to vaccinate against mpox, and 485 (19.7%, 95% CI 18.1–21.3%) had received (initial) mpox vaccination (see Table 3). In the weighted sample, 13.1% (95% CI 11.8–14.4%) had received an invitation for mpox vaccination, and 7.8% (95% CI 6.8–8.9%) had received (initial) mpox vaccination.
In univariable analyses, (initial) mpox vaccination was significantly associated with all potential covariables, except living with HIV (see Table 4). The backward procedure resulted in the elimination of three out of the seven terms from the model (group sex in the past 2 months, chemsex in the past 2 months and sex in sex venues or at sex parties in the past 2 months) due to their lack of independent association with mpox diagnosis. In the final multivariable model, mpox vaccination was significantly independently associated with current HIV-PrEP use (aOR = 8.84, 95% CI 6.71–11.66, p < 0.001), living with HIV (aOR = 2.43, 95% CI 1.63–3.60, p < 0.001), an STI diagnosis in the past 12 months (aOR = 1.86, 95% CI 1.45–2.39, p < 0.001), having had 10–19 sex partners in the past 12 months (aOR = 1.54, 95% CI 1.14–2.08, p = 0.005) and having had ≥20 sex partners in the past 12 months (aOR = 1.65, 95% CI 1.25–2.18, p < 0.001). The multivariate model explained nearly a third of the variance in the uptake of mpox vaccination (Nagelkerke R-squared = 0.30).
The proportion of participants reporting zero, one, two or three IRFs for mpox diagnosis (i.e., current HIV-PrEP use or living with HIV, ≥20 sex partners in the past 12 months, or sex in sex venues or at parties in the past 2 months) is shown in Table 5.
Of the 2,460 participants in the non-weighted sample, 1,104 (44.9%, 95% CI 42.9–46.8%) presented no IRF, 746 (30.3%, 95% CI 28.5–32.1%) presented one IRF, 392 (15.9%, 95% CI 14.5–17.4%) two IRFs, and 218 (8.9%, 95% CI 7.7–10.0%) three IRFs (Table 5). In the weighted sample, these proportions were 72.8% (95% CI 71.0–74.6%), 19.3% (95% CI 17.7–20.8%), 6.3% (95% CI 5.3–7.2%), and 1.6% (95% CI 1.1–2.1%), respectively.
In total, 1,356 of the 2,460 participants in the non-weighted sample (55.1%, 95% CI 53.2–57.1%) presented at least one of the four IRFs for mpox diagnosis. This proportion was smaller (27.2, 95% CI 25.4–29.0%) in the weighted sample.
Of the 1,356 participants with one or more IRFs in the unweighted sample, 61 (4.5%, 95% CI 3.4–5.6%) had been diagnosed with mpox, 181 (13.3%, 95% CI 11.5–15.2%) had presumably received one dose of mpox vaccine while being vaccinated against smallpox, and 251 (18.5%, 95% CI 16.4–20.6%) had presumably received one dose of mpox vaccine but no smallpox vaccination (Table 6). Based on the proportions of participants reporting the first two situations, 17.8% (95% CI 15.8–19.9%) of the 1,356 participants with one or more IRFs, would have presumably achieved adequate immunity against mpox. The overall proportion of presumed adequate immunity varied according to the number of independent risk factors reported, from 12.4% (95% CI 10.1–14.8%) of the 746 participants with one IRF, 20.4% (95% CI 16.4–24.4%) of the 392 participants with two IRFs and 31.7% (95% CI 25.4–37.9%) of the 218 participants with three IRFs.
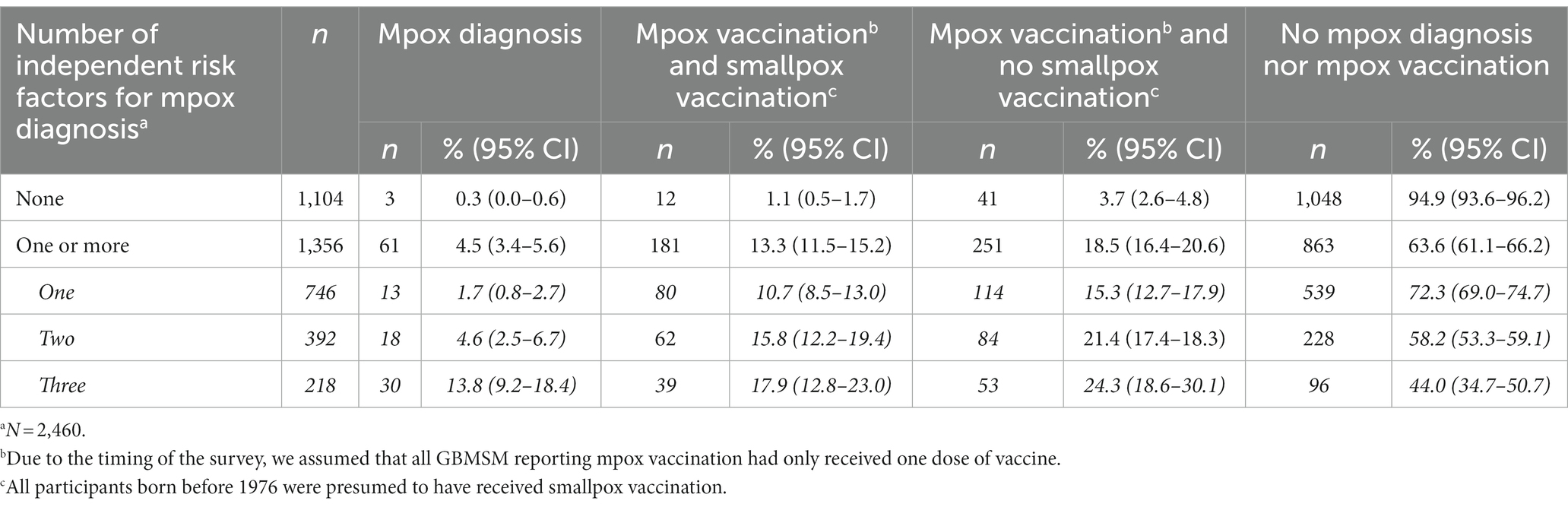
Table 6. Level of presumed adequate immunity towards mpox according to GBMSM’s numbers of independent risk factors for mpox diagnosis (unweighted dataa).
Our findings provide novel insights into the extent and covariables of the risk of mpox diagnosis as well as the uptake and need of mpox vaccination among GBMSM in the Netherlands. Of the participants in this study, 3.0% had been diagnosed with mpox and a fifth (19.7%) had received (initial) mpox vaccination since the start of the mpox outbreak. Using data weighting to attenuate participation bias, we found that an estimated 1.1% of the sexually active GBMSM population 18–80 years in the Netherlands had been diagnosed with mpox and less than a tenth (7.8%) had received (initial) mpox vaccination. Our estimates seem to surpass those derived from the national records of mpox cases and first dose mpox vaccination at the time the survey recruitment ended. By September 1st, 2022, 1,080 GBMSM mpox cases (11) and 12,820 first vaccine doses (29) had been recorded nationally. Extrapolating these figures to a population of 310,000 GBMSM yields an mpox prevalence estimate of 0.3% and an mpox vaccination prevalence estimate of 4.1%.
Our multivariable assessment of covariables found commonalities and differences in the independent covariables of mpox diagnosis and mpox vaccination. Mpox diagnosis and mpox vaccination were each independently associated with HIV-PrEP use, living with HIV and reporting ≥20 sex partners in the past 12 months. STI diagnosis in the past 12 months and 10–19 partners in the past 12 months were independently associated with mpox vaccination but not with mpox diagnosis. Sex in sex venues or at sex parties in the past 2 months was independently associated with mpox diagnosis but not with mpox vaccination. Neither group sex nor chemsex in the past 2 months were independently associated with mpox diagnosis or vaccination, although associations were observed in univariable analyses.
In contexts where the vaccination of all sexually active GBMSM is not possible, mpox vaccination is best targeted at GBMSM most at risk for mpox, as also recommended by ECDC (24), and our findings provide novel guidance to further improve the alignment between the eligibility criteria for mpox vaccination and the evidence regarding risk factors for mpox. Our findings underscore the importance of four IRFs for mpox diagnosis: current use of HIV-PrEP, living with HIV, a higher number (≥20) of sex partners in the past 12 months, and having sex in sex venues or at sex parties in the past 2 months.
These findings confirm the importance of HIV-PrEP use as an eligibility criterion for mpox vaccination among GBMSM in the Netherlands (22), and more broadly in Europe (24). Our results also suggest the importance of maintaining living with HIV as eligibility criterion in the Netherlands (22), although this was not listed by ECDC (24). The findings also provide new insights into the ways in which sexual activity affects GBMSM’s vulnerability to mpox. The finding that both a high number of sex partners and having sex in sex venues or at sex parties were independently associated with mpox diagnosis is noteworthy. This suggests that GBMSM’s susceptibility to mpox does not merely result from their number of sex partners (and sex contacts) but also from engaging in specific sexual activities and networks.
The findings on IRFs for mpox diagnosis can help refine the eligibility criteria for mpox vaccination, starting with the criteria used in the Netherlands. Having multiple sex partners was noted as a potential risk factor for mpox in ECDC guidance (24). During the 2022 vaccination campaign in the Netherlands, the number of partners was not used as a primary eligibility criteria. However, GBMSM known from the sexual health centres as being at higher risk of STIs had to have more than 3 partners in the last 6 months to be invited for vaccination (22). In light of our results on the number of partners from which the risk for mpox become significantly higher, the threshold of three partners per semester seems relatively low. We hence suggest using ≥10 partners in the past 6 months as an independent eligibility criterion for vaccinating most at risk GBMSM in the Netherlands. This criterion derives from an adaptation of the ≥20 partners threshold established in this study to the 6-month timeframe used for the recording national indicator data.
Our findings also confirm ECDC guidance that attending sex venues is a risk factor for mpox (24), and this should be included as an eligibility criterion for mpox vaccination among GBMSM in the Netherlands. However, unlike suggested by ECDC (24), we did not find that engaging in group sex, chemsex, or having a recent history of STI were independent risk factors for mpox. Nevertheless, as these variables were associated with mpox diagnosis in univariate analyses, they could potentially offer additional insights in determining the need for mpox vaccination, particularly when data concerning other eligibility criteria, such as having sex at sex venues or at sex parties, are not available.
Our assessment enabled estimating the proportions of GBMSM most at risk for mpox and in need of vaccination. In the non-weighted sample, more than half of the participants (55.1%) presented at least one of the four IRFs for mpox. This proportion was reduced to over a quarter (27.2%) in the weighted sample, equating to approximately 83,700 individuals when extrapolated to an estimated 310,000 adult GBMSM.
As the number of IRFs accumulated increased, the size of the high-risk groups decreased. Specifically, the presumed core transmission group, consisting in this study of GBMSM with three IRFs, accounted for less than a tenth (8.9%) of participants in the non-weighted sample and less than 2% (1.6%) in the weighted sample. The study also contributed to estimating the proportion of GBMSM at higher risk who had presumably achieved adequate immunity towards mpox at the time of the survey. Among the participants with one or more IRFs, less than one in five (17.8%) were presumed to have adequate immunity either due to infection or vaccination. The proportion of GBMSM with presumed adequate immunity varied according to the number of IRFs reported, from above a tenth (12.5%) among participants with one IRF, to a fifth (20.4%) among participants with two IRFs and near a third (31.7%) among participants with three IRFs. If all GBMSM born after 1975, who have not been vaccinated against smallpox but had presumably received only one dose of mpox vaccine at the time of the survey, were to receive a second dose—acknowledging the challenges of achieving full compliance with the recommendation to obtaining two doses—the resultant presumed immunity levels would approximate 27.8%, 41.8%, and 56% among GBMSM with one, two, and three IRFs for mpox, respectively.
These findings show that the presumed level of adequate immunity against mpox achieved at the time of the survey and potentially achieved if second doses are received after the time of the survey, was highest amongst GBMSM reporting three IRFs. As modelling has shown (15), a vaccination strategy targeting those most at risk may contribute to effectively curbing mpox outbreaks. It is, however, possible that mpox is reintroduced among lower risk and less vaccinated and immune GBMSM, for instance those who present one or two IRFs for mpox. Vaccination of at least all GBMSM at risk presenting one or more IRFs is hence needed (21).
This study has unique strengths. To the best of our knowledge, this is the first study to assess the extent of mpox diagnoses and vaccination in a community sample of GBMSM, and to assess and compare commonalities and differences in covariables for mpox diagnosis and mpox vaccination. Also, we recruited a large sample of GBMSM and achieved a high survey completion rate. Furthermore, we provided an updated estimate of the size of the sexually active adult GBMSM population in the Netherlands and used weighting procedures to reduce some recruitment biases. Our study also has limitations that are common to survey research in GBMSM, including potential memory and social desirability biases related to self-report. We mitigated these biases by clearly specifying reporting periods and ensuring anonymity of responses. Furthermore, we used convenience sampling and, despite the use of weighting procedures, the findings may not be representative of the population of GBMSM in the Netherlands. The survey’s focus may have led to an overrepresentation of GBMSM interested in mpox, including individuals who had mpox or had obtained vaccination. Furthermore, as mpox is a relatively rare condition, the number of participants diagnosed with mpox was limited, despite the large sample size. This low number led to the risk of not being able to detect associations with covariables of mpox diagnosis. Our analyses on covariables of mpox diagnosis would therefore need to be replicated in a sample that includes a larger number of participants diagnosed with mpox, including people living with HIV.
Also, some information on specific eligibility criteria for mpox vaccination was not assessed in the survey, notably being on a waiting list for PrEP and regular HCV screening among participants living with HIV. In addition, the survey did not allow for differentiating between vaccination pre- or post-exposure to mpox, albeit that uptake of post-exposure vaccinations remains very limited in the Netherlands. Furthermore, the survey assessed mpox diagnoses and vaccination uptake and covariables only during August 2022, which was merely 1 month into the vaccination program. However, while the reach of the vaccination program has increased thereafter, vaccinations mostly occurred at the start of the program, and GBMSM at highest risk for mpox were invited first.
The prospect of mpox reintroduction from endemic regions remains a possibility, carrying with it the potential for novel (sporadic) mpox outbreaks in the Netherlands. To prevent future reintroductions from giving rise to new outbreaks, and to ensure the sustained eradication of mpox (18), even in the absence of such introductions, the vaccination of all GBMSM and transgender persons, who constitute another group at risk for mpox in the Netherlands, may prove necessary. However, the comprehensive vaccination of entire groups or populations can be constrained by various factors and considerations, including in relation to costs, or by a diminishing enthusiasm for mpox vaccination among the targeted groups when the number of mpox cases becomes negligible or nil. In situations where universal vaccination is not feasible or chosen, our research findings play a crucial role in determining individuals most susceptible to mpox, thereby offering a targeted approach to mpox vaccination.
The findings of our study underscore the importance of an evidence-based approach to identifying risk factors for mpox diagnosis and guiding the selection of eligibility criteria for mpox vaccination. Our approach has already contributed to a refinement of vaccination eligibility criteria applied as of May 2023 in the Netherlands (16). Both ≥10 partners in the past 6 months and having sex at sex venues or at sex parties were added to the list of eligibility criteria (16). Our findings have also contributed to the policy change to provide access to vaccination to a larger number of GBMSM than initially planned (30). As of May 2023, unvaccinated GBMSM who self-identify as high-risk for mpox and GBMSM born after 1975 who received only a single vaccine dose have the possibility to contact their healthcare service to discuss mpox vaccination (16). This extension of the scope of the vaccination program would provide valuable health benefits to a larger group of GBMSM at risk of mpox. However, it will be essential to monitor progress in achieving sufficient vaccination coverage and immunity levels among the overall population of GBMSM and within MSM priority groups. This is particularly important for the new GBMSM population groups who became eligible for vaccination in 2023, such as GBMSM who frequent sex venues, and who do not access HIV treatment, HIV-PrEP, or sexual health programs. Ensuring that these GBMSM engage with health services to obtain vaccination may require specific communication strategies and initiatives. In case the uptake of vaccination in this group remains low, exploring the benefits of outreach vaccination, in particular offering mpox vaccination in sex venues, would become pertinent, particularly in the context of a potential resurgence of mpox. While the number of mpox cases is currently negligible or nil in the Netherlands, the future spread of mpox remains uncertain. The most effective safeguard against the risk of potential future outbreaks is to continue to offer mpox vaccination over the next years to vaccinate the largest possible number of GBMSM, starting with MSM population groups most at risk as identified in this study.
The data that support the findings of this study are available from the corresponding author, JBFdW, upon reasonable request.
This study involving humans was approved by Faculty Ethics Review Board, Faculty of Social and Behavioural Sciences, Utrecht University. The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their electronic informed consent to participate in this study.
PA developed the idea for this study, conducted the statistical analyses, and wrote the first draft of the manuscript together with JW. EC and MX provided important additions to the manuscript, including information and insights on the mpox outbreak and vaccination program in the Netherlands. PZ, HB, CB, IK, SD, and MW reviewed the manuscript and contributed further revisions. All authors have read the final manuscript and accepted authorship.
The author(s) declare financial support was received for the research, authorship, and publication of this article. The National Institute for Public Health and the Environment (RIVM) and Soa Aids Nederland provided financial support for this research study.
The authors thank Manon Haverkate, Lisette Kuyper, and Hanneke de Graaf for sharing information and insights, Arjan van Bijnen and Laurian Kuipers for their contribution to the recruitment, and all participants to the survey.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1194844/full#supplementary-material
1. World Health Organization [Internet]. (2023) WHO recommends new name for monkeypox disease [about 4 screens]. Geneva: World Health Organization. Available at: https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease
2. Marennikova, SS, Seluhina, EM, Mal'ceva, N, Cimiskjan, KL, and Macevic, GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. (1972) 46:599–611.
3. Sukhdeo, S, Mishra, S, and Walmsley, S. Human monkeypox: a comparison of the characteristics of the new epidemic to the endemic disease. BMC Infect Dis. (2022) 22:928. doi: 10.1186/s12879-022-07900-7
4. Bunge, EM, Hoet, B, Chen, L, Lienert, F, Weidenthaler, H, Baer, LR, et al. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl Trop Dis. (2022) 16:e0010141. doi: 10.1371/journal.pntd.0010141
5. Vivancos, R, Anderson, C, Blomquist, P, Balasegaram, S, Bell, A, Bishop, L, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. (2022) 27:2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422
6. Thornhill, JP, Barkati, S, Walmsley, S, Rockstroh, J, Antinori, A, Harrison, LB, et al. Monkeypox virus infection in humans across 16 countries – April-June 2022. N Engl J Med. (2022) 387:679–91. doi: 10.1056/NEJMoa2207323
7. Vaughan, AM, Cenciarelli, O, Colombe, S, Alves de Sousa, L, Fischer, N, Gossner, CM, et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European region, 7 March to 23 August 2022. Euro Surveill. (2022) 27:2200620. doi: 10.2807/1560-7917.ES.2022.27.36.2200620
8. World Health Organization [Internet]. (2023) 2022-23 Mpox (Monkeypox) outbreak: Global trends [about 25 screens]. Geneva: World Health Organization. Available at: https://worldhealthorg.shinyapps.io/mpx_global/#section-fns
9. Selb, R, Werber, D, Falkenhorst, G, Steffen, G, Lachmann, R, Ruscher, C, et al. A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Euro Surveill. (2022) 27:2200499. doi: 10.2807/1560-7917.ES.2022.27.27.2200499
10. Vusirikala, A, Charles, H, Balasegaram, S, Macdonald, N, Kumar, D, Barker-Burnside, C, et al. Epidemiology of early monkeypox virus transmission in sexual networks of gay and bisexual men, England, 2022. Emerg Infect Dis. (2022) 28:2082–6. doi: 10.3201/eid2810.220960
11. van Ewijk, C, Miura, F, van Rijckevorsel, G, de Vries, H, Welkers, M, van den Berg, O, et al. Mpox outbreak in the Netherlands, 2022: public health response, characteristics of the first 1,000 cases and protection of the first-generation smallpox vaccine. Eur Secur. (2023) 28:2200772. doi: 10.2807/1560-7917.ES.2023.28.12.2200772
12. Payne, AB, Ray, LC, Kugeler, KJ, Fothergill, A, White, EB, Canning, M, et al. Incidence of monkeypox among unvaccinated persons compared with persons receiving ≥1 JYNNEOS vaccine dose — 32 U.S. jurisdictions, July 31–September 3, 2022. Morb Mortal Wkly Rep. (2022) 71:1278–82. doi: 10.15585/mmwr.mm7140e3
13. World Health Organization. Vaccines and immunisation for monkeypox. Interim guidance 16 November 2022. Geneva: World Health Organization (2022) 20 p.
14. Martín-Delgado, MC, Martín Sánchez, FJ, Martínez-Sellés, M, Molero García, JM, Moreno Guillén, S, Rodríguez-Artalejo, FJ, et al. Monkeypox in humans: a new outbreak. Rev Esp Quimioter. (2022) 35:509–18. doi: 10.37201/req/059.2022
15. Knight, J, Tan, DHS, and Mishra, S. Maximising the impact of limited vaccine supply under different early epidemic conditions: a 2-city modelling analysis of monkeypox virus transmission among men who have sex with men. CMAJ. (2022) 194:E1560–7. doi: 10.1503/cmaj.221232
16. National Institute for Public Health and the Environment [Internet]. (2023). Mpox infections [about 8 screens]. Bilthoven, the Netherlands: National Institute for Public Health and the Environment. Available at: https://www.rivm.nl/en/monkeypox
17. Statistics Netherlands [Internet]. (2023) Population dashboard. Up to date figures on the Dutch population [about 3 screens]. Den Haag, the Netherlands: Statistics Netherlands. Available at: https://www.cbs.nl/en-gb/visualisations/dashboard-population
18. European Centre for Disease Prevention and Control [Internet]. (2023). Mpox (formerly named monkeypox) situation update, as of 14 February 2023. Stockholm, Sweden: European Centre for Disease Prevention and Control. Available at: https://www.ecdc.europa.eu/en/news-events/monkeypox-situation-update
19. World Health Organization [Internet]. (2023). An mpox resurgence in the European region this spring and summer? To prevent that, key measures must continue [about 1 screen]. Geneva: World Health Organization. Available at: https://www.who.int/europe/news/item/17-05-2023-an-mpox-resurgence-in-the-european-region-this-spring-and-summer--to-prevent-that--key-measures-must-continue
20. Xiridou, M, Miura, F, Adam, P, Op de Coul, E, de Wit, J, and Wallinga, J. The fading of the mpox outbreak among men who have sex with men: a mathematical modelling study. J Infect Dis. (2023). doi: 10.1093/infdis/jiad414
21. World Health Organization [Internet]. (2023) Mpox – It hasn't gone away [about 6 screens]. Geneva: World Health Organization. Available at: https://www.who.int/europe/news/item/15-02-2023-as-the-mpox--emergency--continues--the-united-kingdom-shows-how-achieving-and-sustaining-disease-elimination-has-to-be-the-next-priority
22. Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment] [Internet]. Mpoxvaccinatie Uitvoeringsrichtlijn [Mpox vaccination guidelines] [about 29 screens]. Bilthoven, the Netherlands: Rijksinstituut voor Volksgezondheid en Milieu. Dutch. Available at: https://lci.rivm.nl/richtlijnen/monkeypoxvaccinatie#1-over-deze-richtlijn
23. Leenstra, T. (2022) (Landelijke Coördinatie Infectieziektebestrijding, Rijksinstituut voor Volksgezondheid en Mileau [National Coordination Centre for Communicable Disease Control, National Institute for Public Health and the Environment]). Letter to: Marjolein Sonnema (Ministerie van Volksgezondheid, Welzijn en Sport [Ministry of Health, Welfare and Sport]), 5p. Dutch. Available at: https://open.overheid.nl/repository/ronl-614a46ff6591d770978a2bae54768ae183cd7468/1/pdf/adviesbrief-mpx-prep-vaccinatie-6-juli-2022.pdf
24. European Centre for Disease Prevention and Control. Monkeypox multi-country outbreak – Second update, 18 October 2022. Stockholm: ECDC (2022).
25. Van Sighem, A., Rokx, C, and Op De Coul, E. HIV in the Netherlands. In: A SighemVan, F Wit, A Boyd, C Smit, A Matser, and M ValkVan De. Human immunodeficiency virus (HIV) infection in the Netherlands. Monitoring report 2022. Amsterdam, the Netherlands: Stichting Hiv Monitoring; (2023), 11–92.
26. Op de Coul, EL, Schreuder, I, Conti, S, van Sighem, A, Xiridou, M, Van Veen, MG, et al. Changing patterns of undiagnosed HIV infection in the Netherlands: who benefits most from intensified HIV test and treat policies? PLoS One. (2015) 10:e0133232. doi: 10.1371/journal.pone.0133232
27. Kuyper, L. Seksualiteit en seksuele gezondheid bij homo- en biseksuelen [Sexuality and sexual health of gay and bisexual persons] In: F Bakker and I Vanwesenbeeck, editors. Seksuele gezondheid in Nederland 2006. Delft, the Netherlands: Eburon (2006). 167–88.
28. Statistics Netherlands [Internet] (2023). Population growth. How fast is the population of the Netherlands growing? [About 2 screens]. Den Haag, the Netherlands: Statistics Netherlands. Available at: https://www.cbs.nl/en-gb/visualisations/dashboard-population/population-dynamics/population-growth (Accessed February 24, 2023)
29. National Institute for Public Health and the Environment (2022). Bilthoven, the Netherlands: National record of mpox vaccination [Osiris and iMPeX databases]. Unpublished raw data.
30. Van Dissel, J. T. (2022). (Centrum Infectieziektebestrijding, Rijksinstituut voor Volksgezondheid en Mileu [Centre for Infectious Disease Contro, National Institute for Public Health and the Environment]). Letter to: Marjolein Sonnema (Ministerie van Volksgezondheid, Welzijn en Sport [Ministry of Health, Welfare and Sport]). 11p. Dutch. Available at: https://open.overheid.nl/Details/ronl-b7a448c0a585892110380aa55cb35a558e75b34f/1
Keywords: mpox, monkeypox, MPXV infection, mpox vaccination, risk factors, men who have sex with men (MSM), gay, bisexual and other men who have sex with men (GBMSM)
Citation: Adam PCG, Op de Coul ELM, Zantkuijl P, Xiridou M, Bos H, Blom C, Ketsuwan I, te Wierik MJM, David S and de Wit JBF (2024) A survey-based assessment of rates and covariates of mpox diagnosis and vaccination provides evidence to refine eligibility criteria for mpox vaccination among gay, bisexual and other men who have sex with men in the Netherlands. Front. Public Health. 12:1194844. doi: 10.3389/fpubh.2024.1194844
Received: 27 March 2023; Accepted: 04 January 2024;
Published: 30 January 2024.
Edited by:
Miguel Angel Sanchez-Aleman, National Institute of Public Health, MexicoReviewed by:
Rodney Hicks, Western University of Health Sciences, United StatesCopyright © 2024 Adam, Op de Coul, Zantkuijl, Xiridou, Bos, Blom, Ketsuwan, te Wierik, David and de Wit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John B. F. de Wit, ai5kZXdpdEB1dS5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.