
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Public Health, 12 January 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1316531
This article mentions parts of:
Wastewater-based surveillance identifies start to the pediatric respiratory syncytial virus season in two cities in Ontario, Canada
Respiratory syncytial virus (RSV) is the leading viral cause of childhood bronchiolitis and pneumonia causing over 3 million hospitalizations and 100,000 deaths in children under 5 years of age annually. Wastewater-based surveillance (WBS) has proven an effective early warning system for high-consequence pathogens, including SARS-CoV-2, polio, mpox, and influenza, but has yet to be fully leveraged for RSV surveillance. A model predicated on the Canadian province of Ontario demonstrates that implementation of a WBS system can potentially result in significant cost savings and clinical benefits when guiding an RSV preventive program with a long-acting monoclonal antibody. A network of integrated WBS initiatives offers the opportunity to help minimize the devastating global burden of RSV in children by optimizing the timing of preventive measures and we strongly advocate that its benefits continue to be explored.
The benefits of a global, integrated wastewater-based surveillance (WBS) system was recently reported to provide an early warning of high-consequence pathogens, such as SARS-CoV-2, polio, mpox, and influenza (1). We unreservedly agree with this proposal and strongly advocate for respiratory syncytial virus (RSV) to be included as a priority pathogen in the evaluation of integrated WBS systems.
The World Health Organization has long recognized severe RSV infection as a major health care burden, causing greater than 3 million RSV related hospitalizations (RSVHs) and 100,000 deaths every year in children aged less than 5 years as well as a substantial burden of illness in older adults, greater than 60 years of age (2–4). Until recently, the options to prevent severe RSV infection have been limited to basic hygiene measures and selective prophylaxis of the most vulnerable pediatric populations with the short-acting monoclonal antibody, palivizumab. Newer preventive options that include long-acting monoclonal antibodies, such as nirsevimab, and maternal, child and adult vaccines (5), have the potential to profoundly impact the global burden of RSV. Accurate knowledge on the timing of community surges in RSV infection is essential to guide the planning, implementation, and real-time assessment of prophylaxis and vaccine programs, thereby ensuring maximum coverage and protection against RSV.
A Canadian study undertaken in Ontario recently reported that RSV-WBS resulted in a 12-day lead time in pediatric RSVH surge and 36-day lead time in defining the start to the pediatric RSV season vs. clinical surveillance alone (CS) (6). These results suggest that WBS may provide an important, population-level, early warning signal to prime policy decision-makers to plan and implement timely RSV prevention strategies.
To explore the potential impact and costs associated with WBS, we developed a cost-consequence model that compared WBS- vs. CS-guided nirsevimab prophylaxis programs for infants in Ontario aged <6 months at the start of the RSV season (Figure 1), where RSV epidemics were traditionally seasonal (November to March) in the pre-pandemic COVID-19 era. Currently, the start of the RSV season is determined by CS, typically defined as the first 2 consecutive weeks when >10% of the total samples tested for respiratory pathogens are positive for RSV (7). Similarly, the end of the season is defined when the proportion of RSV-positive tests is <10% for 2 consecutive weeks (7). Testing for RSV infection primarily occurs among children requiring hospitalization, which inherently reflects a lag in determining the community incidence of RSV. This form of CS also relies on timely submission of test results to public health agencies that will determine the start and end of the pediatric RSV season and initiation of immunoprophylaxis.
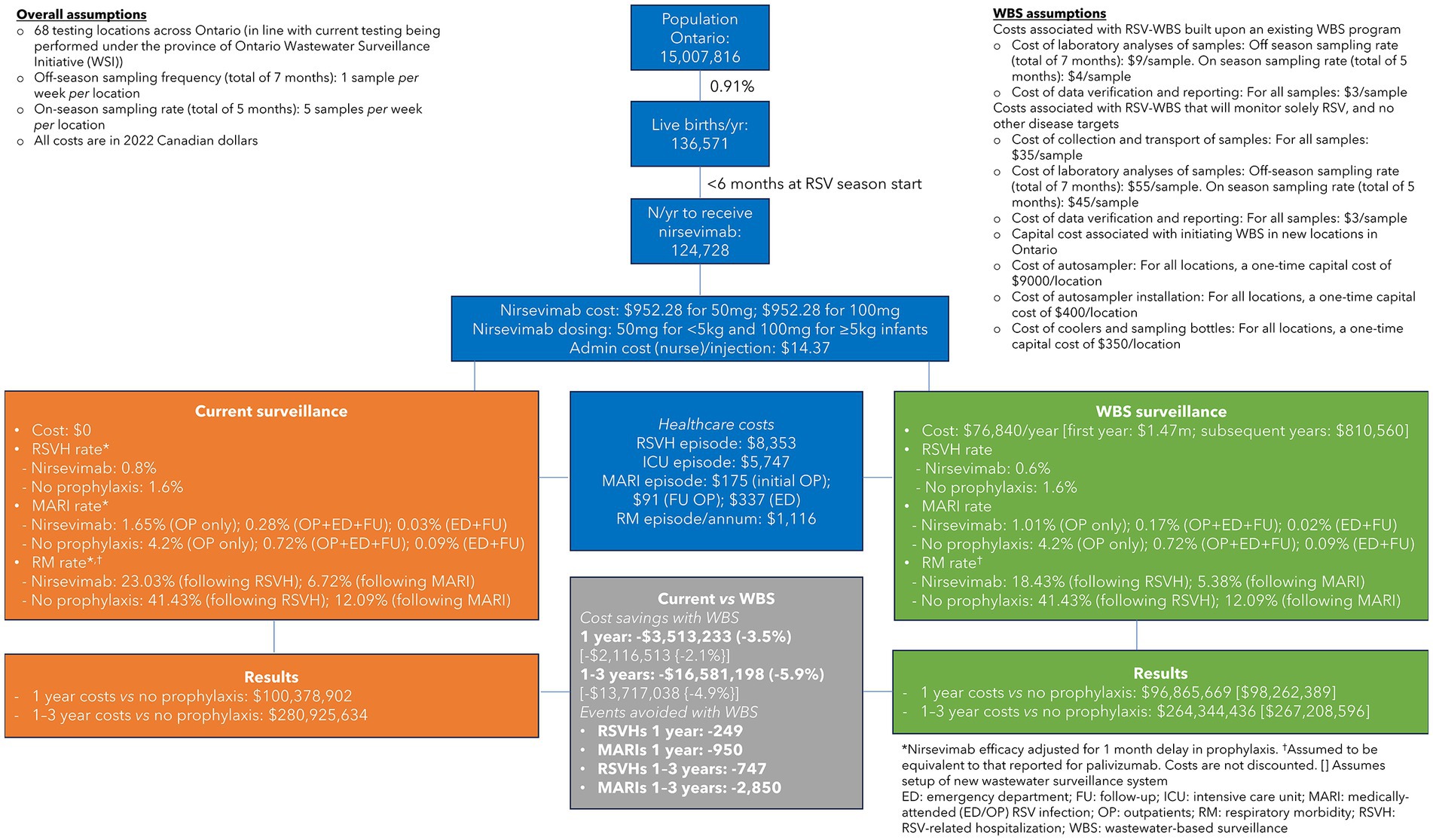
Figure 1. Cost-consequence model comparing wastewater-based surveillance (WBS)- vs. clinical surveillance (CS)-guided respiratory syncytial virus (RSV) prophylaxis programs in Ontario, Canada.
Nirsevimab was chosen for our cost analysis as it is indicated for all infants <8 months of age at the start of the RSV season (8–10) and could have a dramatic impact on reducing the intensity of RSV epidemic waves and concomitant capacity surges on pediatric acute care systems. Based on the Canadian data, we assumed that the RSV season was detected 1 month earlier using WBS than CS and, in both scenarios, spanned 5 months (6). Nirsevimab efficacy in the CS scenario was adjusted by having 1 month without prophylaxis to account for the earlier implementation of the RSV prophylaxis program with WBS. For example, the RSVH rate was 0.6% for WBS based on the nirsevimab registrational trial (9) vs. 0.8 and 1.6% for CS in infants who, respectively, did or did not receive nirsevimab. Efficacy rates for nirsevimab in the prevention of medically-attended emergency room/outpatient RSV infections (MARI) and subsequent respiratory morbidity were similarly adjusted.
It was assumed, based on current WBS systems, that off-season sampling would take place at a frequency of one sample per week per location (CAN 9/sample) for a total of 7 months, increasing to five samples per week (CAN 4/sample) during the RSV season, across 68 testing locations in Ontario. When RSV testing was added to an existing WBS system, we calculated that the costs incurred would be CAN 76,840 in the first year, which increased to CAN 1.47 m when considering the capital costs associated with setting-up a new provincial WBS system. Once established, the cost of a new system decreased to CAN 810,560 in subsequent years. No additional costs were assumed for current CS. Other costs included in the analysis were for nirsevimab (infants <5 kg: CAN 952.28 for 50 mg; infants ≥5 kg: CAN 952.28 for 100 mg), RSVHs (CAN 8,353/episode), intensive care unit stays (CAN 5,747/episode), MARIs (CAN 175–$337/episode), and respiratory morbidity (CAN 1,116/annum/episode) (11), all of which were equivalent for both WBS and CS. Costs in the model were calculated based on 136,571 annual live births in Ontario, of which 124,728 infants were aged <6 months at the start of the RSV season and thereby eligible for prophylaxis.
The results of the model found that WBS was associated with savings of CAN 2.1–3.5 m in the first year and CAN 13.7–16.6 m over 3 years, with larger savings accrued when RSV testing was added to an existing WBS system. These savings translate into a benefit–cost ratio of 45.7 in the first year and 71.9 over 3 years, when RSV is added to an existing WBS system. This implies that for every CAN 1 spent on RSV-WBS, there is CAN 45.7–71.9 return on investment. The corresponding ratios for a new RSV-WBS are 1.4 for year 1 and 4.4 over 3 years (CAN 1.4–4.4 return for every CAN 1 invested). Importantly, WBS-guided prophylaxis resulted in 249 fewer RSVHs and 950 fewer MARIs per year vs. CS-guided prophylaxis.
In a sensitivity analysis, the advantage of WBS over CS was shortened to a 2-week earlier detection of the RSV season, which resulted in a saving of CAN 321 k–1.7 m in the first year and CAN 5.3–8.2 m over 3 years. Under this scenario, WBS-guided prophylaxis resulted in 125 fewer RSVHs and 475 fewer MARIs per year versus CS-guided prophylaxis.
This simple model, covering one Canadian province and one preventive strategy, highlights the substantial benefit that WBS can confer in this new era of RSV prevention once the strategy is proven to be reproducible and valid throughout sequential RSV seasons and generalizable across communities. A fully integrated and publicly-funded network of WBS initiatives across high-, middle-, and low-income countries, affords a real opportunity for policy decision makers and public health agencies to intervene early and substantially reduce the impact on already strained pediatric bed capacities and associated hospital costs at the onset of the RSV season. WBS also provides a unique opportunity to minimize the devastating global burden of RSV in children by optimizing the timing of preventive measures.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
NT: Conceptualization, Writing – original draft, Writing – review & editing. EM: Conceptualization, Writing – original draft, Writing – review & editing. BP: Conceptualization, Writing – original draft, Writing – review & editing. JE: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. BR-G: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. RD: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
RD and EM have no conflicts to declare regarding this study but hold leadership positions in a company (Advanced Environmental Molecular Analytics Ltd.) with work non-relevant to the work presented. BP has received research funding and/or compensation as advisor/lecturer from AstraZeneca and Sanofi outside the scope of this study. BR-G and JE were employed by the company Violicom Medical Limited.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Keshaviah, A, Diamond, MB, Wade, MJ, and Scarpino, SV. Global wastewater action group. Global wastewater action group. Wastewater monitoring can anchor global disease surveillance systems. Lancet Glob Health. (2023) 11:e97681. doi: 10.1016/S2214-109X(23)00170-5
2. Wright, PF, and Cutts, FT (2000). Generic protocol to examine the incidence of lower respiratory infection due to respiratory syncytial virus in children less than five years of age: field test version. World Health Organization, Geneva. WHO document WHO/V&B/00.08. Available at: https://iris.who.int/handle/10665/66276?locale-attribute=pt&show=full (Accessed October 03, 2023).
3. Li, Y, Wang, X, Blau, DM, Caballero, MT, Feikin, DR, Gill, CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0
4. Shi, T, Vennard, S, Jasiewicz, F, Brogden, R, and Nair, H, RESCEU investigators. Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis. (2022) 226:S17–21. doi: 10.1093/infdis/jiab040
5. PATH (2023). RSV vaccine and mAb snapshot. Updated September 21, 2023. Available at: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (Accessed October 03, 2023).
6. Mercier, E, Pisharody, L, Guy, F, Wan, S, Hegazy, N, D’Aoust, PM, et al. Wastewater-based surveillance identifies start to the pediatric respiratory syncytial virus season in two cities in Ontario. Front Public Health. (2023) 11:1261165. doi: 10.3389/fpubh.2023.1261165
7. Obando-Pacheco, P, Justicia-Grande, AJ, Rivero-Calle, I, Rodríguez-Tenreiro, C, Sly, P, Ramilo, O, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. (2018) 217:1356–64. doi: 10.1093/infdis/jiy056
8. Griffin, MP, Yuan, Y, Takas, T, Domachowske, JB, Madhi, SA, Manzoni, P, et al. Single-dose Nirsevimab for prevention of RSV in preterm infants. N Engl J Med. (2020) 383:415–25. doi: 10.1056/NEJMoa1913556
9. Hammitt, LL, Dagan, R, Yuan, Y, Baca Cots, M, Bosheva, M, Madhi, SA, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. (2022) 386:837–46. doi: 10.1056/NEJMoa2110275
10. Jones, JM, Fleming-Dutra, KE, Prill, MM, Roper, LE, Brooks, O, Sánchez, PJ, et al. Use of Nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices - United States, 2023. MMWR Morb Mortal Wkly Rep. (2023) 72:920–5. doi: 10.15585/mmwr.mm7234a4
11. Rodgers-Gray, BS, Fullarton, JR, Xavier Carbonell-Estrany, X, Keary, IP, Tarride, JE, and Paes, BA. Impact of using the international risk scoring tool on the cost-utility of palivizumab for preventing severe respiratory syncytial virus infection in Canadian moderate-to-late preterm infants. J Med Econ. (2023) 26:630–43. doi: 10.1080/13696998.2023.2202600
Keywords: respiratory syncytial virus, wastewater-based surveillance, economics, pediatric hospitalization, community incidence, season start date, passive immunoprophylaxis, hospital level preparedness
Citation: Thampi N, Mercier E, Paes B, Edwards JO, Rodgers-Gray B and Delatolla R (2024) Perspective: the potential of wastewater-based surveillance as an economically feasible game changer in reducing the global burden of pediatric respiratory syncytial virus infection. Front. Public Health. 11:1316531. doi: 10.3389/fpubh.2023.1316531
Received: 02 November 2023; Accepted: 13 December 2023;
Published: 12 January 2024.
Edited by:
Marwan Osman, Yale University, United StatesReviewed by:
Hiam Chemaitelly, Weill Cornell Medicine-Qatar, QatarCopyright © 2024 Thampi, Mercier, Paes, Edwards, Rodgers-Gray and Delatolla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nisha Thampi, bnRoYW1waUBjaGVvLm9uLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.