
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Public Health , 06 December 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1305655
This article is part of the Research Topic World TB Day 2023: Yes! We can end TB View all 30 articles
 Aliabbas A. Husain
Aliabbas A. Husain Rajpal Singh Kashyap*
Rajpal Singh Kashyap*Antimicrobial resistance (AMR) has been regarded as a global health threat by the WHO (1). The rise of general AMR to multiple drugs in the Indian population poses a formidable challenge to the TB control strategies, especially for drug-resistant tuberculosis (DR-TB). India has been regarded as having one of the highest burdens of drug resistant (DR) pathogens, including multi-drug-resistant TB (MDR-TB), which accounts for a quarter of the global burden (2). A 6-year observational study in south India on the prevalence of DR-TB indicated a high proportion (11%) of rifampicin and isoniazid mono-resistance in new cases (3). Increasing trends of DR-TB in new cases highlight potential public health concerns, as globally, 78% of rifampicin-resistant TB (RR-TB) cases were identified to be MDR-TB (3). In India, major mediating factors for the high incidence of DR-TB in new cases include transmission of AMR strains in endemic communities and acquired DR due to misuse and overuse of antimicrobials.
A compounding effect of COVID-19 on TB care has been extensively reported earlier in various countries (4–6). According to a recent WHO global TB report, India was the largest contributor to the global shortfall in TB case notification in 2020 compared to other high-burden countries (7). A significant impact of reduced TB case notification was directly observed on the incidence of TB and DR-TB in India, which showed a significant decline in 2020 (8) compared to previous years, which should be mainly attributable to restricted access to healthcare facilities amid lockdown measures in the country (5, 9). Available reports suggest MDR-TB patients co-infected with COVID-19 pose formidable challenges in treatment, as COVID-19 can favor bacterial replication in the lungs through interference in intestinal homeostasis (10, 11). The empirical use of antibiotics is considered to be an important risk factor for the development of resistance (11–13). As per a recent modeling analysis report, COVID-19 in India contributed to about 216 million excess doses of antibiotics in between epidemic peaks (14). Reports from a meta-analysis by Langford et al. (15) showed high antibiotic usage (~71.9%) in hospitalized COVID-19 patients, even though only 6–9% of them were confirmed to have bacterial infection. Studies also indicate high antibiotic prescribing patterns of almost 82% in Southeast-Asian countries (excluding China) during the pandemic period (16). As a result, the incidence of MDR infections in COVID-19 patients is estimated to be around 32–50% (17). Additionally, oral glucocorticoids such as methylprednisolone and intravenous monoclonal antibody tocilizumab therapy to control IL-6-induced cytokine release syndrome (CRS) were used rampantly in India during the second wave, despite a stringent WHO advisory on their administration (18). A case report by Khayat et al. indicated that prolonged corticosteroid therapy in COVID-19 leads to significant CD4 and CD8 T-cell depletion and may promote the development of active infection in latent close contacts of TB cases (19). This represents a significant public health concern, particularly for COVID-infected close contacts of MDR-TB cases, who might be at high risk for conversion to active infection due to depletion and dysfunction of T-cells similar to HIV.
India has been regarded as the global capital of AMR (2). According to the national anti-TB DR survey conducted by the Indian government in collaboration with the WHO and the United States Agency for International Development (USAID), it was found that around 23% of new cases showed resistance to any drug, with MDR-TB detected in 3% (20). In addition, ~36% of MDR-TB cases tested for resistance to second-line anti-tuberculosis medications showed resistance to fluoroquinolones (2). One of the major drivers of AMR in India is the irrational consumption of non-prescribed drugs taken as self-medication (21). India is regarded as the top consumer of antibiotics for humans among all other countries (22). Between 2005 and 2009, India witnessed a 40% rise in the sale of antibiotics, with sales of new-generation cephalosporin increasing by almost 60% (23). According to one survey, ~52% of the Indian population is estimated to be self-medicating (24). Despite regulations on the sale of schedule H1 and X drugs in India, second-line drugs like fluoroquinolones are readily available over the counter (OTC) in pharmacies due to their rampant empirical use against a wide variety of infections. Such OTC practices are even more prevalent in peripheral and rural zones of India, wherein mostly experienced and untrained pharmaceutical staff can prescribe the wrong combination and duration of drugs to the locals. As a result, it is estimated that the majority of people consuming OTC drugs generally under-dose themselves, exposing bacteria to non-lethal drug concentrations and thereby developing DR. Figures 1A, B shows general drug resistant pattern of carbapenem, fluoroquinolones and aminoglycosides on common invasive isolates of E. coli, P. aeruginosa in India and China. In addition to self-medication, high prescription and consumption patterns of second- and third-line antibiotics (Figure 1C) and mismanagement of TB presumptive cases by private physicians and pharmacists at the primary care level have been attributed to bolstering AMR cases in Indian communities (24). A study published by Arinaminpathy et al. (25) shows mismanagement and a lack of standard of care followed by private practitioners among presumptive TB cases as one of the major drivers for MDR-TB development. Similar studies by Kwan et al., based on referral rates of patients to healthcare centers, have shown a significant impact of urban pharmacies on TB control (26). Their study shows low referral rates of presumptive cases by urban pharmacies as a major compounding factor for low detection rates and disease control.
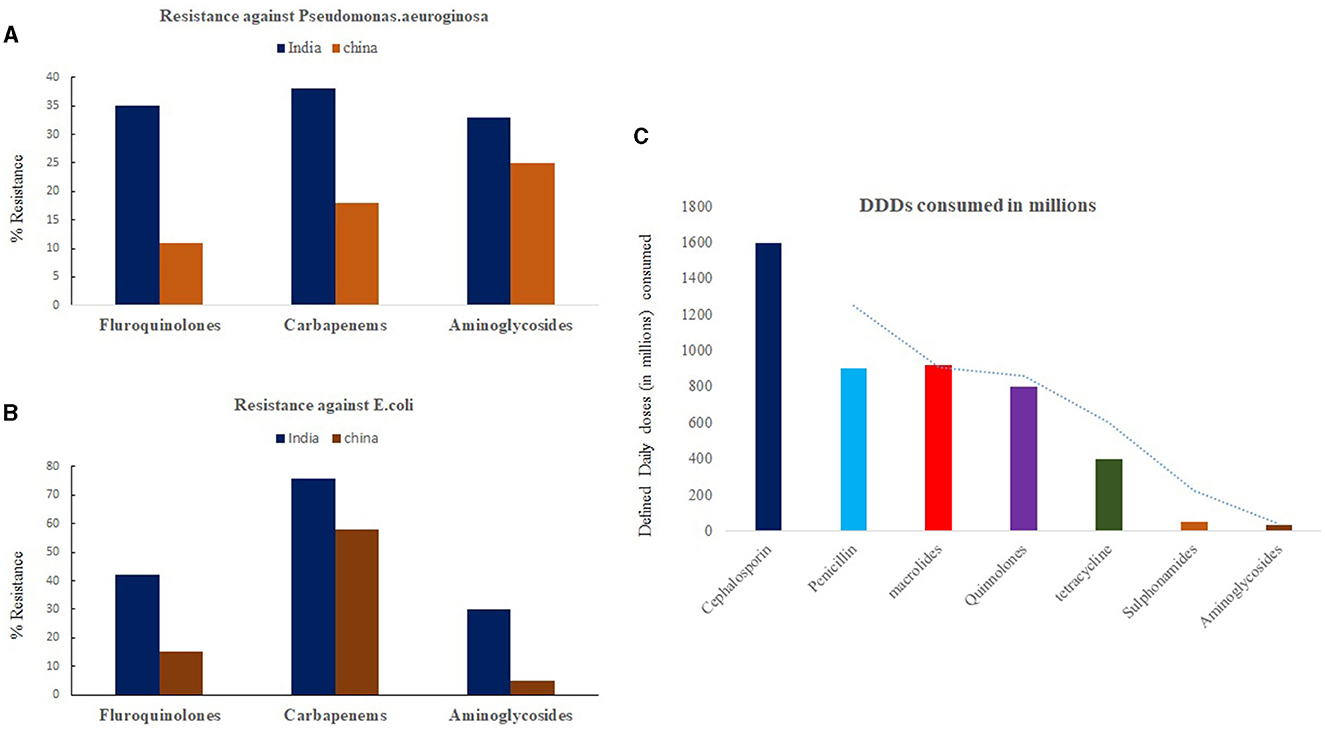
Figure 1. Trend of Antibiotic resistance (2019–20) among second line drugs against invasive isolates of (A) Pseudomonas aeruginosa and (B) E. coli in India and China. (C) Represents trend of antibiotic consumption pattern of common drugs in Indian population represented as defined daily dose (DDDs) per million populations. Source: One Health Trust. Resistance Map: Antibiotic resistance 2023. https://resistancemap.onehealthtrust.org/AntibioticResistance.php (accessed May 31, 2023).
To combat AMR, India introduced several measures, including the “Red Line” campaign in 2016 and the National Action Plan (NAP) on AMR in 2017 (27). Additionally, the Cosmetics and Drug Act launched in 2013 prohibited the sale of Schedule H1 drugs without prescription in India, which includes second- and third-generation antibiotics and anti-TB drugs. With due cognizance of AMR, the National Center for Disease Control under the Ministry of Health and Family Welfare, Government of India, developed a national plan on AMR containment with the objectives of improving awareness and surveillance of AMR in Indian health settings. In 2017, India launched its revised national strategic plan (NSP) with the ambitious goal of ending TB by 2025. Under its revised NSP (2017–25), the Government of India introduced several aggressive steps, such as decentralization of DR-TB services for better accessibility under the private sector, universal drug susceptibility testing (U-DST) for presumptive patients from MDR-TB hotspots, and scaling up diagnostic services of CBNAAT, True NAT, and line probe assays in low-resource settings for better surveillance and diagnosis (28).
Despite the aggressive efforts made under the revised NSP, DR-TB cases continue to bolster in Indian health settings. Improved commitments are needed to integrate antimicrobial stewardship programs under pandemic response to frontline providers for the management of TB patients co-infected with COVID-19. Integration of TB health services with AMR programs can be done to leverage the expertise of TB control planners for strengthening diagnostic capacity for AMR testing, surveillance, better quality assurance, record keeping, and logistics. Expanding the diagnostic scope of TB testing laboratories for AMR surveillance in high-endemic spots for TB can be useful for developing important public health measures for combating AMR. Joint efforts and funding commitments from major TB stakeholders are needed globally to develop improved diagnostics and surveillance tools to detect AMR in second- and third-line drugs in high-TB settings. Similarly, efforts are needed to develop diagnostic stewardship programs that include mandatory U-DST for second-line drugs in COVID health care settings, COVID-19 hotspots and TB-endemic settings for early diagnosis and treatment outcomes. Accelerated surveillance and vigilance at local pharmacies is needed to minimize the sale of OTC schedule H1 and X drugs. The development of an online, multilingual, free consultation system for primary care physicians with TB health care providers with expertise in clinical and management experience can improve AMR stewardship for DR-TB. The European respiratory society has a similar web-based free consultation system for South Africa (29), which can be extended to highly endemic South Asian countries for better DR-TB management.
The alarming rise of AMR in Indian communities in the aftermath of waves combined is a major risk driver for future MDR-TB epidemics in India. The country needs immediate attention on surveillance of DR in COVID hotspots to minimize future transmission of MDR in communities. Additionally, increased political commitments and advocacy are needed to bolster antimicrobial stewardship in COVID care hospitals and quarantine zones to minimize the irrational use of empirical regimens. Such advocacy is needed to prevent the risk of the development of AMR in Indian communities and improve treatment outcomes for cases of MDR-TB in India in the near future.
RK: Writing – original draft, Writing – review & editing. AH: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World health Organisation. Antimicrobial Resistance. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed May 23, 2023).
2. Taneja N, Sharma M. Antimicrobial resistance in the environment: the Indian scenario. Indian J Med Res. (2019) 149:119–28. doi: 10.4103/ijmr.IJMR_331_18
3. Shivekar SS, Kaliaperumal V, Brammacharry U, Sakkaravarthy A, Raj CKV, Alagappan C, et al. Prevalence and factors associated with multi-drug-resistant tuberculosis in South India. Sci Rep. (2020) 10:175–52. doi: 10.1038/s41598-020-74432-y
4. Aznar ML, Espinosa-Pereiro J, Saborit N, Jové N, Sánchez Martinez F, Pérez-Recio S, et al. Impact of the COVID-19 pandemic on tuberculosis management in Spain. Int J Infect Dis. (2021) 108:300–5. doi: 10.1016/j.ijid.2021.04.075
5. Husain AA, Monaghan TM, Kashyap RS. Impact of COVID-19 pandemic on tuberculosis care in India. Clin Microbiol Infect. (2021) 27:293–4. doi: 10.1016/j.cmi.2020.08.014
6. Glaziou P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. (2020) MedRxiv. doi: 10.1101/2020.04.28.20079582
7. World Health Organisation Global Tuberculosis Report. (2021). Available online at: file:///D:/Data%20from%20C%20drive/Downloads/9789240037021-eng.pdf (accessed June 1, 2023).
8. Central Tuberculosis Division, Government Government of India. India Tuberculosis Report 2021. India: Revised National Tuberculosis Control Program. (2021). Available online at: https://tbcindia.gov.in/showfile.php?lid=3587 (accessed December 10, 2021).
9. Stop TB Partnership. The Potential Impact of the Covid-19 Response on Tuberculosis in High-Burden Countries: A Modelling Analysis. (2020). Available online at: http://www.stoptb.org/assets/documents/news/Modeling%20Report_1%20May%202020_FINAL.pdf (accessed November 10, 2021).
10. Marwah V, Peter DK, Ajai Kumar T, Bhati G, Kumar A. Multidrug-resistant tuberculosis in COVID-19: double trouble. Med J Armed Forces India. (2021) 77(Suppl. 2):S479–82. doi: 10.1016/j.mjafi.2021.05.002
11. Pelfrene E, Botgros R, Cavaleri M. Antimicrobial multidrug resistance in the era of COVID-19: a forgotten plight. Antimicrob Resist Infect Control. (2021) 10:21. doi: 10.1186/s13756-021-00893-z
12. Kumar G, Mukherjee A, Sharma RK, Menon GR, Sahu D, Wig N, et al. National clinical registry for COVID-19 team. Clinical profile of hospitalised COVID-19 patients in first & second wave of the pandemic: insights from an Indian registry based observational study. Indian J Med Res. (2021) 0153:619–28. doi: 10.4103/ijmr.ijmr_1628_21
13. Adebisi YA, Jimoh ND, Ogunkola IO, Uwizeyimana T, Olayemi AH, Ukor NA, et al. The use of antibiotics in COVID-19 management: a rapid review of national treatment guidelines in 10 African countries. Trop Med Health. (2021) 23:49–51. doi: 10.1186/s41182-021-00344-w
14. Sulis G, Batomen B, Kotwani A, Pai M, Gandra S. Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: an interrupted time series analysis. PLoS Med. (2021) 18:e1003682. doi: 10.1371/journal.pmed.1003682
15. Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta- analysis. Clin Microbiol Infect. (2020) 26:1622–9. doi: 10.1016/j.cmi.2020.07.016
16. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned best practices, and future implications. Int J Infect Dis. (2021) 113:103–8. doi: 10.1016/j.ijid.2021.10.001
17. Pasero D, Cossu AP, Terragni P. Multi-drug resistance bacterial infections in critically ill patients admitted with COVID-19. Microorganisms. (2021) 9:1773. doi: 10.3390/microorganisms9081773
18. Chaudhry D, Singh PK. Tocilizumab and COVID-19. Indian J Crit Care Med. (2020) 24:741–3. doi: 10.5005/jp-journals-10071-23608
19. Khayat M, Fan H, Vali Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: a case report. Respir Med Case Rep. (2021) 32:101344. doi: 10.1016/j.rmcr.2021.101344
20. Ministry of Health and Family Welfare GoI. Report of the First National Anti-tuberculosis Drug Resistance Survey: India 2014–16. (2018). Available online at: https://tbcindia.gov.in/showfile.php?lid=3315 (accessed December 10, 2022).
21. Kashyap RS, Husain AA. Over-the-counter drug distribution and tuberculosis control in India. Lancet Infect Dis. (2016) 16:1208–9. doi: 10.1016/S1473-3099(16)30262-6
22. Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect Dis. (2014) 14:742–50. doi: 10.1016/S1473-3099(14)70780-7
23. Marathe PA, Kamat SK, Tripathi RK, Raut SB, Khatri NP. Over-the-counter medicines: global perspective and Indian scenario. J Postgrad Med. (2020) 66:28–34. doi: 10.4103/jpgm.JPGM_381_19
24. Dixit A, Kumar N, Kumar S, Trigun V. Antimicrobial resistance: progress in the decade since emergence of New Delhi Metallo-β-lactamase in India. Indian J Community Med. (2019) 44:4–8. doi: 10.4103/ijcm.IJCM_217_18
25. Arinaminpathy N, Batra D, Khaparde S, Vualnam T, Maheshwari N, Sharma L, et al. The number of privately treated tuberculosis cases in India: estimation from drug sales data. Lancet Infect Dis. (2016) 16:1255–60. doi: 10.1016/S1473-3099(16)30259-6
26. Kwan A, Daniels B, Bergkvist S, Das V, Pai M, Das J. Use of standardized patients for healthcare quality research in low- and middle-income countries. BMJ Global Health. (2019) 4:e001669. doi: 10.1136/bmjgh-2019-001669
27. Ranjalkar J, Chandy SJ. India's National Action Plan for antimicrobial resistance - An overview of the context, status, and way ahead. J Family Med Prim Care. (2019) 8:1828–34. doi: 10.4103/jfmpc.jfmpc_275_19
28. Husain AA, Kupz A, Kashyap RS. Controlling the drug-resistant tuberculosis epidemic in India: challenges and implications. Epidemic Health. (2021) 43:e2021022. doi: 10.4178/epih.e2021022
29. Foster I, Sullivan A, Makanda G, Schoeman I, Tisile P, van der Westhuizen HM, et al. The role of counselling in tuberculosis diagnostic evaluation and contact tracing: scoping review and stakeholder consultation of knowledge and research gaps. BMC Public Health. (2022) 22:190. doi: 10.1186/s12889-022-12556-8
Keywords: TB, MDR-TB, India, antimicrobial resistance, COVID-19
Citation: Husain AA and Kashyap RS (2023) Double trouble: compounding effects of COVID-19 pandemic and antimicrobial resistance on drug resistant TB epidemiology in India. Front. Public Health 11:1305655. doi: 10.3389/fpubh.2023.1305655
Received: 02 October 2023; Accepted: 13 November 2023;
Published: 06 December 2023.
Edited by:
Adwoa Asante-Poku, University of Ghana, GhanaReviewed by:
Felix Khuluza, Kamuzu University of Health Sciences (formerly College of Medicine-University of Malawi), MalawiCopyright © 2023 Husain and Kashyap. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajpal Singh Kashyap, cmFqX2NpaW1zQHJlZGlmZm1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.