
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 04 December 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1301492
This article is part of the Research TopicLearning from Cutaneous Manifestations in Systemic Infectious DiseasesView all 8 articles
 Imama N. Butt
Imama N. Butt Charmaine van Eeden
Charmaine van Eeden Katharina Kovacs Burns
Katharina Kovacs Burns Lynora Saxinger
Lynora Saxinger Alison Clifford
Alison Clifford Desiree Redmond
Desiree Redmond Jan Willem Cohen Tervaert
Jan Willem Cohen Tervaert Elaine Yacyshyn*
Elaine Yacyshyn*Objective: To identify the factors that impact COVID-19 vaccine decision-making in vaccine-hesitant vasculitis patients, and compare their perceptions with other rheumatology patients, given existence of data suggesting rheumatology patients may have disease-specific factors that influence their COVID-19 vaccine decision-making.
Methods: This cross-sectional study surveyed adult rheumatology patients from the Kaye Edmonton Clinic Rheumatology Clinic, in Canada, between June and August 2021, using an anonymous online questionnaire. Survey responses were analyzed for statistical differences using chi-square analysis.
Results: The COVID-19 Vaccine Perceptions Survey had a response rate of 70.9%. Of the total 231 respondents, 103 patients were diagnosed with vasculitis. At the time of the survey, 10.6% of vasculitis patients refused to receive a COVID-19 vaccine compared to 6.3% for other rheumatology patients. Compared to other rheumatology patients, vaccine-hesitant vasculitis patients were significantly more concerned about almost every aspect of available COVID-19 vaccines [e.g., safety (p < 0.001), components (p < 0.001)], and feared that they could contract SARS-CoV-2 from a vaccine (p < 0.001). These vaccine-hesitant patients were also significantly less pleased with the government's pandemic response, less confident in healthcare team-provided information (p < 0.001), and more likely to report that healthcare providers had no role in their COVID-19 vaccine decision-making (p < 0.001).
Conclusion: Vaccine-hesitant vasculitis patients may have multiple considerations influencing COVID-19 vaccine hesitancy, including vaccine and disease-specific concerns, along with unfavorable perceptions of the healthcare system (government and healthcare providers). Healthcare providers can address some of these concerns by initiating patient-centered discussions around immunizations to help support educated decision-making.
Vaccination against SARS-CoV-2 is an important tool in the management of the COVID-19 pandemic (1). It is critical to optimize vaccination uptake amongst the public, particularly in vulnerable populations, at greater risk for contracting infectious diseases, including those with rheumatic conditions, associated comorbidities, and therapies used for disease management (2). Relatedly, some rheumatology patients can be at greater risk of contracting SARS-CoV-2 (3, 4), and some data suggests increased risk of poor outcomes after developing COVID-19 (3–6). Specifically, vasculitis patients are at a potentially elevated risk, as preliminary data suggests that these individuals can develop severe COVID-19 disease, with high morbidity and mortality due to underlying conditions and treatments, compared to the general public (7, 8). Additionally, patients with vasculitis have reported stronger negative beliefs about their disease and its implications on their emotional and physical wellbeing compared with other chronic illnesses (9). Regarding the COVID-19 pandemic, patients with vasculitis described high concerns about the pandemic, because of their underlying vasculitis diagnosis (10). Despite increased concerns, many vasculitis patients engaged in harmful health-related behaviors, such as avoiding doctors' office visits, laboratory tests, along with stopping or delaying immunosuppressive medications, often without consulting their healthcare providers (10). Therefore, vaccination against SARS CoV-2 is an important tool to help manage the substantial risk that COVID-19 poses to vasculitis patients' overall health.
Previous international studies have demonstrated significant vaccine hesitancy in rheumatology patients (11, 12). Recent studies indicated that patients with autoimmune and inflammatory rheumatic diseases (AIIRDs), undergoing treatment, were less likely to be vaccinated (76.9 vs. 87.0%) (13), and 32% less likely to receive a COVID-19 vaccine, compared to non-AIIRDs patients (14). Multiple factors could be contributory, but some vasculitis patients are noted to have specific concerns that vaccines may have triggered or exacerbated their autoimmune condition (15, 16). However, recent systematic reviews show limited evidence to support de novo systemic vasculitis post-COVID-19 vaccination (17). Although there are case reports of small-vessel vasculitis as an adverse reaction to SARS-CoV-2 immunization, this is a rare phenomenon, and is typically cutaneous—or renally—limited, transient, with good prognosis (17). Finally, while additional knowledge is emerging, initial COVID-19 vaccine clinical trials excluded immunocompromised patients, which could contribute to rheumatology patient reluctance because of uncertainty around safety or effectiveness of the vaccines (18).
The above factors, along with vaccine misinformation and disinformation, challenge the success of COVID-19 immunization programs (19), emphasizing the need to understand vaccine-hesitant rheumatology patients' perceptions. This knowledge can help healthcare providers in discussions with patients to alleviate concerns, support educated medical decision-making, and potentially encourage vaccine uptake.
The objective of this study was to identify the factors that impact COVID-19 vaccine decision-making in vaccine-hesitant vasculitis patients compared with other rheumatology patients, given that vasculitis patients demonstrated increased COVID-19 related concerns and harmful health-related behaviors, which could exacerbate their pre-existing risk for contracting and developing poor outcomes from SARS CoV-2.
This cross-sectional descriptive survey aimed to explore perceptions of COVID-19 vaccines among rheumatology patients. This study compared factors that influence vaccine decision-making in a sub-group of patients diagnosed with vasculitis, compared to other rheumatology patients. Anonymous participants responded to an online questionnaire using the REDCap platform, between June and August 2021.
This study was conducted at the Kaye Edmonton Clinic (KEC) Rheumatology Clinic, in Edmonton, Alberta, Canada. Patients seen in the Rheumatology Clinic are by referral only, and include individuals living anywhere in Edmonton, and surrounding areas.
Patients were sequentially recruited from a convenience sample of adult (≥18 years) rheumatology patients (diagnosed with one or more rheumatologic condition) seen in-clinic at the KEC between June and August 2021. All participants were recruited from the same physician clinics. Potential participants were informed of the research study and its purpose when they attended a scheduled appointment. Interested patients voluntarily provided their email address and were subsequently forwarded a link to an anonymous online survey. Participants were required to have their own device with reliable internet access. Participants were informed that they could respond to some or all questions and could also withdraw entirely by not submitting the survey.
Vasculitis patients made up 45% of all rheumatology patient participants, permitting relatively similar sample sizes for analysis and comparison. Rheumatic conditions included spondyloarthropathies (ankylosing spondylitis, psoriatic arthritis), rheumatoid arthritis, fibromyalgia, gout, lupus, myositis, scleroderma, polymyalgia rheumatica (PMR), sarcoidosis, tendonitis/bursitis, and osteoporosis. Vasculitis diagnoses included large vessel vasculitis (Giant Cell Arteritis, Takayasu's arteritis), ANCA-associated vasculitis (GPA, MPA, EGPA), and small vessel vasculitis (IgA vasculitis).
The COVID-19 Vaccine Perceptions Survey has been previously described (20), and was a 44-item online REDCap survey, that included quantitative and qualitative items. The survey was based on a review of vaccine hesitancy literature as well as circumstances and messaging regarding vaccination at the time (11, 12, 21). To identify factors that could impact decision to vaccinate, the survey included demographics (22–25), patient self-reported medical condition(s), and treatment, as well as views around contracting SARS-CoV-2, COVID-19 vaccine concerns, views of the government's role in handling the COVID-19 pandemic (26), and questions regarding informed decision-making. As previous work suggested the influence of healthcare teams in promoting vaccine acceptance (27–29), the final survey section also asked participants about their perceptions of their healthcare team. The survey was pilot tested for a grade eight reading and comprehension level. Patients were provided a unique link to the online survey, determined to take 20 min to complete based on pilot-testing.
All quantitative questions were descriptively analyzed (i.e., percentages and frequencies) along with chi-square analysis to determine statistical significance (p < 0.05) using STATA 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). Vaccine-hesitant vasculitis patients were defined as those who indicated that they had been diagnosed with vasculitis and stated that they did not want a COVID-19 vaccine at the time of the survey. Responses from the vaccine-hesitant vasculitis patients were analyzed separately and compared to other non-vaccine-hesitant vasculitis responders.
This study received ethics approval from the Health Research Ethics Board at the University of Alberta (Pro00108774).
The COVID-19 Vaccine Perceptions Survey had a response rate of 70.9% across all rheumatology patient responders, with 326 survey invitations sent out. Of the 231 patient participants, 103 responders (44.6% of total survey participants) indicated that they had been diagnosed with vasculitis [Takayasu's arteritis, Giant cell arteritis (GCA), ANCA-associated vasculitis, or IgA vasculitis].
Table 1 lists demographic characteristics of vasculitis patients compared to other rheumatic patient respondents. There were no significant differences in gender, age, education level, or employment status. The majority of survey participants were female (63.1% vasculitis; 75.2% other rheumatic conditions) between the ages of 40 and 64 years old (51.4% vasculitis; 55.5% other rheumatic conditions), who had completed post-secondary education (46.0% vasculitis; 60.3% other rheumatic conditions), and employed (66.0% vasculitis; 74.6% other rheumatic conditions). There were no significant differences in medical history characteristics between the groups.
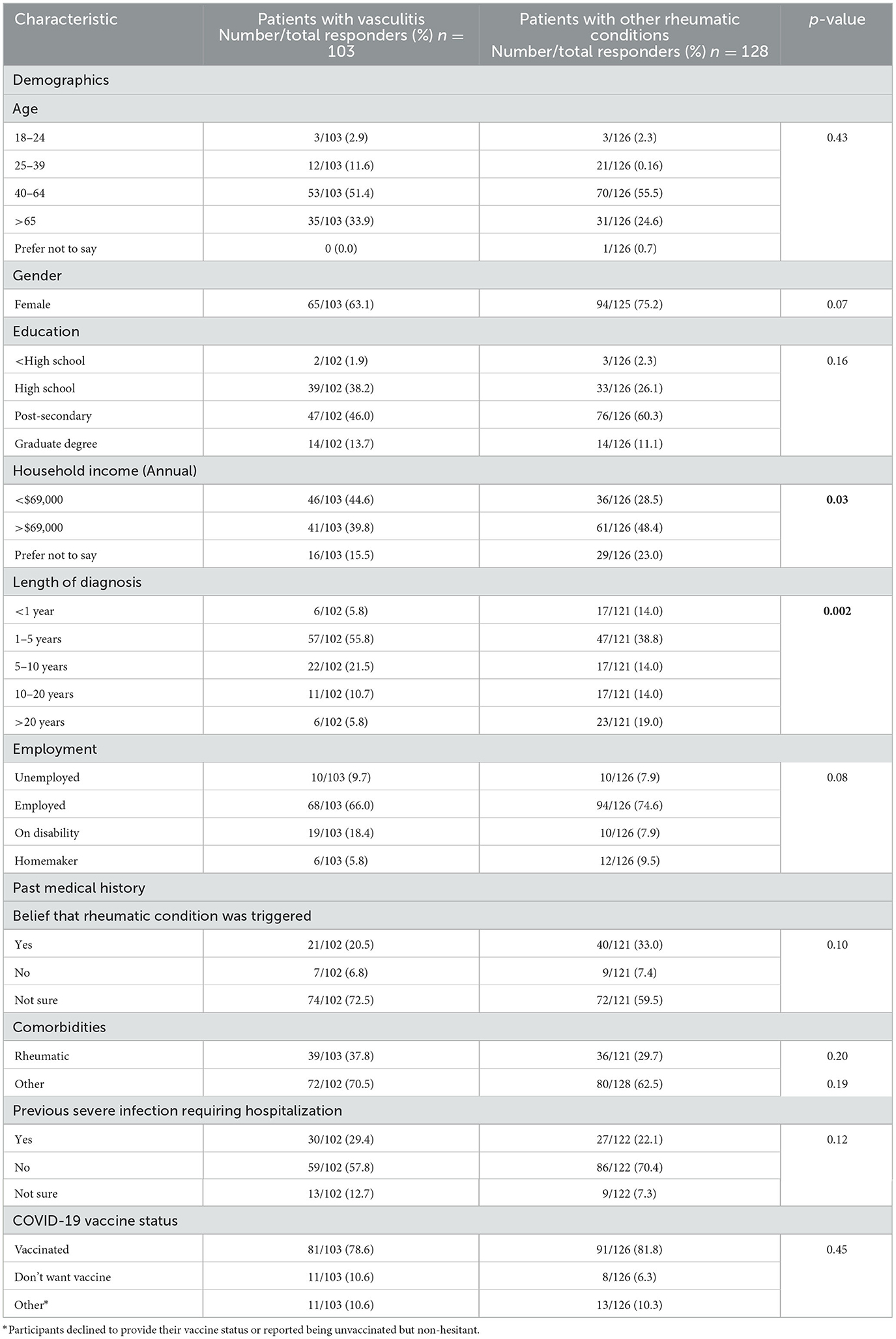
Table 1. Demographic and past medical characteristics of vasculitis patients compared to other rheumatic patients who participated in the COVID-19 vaccine perceptions survey.
The vasculitis subgroup had a higher proportion of patients with annual household income of CAD 69,000 or less (44.6% vasculitis; 28.5% other), compared to other rheumatologic conditions (p = 0.03), with a shorter duration of diagnosed illness (p = 0.002), with 55.8% of patients (38.8% other) diagnosed for 1 to 5 years.
At the time of the survey (June–August 2021), 78.6% of vasculitis respondents had received at least one COVID-19 vaccine dose, compared with 81.8% of other rheumatology patients. However, vasculitis patients had non-significantly higher hesitancy rates compared to other participants with other rheumatic conditions (10.6% vasculitis; 6.3% other).
Survey responses from vaccine-hesitant vasculitis patients were further analyzed to identify the factors implicated in a decision to refuse COVID-19 vaccines, in individuals who had never been immunized against SARS-CoV-2, as listed in Tables 2–4.
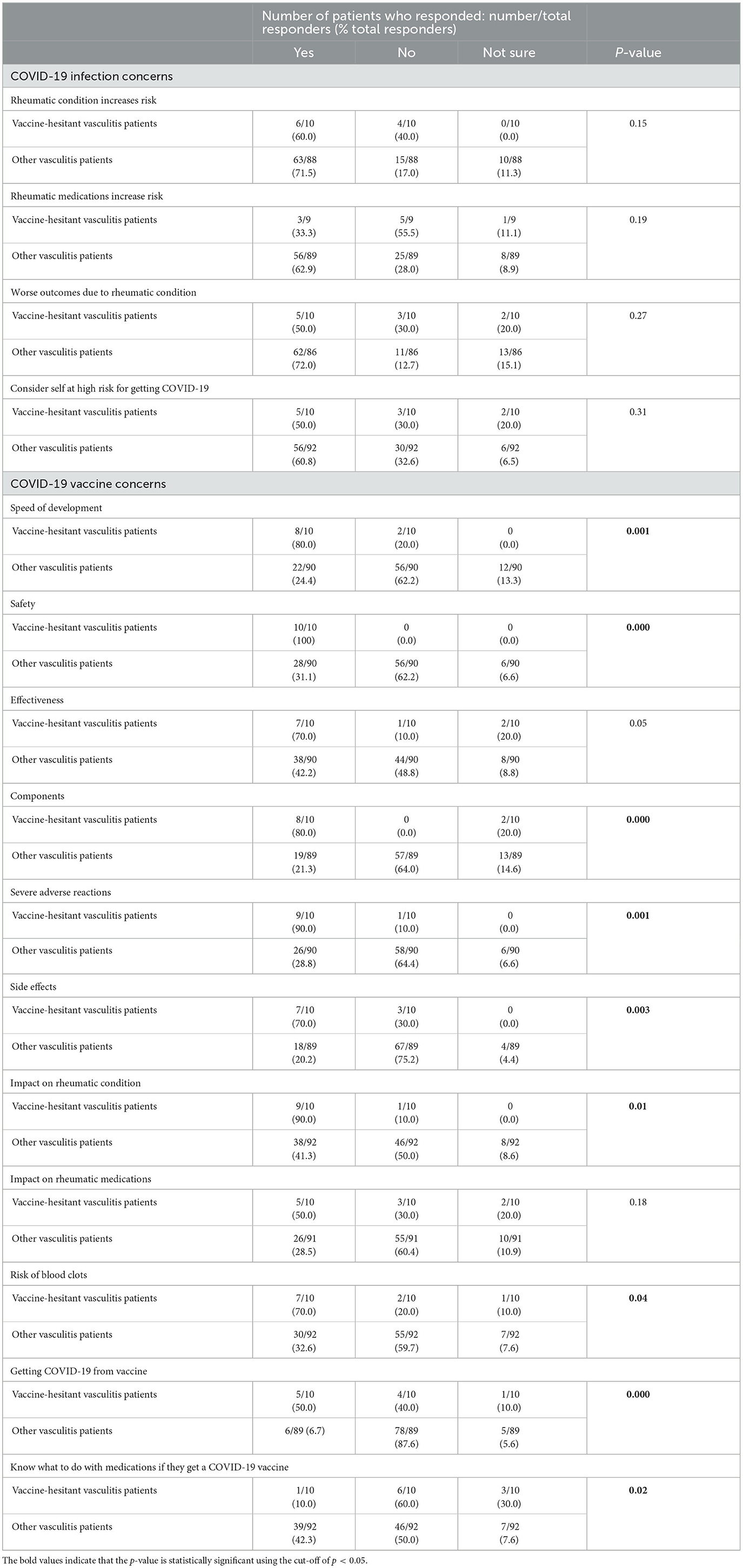
Table 2. Vaccine-hesitant vasculitis patients' concerns around COVID-19 and COVID-19 vaccination in patients who participated in the COVID-19 vaccine perceptions survey.
Table 2 examines concerns related to the impact of rheumatic disease or medications on contracting SARS-CoV-2 or developing worse outcomes, which did not significantly differ between vaccine-hesitant vasculitis and other non-hesitant vasculitis patients. However, vaccine-hesitant vasculitis patients were significantly more concerned about almost every aspect of available COVID-19 vaccines, including safety (p < 0.001), components (p < 0.001), speed of development (p = 0.001), risk of severe adverse reactions (p = 0.001), side effects (p = 0.003), impact on rheumatology condition (p = 0.01), risk of blood clots (p = 0.04), and fears that they could develop COVID-19 from COVID-19 vaccines (p < 0.001). Vaccine-hesitant vasculitis patients tended to be more concerned about vaccine effectiveness compared to other non-hesitant vasculitis responders, although this was not significantly increased (p = 0.05). While vaccine-hesitant vasculitis patients did not significantly differ from other responders regarding concerns about the potential impact of a COVID-19 vaccine on their rheumatology medications (p = 0.18), this subgroup was significantly less informed about how to manage rheumatology medications when receiving a COVID-19 vaccine (p = 0.02). Additionally, there were no significant differences in responses between vaccine-hesitant vasculitis patients compared to vaccine-hesitant patients with other (non-vasculitis) rheumatic conditions (Supplementary Tables A, B).
Table 3 lists vaccine-hesitant vasculitis patients' perceptions of the government's role in handling the COVID-19 pandemic, showing less approval of the government's COVID-19 response, and concerns that publicly available COVID-19 vaccines were not of the highest quality (p = 0.005). There was a trend to believing that the government did not give clear details on available vaccines (p = 0.02), less trust of government-provided reports on vaccines (p = 0.04) and COVID-19 (p = 0.04). Vaccine-hesitant vasculitis patients were like other responders regarding effectiveness of the government's vaccine rollout plan (p = 0.40) and the public health measures (p = 0.31).
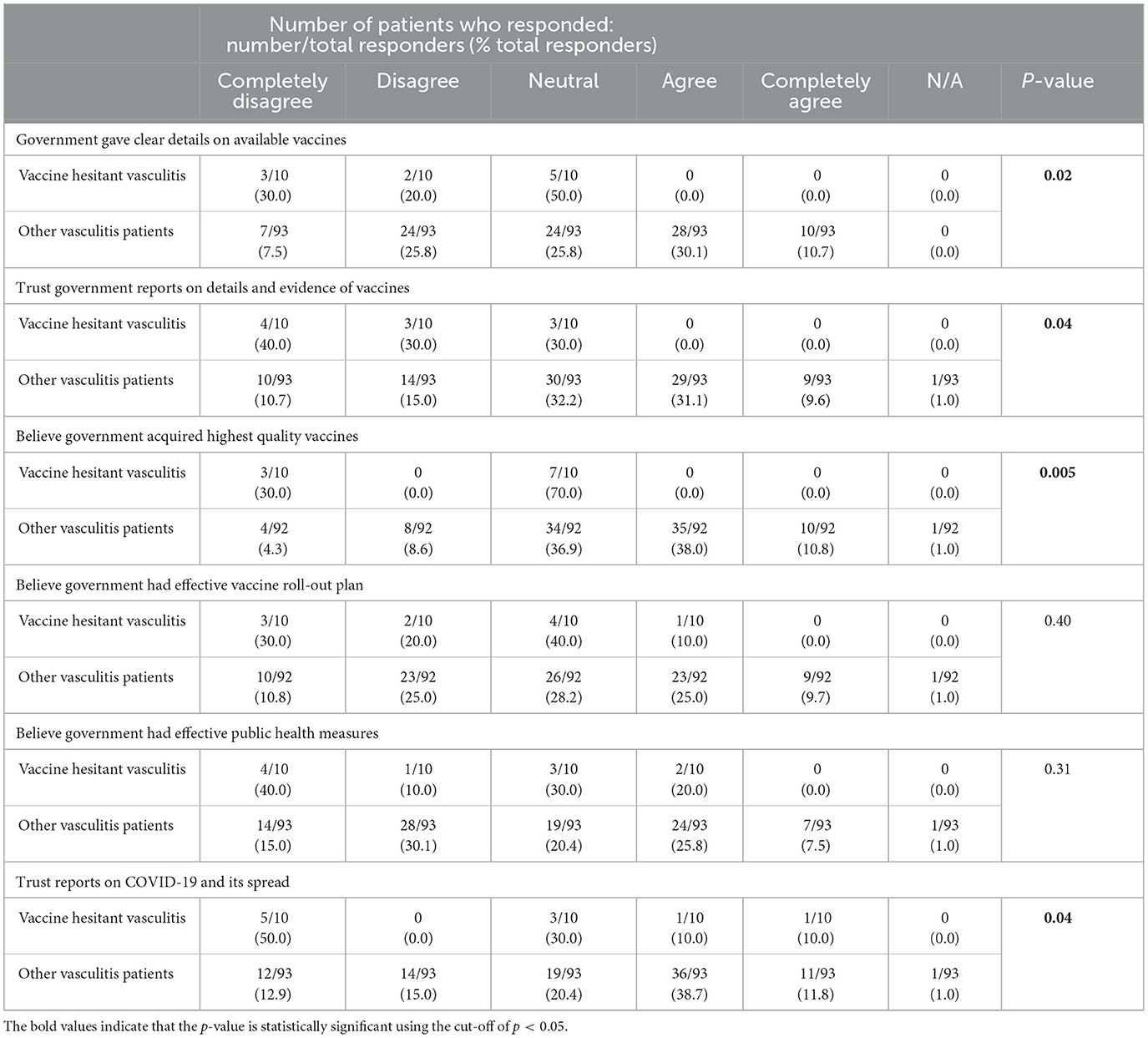
Table 3. Vaccine-hesitant vasculitis patients' perceptions on the government during the COVID-19 pandemic in patients who participated in the COVID-19 vaccine perceptions survey.
Table 4 reports vaccine-hesitant vasculitis patients' views of the healthcare providers involved in their care. Compared to 84.9% of non-hesitant vasculitis responders, only 50% of vaccine-hesitant vasculitis patients indicated having discussed COVID-19 vaccines with their providers (p = 0.01), and 40% felt that their healthcare team was able to answer their questions on SARS-CoV-2 vaccines vs. 81.5% of other vasculitis patients (p = 0.008). Additionally, these vaccine-hesitant participants were more likely to report that their healthcare providers had no role in their immunization decision-making (100% vaccine-hesitant vs. 8.9% other vasculitis) (p < 0.001), had less confidence in the information provided to them by their healthcare team (30% vaccine-hesitant vs. 2.1% other vasculitis) (p < 0.001). Only 40% of these patients indicated that they would involve healthcare providers in their immunization decisions, compared to 76.3% other vasculitis (p = 0.01), and were much more likely (80% vaccine-hesitant vs. 32.2% other vasculitis) to make their decisions independently (p = 0.003). Despite this, vaccine-hesitant vasculitis patients were like other vasculitis responders regarding having spoken to healthcare professionals about COVID-19 vaccine risks and benefits (p = 0.08) and being encouraged to get a COVID-19 vaccine (p = 0.25).
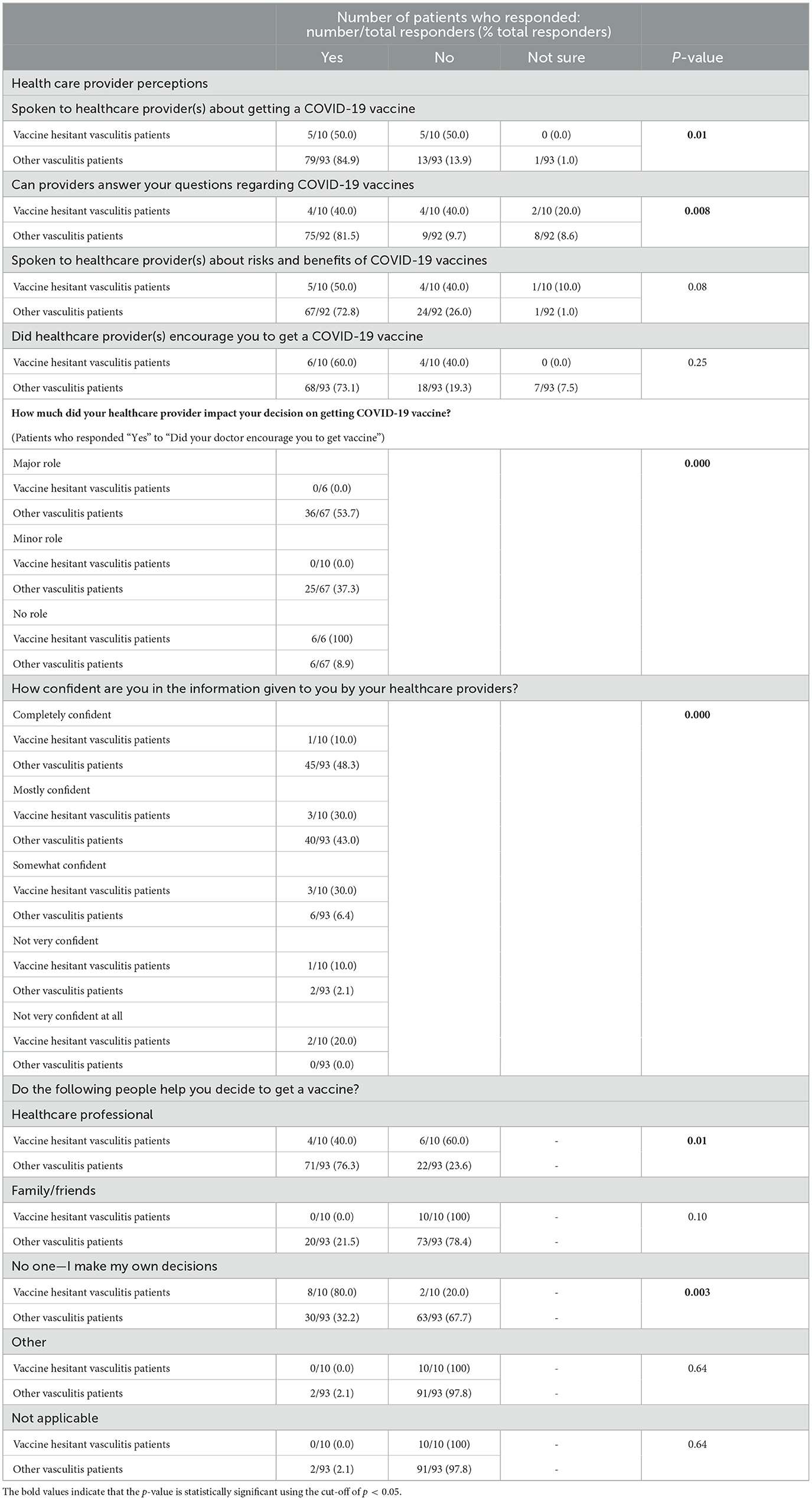
Table 4. Vaccine-hesitant vasculitis patients' perceptions of their healthcare providers in patients who participated in the COVID-19 vaccine perceptions survey.
This study identified factors that vaccine-hesitant vasculitis patients indicated influenced their decision-making around COVID-19 vaccination. To our knowledge, this is the first study specifically analyzing COVID-19 vaccine perceptions in vasculitis patients, a group identified to have increased COVID-19-related concerns and harmful health-related behavior (10), in the context of increased risk of poor COVID-19 outcomes (30).
This survey was administered between June and August 2021, between the third and fourth waves of the COVID-19 pandemic. COVID-19 vaccines were publicly available starting early 2021, with second doses widely accessible in June 2021. At the time of the survey, 78.6% of vasculitis patients had received at least one dose of an approved COVID-19 vaccine, compared with 81.8% of other rheumatology patients. However, slightly more vasculitis patients (10.6%) refused vaccination compared to other rheumatology patients (6.3%), although this was not significantly increased. Similarly, a previous study demonstrated that SARS CoV-2 vaccine acceptance was not significantly different in a subgroup of vasculitis and SLE patients compared to other rheumatic conditions (31).
In this study, vasculitis participants were more likely to have a lower annual household income. Previous research has shown that lower income is associated with increased COVID-19 vaccine hesitancy (32, 33). Additionally, vasculitis patients had shorter length of rheumatic disease diagnosis compared to other rheumatology responders. Although further studies are required to specifically determine if length of disease diagnosis impacts SARS CoV-2 immunization decision-making, a study investigating COVID-19 vaccine acceptance in Takayasu's arteritis patients showed that vaccinated individuals were more likely to have longer disease duration (34).
An earlier report from this dataset demonstrated that rheumatology patients have disease-specific factors that influence their COVID-19 vaccine decision-making, including concerns around vaccine adverse effects, efficacy, and risk of contracting SARS CoV-2 from a COVID-19 vaccine (20). Analysis of the vaccine-hesitant vasculitis patients' responses revealed similar concerns, along with three additional major themes potentially influencing their COVID-19 vaccine refusal. These included significantly greater concerns around COVID-19 vaccines, unfavorable perceptions of healthcare providers, and negative views of the government's role in the COVID-19 pandemic.
Vaccine-hesitant vasculitis patients were significantly more concerned about almost every aspect of COVID-19 vaccines. Additionally, similar to previous studies in rheumatology patients, vaccine-hesitant vasculitis patients were concerned about safety (35), side effects (36), risk of severe adverse events, and risk of thrombosis (35). Previous studies demonstrated that fears around speed of development, safety, and severe adverse events are significant in contributing to vaccine hesitancy (37–39). Vaccine-hesitant vasculitis patients also expressed greater concerns around COVID-19 vaccines' impact on rheumatic condition, which could be related to fears around potential disease flare after exposure to vaccine components (35). Patients can be advised that there are rare case reports of COVID-19 vaccine—exacerbated vasculitis flare (40), although the evidence is limited and does not consistently demonstrate that SARS-CoV-2 immunization induces vasculitis (17).
Vaccine-hesitant vasculitis patients were also less confident and less likely to involve their healthcare providers in COVID-19 vaccine decision-making compared to other responders, and reported feeling that their medical teams were unable to answer their COVID-19 vaccine-related questions. These negative perceptions could be furthering mistrust in healthcare providers and the healthcare system and contributing to vaccine hesitancy. Previous studies have demonstrated that healthcare teams are significant in promoting vaccine acceptance (27–29), and increased willingness to get a SARS-CoV-2 vaccine, if recommended by their doctor (41, 42). Additionally, rheumatology patients have disease-specific concerns (20), which can also be better addressed and managed with healthcare provider support. Given that vaccine-hesitant vasculitis patients in this study were significantly more likely to make their vaccine decisions independently, one intervention may be to provide these individuals with accurate, accessible information after a clinic visit for independent decision-making.
Healthcare providers should counsel patients that approved COVID-19 vaccines are effective, safe, and recommended in rheumatology patients, although a potential for disease exacerbation exists (43, 44). Specifically, patients should be reminded that current evidence does not demonstrate consistently increased risk of disease flare after SARS-CoV-2 immunization, but patients with active disease may be at greater risk for exacerbation (44). Additionally, most disease flares post-COVID-19 vaccination are mild, requiring minimal treatment changes (44). Patients should be informed that particular immunosuppressive therapies used in rheumatic disease management reduce the immune response to vaccination, but vaccination protection is still beneficial and important in disease management (43). Therefore, medication management in the context of SARS CoV-2 immunization should be discussed with rheumatology patients, especially since this study demonstrated that vaccine-hesitant vasculitis patients were less likely to know what to do with their medication when receiving a COVID-19 vaccine. Surprisingly, vaccine-hesitant patients reported being more concerned around the possibility of developing COVID-19 from an approved vaccine, so patients should be informed that approved COVID-19 vaccines do not contain live virus and cannot lead to the development of a full SARS-CoV-2 infection (45).
Vaccine-hesitant vasculitis patients reported being more displeased and less trusting of the government's COVID-19 pandemic role, compared to other responders, which could contribute to vaccine-hesitancy, as trust in the overall health system is critical to vaccine acceptance (46). Additionally, vaccine-hesitant patients found government-provided vaccine information to be unclear, further undermining patient confidence in vaccination decision-making. These patients also reported concerns around the quality of government-acquired COVID-19 vaccines, possibly due to concerns about nearly every aspect of available vaccines, combined with their mistrust in the government's response, and/or potential exposure to vaccine misinformation. Overall, our survey results demonstrate a clear confidence gap between vaccine-hesitant vasculitis patients and the government. Ultimately, this study promotes better understanding of the concerns of a vulnerable patient population, known to have increased COVID-19 related concerns and harmful health-related behaviors, in a background of elevated risk for SARS CoV-2 (10).
This study had the limitations inherent in the cross-sectional design and self-reported survey methods (47). Data captured in this study considers a specific timeframe (i.e., between June and August 2021), and is not generalizable beyond that period. Additionally, survey responses are self-reported by voluntary participants, and could be influenced by personal biases, recollection errors, or misunderstanding questions (48). The in-clinic convenience sample of patients is also a limitation, because only rheumatology patients seen in-clinic at that time were invited to complete the survey. Additionally, survey participation required internet access, and computer literacy, which could limit representation of disadvantaged populations. Despite these limitations, the study had a 70.9% response rate (n = 231) over a two-month period between the third and fourth waves of COVID-19 (i.e., between June and August 2021).
This study demonstrated that vaccine-hesitant patients may have multiple themes (e.g., SARS CoV-2 vaccine concerns, unfavorable perceptions of the healthcare system) implicated in their decision to refuse COVID-19 vaccination. Therefore, it is crucial that healthcare providers initiate patient-centered discussions around SARS CoV-2 immunization to help support educated decision-making. Attempts should be made to bridge the confidence gap between vaccine-hesitant patients and healthcare teams through open, respectful and transparent conversation around the evidence, as well as risks and benefits of vaccination, especially as it relates to the patient's particular rheumatic condition and disease management status. Additionally, further studies investigating perceptions in vasculitis patients are needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study received ethics approval from the Health Research Ethics Board at the University of Alberta (Pro00108774). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because potential participants were informed of the research study and its purpose when they attended a scheduled doctor's appointment. Interested patients voluntarily provided their email address and were subsequently forwarded a link to an anonymous online survey. Participants were informed that they could respond to some or all questions and could also withdraw entirely by not submitting the survey, and participation and submission of the survey was considered implied consent.
IB: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. CE: Data curation, Formal analysis, Writing – review & editing. KK: Investigation, Methodology, Writing – review & editing. LS: Formal analysis, Supervision, Validation, Writing – review & editing. AC: Formal analysis, Writing – review & editing, Validation. DR: Data curation, Methodology, Writing – review & editing. JC: Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. EY: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1301492/full#supplementary-material
1. Graham BS. Rapid COVID-19 vaccine development. Science. (2020) 368:945–6. doi: 10.1126/science.abb8923
2. Hazlewood GS, Pardo JP, Barnabe C, Schieir O, Barber CEH, Bernatsky S, et al. Canadian rheumatology association recommendation for the use of COVID-19 vaccination for patients with autoimmune rheumatic diseases. J Rheumatol. (2021) 48:1330–9. doi: 10.3899/jrheum.210288
3. Conway R, Grimshaw AA, Konig MF, Putman M, Duarte-García A, Tseng LY, et al. SARS–CoV-2 infection and COVID-19 outcomes in rheumatic diseases: a systematic literature review and meta-analysis. Arthr Rheumatol. (2022) 74:766–75. doi: 10.1002/art.42030
4. Li H, Wallace ZS, Sparks JA, Lu N, Wei J, Xie D, et al. Risk of COVID-19 among unvaccinated and vaccinated patients with rheumatoid arthritis: a general population study. Arthr Care Res. (2023) 75:956–66. doi: 10.1002/acr.25028
5. Grainger R, Kim AHJ, Conway R, Yazdany J, Robinson PC. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol. (2022) 18:191–204. doi: 10.1038/s41584-022-00755-x
6. Bruera S, Lei X, Zhao H, Yazdany J, Chavez-Macgregor M, Giordano SH, et al. Risks of mortality and severe coronavirus disease 19 (COVID-19) outcomes in patients with or without systemic lupus erythematosus. Lupus Sci Med. (2023) 10:e000750. doi: 10.1136/lupus-2022-000750
7. Kronbichler A, Geetha D, Smith RM, Egan AC, Bajema IM, Schönermarck U, et al. The COVID-19 pandemic and ANCA-associated vasculitis – reports from the EUVAS meeting and EUVAS education forum. Autoimmun Rev. (2021) 20:102986. doi: 10.1016/j.autrev.2021.102986
8. Sattui SE, Conway R, Putman MS, Seet AM, Gianfrancesco MA, Beins K, et al. Outcomes of COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica from the COVID-19 global rheumatology alliance physician registry: a retrospective cohort study. Lancet Rheumatol. (2021) 3:e855–64. doi: 10.1016/S2665-9913(21)00316-7
9. Grayson PC, Amudala NA, McAlear CA, Leduc RL, Shereff D, Richesson R, et al. Illness perceptions and fatigue in systemic vasculitis. Arthr Care Res. (2013) 65:1835–43. doi: 10.1002/acr.22069
10. Banerjee S, George M, Young K, Venkatachalam S, Gordon J, Burroughs C, et al. Effects of the COVID-19 pandemic on patients living with vasculitis. ACR Open Rheumatol. (2021) 3:17–24. doi: 10.1002/acr2.11204
11. Priori R, Pellegrino G, Colafrancesco S, Alessandri C, Ceccarelli F, Di Franco M, et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis. (2021) 80:953–4. doi: 10.1136/annrheumdis-2021-220059
12. Gaur P, Agrawat H, Shukla A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int. (2021) 41:1601–5. doi: 10.1007/s00296-021-04938-9
13. Ledbetter SS, Xie F, Cutter G, Saag KG, Jackson L, Danila MI, et al. COVID-19 vaccine uptake and vaccine hesitancy in rheumatic disease patients receiving immunomodulatory therapies in community practice settings. Arthr Rheumatol. (2022) 74:1091. doi: 10.1002/art.42067
14. Holladay EE, Mudano AS, Xie F, Stewart P, Jackson LE, Danila MI, et al. COVID-19 vaccine uptake, hesitancy, and flare in a large rheumatology practice network. Arthr Care Res. (2023) 25:241. doi: 10.1002/acr.25241
15. Stassen PM, Sanders JSF, Kallenberg CGM, Stegeman CA. Influenza vaccination does not result in an increase in relapses in patients with ANCA-associated vasculitis. Nephrol Dialysis Transpl. (2008) 23:654–8. doi: 10.1093/ndt/gfm640
16. Jeffs LS, Nitschke J, Tervaert JWC, Peh CA, Hurtado PR. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin Rheumatol. (2016) 35:943–51. doi: 10.1007/s10067-015-3073-0
17. Tang X, Liu F, Li Q, Fu H, Wang J, Mao J, et al. Novo vasculitis after COVID-19 vaccination. Curr Rheumatol Rev. (2023) 19:151–8. doi: 10.2174/1573397118666220817092235
18. Public Health Agency of Canada. Recommendations on the use of COVID-19 vaccines (2021). Available online at: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html (accessed September 4, 2021).
19. MacDonald NE, Comeau J, Dubé È, Graham J, Greenwood M, Harmon S, et al. Royal society of Canada COVID-19 report: enhancing COVID-19 vaccine acceptance in Canada. Facets. (2021) 6:1184–246. doi: 10.1139/facets-2021-0037
20. Butt IN, van Eeden C, Kovacs Burns K, Saxinger L, Clifford A, Cohen Tervaert JW, et al. COVID-19 vaccination perceptions in rheumatology patients: a cross-sectional online survey. J Rheumatol. (2022) 50:690–6. doi: 10.3899/jrheum.220765
21. Yurttas B, Poyraz BC, Sut N, Ozdede A, Oztas M, Ugurlu S, et al. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol Int. (2021) 41:1105–14. doi: 10.1007/s00296-021-04841-3
22. Kumar D, Chandra R, Mathur M, Samdariya S, Kapoor N. Vaccine hesitancy: understanding better to address better. Isr J Health Policy Res. (2016) 5:1–8. doi: 10.1186/s13584-016-0062-y
23. Gatwood J, Shuvo S, Hohmeier KC, Hagemann T, Chiu CY, Tong R, et al. Pneumococcal vaccination in older adults: an initial analysis of social determinants of health and vaccine uptake. Vaccine. (2020) 38:5607–17. doi: 10.1016/j.vaccine.2020.06.077
24. Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis. (2014) 209:S114–9. doi: 10.1093/infdis/jiu066
25. Lazarus J V, Wyka K, Rauh L, Rabin K, Ratzan S, Gostin LO, et al. Hesitant or not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: a country-level analysis. J Health Commun. (2020) 25:799–807. doi: 10.1080/10810730.2020.1868630
26. Lazarus J V, Ratzan S, Palayew A, Billari FC, Binagwaho A, Kimball S, et al. COVID-SCORE: a global survey to assess public perceptions of government responses to COVID-19 (COVID-SCORE-10). PLoS ONE. (2020) 15:e0240011. doi: 10.1371/journal.pone.0240011
27. Nganga SW, Otieno NA, Adero M, Ouma D, Chaves SS, Verani JR, et al. Patient and provider perspectives on how trust influences maternal vaccine acceptance among pregnant women in Kenya. BMC Health Serv Res. (2019) 19:1–13. doi: 10.1186/s12913-019-4537-8
28. Moss JL, Reiter PL, Rimer BK, Brewer NT. Collaborative patient-provider communication and uptake of adolescent vaccines. Soc Sci Med. (2016) 159:100–7. doi: 10.1016/j.socscimed.2016.04.030
29. Mergler MJ, Omer SB, Pan WKY, Navar-Boggan AM, Orenstein W, Marcuse EK, et al. Association of vaccine-related attitudes and beliefs between parents and health care providers. Vaccine. (2013) 31:4591–5. doi: 10.1016/j.vaccine.2013.07.039
30. Furer V, Rondaan C, Agmon-Levin N, Van Assen S, Bijl M, Kapetanovic MC, et al. Point of view on the vaccination against COVID-19 in patients with autoimmune inflammatory rheumatic diseases. RMD Open. (2021) 7:e001594. doi: 10.1136/rmdopen-2021-001594
31. Boekel L, Hooijberg F, van Kempen ZLE, Vogelzang EH, Tas SW, Killestein J, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. (2021) 3:e241–3. doi: 10.1016/S2665-9913(21)00037-0
32. Lazarus J V, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. doi: 10.1038/s41591-020-1124-9
33. Lin C, Tu P, Beitsch LM. Confidence and receptivity for covid-19 vaccines: a rapid systematic review. Vaccines. (2021) 9:1–16. doi: 10.3390/vaccines9010016
34. Kong X, Dai X, Ma L, Wang J, Sun Y, Jiang L. COVID-19 vaccine uptake, hesitancy and clinical effects on patients with Takayasu's arteritis: a web-based questionnaire survey from a large cohort. Front Immunol. (2023) 14:1030810. doi: 10.3389/fimmu.2023.1030810
35. Fragoulis GE, Bournia VK, Mavrea E, Evangelatos G, Fragiadaki K, Karamanakos A, et al. COVID-19 vaccine safety and nocebo-prone associated hesitancy in patients with systemic rheumatic diseases: a cross-sectional study. Rheumatol Int. (2022) 42:31–9. doi: 10.1007/s00296-021-05039-3
36. Mohanasundaram K, Santhanam S, Natarajan R, Murugesan H, Nambi T, Chilikuri B, et al. Covid-19 vaccination in autoimmune rheumatic diseases: a multi-center survey from southern India. Int J Rheum Dis. (2022) 25:1046–52. doi: 10.1111/1756-185X.14378
37. Dror AA, Eisenbach N, Taiber S, Morozov NG, Mizrachi M, Zigron A, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. (2020) 35:775–9. doi: 10.1007/s10654-020-00671-y
38. Kricorian K, Civen R, Equils O. COVID-19 vaccine hesitancy: misinformation and perceptions of vaccine safety. Hum Vaccin Immunother. (2021) 18:1950504. doi: 10.1080/21645515.2021.1950504
39. Kaplan RM, Milstein A. Influence of a COVID-19 vaccine's effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. (2021) 118:e2021726118. doi: 10.1073/pnas.2021726118
40. Fillon A, Sautenet B, Barbet C, Moret L, Thillard EM, Jonville-Béra AP, et al. De novo and relapsing necrotizing vasculitis after COVID-19 vaccination. Clin Kidney J. (2022) 15:560–3. doi: 10.1093/ckj/sfab285
41. Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. (2021) 3:e243–5. doi: 10.1016/S2665-9913(21)00039-4
42. Putman M, Kennedy K, Sirotich E, Liew JW, Sattui SE, Moni TT, et al. COVID-19 vaccine perceptions and uptake: results from the COVID-19 global rheumatology alliance vaccine survey. Lancet Rheumatol. (2022) 4:e237–40. doi: 10.1016/S2665-9913(22)00001-7
43. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 5. Arthr Rheumatol. (2023) 73:e60–75. doi: 10.1002/art.41928
44. Xie Y, Liu Y, Liu Y. The flare of rheumatic disease after SARS-CoV-2 vaccination: a review. Front Immunol. (2022) 13:919979. doi: 10.3389/fimmu.2022.919979
45. Francis AI, Ghany S, Gilkes T, Umakanthan S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad Med J. (2021) 98:389–94. doi: 10.1136/postgradmedj-2021-140654
46. Larson HJ, Clarke RM, Jarrett C, Eckersberger E, Levine Z, Schulz WS, et al. Measuring trust in vaccination: a systematic review. Hum Vacc Immunother. (2018) 14:1599–609. doi: 10.1080/21645515.2018.1459252
47. Wang X, Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. (2020) 158:S65–71. doi: 10.1016/j.chest.2020.03.012
Keywords: vaccine hesitancy, COVID-19 vaccines, vasculitis, patient perceptions, SARS CoV-2
Citation: Butt IN, van Eeden C, Kovacs Burns K, Saxinger L, Clifford A, Redmond D, Cohen Tervaert JW and Yacyshyn E (2023) Understanding COVID-19 vaccine hesitancy in vasculitis patients. Front. Public Health 11:1301492. doi: 10.3389/fpubh.2023.1301492
Received: 25 September 2023; Accepted: 13 November 2023;
Published: 04 December 2023.
Edited by:
Sebastiano Recalcati, Alessandro Manzoni Hospital, ItalyReviewed by:
S. Mushtaq, GMC & Associated Hospitals, University of Jammu, IndiaCopyright © 2023 Butt, van Eeden, Kovacs Burns, Saxinger, Clifford, Redmond, Cohen Tervaert and Yacyshyn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elaine Yacyshyn, ZXlhY3lzaHluQHVhbGJlcnRhLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.