- 1Center for Obesity and Metabolic Health, Department of General Surgery, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University, Chengdu, China
- 2Department of General Surgery, Center for Obesity and Metabolic Health, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University, Chengdu, China
- 3Mianyang Central Hospital, Mianyang, China
- 4First Affiliated Hospital of Air Force Military Medical University, Xi’an, China
- 5Dazhou Central Hospital, Dazhou, China
Objectives: This multicenter, cross-sectional study aimed to investigate whether sex differences persist among patients who have undergone bariatric surgery and tested positive for the coronavirus disease (COVID-19).
Methods: We conducted a multicenter cross-sectional study via an online electronic questionnaire to collect data. Categorical data were presented as absolute and relative frequencies. Data for continuous variables were expressed as mean and standard deviation (SD) or median [interquartile range (IQR)]. We employed ordered logistic regression to assess whether females had higher odds of an increased self-reported duration of the most severe symptom compared to males. Using a modified Poisson regression model with robust standard errors to assess the differences in clinical characteristics among COVID-19 cases.
Results: Statistical analysis revealed significant differences in the prevalence rates of various comorbidities. Among participants who reported their temperature during COVID-19 infection, more than half engaged in vitamin supplementation and regular exercise, while 4.2% remained asymptomatic. The probability of females experiencing a longer duration of severe symptoms increased compared to males [adjusted Odds Ratio (aOR) = 1.92, 95% confidence interval (CI) 1.73–2.12]. In the multivariate mixed-effects Poisson regression analysis, compared to males, females exhibited a lower prevalence rate of asymptomatic infection [adjusted prevalence ratio (aPR 0.40, 95% CI 0.28–0.58), lower prevalence of infection without therapeutic medication use (aPR 0.76, 95% CI 0.70–0.82), and lower prevalence of multiple infections (aPR 0.39, 95% CI 0.20–0.74)].
Conclusion: This cross-sectional study indicates the persistence of sex differences among patients with COVID-19 who have undergone bariatric surgery. Further research is needed to explore the underlying factors contributing to this disparity.
1 Introduction
As of November 2023, the novel coronavirus disease (COVID-19) has resulted in over 700 million confirmed cases and approximately 7 million deaths worldwide (1). Studies have shown that men are more likely to experience severe symptoms and a higher mortality rate from COVID-19 than women (2–4). To address this issue, the World Health Organization (WHO) has recommended collecting data disaggregated by sex in the COVID-19 Strategic Preparedness and Response Plan (SPRP) (5).
The relationship between a history of bariatric surgery, sex differences, and severe outcomes in COVID-19-positive patients is still under investigation. Currently, many clinical and epidemiological studies have demonstrated disproportionate sex-based discrepancy in the severity of COVID-19 symptoms, with men twice as likely to develop severe symptoms or die from the virus compared to women (6, 7). The cumulative effects of biological factors, lifestyle factors, social structural factors, etc., render males a high-risk population (4, 8, 9). However, one study reported no statistically significant differences in symptom presence, hospitalization requirement, intensive care unit (ICU) admission, and invasive ventilation among patients infected with COVID-19 before and after sleeve gastrectomy (10). Another study conducted in western Sweden reported that the risk of ICU admission was associated with a higher body mass index (BMI) only among women (11). Interestingly, studies have also shown that patients with a history of bariatric surgery have lower risks of emergency admission, mechanical ventilation, prolonged ICU stay, and mortality in the context of COVID-19 infection (12–14).
However, sex-specific studies in Chinese post-bariatric patients are still limited. Our study aimed to explore whether sex differences persist among patients with COVID-19 after bariatric surgery by conducting a questionnaire survey based on the basic characteristics, comorbidities, infection, and living habits.
2 Methods
2.1 Study population and design
This is a multicenter, cross-sectional study involving eight medical centers, conducted from December 1, 2022, to January 30, 2023. Each patient undergoing bariatric surgery treatment was identified by a nursing staff member at each site using the hospital’s patient management system. They were invited via telephone to participate in an online electronic questionnaire. The collected data were stored in an online database that was protected and accessible only to authorized personnel. The data supporting this study’s findings are available from the corresponding author upon reasonable request.
The survey included three main parts: (1) basic information about patients, including age, sex, preoperative BMI and postoperative BMI, and date of surgery; (2) COVID-19-related information, including COVID-19 vaccination status, symptoms after infection, drug use, time to negative conversion, etc.; (3) changes in body weight, physical exercise habits, and nutrient supplementation during infection. The questions about the questionnaire are detailed in Supplementary Table S1. Inclusion Criteria: (1) The individuals included in the study are those who have undergone weight loss surgery and subsequently confirmed to be infected with COVID-19 through laboratory testing, either by real-time (Polymerase Chain Reaction) PCR fluorescence method or Antigen Detection; (2) Due to a significant increase in the number of infections in a short period after the Chinese government lifted the lockdown measures for COVID-19 after December 1, 2022, we only analyzed individuals infected after December 1, 2022, to minimize recall bias. Exclusion Criteria: (1) Participants who did not provide informed consent to participate in the study; (2) Individuals who incompletely filled out the questionnaire.
The disease characteristics of COVID-19 in these patients were evaluated. Out of the 1,710 questionnaires distributed, 1,406 survey reports were collected, resulting in a response rate of 82.22%. After excluding questionnaires with missing data on COVID-19 and outliers (Figure 1), the final analysis included 1,134 participants, representing 66.32% of the total initial sample.
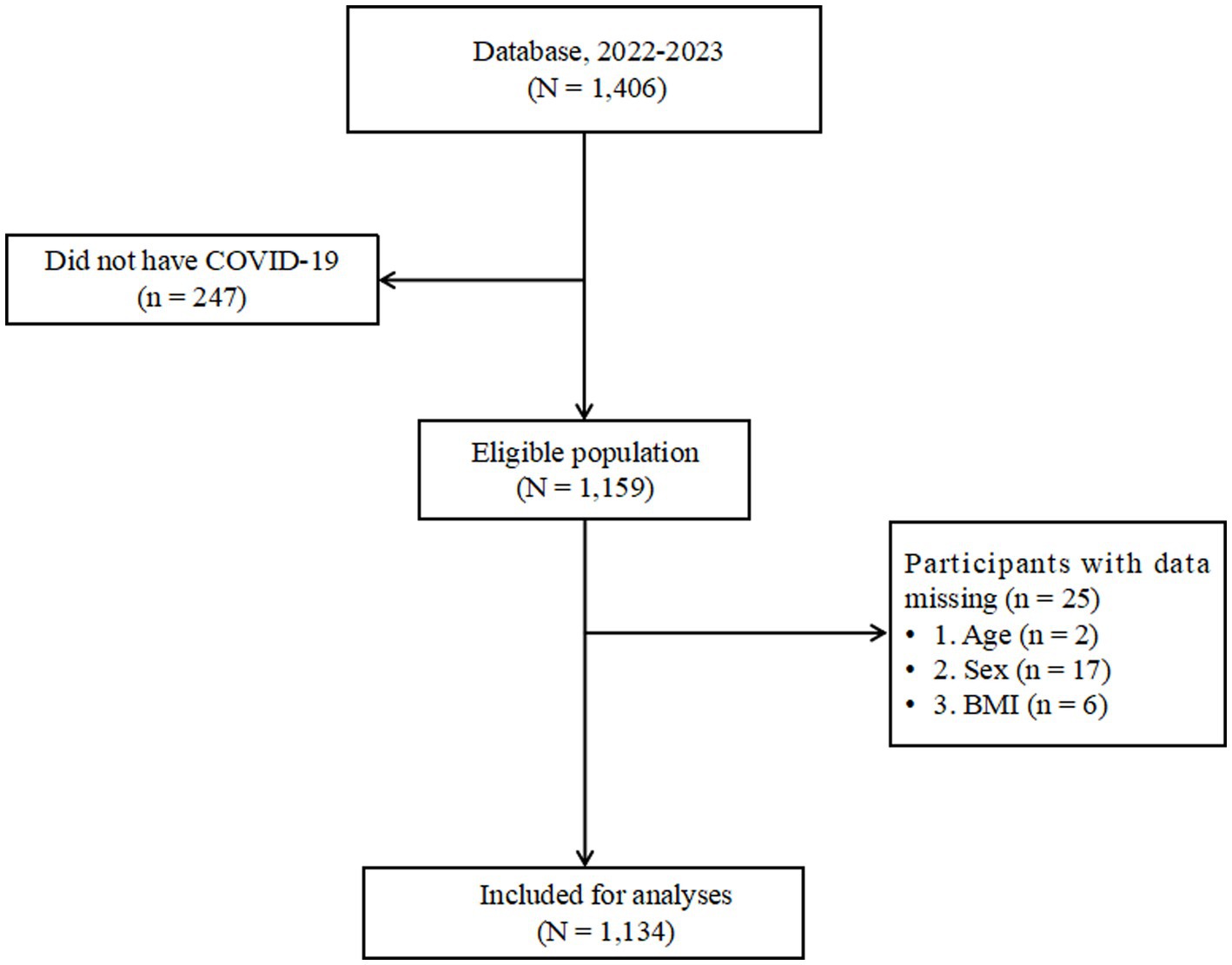
Figure 1. Flowchart of eligibility of study participants in the Database. BMI, body mass index; Database, Database of Weight Loss and Metabolism Center.
2.2 Definition of outcomes, exposures, and covariates
This study focuses on several outcomes of interest. The duration of self-reported severe symptoms, considered the primary endpoint of our investigation, was examined. Specifically, following the pre-designed questionnaire, participants were asked the following specific question during the survey: “How long did the most severe symptoms you experienced during the infection persist? Please choose: A. Less than 24 h, B. One day, C. Two days, D. Three days, E. More than 3 days.” Secondary outcomes include investigating vaccination status, participation in physical activities during the infection, vitamin C supplementation, composite vitamin supplementation, protein powder supplementation, asymptomatic infection status, use of COVID-19-related medications during the infection, and the total number of reported COVID-19 infections by participants at the time of data submission. The exposure variable is sex, with males serving as the reference group in all regression analyses.
In our study, the primary focus revolved around the severity of participants’ infection with COVID-19. Therefore, relevant variables were identified based on professional knowledge (15, 16), and their associations with the self-reported duration of severe symptoms of COVID-19 were examined as the main outcome. Employing a historical confounding approach, a multivariate regression model incorporated age (years) (17), BMI group (18), education level (19), marital status (20), smoking status (21), alcohol consumption (22), bariatric surgery type (SG, RYGB, and others) (23), hypertension (24), and diabetes (25). Due to the skewed distribution of BMI data, we grouped the values into intervals with uniform spacing of 5, namely (<20, 20–25, 25–30, 30–35, 35–40, 45–50, 50–55, and 55–60 kg/m2). It should be emphasized that we combined the <20 category with the BMI 20–25 category, creating a <25 BMI category, which was used as our reference category. Due to limited sample size in certain categories, making it challenging to achieve sufficient fit in regression modeling, the education level was grouped into four categories: high school and below (including high school, junior high, and elementary), college, undergraduate, and graduate degree. Similarly, surgical procedures were categorized into four groups based on current mainstream techniques: SG, RYGB, and others (SG + JJB, OAGB, BIDs, and unknown) (26).
2.3 Statistical analysis
Categorical data were presented as absolute and relative frequencies. Continuous variables were presented as mean and standard deviation (SD). Due to the non-normal distribution of BMI, percentage of excess weight loss (EWL%), percentage of total weight loss (TWL%), and the time to negative variables (the time participants self-reported from the onset of self-discovered COVID-19 infection to a negative result in COVID-19 real-time PCR fluorescence method or Antigen Detection), these variables are expressed as median [interquartile range (IQR)]. For BMI, EWL%, TWL% and the time to negative variables, the Wilcoxon rank-sum test (ranksum test) was employed for independent sample comparison. The TWL% and EWL% can be utilized to the indirectly approximate effectiveness of bariatric surgery, offering a quantitative measure of the extent of individual weight reduction. Continuous variables were analyzed using Student’s t-test with sex as the grouping variable. For categorical variables such as education level, marital status, alcohol consumption, smoking status, and comorbidities (excluding coronary heart disease, hypothyroidism, and osteoarthritis), Pearson’s chi-square test was employed. Additionally, for categorical variables such as coronary heart disease, hypothyroidism, and osteoarthritis, Fisher’s exact test was utilized to compare baseline characteristics and infection status, given that theoretical frequencies were less than 5 in some categories.
In the analysis of the primary outcome, we employed ordered logistic regression to assess whether females had higher odds of an increased self-reported duration of the most severe symptom compared to males. The Parallel Lines Assumption was assessed using the Brant test, where p > 0.05 was considered indicative of the suitability for adopting the ordered logistic regression (27). Under the assumption of parallel lines, and recognizing the unique characteristics of patients from each center, which render them not entirely independent, we utilized a mixed-effects model to adjust for the impact of center-specific effects. Therefore, for our primary outcome, we applied a mixed-effects ordered regression model. The effect size in the univariate mixed-effects ordered regression model was expressed as Odds Ratio (OR), and 95% confidence intervals (CIs). In the multivariate mixed-effects ordered regression model, the effect size was represented as Adjusted OR (aOR), and 95% CIs. In Model 1, the association between gender and self-reported duration of severe symptoms was assessed without controlling for any variables. In Model 2, age (years), BMI group were added based on Model 1. Model 3 further included education level, marital status, smoking status, and alcohol consumption, bariatric surgery type, hypertension, and diabetes.
The secondary outcomes were exploratory. Following univariate analysis, variables showing differences in clinical characteristics between males and females after infection were explored. These variables included self-reported asymptomatic infection, self-reported non-usage of therapeutic drugs at the time of infection, and the frequency of COVID-19 infections. In accordance with our research design, utilizing the Prevalence ratio (PR) for analysis is more intuitive, as it allows for a direct comparison of raw morbidity rates (28). Since patients from each center possess unique characteristics, rendering them not entirely independent, we employed a mixed-effects model to adjust for the impact of center-specific effects. Ultimately, our regression analysis utilized a modified Poisson regression model with robust standard errors to evaluate the effect size of infection status between males and females. In the multivariate mixed-effects Poisson regression model, the effect size was denoted as Adjusted PR (aPR) with associated 95% CIs. Adjusted for age (years), BMI group, education level, marital status, smoking status, alcohol consumption, bariatric surgery type, hypertension, and diabetes.
All statistical tests were two-tailed and p < 0.05 was considered statistically significant. All statistical analyses were performed using STATA 17.0 (Stata Corp LLC, College Station, Texas, US).
3 Results
3.1 Baseline characteristics of the participants
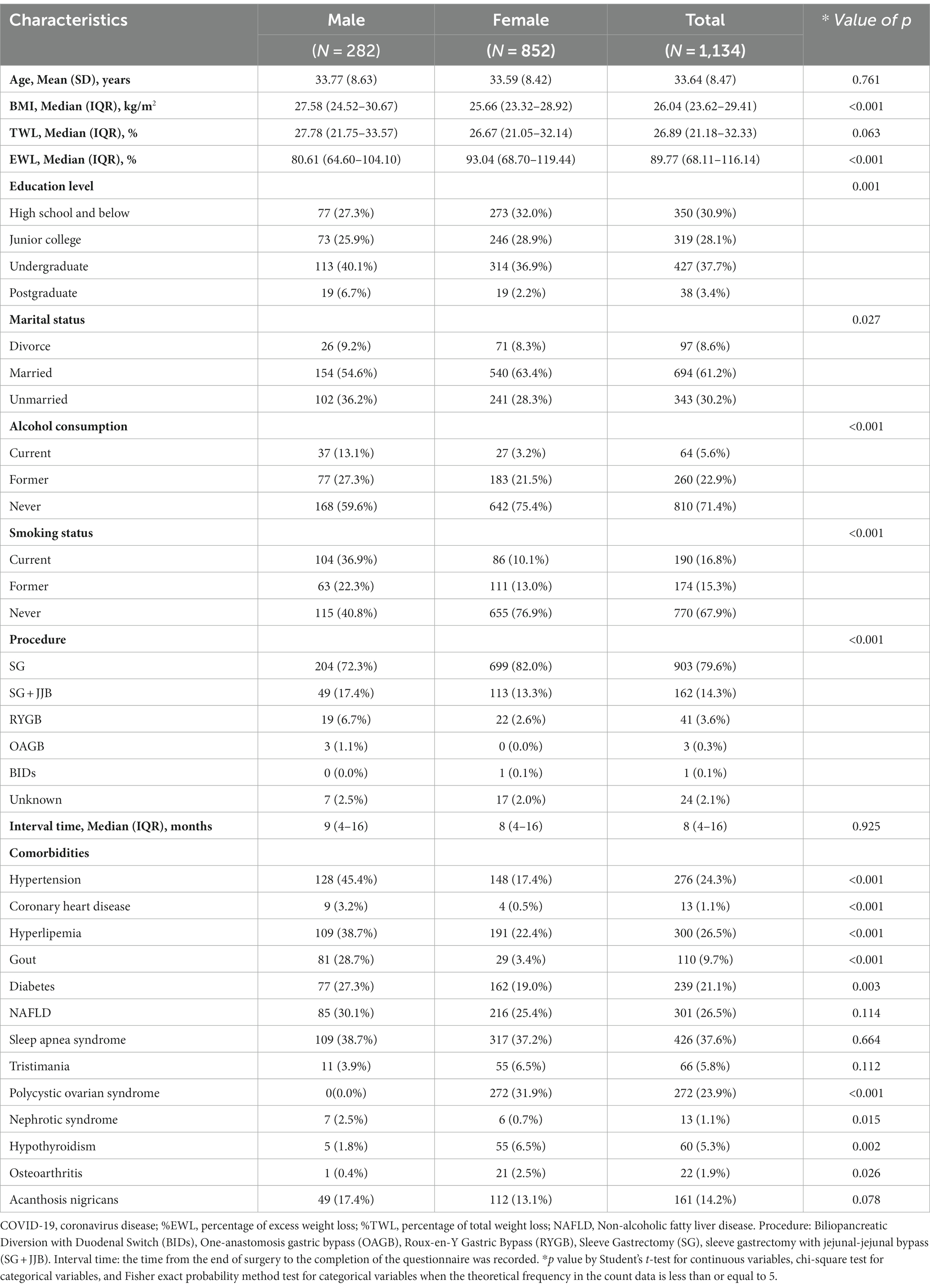
Of 1,134 participants, the mean age was 33.64 (SD, 8.47) years including 852 females (75.1%) and 282 males (24.9%) (Table 1). The overall BMI of the participants ranged from 17.99 to 58.95 kg/m2, with a median of 26.04 (IQR: 23.62–29.41). Survey found that among the participants, 61.2% were married, 37.7% had a bachelor’s degree, 67.9% had never consumed alcohol, and 71.4% had never smoked. The median interval between the completion of surgery and the most recent occurrence of COVID-19 is 9 months (IQR: 4–16). Among the participants, the highest incidence was observed for hypertension, reaching 45.4%. This was followed by hyperlipemia and sleep apnea syndrome, with incidences of 38.7%. Most commonly performed bariatric surgical technique was sleeve gastrectomy (SG) (79.6%). The majority of participants included in the study were from The Third People’s Hospital of Chengdu (n = 668), with only 14 individuals from the First Affiliated Hospital of Air Force Military Medical University and Mianyang Central Hospital, as illustrated in Supplementary Figure S1.
Single-factor analysis revealed that, compared to male participants, female participants had lower BMI values, higher EWL% indices, were more likely to be married, and had no history of alcohol consumption and smoking. In the analysis of comorbidities, female participants showed a relatively higher incidence of Hypothyroidism and Osteoarthritis compared to male participants. However, the incidence rates of Hypertension, Coronary heart disease, Hyperlipemia, Gout, Diabetes, and Nephrotic syndrome were lower in female participants than in male participants (all p < 0.001).
3.2 Effects of COVID-19 on the bariatric surgery population
3.2.1 A comparison of outcomes in male and female COVID-19 cases
In 1,134 patients with COVID-19, only 806 (71.1%) reported their body temperature, including 199 males and 607 females (Table 2). 98.6% of respondents reported having been infected with the virus, 1.2% reported being infected twice, and 0.2% reported being infected three times. Over half of the participants reported taking vitamin C, multivitamins, or engaging in physical exercise during infection (58.2, 54.1, and 57.7%, respectively). However, only 22.5% of respondents consistently took protein powder during the pandemic. In our surveyed population, 3.4% of respondents stated that they had never received any brand of COVID-19 vaccine. Interestingly, 4.2% of the infected individuals were asymptomatic, and 16.9% did not take any COVID-19 medications. The reported symptom intensity was based on their self-assessment. The overall median days to negative were 20 (IQR: 16–22) days, and there was no significant difference observed in the median days to negative between the two groups. Figure 2 displays a bar chart of relative frequencies grouped by sex. It can be observed that females have the highest prevalence rates of severe symptom duration exceeding 3 days, at 41.5%. In contrast, males report the highest prevalence rates for a duration of 3 days, reaching 24.1%. Female participants exhibit the highest prevalence rates in the BMI <25 kg/m2 range, at 44.40%, while males have the highest prevalence rates in the BMI (25–30 kg/m2) range, at 41.8%.
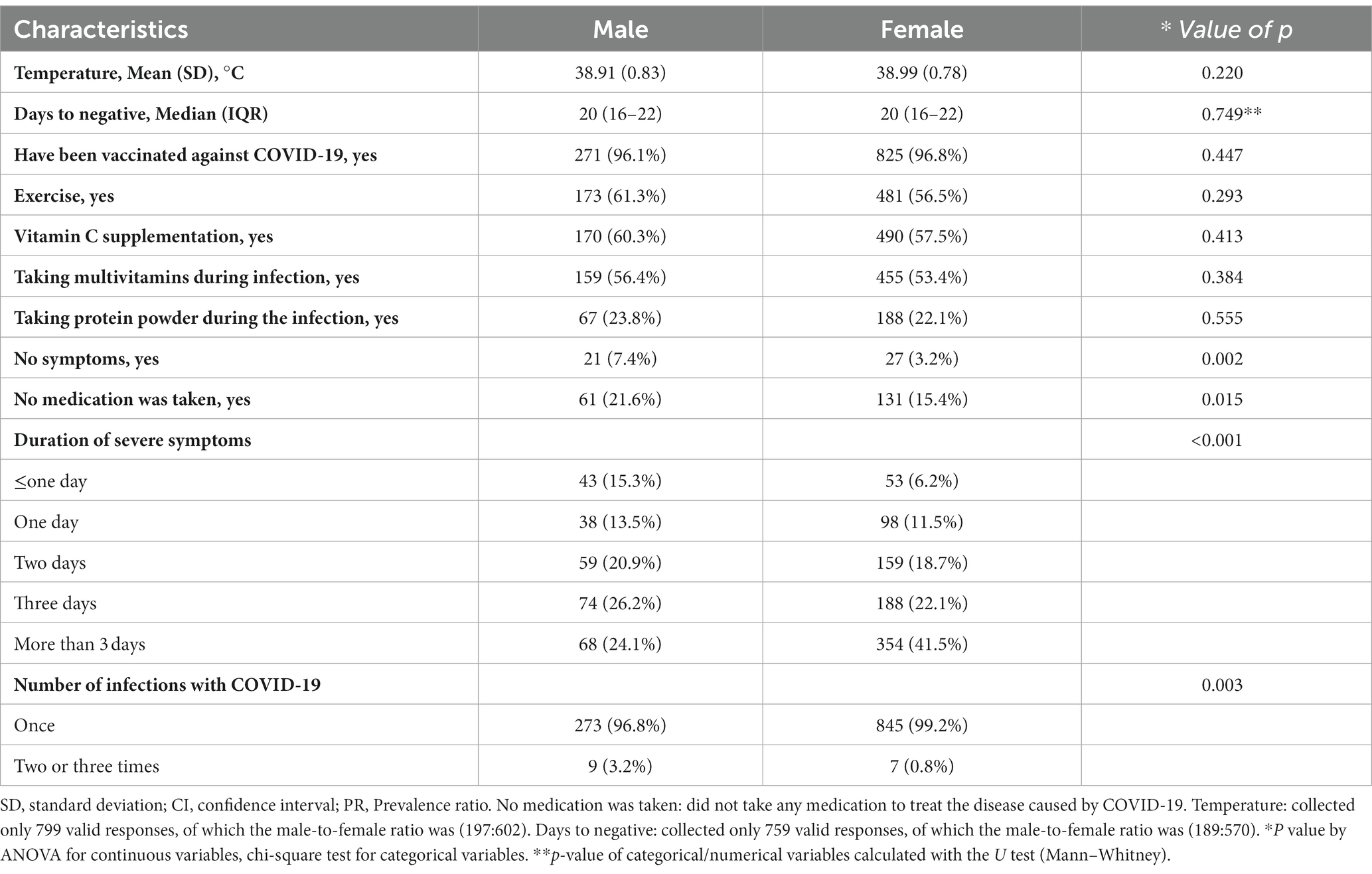
Table 2. A comparison of outcomes in male and female COVID-19 cases on the bariatric surgery population in China.

Figure 2. Relative frequency bar charts. (A) Sex-specific relative frequency bar chart for self-reported symptom duration. (B) Sex-specific relative frequency bar chart for different BMI groups.
3.2.2 Sex differences in the duration of severe symptoms after self-reported infection: a mixed-effects ordered logistic regression analysis
Table 3 reports the association between gender and the duration of self-reported severe symptoms. Without controlling for any covariates, the probability of females experiencing a longer duration of severe symptoms increased compared to males (OR = 1.99, 95% CI 1.84–2.15, Model 1). Even after controlling for age and BMI, this relationship persisted (aOR = 2.02, 95% CI 1.86–2.18, Model 2). After controlling for age (years), BMI group, education level, marital status, smoking status, alcohol consumption, bariatric surgery type, hypertension, and diabetes, this finding remained robust (aOR = 1.92, 95% CI 1.73–2.12, Model 3).
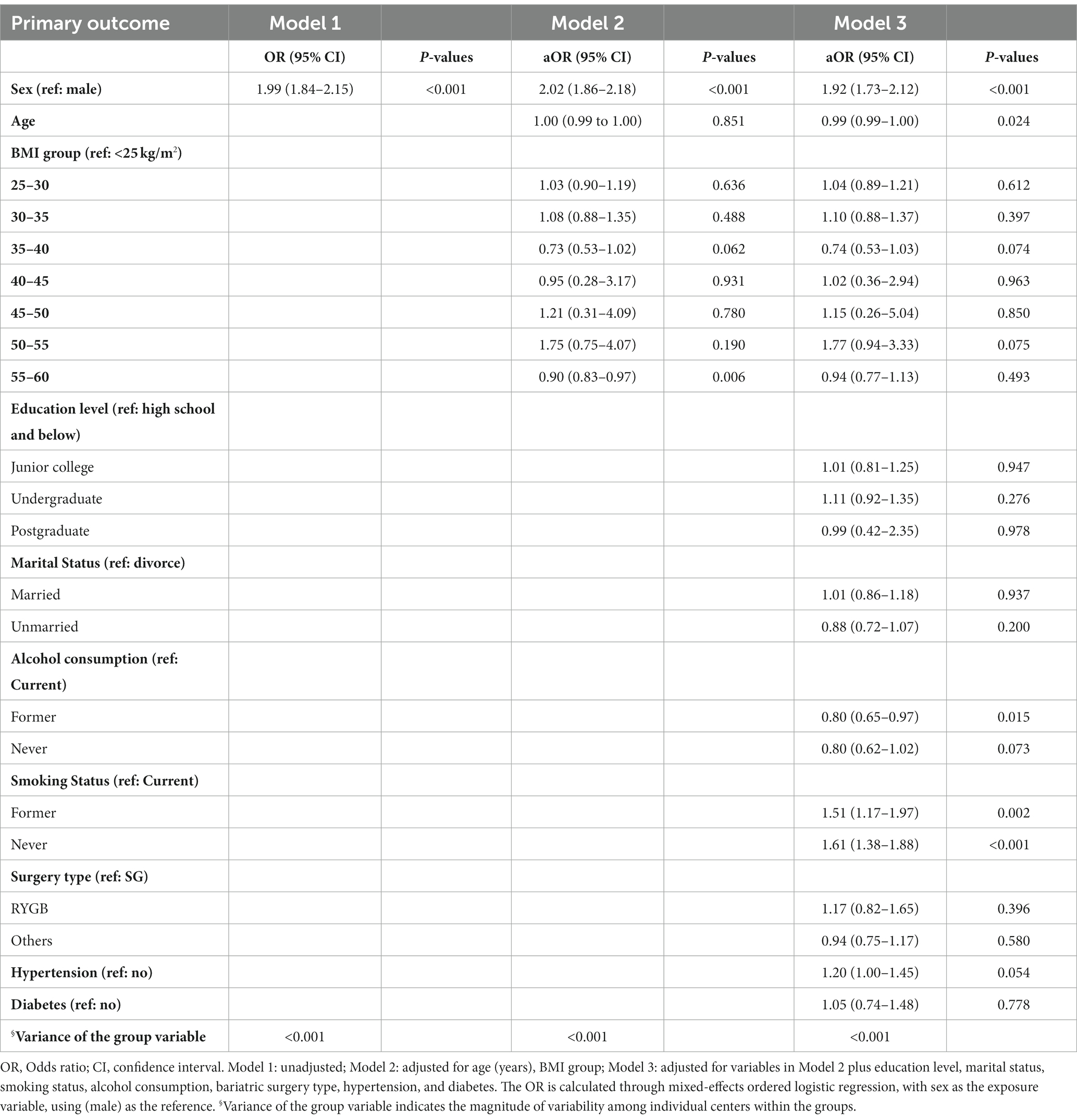
Table 3. Mixed-effects ordered logistic regression analysis of duration of severe symptoms among COVID-19 cases in different sex in China.
3.2.3 Secondary outcomes of mixed-effects Poisson regression analysis on clinical characteristics of COVID-19 cases
Table 4 presents the results of multivariate mixed-effects Poisson regression analysis for secondary outcomes. The study revealed that, compared to males, females had a lower aPR for asymptomatic infection (aPR 0.40, 95% CI 0.28–0.58), lower prevalence of infection without therapeutic medication use (aPR 0.76, 95% CI 0.70–0.82), and lower prevalence of multiple infections (aPR 0.39, 95% CI 0.20–0.74). These findings remained robust after controlling for covariates (Supplementary Table S2).
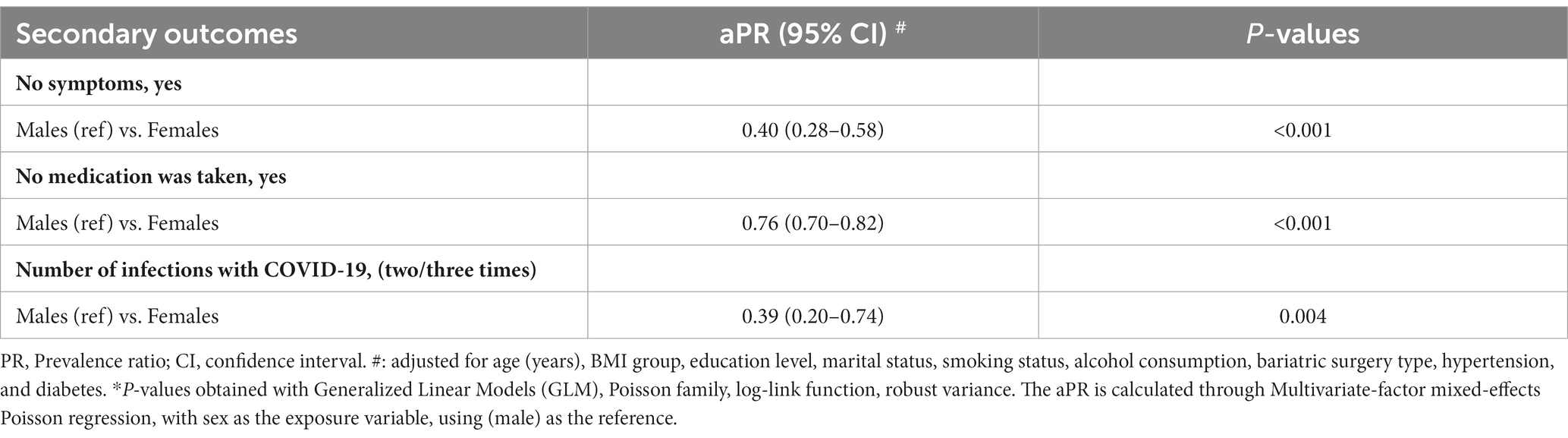
Table 4. Mixed-effects Poisson regression analysis of clinical characteristics among COVID-19 cases in different sex in China.
3.2.4 Symptoms of COVID-19 infection
According to Figure 3, we can find that soreness, cough, and fatigue are the most common symptoms of infection reported. Of the reports received on the characteristics of symptoms following COVID-19 infection, 4.2% reported not having any symptoms while infected (Supplementary Table S3). Diarrhea, fatigue, chest tightness or pain, dyspnea, palpitation, and tinnitus had no significant difference between men and women in univariate analysis, while cough, sore throat, vomiting, soreness, loss of taste and smell, nasal congestion and runny nose, dizziness, headache, heart fatigue, increased appetite, and decreased appetite had significant difference between men and women in univariate analysis.
4 Discussion
In this survey, we investigated Chinese bariatric surgery patients with COVID-19 and identified persistent sex differences, emphasizing a greater risk of prolonged severe symptoms in females. Compared to males, females exhibited a lower prevalence of asymptomatic infections, a lower prevalence of infection without therapeutic medication use, and a lower prevalence of multiple infections.
From the perspective of strict weight loss, our data showed that %EWL and %TWL reached the expected targets, suggesting that the surgical procedure was successful (29, 30). In this context, it is reasonable to explore the impact of bariatric surgery on people with COVID-19 (31). The relationship between social factors (education level, marital status) and COVID-19 outcomes has been previously studied. Low education level (32, 33) and being single (34) is associated with severe COVID-19 severity. This feature is reflected in our report. Of note, whether lifestyle factors influence the severity of COVID-19 is controversial. One study in China reported prevalence rates of smoking, alcohol consumption, and Betel nut chewing of 26.4, 7.1, and 19.0%, respectively, with no significant association between these lifestyle factors and the severity of COVID-19 (35, 36).
However, some studies support the impact of lifestyle-related factors on individuals with COVID-19. A community-based cohort study of 387,109 adults in the United Kingdom showed that simple lifestyle changes can reduce the risk of serious infection (37). Another study suggested that genetic liability to smoking may contribute to an increased risk for a severe course of COVID-19 (38). Our findings found sex differences in the prevalence of smoking, and alcohol consumption reported by patients. However, we believe this difference in lifestyle may strengthen the effect of lifestyle factors after discussion by sex (4, 39, 40). There was also a significant sex shift in comorbidities in the population we investigated, with a greater prevalence of comorbidities in males than in females. Several studies have proved that comorbidities affect the prognosis of COVID-19 (41–43). From this point of view, in the population after weight loss, more attention should be paid to men.
During COVID-19, there was no difference between men and women in adherence to exercise or regular nutritional supplements. Generally, the proportion of vitamin C and multivitamin supplementation was higher than that of protein powder. Several studies in populations with previous bariatric surgery have reported adherence rates ranging from 60 to 92% (44–46). Our reported adherence was below this level, indicating that COVID-19 hurt adherence to nutritional supplements in this population. Notably, 7.4% of men reported no symptoms during infection, compared to a smaller proportion of women (3.2%). Our results are not consistent with published studies. A recent meta-analysis showed that children and women are more likely to present asymptomatic cases of COVID-19 and may be unknown carriers of SARS-CoV-2 (47). Interestingly, 16.9% reported not taking any medication during infection, with a male bias indicating that some symptomatic infected people did not take any medicine to treat Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. As there are no excellent comparative cases, the adoption of this result requires caution. However, we emphasize that we should not reduce our attention to asymptomatic infection in men after bariatric surgery (48).
Moreover, compared to males, females have a higher risk of experiencing prolonged severe symptoms. Although this cannot be directly equated to assessing the severity of the infection, it indirectly confirms the vulnerability of females. Our study findings align with the research conducted by Francesca Fortunato and colleagues (49). Studies have shown that women’s immune system differs from men’s, which will produce a stronger immune response, leading to virus clearance (50). But from the perspective of long-term symptoms, women are more likely to occur than men (51, 52). In our report, women had a higher incidence of typical symptoms (cough, sore throat, nasal congestion, runny nose, etc.) than men, but there was no difference in severe symptoms (dyspnea) between men and women. In contrast, results from the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) prospective multinational observational study showed that women were less likely to present with typical symptoms (53). This difference, we believe, may be attributed in part to sample size and ethnic differences.
Our results highlight the need for healthcare providers to be aware of the sex-specific aspects of managing populations receiving weight-loss therapy during the pandemic and obesity epidemics. At present, there are data available for reference and specific differences from the general population, so it is urgent to increase the attention of this population. Furthermore, the observed sex differences in COVID-19 vulnerability highlight the need to understand better the impact of sex on disease incidence and case fatality rate and to tailor treatment approaches to sex. Ongoing and planned preventive and therapeutic studies should include prospective analyses sensitive to sex.
In our study, we conducted an analysis controlling for center effects, encompassing both primary and secondary outcomes. The results indicated that the impact of center effects on study outcomes was minimal, with relatively limited variability observed across individual centers. Specifically, we found that the size of center effects was not statistically significant, suggesting a minor influence. This finding is, in part, attributable to the fact that the primary study population was drawn from several hospitals in Sichuan, China (Dazhou Central Hospital, Mianyang Central Hospital, and The Third People’s Hospital of Chengdu), totaling 869 individuals. In the same geographic region, similarities in technological advancements and the population undergoing bariatric surgery helped mitigate the potential impact of center effects. However, it is important to note that this conclusion is context-specific to our study. Future research should remain cognizant of the potential variations in center effects in different contexts and continue to address and explore this issue.
This study has some limitations. Firstly, the study was cross-sectional and causality could not be determined. Secondly, due to the inconsistent time intervals between participants’ surgeries and SARS-CoV-2 infection, the study acknowledges the presence of design biases. Additionally, the duration of self-reported severe symptoms by participants might not have been comprehensively considered at the design stage, limiting this open-ended topic to predefined choices. Thirdly, improvement in patients’ comorbidities was not collected, which may somewhat underestimate the benefit of bariatric surgery (54, 55). Fourthly, the investigation depended on self-reported data, thereby potentially susceptible to recall and social desirability bias. Lastly, the risk of finding false positives (Type I error) may increase due to multiple comparisons in our study, necessitating cautious interpretation of our results.
5 Conclusion
This cross-sectional study indicates the persistence of sex differences among patients with COVID-19 who have undergone bariatric surgery. Further research is needed to explore the underlying factors contributing to this disparity.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://figshare.com/s/0ea83dbf1c4cf610037d.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Third People’s Hospital of Chengdu City. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The studies involving humans were approved by the Ethics Committee of the Third People.
Author contributions
SlW: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. QbJ: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. HW: Investigation. YnY: Investigation. MR: Investigation. JY: Investigation. XyL: Investigation. DL: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Chengdu High-level Key Clinical Specialty Construction Project.
Acknowledgments
This study is a cross-sectional study with multi-center participation. We would like to thank the following centers: (1) Thanks to the staff and participants of the risk survey in the First Affiliated Hospital of Air Force Military Medical University. (2) Thanks to the staff and participants of the risk survey in the Affiliated Xiaolan Hospital, Southern Medical University. (3) Thanks to the staff and participants of the risk survey in the First People’s Hospital of Yunnan Province. (4) Thanks to the staff and participants of the risk survey in the First Affiliated Hospital of Jinan University. (5) Thanks to the staff and participants of the risk survey in the Yan’an Hospital affiliated with Kunming Medical University. (6) Thanks to the staff and participants of the risk survey in the Mianyang Central Hospital. (7) Thanks to the staff and participants of the risk survey in the Dazhou Central Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1293318/full#supplementary-material
References
1. WHO. World Health Organization coronavirus (COVID-19). World Health Organization; (2019). Available at: https://covid19.who.int/
2. Yang, X, Yu, Y, Xu, J, Shu, H, Xia, J, Liu, H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
3. Meng, Y, Wu, P, Lu, W, Liu, K, Ma, K, Huang, L, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. (2020) 16:e1008520. doi: 10.1371/journal.ppat.1008520
4. Gebhard, C, Regitz-Zagrosek, V, Neuhauser, HK, Morgan, R, and Klein, SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. (2020) 11:29. doi: 10.1186/s13293-020-00304-9
5. WHO. COVID-19 strategic preparedness and response plan (SPRP 2021). World Health Organization; (2021). Available at: https://www.who.int/publications/i/item/WHO-WHE-2021.02
6. Griffith, DM, Sharma, G, Holliday, CS, Enyia, OK, Valliere, M, Semlow, AR, et al. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Prev Chronic Dis. (2020) 17:E63. doi: 10.5888/pcd17.200247
7. Barber, SJ, and Kim, H. COVID-19 worries and behavior changes in older and younger men and women. J Gerontol B Psychol Sci Soc Sci. (2021) 76:e17–23. doi: 10.1093/geronb/gbaa068
8. Li, Y, Ashcroft, T, Chung, A, Dighero, I, Dozier, M, Horne, M, et al. Risk factors for poor outcomes in hospitalised COVID-19 patients: a systematic review and meta-analysis. J Glob Health. (2021) 11:10001. doi: 10.7189/jogh.11.10001
9. Bechmann, N, Barthel, A, Schedl, A, Herzig, S, Varga, Z, Gebhard, C, et al. Sexual dimorphism in COVID-19: potential clinical and public health implications. Lancet Diabetes Endocrinol. (2022) 10:221–30. doi: 10.1016/S2213-8587(21)00346-6
10. Santa-Cruz, F, Siqueira, LT, Coutinho, LR, Leao, LHA, Almeida, ACA, Kreimer, F, et al. Is COVID-19 severity impacted by bariatric surgery in the early postoperative period? Obes Surg. (2022) 32:1178–83. doi: 10.1007/s11695-022-05915-2
11. Lindgren, M, Toska, T, Alex, C, Lundberg, CE, Cronie, O, Rosengren, A, et al. BMI, sex and outcomes in hospitalised patients in western Sweden during the COVID-19 pandemic. Sci Rep. (2022) 12:4918. doi: 10.1038/s41598-022-09027-w
12. Purdy, AC, Hohmann, SF, and Nguyen, NT. Outcomes of obese patients hospitalized with COVID-19: the impact of prior bariatric surgery. Surg Obes Relat Dis. (2022) 18:35–40. doi: 10.1016/j.soard.2021.08.027
13. Iannelli, A, Bouam, S, Schneck, AS, Frey, S, Zarca, K, Gugenheim, J, et al. The impact of previous history of bariatric surgery on outcome of COVID-19. A Nationwide medico-administrative French study. Obes Surg. (2021) 31:1455–63. doi: 10.1007/s11695-020-05120-z
14. Hung, KC, Chen, HT, Hsing, CH, Jinn-Rung, K, Ho, CN, Lin, YT, et al. Impact of prior bariatric surgery on risk and severity of COVID-19 infection: a meta-analysis of observational studies. Obes Res Clin Pract. (2022) 16:439–46. doi: 10.1016/j.orcp.2022.10.005
15. Becerra-Munoz, VM, Nunez-Gil, IJ, Eid, CM, Garcia Aguado, M, Romero, R, Huang, J, et al. Clinical profile and predictors of in-hospital mortality among older patients hospitalised for COVID-19. Age Ageing. (2021) 50:326–34. doi: 10.1093/ageing/afaa258
16. Li, J, Huang, DQ, Zou, B, Yang, H, Hui, WZ, Rui, F, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. (2021) 93:1449–58. doi: 10.1002/jmv.26424
17. Lohr, P, Schiele, S, Arndt, TT, Grutzner, S, Claus, R, Rommele, C, et al. Impact of age and gender on lymphocyte subset counts in patients with COVID-19. Cytometry A. (2023) 103:127–35. doi: 10.1002/cyto.a.24470
18. Robertson, J, Adiels, M, Lissner, L, Mehlig, K, Af Geijerstam, A, Lindgren, M, et al. BMI in early adulthood is associated with severe COVID-19 later in life: a prospective cohort study of 1.5 million Swedish men. Obesity (Silver Spring). (2022) 30:779–87. doi: 10.1002/oby.23378
19. NeJhaddadgar, N, Pirani, N, Heydarian, N, Ebadi Fard Azar, AA, Yazdi, F, Toghroli, R, et al. Knowledge, attitude, and practice toward the COVID-19 infection among adults Iran: a cross-sectional study. J Public Health Res. (2022) 11:227990362211293. doi: 10.1177/22799036221129370
20. Jafari-Oori, M, Ebadi, A, Moradian, ST, Jafari, M, Dehi, M, and Ghasemi, FF. Psychiatric distress in family caregivers of patients with COVID-19. Arch Psychiatr Nurs. (2022) 37:69–75. doi: 10.1016/j.apnu.2021.07.005
21. Watase, M, Masaki, K, Chubachi, S, Namkoong, H, Tanaka, H, Lee, H, et al. Impact of accumulative smoking exposure and chronic obstructive pulmonary disease on COVID-19 outcomes: report based on findings from the Japan COVID-19 task force. Int J Infect Dis. (2023) 128:121–7. doi: 10.1016/j.ijid.2022.12.019
22. Lewis, SA, Cinco, IR, Doratt, BM, Blanton, MB, Hoagland, C, Newman, N, et al. Chronic alcohol consumption dysregulates innate immune response to SARS-CoV-2 in the lung. EBioMedicine. (2023) 97:104812. doi: 10.1016/j.ebiom.2023.104812
23. Rim, DS, Kim, BS, Sharma, K, Shin, JH, and Kim, DW. Prior bariatric surgery and risk of poor in-hospital outcomes in COVID-19: findings from a National Inpatient Sample. Surg Obes Relat Dis. (2023) 19:1435–43. doi: 10.1016/j.soard.2023.07.006
24. Jackson, SL, Woodruff, RC, Nagavedu, K, Fearrington, J, Rolka, DB, Twentyman, E, et al. PCCA. Association between hypertension and diabetes control and COVID-19 severity: National Patient-Centered Clinical Research Network, United States, march 2020 to February 2022. J Am Heart Assoc. (2023) 12:e030240. doi: 10.1161/JAHA.122.030240
25. Conte, C, Cipponeri, E, and Roden, M. Diabetes mellitus, energy metabolism and COVID-19. Endocr Rev. (2023) 2:bnad032. doi: 10.1210/endrev/bnad032
26. Sharma, A, Shanti, H, Nageswaran, H, Best, LMJ, and Patel, AG. Role of Ursodeoxycholic acid in the prevention of gallstones formation in bariatric patients-a systematic review and Meta-analysis of randomised trials. Obes Surg. (2023) 33:4115–24. doi: 10.1007/s11695-023-06893-9
27. Tesema, GA, Worku, MG, Tessema, ZT, Teshale, AB, Alem, AZ, Yeshaw, Y, et al. Prevalence and determinants of severity levels of anemia among children aged 6-59 months in sub-Saharan Africa: a multilevel ordinal logistic regression analysis. PLoS One. (2021) 16:e0249978. doi: 10.1371/journal.pone.0249978
28. Ando, M, Yazawa, A, and Kawachi, I. Socioeconomic disparities in mammography screening in the United States from 2012 to 2020. Soc Sci Med. (2023) 340:116443. doi: 10.1016/j.socscimed.2023.116443
29. Rucker, RD Jr, Horstmann, J, Schneider, PD, Varco, RL, and Buchwald, H. Comparisons between jejunoileal and gastric bypass operations for morbid obesity. Surgery. (1982) 92:241–9.
30. Majid, SF, Davis, MJ, Ajmal, S, Podkameni, D, Jain-Spangler, K, Guerron, AD, et al. Current state of the definition and terminology related to weight recurrence after metabolic surgery: review by the POWER task force of the American Society for Metabolic and Bariatric Surgery. Surg Obes Relat Dis. (2022) 18:957–63. doi: 10.1016/j.soard.2022.04.012
31. Aminian, A, Tu, C, Milinovich, A, Wolski, KE, Kattan, MW, and Nissen, SE. Association of Weight Loss Achieved through Metabolic Surgery with Risk and Severity of COVID-19 infection. JAMA Surg. (2022) 157:221–30. doi: 10.1001/jamasurg.2021.6496
32. Feldman, JM, and Bassett, MT. Variation in COVID-19 mortality in the US by race and ethnicity and educational attainment. JAMA Netw Open. (2021) 4:e2135967. doi: 10.1001/jamanetworkopen.2021.35967
33. Chen, YH, Matthay, EC, Chen, R, DeVost, MA, Duchowny, KA, Riley, AR, et al. Excess mortality in California by education during the COVID-19 pandemic. Am J Prev Med. (2022) 63:827–36. doi: 10.1016/j.amepre.2022.06.020
34. Kudoh, R, Komiya, K, Shinohara, A, Kageyama, T, Hiramatsu, K, and Kadota, JI. Marital status and post-COVID-19 conditions. Respir Investig. (2023) 61:181–5. doi: 10.1016/j.resinv.2023.01.001
35. Zhong, R, Chen, L, Zhang, Q, Li, B, Qiu, Y, Wang, W, et al. Which factors, smoking, drinking alcohol, betel quid chewing, or underlying diseases, are more likely to influence the severity of COVID-19? Front Physiol. (2020) 11:623498. doi: 10.3389/fphys.2020.623498
36. Ho, KS, Narasimhan, B, Sheehan, J, Wu, L, and Fung, JY. Controversy over smoking in COVID-19-a real world experience in New York city. J Med Virol. (2021) 93:4537–43. doi: 10.1002/jmv.26738
37. Hamer, M, Kivimaki, M, Gale, CR, and Batty, GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. (2020) 87:184–7. doi: 10.1016/j.bbi.2020.05.059
38. Rao, S, Baranova, A, Cao, H, Chen, J, Zhang, X, and Zhang, F. Genetic mechanisms of COVID-19 and its association with smoking and alcohol consumption. Brief Bioinform. (2021) 22:bbab284. doi: 10.1093/bib/bbab284
39. Abate, BB, Kassie, AM, Kassaw, MW, Aragie, TG, and Masresha, SA. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. (2020) 10:e040129. doi: 10.1136/bmjopen-2020-040129
40. Capuano, A, Rossi, F, and Paolisso, G. Covid-19 kills more men than women: an overview of possible reasons. Front Cardiovasc Med. (2020) 7:131. doi: 10.3389/fcvm.2020.00131
41. Zheng, Z, Peng, F, Xu, B, Zhao, J, Liu, H, Peng, J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. (2020) 81:e16–25. doi: 10.1016/j.jinf.2020.04.021
42. Ejaz, H, Alsrhani, A, Zafar, A, Javed, H, Junaid, K, Abdalla, AE, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. (2020) 13:1833–9. doi: 10.1016/j.jiph.2020.07.014
43. Alkhouli, M, Nanjundappa, A, Annie, F, Bates, MC, and Bhatt, DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. (2020) 95:1613–20. doi: 10.1016/j.mayocp.2020.05.014
44. Spetz, K, Svedjeholm, S, Roos, S, Grehn, S, Olbers, T, and Andersson, E. Adherence to vitamin and mineral supplementation after bariatric surgery - a two-year cohort study. Obes Res Clin Pract. (2022) 16:407–12. doi: 10.1016/j.orcp.2022.09.001
45. James, H, Lorentz, P, and Collazo-Clavell, ML. Patient-reported adherence to empiric vitamin/mineral supplementation and related nutrient deficiencies after roux-en-Y gastric bypass. Obes Surg. (2016) 26:2661–6. doi: 10.1007/s11695-016-2155-7
46. Ben-Porat, T, Elazary, R, Goldenshluger, A, Sherf Dagan, S, Mintz, Y, and Weiss, R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy-are supplements required for a lifetime? Surg Obes Relat Dis. (2017) 13:1138–44. doi: 10.1016/j.soard.2017.02.021
47. Syangtan, G, Bista, S, Dawadi, P, Rayamajhee, B, Shrestha, LB, Tuladhar, R, et al. Asymptomatic SARS-CoV-2 carriers: a systematic review and Meta-analysis. Front Public Health. (2020) 8:587374. doi: 10.3389/fpubh.2020.587374
48. Gao, Z, Xu, Y, Sun, C, Wang, X, Guo, Y, Qiu, S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. (2021) 54:12–6. doi: 10.1016/j.jmii.2020.05.001
49. Fortunato, F, Martinelli, D, Lo Caputo, S, Santantonio, T, Dattoli, V, Lopalco, PL, et al. Sex and gender differences in COVID-19: an Italian local register-based study. BMJ Open. (2021) 11:e051506. doi: 10.1136/bmjopen-2021-051506
50. Jin, JM, Bai, P, He, W, Wu, F, Liu, XF, Han, DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. (2020) 8:152. doi: 10.3389/fpubh.2020.00152
51. Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. (2021) 27:28–33. doi: 10.1038/s41591-020-01202-8
52. Marasco, G, Cremon, C, Barbaro, MR, Salvi, D, Cacciari, G, Kagramanova, A, et al. Prevalence of gastrointestinal symptoms in severe acute respiratory syndrome coronavirus 2 infection: results of the prospective controlled multinational GI-COVID-19 study. Am J Gastroenterol. (2022) 117:147–57. doi: 10.14309/ajg.0000000000001541
53. Group ICC. COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection. (2021) 49:889–905. doi: 10.1007/s15010-021-01599-5
54. Tignanelli, CJ, Bramante, CT, Dutta, N, Tamariz, L, Usher, MG, and Ikramuddin, S. Metabolic surgery may protect against admission for COVID-19 in persons with nonalcoholic fatty liver disease. Surg Obes Relat Dis. (2021) 17:1780–6. doi: 10.1016/j.soard.2021.05.029
Keywords: obesity, bariatric surgery, sex, COVID-19, cross-sectional study
Citation: Wang S, Jang Q, Wang H, Yang Y, Ruan M, Yu J, Li X and Luo D (2024) Sex differences in patients with COVID-19 after bariatric surgery: a multicenter cross-sectional study. Front. Public Health. 11:1293318. doi: 10.3389/fpubh.2023.1293318
Edited by:
Edda Cava, San Camillo Forlanini Hospital, ItalyReviewed by:
Javier Mancilla-Galindo, Utrecht University, NetherlandsStyliani A. Geronikolou, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Wang, Jang, Wang, Yang, Ruan, Yu, Li and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Luo, TFVPREFOMTc4NjI4MTMxQHFxLmNvbQ==
†These authors have contributed equally to this work
 Senlin Wang
Senlin Wang Qiubai Jang2†
Qiubai Jang2†