- 1Faculty of Nursing, Phenikaa University, Hanoi, Vietnam
- 2School of Nursing, The Hong Kong Polytechnic University, Hong Kong SAR, China
- 3Hoan My Da Lat Hospital, Dalat, Vietnam
- 4Department of Nursing and Midwifery, Hanoi Medical University, Hanoi, Vietnam
- 5School of Preventive Medicine and Public Health, Hanoi Medical University, Hanoi, Vietnam
- 6Hanoi Oncology Hospital, Hanoi, Vietnam
- 7Center for Ageing Research & Education, Duke-NUS Medical School, Singapore, Singapore
Objectives: Insomnia is a common symptom after COVID-19 infection; however, its current evidence was among hospitalized COVID-19 patients. This study aimed to assess the prevalence of insomnia and identify its association with depression and anxiety among non-hospitalized COVID-19 recovered population.
Methods: We conducted a cross-sectional online survey of 1,056 COVID-19 survivors within 6 months of initial COVID-19 infection and retrieved did not require hospitalization. The Insomnia Severity Index, and Depression Anxiety and Stress Scale-14 were used. Multivariate logistic regression was used to examine the associations between depressive and anxiety score, and participants’ insomnia level.
Results: The prevalence of insomnia was 76.1%, and among those, 22.8% of participants scored for severe insomnia. One third of participants reported worse sleep quality, shorter sleep duration, and harder to fall asleep, half reported more awaken nights after COVID-19 infection. Participants with depressive (OR 3.45; 95%CI 1.87–6.34) or anxiety (OR 3.93; 95%CI 2.52–6.13) had significantly higher odds of developing insomnia. Other risk factors of insomnia included pre-existing chronic conditions and higher education level, while COVID-19 symptoms and duration were not significantly associated.
Conclusion: Our study highlights the substantial burden of insomnia among non-hospitalized COVID-19 survivors and the significant association of depression and anxiety on the development of this long-term effect of COVID-19. These findings underscore the need for comprehensive interventions that address both sychological and sleeping health in this population.
Introduction
Since late 2019, COVID-19 pandemic had reached every region of the world and infected over 767 million people globally (1). As of mid-2023, about 90% of COVID-19 infected cases had recovered from the disease (2). Most common symptoms reported during the acute phases of COVID-19 were cough, fatigue, fever, dyspnea, musculoskeletal disorders, gastrointestinal symptoms, anosmia, dysgeusia, and vertigo (3–5). As the results, COVID-19 patients face many unpleasant symptoms after recovering. Post COVID-19 condition, commonly known as long COVID has been defined as symptoms experienced by COVID-19 patients after the initial SARS-CoV-2 infection, these symptoms can affect anyone exposed to SAR-CoV-2, regardless the severity of original symptoms (6).
Although the pandemic is expected to become an endemic disease, prolonged physical and psychological problems after COVID-19 infection should be considered an important public health issue that needs addressing promptly. Among 200 different symptoms after COVID-19 infection, insomnia is one of the most common symptoms (7–9). Two recent systematic reviews have revealed a wide prevalence of insomnia among COVID-19 recovered patients ranging from 5.4 to 66.67% (8, 9). In general population, when being measured by the standard scale, this prevalence ranged from 10.1 to 66.67% (10–12), while using the standardized classification system of diagnosis codes (ICD-10) it ranged from 5.4 to 36.5% (13, 14). Longitudinal studies among COVID-19 patients after 6 months discharged from hospital reported the proportion of insomnia ranging widely from 10.1 to 45.1% (12, 15, 16).
Several factors have been reported as high-risk factors for insomnia among COVID-19 survivors, such as being female, younger age, higher employment and education status (17). Fu et al. and Campo-Arias et al. both showed anxiety, depression, post-traumatic stress disorders were associated with higher incidence of post-COVID-19 insomnia in general population (15, 18). Research has demonstrated that poor mental health is closely tied to inadequate sleep conditions. Additionally, patients infected with COVID-19 are at risk of developing anxiety and depression, as a result of the severity and isolation. In addition, chronic disease such Obstructive Sleep Apnea (OSA) impact the development of incident glycemic, neurocognitive impairment, and abnormal functional pulmonary changes that persist up to 1 year after COVID-19 recovering (19). Considering the extended recovery period for COVID-19, its impact on patients’ mental health may lead to further negative effects on their sleep quality.
While research has been conducted on sleep issues among those who have recovered from COVID-19, there is still limited information on sleep disruptions in COVID-19 survivors, regardless of whether they were hospitalized or not (17). Currently literature focused on COVID-19 hospitalized patients, which the environment of their treatment and quarantine would differ greatly from those with milder symptoms of COVID-19. Hence, this would impact the COVID-19 survivors who quarantined and received treatment out of hospital, both in terms of psychological impacts and long COVID-19 symptoms. Therefore, we conducted this study to (i) assess the prevalence of insomnia among COVID-19 survivors with no or mild symptoms that not required hospitalization in the recovery period (6 months), and (ii) identify its associated factors. Data from this study would highlight the extent of the problem and inform the development of future intervention trials.
Methods
Study design
A cross-sectional survey was conducted.
Participants
Participants were COVID-19 survivors (recovered as confirmed by Polemerase Chain Reaction test) in Vietnam general population. Participants were 18 years old or above who had recovered from COVID-19 within 6 months, and not require hospitalization due to COVID-19 symptoms. Any participants with medical history of diagnosed insomnia and/or psychiatric disorder prior to infection (self-report) were excluded from the study.
Measurements
Data related to COVID-19 severity
Collected variables on socio-demographic characteristics were age, sex, marital status, employment status, education level, occupation, and pre-existing chronic conditions (Yes if participants answered “Yes” for cardiovascular diseases, cancer, metabolic diseases (i.e., diabetes, high blood lipids), immunocompromised and/or allergic conditions, other). Participants were asked to rate the severity of their COVID-19 infection (asymptomatic or symptomatic), and number of days infected (calculated by days from the first positive COVID-19 test to the first negative COVID-19 test).
Insomnia
The Vietnamese version of the Insomnia Severity Index (ISI) was used to measure insomnia severity among participants. The ISI contains seven items reporting the nature, severity, and impact of insomnia. Each question is given a 5-point Likert scale from 0 (no problem) to 4 (very severe problem), which yields a final score ranging from 0 to 28 for each participant, higher score indicating more severe insomnia level. A score of 10 and above indicated the participants had insomnia. This cut-off point allows the most optimal sensitivity (86.2%) and specificity (87.7%) to detect insomnia in the community (20). Insomnia severity is interpreted as follows: mild insomnia (11–14), moderate insomnia (ISI score of 15–21) and severe insomnia (ISI score of 22–28) (20). In the current study, the ISI had high reliability with Cronbach’s Alpha = 0.93.
In addition, the respondents were also asked to compare their sleep quality, sleep initiation, and total sleep duration in the recent 2 weeks with the time before confirming COVID-19 infection on a 3-point Likert-like scale (3 “worse/less than before,” 2 “about the same,” 1 “better/more than before”).
Other symptoms
The Vietnamese version of Depression, Anxiety, and Stress Scale (DASS-14) was used to measure participants’ mental health. DASS-14 is the modified version of the DASS-21. It is a self-report tool containing 14 items that measure the level of depression and anxiety in the preceding week of participants (7 items per subscale). Participants answered the items using a 4-point Likert scale ranging from 0 (Did not apply at all) to 3 (Applied very much, or most of the time). The total score was calculated by the sum of all items multiplied by two, ranging from 0 to 42 for each of the sub-scale. A cut-off point of 33 on each sub-scale was used to create two binary variables of “Participants with depressive/anxiety symptoms” or without depressive/anxiety symptoms (sensitivity 79.1% and specificity 77.0%) (21). In this study, the internal consitency was reported to be high, with the overall scale having Cronbach’s Alpha = 0.9, the depression subscale’s Cronbach’s Alpha = 0.9, and the anxiety subscale’s Cronbach’s Alpha = 0.81.
Sample size calculation
Sample size calculation was based on the estimated prevalence of insomnia (50%) with a 3% margin of error (10, 14). An estimated sample of 1,068 was suggested under the 95% confidence interval.
Data collection
The questionnaire was created using Qualtrics CoreXM. The survey was opened from June to September 2022. An open invitation to participate was sent to all the member of COVID-19 patients’ network (organized by the Vietnamese government) to approach infected participants. The invitation was sent to either email address or social account (Facebook and Zalo – two most popular social media platforms) of the potential participants.
Data analysis
Data were extracted from the Qualtrics into Stata 15.0 for cleaning and analysis. First, we tabulated participants’ sociodemographic, health, sleep quality assessment, depressive and anxiety level, stratified by insomnia score and binary level. Then the Wilcoxon rank-sum, Pearson’s chi-squared tests and Fisher’s exact tests were used to detect statistically significant differences between participants’ characteristics. Thirdly, we included depressive level, anxiety level, and each covariate into the univariate logistic regression to estimate the association with participants’ insomnia status. The univariate and multivariable logistic regression was employed to estimate odds ratios (ORs) and 95%CIs of the associations between depressive and anxiety level, and participants’ insomnia, controlling for all covariates adjusted for participants’ socio-demographic characteristics, the severity of their COVID-19 infection, and number of days infected.
Results
The research team sent out 34,850 invitation to targeted population and a total of 1,090 participants responded to the survey. However, 34 participants did not complete the ISI and DASS-14 leaving the final sample size of the study was 1,056. Table 1 shows descriptive values of sociodemographic characteristics, COVID-19 infection, and mental health indicators of participants, summarized by ISI score and stratified by insomnia status. Majority of participants were female (68.5%), mean age of 33.6 ± 10.1, married (64%), and had received university education (68.7%). 20.1% of our participants were healthcare workers, 16.1% were students, and 3.4% were not working at time of survey. In addition, 11.7% of them had pre-existing chronic conditions (cardiovascular diseases: 4.5%, metabolic disorders: 3.5%, cancers: 2.2%, and other chronic diseases such as degenerative spine, adiposis hepatica, gout: 1.5%). Participants who were female, divorced/widowed, had post-graduate education, not currently working, or had chronic diseases scored higher ISI score on average than their counterparts, but only those with chronic conditions had significantly higher score (18.46 ± 6.03, p < 0.01) than those without.
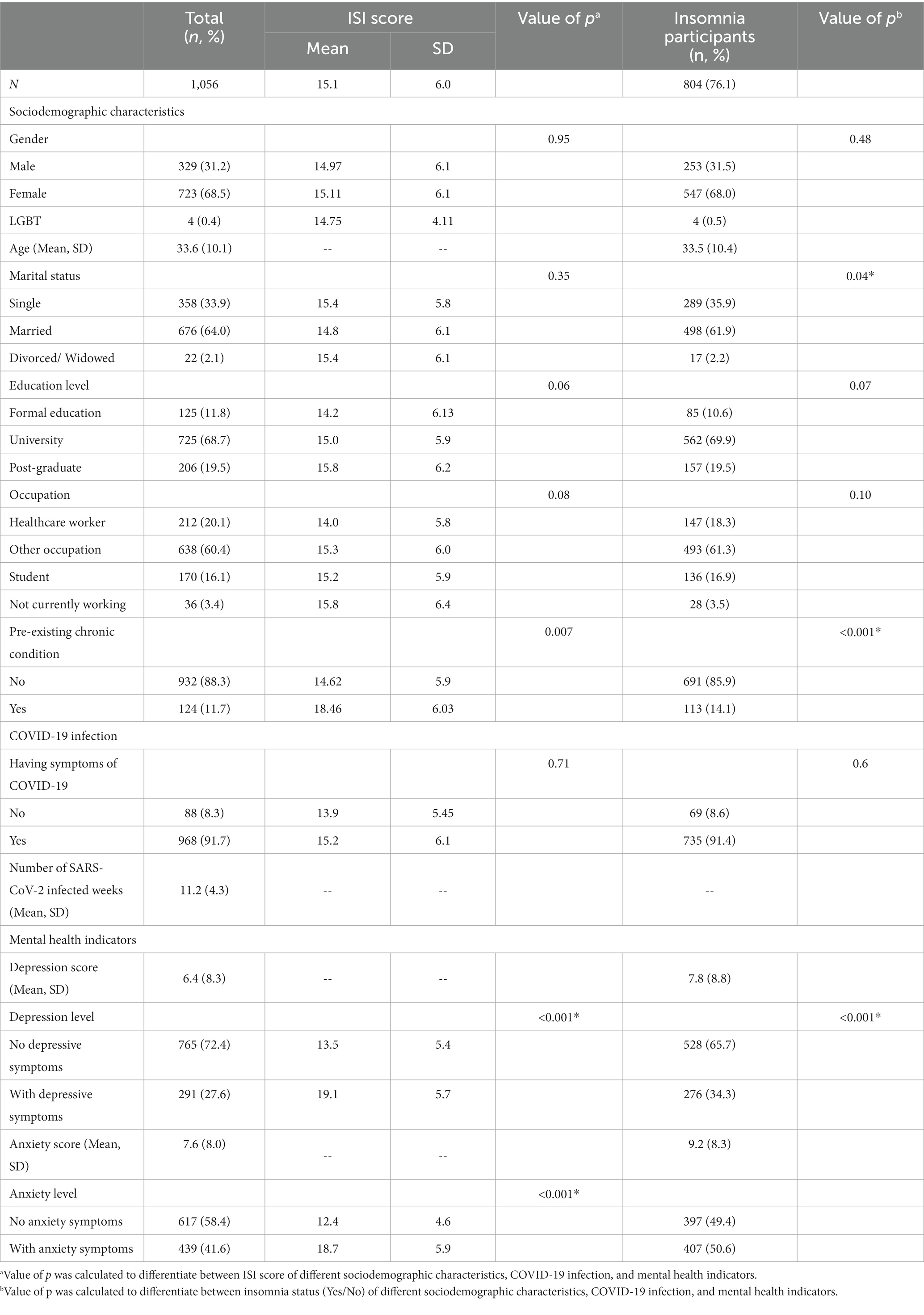
Table 1. Descriptive analyses of sociodemographic characteristics, COVID-19 infection, and mental health indicators of participants.
Regarding COVID-19 infection, 92% of participants were symptomatic during their period of infection (on average 11.2 ± 4.3 weeks). These symptomatic participants also scored higher on ISI (15.2 ± 6.1), yet no statistically significant was found compared to asymptomatic participants. The mean score of depression was 6.4 ± 8.3, and the mean score of anxiety was (7.6 ± 8.0). This resulted in 291 (27.6%) and 439 (41.6%) of participants with relevant depressive and anxiety symptoms, respectively. Participants with depressive symptoms (19.1 ± 5.7) and with anxiety symptoms (18.7 ± 5.9) also had significantly higher ISI score than those without (13.5 ± 5.4 and 12.4 ± 4.6, respectively, p < 0.001).
The average ISI score of the participants was 15.1 ± 6.0. The prevalence of insomnia was 76.1% (804/1056 participants) (mean score of ISI among insonnia participants = 17.32 ± 5.1). Among those who experienced insomnia, 41.5% reported moderate, and 22.8% reported severe insomnia. Characteristics of insomnia participants were comparable to the whole sample size. In addition, there were significant differences in marital status (p < 0.04), pre-existing chronic condition (p < 0.001), depression and anxiety level (p < 0.001) between participants with and without insomnia. The participants with insomnia had higher depression scores (7.8 ± 8.8) and anxiety scores (9.2 ± 8.3) than the average scores of the entire sample. Figure 1 shows participants’ subjective assessment of sleep condition after COVID-19 infection. About one third of participants consistently reported their sleep quality, duration, and initiation was worse than before they were infected with COVID-19. More than half of participants (52.9%) reported higher number of night being awake after recovering from COVID-19 compared to before being infected.
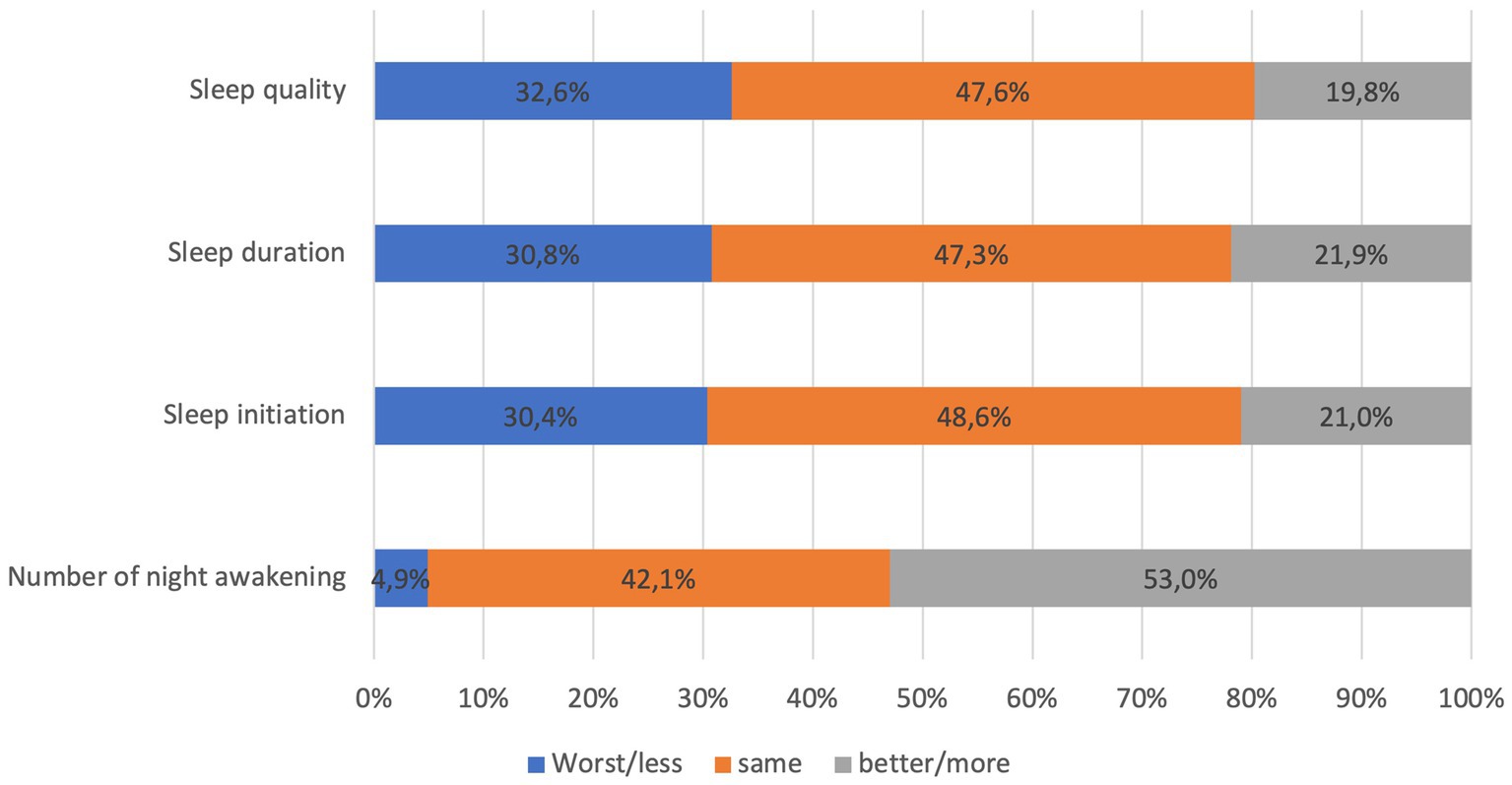
Figure 1. subjective assessment of some sleep parameters after COVID-19 infection of 1,056 participants.
Table 2 shows univariate and multivariable logistic regression of factors associated with insomnia among participants. In univariate regression, participants who were married [Odds ratio (OR) 0.68,95% CI: 0.49–0.91, p < 0.05] and had university education (OR 1.62, 95% CI: 1.07–2.45, p < 0.05) had significantly lower odds of having insomnia than those who were single or had formal education. Participants who were students (OR 1.77, 95% CI: 1.10–2.85, p < 0.05) had significantly higher odds of having insomnia than healthcare workers. Participants who had pre-existing chronic conditions (OR 3.58, 95% CI: 1.90–6.77, p < 0.001) had significantly higher odds of insomnia after their COVID-19 infection than those without chronic diseases. Concerning COVID-19 infection, there was no significant likelihood of having insomnia post-COVID-19 infection between symptomatic status or time of infection. On the other hand, participants with depressive symptoms (OR 8.26, 95% CI: 4.80–14.19, p < 0.001) or anxiety symptoms (OR 7.05, 95% CI: 4.74–10.47, p < 0.001) had significantly higher proportion of insomnia than those without.
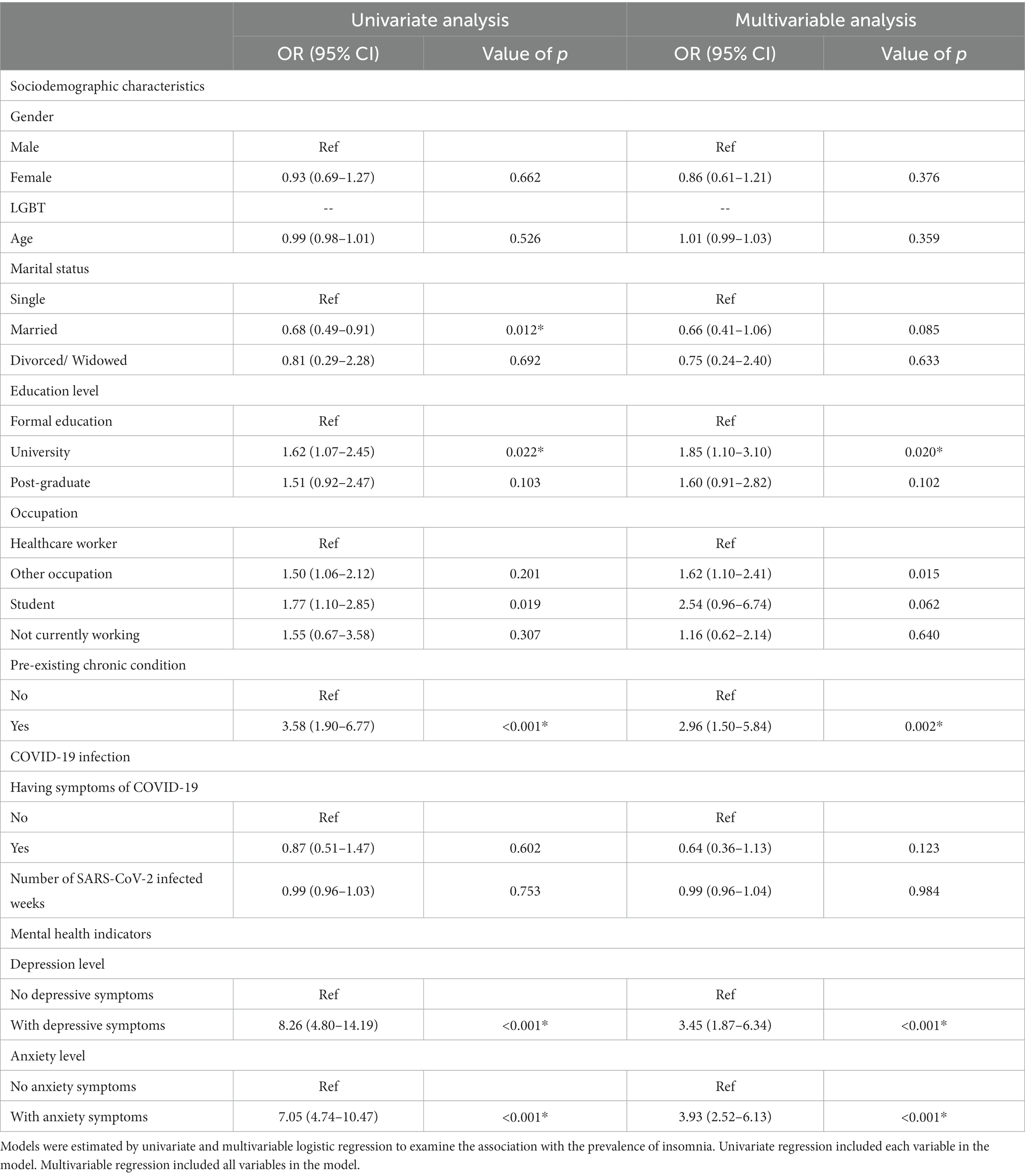
Table 2. Univariate and multivariable logistic regression of factors associated with insomnia among 1,056 participants.
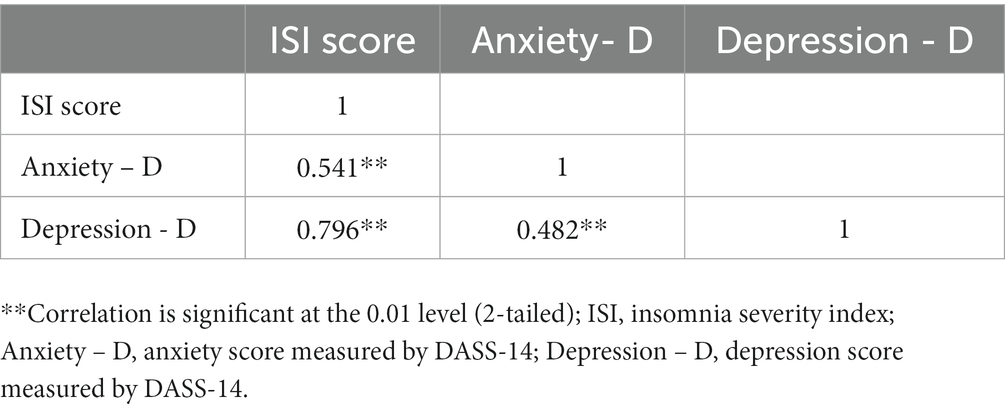
The multivariable regression confirmed the significant association between education level, pre-existing chronic conditions, and the prevalence of insomnia. Participants who had university education had 1.85-fold (95% CI: 1.10–3.10) increase in the odds of experiencing insomnia after their COVID-19 infection compared to those with formal education (primary, junior high, and high school). Participants with chronic conditions had 2.96 (95% CI: 1.50–5.84) times higher odd of having insomnia than those without. After adjusting for covariates, healthcare workers had significantly higher odd of having insomnia (OR 1.62, 95% CI: 1.10–2.41) than workers in other professions, but no significances as compared to those who were not working or were students. Multivariable regression also showed significantly higher odds of having insomnia in participants with depressive symptoms (OR 3.45, 95% CI: 1.87–6.34) and anxiety symptoms (OR 3.93, 95% CI: 2.52–6.13) than those without, but these associations were weaker than in the univariate model. The correlations between insomnia, anxiety, and depression were strong to moderate and significant (r rank from 0.5 to 0.8, p = 0.01) (Table 3).
Discussion
Results from the study indicated that more than 75% of participants had insomnia, which is remarkably higher than previously reported among general population (10–20%) (22, 23). This proportion is also much higher than the prevalence of insomnia among hospitalized COVID- 19 survivors presented in previous systematic reviews (12–47%) (4, 24, 25). This can be explained by the selection criteria (participants had recovered from COVID-19 within 6 months) so insomnia was expected to be more prevalent. Newly covered from an infection, especially a novel viral epidemic, can cause survivors to have increased risk of chronic pain, chronic health, and psychiatric disorders, which can lead to increased insomnia (26). As in our findings, we also found that elevated depressive and stress symptoms associated with insomnia risk. In addition, insomnia is often underreported in general population (27) and often higher in people with chronic diseases or health problems, hence, the findings can reflect a heightened sense of personal health among COVID-19 survivors regarding their sleep quality. As insomnia is also one of the most frequently reported symptoms among COVID-19 survivors (25), public health researchers should expect higher prevalence of insomnia and sleep problems reported among this population, which can remain among one-third recovered patients till 1 year after infection according to a longitudinal research (16).
It was observed that more than two-third of participants reported insomnia, however, only one-third reported their sleep quality, duration, and ability to initiate sleep were worse than before they were infected with COVID-19. Notably, more than half of participants reported an increase of night awakenings. Insomnia was related to the amount of delta sleep (the deepest form of sleep) and improper estimation of sleep parameters such as the total sleep time, the sleep latency, and wakening after sleep onset (28, 29). As such, an intervention that help participants to change their perception of sleep parameters may be helpful to improve insomnia.
In general population, being single or divorced have been found to be positively associated with insomnia (30–33). Other studies have also shown that the proportion of COVID-19 survivors with insomnia is higher among single or divorced individuals than among those with others marital status, which corresponds with the results of the present studies (9, 18, 34). We did not find a significant risk of insomnia of healthcare workers compared to other occupation, which is contradicting previous studies (35). As there are various aspects of healthcare workers – direct involvement with patients, shift working, clinical work versus public health work – that can impact their insomnia level. At time of the survey, COVID-19 epidemic in Vietnam was more under control, so healthcare workers might not be subjected to additional stress of the pandemic. Also in 2022, many companies, schools, and workplaces reopens, and the readjustment from working from home to office working might put a higher toll on sleep health of participants of other occupations than healthcare workers – who work in hospital throughout the pandemic.
Chronic diseases had increased the severe COVID-19 infection (36, 37). Additionally, patients with chronic illnesses are more likely to develop insomnia (38). Even though there has no conclusive on the reciprocal relationship between pre-existing chronic diseases and insomnia after COVID-19, it is possible that the severity of the infection and/or the mental burden of the disease could contribute to the onset of insomnia. The current study excluded people with a history of insomnia prior to expose with COVID-19. As such the results confirm the vulnerability of individuals with chronic diseases to insomnia after infection. This highlights the need for health monitoring and services specifically designed for high-risk groups following their infection.
This study confirmed anxiety and depression were significant associated with insomnia among recovered COVID19 patients, which is in line with previous studies (10, 36, 37, 39, 40). As COVID-19 infected patients were at higher risk of developing mental health problems (41), the psychological burden of being infected and quarantine would much likely to negatively impact their sleep quality even after their infection. Insomnia and sleep disturbances were also often cited as one of the co-occurrence symptoms of depression and anxiety. The symptom cluster of insomnia, depression, and anxiety has been reported in different populations (42–46). Therefore, more researchs must be implemented to further investigate the relationship between this three symtopms during the post-COVID-19 period.
In this study, the correlations between the insomnia, depression, and anxiety were strong to moderate in the correlational analysis. In addtion, bidirectional relationships between these three symptoms had been emphasized in systematic reviews indicating that an intervention to improve sleep should also address depression and anxiety (47, 48). This supports the use of antidepressant and/or benzodiazepine as potential treatments for improving sleep in this population when anxiety and/or depression are present, striking a good balance between the potencial possitive benefit and adverse effects and polypharmacy. However, given the potential adverse effects of pharmacological treatment non-pharmacological treatments, such as self-acupressure, cognitive behavioral therapy, mind subtraction meditation, and Cranial Electrotherapy Stimulation should be considered as they may alleviate the three symptoms (49–53). Public health agencies should consider these interventions to mitigate the long-term negative impact of the pandemic on its growing infected population.
The large sample size and the used of standardized questionnaires to measure study’s outcomes are strengths of the study. Authors acknowledge that the current study has several limitations. Since this is a cross-sectional study, the direct causation or the impact of anxiety and depression on insomnia cannot be made. In addition, due to the online collecting data and convenient sampling method, recalled bias and selection bias may occur. However, due to the situation in Vietnam at that time, collecting data via electronic invitation and convenience sampling was the most efficient and feasible strategy. In addition, some sleep parameters, vaccination status, and physical symptoms of COVID-19 were not measured in this survey but subjected to reporting bias, which can confound the findings. As severity levels and COVID-19 vaccination status might impact COVID-19 patients’ health and well-being, inclusion of other COVID-19 infection conditions might improve the accuracy of our findings and detect the high-risk population for insomnia among COVID-19 survivors. At time of survey, COVID-19 vaccination rate in Vietnam for fully vaccinated dose was at least 70% at all provinces and cities (54) so we suspect this lack of data would impact on our findings.
Conclusion
The results of the current study confirm the high prevalence of insomnia COVID-19 survivors with none or mild symptoms who did not require hospitalization. Pre-existing chronic conditions, depression, and anxiety were associated with higher risk of developing insomnia during recovery of COVID-19. Our findings add to current literature on insomnia after COVID-19 infection and underscores the crucial need to implement comprehensive interventions to address the psychological and sleep health of COVID-19 patients after recovery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the study obtained ethical approval from the Human Subject Ethics Board, Nam Dinh Nursing University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HH: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. WY: Conceptualization, Methodology, Writing – review & editing. QT: Data curation, Formal analysis, Investigation, Writing – review & editing. CL: Formal analysis, Investigation, Methodology, Writing – review & editing. CL: Formal analysis, Investigation, Methodology, Writing – review & editing. AB: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. QB: Investigation, Methodology, Project administration, Writing – review & editing. QL: Investigation, Project administration, Writing – review & editing. LQ: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank all study subjects for participating in this study. We would like to thank our data collection team (Anh H. Vu, Loan T. Dang, Nhung T. Le, Giang T. Nguyen) and research technological team (Trang H. Nguyen, Vi Do) for their contribution to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Worldometer. Coronavirus Update (Live). COVID-19 virus pandemic: worldometer. (2023). Available at: https://www.worldometers.info/coronavirus/ (Accessed April 21, 2022).
3. Pavli, A, Theodoridou, M, and Maltezou, HC. Post-COVID syndrome: incidence, clinical Spectrum, and challenges for primary healthcare professionals. Arch Med Res. (2021) 52:575–81. doi: 10.1016/j.arcmed.2021.03.010
4. Jennings, G, Monaghan, A, Xue, F, Mockler, D, and Romero-Ortuño, R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. (2021) 10:5913. doi: 10.3390/jcm10245913
5. Daker, LI, Elshafei, RR, Bahi, M, Mohammed, A, Erfan, R, and Gomaa, M. Could vertigo be a post-COVID-19 sequela or presenting symptom? Egypt J Neurol Psychiatr Neurosurg. (2023) 59:65. doi: 10.1186/s41983-023-00659-x
6. World Health Organization. Post COVID-19 condition (Long COVID) (2022). Available at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition
7. Akbarialiabad, H, Taghrir, MH, Abdollahi, A, Ghahramani, N, Kumar, M, Paydar, S, et al. Long COVID, a comprehensive systematic scoping review. Infection. (2021) 49:1163–86. doi: 10.1007/s15010-021-01666-x
8. Alkodaymi, MS, Omrani, OA, Fawzy, NA, Shaar, BA, Almamlouk, R, Riaz, M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. (2022) 28:657–66. doi: 10.1016/j.cmi.2022.01.014
9. Bourmistrova, NW, Solomon, T, Braude, P, Strawbridge, R, and Carter, B. Long-term effects of COVID-19 on mental health: a systematic review. J Affect Disord. (2022) 299:118–25. doi: 10.1016/j.jad.2021.11.031
10. Pappa, S, Barmparessou, Z, Athanasiou, N, Sakka, E, Eleftheriou, K, Patrinos, S, et al. Depression, insomnia and post-traumatic stress disorder in COVID-19 survivors: role of gender and impact on quality of life. J Pers Med. (2022) 12:486. doi: 10.3390/jpm12030486
11. Ahmed, GK, Khedr, EM, Hamad, DA, Meshref, TS, Hashem, MM, and Aly, MM. Long term impact of Covid-19 infection on sleep and mental health: a cross-sectional study. Psychiatry Res. (2021) 305:114243. doi: 10.1016/j.psychres.2021.114243
12. Choudhry, AA, Shahzeen, F, Choudhry, SA, Batool, N, Murtaza, F, Dilip, A, et al. Impact of COVID-19 infection on quality of sleep. Cureus. (2021) 13:e18182. doi: 10.7759/cureus.18182
13. González-Andrade, F. Post-COVID-19 conditions in Ecuadorian patients: an observational study. Lancet Reg Health Am. (2022) 5:100088. doi: 10.1016/j.lana.2021.100088
14. Park, HY, Song, IA, and Oh, TK. Insomnia disorder among coronavirus disease survivors: a south Korean Nationwide cohort study. Psychiatry Investig. (2021) 18:1082–90. doi: 10.30773/pi.2021.0223
15. Fu, L, Fang, Y, Luo, D, Wang, B, Xiao, X, Hu, Y, et al. Pre-hospital, in-hospital and post-hospital factors associated with sleep quality among COVID-19 survivors 6 months after hospital discharge: cross-sectional survey in five cities in China. BJPsych Open. (2021) 7:e191. doi: 10.1192/bjo.2021.1008
16. Fumagalli, C, Zocchi, C, Tassetti, L, Silverii, MV, Amato, C, Livi, L, et al. Factors associated with persistence of symptoms 1 year after COVID-19: a longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med. (2022) 97:36–41. doi: 10.1016/j.ejim.2021.11.018
17. Bhat, S, and Chokroverty, S. Sleep disorders and COVID-19. Sleep Med. (2021) 91:253–61. doi: 10.1016/j.sleep.2021.07.021
18. Campo-Arias, A, Pedrozo-Pupo, JC, and Caballero-Domínguez, CC. Relation of perceived discrimination with depression, insomnia and post-traumatic stress in COVID-19 survivors. Psychiatry Res. (2022) 307:114337. doi: 10.1016/j.psychres.2021.114337
19. Labarca, G, Henríquez-Beltrán, M, Lamperti, L, Nova-Lamperti, E, Sanhueza, S, Cabrera, C, et al. Impact of obstructive sleep Apnea (OSA) in COVID-19 survivors, symptoms changes between 4-months and 1 year after the COVID-19 infection. Front Med. (2022) 9:884218. doi: 10.3389/fmed.2022.884218
20. Morin, CM, Belleville, G, Bélanger, L, and Ivers, H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. (2011) 34:601–8. doi: 10.1093/sleep/34.5.601
21. Tran, TD, Tran, T, and Fisher, J. Validation of the depression anxiety stress scales (DASS) 21 as a screening instrument for depression and anxiety in a rural community-based cohort of northern Vietnamese women. BMC Psychiatry. (2013) 13:24. doi: 10.1186/1471-244X-13-24
23. Neubauer, DN, Pandi-Perumal, SR, Spence, DW, Buttoo, K, and Monti, JM. Pharmacotherapy of insomnia. J Central Nerv Syst Dis. (2018) 10:117957351877067. doi: 10.1177/1179573518770672
24. Tarsitani, L, Vassalini, P, Koukopoulos, A, Borrazzo, C, Alessi, F, Di Nicolantonio, C, et al. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J Gen Intern Med. (2021) 36:1702–7. doi: 10.1007/s11606-021-06731-7
25. Han, Q, Zheng, B, Daines, L, and Sheikh, A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. (2022) 11:269. doi: 10.3390/pathogens11020269
26. Katic, B, Heywood, J, Turek, F, Chiauzzi, E, Vaughan, TE, Simacek, K, et al. New approach for analyzing self-reporting of insomnia symptoms reveals a high rate of comorbid insomnia across a wide spectrum of chronic diseases. Sleep Med. (2015) 16:1332–41. doi: 10.1016/j.sleep.2015.07.024
27. Leger, D, and Poursain, B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. (2005) 21:1785–92. doi: 10.1185/030079905X65637
28. O'Donnell, JF. Insomnia in cancer patients. Clin Cornerstone. (2004) 6:S6–S14. doi: 10.1016/S1098-3597(05)80002-X
29. Kay, DB, Karim, HT, Soehner, AM, Hasler, BP, James, JA, Germain, A, et al. Subjective–objective sleep discrepancy is associated with alterations in regional glucose metabolism in patients with insomnia and good sleeper controls. Sleep. (2017) 40:zsx155. doi: 10.1093/sleep/zsx155
30. Nugent, CN, and Black, LI. Sleep duration, quality of sleep, and use of sleep medication, by sex and family type, 2013-2014. NCHS Data Brief. (2016) 230:1–8.
31. Kawata, Y, Maeda, M, Sato, T, Maruyama, K, Wada, H, Ikeda, A, et al. Association between marital status and insomnia-related symptoms: findings from a population-based survey in Japan. Eur J Pub Health. (2019) 30:144–9. doi: 10.1093/eurpub/ckz119
32. Yu, BY, Yeung, WF, Lam, JC, Yuen, SC, Lam, SC, Chung, VC, et al. Prevalence of sleep disturbances during COVID-19 outbreak in an urban Chinese population: a cross-sectional study. Sleep Med. (2020) 74:18–24. doi: 10.1016/j.sleep.2020.07.009
33. Xiang, YT, Ma, X, Cai, ZJ, Li, SR, Xiang, YQ, Guo, HL, et al. The prevalence of insomnia, its sociodemographic and clinical correlates, and treatment in rural and urban regions of Beijing, China: a general population-based survey. Sleep. (2008) 31:1655–62. doi: 10.1093/sleep/31.12.1655
34. Liu, C, Liu, D, Huang, N, Fu, M, Ahmed, JF, Zhang, Y, et al. The combined impact of gender and age on post-traumatic stress symptoms, depression, and insomnia during COVID-19 outbreak in China. Frontiers. Public Health. (2021) 8:8. doi: 10.3389/fpubh.2020.620023
35. Wu, T, Jia, X, Shi, H, Niu, J, Yin, X, Xie, J, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. (2021) 281:91–8. doi: 10.1016/j.jad.2020.11.117
36. Geng, J, Yu, X, Bao, H, Feng, Z, Yuan, X, Zhang, J, et al. Chronic diseases as a predictor for severity and mortality of COVID-19: a systematic review with cumulative meta-analysis. Front Med. (2021) 8:8. doi: 10.3389/fmed.2021.588013
37. Hacker, K, Briss, P, Richardson, LW, Wright, J, and Petersen, R. COVID-19 and chronic disease: the impact now and in the future. Prev Chronic Dis. (2021) 18:18. doi: 10.5888/pcd18.210086
38. Ancoli-Israel, S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. (2006) 12:S221.
39. Al-Aly, Z, Xie, Y, and Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. (2021) 594:259–64. doi: 10.1038/s41586-021-03553-9
40. Premraj, L, Kannapadi, NV, Briggs, J, Seal, SM, Battaglini, D, Fanning, J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. (2022) 434:120162. doi: 10.1016/j.jns.2022.120162
41. Xie, Y, Xu, E, and Al-Aly, Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ. (2022) 376:e068993. doi: 10.1136/bmj-2021-068993
42. Ji, X, Ivers, H, Beaulieu-Bonneau, S, and Morin, CM. Complementary and alternative treatments for insomnia/insomnia-depression-anxiety symptom cluster: meta-analysis of English and Chinese literature. Sleep Med Rev. (2021) 58:101445. doi: 10.1016/j.smrv.2021.101445
43. Conley, S, Jeon, S, Breazeale, S, O’Connell, M, Hollenbeak, CS, Jacoby, D, et al. Symptom cluster profiles among adults with insomnia and heart failure. Behav Sleep Med. (2023) 21:150–61. doi: 10.1080/15402002.2022.2060226
44. Gehrman, PR, Garland, SN, Matura, LA, and Mao, J. Insomnia in breast cancer: independent symptom or symptom cluster? Palliat Support Care. (2017) 15:369–75. doi: 10.1017/S1478951516000900
45. Maniaci, A, Ferlito, S, Lechien, JR, Di Luca, M, Iannella, G, Cammaroto, G, et al. Anxiety, depression and sleepiness in OSA patients treated with barbed reposition pharyngoplasty: a prospective study. Eur Arch Otorhinolaryngol. (2022) 279:4189–98. doi: 10.1007/s00405-022-07369-9
46. Hoang, HTX, Molassiotis, A, Chan, CW, Nguyen, TH, and Liep, NV. New-onset insomnia among cancer patients undergoing chemotherapy: prevalence, risk factors, and its correlation with other symptoms. Sleep Breath. (2020) 24:241–51. doi: 10.1007/s11325-019-01839-x
47. Alvaro, PK, Roberts, RM, and Harris, JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. (2013) 36:1059–68. doi: 10.5665/sleep.2810
48. Fang, H, Tu, S, Sheng, J, and Shao, A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. (2019) 23:2324–32. doi: 10.1111/jcmm.14170
49. Yun, MR, Song, M, Jung, KH, Yu, BJ, and Lee, KJ. The effects of mind subtraction meditation on breast cancer Survivors' psychological and spiritual well-being and sleep quality: a randomized controlled trial in South Korea. Cancer Nurs. (2017) 40:377–85. doi: 10.1097/NCC.0000000000000443
50. Kwekkeboom, KL, Abbott-Anderson, K, Cherwin, C, Roiland, R, Serlin, RC, and Ward, SE. Pilot randomized controlled trial of a patient-controlled cognitive-behavioral intervention for the pain, fatigue, and sleep disturbance symptom cluster in cancer. J Pain Symptom Manag. (2012) 44:810–22. doi: 10.1016/j.jpainsymman.2011.12.281
51. Fleming, L, Randell, K, Harvey, CJ, and Espie, CA. Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psychooncology. (2014) 23:679–84. doi: 10.1002/pon.3468
52. Hoang, HTX, Molassiotis, A, Chan, CW, Vu, AH, and Bui, PT. Pilot randomized sham-controlled trial of self-acupressure to manage the symptom cluster of insomnia, depression, and anxiety in cancer patients undergoing chemotherapy. Sleep Breath. (2022) 26:445–56. doi: 10.1007/s11325-021-02370-8
53. Yeung, WFYB, Chung, KF, Zhang, ZJ, Lao, L, Ho, FY, Suen, LK, et al. Self-administered acupressure for insomnia disorder: a randomized controlled trial. Phytomedicine. (2022) 99:153993. doi: 10.1016/j.phymed.2022.153993
54. World Health Organization. WHO Coronavirus (COVID-19) Dashboard (2023). Available at: https://covid19.who.int/table.
Keywords: post COVID-19, insomnia, depression, anxiety, ISI, DASS-14
Citation: Hoang HTX, Yeung WF, Truong QTM, Le CT, Bui ATM, Bui QV, Le QTL and Quach LH (2024) Sleep quality among non-hospitalized COVID-19 survivors: a national cross-sectional study. Front. Public Health. 11:1281012. doi: 10.3389/fpubh.2023.1281012
Edited by:
Akiyoshi Shimura, Tokyo Medical University, JapanReviewed by:
Antonino Maniaci, University of Catania, ItalyVinh Nhu Nguyen, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam
Kentaro Matsui, National Center of Neurology and Psychiatry, Japan
Copyright © 2024 Hoang, Yeung, Truong, Le, Bui, Bui, Le and Quach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huong Thi Xuan Hoang, huong.hoangthixuan@phenikaa-uni.edu.vn
 Huong Thi Xuan Hoang
Huong Thi Xuan Hoang Wing Fai Yeung
Wing Fai Yeung Quyen Thi Mai Truong3
Quyen Thi Mai Truong3 Anh Thi My Bui
Anh Thi My Bui Quang Vinh Bui
Quang Vinh Bui Linh Ha Quach
Linh Ha Quach