- 1Division of Microbiology, National Center for Toxicological Research, U.S. Food and Drug Administration, Jefferson, AR, United States
- 2Office of Cosmetics and Colors, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, College Park, MD, United States
In this study, we collected voluntary recall records of tattoo and permanent makeup ink from the U.S. Food and Drug Administration (US FDA) Enforcement Report Database. The recall records contain information, such as recall date, manufacturer, ink color, reason for recall, and the microorganisms detected from the ink samples. Between 2003 and 2021, a total of 15 voluntary tattoo ink recalls occurred in the U.S. market, involving over 200 tattoo inks marketed by 13 manufacturers and one distributor. Fourteen recalls were due to microbial contamination, and one recall was due to allergic reaction. As follow-up, a microbiological survey of 28 tattoo inks of new batches from seven manufacturers having products that were previously recalled was conducted. Aerobic plate count (APC) and enrichment culture methods based on the FDA’s Bacteriological Analytical Manual (BAM) were used to detect microbial contamination. The results revealed that six out of 28 tattoo inks were contaminated with bacteria and were produced by two manufacturers. The level of microbial contamination was less than 250 CFU/g in three of the tattoo inks and between 1 × 103 and 1 × 105 CFU/g in the other three inks. Eleven bacterial isolates were identified, including spore-forming Bacillus-related species and potentially pathogenic species. Overall, this study shows that some tattoo ink products produced by manufacturers with a recall history continue to be contaminated with microorganisms. This highlights the need for ongoing monitoring and quality control of such products.
1. Introduction
The popularity of tattooing has increased in the United States among adults. Approximately 21 percent of adults in 2012 had at least one tattoo and over 30 percent in 2019 (1, 2). The incidence of tattoo-related complications is also increasing (3–5). Among the various tattoo-related complications reported are microbial infections, and inflammatory or hypersensitivity allergic reactions (6–8). Microbial infections can result from insufficient hygiene practices, such as the use of nonsterile tattoo instruments, or from tattoo inks being or becoming contaminated with pathogenic microorganisms (7, 8). Previously, a series of outbreaks involving Staphylococcus aureus and nontuberculous mycobacteria (NTM), including Mycobacterium chelonae, a causative agent of skin infection, were linked to contaminated tattoo inks (9–14). Studies have reported that 10–86% of marketed tattoo inks are microbially contaminated, including potentially pathogenic microorganisms (15–21).
Recently, updates on the tattoo ink FDA webpage1 revealed that manufacturers conducted 18 voluntary recalls of tattoo inks contaminated with microorganisms between 2003 and 2023 (22). That is, tattoo inks associated with microbial infections are removed from the U.S. market by their manufacturers and distributors. The 2003 recall was the first tattoo ink recall recorded in the U.S. The contaminated tattoo inks associated with the NTM outbreaks in 2012 and 2015 in the U.S. led to nationwide voluntary recalls (12, 13). However, little is known about the current safety of tattoo ink products which are manufactured and/or distributed by facilities that previously underwent voluntary recalls. To our knowledge, no follow-up information is available about the microbial safety of these products.
In this survey, we initially reviewed retrospective recall records of tattoo inks from 2003 to 2021 to retrieve information regarding: how many tattoo ink recalls were issued, reasons for the recalls, including any known illnesses or adverse events associated with recalled products, and by whom and when the problem was initially recognized. Next, we assessed the current microbial contamination in new lots of tattoo inks from facilities that were previously involved in recalls.
2. Materials and methods
2.1. Information retrieval
Information on recalls of tattoo and permanent makeup (PMU) inks was obtained from US FDA website2,” warning letters on recalled tattoo inks, and Enforcement Report database.3 The recall information was reviewed to determine the following: the recall year; the manufacturer, brand and ink names; ink color; manufacturer’s country of origin; recall classification Class (I, II, or III) that identifies the degree of health hazard presented by the product; reason for recall; number of inks involved in each recall; the microbes identified (if applicable); and any additional information obtained by the agency, such as who recognized the problem for which the product was recalled, if available.
2.2. Ink sampling
We purchased 28 tattoo and PMU inks from seven manufacturers whose products had previously been recalled. We purchased 3–6 bottles of each individual ink with the same lot number, confirmed that the bottle packaging was intact and sealed upon arrival, and stored them in a stainless-steel storage cabinet at room temperature. For each bottle of tattoo ink, we recorded the lot number, ingredients, sterility claim(s), manufacturing location, and expiration date, if available, from the product label or material safety data sheet.
2.3. Microbiological analysis
Tattoo inks were analyzed for bacterial and fungal contamination based on the analytical methods described in the FDA’s Bacteriological Analytical Manual (BAM) chapter 23, “Methods for Cosmetics” (23), and BAM chapters 3, “Aerobic Plate Count,” was used for the enumeration of aerobic plate counts (24). Briefly, modified Letheen agar (MLA) and potato dextrose agar (PDA) with chlortetracycline (40 μg mL−1) were used for the detection of bacteria and fungi, respectively. Ink samples (1 gram) were serially diluted using modified Letheen broth (MLB) up to 10−3. One mL of 10−1 dilution was plated on 2 MLA plates (500 μL each) and 100 μL (×2) of each 10−1, 10−2, and 10−3 dilutions were additionally plated on MLA plates. Diluted samples were also enriched for up to 7 days and then streaked (~5 μL) on MLA plates to detect the presence of microorganisms according to BAM Chapter 23. For quality controls, plates and culture media, spiking with and without test microorganisms including Staphylococcus aureus (ATCC 25923), Candida albicans (ATCC 10231), Pseudomonas aeruginosa (ATCC 27853), and Klebsiella pneumoniae (ATCC 13883), were analyzed.
2.4. Identification of bacterial isolates
Isolates from the original MLA plates were sub-cultured before identity testing via the automated micro-identification system, VITEK 2 Compact System (BioMerieux, Inc., Durham, NC), with GN, GP, and BCL colorimetric cards. These cards identify Gram negatives, Gram positives, and Bacillus species, respectively. The inoculum suspension was prepared in 0.45% saline, giving the equivalent of a 0.5 McFarland turbidity. The respective VITEK test cards were filled with the cell suspension according to the manufacturer’s instruction. Sequencing of 16S rRNA genes was also used to identify bacteria using standard methods (25). Briefly, we used a colony PCR amplification with the 16S rRNA gene primers 27f and 1492r (25). PCR products were purified with ExoSAP-IT (USB Corporation, Cleveland, OH), as recommended by the manufacturer. DNA sequences were determined at the sequencing core at the University of Arkansas for Medical Sciences in Little Rock, AR,4 and sequence search and comparison was performed using NCBI BLAST.
3. Results
3.1. U.S. recall records of tattoo inks
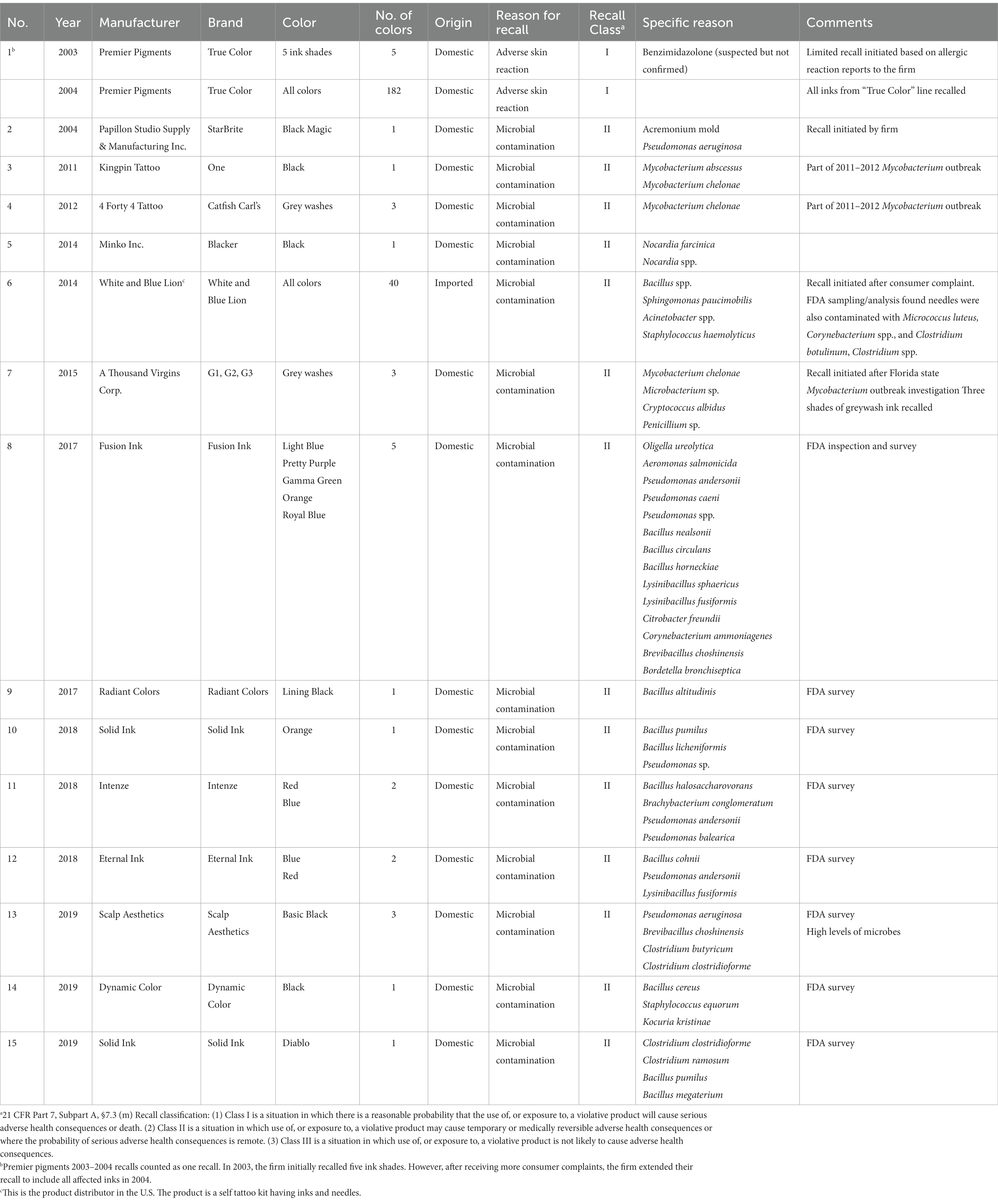
A total of 15 voluntary tattoo ink recalls involving 13 manufacturers and one distributor were tracked from 2003 through 2021 (Table 1). One manufacturer, Solid Ink, had two recalls, in 2018 and 2019, respectively. In 2003, Premier Pigments initiated a voluntary recall of five ink colors used in PMU; however, the firm extended its recall to include all affected inks in that line in 2004 due to continuing adverse events submitted to the firm. Premier Pigments products were the PMU inks among recalled inks; all others were tattoo inks. Recalls were not concentrated on any particular ink color, but outbreaks before 2017 were frequently associated with black or gray inks. While in seven instances only a single ink color was recalled, in the other eight instances, multiple ink colors up to 182 inks were recalled. There was no information available on the actual number of ink bottles included in each recall from the U.S. market. Only one recall was conducted for an imported tattoo kit, which included inks and needles (White and Blue Lion in 2014) and testing results showed that both inks and needles were contaminated with multiple microorganisms.
3.1.1. Reasons for recalls
The FDA records showed that six of the seven recalls that occurred prior to 2015 were triggered by microbial infection or adverse skin reactions, reported by consumers to manufacturers or regulatory authorities (i.e., states and FDA). Since then, recalls were initiated as a result of FDA’s surveillance and inspection programs (eight recalls) find microorganism contaminated inks available on the U.S. market. This effort led to voluntary recalls by the firm. Most voluntary recalls were due to microbial contamination but the recall of ink products from Premier Pigments was initiated due to allergic reactions reported to the firm. Among recalls associated with microbial contamination, three recalls involved outbreaks of NTM skin infections in multiple states, including New York, Washington, Iowa, Colorado, and Florida, during 2011–2012 and 2015. All but one recall was assessed as Class II, indicating that products may cause temporary or reversible adverse health consequences or where the risk of serious harm is remote. One recall (Premier Pigments ink products) was assessed as Class I, the most serious class with a reasonable probability of causing serious adverse health outcomes or death (26). The recall was initiated due to reports of adverse events related to the ink products, which caused swelling, cracking, peeling, blistering, scarring, and granuloma formation. In some instances, the adverse reactions resulted in serious disfigurement, leading to difficulties with eating and speaking (27).
3.1.2. Microorganisms detected from the recalled inks
The 15 recalls identified 51 microorganisms, including 48 bacteria and three fungi (Table 1). The bacterial isolates belonged to 18 genera and 33 species. The genus Bacillus was the most prevalent (12 isolates, 24%). When the genera Brevibacillus (2 isolates) and Lysinibacillus (2 isolates) were included, spore-forming bacillus-related isolates accounted for nearly 30% of the total (16 isolates). The next two most common isolates belonged to the genera Pseudomonas (9 isolates, 18%) and Mycobacterium (4 isolates, 8%). The fungi were identified as Acremonium, Cryptococcus, and Penicillium.
3.2. Microbiological survey of tattoo inks
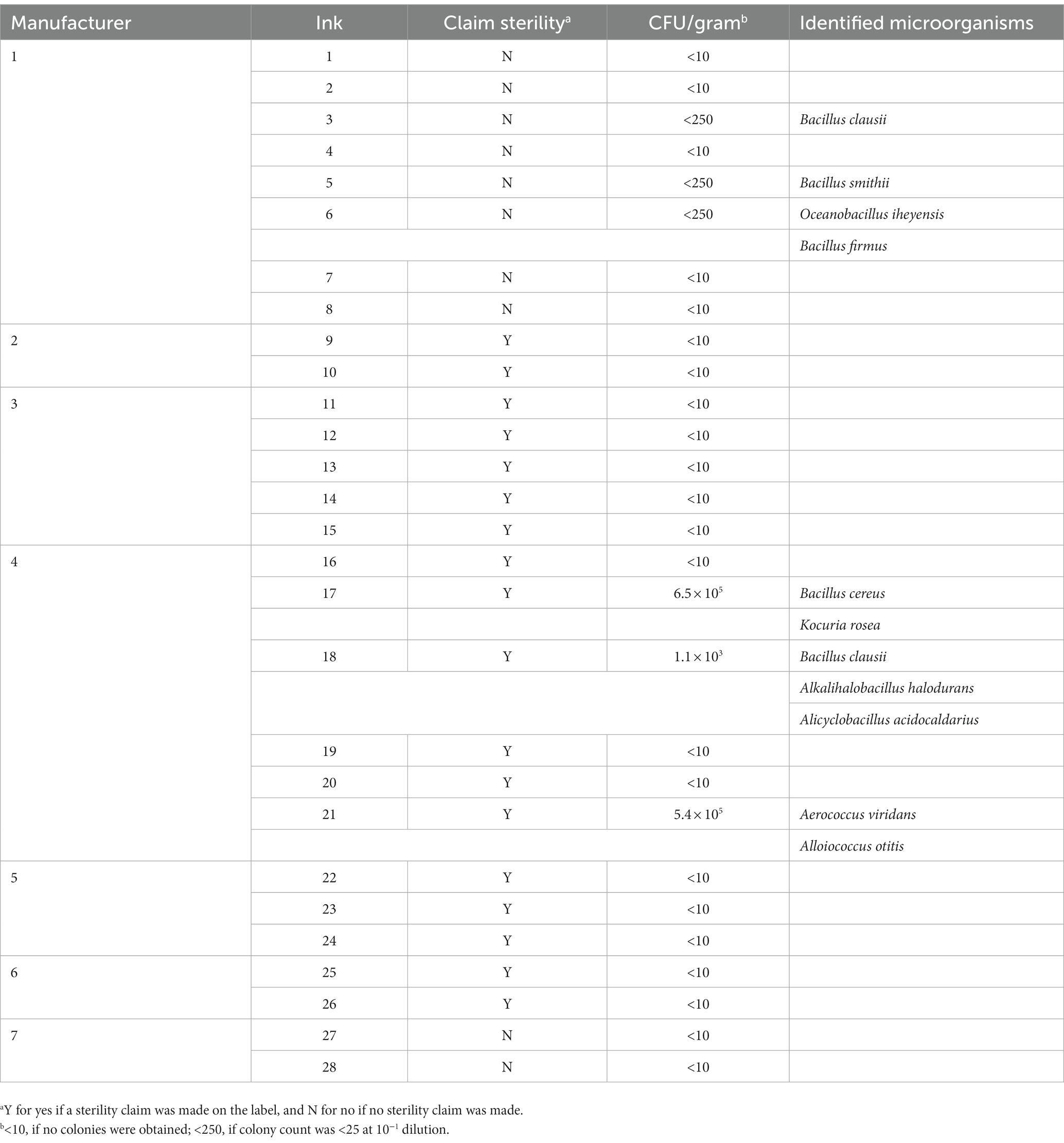
We evaluated microbial contamination in 28 sealed tattoo and PMU inks from seven manufacturers whose products had previously been recalled (Table 2). Tattoo ink product labels from five manufacturers (#2, #3, #4, #5, and #6) claimed the products were sterile; whereas sterility claims for the inks from manufacturers #1 and #7 were not available. Overall APC and enrichment culture analysis revealed that six inks (21%) showed microbial contamination: three inks each from manufacturer #1 and manufacturer #4 (Table 2). No microbial contamination was detected in the tested ink products from the other five manufacturers. Total microbial counts were below 250 CFU per gram in three ink samples and higher than 1 × 103 CFU per gram in another three ink samples, with the highest count being 2.5 × 106 CFU per gram. Eleven bacterial species, including 5 species belonging to Bacillus, were identified. Possibly pathogenic bacteria, such as Kocuria rosea, Aerococcus viridans, and Alloiococcus otitis, were identified (Table 2). No fungi were detected.
4. Discussion
In recent years, the popularity of tattoos has increased, resulting in a corresponding increase in tattoo-related complications. According to a 2015 online poll, 29% of adults in the U.S. reported having a tattoo. This marked a substantial increase from 21% in 2012, 16% in 2008, and 14% in 2003 (28). Along with this trend, the U.S. FDA has observed an increase in the number of tattoo ink recalls between 2003 and 2023. In total, 18 voluntary recalls have been recorded in the U.S. market ((22); a), commencing with the initial recalls in 2003 and 2004, prompted by adverse events reported by consumers (29). Of note, in the time frame of interest in this survey, 2003–2021, there were 15 voluntary recalls. More than half of these (8 recalls) occurring from 2017 to 2021 were prompted by the FDA’s surveillance programs, that included sampling and microbiological analysis of tattoo inks, and were followed by voluntary recalls by manufacturers. The results of this surveillance efforts highlight the ongoing efforts to address the safety and quality of tattoo ink products and the importance of this activity to reduce the risk to consumers (30).
The recall records show that there are two main reasons for recall: microbial contamination and allergic reactions. In 14 out of the 15 recall cases, the main reason was associated with microbial contamination of tattoo ink. One recall was related to allergic reactions, but the causative agent was not confirmed.
The recall records provide taxonomical identification of microbial contaminants found in tattoo inks which can help identify potential sources of contamination and improve surveillance and preventive programs. The recall records have shown that Bacillus species and other spore-forming bacilli are the most commonly identified groups of microbial contaminants. This is consistent with the findings of previous microbial surveys of tattoo inks (15–21).
As shown in this study, 6 out of 28 ink samples, from two of the seven manufacturers’ inks samples demonstrated microbial contamination. These results indicate that despite previous surveys and publications reporting this problem since 2004 (31), microbial contamination continues to be an issue. These results also emphasize the importance of follow-up monitoring of the manufacturers of tattoo inks.
In conclusion, we analyzed tattoo ink recall records spanning almost 20 years. Microbial contamination was the leading cause of voluntary tattoo ink recalls in the U.S. According to our microbiological survey results, some tattoo ink products from manufactures with a previous recall history were still contaminated with microorganism. These findings emphasize the importance of raising consumer awareness about the public health and safety of tattoo inks, as well as the continued monitoring of tattoo ink manufacturers having previous recalls.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SY: Investigation, Writing – original draft. SK: Investigation, Writing – original draft. MM: Investigation, Resources, Writing – review & editing. M-CH: Writing – review & editing. GP: Writing – review & editing. SF: Writing – review & editing. OK: Conceptualization, Supervision, Writing – review & editing. S-JK: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Kidon Sung, Miseon Park, and Linda Katz for critical review of the manuscript. This work was supported in part by an appointment to the Postgraduate Research Fellowship Program at the National Center for Toxicological Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U. S. Department of Energy and the U. S. Food and Drug Administration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://www.fda.gov/cosmetics/cosmetics-news-events/fda-issues-draft-guidance-tattoo-inks
2. ^https://www.fda.gov/cosmetics/cosmetic-products/tattoos-temporary-tattoos-permanent-makeup
3. ^https://www.accessdata.fda.gov/scripts/ires/index.cfm#tabNav_advancedSearch
4. ^http://mbim.uams.edu/research-cores/dna-sequencing-core-facility
References
1. Kluger, N, Seite, S, and Taieb, C. The prevalence of tattooing and motivations in five major countries over the world. J Eur Acad Dermatol Venereol. (2019) 33:e484–6. doi: 10.1111/jdv.15808
2. The Ipsos Poll (2019). More Americans have tattoos today than seven years ago. Available at: https://www.ipsos.com/en-us/news-polls/more-americans-have-tattoos-today (Accessed 2023).
3. Islam, PS, Chang, C, Selmi, C, Generali, E, Huntley, A, Teuber, SS, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. (2016) 50:273–86. doi: 10.1007/s12016-016-8532-0
4. Laux, P, Tralau, T, Tentschert, J, Blume, A, Dahouk, SA, Baumler, W, et al. A medical-toxicological view of tattooing. Lancet. (2016) 387:395–402. doi: 10.1016/S0140-6736(15)60215-X
5. Wenzel, SM, Rittmann, I, Landthaler, M, and Baumler, W. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatol. (2013) 226:138–47. doi: 10.1159/000346943
6. Dieckmann, R, Boone, I, Brockmann, SO, Hammerl, JA, Kolb-Maurer, A, Goebeler, M, et al. The risk of bacterial infection after tattooing. Dtsch Arztebl Int. (2016) 113:665–71. doi: 10.3238/arztebl.2016.0665
7. LeBlanc, PM, Hollinger, KA, and Klontz, KC. Tattoo ink-related infections--awareness, diagnosis, reporting, and prevention. N Engl J Med. (2012) 367:985–7. doi: 10.1056/NEJMp1206063
8. Serup, J, Carlsen, KH, and Sepehri, M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. (2015) 48:48–60. doi: 10.1159/000369645
9. Centers for Disease Control. Methicillin-resistant Staphylococcus aureus skin infections among tattoo recipients-Ohio, Kentucky, and Vermont, 2004–2005. Morb Mortal Wkly Rep. (2006) 55:677–9.
10. Drage, LA, Ecker, PM, Orenstein, R, Phillips, PK, and Edson, RS. An outbreak of Mycobacterium chelonae infections in tattoos. J Am Acad Dermatol. (2010) 62:501–6. doi: 10.1016/j.jaad.2009.03.034
11. Goldman, J, Caron, F, de Quatrebarbes, J, Pestel-Caron, M, Courville, P, Dore, MX, et al. Infections from tattooing. Outbreak of Mycobacterium chelonae in France. BMJ. (2010) 341:c5483. doi: 10.1136/bmj.c5483
12. Griffin, I, Schmitz, A, Oliver, C, Pritchard, S, Zhang, G, Rico, E, et al. Outbreak of tattoo-associated nontuberculous mycobacterial skin infections. Clin Infect Dis. (2019) 69:949–55. doi: 10.1093/cid/ciy979
13. Kennedy, BS, Bedard, B, Younge, M, Tuttle, D, Ammerman, E, Ricci, J, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. N Engl J Med. (2012) 367:1020–4. doi: 10.1056/NEJMoa1205114
14. Kluger, N, Muller, C, and Gral, N. Atypical mycobacteria infection following tattooing: review of an outbreak in 8 patients in a French tattoo parlor. Arch Dermatol. (2008) 144:941–2. doi: 10.1001/archderm.144.7.941
15. Baumgartner, A, and Gautsch, S. Hygienic-microbiological quality of tattoo- and permanent make-up colours. J Verbrauch Lebensm. (2011) 6:319–25. doi: 10.1007/s00003-010-0636-5
16. Bonadonna, L. Survey of studies on microbial contamination of marketed tattoo inks. Curr Probl Dermatol. (2015) 48:190–5. doi: 10.1159/000369226
17. Charnock, C. Tattooing dyes and pigments contaminated with bacteria. Tidsskr Nor Laegeforen. (2004) 124:933–5.
18. Hogsberg, T, Saunte, DM, Frimodt-Moller, N, and Serup, J. Microbial status and product labelling of 58 original tattoo inks. J Eur Acad Dermatol Venereol. (2013) 27:73–80. doi: 10.1111/j.1468-3083.2011.04359.x
19. Nho, SW, Kim, M, Kweon, O, Kim, SJ, Moon, MS, Periz, G, et al. Microbial contamination of tattoo and permanent makeup inks marketed in the US: a follow-up study. Lett Appl Microbiol. (2020) 71:351–8. doi: 10.1111/lam.13353
20. Nho, SW, Kim, SJ, Kweon, O, Howard, PC, Moon, MS, Sadrieh, NK, et al. Microbiological survey of commercial tattoo and permanent makeup inks available in the United States. J Appl Microbiol. (2018) 124:1294–302. doi: 10.1111/jam.13713
21. Yoon, S, Kondakala, S, Nho, SW, Moon, MS, Huang, MCJ, Periz, G, et al. Microbiological survey of 47 permanent makeup inks available in the United States. Microorganisms. (2022) 10:820. doi: 10.3390/microorganisms10040820
22. U.S. Food and Drug Administration (2023b). FDA issues draft guidance on tattoo inks. Available at: https://www.fda.gov/cosmetics/cosmetics-news-events/fda-issues-draft-guidance-tattoo-inks (Accessed 2023).
23. Huang, J, Hitchins, AD, Tran, TT, and McCarron, JE. Bacteriological analytical manual (BAM) chapter 23: methods for cosmetics. Silver Spring, MD, USA: U.S. Food and Drug Administration (2017).
24. Maturin, L, and Peeler, J. Bacteriological analytical manual (BAM) chapter 23: aerobic plate count. 8th ed. Silver Spring, MD, USA: U.S. Food and Drug Administration (1998).
25. Weisburg, WG, Barns, SM, Pelletier, DA, and Lane, DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. (1991) 173:697–703. doi: 10.1128/jb.173.2.697-703.1991
26. U.S. Food and Drug Administration, H. (2000). 21 CFR part 7 – Enforcement Policy, Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.), (ed.) U.S. Food and Drug Administration. Washington, DC: U.S. Government Printing Office
27. Klontz, KC, Lambert, LA, Jewell, RE, and Katz, LM. Adverse effects of cosmetic tattooing: an illustrative case of granulomatous dermatitis following the application of permanent makeup. Arch Dermatol. (2005) 141:918–9. doi: 10.1001/archderm.141.7.918
28. Shannon-Missal, L. Tattoo takeover: three in ten Americans have tattoos, and most don’t stop at just one. Harris Poll. (2016) 12:1–15.
29. U.S. Food and Drug Administration (2004). FDA alerts consumers about adverse events. Associated with permanent makeup FDA talk paper T04-20. Available at: http://www.fda.gov/bbs/topics/answers/2004/ANS01295.html (Accessed 2023).
30. U.S. Food and Drug Administration (2017). Think before you ink: are tattoos safe? Available at: https://www.fda.gov/consumers/consumer-updates/think-you-ink-are-tattoos-safe (Accessed 2023).
31. U.S. Food and Drug Administration (2023a). Enforcement report. Available at: https://www.accessdata.fda.gov/scripts/ires/index.cfm#tabNav_advancedSearch (Accessed 2023).
Keywords: tattoo ink recall, microbial contamination, microbiological survey, bacterial identification, US market
Citation: Yoon S, Kondakala S, Moon MS, Huang M-CJ, Periz G, Foley SL, Kweon O and Kim S-J (2023) Recalls of tattoo and permanent makeup inks in the United States and a follow-up microbiological survey of inks with a previous recall history. Front. Public Health. 11:1279884. doi: 10.3389/fpubh.2023.1279884
Edited by:
Paola Minghetti, University of Milan, ItalyReviewed by:
Jean L. Schoeni, Eurofins Microbiology Laboratory, United StatesSara Manellari, University of Milan, Italy
Copyright © 2023 Yoon, Kondakala, Moon, Huang, Periz, Foley, Kweon and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ohgew Kweon, b2gtZ2V3Lmt3ZW9uQGZkYS5oaHMuZ292; Seong-Jae Kim, c2VvbmctamFlLmtpbUBmZGEuaGhzLmdvdg==
†These authors share first authorship
 Sunghyun Yoon
Sunghyun Yoon Sandeep Kondakala
Sandeep Kondakala Mi Sun Moon2
Mi Sun Moon2 Steven L. Foley
Steven L. Foley Ohgew Kweon
Ohgew Kweon Seong-Jae Kim
Seong-Jae Kim