- 1Department of Cardiological, Thoracic, Vascular Sciences and Public Health, University of Padua, Padua, Italy
- 2Veneto Tumour Registry, Azienda Zero, Padua, Italy
- 3Soft-Tissue, Peritoneum, and Melanoma Surgical Oncology Unit, Veneto Institute of Oncology IOV – IRCCS, Padua, Italy
- 4Department of Surgery, Oncology, and Gastroenterology – DISCOG, University of Padova, Padua, Italy
- 5Veneto Institute of Oncology IOV – IRCCS, Padua, Italy
- 6Pathology and Cytopathology Unit, Department of Medicine-DIMED, University of Padua, Padua, Italy
Background: This observational study considers the sex-specific incidence of the most incident cancers as recorded in the population-based Veneto Regional Cancer Registry over a period of more than 30 years (1987-2019).
Methods: The Veneto Regional Cancer Registry collected data for the time interval 1987–2019. Significant changes in incidence trends calculated on age-standardized incidence rates (Annual Percent Change—APC) were identified by join point regression analysis.
Results: Overall, the incidence trend for all cancers decreased in males and remained stable in females. In nine cancer sites, the incidence trends showed consistent differences by sex (oral cavity, esophagus, colon rectum and anus, liver, larynx, lung, cutaneous malignant melanoma, bladder, and thyroid gland). Other malignancies did not show significant sex-related differences (stomach, pancreas, biliary tract, kidney/urinary tract, central nervous system, multiple myeloma, non-Hodgkin lymphoma, and leukemia).
Conclusion: In the period 1987–2019, this study revealed sex-related differences in cancer incidence trends. Over time, cancer incidence remained higher in males, with a decreasing epidemiological impact, plausibly resulting from prevention campaigns against environmental cancer risk factors, as tobacco and alcohol. Conversely, a significant decrease was not observed in the incidence trend in females. These findings contribute essential insights for profiling the epidemiological map of cancer in a large Italian population, allowing comparison with other European cancer epidemiology studies and providing updated data supporting sex-related primary and secondary cancer prevention strategies.
1 Introduction
Globally, 18,094,716 million cancer cases were diagnosed in 2020. Among these, the incidence rate for all cancers combined was 19% higher in males (222.0 per 100,000) than in females (186.0 per 100,000) (1). Previous studies have also reported higher cancer incidence rates in males at nearly all ages and in most countries (2–4). In Italy, the combined incidence rate was 16% higher in males (317.5 per 100,000) than in females (274.8 per 100,000) (1). The available Italian data on cancer incidence trends by sex support a significant decrease in males and a stable trend in females (5).
Sex and age play an important role in the epidemiology of cancer but they are not always considered in the cancer management. Few recent, large-scale estimates specifically focus on cancer incidence trends by sex across all cancer site. Many of them use less comprehensive dataset due to the limited number of tumor site (6) or time horizon (7–9) or are limited to a specific age (i.e. childhood, young adults and older adults) (10–12). Systematic assessment of sex-specific incidence trends over a large time horizon can provide important information about sex-specific cancer epidemiology, its temporal variability, and the priorities for gender-tailored preventive interventions.
In the north east of Italy, the Veneto region covers approximately 18,345 km2, with a resident population of over 4.8 million. Mortality, measured by the standardized death rate, is lower than the national average (7.9 vs. 8.2 per 1,000 inhabitants in 2016) (13). The main causes of death are cancer and cardiovascular disease (14).
The Regional healthcare system is based on the fundamental values of universality, free access, freedom of choice, pluralism of supply, and equity (14).
This population-based cohort study focused on sex-related differences in the incidence trends of malignancy by site, during a time span of just over 30 years (1987–2019), in a large northern Italian population.
2 Materials and methods
2.1 Data sources
This retrospective population-based cohort study draws on epidemiological data of cancer incidence recorded by the Veneto Cancer Registry (RTV-Registro Tumori del Veneto) from 1987 to 2019. The RTV was certified by the International Agency for Research on Cancer with excellent quality indicators. In the last publication the percentage of cases microscopically verified was greater than 88% and the percentage of cases with death certificate only was less than 1% (15). Population coverage increased from 1,154,000 inhabitants in 1987 to nearly five million (encompassing the entire regional population) since 2014 (see Supplementary Table S1).
Cancer registration methods have also been updated during the period from the International Classification of Diseases, 9th Revision (ICD-9) to ICD-10, and from ICD-O-2 to ICD-O-3 (16). The temporal trends in cancer incidence (standardized for the European population in 2013) were calculated based on the tumor and sex of the patient.
RTV records all malignancies—considering only invasive cutaneous malignant melanoma (CMM) among the skin malignancies—and non-malignant tumors of the urinary bladder, identified according to ICD-10 (17).
Official population data are made available from Italian National Institute of Statistics (18).
2.2 Statistics
A joinpoint regression analysis was performed to identify any significant changes in the annual trends (using the 2013 European Standard Population) of standardized incidence rates (ASR) for the most frequent cancer sites, stratified by sex (19). For each identified trend, the annual percent change (APC) was calculated by fitting a regression line to the natural logarithm of the rates, using the calendar year as a regression variable. The graph for each tumor model depicts statistically significant changes in trend.
To verify the cancer incidence homogeneity among different areas covered form the Registry in the whole period analyzed we calculated the rate ratio (RR), i.e., the ratio between the ASR of the Region and the ASR of the historical area by sex and sites in the last 5 years analyzed. Historical area (HA) is defined as the population covered by the Registry since 1990, representing 45% of the whole regional population. RR was also used to test differences in ASR between sexes.
The significance level is set to 0.05 for all tests.
The statistical analysis was performed using Joinpoint Regression Program, 4.6.0 version (2018) and SEER*Stat, 8.4.2 version (2023).
3 Results
3.1 Current epidemiological profile
In 2019, the RTV recorded 31,925 incident malignancies [males (M) = 16,725; females (F) = 15,200; Table 1]. In males, the five most frequent cancer sites (prostate, lung/bronchus/trachea, colon/rectum/anus, urinary bladder, and CMM) accounted for 59.5% of all malignancies. In females, breast malignancies accounted for 34% of all incident cancers, followed by the colorectum, lung, corpus uteri, and thyroid (overall frequency = 60%).
Age-standardized incidence rates were significantly higher in males, except for thyroid cancer (p < 0.001).
3.2 Temporal trends in incidence
From 1987 to 2019, the regional population ranged from 4,498,402 to 4,906,000. From its establishment in 1987 until 2013, cancer registration included about half of the population, reaching full regional population coverage from 2014 onwards (see Supplementary Table S1). Supplementary Table S2 shows that the historical registration area yielded incidence estimates for different cancer sites which have proven comparable with those available for the whole population. Only for the Urinary Bladder in women was a significant difference found (p = 0.04). The standardized rate of all incident malignancies in the interval times of 1987–2013 and 2013–2019 was 690.0 vs. 690.7 for males, and 516.5 vs. 512.8 for females, with rate ratios of 1.001 and 0.9929, respectively (both p > 0.05).
During the considered time interval, the cancer incidence trends by site showed significant sex-related differences both in not sex-specific malignancies (Figure 1; Table 2) and sex-linked hormone-dependent cancers (Figure 2A; Table 2).
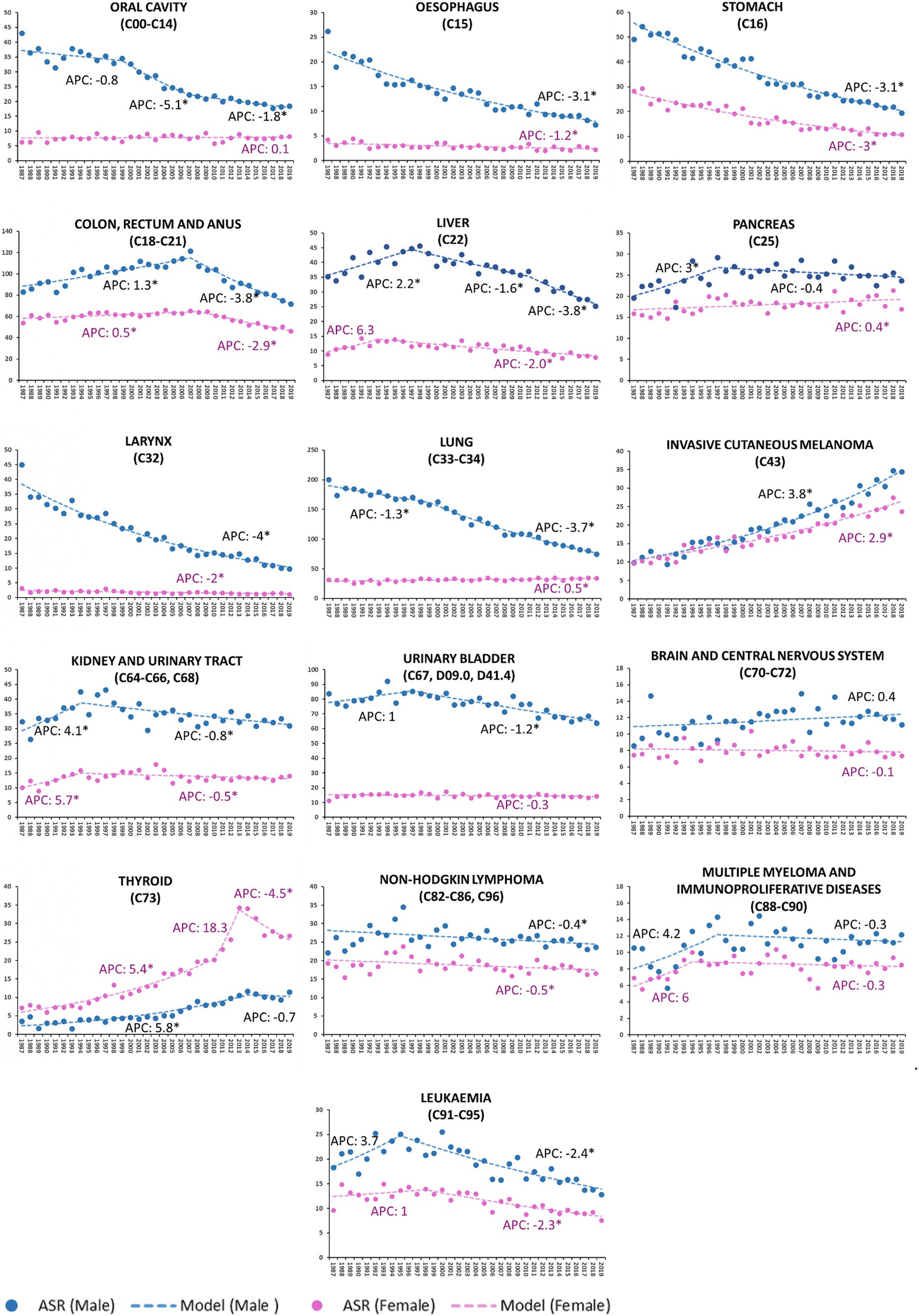
Figure 1. Age-standardized incidence rate (ASR) per 100,000 residents (2013 European standard population) and annual percent change (APC) by tumor site and by sex in Veneto Region, Italy from 1987 to 2019. Cancers are listed according to ICD-10 classification. *Statistically significant value (p < 0.05).
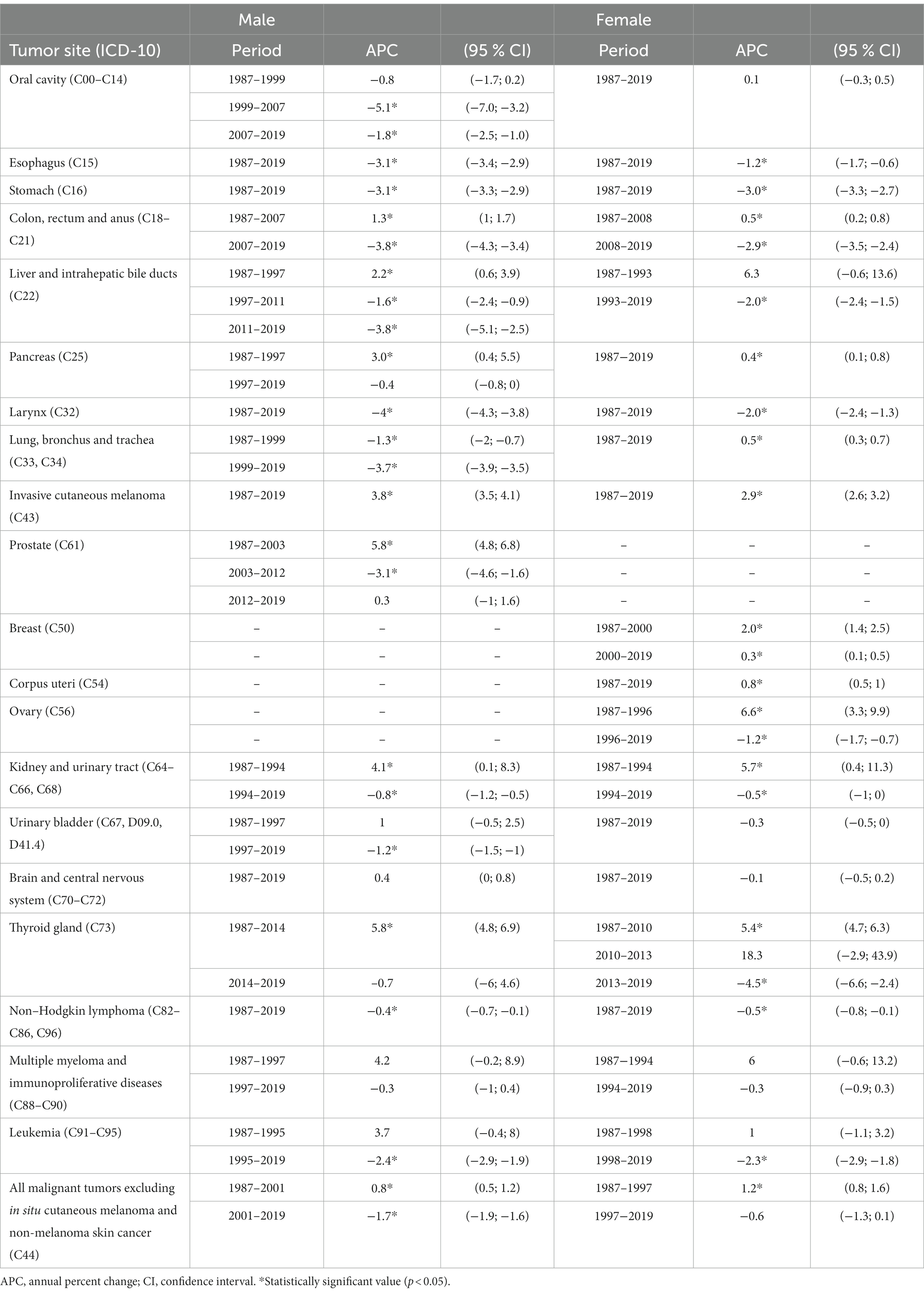
Table 2. Joinpoint regression models of individual cancer diagnoses by tumor site and by sex in Veneto Region, Italy
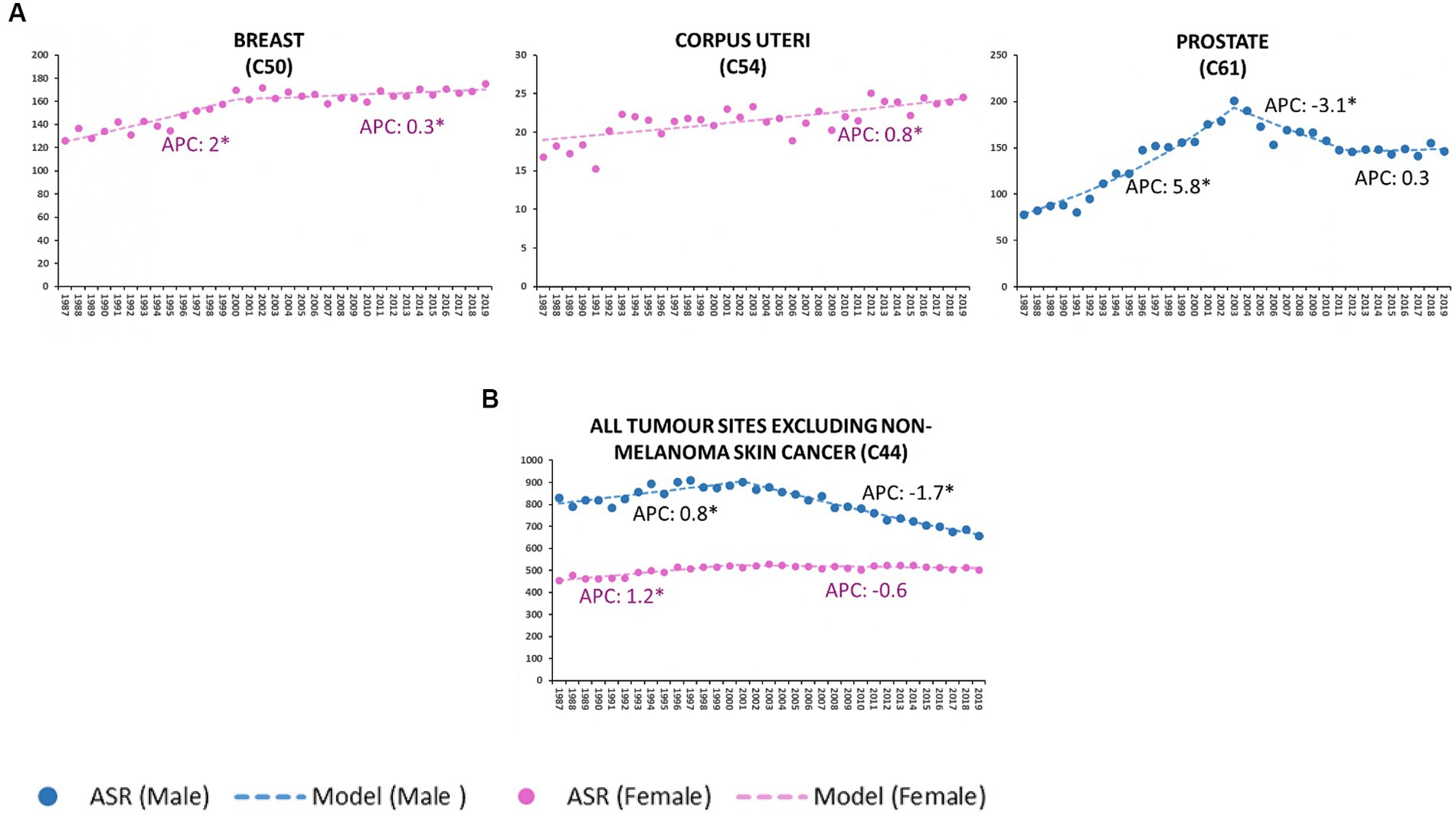
Figure 2. Age-standardized incidence rate (ASR) per 100,000 residents (2013 European standard population) and annual percent change (APC) of breast, corpus uteri, prostate sites (A), all malignant tumors except in situ cutaneous melanoma and non-melanoma skin cancer (C44) (B). Veneto Region, Italy from 1987 to 2019. *Statistically significant value (p < 0.05).
From 2001 onwards, the incidence of all-site malignancies in males declined steadily (M-APC: −1.7, 95%C.I.: −1.9, −1.6); since 1997, no significant changes in incidence have been recorded in females (Figure 2B).
The incidence of oral cavity cancer consistently decreased in males only (M-APC: −1.8, 95%C.I.: −2.5; −1.0 [2007–2019] vs. F-APC: 0.1, 95%C.I.: −0.3; 0.5 [1987–2019]).
From 1987 to 2019, the decline of APC in esophageal cancer was three times higher in males than females (M-APC: −3.1, 95%C.I.: −3.4; −2.9 vs. F-APC: −1.2, 95%C.I.: −1.7; −0.6).
Colorectal and anal cancers decreased more significantly in males (M-APC: −3.8, 95%C.I.: −4.3; −3.4 [2007–2019] vs. F-APC −2.9, 95%C.I. −3.5; −2.4 [2008–2019]).
The incidence of liver malignancies fell more significantly among males (M-APC: −3.8, 95%C.I.: −5.1; −2.5, [2011–2019] vs. F-APC −2.0, 95%C.I.: −2.4; −1.5 [1993–2019]).
Over the time interval 1987–2019, laryngeal cancers consistently displayed a decreasing incidence trend, and the decline of APC was twofold higher in males than in females (M-APC: −4.0, 95%C.I.: −4.3; −3.8 vs. F-APC −2.0, 95%C.I.: −2.4; −1.3).
Lung cancer decreased in males (M-APC: −3.7, 95%C.I.: −3.9; −3.5, [1999–2019]), while a slight, but significant increase was recorded in females (F-APC: 0.5, 95%C.I.: 0.3; 0.7 [1987–2019]).
During the reported time interval (1987–2019), invasive cutaneous melanoma showed a growing trend, particularly in males (M-APC: 3.8, 95%C.I.: 3.5; 4.1 vs. F-APC: 2.9, 95%C.I.: 2.6; 3.2).
Urinary bladder malignancies in males consistently showed a decreasing trend (M-APC: −1.2, 95%C.I.: −1.5; −1.0 [1997–2019]); since 1987, no significant modifications in incidence were recorded in females.
From 1987 to 2014, the incidence of thyroid cancer rose steadily in males (M-APC: 5.8, 95%C.I.: 4.8; 6.9) and subsequently stabilized. Incidence in females showed a two-phase increasing trend up to 2013 (APC: 5.4, 95%C.I.: 4.7; 6.3 [1987–2010]; APC: 18.3, 95%C.I.: −2.9; 43.9 [2010–2013]), followed by a steep decline (APC: −4.5, 95%C.I.: −6.6; −2.4 [2013–2019]).
Between 2000 and 2019, the incidence of breast cancer displayed a slight but steady increase (APC: 0.3, 95%C.I.: 0.1; 0.5), differing substantially from the sharp rise recorded in the period 1987–2000 (APC: 2.0, 95%C.I.: 1.4; 2.5).
Throughout the considered time interval (1987–2019), the incidence of corpus uteri malignancies consistently increased (APC: 0.8, 95%C.I.: 0.5; 1).
Prostate cancer rose significantly between 1987 and 2003 (APC: 5.8, 95%C.I.: 4.8, 6.8), followed by a downturn in the decade 2003–2012 (APC: −3.1, 95C.I.: −4.6, −1.6); no notable changes were observed in the last 10 years.
4 Discussion
This study explores cancer incidence trends by site and sex as recorded in the population-based Veneto Regional Cancer Registry from 1987 to 2019. Overall cancer incidence declined in both sexes, and the more significant decrease observed in men lowered the “historical” gap between males and females (20).
4.1 Divergent incidence trends by sex
As in other high-income countries (21–24), environmental cancers, particularly those related to tobacco exposure, showed a divergent trend of incidence by sex. The incidence of lung cancer decreased significantly in males, while marginally increasing in females. These features are consistent with the decline in tobacco consumption recorded among Italian males since 1970. During the same time interval, the percentage of female smokers steadily rose (25). Moreover, the decreasing incidence in males, as recorded by RTV since the late 1980s, is biologically consistent with the 20–30-year latency of tobacco-related cancer development reported in one other study (26).
Similar opposing sex-related trends were observed for cancers arising from the oral cavity and urinary bladder (27, 28), both sharing the same environmental risk factor(s) (29–31).
4.2 Decreasing incidence trends in both sexes
Many cancer sites showed consistently decreasing trends in both sexes, even at different magnitudes.
Globally, the incidence of thyroid cancer proved considerably higher among females. In 2020, the age-standardized incidence rates of thyroid malignancies were 10.1 and 3.1 per 100,000 among females and males, respectively (32). From 1987 to 2014, RTV recorded a steadily increasing incidence in males, followed by a slight decline. From 1987 to 2013, the incidence of female thyroid malignancies rose significantly, followed by a dramatic sixfold decrease compared to males. Similar findings were reported in other studies (33, 34), being critically interpreted to stem from newly established (more stringent) diagnostic criteria rather than from changes in clinical-biological risk factors (35–39). The proportion of thyroid cancer due to overdiagnosis between 2008 and 2012 was in fact estimated to be higher than 70% in Italy (40, 41) and also relevant globally (40). As a consequence, the guidelines do not recommend screening for thyroid cancer.
Alcohol is a well-recognized risk factor for esophageal and liver cancers. In Italy, between 1970 and 2014, the average alcohol consumption per capita (liters of ethanol per person per year) dropped from 14 to slightly over 6 L (42); the prevalence of drinking was significantly lower in women compared to men [2016 estimates M = 81% vs. F = 54% (43)]. Interestingly, the decline in alcohol consumption in both sexes correlated with the falling trend observed for esophageal and laryngeal malignancies (44, 45). It is therefore conceivable that the decline in the incidence rate recorded by RTV for esophageal and liver cancers is linked to the decrease in alcohol and tobacco use.
The decreasing trend observed for laryngeal cancer was almost twofold in males compared to females, whose higher smoking rates potentially reduced the benefits associated with lower exposure to tobacco genotoxicity. The observed decrease among women, which seems inconsistent with the reported increase in female smoking, could be explained by a reduction in the fraction of cases attributed to alcohol consumption (46). Alcohol was found to have a multiplicative effect on the risk of developing these cancers when combined with smoking (47–50). Hence, the decrease in alcohol consumption may have lessened this multiplicative effect.
Italy has one of the highest rates of liver cancer in the world, outside of Asia (51). In the addressed population, the burden of liver malignancy was considerably higher among males. The increasing incidence recorded in the decade 1987–1997, was followed by a continuing decline from the late 1990s. The lowering incidence rate was clearly more significant in males. Environmental infectious (i.e., viruses) and chemical/hepatotoxic factors (alcohol in particular) are the main etiological agents of cirrhosis and ultimately of its progression to cancer (i.e., hepatocarcinoma). Intrahepatic cholangiocarcinoma (by far less incident) and hepatocarcinoma are recorded together by RTV (i.e., C22). Routine screening in blood transfusions and the advent of the anti-hepatitis B virus (HBV) vaccination in 1980 (52) conceivably played a crucial role in reducing the risk of infectious cirrhosis (HBV, HCV), ultimately lowering liver cancer incidence. As for the etiological role of alcohol intake, Veneto has one of the three regional populations with the highest alcohol intake per capita in Italy (53). However, national data point to a decline in alcohol consumption (70% lower) from 1970 to 2010 (54), to which the declining trend in liver cancer incidence during the same time period could be attributed.
Since 2007, both sexes showed a decreasing incidence of colorectal (CRC) and anal malignancies, with a slightly earlier, more pronounced downward trend in males. In the early 2000s, the regional public health system established a free-access project for secondary prevention of CRC, consisting of a two-step procedure combining a fecal immunochemical test (FIT) with a second-step colonoscopy for FIT-positive patients. The reduction in CRC risk resulting from endoscopic removal of adenomas may explain the declining trend observed from 2007 onwards. Notably, the decrease in the incidence of CRC observed in the current study started earlier in males than females and was also more evident, apparently contrasting with higher compliance with screening among women in our region (55). Underpinning this unanticipated epidemiological evidence are several biological and clinical reasons, which should be addressed by cancer screening guidelines (56–61).
4.3 Increasing incidence trends in both sexes
Several cancer sites showed increasing incidence trends of varying magnitudes in both sexes. Cutaneous malignant (invasive) melanoma (CMM) showed the sharpest increase in the years considered in the study (1987–2019), with males experiencing the greatest annual change. The present findings are consistent with previous Italian and European studies, which reported a steadily increasing incidence in both sexes from 1990 to 2015 (62, 63). The more significant upward trend in males has been interpreted to stem from the potential removal of pre-invasive lesions associated with women’s attitude toward CMM prevention (use of sunscreen and “skin awareness”) and their higher propensity to perform skin self-examination (64–67). Recent evidence, however, supports the hypothesis that host-related biological factors (hormones, immune homeostasis, oxidative stress response, and X-linked genes) may play a sex-dependent “protective” role (68–70).
4.4 Sex-linked hormone-dependent cancers
Hormone-dependent malignancies contribute substantially to the overall incidence trends.
The incidence of breast cancer, accounting for more than one-third of female cancers in 2019, displayed a slight but steady increase from 1987 to 2019. Similar findings were observed in the UK (71), Germany, and other European countries (72) in the same period.
The different Local Health Units of the Veneto Region started breast cancer screening programs between 1999 and 2009. However, according to a survey by the National Institute of Statistics in 1999–2000, 67% of 50–69-year-old women referred that they had undergone at least one mammography in their life, in the absence of signs or symptoms (73). This is in line with the increase in incidence rates observed until 2000. The coverage of the regional female population with mammography after the spread population-based screening programs increased up to values higher than 80% (74).
Corpus uteri cancer, the fourth most common malignancy in women in 2019, showed constant growth over the whole-time interval considered. Similar trends were reported in other high-income countries, due mainly to the increasing incidence of endometrial cancer (75–77). This growing incidence is—at least in part—due to “epidemic” risk factors such as obesity and physical inactivity (78–83).
As in other European countries and worldwide (84), the incidence of prostate cancer rose significantly from 1987 to 2003, followed by a rapid decrease and a plateau, which remained unchanged throughout the 2010s. As seen in thyroid cancer, the marked change in prostate cancer incidence reported in the present study is conceivably the result of modifications in diagnostic criteria and clinical strategies for patient management. The increasing incidence resulting from the widespread spontaneous uptake of prostate-specific antigen (PSA) testing rapidly declined when the diagnostic reliability of PSA testing was clinically reconsidered (84–86).
4.5 All tumor sites
Consistently with long-term trends reported in Europe (5), the USA and Canada (87, 88), the present results show a decreasing trend in incidence for all cancer sites in males; the stable trends in females have consequently narrowed the sex gap in cancer incidence.
4.6 Limitations
Some limitations need to be considered when interpreting the findings of the present study. To start with, the present study focused only on cancer incidence trends in northern Italian region and may not be representative of other regions or on a global scale. However, its population-based design minimizes the risk of selection bias and the use of standardized algorithms reduced measurement variability, hence increasing the reliability of the values. Secondly, error in diagnosis is possible, but probability is very small given the high percentage of microscopically verified cases (88%) and the low percentage of death certificate only cases (<1%). At last, we fixed a significance level of 0.05 for each test, so some results might be statistically significant due to chance.
5 Conclusion
In the Veneto Region, the risk of lung cancer continues to increase in women, similar to other cancers associated to smoking, such as bladder and oral cancer. Greater efforts should be put in place to promote effective interventions to avoid and quit smoking, particularly in women. Prevention activities need to take into account sex differences in the psychological attitude that leads to smoking (for example, women are more likely to use tobacco to cope with negative feelings), in the propensity to quit smoking (e.g., women show more severe withdrawal symptoms and cravings to smoke, especially in relation to the luteal phase of the menstrual cycle), and in the level of smoking cessation (e.g., pregnancy promotes smoking cessation among women to a greater extent than becoming a parent does among men) (89).
Our data suggest the utmost importance of health promotion strategies for the prevention of melanoma in both sexes, aimed at spreading the knowledge on risk factors (i.e., ultraviolet (UV) radiation) and on protection strategies, including the UV index (90).
Finally, the growing trend of breast cancer suggests promoting both population-based educational interventions, such as increasing physical activity and reducing BMI and alcohol intake, and an approach with systematic targets or precision prevention(91).
The marked decline in the incidence of several cancer sites (e.g., esophagus, stomach, liver, larynx, oral cavity, lung in males, bladder, and leukemia) plausibly stems from the lessening impact of environmental risk factors. The secondary prevention strategy adopted for CRC has shown its well-recognized beneficial effect. In a minority of cancer sites (e.g., thyroid, prostate), the downturn in trend has been due mostly to changes in diagnostic criteria.
The findings of the present study contribute to understanding the background to different trends in cancer incidence by sex, and prompt the promotion of high-resolution cancer registration. Combining incidence and mortality rates with high-resolution patient profiles has the potential to crucially inform sex-tailored strategies for primary and secondary cancer prevention.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Figshare (https://figshare.com/s/2b061e3d3825a3556154).
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
AB: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. GL: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. MZ: Data curation, Formal analysis, Writing – review & editing. EB: Data curation, Formal analysis, Writing – review & editing. SM: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. CT: Conceptualization, Methodology, Visualization, Writing – review & editing. VB: Conceptualization, Methodology, Supervision, Writing – review & editing. SG: Data curation, Formal analysis, Writing – review & editing. MR: Data curation, Formal analysis, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research has received “Current Research” funds from the Italian Ministry of Health to cover publication costs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1267534/full#supplementary-material
References
1. Sung, H , Ferlay, J , Siegel, RL , Laversanne, M , Soerjomataram, I , Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kesteloot, H . On the sex ratio of all-cause and disease-specific mortality rates worldwide. Verh K Acad Geneeskd Belg. (2007) 69:105–30.
3. Benigni, R . Social sexual inequality and sex difference in cancer incidence. Environ Res. (2007) 104:128–34. doi: 10.1016/j.envres.2006.09.007
4. Arbeev, KG , Ukraintseva, SV , Arbeeva, LS , and Yashin, AI . Difference between male and female cancer incidence rates: how can it be explained? In: M Nikulin, D Commenges, and C Huber, editors. Probability, statistics and modelling in public health. Boston, MA: Springer (2006). 12–22.
5. Buzzoni, C , Crocetti, E , Guzzinati, S , Dal Maso, L , and Francisci, S . Cancer incidence and mortality trends from 2003 to 2014 in Italy. Tumori. (2019) 105:121–37. doi: 10.1177/0300891619839844
6. Wanner, M , Matthes, KL , Karavasiloglou, N , Limam, M , Korol, D , and Rohrmann, S . 37-year incidence and mortality time trends of common cancer types by sex, age, and stage in the canton of Zurich. Swiss Med Wkly. (2020) 150:w20388. doi: 10.4414/smw.2020.20388
7. Dong, M , Cioffi, G , Wang, J , Waite, KA , Ostrom, QT , Kruchko, C, et al. Sex differences in cancer incidence and survival: a pan-cancer analysis. Cancer Epidemiol Biomarkers Prev. (2020) 29:1389–97. doi: 10.1158/1055-9965.EPI-20-0036
8. Arias-Ortiz, N , and Rodríguez-Betancourt, JD . Trends in cancer incidence and mortality in Manizales, Colombia, 2008-2017. Colomb Med. (2022) 53:e2044920. doi: 10.25100/cm.v53i1.4920
9. Koon Sun Pat, M , Manraj, M , Fauzee, J , Sewsurn, S , Parkin, DM , and Manraj, S . Trends in cancer incidence in the Republic of Mauritius, 1991-2015. Cancer Epidemiol. (2019) 63:101616. doi: 10.1016/j.canep.2019.101616
10. Liu, Z , Yang, Q , Cai, N , Jin, L , Zhang, T , and Chen, X . Enigmatic differences by sex in cancer incidence: evidence from childhood cancers. Am J Epidemiol. (2019) 188:1130–5. doi: 10.1093/aje/kwz058
11. Ward, EM , Sherman, RL , Henley, SJ , Jemal, A , Siegel, DA , Feuer, EJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20-49 years. J Natl Cancer Inst. (2019) 111:1279–97. doi: 10.1093/jnci/djz106
12. Pilleron, S , Alqurini, N , Ferlay, J , Haase, KR , Hannan, M , Janssen-Heijnen, M, et al. International trends in cancer incidence in middle-aged and older adults in 44 countries. J Geriatr Oncol. (2022) 13:346–55. doi: 10.1016/j.jgo.2021.11.011
13. ISTAT . Tavole di mortalità della popolazione residente - Serie storica per ripartizione/regione/provincia. Available at: http://demo.istat.it/tvm2016/index.php?lingua=ita (Accessed December 13, 2022).
14. Toniolo, F , Mantoan, D , and Maresso, A . Veneto Region, Italy. Health system review. Health Syst Transit. (2012) 14:i–xix,:1–138.
15. All sites except non–melanoma skin cancer (C00–96/C44) Data quality table. Cancer Incidence in Five Continents, Vol. XII. Available at: https://gco.iarc.fr/media/ci5/data/vol12/Indices/C00-96%20excl%20C44.pdf
16. Guzzinati, S , Battagello, J , Bovo, E , Baracco, M , Baracco, S , Carpin, E, et al. Quality control on digital cancer registration. PLoS One. (2022) 17:e0279415. doi: 10.1371/journal.pone.0279415
17. World Health Organization . International statistical classification of diseases and related health problems World Health Organization (2015) Available at: https://apps.who.int/iris/handle/10665/246208.
18. Demo - Demographic statistics. Available at: https://demo.istat.it/?l=en (Accessed November 30, 2023).
19. Joinpoint regression program. Available at: https://surveillance.cancer.gov/joinpoint/ (Accessed July 9, 2021).
20. Cook, MB , Dawsey, SM , Freedman, ND , Inskip, PD , Wichner, SM , Quraishi, SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. (2009) 18:1174–82. doi: 10.1158/1055-9965.EPI-08-1118
21. Gilhodes, J , Belot, A , Bouvier, A-M , Remontet, L , Delafosse, P , Ligier, K, et al. Incidence of major smoking-related cancers: trends among adults aged 20-44 in France from 1982 to 2012. Cancer Epidemiol. (2015) 39:707–13. doi: 10.1016/j.canep.2015.07.001
22. Fidler-Benaoudia, MM , Torre, LA , Bray, F , Ferlay, J , and Jemal, A . Lung cancer incidence in young women vs. young men: a systematic analysis in 40 countries. Int J Cancer. (2020) 147:811–9. doi: 10.1002/ijc.32809
23. Huang, J , Deng, Y , Tin, MS , Lok, V , Ngai, CH , Zhang, L, et al. Distribution, risk factors, and temporal trends for lung cancer incidence and mortality: a global analysis. Chest. (2022) 161:1101–11. doi: 10.1016/j.chest.2021.12.655
24. Chen, X , Mo, S , and Yi, B . The spatiotemporal dynamics of lung cancer: 30-year trends of epidemiology across 204 countries and territories. BMC Public Health. (2022) 22:987. doi: 10.1186/s12889-022-13281-y
25. Trama, A , Boffi, R , Contiero, P , Buzzoni, C , Pacifici, R , Mangone, L, et al. Trends in lung cancer and smoking behavior in Italy: an alarm bell for women. Tumori. (2017) 103:543–50. doi: 10.5301/tj.5000684
26. Weiss, W . Cigarette smoking and lung cancer trends. A light at the end of the tunnel? Chest. (1997) 111:1414–6. doi: 10.1378/chest.111.5.1414
27. Cumberbatch, MG , Rota, M , Catto, JWF , and La Vecchia, C . The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. (2016) 70:458–66. doi: 10.1016/j.eururo.2015.06.042
28. Petti, S . Lifestyle risk factors for oral cancer. Oral Oncol. (2009) 45:340–50. doi: 10.1016/j.oraloncology.2008.05.018
29. Antoni, S , Ferlay, J , Soerjomataram, I , Znaor, A , Jemal, A , and Bray, F . Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. (2017) 71:96–108. doi: 10.1016/j.eururo.2016.06.010
30. Bosetti, C , Carioli, G , Santucci, C , Bertuccio, P , Gallus, S , Garavello, W, et al. Global trends in oral and pharyngeal cancer incidence and mortality. Int J Cancer. (2020) 147:1040–9. doi: 10.1002/ijc.32871
31. Tian, Y-Q , Yang, J-C , Hu, J-J , Ding, R , Ye, D-W , and Shang, J-W . Trends and risk factors of global incidence, mortality, and disability of genitourinary cancers from 1990 to 2019: systematic analysis for the Global Burden of Disease Study 2019. Front Public Health. (2023) 11:1119374. doi: 10.3389/fpubh.2023.1119374
32. Pizzato, M , Li, M , Vignat, J , Laversanne, M , Singh, D , La Vecchia, C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diab Endocrinol. (2022) 10:264–72. doi: 10.1016/S2213-8587(22)00035-3
33. Brito, JP , and Davies, L . Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes. (2014) 21:405–8. doi: 10.1097/MED.0000000000000094
34. Franceschi, S , and Vaccarella, S . Thyroid cancer: an epidemic of disease or an epidemic of diagnosis? Int J Cancer. (2015) 136:2738–9. doi: 10.1002/ijc.29311
35. Kitahara, CM , and Sosa, JA . The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
36. Raposo, L , Morais, S , Oliveira, MJ , Marques, AP , Bento, MJ , and Lunet, N . Trends in thyroid cancer incidence and mortality in Portugal. Eur J Cancer Prevent. (2017) 26:135–43. doi: 10.1097/CEJ.0000000000000229
37. LeClair, K , Bell, KJL , Furuya-Kanamori, L , Doi, SA , Francis, DO , and Davies, L . Evaluation of gender inequity in thyroid cancer diagnosis: differences by sex in US thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Internal Med. (2021) 181:1351–8. doi: 10.1001/jamainternmed.2021.4804
38. Haymart, MR , Banerjee, M , Reyes-Gastelum, D , Caoili, E , and Norton, EC . Thyroid ultrasound and the increase in diagnosis of low-risk thyroid cancer. J Clin Endocrinol Metab. (2019) 104:785–92. doi: 10.1210/jc.2018-01933
39. Kim, BW , Yousman, W , Wong, WX , Cheng, C , and McAninch, EA . Less is more: comparing the 2015 and 2009 american thyroid association guidelines for thyroid nodules and cancer. Thyroid. (2016) 26:759–64. doi: 10.1089/thy.2016.0068
40. Li, M , Dal Maso, L , and Vaccarella, S . Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. (2020) 8:468–70. doi: 10.1016/S2213-8587(20)30115-7
41. Dal Maso, L , Panato, C , Franceschi, S , Serraino, D , Buzzoni, C , Busco, S, et al. The impact of overdiagnosis on thyroid cancer epidemic in Italy,1998-2012. Eur J Cancer. (2018) 94:6–15. doi: 10.1016/j.ejca.2018.01.083
42. Ritchie, H , and Roser, M . Alcohol consumption. Our World in Data (2018). Available at: https://ourworldindata.org/alcohol-consumption (Accessed April 14, 2023).
43. Share of men vs. share of women who drank alcohol in last year. Our World in Data. Available at: https://ourworldindata.org/grapher/males-vs-females-who-drank-alcohol-in-last-year (Accessed April 14, 2023).
44. Pelucchi, C , Gallus, S , Garavello, W , Bosetti, C , and La Vecchia, C . Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Res Health. (2006) 29:193–8.
45. Abnet, CC , Arnold, M , and Wei, W-Q . Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. (2018) 154:360–73. doi: 10.1053/j.gastro.2017.08.023
46. Rumgay, H , Shield, K , Charvat, H , Ferrari, P , Sornpaisarn, B , Obot, I, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. (2021) 22:1071–80. doi: 10.1016/S1470-2045(21)00279-5
47. Thomas, DB . Alcohol as a cause of cancer. Environ Health Perspect. (1995) 103:153–60. doi: 10.1289/ehp.95103s8153
48. Castellsagué, X , Muñoz, N , De Stefani, E , Victora, CG , Castelletto, R , Rolón, PA, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. (1999) 82:657–64. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c
49. Talamini, R , Bosetti, C , La Vecchia, C , Dal Maso, L , Levi, F , Bidoli, E, et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: a case–control study. Cancer Causes Control. (2002) 13:957–64. doi: 10.1023/A:1021944123914
50. Fan, Y , Yuan, J-M , Wang, R , Gao, Y-T , and Yu, MC . Alcohol, tobacco and diet in relation to esophageal cancer: the Shanghai cohort study. Nutr Cancer. (2008) 60:354–63. doi: 10.1080/01635580701883011
51. Petrick, JL , Florio, AA , Znaor, A , Ruggieri, D , Laversanne, M , Alvarez, CS, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. (2020) 147:317–30. doi: 10.1002/ijc.32723
52. Romano, L , and Zanetti, AR . Hepatitis B vaccination: a historical overview with a focus on the Italian achievements. Viruses. (2022) 14:1515. doi: 10.3390/v14071515
53. EpiCentro . Consumo di alcol - Dati di popolazione adulta. Available at: https://www.epicentro.iss.it/passi/dati/alcol (Accessed April 14, 2023).
54. La Vecchia, C , Bosetti, C , Bertuccio, P , Castro, C , Pelucchi, C , and Negri, E . Trends in alcohol consumption in Europe and their impact on major alcohol-related cancers. Eur J Cancer Prev. (2014) 23:319–22. doi: 10.1097/CEJ.0b013e32836562f1
55. Registro Tumori del Veneto . I programmi di screening oncologici del Veneto. Rapporto 2012-2013. Available at: https://www.regione.veneto.it/web/sanita/screening-oncologici1 (Accessed May 10, 2023).
56. Brenner, H , Haug, U , and Hundt, S . Sex differences in performance of fecal occult blood testing. Am J Gastroenterol. (2010) 105:2457–64. doi: 10.1038/ajg.2010.301
57. Chacko, L , Macaron, C , and Burke, CA . Colorectal cancer screening and prevention in women. Dig Dis Sci. (2015) 60:698–710. doi: 10.1007/s10620-014-3452-4
58. Lash, RH , Genta, RM , and Schuler, CM . Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. (2010) 63:681–6. doi: 10.1136/jcp.2010.075507
59. Murakami, T , Sakamoto, N , and Nagahara, A . Clinicopathological features, diagnosis, and treatment of sessile serrated adenoma/polyp with dysplasia/carcinoma. J Gastroenterol Hepatol. (2019) 34:1685–95. doi: 10.1111/jgh.14752
60. Saunders, BP , Fukumoto, M , Halligan, S , Jobling, C , Moussa, ME , Bartram, CI, et al. Why is colonoscopy more difficult in women? Gastrointest Endosc. (1996) 43:124–6. doi: 10.1016/s0016-5107(06)80113-6
61. Kaku, E , Oda, Y , Murakami, Y , Goto, H , Tanaka, T , Hasuda, K, et al. Proportion of flat- and depressed-type and laterally spreading tumor among advanced colorectal neoplasia. Clin Gastroenterol Hepatol. (2011) 9:503–8. doi: 10.1016/j.cgh.2011.03.018
62. Rossi, S , Crocetti, E , Capocaccia, R , and Gatta, G, AIRTUM Working Group . Estimates of cancer burden in Italy. Tumori. (2013) 99:416–24. doi: 10.1700/1334.14807
63. Sacchetto, L , Zanetti, R , Comber, H , Bouchardy, C , Brewster, DH , Broganelli, P, et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer. (2018) 92:108–18. doi: 10.1016/j.ejca.2017.12.024
64. White, LP . Studies on melanoma. N Engl J Med. (1959) 260:789–97. doi: 10.1056/NEJM195904162601601
65. Buja, A , Rugge, M , Damiani, G , Zorzi, M , De Toni, C , Vecchiato, A, et al. Sex differences in cutaneous melanoma: incidence, clinicopathological profile, survival, and costs. J Women Health. (2022) 31:1012–9. doi: 10.1089/jwh.2021.0223
66. Raimondi, S , Suppa, M , and Gandini, S . Melanoma EPIDEMIOLOGY AND SUN EXposure. Acta Derm Venereol. (2020) 100:adv00136. doi: 10.2340/00015555-3491
67. Gamba, CS , Clarke, CA , Keegan, THM , Tao, L , and Swetter, SM . Melanoma survival disadvantage in young, non-hispanic white males compared with females. JAMA Dermatol. (2013) 149:912–20. doi: 10.1001/jamadermatol.2013.4408
68. Cosci, I , Grande, G , Di Nisio, A , Rocca, MS , Del Fiore, P , Benna, C, et al. Cutaneous melanoma and hormones: focus on sex differences and the testis. Int J Mol Sci. (2023) 24:599. doi: 10.3390/ijms24010599
69. Watts, CG , McLoughlin, K , Goumas, C , van Kemenade, CH , Aitken, JF , Soyer, HP, et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. (2021) 157:1425–36. doi: 10.1001/jamadermatol.2021.3884
70. Klein, SL , and Flanagan, KL . Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
71. Lei, S , Zheng, R , Zhang, S , Wang, S , Chen, R , Sun, K, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. (2021) 41:1183–94. doi: 10.1002/cac2.12207
72. Huang, J , Chan, PS , Lok, V , Chen, X , Ding, H , Jin, Y, et al. Global incidence and mortality of breast cancer: a trend analysis. Aging. (2021) 13:5748–803. doi: 10.18632/aging.202502
73. Istituto Nazionale di Statistica . La Prevenzione dei Tumori Femminili in Italia: Il Ricorso a Pap Test e Mammografia (Anni 2004–2005). Rome: Istituto Nazionale di Statistica. (2006).
74. PASSI reportistica - Regione del Veneto. Available at: https://www.regione.veneto.it/web/sanita/passi-reportistica (Accessed November 30, 2023).
75. Li, S , Chen, H , Zhang, T , Li, R , Yin, X , Man, J, et al. Spatiotemporal trends in burden of uterine cancer and its attribution to body mass index in 204 countries and territories from 1990 to 2019. Cancer Med. (2022) 11:2467–81. doi: 10.1002/cam4.4608
76. Gu, B , Shang, X , Yan, M , Li, X , Wang, W , Wang, Q, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol Oncol. (2021) 161:573–80. doi: 10.1016/j.ygyno.2021.01.036
77. Crosbie, EJ , Kitson, SJ , McAlpine, JN , Mukhopadhyay, A , Powell, ME , and Singh, N . Endometrial cancer. Lancet. (2022) 399:1412–28. doi: 10.1016/S0140-6736(22)00323-3
78. Calle, EE , and Thun, MJ . Obesity and cancer. Oncogene. (2004) 23:6365–78. doi: 10.1038/sj.onc.1207751
79. Fader, AN , Arriba, LN , Frasure, HE , and von Gruenigen, VE . Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. (2009) 114:121–7. doi: 10.1016/j.ygyno.2009.03.039
80. Devericks, EN , Carson, MS , McCullough, LE , Coleman, MF , and Hursting, SD . The obesity-breast cancer link: a multidisciplinary perspective. Cancer Metastasis Rev. (2022) 41:607–25. doi: 10.1007/s10555-022-10043-5
81. Tewari, S , Vargas, R , and Reizes, O . The impact of obesity and adipokines on breast and gynecologic malignancies. Ann N Y Acad Sci. (2022) 1518:131–50. doi: 10.1111/nyas.14916
82. Frank, J . Origins of the obesity pandemic can be analysed. Nature. (2016) 532:149–9. doi: 10.1038/532149a
83. Popov, VB , Aytaman, A , and Alemán, JO . Obesity: the forgotten pandemic. Offic J Am Coll Gastroenterol. (2022) 117:7–10. doi: 10.14309/ajg.0000000000001553
84. Culp, MB , Soerjomataram, I , Efstathiou, JA , Bray, F , and Jemal, A . Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. (2020) 77:38–52. doi: 10.1016/j.eururo.2019.08.005
85. Loeb, S , and Catalona, WJ . Prostate-specific antigen in clinical practice. Cancer Let. (2007) 249:30–9. doi: 10.1016/j.canlet.2006.12.022
86. Vickers, AJ . Redesigning prostate cancer screening strategies to reduce overdiagnosis. Clin Chem. (2019) 65:39–41. doi: 10.1373/clinchem.2018.287094
87. Siegel, RL , Miller, KD , Fuchs, HE , and Jemal, A . Cancer statistics, 2022. CA. (2022) 72:7–33. doi: 10.3322/caac.21708
88. Brenner, DR , Poirier, A , Woods, RR , Ellison, LF , Billette, J-M , Demers, AA, et al. Projected estimates of cancer in Canada in 2022. CMAJ. (2022) 194:E601–7. doi: 10.1503/cmaj.212097
89. Baldacci, S , Maio, S , Meschi, C , Chimera, D , Tagliaferro, S , Angino, A, et al. A narrative review of epidemiology and prevention of lung cancer: sex/gender differences? Precis Cancer Med. (2021) 5. doi: 10.21037/pcm-21-54
90. Heckman, CJ , Liang, K , and Riley, M . Awareness, understanding, use, and impact of the UV index: a systematic review of over two decades of international research. Prev Med. (2019) 123:71–83. doi: 10.1016/j.ypmed.2019.03.004
Keywords: epidemiology, cancer, sex characteristics, observational study, cohort studies, incidence, registry
Citation: Buja A, De Luca G, Zorzi M, Bovo E, Mocellin S, Trevisiol C, Bronte V, Guzzinati S and Rugge M (2024) Thirty-two-year trends of cancer incidence by sex and cancer site in the Veneto Region from 1987 to 2019. Front. Public Health. 11:1267534. doi: 10.3389/fpubh.2023.1267534
Edited by:
Ingmar Schäfer, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Annalisa Quattrocchi, University of Nicosia, CyprusYufei Liu, Shenzhen Second People's Hospital, China
Copyright © 2024 Buja, De Luca, Zorzi, Bovo, Mocellin, Trevisiol, Bronte, Guzzinati and Rugge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandra Buja, YWxlc3NhbmRyYS5idWphQHVuaXBkLml0
 Alessandra Buja
Alessandra Buja Giuseppe De Luca
Giuseppe De Luca Manuel Zorzi
Manuel Zorzi Emanuela Bovo
Emanuela Bovo Simone Mocellin3,4
Simone Mocellin3,4 Chiara Trevisiol
Chiara Trevisiol Stefano Guzzinati
Stefano Guzzinati Massimo Rugge
Massimo Rugge