
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 20 September 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1251440
Purpose: This study aims to address the existing data gap regarding the status of high-risk human papillomavirus (HR-HPV) infection and the distribution of HR-HPV subtypes among women in rural areas of Nyingchi City, Tibet. The research objectives include providing insights for HPV vaccine development.
Methods: The research collected data from two rounds of cancer screening conducted among rural women in Nyingchi City, Tibet, from 2019 to 2022. HR-HPV subtype gene detection was performed using the PCR fluorescence method on the collected samples. And then analyzed the HR-HPV infection rate among rural women in Nyingchi City, Tibet, as well as the infection rate of different HR-HPV subtypes and their distribution across different age groups. A comparison was made between the infection rates of women in rural areas outside the Qinghai-Tibet Plateau and those in Nyingchi City.
Results: A total of 15,687 cases included. The overall HR-HPV infection rate among women in rural areas of Nyingchi City, Tibet, was 13.00% (2040/15,687), which was significantly higher than the rate among women in rural areas outside the Qinghai-Tibet Plateau (7.82% (9,249/118,237); χ2 = 635.7, p < 0.001). The highest HPV infection rate was observed in the 35–39 age group, with a rate of 15.31% (499/3260), which was significantly higher than the rate of 7.22% (1827/25,322) among women in the same age group in rural areas outside the Qinghai-Tibet Plateau (χ2 = 253.00, p < 0.001). The lowest HPV infection rate was found in the 50–54 age group, with a rate of 9.69% (246/2540), which was statistically different from the rate of 8.14% (1,604/19,698) among women in the same age group outside the Qinghai-Tibet Plateau (χ2 = 17.68, p < 0.001). The top three HR-HPV subtypes among women in rural areas of Nyingchi City, Tibet, were HPV52 (20.15%, 411/2040), HPV16 (12.45%, 254/2040), and HPV58 (11.96%, 244/2040). These findings align with the top three HR-HPV subtypes among women in rural areas outside the Qinghai-Tibet Plateau. Furthermore, the top three HR-HPV subtypes among women aged 35–39, 40–44, and 45–49 in rural areas of Nyingchi City, Tibet, were HPV52, HPV16, and HPV58. In conclusion, the HR-HPV infection rate among women in rural areas of Nyingchi City, Tibet, is significantly higher compared to women in rural areas outside the Qinghai-Tibet Plateau, with consistent patterns observed in the distribution of the top three HR-HPV subtypes between the two regions.
In 2018, cervical cancer accounted for approximately 570,000 new cases worldwide, resulting in over 310,000 deaths (1, 2). A comprehensive epidemiological study conducted in 1999 by the International Agency for Research on Cancer involving 22 countries revealed a close association between human papillomavirus (HPV) and 99.7% of cervical cancer cases (3). Persistent infection with high-risk human papillomavirus (HR-HPV) subtypes is the primary cause of cervical precancerous lesions and cervical cancer (4). Notably, 13 HR-HPV subtypes, including HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, have been identified as predominantly responsible for cervical cancer (5).
Research on HR-HPV subtype infections among different regions and races in China has been limited to specific provinces or cities in the eastern and central regions, with significant variations observed in the distribution of HR-HPV subtypes across different regions (6–11). Previous studies have reported an overall HR-HPV infection rate of 9.19% among women residing in urban areas of Lhasa, Shigatse, and Nagqu in Tibet, and the most prevalent HR-HPV subtypes include HPV 16, HPV 33, HPV 58, HPV 52, and HPV 31 (12). However, precise data regarding HR-HPV infection among women in rural areas of the Qinghai-Tibet Plateau, including epidemiological data on HR-HPV subtypes, remain lacking.
In addition to the well-established correlation between HR-HPV strains and cervical abnormalities or cervical carcinoma, the noteworthy impact of low-risk human papillomavirus (LR-HPV), particularly HPV 6 and HPV 11, in causing genital warts has attracted considerable attention. The development of HPV vaccines, notably the multi-valent variant, emphasizes the importance of including protection against HPV 6 and HPV 11 (13). However, there is a scarcity of epidemiological data pertaining to LR-HPV, particularly in Qinghai-Tibet Plateau (14–16). This study draws on cervical cancer screening results as its primary data source, concentrating exclusively on HR-HPV strains, while not encompassing pertinent data regarding LR-HPV.
This information is crucial given the limited cross-protection provided by existing HPV vaccines against HR-HPV subtypes prevalent in rural areas of China. Therefore, conducting further research on the prevalence of HR-HPV infection and HR-HPV subtype distribution among women in rural areas of Nyingchi City, Tibet is essential to enhance our understanding of HR-HPV epidemiology and to facilitate the development of domestic HPV vaccines.
Situated in the southeastern part of the Tibet Autonomous Region, Nyingchi City enjoys an average elevation of under 3,000 meters, placing it below higher-altitude areas like Lhasa, Shigatse, Nagqu, and Shannan. This geographical distinction results in a consistent abundance of rainfall and flourishing vegetation throughout the year, earning Nyingchi the epithet “Plateau Jiangnan.” Given the dearth of HPV infection data in Nyingchi City, it becomes imperative to meaningfully compare the HPV infection distribution in this “Plateau Jiangnan” with rural areas outside the Qinghai-Tibet Plateau, especially considering existing data from high-altitude regions in Tibet (14–16).
According to the requirements of the national “Screening of Cervical cancer and Breast cancer Program” in China, this study focused on women aged 35–64 with rural household registration and a history of sexual activity. The research collected cervical cancer screening data (HPV results) from Bomi County, Chayu County, Motuo County, GongbuJiangda County, Lang County, and Bayi District in Nyingchi City, Tibet, spanning the period from 2019 to 2022. Inclusion criteria encompassed women aged 35–64 with rural household registration, residing continuously for over 1 year, and willingly participating in HR-HPV subtype genetic testing. Exclusion criteria included individuals who had undergone hysterectomy for non-cervical cancer or non-cervical diseases. Informed consent was obtained from all research subjects. A total of 15,687 rural women underwent HR-HPV subtype genetic testing, distributed as follows: 3,235 from Bomi County, 2,795 from Chayu County, 1,271 from Motuo County, 4,612 from Gongbujiangda County, 50 from Lang County, and 3,724 from Bayi District (Figure 1). The Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University granted approval for this study.
The gynecological examination involved assessing the vagina, checking for visible abnormalities on the cervix, and conducting a bimanual pelvic examination. HR-HPV subtype gene detection was performed using the PCR fluorescence method, employing detection reagents for the 13 HR-HPV subtypes recommended by the World Health Organization (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). This allowed for clear identification of specific HR-HPV subtypes. The HPV infection rate, the infection rate of different HR-HPV subtypes, and the infection rate of HR-HPV subtypes among different age groups of rural women outside the Qinghai-Tibet Plateau were compared based on the reference (15).
Statistical analysis was conducted using Graphpad Prism 9.0 software. The age distribution of the research subjects conformed to a normal distribution, represented as x̄ ± s. The χ2 test was utilized to compare the HPV infection rates across various age groups and HR-HPV subtypes. A significance level of α = 0.05 was set for the comparison tests, and a p-value of <0.01 was considered statistically significant.
Among the 15,687 eligible rural women, the average age was 46.02 ± 8.31 years. The largest proportion of participants was in the 35–39 age group, accounting for 20.78% (3,260/15,687), while the smallest proportion was in the 60–64 age group, accounting for 10.80% (1,694/15,687). The majority of the research subjects, 97.5%, were Tibetan, while the remaining participants were of Han ethnicity.
The overall HR-HPV infection rate among women in rural areas was 13.00% (2,040/15,687). Among the different age groups, the 35–39 age group exhibited the highest infection rate of 15.31% (499/3,260), while the 50–54 age group showed the lowest infection rate of 9.69% (246/2,540). Notably, the HR-HPV infection rate decreased with increasing age, with the 55–59 age group having an infection rate of 12.23% (222/1,815), and the 60–64 age group having an infection rate of 10.92% (185/1,694). These differences in HR-HPV infection rates among age groups were found to be statistically significant (Table 1).
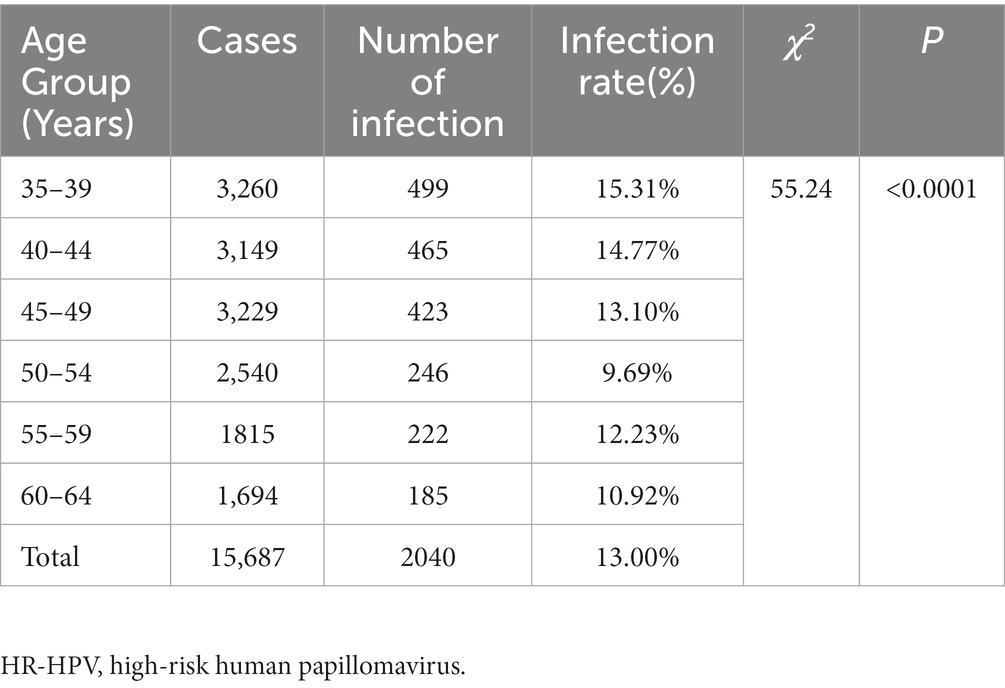
Table 1. HR-HPV infection status of women of different ages group in rural areas of Nyingchi City, Tibet.
The overall HR-HPV infection rate in rural areas of Nyingchi City, Tibet was significantly higher at 13.00% (2,040/15,687) compared to rural areas outside the Qinghai-Tibet Plateau at 7.82% (9,249/118,237; χ2 = 635.7, p < 0.001). Specifically, when analyzing the HR-HPV infection rates in different age groups, including the 35–39, 40–44, 45–49, 50–54, and 55–59 age groups, the rates in rural areas of Nyingchi City, Tibet were significantly higher than those in rural areas outside the Qinghai-Tibet Plateau (χ2 = 253.0, 227.7, 114.0, 7.015, and 17.68, respectively, all p < 0.001). However, in the 60–64 age group, there was no significant difference in the HR-HPV infection rate between the two regions (χ2 = 1.622, p = 0.2028; Table 2).
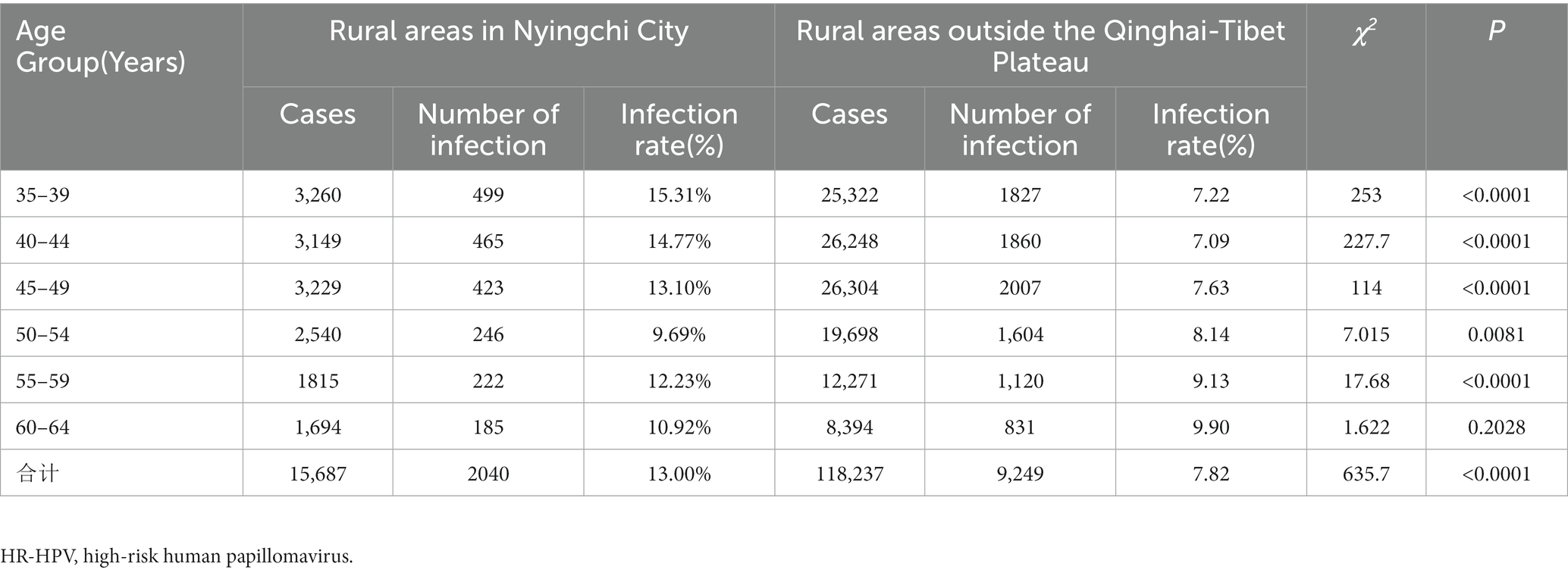
Table 2. Comparison of HR-HPV infection among women of different ages in Nyingchi City, Tibet and rural areas outside the Qinghai-Tibet Plateau.
A total of 2,040 cases of HR-HPV infection were observed among women in rural areas of Nyingchi City, Tibet. The three most prevalent HR-HPV subtypes were HPV52 (20.15%, 411/2,040), HPV16 (12.45%, 254/2,040), and HPV58 (11.96%, 244/2,040). Similarly, among women in rural areas outside the Qinghai-Tibet Plateau, the top three HR-HPV subtypes were HPV52 (20.98%, 1,432/6,824), HPV16 (18.23%, 1,244/6,824), and HPV58 (11.06%, 755/6,824). The ranking of HR-HPV subtypes was consistent between the two regions. However, the HPV16 infection rate differed significantly at (12.45%, 254/2,040) in rural areas of Nyingchi City, Tibet and (18.23%, 1,244/6,824) in rural areas outside the Qinghai-Tibet Plateau (χ2 = 37.34, p < 0.001; Table 3).
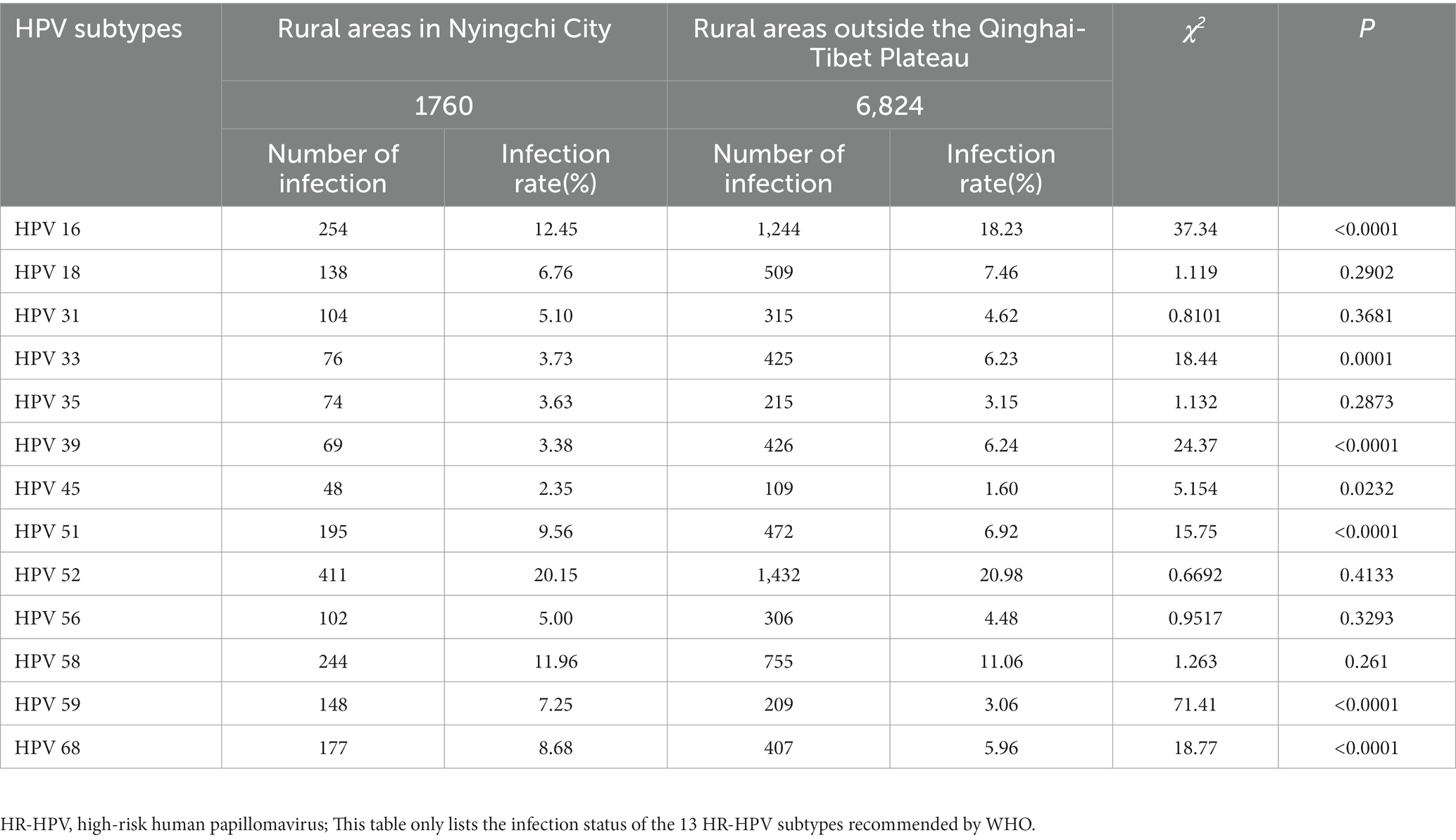
Table 3. Comparison of infection status of different HR-HPV subtypes among women in Nyingchi City, Tibet and rural areas outside the Qinghai-Tibet Plateau.
The distribution of HR-HPV subtype infection varied among different age groups of women. In the 35–39 age group, the top three HR-HPV subtypes were HPV52, HPV16, and HPV58. Similarly, in the 40–44, 45–49, and 50–54 age groups, the predominant HR-HPV subtypes were HPV52, HPV16, and HPV58. Among women aged 55–59, the top three HR-HPV subtypes were HPV52 (16.22%, 36/222), HPV58 (10.36%, 23/222), and a combination of HPV51 and HPV16 (9.91%, 22/222). In the 60–64 age group, the most prevalent HR-HPV subtypes were HPV58 (15.14%, 28/185), HPV52 (13.51%, 22/185), and HPV59 (13.51%, 22/185; Table 4).
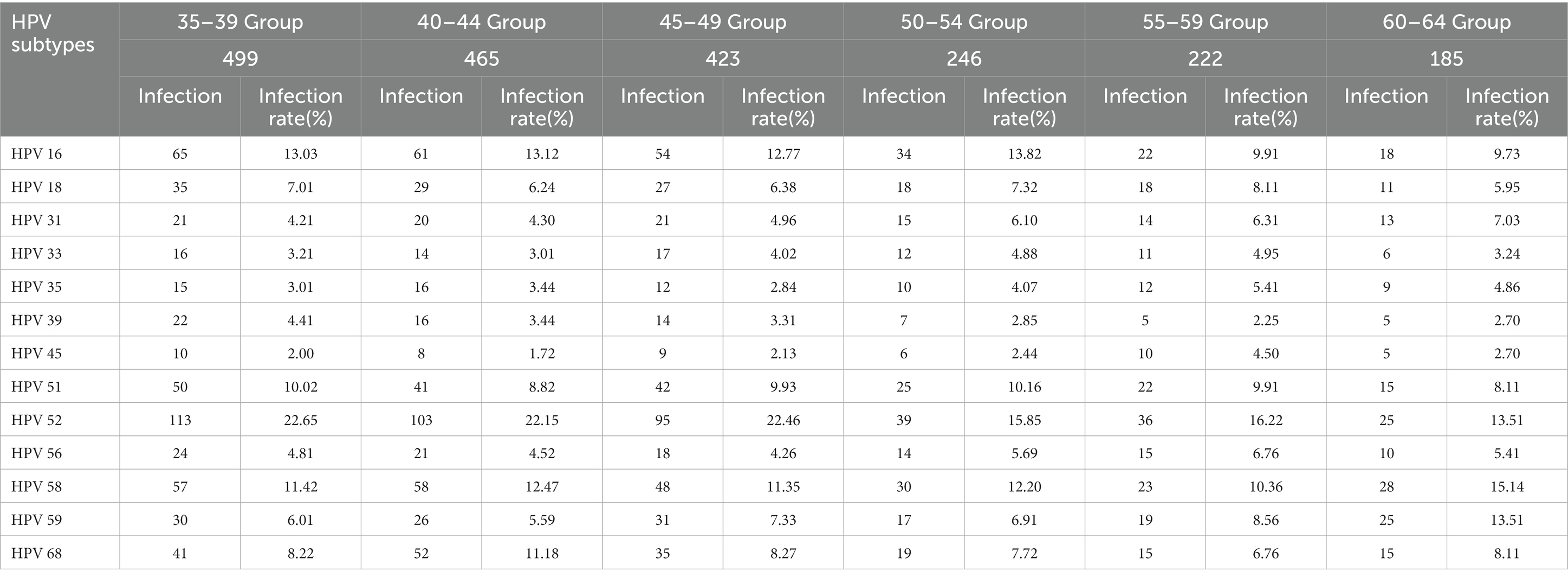
Table 4. Comparison of infection status of different HR-HPV subtypes among women of different ages in rural areas of Nyingchi City, Tibet.
Our survey revealed that the overall HR-HPV infection rate among women in rural areas of Nyingchi City, Tibet, was 13.00%, which was significantly higher than the infection rate among women in rural areas outside the Qinghai-Tibet Plateau (7.82%; χ2 = 635.7, p < 0.001). Furthermore, the infection rate in Nyingchi City was also significantly higher than the overall HR-HPV infection rate (9.19%) observed in Lhasa, Shigatse, and Nagqu in Tibet (15). Altitude was found to potentially impact the HPV infection rate, with Lhasa’s average altitude of 3,650 meters correlating to a 9.16% infection rate, and Shigatse’s average altitude of 3,836 meters correlating to an 8.86% infection rate (16). However, when compared with other regions, the overall HR-HPV infection rate among women in rural areas of Nyingchi City, Tibet, was lower than that reported in Shenzhen City (23.2%) (17), Shanxi Province (14.8%) (18), Taiwan (29.4%) (19), and certain areas of Hubei (20).
Regarding age groups, a “U”-shaped distribution pattern was observed in the HR-HPV infection rate (21), with the lowest rate recorded in the 50–54 age group at 9.69% (246/2540). The infection rate in women aged 35–54 showed a declining trend from 15.31 to 9.69%. Among women aged 55–59, the HR-HPV infection rate was 12.23% (222/1815), and among those aged 60–64, it was 10.92% (185/1694), indicating a consistent downward trend. This trend aligns with similar research findings on age distribution in other countries. A domestic study (22) similarly observed the phenomenon: the graphical representation of the positive infection rate of high-risk (HR) HPV follows a U-shaped function with respect to age. The peak occurs among females under 30 years old, with a second peak appearing among females over 50 years old. Women who are sexually active have a higher risk of HR-HPV infection compared to perimenopausal women (23, 24), while older adult women may experience a decline in immune function due to reduced postmenopausal ovarian sex hormone levels, increasing their susceptibility to HR-HPV infection or reducing their capacity for self-clearance of HR-HPV infection (25). Moreover, the HR-HPV infection rate among women in rural areas of Nyingchi City, Tibet, was significantly higher than that among women in rural areas outside the Qinghai-Tibet Plateau across all age groups, with a statistically significant difference (p < 0.001).
Previous studies have identified HPV types 16, 18, 31, 58, 52, 51, and 33 as the most common HR-HPV subtypes in the female population, with variations in their distribution and order of infection (26). In our study, the top three HR-HPV subtypes were HPV52 (20.15%, 411/2040), HPV16 (12.45%, 254/2040), and HPV58 (11.96%, 244/2040), which were consistent with the top three subtypes among women in rural areas outside the Qinghai-Tibet Plateau (12). Furthermore, our findings confirm that HPV52 is the most prevalent and common HR-HPV subtype in rural areas of China and even across Asia (26). Additionally, our study revealed a consistent distribution of the top three HR-HPV subtypes (HPV52, HPV16, and HPV58) among the 35–39, 40–44, and 45–49 age groups. In the 60–64 age group, the top three subtypes were HPV58 (15.14%, 28/185), HPV52 (13.51%, 22/185), and HPV59 (13.51%, 22/185).
In summary, our study provides valuable data on the composition and distribution of the top three HR-HPV subtypes among women in rural China and highlights the need for HPV infection prevention and the development of HPV vaccines in China. However, it is important to note that this study focused solely on rural areas of Nyingchi City and may not represent the overall situation in the Qinghai-Tibet region. Therefore, future research should employ rigorous study designs, expand the research scope, and increase the sample size for comprehensive investigation and analysis.
Furthermore, building upon this study, it would be beneficial to explore the relationship between HR-HPV subtype infection and cervical lesions and cervical cancer among women in rural areas of Nyingchi City, thus providing data support for reducing the incidence of cervical lesions and cervical cancer in this region.
However, the data collected exhibit limitations. Researchers have access to information such as marriage timing, commencement of sexual activity, the interval between screening and initial sexual activity, and childbirth history solely from Bomê County. Conversely, this data is unavailable for the remaining counties. Moreover, the data pertaining to low-risk HPV infection rates and multiple HPV infections in Nyingchi City is incomplete, with only partial data accessible from the Bayi District. Consequently, a thorough analysis and inclusion of comprehensive low-risk HPV infection rates and multiple HPV infections in this study’s outcomes are unfeasible, signifying a significant research gap and a regrettable limitation.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University. All participants signed the informed consent.
JL and XS: conceptualization, funding acquisition, and writing– review and editing. JL: formal analysis. XL: methodology. JL and XL: writing–original draft. All authors contributed to the article and approved the submitted version.
This research was funded by Science and Technology Planning Project of Guangzhou (funding No. 202102010003); the Project for Key Medicine Discipline Construction of Guangzhou Municipality (No. 2021–2023-17) and Guangzhou Health Science and Technology Project (No. 20231A011091).
Thanks to the obstetrician and gynecologist of People’s Hospital of Bomi for providing the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, and Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Arbyn, M, Weiderpass, E, Bruni, L, de Sanjosé, S, Saraiya, M, Ferlay, J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
3. Walboomers, JM, Jacobs, MV, Manos, MM, Bosch, FX, Kummer, JA, Shah, KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. (1999) 189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
4. World Health Organization. Comprehensive cervical Cancer control: A guide to essential practice. 2nd ed. Geneva: World Health Organization (2014).
5. Clifford, G, Franceschi, S, Diaz, M, Muñoz, N, and Villa, LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. (2006) 24:S26–34. doi: 10.1016/j.vaccine.2006.05.026
6. Zhang, J, Cheng, K, and Wang, Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. (2020) 302:1329–37. doi: 10.1007/s00404-020-05787-w
7. Baloch, Z, Yue, L, Yuan, T, Feng, Y, Tai, W, Liu, Y, et al. Status of human papillomavirus infection in the ethnic population in Yunnan Province, China. Biomed Res Int. (2015) 2015:314815:1–10. doi: 10.1155/2015/314815
8. Li, X, Ding, L, Song, L, Gao, W, Wang, L, and Wang, J. Effects of exposure to polycyclic aromatic hydrocarbons combined with high-risk human papillomavirus infection on cervical intraepithelial neoplasia: a population study in Shanxi Province, China. Int J Cancer. (2020) 146:2406–12. doi: 10.1002/ijc.32562
9. Liu, SS, Chan, KY, Leung, RC, Chan, KK, Tam, KF, Luk, MH, et al. Prevalence and risk factors of human papillomavirus (HPV) infection in southern Chinese women-a population-based study. PLoS One. (2011) 6:e19244. doi: 10.1371/journal.pone.0019244
10. Ye, J, Cheng, X, Chen, X, Ye, F, Lü, W, and Xie, X. Prevalence and risk profile of cervical human papillomavirus infection in Zhejiang Province, Southeast China: a population-based study[J]. Virol J. (2010) 7:66. doi: 10.1186/1743-422X-7-66
11. Zhao, FH, Lewkowitz, AK, Hu, SY, Chen, F, Li, LY, Zhang, QM, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. (2012) 131:2929–38. doi: 10.1002/ijc.27571
12. Jin, Q, Shen, K, Li, H, Zhou, XR, Huang, HF, and Leng, JH. Age-specific prevalence of human papillomavirus by grade of cervical cytology in Tibetan women. Chin Med J. (2010) 123:2004–11. doi: 10.3760/cma.j.issn.0366-6999.2010.15.010
13. Rahangdale, L, Mungo, C, O'Connor, S, Chibwesha, CJ, and Brewer, NT. Human papillomavirus vaccination and cervical cancer risk. BMJ. (2022) 15:e070115. doi: 10.1136/bmj-2022-070115
14. Feng, D, Wei, S, Chen, J, Yu, Z, Lhamo, Y, Wang, H, et al. Human papillomavirus prevalence and genotype distribution landscapes in Shannan City, Tibet Tibetan autonomous region, China. Virol J. (2022) 19:46. doi: 10.1186/s12985-022-01775-5
15. Di, JL, Luo, XM, Wu, JL, Song, B, and Ma, L. Population-based study on infection and genotype distribution of high-risk human among women in rural areas of China, 2014. Chin J Prev Med. (2017) 51:325–31. doi: 10.3760/cma.j.issn.0253-9624.2017.04.009
16. Jin, Q, Shen, K, Li, H, Zhou, XR, Huang, HF, Leng, JH, et al. Prevalence of human papillomavirus infection in women in Tibet autonomous region of China. Chin J Obstet Gynecol. (2009) 44:898–902.
17. Tingyu, R, Yilang, L, Tingting, H, Jing, W, Xiaowei, X, Wei, W, et al. Detection and infection of HPV infection in women of Shenzhen, Guangdong province. J Pract Med. (2020) 36:969–73. doi: 10.3969/j.issn.1006-5725.2020.07.027
18. Dai, M, Bao, YP, Li, N, Clifford, GM, Vaccarella, S, Snijders, PJF, et al. Human papillomavirus infection in Shanxi Province, People’s Republic of China: a population-based study. Br J Cancer. (2006) 95:96–101. doi: 10.1038/sj.bjc.6603208
19. Strong, C, Zou, H, Ko, NY, Liang, YL, Ku, WW, and Lee, CW. Prevalence and risk factors of anogenital human papillomavirus infection in a community sample of men who have sex with men in Taiwan: baseline findings from a cohort study. Sex Transm Infect. (2020) 96:62–6. doi: 10.1136/sextrans-2018-053629
20. Weipeng, W. Genotype distribution of high-risk human papillomavirus in women with cervical infection in our hospital. J Pract Med. (2007) 23:2040–1. doi: 10.3969/j.issn.1006-5725.2007.13.040
21. Castle, PE, Schiffman, M, Herrero, R, Hildesheim, A, Rodriguez, AC, Bratti, MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. (2005) 191:1808–16. doi: 10.1086/428779
22. Xue, H, Lin, X, Li, T, Yan, X, Guo, K, and Zhang, Y. Prevalence and genotype distribution of human papillomavirus infection in asymptomatic women in Liaoning province. China J Med Virol. (2015) 87:1248–53. doi: 10.1002/jmv.24029
23. Velicer, C, Zhu, X, Vuocolo, S, Liaw, KL, and Saah, A. Prevalence and incidence of HPV genital infection in women. Sex Transm Dis. (2009) 36:696–703. doi: 10.1097/OLQ.0b013e3181ad25ff
24. Zhao, FH, Tiggelaar, SM, Hu, SY, Xu, LN, Hong, Y, Niyazi, M, et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: suggestions for optimal age of human papillomavirus vaccination in China. Cancer Epidemiol. (2012) 36:384–90. doi: 10.1016/j.canep.2012.01.009
25. González, P, Hildesheim, A, Rodríguez, AC, Schiffman, M, Porras, C, Wacholder, S, et al. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol Biomark Prev. (2010) 19:3044–54. doi: 10.1158/1055-9965.EPI-10-0645
Keywords: HR-HPV, HR-HPV subtypes, rural women, Qinghai-Tibet plateau, Tibet (China)
Citation: Li J, Li X and Sheng X (2023) Four-year analysis of high-risk human papillomavirus infection among women in rural areas of Nyingchi City, Tibet. Front. Public Health. 11:1251440. doi: 10.3389/fpubh.2023.1251440
Received: 01 July 2023; Accepted: 01 September 2023;
Published: 20 September 2023.
Edited by:
Antoinette van der Kuyl, University of Amsterdam, NetherlandsReviewed by:
Abdoul Karim Ouattara, University Norbert Zongo, Burkina FasoCopyright © 2023 Li, Li and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujie Sheng, MjAwODY5MTE1MEBnemhtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.