- 1Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
- 2Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
Tuberculosis (TB) in young infants (< 3 months of age), often referred to as perinatal TB, is underdiagnosed, leading to severe morbidity and high mortality. Perinatal TB includes both congenital and postnatal transmission of Mycobacterium tuberculosis. We aimed to increase an awareness of TB in neonates and young infants and to provide guidance on the assessment and management when in contact with mothers with TB during or soon after pregnancy. Approximately 217,000 pregnant women develop TB annually; if they are not diagnosed and treated during pregnancy, their infants are at high risk of adverse birth outcomes and TB disease. Although safe and effective antituberculosis treatment regimens are available during pregnancy, the diagnosis of TB is challenging. Infants born to mothers newly diagnosed with TB, not receiving any effective treatment or with cultures not yet negative, should be assessed for TB disease or M. tuberculosis infection. TB preventive therapy should be instituted if the infant is clinically well but exposed to TB, while prompt initiation of TB treatment is essential if TB disease is presumed. HIV status of mother and infant should be considered as this will affect the management. Further research is needed for the diagnosis and prevention of TB during pregnancy, an early diagnosis of TB in infants, and antituberculosis drug pharmacokinetics in young infants.
Introduction
Tuberculosis (TB) remains the infectious disease with the highest mortality globally. In 2021, an estimated 10.6 million new TB cases occurred, including 1.17 million children. Of the 1.59 million deaths in 2021, 14% were in children aged 0–14 years (1). Mathematical models for 2015 estimated that of the 239,000 (95% uncertainty interval [UI]: 194,000–298,000) TB-related deaths in children aged 0–14 years, 191,000 (80%; 95% UI 132,000–257,000) occurred in children younger than 5 years of age. The per-person TB mortality in children is highest in sub-Saharan African countries with high annual TB incidences and almost all (228,000; 96%) deaths occurring in children not on treatment (2).
Immune immaturity related to young age is associated with a high risk of developing TB disease following infection. Infants (<12 months) experience the highest risk of developing TB (50%) after infection in the absence of preventive measures; among these, up to 30% develop progressive pulmonary or disseminated (miliary) TB (3). Although vertical transmission prevention programmes are successful in preventing perinatal and breastfeeding-related HIV infection, infants born to mothers living with HIV remain at an increased risk of exposure to TB because of the high TB prevalence in people living with HIV (4, 5). Maternal TB also increases the risk of HIV transmission (5).
TB in infants of <3 months of age can either be congenital or postnatal in origin. It is challenging to clinically distinguish between congenital TB, transmitted in utero by haematogenous spread through the umbilical vein or ingestion/aspiration of Mycobacterium tuberculosis-infected amniotic fluid during birth, and postnatal transmission that occurs by the inhalation of M. tuberculosis bacilli spread by the airborne route from a mother or other close source patient with infectious pulmonary TB early after birth; therefore, the term perinatal TB is preferred (i.e., infants likely infected congenitally or within the 1st days of life postnatally and presenting within the first 3 months of life) (6). The focus of this minireview was to increase awareness of TB in neonates (<28 days) and young infants (<3 months), describe the clinical presentation, and provide guidance on the investigation and the management of young infants in contact with mothers with TB during or after pregnancy.
Epidemiology
Due to immunological changes, women are at higher risk of developing TB disease after M. tuberculosis infection during pregnancy and in the immediate postpartum period (7). HIV infection further increases this risk (6–8). A review in 2012 estimated the TB prevalence among pregnant women to range from 0.06–0.25% in low-burden countries to 0.07–0.5% in high-burden countries (9). The documented rates were 0.07–0.5% in HIV-negative women and 0.7–11% in HIV-positive women (9). Epidemiological modeling estimated that in 2011, there were 216,500 (95% uncertainty range 192,000–247,000) pregnant women with TB globally (10).
TB in pregnancy is associated with poor pregnancy outcomes, and undiagnosed or untreated TB during pregnancy and postpartum holds a high risk for perinatal M. tuberculosis infection and disease in infants. Compared with pregnant women without TB, pregnant women with TB disease had an increased risk of preterm birth (OR 1.7, 95% CI 1.2–2.4), giving birth to low birth weight infants (OR 1.7, 95% CI 1.2–2.4), birth asphyxia (OR 4.6, 95% CI 2.4–8.6), and perinatal death (OR 4.2, 95% CI 1.5–11.8) (11). The multitude of case reports, several case series, the likelihood that many cases are undiagnosed and not reported, and data from high HIV-burden settings suggest that congenital TB is not as rare as most reports suggest (6, 7). However, postnatal transmission of M. tuberculosis remains more common than congenital TB and in the first 3 months of life often presents with severe disease because of the immature immune system. Over a 12.3-year period in a high TB-incidence setting, 106 of 2017 (5%) children under 13 years of age with culture-confirmed TB were infants under 3 months of age (12).
Possible risk factors for congenital TB are as follows: (1) Infants born to mothers who have primary TB, which often has a haematogenous (bacillaemic) phase (see Clinical presentation) (6), (2) mothers co-infected with TB and HIV, and (3) mothers who had undergone in vitro fertilization (IVF) with TB as the cause of infertility (13).
Prevention of TB in mothers and their infants
Prevention of TB disease in pregnant women and prevention of TB in infants of pregnant women with TB are paramount. This has four components: (1) primary prevention of TB among pregnant women, especially by providing TB preventive therapy (TPT) after infection or high-risk TB exposure (14, 15); (2) preventing unintended pregnancies in women receiving TB therapy; (3) early identification and treatment of TB with treatment support in women during pregnancy and postpartum; and (4) providing appropriate post-TB-exposure TPT to infants when indicated (16). The risk of untreated TB disease to the pregnant woman and her fetus is much greater than the risks of antituberculosis treatment. Appropriate and safe regimens for both drug-susceptible and drug-resistant TB treatment in pregnancy are available (15).
TB of the genitourinary tract is a common cause of infertility; several cases of congenital TB were reported in infants of mothers who had undergone IVF; therefore, TB should be excluded and treated before IVF is performed to prevent congenital TB in their infants (13).
TB preventive therapy in exposed/infected neonates and young infants
When mothers are diagnosed and/or treated for TB in pregnancy, their neonates should be screened for TB at birth or within the 1st days of life (Figure 1) (17–19). However, many young infants with TB are the index (first diagnosed) patients, and after their diagnosis, a source case should be sought. In most cases, this is the mother who may have been asymptomatic in pregnancy, previously undiagnosed, or her symptoms were not identified as related to possible TB (20, 21).
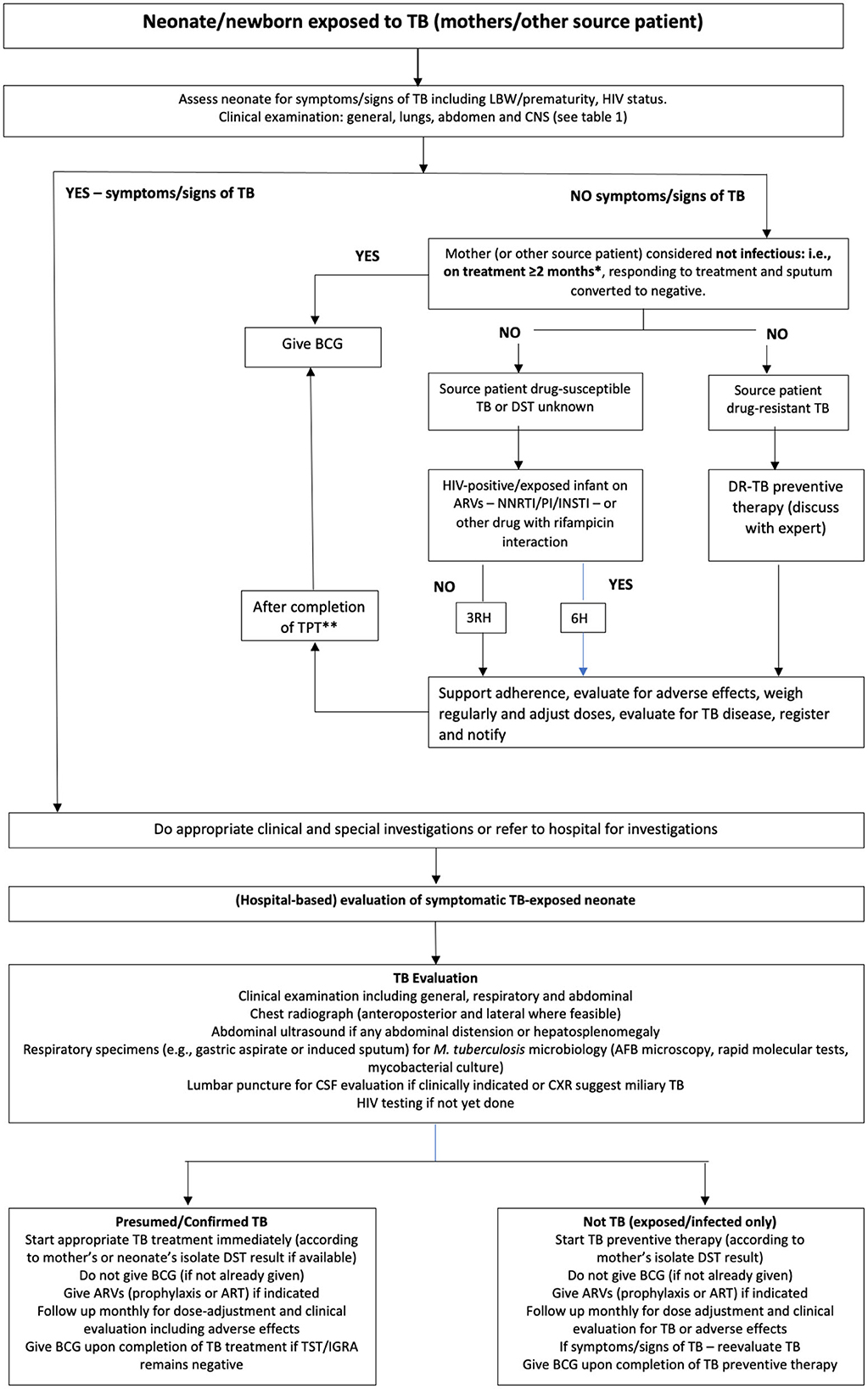
Figure 1. An algorithm for the assessment and management of newborn infants/neonates in contact with mothers (or other source cases) with tuberculosis during pregnancy or directly after birth. (Adapted from Van der Westhuizen et al. (17) and National Department of Health, South Africa (18) with permission). *Some experts recommend mothers to only be on treatment for 2 weeks during pregnancy, but this duration is insufficient to prevent congenital exposure and postnatal exposure if still microbiologically positive for Mycobacterium tuberculosis on sputum. **If tuberculin skin test (or interferon-gamma release assay) is negative or not available. AFB, acid-fast bacilli; ARV, antiretroviral; ART, antiretroviral treatment; BCG, Calmette–Guérin; CNS, central nervous system; CXR, chest x-ray; DR-TB, drug-resistant tuberculosis; DST, drug susceptibility testing; INSTI, integrase strand transfer inhibitor; LBW, low birth weight; M. tuberculosis, Mycobacterium tuberculosis; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TPT, tuberculosis preventive therapy; 3HR, 3 months of isoniazid plus rifampicin; 6H, 6 months of isoniazid.
In neonates screened for TB with known exposure or infection, TPT should be provided once TB disease has been excluded (15, 22).
The choice of TPT depends on the confirmed or presumed susceptibility pattern of the source patient and the possibility of other drug interactions. In case of exposure to drug-susceptible TB, 6 months of isoniazid (including in HIV-exposed infants on antiretroviral drugs) or 3 months of isoniazid–rifampicin combination should be provided; in isoniazid mono-resistant TB exposure, a 4-month rifampicin regimen should be used, while in rifampicin-resistant (RR) or multidrug-resistant TB (MDR-TB; resistance to isoniazid plus rifampicin) exposure, levofloxacin alone or in combination with another drug with known or presumed susceptibility should be used (13, 23, 24). With exposure to MDR strains with fluoroquinolone resistance (pre-extensive drug-resistant [pre-XDR] TB), or XDR-TB (pre-XDR-TB plus resistance to bedaquiline or linezolid), TPT may not be possible and expert opinion should be sought. In infants exposed to pre-XDR/XDR-TB source patients with low-level isoniazid resistance, as conferred by an inhA mutation only, high-dose isoniazid at 15 mg/kg for 6 months could be used as TPT (25). Delamanid as a TPT option is currently being investigated for contacts of MDR- to XDR-TB patients (26). In all exposed/infected infants, irrespective of TPT, close long-term follow-up for at least 1 year is essential for early TB disease detection.
On completion of TPT, the Bacillus Calmette–Guérin (BCG) vaccine should be administered to all infants who did not convert TST or IGRA (or if TST/IGRA is not available) and who live in countries where BCG is part of the routine vaccine policy.
Separation of infants from mothers and other preventive measures
In low-income settings, infants are rarely separated from their mothers after birth even if the mothers are known to still be contagious. Establishing breastfeeding and bonding with the newborn infant is important. Care for the infant includes advice on infection prevention and control measures, such as wearing a mask when handling or feeding the infant until the mother is on effective therapy for at least 2 weeks and/or sputum smear-negative for acid-fact bacilli, sleeping in a separate room, allowing ventilation in the home, and adhering to mothers' own treatment and infant's TPT (6).
Temporarily separating infants from infectious mothers until they are non-infectious may be indicated in mothers with MDR-, pre-XDR-, or XDR-TB if no TPT options are available; however, this is only possible if an alternative reliable caregiver is available (22).
With the possible exception of bedaquiline, where high concentrations were reported in only one breastfeeding infant, very little drug is excreted in breast milk, and breastfeeding is not contraindicated (14, 22, 27–29). Electrocardiogram (ECG) monitoring of breastfed infants should be done if mothers are on bedaquiline.
Clinical presentation
Congenital TB is the result of the haematogenous spread of bacilli to the endometrium: Such spread occurs mainly in primary TB (e.g., pleural effusions) or disseminated TB (e.g., miliary TB or TB meningitis), while postnatal transmission occurs mainly from mothers or other source patients with cavitary adult-type TB (6). The Cantwell revised criteria for congenital TB can assist in distinguishing congenital TB from postnatal transmitted TB: Any young infant (<3 months) with a confirmed TB lesion and one or more of the following most likely has congenital TB: (1) presents within the 1st week of life, (2) a primary hepatic complex or caseating hepatic granuloma, (3) Mycobacterial bacilli identified in the placenta or endometrial TB in the mother, or (4) excluding possible postnatal transmission by excluding TB in other contacts (30).
Infants with congenital TB may be symptomatic within the 1st days of life, but mostly present between 2 and 4 weeks of age, and could be diagnosed as late as up to 5 months of age (6, 21). Symptom onset in young infants is often acute over days, rather than chronic over weeks. Initial complaints can be non-specific and vague. The clinical picture may vary from asymptomatic to severe acute or chronic illness. Infants are often misdiagnosed with acute bacterial or viral infections. Often, several courses of antibiotics are given, with poor response (31). Given the high risk of TB disease, the non-specific clinical presentation of TB in infants, and high morbidity and mortality, there should be a low threshold for rapid referral of unwell TB-exposed neonates and young infants with any (acute or chronic) symptoms or signs indicative of TB. The most common symptoms and signs of congenital TB in early infancy are summarized in Table 1 (6, 21, 22, 30, 32).
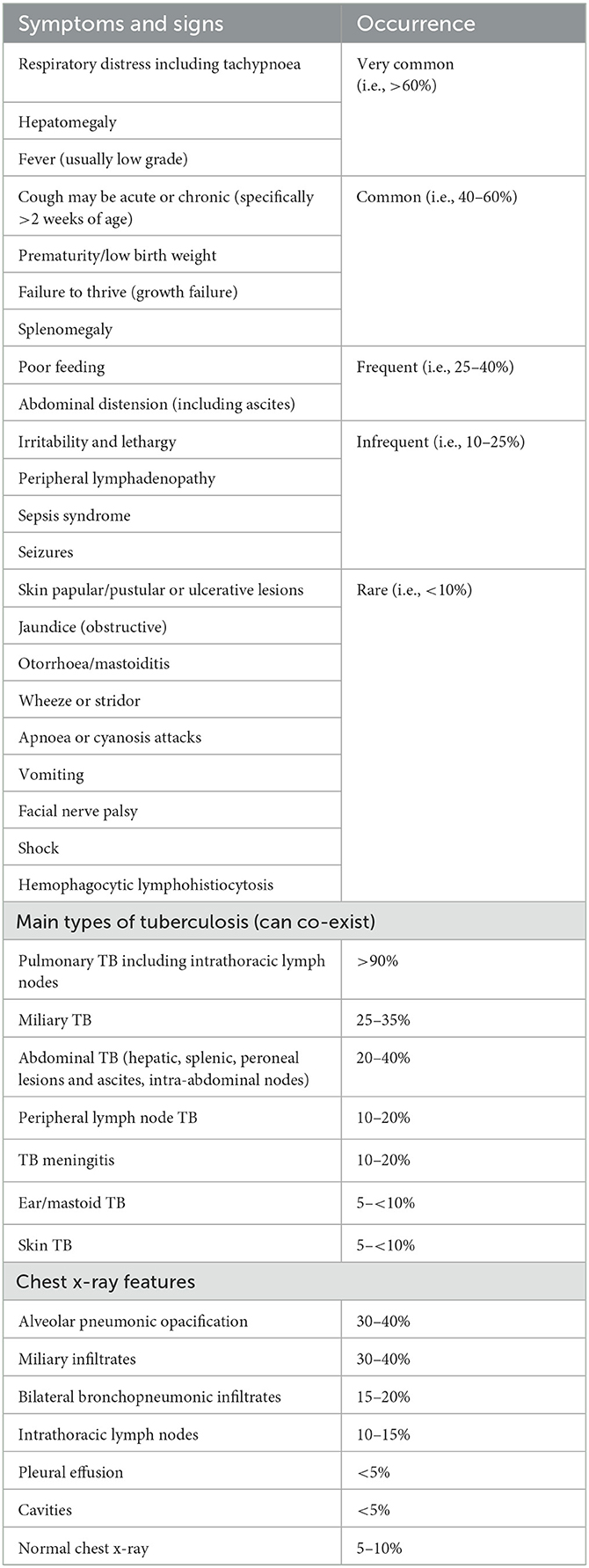
Table 1. Symptoms and signs, types of tuberculosis, and chest X-ray features in congenital TB [adapted from Schaaf et al. (6), added information from Du et al. (20), Li et al. (21), Cantwell et al. (30), Saramba and Zhao (31)].
Diagnostic approach
Awareness of TB as a possible diagnosis and a high index of suspicion for TB is essential, especially in high TB (and HIV)-incidence settings. An algorithmic approach for neonates exposed to TB is proposed in Figure 1.
TB should always be considered in neonates and young infants if mothers (or other source patients) have TB, particularly in infants with pneumonia not responding to antibiotics, unremitting fever and/or hepatosplenomegaly with or without jaundice, abdominal distension with ascites, or high lymphocyte count in cerebrospinal fluid (CSF).
TB in infants is often diagnosed by a constellation of history, clinical features, and special investigations. In the history, in vitro fertilization and contact with an adult with TB, particularly the mother are red flags (33, 34). The history and other assessments are put together like pieces of a puzzle to decide whether a young infant has TB or not (35). However, the threshold for treating young infants for TB should be low, especially if the mother/caregiver has TB.
Clinical evaluation should assess growth (e.g., prematurity, small for gestational age, low birth weight, weight gain over time) and a full general examination and systems assessment (Table 1). Postnatal TB may differ in presentation from congenital TB: Postnatal TB often presents with cough, tachypnoea, wheeze, or stridor, while congenital TB patients more often present with acute respiratory distress, hepatomegaly, and/or abdominal signs; other presenting symptoms and signs such as poor weight gain are similar (6). Rare late presentations could even include osteoarticular TB (36).
Chest X-ray (CXR) is the most common imaging modality; CXR findings of congenital TB are summarized in Table 1. CXRs in postnatal acquired TB can look similar to congenital TB but mediastinal lymphadenopathy, large airway compression, lobar or unilateral hyperinflation, or collapse due to partial or complete large airway obstruction by enlarged mediastinal lymph nodes, respectively, and Ghon foci are more common in postnatal acquisition (6, 12). Where available, chest computed tomography (CT) scans are often used for better identification of intrathoracic lymph nodes and differentiating TB from other conditions including congenital abnormalities (20, 37).
Abdominal ultrasound is important in presumed congenital TB; ultrasound can identify hypoechoic lesions in the liver and spleen, confirm ascites in infants with abdominal distension, and identify intra-abdominal or retroperitoneal lymphadenopathy (6). Ultrasound-guided biopsy from hepatic lesions may confirm the diagnosis if microbiologically positive or granulomatous lesions are found (38). Abdominal CT scans are sometimes advised (21) but are unlikely to yield more information than abdominal ultrasound in congenital TB patients.
Central nervous system (CNS) TB, such as TB meningitis and tuberculomas, should be excluded—lumbar puncture for obtaining CSF for chemistry, cell count, and microbiological testing is important in all young infants presenting with presumed TB (39). Brain CT or magnetic resonance imaging (MRI) scans are important if CNS involvement is considered or if CXR shows miliary TB.
Tests of M. tuberculosis infection [tuberculin skin test (TST) and interferon-gamma release assay (IGRA)] can be helpful but are less often positive in young infants as it can take 6–8 weeks to convert to positive after becoming infected.
Microbiological confirmation should be sought wherever possible. Asymptomatic newborn infants may yield positive microbiological tests from gastric aspirates most likely due to ingestion of infected amniotic fluid (6, 21, 40). Specimens should be obtained from any affected site, such as respiratory secretions (gastric aspirates, tracheal aspirates, induced sputum, and bronchoalveolar lavage or stool), CSF, pleural or ascitic fluid, urine (if disseminated TB), ear swabs, and biopsy specimens of mainly lymph nodes, as well as liver and skin where feasible. Although smear-microscopy for acid-fast bacilli (AFB) may be positive, the nucleic acid amplification-based rapid molecular tests (NAATs) for M. tuberculosis complex (e.g., Xpert MTB/RIF Ultra and Truenat assay) are much more sensitive. Obtaining repeated specimens for testing may be necessary to confirm the diagnosis (21). Specimens should be sent for mycobacterial culture as drug susceptibility testing (DST) of children's specimens is often only confirmed on cultured isolates through molecular or phenotypic DST. The microbiological confirmation rate of M. tuberculosis in young infants with TB is generally high, typically 70–80% (6, 41). This is likely because of high bacillary load due to late identification of disease or uncontrolled multiplication of bacilli in the absence of a well-developed immune system. There are no data on the use of Xpert MTB/RIF Ultra on stool or urine lipoarabinomannan (LAM) in congenital TB.
To confirm congenital TB, examining the placenta may be helpful (42), and CXR and sputum (or other relevant specimen) microbiological examination for M. tuberculosis should be performed in the mother if not already done. In addition, an endometrial biopsy of the mother as a likely source patient is necessary if other assessments are negative (43).
Treatment
Prompt treatment initiation is essential when TB is presumed in any neonate or young infant. Some experts advise full treatment if culture (or NAAT) for M. tuberculosis is positive in a young infant even if the infant is clinically well and CXR is normal (44).
Pharmacokinetic studies of first-line antituberculosis drugs in young infants are limited. Studies show that isoniazid concentrations at the current WHO-recommended dose of 7–15 mg/kg/day are adequate, but in preterm and low birth weight infants where the infant's N-acetyltransferase-2 (NAT-2) acetylation enzyme system may be immature and of slow or intermediate acetylator status, 10 mg/kg/day should not be exceeded if therapeutic drug monitoring is not available (45, 46). The current recommended rifampicin dose of 10–20 mg/kg seems too low, as very low peak concentrations (Cmax) and area under the time–concentration curves (AUCs) are reported, particularly when using liquid formulations (47, 48). Current pyrazinamide dosing at 30–40 mg/kg/day is adequate (47, 48). The ethambutol Cmax and AUC are generally very low at the current recommended doses of 15–25 mg/kg/day; however, the risk for optic neuritis precludes higher doses (48). Ethionamide doses of 20 mg/kg are considered safe and effective across all ages of children, but there are few data in neonates (49). Pharmacokinetics of almost all second-line drugs are limited, but recent (2022) WHO-published provisional dosing recommendations for second-line drugs, including bedaquiline and delamanid, are based on available pharmacokinetic data and modeling (23).
Treatment regimens of infants with congenital or postnatal acquired TB are similar to childhood TB treatment: however, the new 4-month regimen for non-severe drug-susceptible TB is not recommended for infants of <3 months of age (23, 50). The site and severity of the disease as well as the source case or infant's M. tuberculosis isolate DST result should be considered when making treatment decisions. A combination of isoniazid, rifampicin, and pyrazinamide with (21) or without (37) a fourth drug, such as ethambutol, an aminoglycoside (streptomycin or amikacin), levofloxacin, ethionamide, or even linezolid (20), has mainly been used for the intensive phase in drug-susceptible TB, with a continuation phase of isoniazid and rifampicin ranging from 4 to 10 months depending on disease severity and response to treatment (6, 21, 37). The aminoglycosides should be avoided because of the risk of permanent hearing loss and the need to give it parenterally. Despite concerns about optic neuritis, ethambutol at the current recommended dose is rarely toxic, but visual acuity cannot be evaluated. Ethionamide or levofloxacin is preferred because of the high rate of miliary TB and CNS involvement—in such cases, the intensive phase with all four drugs should be continued throughout treatment (23, 51). Baseline liver function tests should be done, as both TB and other conditions may affect the liver in young infants, which may be misinterpreted as drug-related hepatotoxicity; liver function tests should be carefully monitored in young infants receiving hepatotoxic drugs (52).
All infants co-infected with HIV require urgent initiation of antiretroviral treatment (ART) to prevent early HIV-associated mortality (53). TPT or TB treatment should not delay initiation of ART although regimen choice may be challenging in the youngest and smallest infants. Currently, WHO recommends initiation of ART within 2 weeks, unless meningitis is present (54). All co-infected infants of more than 4 weeks of age should receive co-trimoxazole preventive therapy against Pneumocystis jirovecii pneumonia, irrespective of CD4 count (54). Pyridoxine levels were consistently low in children living with HIV; therefore, these children should receive pyridoxine to prevent isoniazid toxicity (55).
Infants in contact with mothers or other source patients with drug-resistant TB should be managed as being infected or having developed disease with the same resistant strain; therefore, they should be treated according to the DST result of the adult source patient and not wait for the infant's own culture and DST results (56). However, microbiological confirmation and DST on the infant's own M. tuberculosis strain should be attempted to confirm drug susceptibility or resistance.
Corticosteroids are indicated in infants with TB lymph node compression of the large airways or in TB meningitis.
After completion of treatment, BCG vaccination should be considered in infants living in high TB-burden settings if the TB diagnosis was unconfirmed, including infants living with HIV who are clinically and immunologically stable on ART (CD4 > 25%) (57). Clinical follow-up for recurrence of TB, either relapse or reinfection, and post-TB disease is important (58).
Outcome
Mortality in congenital TB remains high at 15–50% (6, 21); however, some case series report 100% survival, which may be due to publication bias (presenting only patients who survived) or early diagnosis and treatment. Outcomes in published cohorts of infants of <3 months of age, which likely include mainly postnatal acquired TB but also some congenital TB patients, have found mortality between 11 and 13% (6, 43, 59).
Pulmonary TB in infants may have a long-term effect on lung health outcomes, and neurodevelopmental delay or neurological sequelae may follow severe TB or TB meningitis. Clinical evaluation and CXR (if severe lung involvement) should be done at the end of treatment to exclude post-TB disease, and follow-up and multidisciplinary management should be provided where indicated (58).
Discussion
Neonates and young infants are at high risk of developing TB after M. tuberculosis infection, but the diagnosis is often missed, or TB is diagnosed late, leading to high morbidity and mortality. A high index of suspicion for TB in mothers and their infants is required, especially in high TB or TB/HIV-burden settings. TB in neonates/infants may have an atypical, often acute presentation.
Diagnosis of TB in neonates and infants is challenging. The role of tests such as IGRA, urinary LAM, and stool NAAT in this age group needs further evaluation. Furthermore, alternative imaging modalities, such as chest CT, should be evaluated in infants with pulmonary infiltrates not responding to antibiotics, who have complicated intrathoracic lymph node disease or possible congenital abnormalities.
If TB is diagnosed in an infant, the mother should primarily be screened and endometrial TB considered, especially in neonatal TB. TB bacteriological investigations including DST in the mother (or other source patient) should guide management of the infant in the absence of microbiological confirmation in the infant. If TB infection or disease is presumed in an infant, TB preventive therapy and treatment, respectively, should not be delayed, as progression to TB disease or disseminated TB is very high, with high morbidity and mortality.
Although enrolment may be complex and drug formulations limited, neonates and young infants should be included in pharmacokinetic and safety studies evaluating new and better TB drugs and regimens, including new strategies, as they become available (60–62). Although optimal dosing of antituberculosis drugs in young infants is still being evaluated, dosing recommendations exist, and drugs should be administered accordingly and adjusted with weight gain (23). Further studies of the transmission of drugs through breast milk are needed.
Finally, it is important to also screen other children and adults in the household if a mother has TB.
Author contributions
HS wrote first draft and AB and HR reviewed the manuscript and gave critical input. All authors agreed on the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Global tuberculosis report 2022. Geneva: World Health Organization (2022). Licence: CC BY-NC-SA 3.0 IGO.
2. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health. (2017) 5:e898–906. doi: 10.1016/S2214-109X(17)30289-9
3. Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JR, et al. The natural history of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. (2004) 8:392–402.
4. Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S, et al. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis. (2008) 12:225–7.
5. Gupta A, Nayak U, Ram M, Bhosale R, Patil S, Basavraj A, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. (2007) 45:241–9. doi: 10.1086/518974
6. Schaaf HS, Collins A, Bekker A, Davies PDO. Tuberculosis at extremes of age. Respirology. (2010) 15:747–63. doi: 10.1111/j.1440-1843.2010.01784.x
7. Bates M, Ahmed Y, Kapata N, Maeurer M, Mwabab P, Zumla A. Perspectives on tuberculosis in pregnancy. Int J Infect Dis. (2015) 32:124–7. doi: 10.1016/j.ijid.2014.12.014
8. Zenner D, Kruijshaar ME, Nick Andrews N, Abubakar I. Risk of tuberculosis in pregnancy a national, primary care–based cohort and self-controlled case series study. Am J Respir Crit Care Med. (2012) 185:779–784. doi: 10.1164/rccm.201106-1083OC
9. Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. (2012) 55:1532–49. doi: 10.1093/cid/cis732
10. Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Glob Health. (2014) 2:e710–6. doi: 10.1016/S2214-109X(14)70330-4
11. Sobhy S, Babiker Z, Zamora J, Khan KS, Kunst H. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: a systematic review and meta-analysis. BJOG. (2017) 124:727–33. doi: 10.1111/1471-0528.14408
12. Frigati L, Bekker A, Stroebele S, Goussard P, Schaaf HS. Culture-confirmed tuberculosis in South African infants younger than 3 months of age: clinical presentation and management of respiratory complications. Pediatr Infect Dis J. (2019) 38:351–4. doi: 10.1097/INF.0000000000002163
13. Zhang X, Zhuxiao R, Xu F, Zhang Q, Yang H, Chen L, et al. Congenital tuberculosis after in vitro fertilization: suggestion for tuberculosis tests in infertile women in developing countries. J Int Med Res. (2018) 46:5316–21. doi: 10.1177/0300060518808179
14. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. Geneva: World Health Organization (2020). Licence: CC BY-NC-SA 3.0 IGO. doi: 10.30978/TB2021-2-86
15. Maugans C, Loveday M, Hlangu S, Waitt C, van Schalkwyk M, van de Water B, et al. Best practices for the care of pregnant people living with TB. Int J Tuberc Lung Dis. (2023) 27:357–66. doi: 10.5588/ijtld.23.0031
16. National Department of Health, South Africa. Guideline for the prevention of mother to child transmission of communicable infections (HIV, hepatitis, listeriosis, malaria, syphilis and TB). (2019). Available online at: https://www.nicd.ac.za/wp-content/uploads/2019/11/Guidelines-for-the-Prevention-of-Transmission-of-Communicable-Diseases-from-mother-to-child_28-October.pdf (accessed May 17, 2023)
17. Van der Westhuizen A, Dramowski A, Schaaf HS, Groenewald M, Bekker A. Hospital-based evaluation of a tuberculosis-exposed neonate: an approach to complement the national guidance. S Afr J Med. (2021) 111:724–8. doi: 10.7196/SAMJ.2021.v111i8.15575
18. National Department of Health, South Africa. National Guidelines on the treatment of tuberculosis infection. (2023). Available online at: https://sahivsoc.org/Files/Health_Latent%20TB%20Infection_2023_web.pdf (accessed May 17, 2023).
19. Bekker A, du Preez K, Schaaf HS, Cotton MF, Hesseling AC. High tuberculosis exposure among neonates in a high tuberculosis and human immunodeficiency virus burden setting. Int J Tuberc Lung Dis. (2012) 16:1040–6. doi: 10.5588/ijtld.11.0821
20. Du J, Dong S, Jia S, Zhang Q, Hei M. Clinical characteristics and post-discharge follow-up analyses of 10 infants with congenital tuberculosis: A retrospective observational study. Pediatr Investig. (2021) 5:86–93. doi: 10.1002/ped4.12266
21. Li C, Liu L, Tao Y. Diagnosis and treatment of congenital tuberculosis: a systematic review of 92 cases. Orphanet J Rare Dis. (2019) 14:131. doi: 10.1186/s13023-019-1101-x
22. Loveday M, Hlangu S, Furin J. Breastfeeding in women living with tuberculosis. Int J Tuberc Lung Dis. (2020) 24:880–91. doi: 10.5588/ijtld.20.0122
23. World Health Organization. WHO operational handbook on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization (2022). Licence: CC BY-NC-SA 3.0 IGO.
24. Migliori GB, Wu SJ, Matteelli A, Zenner D, Goletti D, Ahmedov S, et al. Clinical standards for the diagnosis, treatment and prevention of TB infection. Int J Tuberc Lung Dis. (2022) 26:190–205. doi: 10.5588/ijtld.22.0452
25. Dooley KE, Miyahara S, von Groote-Bidlingmaier F, Sun X, Hafner R, Rosenkranz SL, et al. early bactericidal activity of different isoniazid doses for drug-resistant tuberculosis (inhindsight): a randomized, open-label clinical trial. Am J Respir Crit Care Med. (2020) 201:1416–24. doi: 10.1164/rccm.201910-1960OC
26. ClinicalTrials.gov Identifier: NCT03568383. Protecting Households On Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB). Available online at: clinicaltrials.gov/ct2/show/NCT03568383 (Accessed May 22, 2023).
27. Partosch F, Mielke H, Stahlmann R, Gundert-Remy U. Exposure of nursed infants to maternal treatment with ethambutol and rifampicin. Basic Clin Pharmacol Toxicol. (2018) 123:213–20. doi: 10.1111/bcpt.12995
28. Court R, Gausi K, Mkhize B, Wiesner L, Waitt C, McIlleron H, et al. Bedaquiline exposure in pregnancy and breastfeeding in women with rifampicin-resistant tuberculosis. Brit J Clin Pharmacol. (2022) 88:3548–58. doi: 10.1111/bcp.15380
29. Algharably EA, Kreutz R, Gundert-Remy U. Infant exposure to antituberculosis drugs via breast milk and assessment of potential adverse effects in breastfed infants: critical review of data. Pharmaceutics. (2023) 15:1228. doi: 10.3390/pharmaceutics15041228
30. Cantwell MF, Shehab ZM, Costello AM, Sands L, Green WF, Ewing EP, et al. Congenital Tuberculosis. N Engl J Med. (1994) 330:1051–4. doi: 10.1056/NEJM199404143301505
31. Saramba MI, Zhao D, A. Perspective of the diagnosis and management of congenital tuberculosis. J Pathog. (2016) 2016:8623825. doi: 10.1155/2016/8623825
32. Peng W, Yang J, Liu E. Analysis of 170 cases of congenital TB reported in the literature between 1946 and 2009. Pediatr Pulm. (2011) 46:1215–24. doi: 10.1002/ppul.21490
33. McLaughlin SE, Vora SB, Church EC, Spitters C, Thyer A, LaCourse S, et al. Adverse pregnancy outcomes after in vitro fertilization due to undiagnosed urogenital tuberculosis and proposed screening algorithm for patients from tuberculosis-endemic countries. Fertil Steril Rep. (2022) 3:285–91. doi: 10.1016/j.xfre.2022.07.008
34. Sands A, Santiago MT, Uduwana S, Glater-Welt L, Ezhuthachan ID, Coscia G, et al. Congenital tuberculosis after in vitro fertilization: a case for tuberculosis screening of women evaluated for infertility. Clin Infect Dis. (2023) 76:e982–6. doi: 10.1093/cid/ciac542
35. Schaaf HS, Marais BJ, Carvalho I, Seddon JA. Challenges in childhood tuberculosis. In: ERS Monograph: Tuberculosis, editors Migliori GB, Bothamley G, Duarte R, Rendon A. Sheffield, UK: European Respiratory Society (2018). p. 234–262. doi: 10.1183/2312508X.10021817
36. Manjate N, Ventura J, Nhassengo C, Navaia A, João P, Elias B, et al. Spinal tuberculosis in young HIV-exposed infants: two cases of probable congenital transmission. Pediatr Infect Dis J. (2019) 38:e48–50. doi: 10.1097/INF.0000000000002118
37. Yeh JJ, Lin SC, Lin WC. Congenital tuberculosis in a neonate: a case report and literature review. Front Pediatr. (2019) 7:255. doi: 10.3389/fped.2019.00255
38. Grover SB, Taneja DK, Bhatia A, Chellani H. Sonographic diagnosis of congenital tuberculosis: an experience with four cases. Abdom Imaging. (2000) 25:622–6. doi: 10.1007/s002610000071
39. Chiang SS, Graham SM, Schaaf HS, Marais BJ, Sant'Anna CC, et al. Clinical standards for drug-susceptible tuberculosis in children and adolescents. Int J Tuberc Lung Dis. (2023) 27:584–98. doi: 10.5588/ijtld.23.0085
40. Obringer E, Heald-Sargent T, Hageman JR. Neonatal tuberculosis. Pediatr Ann. (2015) 44:e126–30. doi: 10.3928/00904481-20150512-12
41. Lamb GS, Starke JR. Tuberculosis in infants and children. Microbiol Spectr. (2017) 5:TNMI7. doi: 10.1128/microbiolspec.TNMI7-0037-2016
42. Abramowsky CR, Gutman J, Hilinski JA. Mycobacterium tuberculosis infection of the placenta: a study of the early (innate) inflammatory response in two cases. Pediatr Dev Pathol. (2012) 15:132–6. doi: 10.2350/11-05-1039-CC.1
43. Del Rosal Rabes T, Baquero-Artigao F, Méndez-Echevarría AN, Mellado Peña MJ. Tuberculosis in infants less than 3 months of age. Enferm Infecc Microbiol Clin. (2017) 35:243–5. doi: 10.1016/j.eimce.2017.03.001
44. Donald PR, Ronge L, Demers AM, Thee S, Schaaf HS, Hesseling AC. Positive Mycobacterium tuberculosis gastric lavage cultures from asymptomatic children with normal chest radiography. J Pediatr Infect Dis Soc. (2021) 10:502–8. doi: 10.1093/jpids/piaa113
45. Bekker A, Schaaf HS, Seifart HI, Draper HR, Werely CJ, Cotton MF, et al. The pharmacokinetics of isoniazid in low birth weight and premature infants. Antimicrob Agents Chemother. (2014) 58:2229–34. doi: 10.1128/AAC.01532-13
46. Béranger A, Bekker A, Solans BP, Cotton MF, Mirochnick M, Violari A, et al. Influence of NAT2 genotype and maturation on isoniazid exposure in low-birth-weight and preterm infants with or without human immunodeficiency virus (HIV) exposure. Clin Infect Dis. (2022) 75:1037–45. doi: 10.1093/cid/ciac001
47. Denti P, Wasmann RE, van Rie A, Winckler J, Bekker A, Rabie H, et al. Optimizing dosing and fixed-dose combinations of rifampicin, isoniazid, and pyrazinamide in pediatric patients with tuberculosis: a prospective population pharmacokinetic study. Clin Infect Dis. (2022) 75:141–51. doi: 10.1093/cid/ciab908
48. Bekker A, Schaaf HS, Draper HR, van der Laan L, Murray S, Wiesner L, et al. The pharmacokinetics of rifampicin, isoniazid, pyrazinamide and ethambutol in infants dosed at revised WHO-recommended treatment guidelines. Antimicrob Agents Chemother. (2016) 60:2171–9. doi: 10.1128/AAC.02600-15
49. Bjugård Nyberg H, Draper HR, Garcia-Prats AJ, Thee S, Bekker A, Zar HJ, et al. Population pharmacokinetics and dosing of ethionamide in children with tuberculosis. Antimicrob Agents Chemother. (2020) 64:e01984–19. doi: 10.1128/AAC.01984-19
50. Turkova A, Wills GH, Wobudeya E, Chabala C, Palmer M, Kinikar A, et al. Shorter treatment for nonsevere tuberculosis in African and Indian children. N Engl J Med. (2022) 386:911–22. doi: 10.1056/NEJMoa2104535
51. Van Toorn R, Schaaf HS, Laubscher JA, Elsland SL, Donald PR, Schoeman JF. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J. (2014) 33:248–52. doi: 10.1097/INF.0000000000000065
52. Sanchez-Codez M, Hunt WG, Watson J, Mejias A. Hepatitis in children with tuberculosis: a case report and review of the literature. BMC Pulm Med. (2020) 20:173. doi: 10.1186/s12890-020-01215-6
53. Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. (2008) 359:2233–44. doi: 10.1056/NEJMoa0800971
54. World Health Organization. Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring: March 2021. Geneva: World Health Organization; (2021). Licence: CC BY-NC-SA 3.0 IGO.
55. Cilliers K, Labadarios D, Schaaf HS, Willemse M, Maritz JS, Werely CJ, et al. Pyridoxal-5-phosphate plasma concentrations in children receiving tuberculosis chemotherapy including isoniazid. Acta Paediatr. (2010) 99:705–10. doi: 10.1111/j.1651-2227.2010.01696.x
56. Espiritu N, Aguirre L, Jave O, Sanchez L, Kirwan DE, Gilman RH. Case report: congenital transmission of multidrug-resistant tuberculosis. Am J Trop Med Hyg. (2014) 91:92–5. doi: 10.4269/ajtmh.13-0002
57. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization (2022). Licence: CC BY-NC-SA 3.0 IGO.
58. Nightingale R, Carlin F, Meghji J, McMullen K, Evans D, van der Zalm MM, et al. Post-TB health and wellbeing. Int J Tuberc Lung Dis. (2023) 27:248–83. doi: 10.5588/ijtld.22.0514
59. Schaaf HS, Gie RP, Beyers N, Smuts N, Donald PR. Tuberculosis in infants less than 3 months of age. Arch Dis Child. (1993) 69:371–4. doi: 10.1136/adc.69.3.371
60. Gafar F, Wasmann RE, McIlleron HM, Aarnoutse RE, Schaaf HS, Marais BJ, et al. Global estimates and determinants of antituberculosis drug pharmacokinetics in children and adolescents: a systematic review and individual patient data meta-analysis. Eur Respir J. (2023) 61:2201596. doi: 10.1183/13993003.01596-2022
61. Autmizguine J, Benjamin DK Jr, Smith PB, Sampson M, Ovetchkine P, Cohen-Wolkowiez M, et al. Pharmacokinetic studies in infants using minimal-risk study designs. Curr Clin Pharmacol. (2014) 9:350–8. doi: 10.2174/1574884709666140520153308
Keywords: tuberculosis, perinatal, congenital, postnatal, prevention, diagnosis, treatment
Citation: Schaaf HS, Bekker A and Rabie H (2023) Perinatal tuberculosis—An approach to an under-recognized diagnosis. Front. Public Health 11:1239734. doi: 10.3389/fpubh.2023.1239734
Received: 13 June 2023; Accepted: 13 October 2023;
Published: 07 November 2023.
Edited by:
Tinsae Alemayehu, American Medical Center, EthiopiaReviewed by:
Jombo Namushi, University of Zambia, ZambiaCopyright © 2023 Schaaf, Bekker and Rabie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: H. Simon Schaaf, aHNzQHN1bi5hYy56YQ==
 H. Simon Schaaf
H. Simon Schaaf Adrie Bekker
Adrie Bekker Helena Rabie2
Helena Rabie2