
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 24 November 2023
Sec. Digital Public Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1239231
This article is part of the Research Topic Internet of Things (IoT) and Public Health: An Artificial Intelligence Perspective View all 9 articles
Background: Infectious keratitis (IK) is a sight-threatening condition requiring immediate definite treatment. The need for prompt treatment heavily depends on timely diagnosis. The diagnosis of IK, however, is challenged by the drawbacks of the current “gold standard.” The poorly differentiated clinical features, the possibility of low microbial culture yield, and the duration for culture are the culprits of delayed IK treatment. Deep learning (DL) is a recent artificial intelligence (AI) advancement that has been demonstrated to be highly promising in making automated diagnosis in IK with high accuracy. However, its exact accuracy is not yet elucidated. This article is the first systematic review and meta-analysis that aims to assess the accuracy of available DL models to correctly classify IK based on etiology compared to the current gold standards.
Methods: A systematic search was carried out in PubMed, Google Scholars, Proquest, ScienceDirect, Cochrane and Scopus. The used keywords are: “Keratitis,” “Corneal ulcer,” “Corneal diseases,” “Corneal lesions,” “Artificial intelligence,” “Deep learning,” and “Machine learning.” Studies including slit lamp photography of the cornea and validity study on DL performance were considered. The primary outcomes reviewed were the accuracy and classification capability of the AI machine learning/DL algorithm. We analyzed the extracted data with the MetaXL 5.2 Software.
Results: A total of eleven articles from 2002 to 2022 were included with a total dataset of 34,070 images. All studies used convolutional neural networks (CNNs), with ResNet and DenseNet models being the most used models across studies. Most AI models outperform the human counterparts with a pooled area under the curve (AUC) of 0.851 and accuracy of 96.6% in differentiating IK vs. non-IK and pooled AUC 0.895 and accuracy of 64.38% for classifying bacterial keratitis (BK) vs. fungal keratitis (FK).
Conclusion: This study demonstrated that DL algorithms have high potential in diagnosing and classifying IK with accuracy that, if not better, is comparable to trained corneal experts. However, various factors, such as the unique architecture of DL model, the problem with overfitting, image quality of the datasets, and the complex nature of IK itself, still hamper the universal applicability of DL in daily clinical practice.
Keratitis is a sight-threatening condition that requires rapid and accurate management to prevent irreversible outcomes. Among all etiologies, infectious keratitis (IK) is the most common cause to corneal blindness, with an estimated incidence ranging from 2.5 to 799 per 100,000 population-year (1). Globally, it is among the top five leading causes of vision impairment and blindness, accounting for 1.5–2.0 million cases of blindness yearly (2). IK can be caused by extensive range of microbial organisms, including bacteria, fungi, viruses, parasites and polymicrobial infections. It was once considered as a ‘silent epidemic’ in low-income and middle-income countries (LMICs) with an incidence of 113–799 per 100,000 populations per year compared to high-income countries (HICs) with an incidence rate of 2.5–4.3 per 100,000 populations per year (1, 3, 4). It is very highly likely that occupational factors, such as agricultural work, environmental factors, and abuse of traditional eyedrops or eye wash contribute to the high incidence number in LMICs. These factors precipitated by limited access to primary and secondary care consequently leads to the patients to suffer from intractable infection and at higher risk of losing eyesight (1, 5, 6). Moreover, as IK usually affects patients in their productive years, it has magnified the financial burden of both individuals and the countries affected.
It is critical to note that the outcome of IK depends heavily on prompt treatment following a timely and accurate diagnosis. Current practice of IK diagnosis that involves utilizing slit lamp photography of infectious cornea disease among cornea specialists has been reported to only yield 66.0–75.9% of accuracy in differentiating bacterial and fungal keratitis (7). Corneal scraping and biopsy come as the ‘gold standard’ method for definitive IK diagnosis. However, these approaches are oftentimes dampened by the challenges in LMICSs where there may be a lack of access ophthalmic units with clinical experts and standardized investigating equipment, resulting in reliance in empirical treatment and delay in definitive treatment. Moreover, even when the option of microbiological workups is available, this procedure might also take days before producing any results. These challenges may be held accountable for poorer clinical outcome and higher risk of irreversible sequalae. On the other hand, poorly differentiated clinical feature may also lead to misdiagnosis, which may result in disastrous cascade of inappropriate treatment, increasing the risk of unidentified clinically essential lesions (8), making human decision-making for diagnosis even more difficult.
Artificial intelligence (AI) has captured the attention of medical specialties, such as ophthalmology, dermatology, and radiology, where visual analysis and interpretation skill are highly demanded to create diagnosis. Deep learning (DL), a subfield of AI, has shown promising potential in assisting automated clinical diagnosis and decision-making by performing high-dimensional analyzes, henceforth improving healthcare efficiency. The ophthalmology field is not a stranger to DL itself, with previous studies have reported diagnostic accuracy that, if not better, is comparable to clinical experts in making the diagnosis of posterior segment diseases, including macular degeneration, glaucoma, and diabetic retinopathy (9–14). One DL-based technology has also been reported with remarkable sensitivity and specificity, with the respective number of 91.3 and 91.1% in detecting vision-threatening diabetic retinopathy (14). Apart from its accuracy, availability of DL may also enable clinician to provide better care without needing to accommodate resources in constant purchasing, maintaining, training, and upgrading expensive capital for diagnostic laboratory equipment and technicians. Furthermore, it may also assist ophthalmologists and even empower untrained clinicians to diagnose IK in resource-scarce regions and take the needed actions, therefore diminishing its progressivity to debilitating corneal-related blindness. Despite its promising potential and the number of studies demonstrating high accuracy of DL in IK diagnosis and recognizing IK apart from other ocular diseases (8, 15–20), its diagnostic accuracy remains to be elucidated.
To the best of our knowledge, there is no published systematic review and/or meta-analysis on this topic to this date. Accordingly, we conducted a comprehensive systematic review and meta-analysis aiming to evaluate the accuracy of the available artificial intelligence algorithms to correctly classify infectious keratitis based on the etiology compared to its respective gold standard, i.e., the human counterparts. It is expected that this systematic review and meta-analysis may be a strong basis for the consideration of DL deployment into clinical practice, therefore preventing delay and providing better care for IK patients.
Literature was searched in six online scientific databases (PubMed, Google Scholar, ProQuest, ScienceDirect, Cochrane, and SCOPUS) on December 10th, 2022. Search phrases include a combination of all main keywords and their related terms: “artificial intelligence” and “keratitis,” as described in Table 1. Reference lists of each study were also analyzed for potentially relevant articles. Identification of new studies via other methods was not performed. There was no restriction in the publication year.
Based on the search strategies previously described, articles were considered eligible to be reviewed if the article met the following criteria: (1) study on human; (2) corneal images were based on diffuse slit lamp photography of the eye; (3) validity study on DL performance. The flow chart of the literature search was described in Figure 1 based on preferred reporting items for systematic reviews and meta-analyzes (PRISMA) flow chart.
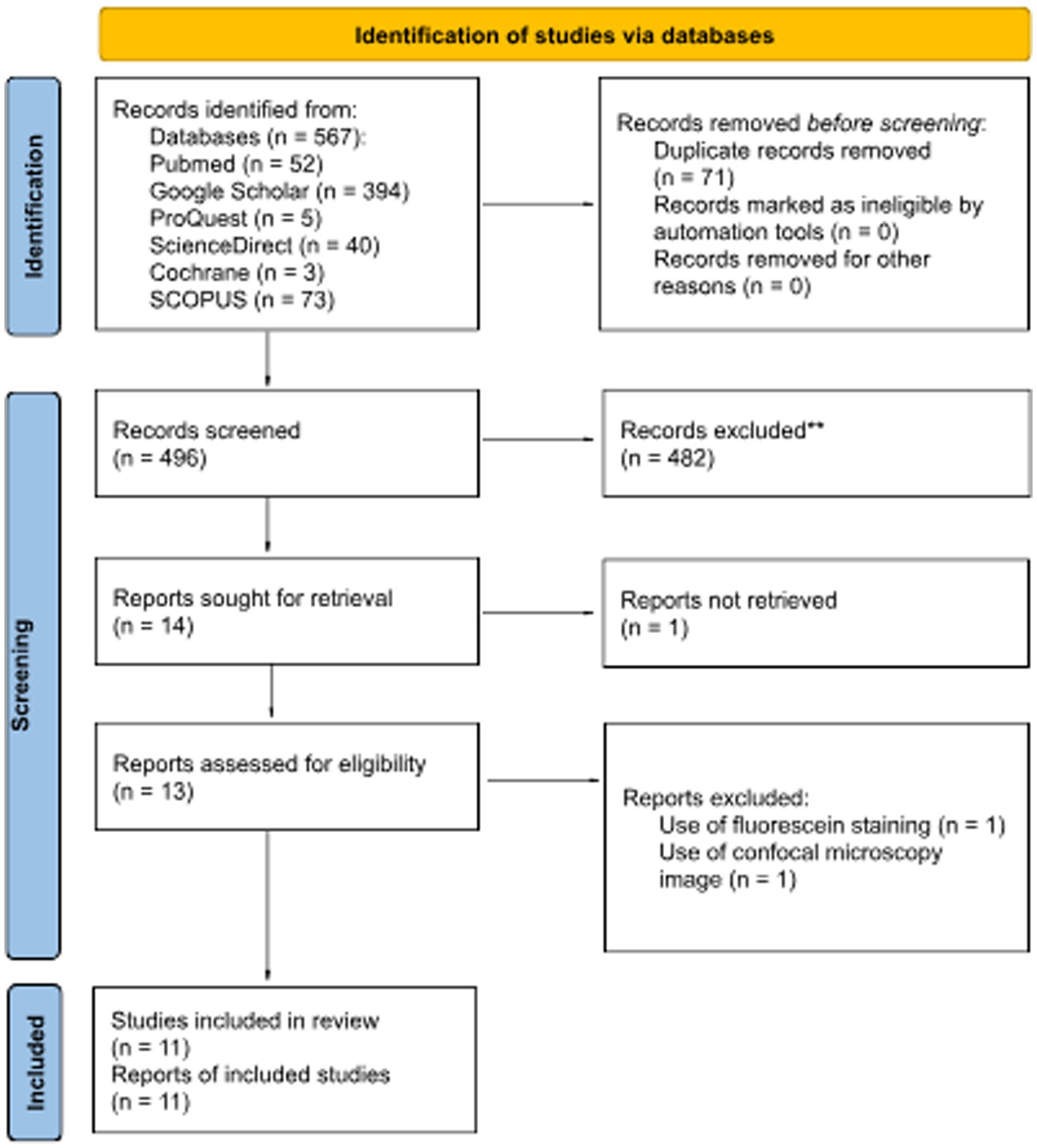
Figure 1. PRISMA 2020 flow diagram for updated systematic reviews that included searches of databases and registers.
Validity assessment of articles previously met our criteria using an appraisal tool for diagnostic study by the Center for Evidence-Based Medicine (CEBM), University of Oxford. Data validity, result, and applicability were assessed in all included studies. A total of 5 parameters were used, each with the results of Yes, No, or Unclear. All authors discussed discrepancies in the assessment results.
The information extracted from each study includes the authors, the year the study was reported or published, baseline characteristics of subjects, number or proportion of dataset and test images, and AI algorithm name. The primary outcomes reviewed were the accuracy and classification capability of the AI/machine learning/deep learning algorithm in terms of Receiver Operator Characteristic (ROC) curve or Area Under the Curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV).
The extracted data were then analyzed with the MetaXL 5.2 Software. All data on accuracy and AUC on the performance of AI on infectious keratitis diagnosis were pooled and shown in the form of forest plots and summary tables for visualization.
A total of eleven articles were included in this review. These articles study the accuracy of various machine learning algorithms in solving the classification of keratitis based on diffuse slit lamp photography as compared with the human counterparts and cross-referenced with the respective laboratory diagnosis as the confirmatory tests.
All eleven relevant articles were assessed for validity using standardized critical appraisal tools for diagnostic study by CEBM before proceeding to data synthesis. Validity assessment based on the CEBM Diagnostic Study Appraisal Worksheet was summarized in Table 2.
Eleven eligible studies published between 2003 and 2022 were assessed (Table 3). Studies were conducted mainly in Asian and Australian populations, with samples acquired from the People’s Republic of China, India, Japan, Thailand, and Australia with 34,070 data from slit-lamp images.
Nine out of the eleven studies classify IK based on two or more etiologies in which all the five studies have bacterial keratitis group; other etiologies of IK, such as fungal, viral, or parasitic are not continually assessed separately. Two studies by Li et al. (15) and Gu et al. (25) did not classify IK based on the causative microorganisms (6,055 and 845 IK images of non-specific etiologies respectively). However, these two studies compared normal cornea, infectious, and non-infectious keratitis (corneal dystrophy, degeneration, or neoplasm). No other study assessed normal corneas, with a striking amount of 6,055 [Li et al. (15)] and 870 [Gu et al. (25)] total images in their dataset. Four studies [Zhang et al. (22), Koyama et al. (16), Kuo et al. (26), Xu et al. (8)] specified viral keratitis as herpes simplex keratitis (HSK), but Sajeev et al. (24) did not specify the specific virus in the classification as the causative agent of the IK. The complete dataset classification can be seen in Figure 2 (Supplementary Table S1).
Seven of the eleven studies compare the performance of AI models with clinicians’ diagnoses made by trained ophthalmologists, corneal specialists, or non-corneal specialists. Redd et al. (21) have twelve local expert cornea specialists performing a remote interpretation of the images used in the same test set to enable direct comparison against CNN performance. Zhang et al. (22) invited three corneal specialty ophthalmologists to assess the external validation set to make a clinical diagnosis. Zhang et al. (22) invited three corneal specialty ophthalmologists to assess the external validation set to make a clinical diagnosis. Saini et al. (20) used only two corneal clinicians as a comparison. Other studies went to a greater extent to assess the human-machine comparison. Xu et al. (8) used 21 ophthalmologists trained with 120 images before the grading process, Gu et al. (25) enrolled 20 trained ophthalmologists as well as two corneal specialists to diagnose slit-lamp images of healthy and abnormal cornea conditions clinically, and Koyama et al. (16) tested 35 board-certified ophthalmologists including 16 faculty members of corneal specialist diagnosis accuracy against their AI algorithm using a diagnostic software named “KeratiTest.” Kuo et al. (26) evaluated the machine learning algorithm against two different levels of human expertise in corneal diseases: assessing a group of 3 non-cornea specialists against three corneal specialists to show if there is any difference in accuracy between the subgroups.
All eleven studies use respective laboratory examinations such as culture or direct microscopy or PCR as the reference standard for diagnosis based on the patient’s medical records in the database and prospective data. Although Sajeev et al. (24) and Gu et al. (25) did not explicitly state that the diagnoses retrieved from their database were based on a confirmatory laboratory test. The article by Saini et al. (20), however, went a step further to evaluate the correct diagnosis of IK based on the clinical improvement of the treated eye as an additional parameter of accurate diagnosis.
The established AI models presented in this review are listed in Table 4 (DenseNet121, DenseNet169, DenseNet201, EfficientNet-b0, EfficientNet-b3, EfficientNet-b5, EfficientNet-b7, Ensemble, GBDT, GoogleNet-V3, Inception-V3, InceptionResNetV2, Mobile-NetV2, ResNext101_32x8d, ResNext101_32x16d, ResNet18, ResNet34, ResNet50, ResNet101, ResNet152V2, VGG16, VGG19, Xception) were all based on convolutional neural network (CNN), a class of artificial neural network, most commonly applied to analyze visual imagery. Three studies by Sajeev et al. (24), Gu et al. (25), and Saini et al. (20) developed their algorithm to evaluate its accuracy or compare it to other established deep learning algorithms in classifying IK. Zhang et al. (22) used model blending technology and constructed KeratitisNet, their final chosen modeling method from the combination of RexNext101_32x16d and DenseNet169. The detailed training and validation process and number of images enrolled in each step were only explicitly documented in four out of ten articles [Zhang et al. (22), Hung et al. (18), Sajeev et al. (24), and Kuo et al. (26)].
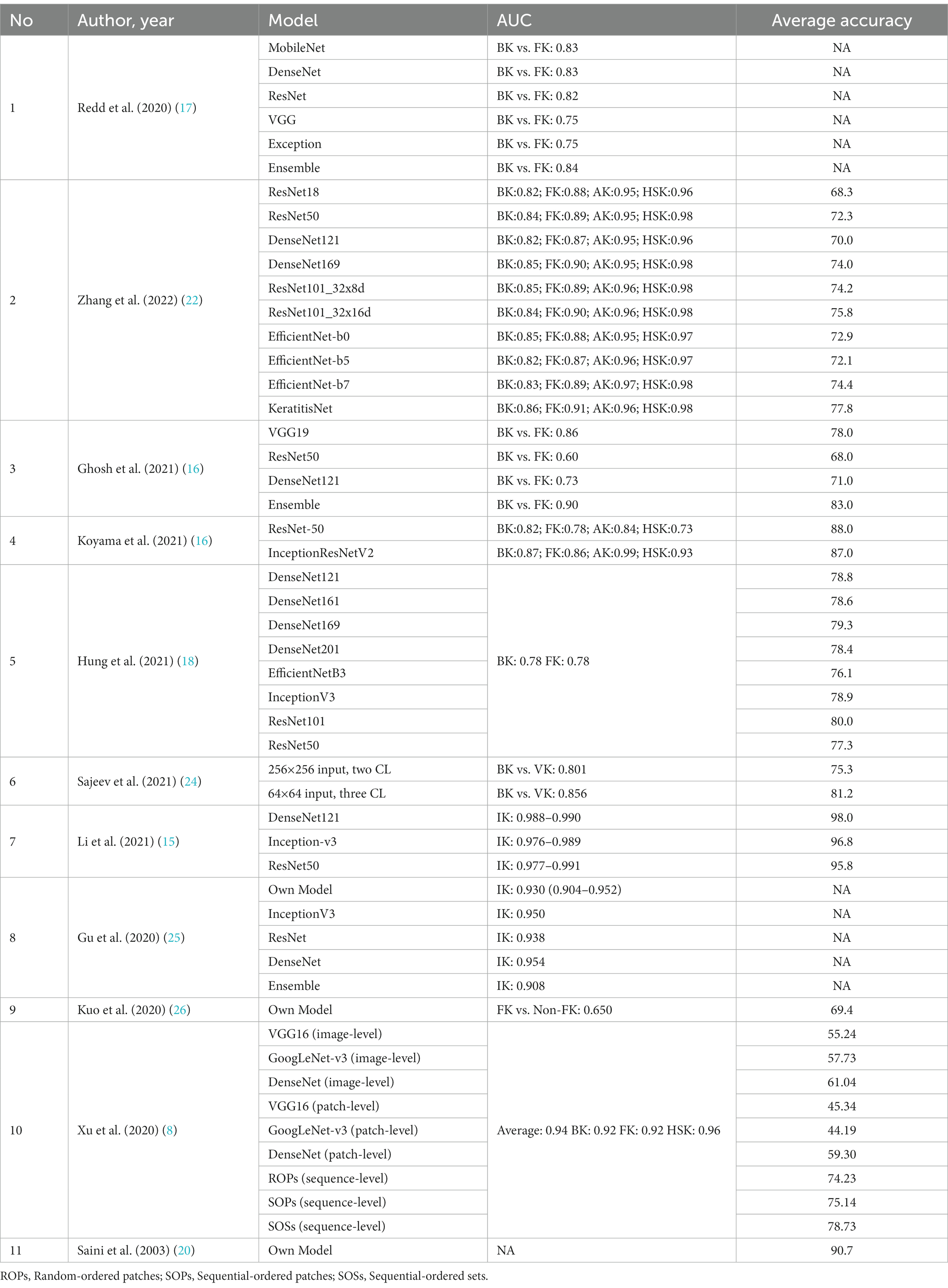
Table 4. AUC and accuracy of different deep learning models used in the classification of IK in each study.
All eleven studies used diffuse illumination slit-lamp photography of the corneal surface as the input. However, Hung et al. (18) removed the conjunctival and palpebral region to focus the observation area on the corneal area.
The number of image datasets used to train and validate the deep learning algorithm in the ten included studies varies greatly. The highest number of images for training per causative microbes’ classification is 1,690 fungal keratitis photographs in Zhang et al. (22). Study by Saini et al. (20) included 63 combination images of IK from bacterial and fungal origin. Li et al. (15) collected 6,055 IK images of unstated microorganism origin.
In the paper by Li et al. (15) and Gu et al. (25), which assessed the performance of DL algorithms to differentiate between IK and non-IK without classifying it based on the causative organism, the highest average accuracy was achieved by DenseNet121 with 98.0% accuracy, and ResNet50 achieved the lowest accuracy of 95.8%. All DL architectures in these two studies could successfully differentiate IK from non-IK with an AUC of 0.908–0.991. In classifying IK based on its respective etiologies, various available models such as InceptionV3, ResNet, and DenseNet had shown a considerable performance of 70–80% accuracy. One of the earliest DL models utilizing CNN to classify BK vs. FK was by Saini et al. (20) in 2003, which had an accuracy rate of 90.7%. The later model achieves 81.2 accuracy at maximum [BK vs. VK by Sajeev et al. (24)]. The latest study by Zhang et al. (22) compared nine model development methods and introduced KeratitisNet, demonstrating the highest average accuracy in this study. Another study by Li et al. (15) also included smartphone macro corneal photography as a dataset. It surprisingly yielded a favorable outcome, with the best algorithm reaching an AUC of 0.967 in keratitis detection. A summary of all the reviewed DL models is presented in Table 4.
Eight of the eleven included studies reviewed the performance of ophthalmologists of varying experience or affiliation and whether additional information or training improved visual diagnostic of IK from the available slit-lamp image data. The results showed that classifying the etiology of IK based on visual clues alone is not trivial for human experts. For instance, the study by Redd et al. (21) with AUC of 0.76 compared to 0.84 in CNN ensemble or Zhang et al. (22) with experts’ diagnostic accuracy of 31–59%. Additional patient history information, IK lesion training, and experience in cornea diseases improve human performance, as found in Li et al. (15) with increased accuracy in 6 years-experienced compared to 3 years-experienced. However, the level of accuracy was not satisfactory in almost all studies, with the highest accuracy performance being only 75% for fungal keratitis by corneal specialists in Kuo et al. (26) and did not exceed any higher than 58% even for ophthalmologists who were actively involved in teaching hospitals in Xu et al. (8). Only in a study by Li et al. (15) can a corneal specialist with 3 to 6 years of experience achieve 96% accuracy.
The AUC and accuracy in the classification of IK by the currently developed deep-learning models were summarized in Table 5. In almost all the studies, the deep-learning model was on par at a minimum compared with humans in classifying IK. In fact, in seven of the eight studies, AI models outperformed their human counterparts.
The data analysis was conducted using the data on AUC and accuracy as the performance metrics. The AUC analysis of bacterial keratitis vs. fungal keratitis used datasets from two included studies. The pooled results have shown the Receiver Operating Characteristics (ROC) curve area of 0.895 (95% CI: 0.512–1.000; p < 0.001), with homogenous data (Figure 2). On the contrary, the data of AUC analysis on infectious keratitis vs. non-infectious keratitis were heterogenous with I2 (inconsistency) of 98.51%. On the random effects analysis, the pooled AUC value was 0.851 (95% CI: 0.693–1.000) (Figures 3, 4). It has shown both studies’ relatively high performance of the artificial intelligence model.
Based on the results of the analysis of accuracy rate, the total accuracy value of the twelve tools tested in two studies was obtained, the study by Hung et al. (18) and Ghosh et al. (23) in differentiating between bacterial keratitis and fungal keratitis amounting to 64.38% (Figure 5). at the same time, the accuracy value of the 3 tools tested by Li et al. (15) was 96.6% (Figure 6).
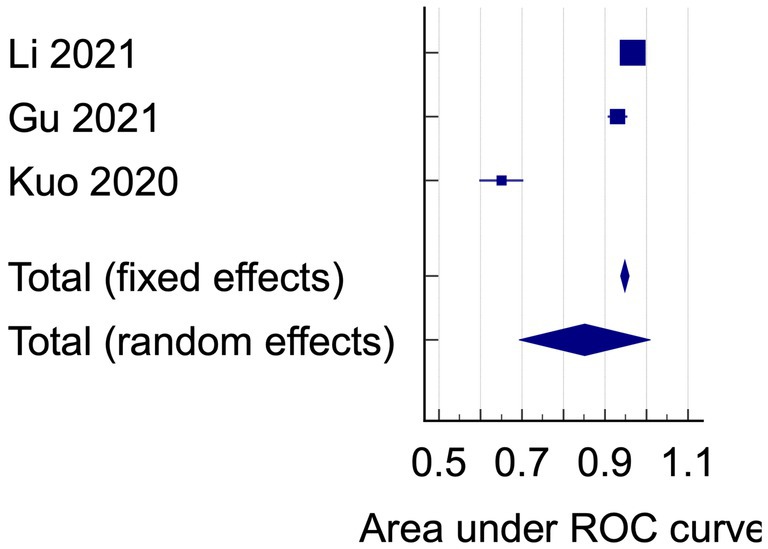
Figure 4. The area under ROC curve pooled analysis on infectious keratitis vs. non-infectious keratitis.
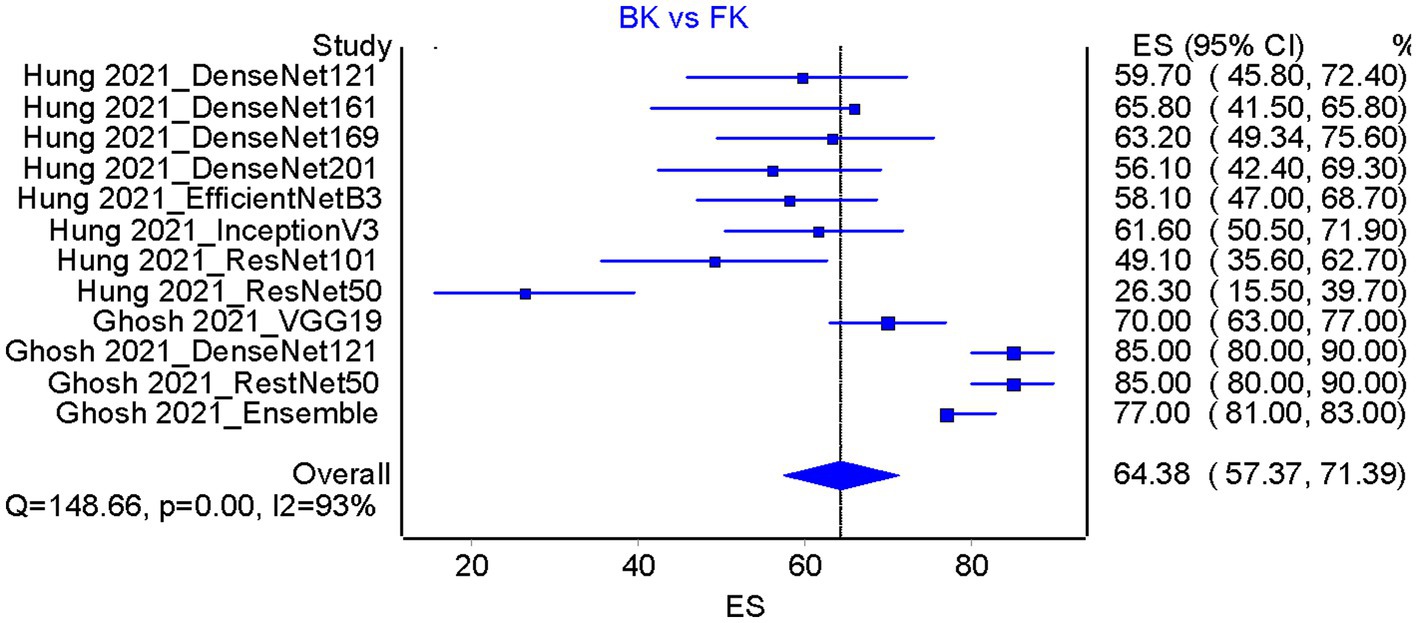
Figure 5. Accuracy pooled analysis on bacterial keratitis vs. fungal keratitis in two included studies [Hung et al. (18) and Ghosh et al. (23)]; ES, Effect size; CI, Confidence interval.
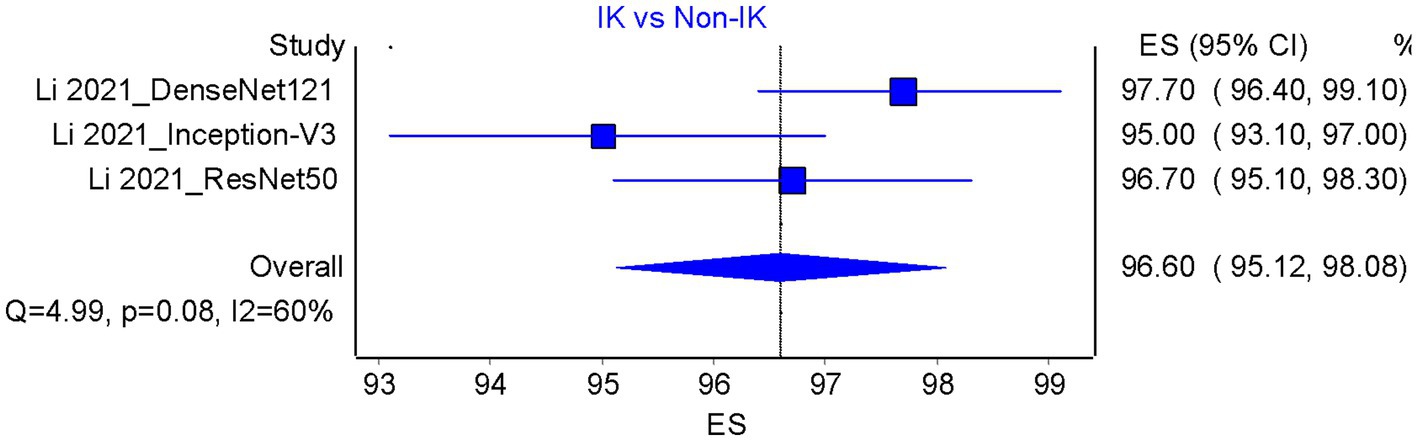
Figure 6. Accuracy pooled analysis on infectious vs. non-infectious keratitis; ES, Effect size; CI, Confidence interval.
Our systematic review has shown the performance of artificial intelligence, particularly the DL model in identifying infectious keratitis. Most models perform fairly well, as seen in high accuracy and AUC analysis. The highest being DenseNet121 by Li et al. (15). Meanwhile, on the comparison between DL and human experts, DL has significantly higher accuracy than human experts in all studies, which shows a good performance of DL in IK diagnosis. On the pooled analysis, an AUC value of 0.895 on identifying bacterial keratitis vs. fungal keratitis, while AUC of 0.851 were retrieved for identifying infectious vs. non-infectious keratitis. On the accuracy analysis, pooled accuracy on BK vs. FK was 64.38%. However, the accuracy in identifying infectious vs. non-infectious keratitis was 96.6%, showing a high DL accuracy rate. The DL-based systems can learn to recognize more complex patterns and therefore have gained a particular reputation for complex applications such as ophthalmology, which require extensive image recognition tasks to identify specific clinical patterns for accurate diagnosis. Despite its growing popularity, the DL framework in ophthalmology is still in its earliest stage (27). Most DL models used to classify IK in the ten included studies used CNNs as the main framework. The CNN is the most popular neural network model for image classification. The main idea behind CNNs is to decompose image understanding into more straightforward mapping, each described by a layer in the model. For example, to recognize an apple, we teach the computer to progressively recognize lines, edges, corners, textures, and eventually apple. This notion brings about the practical advantage of requiring less feature engineering for object identification which eventually results in a more time-efficient and adequate training of the machine learning models.
Finding the best DL model for classifying IK is not trivial. It involves tuning various hyper-parameters that affect the performance and accuracy of the model. However, some studies have shown that more novel architectures, such as DenseNet, outperform other models in this task. DenseNet is a new neural network architecture for visual object recognition with a dense connectivity pattern. It means that each layer is connected to every other layer and reuses them in all the subsequent layers, hence the name Densely Connected Convolutional Network. This feature enhances the model by allowing it to learn more diverse and rich features from the input data. However, DenseNet is one of many possible architectures that can achieve good results on IK classification. Furthermore, models with larger capacities, e.g., deeper or broader networks, can eventually outperform smaller models when more extensive data is provided. Therefore, future works should continuously evaluate multiple architectures and compare their performance and characteristics on this problem.
Even if DenseNet seemed to have the highest average accuracy, some models have better accuracy in performing a specific classification of IK. For example, in the algorithms developed by Xu et al. (8), the sequence-level classification model using ROPs (74.2%) may have a lower average accuracy than SOSs (78.7%). However, ROPs performance in diagnosing bacterial keratitis outperformed SOSs by nearly 10% (75.3% vs. 65.1%, respectively). Hence, using more than one model for each type of IK is one way to push the limit for diagnostic supremacy further. This approach is referred to as model ensemble and is an effective strategy in improving the performance of machine learning algorithms (28). Redd et al. (21) evaluated five CNNs using images of culture-proven IK and reported a higher diagnostic accuracy than cornea experts in diagnosing BK and FK (area under the ROC = 0.84 vs. 0.76). Zhang et al. (22) combine ResNext101_32x16d and DenseNet169, giving birth to the chosen final modeling method named KeratitisNet, which demonstrated the best performance and highest accuracy in diagnosing BK, FK, HSK, and AK compared with 9 other individual models. The danger of overfitting could also threaten the early development of the DL model. The pioneering model by Saini et al. (20) in 2003 correctly classified all 63 cases in the training set and performed significantly better at 90.7% accuracy, while the clinicians could only yield 62.7%. Hence, overfitting could explain how such a model could perform significantly better than the later models with more images despite containing the least number of training datasets. An overfitted function will tend to require more information and be less flexible to be applied to the real-world dataset than an optimal function. Multiple deep learning architectures with better performance are introduced every year. Given enough data, it is likely that more powerful networks will achieve better performance for the task at hand. However, the quest to find the one-model-to-fit-all may be challenging. Classifying a corneal ulcer may not be a straightforward function as the real-world application will not be as simple as bacterial and fungal alone. Duration of onset, other classes of microbial infection, mixed infection, and image quality may add to the complexity of the model.
The overall performance of the deep learning algorithm in the included studies showed that the model outperformed the average ophthalmologist and was comparable to or better than the experience corneal specialist with years of experience in classifying IK, especially for bacterial and viral keratitis. For fungal keratitis, Xu et al. (8) reported diagnostic rates of 83.3% (higher than that of bacterial keratitis of 53.3% accuracy) using a deep sequential-level learning model with slit-lamp photographs. The accuracy of their model exceeded that of the humans by an impressive number (49.3% ± 11.9%) for over 120 test image samples. The study by Zhang et al. (22) showcases that the algorithm outperformed ophthalmologists for all 4 causative types of keratitis (BK, FK, HSK, and AK). Exceptions were found in the study by Kuo et al. (26) in which the average performance of the DL models was not as good as the overall accuracy of experts but still better than the non-corneal specialist ophthalmologist. However, even if we took a pessimistic stance towards DL classification for IK, with its sub-par performance, the DL model can still benefit primary care where most patients with IK presented. Even with a slightly lower accuracy than the experienced ophthalmologist, it will be beneficial to identify fungal keratitis since anti-fungal medications are not the empirical therapy for IK and false-negative diagnosis for FK can be devastating to the visual prognosis.
Diagnosis of less common or more complex IK such as mixed infection requires an experienced ophthalmologist or specially trained physician, or it may be missed. There is a need for further research regarding the deep learning algorithm’s ability to detect such cases which will eventually be faced in primary care settings even if it may be rare. Another critical group of classification will be the poor-quality images. Both poor-quality images and mixed infection are usually excluded in many studies with the primary intention to reduce bias or if included, will be in a small proportion. Interestingly, one paper by Li et al. (15) considered the performance of the best algorithm with poor-quality images and showed just a slightly lower AUC (0.004–0.012 difference); this number may give a hint that the performance of DL system classification even in poor-quality images will still be considered satisfactory. A study by Koyama et al. (16) also tests the algorithm’s robustness by the absence of fluorescein staining and using low-resolution web images, resulting in a minimal decrease in diagnostic efficacy. Currently, there is no minimum sample size for image dataset for a DL system to achieve a minimum acceptable performance; it is generally understood that there is no limit and the more the better, be it in the quantity or the quality of the image for training and validation of a DL architecture. In this review, Li et al. (15) study with over 6,567 images for validation, training, and testing. Using such datasets, DenseNet 121, Inception V3, and ResNet 50 could achieve accuracy between 87.1 and 98.9% in differentiating infectious keratitis vs. normal cornea and other types. This number may appear to be significant in comparison with other conventional diagnostic studies, but this number is minuscule for the development of a DL network.
To further improve DL accuracy, both the quantity and quality of the dataset input are the holy grail. Beyond just the photographs of the cornea, additional clinical data such as patient’s history, visual acuity, intraocular pressure, onset, progression, and corneal sensitivity data may further enhance the diagnostic accuracy of the deep learning model. Integration of multiple modalities may also improve the diagnostic efficacy as shown in study by Zhang et al. (22). The study by Xu et al. (8) which focused on the specific parts of infectious lesions, can yield higher accuracy than seeing the whole eye illustrate this idea well. In Xu’s study, the DenseNet model improved its performance from 60.0 to 66.3% after “voting” in patch-level classification. ROP (random-ordered patches) method can also be an alternative besides voting. However, SOS (inner-outer sequential order classification) is the most promising method to achieve the best accuracy of IK diagnosis based on the slit-lamp image alone. In addition, an AI system fed with additional clinical data or medical history might improve its performance, just like when an ophthalmologist’s image-only diagnostic improves when more patient information is integrated into the decision-making process.
Together with other DL models, such as ResNet, Inception, GoogleNet, and VGGNet, they had been made as an open-source algorithm that enabled any data scientist or layperson with sufficient programming knowledge to reproduce the basic model. This inclusive, open science environment has made the development of DL rapidly bloom and hopefully remains in such a way. However, certain adjustments must be individually modified to refine the accuracy of each model. This unique fine adjustment to the base model is usually achieved by trial and error and may not be suitable for all corneal images with IK worldwide. Hence, some models may perform slightly better than others in different geographical conditions, population groups, and etiology of IK. However, the excellent accuracy of the stated model in the eleven studies comes at a price. It required a good amount of training images, in quantity and quality to yield such results. Despite the open access nature of the currently available DL models, another factor that may also hamper the replicability of the DL study is the availability of the sample images or dataset for validity and training of the DL algorithm.
Increasingly there has also been an open-source dataset for eye disease available to be accessed, such as github.com or kaggle.com. However, this dataset was limited by its variety, mainly sourced from Google image, and most importantly lacked the correct labeling; hence, the dataset was considered unsuitable for serious training purposes. Most models in the eleven included studies utilized well-established datasets from electronic or conventional medical records of an academic institution or hospital in the People’s Republic of China, Australia, or India. The generalizability of models reviewed for use in other populations of IK patients should be concerned, especially if used with eyes with a different anatomical structure (e.g., different ethnicities, other ocular pathology).
Furthermore, these countries had strict regulations regarding personal data protection, especially across borders, which makes replicating such studies using the same data samples impossible by individual or study group without authority. For example, The Australian legislation system has The Privacy Act (1988) which protects the handling of personal information about individuals. It includes the collection, use, storage, and disclosure of personal information in the federal public sector and the private sector. Use of Healthcare Identifiers in Australia, and access to the My Health Record system, are governed by the Healthcare Identifiers Act 2010 (HI Act) and the My Health Records Act 2012, the My Health Records Rule 2016, and the My Health Records Regulation 2012. Whereas in China, the Data Security Law (DSL) sets up a framework that classifies data collected and stored in China based on its potential impact on Chinese national security and regulates its storage and transfer depending on the data’s classification level. China’s Personal Information Protection Law (PIPL) is also in force starting August 2021, laying out ground rules around how data is collected, used, and stored. Multinational corporations (MNCs) that move personal information out of the country also will have to obtain certification on data protection from professional institutions, according to the PIPL. In the case of IK dataset, a center that would like to have a comprehensive DL-aided diagnosis for IK may have to work itself by collecting high-quality training images either from slit-lamp clinical diagnosis made by an experienced corneal specialist or through confirmatory lab tests to obtain the local rate of accuracy for implementation of DL system for IK classification since the commercially available or approved system is not available yet to this date despite its promising development. Another condition that may hamper the application of DL-assisted diagnosis of infectious keratitis is the availability of the respective treatment. In an area with a shortage of qualified diagnosticians or confirmatory laboratory facilities, scarcity of antimicrobial eye drops, be it antibiotic or anti-fungal, will usually co-exist. Unless the same effort to improve the availability of IK treatment has been achieved, the advantage of having a cheaper, faster, or more accurate diagnostic tool may not bring additional benefits to the patient.
The fact that classification of IK is still very challenging even for trained humans with years of experience with IK lesions, developing a deep-learning model to help physicians to gain the upper hand in the war against IK for accurate diagnosis and prompt treatment can be a worthwhile effort to be made especially in countries where establishing multiple laboratory facilities is geographically or financially challenging, or where the availability of expert diagnostician is scarce and may take too long to be trained. Although there has not been any cost-effectiveness study regarding this yet, with the advent of cloud computing and the increasing availability of internet access, it may be more economically sound and efficient to set up one DL-assisted diagnostic model which can cover a wider area at a lower cost to solve the blindness problem arising from delay or mistreatment of IK in less time and with fewer resource than to invest in the high-cost and high-maintenance laboratory facility. However, cost analysis on the use of AI in ophthalmology has shown significant cost-saving nature of the implementation, such as a report by Huang et al. (29), which shows cost-effective screening of diabetic retinopathy using AI compared to traditional screening. Ruamviboonsuk et al. (30) also stated a similar report; however, the research also suggests the cost of AI implementation system in the early years might be enormous and not applicable, particularly in low or middle-income countries (22).
The “black box” nature of deep learning will still be an endless debate. It may deter skeptical future users from using deep learning-assisted diagnostic tools because it lacks an explanation despite their proven accuracy. However, this issue can be addressed with the GRAD-CAM or GRAD-CAM++ (31, 32) which shows a gradient-weighted heat map for a visual explanation of what the CNN observed as a potential corneal lesion, making cross-checking by humans possible. Although currently this system is still far from perfect, with these heatmaps, a certain level of accountability and human ‘trust’ for the machine-generated visual diagnosis will likely improve as better algorithms that adopted in future studies.
Firstly, even with confirmatory lab examinations, there is still room for error in corneal sampling in IK. Not all studies included in this review followed the current standard method of sample collection in IK by collecting 5–6 specimens from each affected eye using various collection devices. This collecting method increases the chance of retrieving a responsible pathogen from the corneal tissue, which has a relatively low microbial count compared to other anatomical parts. Taking multiple corneal scrapes with sharp and uncomfortable instruments is troublesome for patients and clinicians. It also increases the likelihood of contamination from inappropriate techniques, open plates, and specimen handling by non-laboratory-trained personnel (33). Not all studies adhered to the strict standard procedure of specimen collection; hence, this flaw underlies all current gold standard methods of IK diagnosis. In addition to the constraints encountered with direct microscopic pathogen identification as the gold-standard method, clinical or therapeutic diagnoses made by ophthalmologists are even more likely to compromise their accuracy, as demonstrated by some studies mentioned beforehand. Using studies with suitable sampling methods, can reduce the future dependency on a method that had an inherited low-reproducibility and low-reliability method, not to mention expensive to be maintained. Hence, in the real-world setting, using a combination of clinical diagnosis by ophthalmologists, lesion improvement by respective microbial therapy, and direct laboratory identification might still be closer to the ideal standard of IK diagnosis than just relying on one method of diagnosis.
Secondly, the ophthalmologists in each study are of a different experience. Even with the implementation of prior standardized training such as in the study by Zhang et al. (22), Gu et al. (25), and Xu et al. (8), the inter-observer variability data of the clinicians were not explicitly reviewed. Hence, comparing the overall performance of man vs. machine is difficult.
Lastly, this review only included articles or studies from online databases without manual searching. Despite the open science nature of AI-related study, the chance for unpublished material for this topic is still highly possible from the ever-growing nature of the framework and the likelihood of undisclosed research for commercial development.
Our study has demonstrated the promising potential of DL to diagnose and classify IK with high accuracy that competes with trained clinical experts despite the variability within the DL models architectures among the reviewed articles. However, the variability of the architectures, in addition to the nature of IK itself remain a question whether there is one most reliable model to be used in daily clinical practice. It is expected that with the continuous growth of DL advancement and future studies of DL use in IK, debilitating visual impairment or blindness due to IK can be prevented. Therefore, future studies that further analyze the accuracy of DL that also weighs in other factors are still needed.
RanS and YL conceptualized the review. RanS designed the search strategies and drafted the initial manuscript. RanS, YL, and RatS critically reviewed all the included studies and revised the manuscript. AS provided expertise and added the discussion in the Artificial Intelligence or Deep Learning aspect of the study. YL and RatS approved the final manuscript and acted as the guarantor of the study. YL acts as the correspondence of the review and analysis. All authors contributed to the article and approved the submitted version.
The authors received no financial support for the research, authorship, and/or publication of this article.
AS was employed by the company Siemens Healthineers.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1239231/full#supplementary-material
1. Ting, DSJ, Ho, CS, Deshmukh, R, Said, DG, and Dua, HS. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye. (2021) 35:1084–101. doi: 10.1038/s41433-020-01339-3
2. Flaxman, SR, Bourne, RRA, Resnikoff, S, Ackland, P, Braithwaite, T, Cicinelli, MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. (2017) 5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5
3. Ting, DSJ, Ho, CS, Cairns, J, Elsahn, A, Al-Aqaba, M, Boswell, T, et al. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: the Nottingham infectious keratitis study. Br J Ophthalmol. (2021) 105:328–33. doi: 10.1136/bjophthalmol-2020-316128
4. Collier, SA, Gronostaj, MP, MacGurn, AK, Cope, JR, Awsumb, KL, Yoder, JS, et al. Centers for Disease Control and Prevention (CDC). Estimated burden of keratitis--United States, 2010. MMWR Morb Mortal Wkly Rep. (2014) 63:1027–30.
5. Gopinathan, U, Sharma, S, Garg, P, and Rao, G. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. (2009) 57:273–9. doi: 10.4103/0301-4738.53051
6. Ting, DSJ, Cairns, J, Gopal, BP, Ho, CS, Krstic, L, Elsahn, A, et al. Risk factors, clinical outcomes, and prognostic factors of bacterial keratitis: the Nottingham infectious keratitis study. Front Med (Lausanne). (2021) 8:715118. doi: 10.3389/fmed.2021.715118
7. Dalmon, C, Porco, TC, Lietman, TM, Prajna, NV, Prajna, L, Das, MR, et al. The clinical differentiation of bacterial and fungal keratitis: a photographic survey. Invest Ophthalmol Vis Sci. (2012) 53:1787–91. doi: 10.1167/iovs.11-8478
8. Xu, Y, Kong, M, Xie, W, Duan, R, Fang, Z, Lin, Y, et al. Deep sequential feature learning in clinical image classification of infectious keratitis. Engineering. (2021) 7:1002–10. doi: 10.1016/j.eng.2020.04.012
9. De Fauw, J, Ledsam, JR, Romera-Paredes, B, Nikolov, S, Tomasev, N, Blackwell, S, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. (2018) 24:1342–50. doi: 10.1038/s41591-018-0107-6
10. Ting, DSW, Cheung, CY-L, Lim, G, Tan, GSW, Quang, ND, Gan, A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. (2017) 318:2211–23. doi: 10.1001/jama.2017.18152
11. Al-Aswad, LA, Kapoor, R, Chu, CK, Walters, S, Gong, D, Garg, A, et al. Evaluation of a deep learning system for identifying glaucomatous optic neuropathy based on color fundus photographs. J Glaucoma. (2019) 28:1029–34. doi: 10.1097/IJG.0000000000001319
12. Gargeya, R, and Leng, T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology. (2017) 124:962–9. doi: 10.1016/j.ophtha.2017.02.008
13. Goh, JKH, Cheung, CY, Sim, SS, Tan, PC, Tan, GSW, and Wong, TY. Retinal imaging techniques for diabetic retinopathy screening. J Diabetes Sci Technol. (2016) 10:282–94. doi: 10.1177/1932296816629491
14. Bhaskaranand, M, Ramachandra, C, Bhat, S, Cuadros, J, Nittala, MG, Sadda, SR, et al. The value of automated diabetic retinopathy screening with the EyeArt system: a study of more than 100,000 consecutive encounters from people with diabetes. Diabetes Technol Ther. (2019) 21:635–43. doi: 10.1089/dia.2019.0164
15. Li, Z, Jiang, J, Chen, K, Chen, Q, Zheng, Q, Liu, X, et al. Preventing corneal blindness caused by keratitis using artificial intelligence. Nat Commun. (2021) 12:3738. doi: 10.1038/s41467-021-24116-6
16. Koyama, A, Miyazaki, D, Nakagawa, Y, Ayatsuka, Y, Miyake, H, Ehara, F, et al. Determination of probability of causative pathogen in infectious keratitis using deep learning algorithm of slit-lamp images. Sci Rep. (2021) 11:22642. doi: 10.1038/s41598-021-02138-w
17. Tiwari, M, Piech, C, Baitemirova, M, Prajna, NV, Srinivasan, M, Lalitha, P, et al. Differentiation of active corneal infections from healed scars using deep learning. Ophthalmology. (2022) 129:139–46. doi: 10.1016/j.ophtha.2021.07.033
18. Hung, N, Shih, AK-Y, Lin, C, Kuo, M-T, Hwang, Y-S, Wu, W-C, et al. Using slit-lamp images for deep learning-based identification of bacterial and fungal keratitis: model development and validation with different convolutional neural networks. Diagnostics. (2021) 11:1246. doi: 10.3390/diagnostics11071246
19. Li, W, Yang, Y, Zhang, K, Long, E, He, L, Zhang, L, et al. Dense anatomical annotation of slit-lamp images improves the performance of deep learning for the diagnosis of ophthalmic disorders. Nat Biomed Eng. (2020) 4:767–77. doi: 10.1038/s41551-020-0577-y
20. Saini, JS, Jain, AK, Kumar, S, Vikal, S, Pankaj, S, and Singh, S. Neural network approach to classify infective keratitis. Curr Eye Res. (2003) 27:111–6. doi: 10.1076/ceyr.27.2.111.15949
21. Redd, TK, Prajna, NV, Srinivasan, M, Lalitha, P, Krishnan, T, Rajaraman, R, et al. Image-based differentiation of bacterial and fungal keratitis using deep convolutional neural networks. Ophthalmol Sci. (2022) 2:100119. doi: 10.1016/j.xops.2022.100119
22. Zhang, Z, Wang, H, Wang, S, Wei, Z, Zhang, Y, Wang, Z, et al. Deep learning-based classification of infectious keratitis on slit-lamp images. Ther Adv Chronic Dis. (2022) 13:204062232211360. doi: 10.1177/20406223221136071
23. Ghosh, AK, Thammasudjarit, R, Jongkhajornpong, P, Attia, J, and Thakkinstian, A. Deep learning for discrimination between fungal keratitis and bacterial keratitis: DeepKeratitis. Cornea. (2022) 41:616–22. doi: 10.1097/ICO.0000000000002830
24. Sajeev, S, and Prem, SM (2021). Classifying infective keratitis using a deep learning approach. 2021 Australasian computer science week multiconference. New York, NY, USA: ACM.
25. Gu, H, Guo, Y, Gu, L, Wei, A, Xie, S, Ye, Z, et al. Deep learning for identifying corneal diseases from ocular surface slit-lamp photographs. Sci Rep. (2020) 10:17851. doi: 10.1038/s41598-020-75027-3
26. Kuo, M-T, Hsu, BW-Y, Yin, Y-K, Fang, P-C, Lai, H-Y, Chen, A, et al. A deep learning approach in diagnosing fungal keratitis based on corneal photographs. Sci Rep. (2020) 10:14424. doi: 10.1038/s41598-020-71425-9
27. Tahvildari, M, Singh, RB, and Saeed, HN. Application of artificial intelligence in the diagnosis and Management of Corneal Diseases. Semin Ophthalmol. (2021) 36:641–8. doi: 10.1080/08820538.2021.1893763
28. Dietterich, TG . “Ensemble methods in machine learning.” (2000). p. 1–15 Springer, Berlin, Heidelberg
29. Huang, X-M, Yang, B-F, Zheng, W-L, Liu, Q, Xiao, F, Ouyang, P-W, et al. Cost-effectiveness of artificial intelligence screening for diabetic retinopathy in rural China. BMC Health Serv Res. (2022) 22:260. doi: 10.1186/s12913-022-07655-6
30. Ruamviboonsuk, P, Chantra, S, Seresirikachorn, K, Ruamviboonsuk, V, and Sangroongruangsri, S. Economic evaluations of artificial intelligence in ophthalmology. Asia Pac J Ophthalmol. (2021) 10:307–16. doi: 10.1097/APO.0000000000000403
31. Selvaraju, RR, Cogswell, M, Das, A, Vedantam, R, Parikh, D, and Batra, D. Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Comput Vis. (2019) 128:336–59. doi: 10.1007/s11263-019-01228-7
32. Chattopadhay, A, Sarkar, A, Howlader, P, and Balasubramanian, VN. Grad-CAM++: generalized gradient-based visual explanations for deep convolutional networks. 2018 IEEE winter conference on applications of computer vision (WACV). IEEE. (2018).
Keywords: deep learning, artificial intelligence, infectious keratitis, systematic review, accuracy
Citation: Sarayar R, Lestari YD, Setio AAA and Sitompul R (2023) Accuracy of artificial intelligence model for infectious keratitis classification: a systematic review and meta-analysis. Front. Public Health. 11:1239231. doi: 10.3389/fpubh.2023.1239231
Received: 13 June 2023; Accepted: 03 November 2023;
Published: 24 November 2023.
Edited by:
Amit Chhabra, Guru Nanak Dev University, IndiaReviewed by:
Poonam Mudgil, Western Sydney University, AustraliaCopyright © 2023 Sarayar, Lestari, Setio and Sitompul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yeni Dwi Lestari, eWVuaTJsZXN0YXJpQHlhaG9vLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.