- 1Provincial Health Directorate of Ankara, Republic of Türkiye Ministry of Health, Ankara, Türkiye
- 2Department of Medical Microbiology, Medical Faculty, TOBB University of Economics and Technology, Ankara, Türkiye
- 3Department of Clinical Microbiology and Infectious Diseases, Medical Faculty, Ankara Yildirim Beyazit University, Ankara, Türkiye
Emerging Infectious Diseases (EIDs) and Re-Emerging Infectious Diseases (REIDs) constitute significant health problems and are becoming of major importance. Up to 75% of EIDs and REIDs have zoonotic origin. Several factors such as the destruction of natural habitats leading humans and animals to live in close proximity, ecological changes due to natural disasters, population migration resulting from war or conflict, interruption or decrease in disease prevention programs, and insufficient vector control applications and sanitation are involved in disease emergence and distribution. War and natural disasters have a great impact on the emergence/re-emergence of diseases in the population. According to a World Bank estimation, two billion people are living in poverty and fragility situations. Wars destroy health systems and infrastructure, curtail existing disease control programs, and cause population movement leading to an increase in exposure to health risks and favor the emergence of infectious diseases. A total of 432 catastrophic cases associated with natural disasters were recorded globally in 2021. Natural disasters increase the risk of EID and REID outbreaks by damaging infrastructure and leading to displacement of populations. A Generic National Action Plan covering risk assessment, mechanism for action, determination of roles and responsibilities of each sector, the establishment of a coordination mechanism, etc. should be developed.
1. Introduction
Emerging Infectious Diseases (EIDs) are illnesses that are newly defined or have existed but are increasing in incidence or geographic range which poses a threat to the population, either in a particular area or globally (1–3). Conversely, Re-Emerging Infectious Diseases (REIDs) are illnesses that existed in the past but reappear after they have been on a significant decline, and rapidly spread either in terms of incidence or to new geographical areas (2–5).
Many factors such as the destruction of natural habitats, which leads humans and animals to live in close proximity, changing climate and ecosystems, replacing reservoir hosts or intermediate insect vectors, and microbial genetic mutations are involved in the emergence of new diseases (6). Ecological changes due to natural disasters such as flood/drought and earthquakes, population migration resulting from war or conflict, interruption or decrease in disease prevention programs, and insufficient vector control applications and sanitation have great importance in disease emergence (1). Environmental and social determinants resulting from natural disasters, wars, etc. are also of great importance in disease re-emergences as well as viral and microbial factors (4). Major contributing factors to the prevalence of EIDs and REIDs are summarized in Table 1.
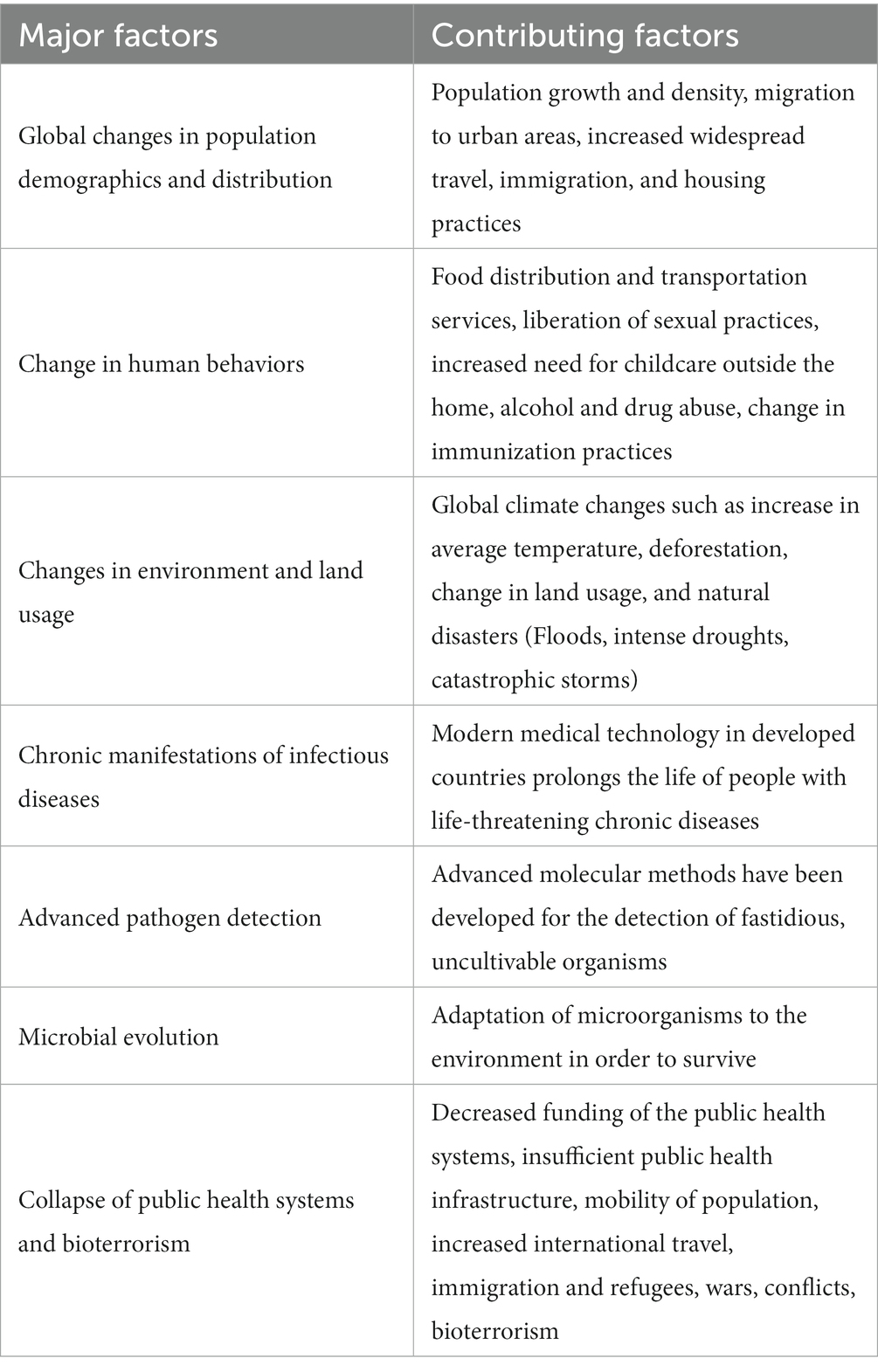
Table 1. Selected major and contributing factors to the prevalence of emerging and re-emerging infectious diseases [Adopted from Church (7)].
Re-emergence of diseases that were considered to have ended at the beginning of the 20th century has begun in recent years (8). Wars and natural disasters have a great impact on the emergence/re-emergence of diseases in the population.
2. Impact of wars on human health
According to a World Bank estimation, two billion people are living in poverty and fragility situations (9). Wars destroy the health systems and infrastructure, curtail the existing disease control programs and cause population movement. Large population displacement into overcrowded camps and temporary shelters leads to an increase in exposure to health risks such as disease vectors favoring the emergence/re-emergence of infectious diseases (10, 11). Outbreaks of typhoid fever, cholera, dysentery, malaria, small-pox, typhus fever and influenza caused a significant number of deaths among soldiers and civilians in war zones during or after World War I and World War II (12, 13).
After a dramatic increase in morbidity/mortality rates in war-involved countries during and after World War I, tuberculosis was recognized as a war disease and became one of the main concerns associated with wars and conflicts (12, 14, 15). The disease re-emerged in Europe as a result of World War I where the incidence was declining (14). As most of current conflicts are prevalent in tuberculosis-endemic countries, tuberculosis continues to be an important threat not only to the people living in conflict areas, but also to the population living in unaffected areas and neighboring countries since huge numbers of people move to safer places internally or internationally (14–17).
One of the examples is the Syrian crisis which led millions of people to migrate to neighboring countries such as Türkiye. The proportion of foreign-country-born tuberculosis cases among total reported cases in Türkiye increased progressively from 1.1% in 2010 to 6.0% in 2014, 7.3% in 2017, and 10.84% in 2018. 587 Syrian cases constituted 53.0% of foreign-country-born cases and 4.87% of the total cases diagnosed in Türkiye in 2017 (18, 19).
The possibility of death in tuberculosis patients who received irregular or no treatment during the conflict was up to three times higher than the ones who received a full course of treatment during peacetime in Guinea-Bissau, West Africa (20, 21).
In a study assessing the impact of war on Ebola transmission and control in the Democratic Republic of the Congo (DRC), it was found that an increase in the number of cases had been observed over and over due to conflict conditions. Violence against healthcare workers and Ebola treatment centers inhibited the rapidity of case isolation, treatment, following up the contacts of cases, and vaccination programs due to continuous conflict events (22). War in the DRC contributed also to an increase in the transmission of sleeping sickness (23), river blindness (22, 24), and pneumonic plague (25–27) in addition to the spread of Ebola.
Destruction of healthcare infrastructure during wars resulting in weakened prevention and treatment programs can cause new strains of infectious diseases to emerge (28). Eradication of Guinea worm, river blindness, and polio programs have been disrupted resulting in challenges to delivering vaccines due to insecurity and conflict in countries experiencing war (28, 29).
As of 20 March 2023, around 8.1 million Ukrainians have moved to other European countries since 24 February 2022 after the attack by Russia on Ukraine, 5 million of whom were recruited into protection programs (30). Owing to the living conditions in the temporary shelters and the conditions they had faced during their movement, these displaced people are likely to acquire certain infectious diseases including EIDs or REIDs, and cases of EIDs or REIDs among this population are not unexpected (31).
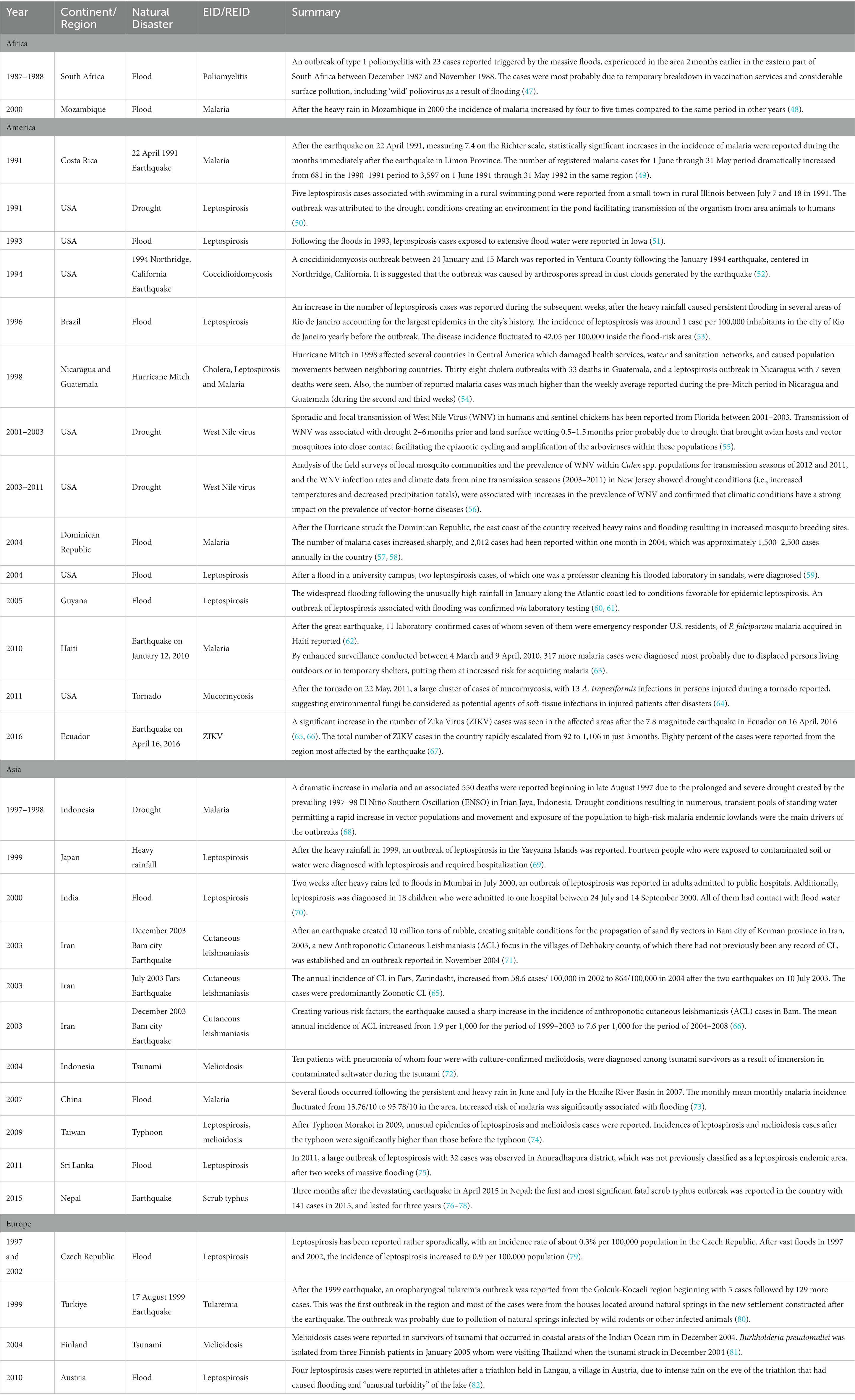
There have been numerous reported outbreaks of conflict/war-associated EIDs/REIDs from a wide range of countries. Selected outbreak reports are presented in Table 2.
3. Impact of natural disasters on human health
Natural disasters increase the risk of infectious disease outbreaks, including EIDs and REIDs, by damaging infrastructure and leading to displacement of populations (41). The population residing in natural disaster-prone areas has risen in most countries due to land shortage, urbanization, poverty, and population growth, and led to an increase in the public health impacts of natural disasters (42, 43).
A total of 432 catastrophic cases associated with natural disasters were recorded globally in 2021 though the average was 357 between 2001 and 2020 (44). The annual number of floods, which is the most common event among natural disasters, has increased from an average of 163 in the 2001–2020 period to 223 in 2021 (44). Additionally, globalization, climate change, and population movement cause natural disasters and their effects to become more complex (41).
Natural disasters are stratified into several categories by experts. Even though one classification divides natural disasters broadly into three general types (hydrometeorological, geophysical, and geo-meteorological disasters), any disaster in one category can have elements of another, for example earthquakes can cause avalanches and tsunamis, which can, in turn, cause flooding (45).
Hydrometeorological disasters such as floods from rains, hurricanes, cyclones, typhoons, and tsunamis are the most common natural disasters followed by geophysical disasters (Earthquakes, volcanic eruptions) while geo-meteorological ones are rarer than the others (45). Sometimes an emergence or re-emergence of an infectious disease agent can be, by itself, a natural disaster. Centre for Research on the Epidemiology of Disasters (CRED) classify these epidemics/pandemics as a biological sub-group of natural disasters (46). Plague outbreaks, especially the 14th century Black Death period and more recently the emergence of SARS-CoV-2 are the best examples. Even though these diseases cannot be attributed to any specific war or natural disaster they did have serious consequences on the control and prevention of other infectious diseases.
There have been numerous reported outbreaks of natural disaster associated EIDs/REIDs from a wide range of countries. Selected outbreak reports are presented in Table 3.
4. Drivers of emerging and re-emerging infectious disease outbreaks in wars and natural disasters
There are several causal factors in emerging and re-emerging infectious disease outbreaks in wars and natural disasters. Population movement and migration, environmental health disruption and ecological changes, displacement of domestic and wild animals, the collapse of health systems and disruption of disease control programs, inadequate surveillance and early warning and response systems, impaired laboratory services and diagnosis, and breakdown in infection control and treatment in effectiveness and development of drug resistance are the most accepted drivers of emerging and re-emerging infectious disease outbreaks in wars and natural disasters.
The impact of wars and natural disasters on emerging and re-emerging infectious disease outbreaks can be direct or indirect.
5. Direct impact with increasing conditions for vector/reservoir proliferation or environmental conditions
Waterborne, rodent-borne, and vector-borne diseases are the main groups of diseases associated with natural disasters. Emergence and increase in the incidence of waterborne diseases due to Campylobacter, Cryptosporidium, Escherichia coli, Giardia, Hepatitis A virus, Norovirus, Shigella, and Salmonella are generally reported from the affected areas (83).
It is widely recognized that natural disasters, especially hydrometeorological ones like floods, hurricanes, and cyclones, have the potential to lead to an increase in vector-borne diseases due to environmental factors (enlargement of vector habitats in number and area) enhancing vector population densities (84, 85). Existing mosquito breeding sites may be washed away at the beginning of flooding, but later on, new breeding sites for mosquitoes can occur due to overflows of rivers or rainfall and therefore enhance the risk of re-emergence of mosquito-borne diseases such as malaria, Chikungunya, and dengue (57, 85–87). There have been also emergence/re-emergence and outbreak reports of Chikungunya virus (CHIKV), Tahyna virus (TAHV), West Nile virus (WNV), and Japanese encephalitis diseases linked to flooding events (83, 88). Flooding can also cause a proliferation of disease vectors and microorganisms by changing the balance in ecosystems and the environment (43, 86). Mosquito-borne diseases, related to ecological changes which are suitable for mosquito breeding, also increase after earthquakes (49). Following the devastating earthquake in Ecuador in 2016, an important increase in the incidence of Zika Virus (ZIKV) was observed in the affected regions (89, 90). After the earthquakes in Iran in 2003, Cutaneous Leishmaniasis (CL) cases fluctuated, new foci emerged and the epidemiology of the disease changed (65, 66, 71, 91).
Mosquito-borne diseases were also reported during drought conditions. Drought causes reduce in the size of water bodies making them stagnant which leads to additional breeding places for mosquitoes (92). Also, the collection of rainwater due to inadequate water supply can increase the stagnant water sources (92). Drought-induced amplifications of the West Nile virus have been reported in the USA (55, 56). Malaria cases and deaths increased excessively due to the drought following an El Niño Southern Oscillation that occurred in Indonesia (68). Drought conditions caused numerous stagnant water puddles in rivers acting as mosquito breeding places (68, 93).
The risk of diseases transmitted by rodents such as leptospirosis and Hantavirus Pulmonary Syndrome increases as the probability of contact with bacteria and their animal hosts rise during heavy rainfall and flooding (94, 95). Flooding catalyzes the transmission of leptospirosis as a result of an increase in the number of infected rodents sheading leptopiras (57, 95). Insufficient garbage collection and management causing the spill of rubbish into streets after natural disasters can lead to an increase in rodent population (96, 97).
Rarely, unusual disease outbreaks such as coccidioidomycosis and scrub typhus have been observed following earthquakes. For example, an outbreak of acute pulmonary coccidioidomycosis related to exposure to high-level airborne dust following landslides after the 1994 earthquake occurred in southern California (52, 98). This outbreak illustrates the relationship between specific environmental conditions and the emergence of infectious diseases (52). After the massive earthquake in Nepal in 2015, scrub typhus emerged and outbreaks were reported from various parts of the country due to rodent infestation of the environment around temporary shelters which led people and rodents to be in close proximity (76, 77, 99).
Also, the destruction of infrastructure such as electricity, water supply, sewage disposal, and gas supply increases the risk of food poisoning and water-borne diseases after natural disasters (100, 101).
Outbreaks of leptospirosis and malaria were reported in Guatemala and Nicaragua after Hurricane Mitch in 1998. The number of diagnosed malaria cases was considerably higher than the number of cases diagnosed before the hurricane (54).
Melioidosis cases were reported in survivors of the tsunami which occurred in coastal areas of the Indian Ocean in December 2004 (72, 81). The risk of waterborne and vector-borne diseases increases due to physical changes in the environment in tropical cyclones, followed by floods and sea surges (102).
Findings from previous El Nino episodes show that rains due to El Nino that result in increased rodent density heightens the risk of human contraction of Hantavirus pulmonary syndrome (103). A previously undescribed disease, subsequently identified as a previously unknown hantavirus (Bunyaviridae), emerged in the Southwest USA in 1993 after El Nino Southern Oscillation events the year before, which had allowed excess precipitation, warmer seasons, and plenty of food for rodents (21, 103). Also, a statistically significant association was demonstrated between malaria epidemics and El Niño most probably due to widespread flooding during El Niño (104).
6. Indirect impact through changes in human behavior
6.1. Population movement and migration
Population displacement is generally observed after natural disasters and wars (105, 106). Population displacement can lead to contact with infectious agents both for the migrating population and for the population at their point of arrival. The migrating population can introduce new infectious agents to the population living in their destination. Conversely, the migrating population can get in touch with new local pathogens. Additionally, the importation of the diseases from endemic regions to non-endemic regions by the migrants (107, 108).
According to UN Refugee Agency, 89.3 million people—of which 27.1 million were refugees—had to leave their homes in 2021 because of conflicts/war, assault, human rights violations, etc. Around 69 percent of people displaced across borders originated from Afghanistan, Myanmar, South Sudan, the Syrian Arab Republic, and Venezuela. The Syrian refugee population constituted 27 percent of the global refugee population with around 7 million and spread to 129 countries. Most of them are hosted in Türkiye (3.7 million), Lebanon (840,900), and Jordan (673,000) (109).
The displaced population is generally housed in temporary settlements or camps with overcrowded shelters, which increases infectious disease transmission and the number of endemic and epidemic-prone disease cases (11, 85). There have been numerous reports of EIDs/REIDs related to population movement associated with the destruction of health services and disease control programs.
Being the largest refugee crisis since World War II, the Syrian crisis is one of the examples of how conflict and refugees change epidemiological patterns of diseases not only in the source country but also in neighboring countries (85, 110). Cutaneous leishmaniasis (CL) has always been reported in Syria, particularly in the western part of the country. CL cases had increased from 17.709 in 2007 to 78.231 (suspected cases 111.144) in 2021 due to decreased public health services, reduced diagnosis and treatment access, population displacements, demolition of infrastructure, and worse living conditions as a result of war. Additionally, the epidemiology of the disease in Syria also changed during wartime; mass population movements from highly endemic areas raised the number of cases in some governorates, which used to report few cases before (111–113). Also, the CL situation in countries that hosted huge numbers of Syrian refugees altered. The disease re-emerged in Lebanon and the number of cases rose to 1.383 in 2014 (39, 114). Even though CL is endemic in Türkiye, the number of CL cases increased from 1.133 in 2008 to 5.362 in 2013 (115).
The risk of outbreaks of norovirus, Salmonella enterica, and chickenpox resulting from post-disaster living conditions increases after earthquakes (41, 100). Diarrhea and respiratory tract infections are the most common diseases reported after earthquakes (116, 117).
Movement of workers from the CL endemic regions to Bam city in order to work on construction projects, provision and creation of suitable conditions for propagation of sand fly vectors and many new resting and breeding sites, changes in the behavior of people following earthquake increasing their exposure to the bites of the vectors, and displacement of the wild mammals acting as the ‘reservoir’ hosts of the parasite (65, 66, 71, 91) were likely to be the main drivers.
In drought conditions both human and animal behavior changes, such as movement to places where water supplies can be found. This increases the likelihood of contact with one another (92, 93). Current theories suggest even the bubonic plague outbreaks in Europe in the 14th century were caused by the drought in Asia which led the introduction of infected rodents to Europe (118, 119).
6.2. Displacement of domestic and wild animals
During natural disasters, war, or conflicts animals can be lost, abandoned, or left unsupervised leading to a high number of stray or owned free-roaming dogs (120). Free-roaming dogs and cats are source of a variety of bacterial, viral, and parasitic infections which pose risks to humans (121). Free-roaming dogs change or increase the risk for emergence and transmission of zoonotic diseases in dogs and people such as rabies, echinococcosis, toxocariasis and even leptospirosis, Chagas disease, and leishmaniasis (122, 123).
Colonization of rodents can be increased by dog foraging activities in accumulated waste in camps (122, 124). Loss of rodent control resulted in the Lassa outbreak in refugee camps in Sierra Leone (106).
The risk of rabies can be increased due to a higher roaming dog population, increased aggression in dogs and dog bites, reduced/low vaccine rates in dogs, and post-exposure prophylaxis in humans (122) in natural disasters and wars. There have been some studies suggesting an increase in the incidence of dog bites following natural disasters (125–128). News stated that cases of exposure to dog bites have increased in the areas of northwestern parts of Syria and rabies cases were reported in 2022 (129).
An increase in contact with wildlife due to population movement is another risk factor for EIDs/REIDs in natural disasters and especially in conflicts (130). Disruption of livelihoods that is likely to increase the consumption of bush meat (131, 132) and increased exposure to animal hosts such as bats, increase the likelihood of zoonotic diseases (106, 130). Evidence suggested that Ebola may have been spread by these population movements in conflict areas (130).
European Centre for Disease Prevention and Control (ECDC) warns the countries accepting Ukrainians about rabies risk as it is still endemic in sylvatic animals and dogs and cats in Ukraine due to the reports describing displaced Ukrainians as fleeing with their pets (31).
7. Indirect impacts through the collapse of health systems and sanitation services
7.1. Collapse of health systems and disruption of disease control programs
The deterioration of the public health systems leads to an interruption in health measures such as immunization, surveillance, prevention and control programs, sanitation, and hygiene services and results in an increase in communicable diseases (105, 133). Disruption of public and animal health care systems has a major impact on the increase in vulnerability of displaced populations to different kinds of infections (85). Beyond the destruction of pre-existing local health facilities such as buildings, medical stores, and laboratories, access to functioning facilities can be blocked due to risks involved in traveling through the conflict zones (11, 134). Moreover, local medical staff are often also affected by the conflict or natural disaster, making them incapacitated (133).
The incidence of vector-borne diseases such as malaria can increase due to the disruption of control activities (135). Re-emerge of malaria in former Soviet Union republics is an excellent example of how the disruption of disease programs due to conflict can alter the disease epidemiology.
Malaria had been eradicated in all the republics of the former Soviet Union with a campaign launched at the end of the 1950s (136). Re-emergence of malaria started in the former Soviet Union republics due to internally displaced people and mass population movement from malaria-endemic neighboring countries, mainly Afghanistan, experiencing armed conflicts and war in the 1990s (137). Beyond this; the deterioration of preventive measures including malaria services due to economic collapse as a result of the disintegration of the former Soviet Union contributed to the situation (137). Malaria re-emerged in Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, the Russian Federation, Tajikistan, Turkmenistan, and Uzbekistan and several outbreaks have been reported afterwards (137). During the civil war in 1992–1993, with the influx of massive population displacement united with deterioration in living conditions and breakdown of the health system, particularly public health programs, and refugees from Afghanistan, a major malaria outbreak reached 29.794 cases in 1997 in Tajikistan, where malaria had been eradicated in 1960. Moreover, P. falciparum malaria was re-established after 35 years in Tajikistan due to mass population movement to and from Afghanistan (36, 137).
Curtailment of vaccination programs is especially crucial. A decline in or suspensions of vaccination programs have serious effects not only on the local disease programs but also globally implemented ones. Conflicts are now causing re-emergences of diseases that were in the eradication phase such as polio (138). The World Health Assembly put in force a resolution for polio eradication globally in 1988 (139). Due to conflict the three doses of polio vaccine coverage dropped to 35% and led to a large polio outbreak in Somalia in 2005 (10, 140).
The last case of wild poliovirus in Syria had been reported in 1999 (141). But in 2013 the disease re-emerged and 36 children of whom most had not been vaccinated against polio were paralyzed (141). In addition, another poliovirus outbreak in 2017 left 74 children paralyzed in Syria (142). The emergence of poliovirus in Syria manifests the public health results of the war (143).
The last case of autochthonous wild polio was reported in 2020 in Africa, and in Malawi the last clinically confirmed wild polio case was reported in 1992 (35). Wild poliovirus type 1 (WPV1) cases were diagnosed in Malawi and in Mozambique on 17 February 2022 and 15 May 2022, respectively, which the WHO considered at high risk of spreading, particularly to the countries of Southeast Africa, owing to their low immunity and surveillance, huge population movements, and decreased immunization rate (35, 144).
Yellow fever outbreaks were reported in West Africa as a result of the curtailment of immunization programs and spontaneous or forced migrations of thousands of people due to conflict. The circulation of the yellow fever virus in Africa has increased significantly between 2000 and 2004 (10, 145). Yellow fever re-emerged in 2020 with two outbreaks in West African countries (Guinea and Senegal) and enlarged to 12 African countries of which most have been facing political instability and insecurity (146). The outbreak continued in nine countries of the WHO African Region (Central African Republic (CAR), Cameroon, Chad, Côte d’Ivoire, DRC, Ghana, Niger, Nigeria, and the Republic of Congo) in 2021 with aggregation of decreased routine immunization and increased population movement (147). A resurgence of yellow fever began additionally in four countries (Kenya, Niger, Sierra Leone, and Uganda) in 2022 (148).
Security problems are another concern in wars, and can hinder the implementation of control programs and also can cause delays in response in cases of disease outbreaks (10).
There have been various reports of outbreaks of plague in DRC. A total of 8,379 cases have been reported between 2004 and 2014 in the country (149). The disease re-emerged in 2020 (25, 26) and the outbreak continued in 2021 (26) and 2022 (150). Worryingly, cases are also reported from areas that have not seen cases for several decades (26). The collapse of control programs impeded the access of the population to health facilities, the insecurity of the area, and population displacement due to a prolonged and escalating conflict situation were the main drivers of the outbreaks (26, 149–151).
7.2. Insufficient surveillance and impaired early warning and response systems
An effective disease surveillance system is essential in order for timely detection of infectious disease outbreaks both in human and animal populations (85, 152). Surveillance is needed to assess the situation of diseases, follow up the alterations in epidemiology, characterize health risks, trace the populations’ health, guide immediate and long-term measures, prioritize of financial resources for health, and complement more targeted epidemiological and laboratory investigations (153). However, surveillance systems are often weak and adversely affected in conflict situations and disasters or are not designed to serve emergency preparedness and response activities, give early warning of diseases, or gather information necessary to assess needs (10, 152, 153).
Existing surveillance systems may not be functioning or reachable due to the conditions (154). For example, in the earthquake in Haiti in 2010, the identification of the emergence of cholera took several days. The surveillance system and infectious diseases control programs took 2 weeks to be set up following the earthquake (154, 155).
7.3. Impaired laboratory services and diagnosis
Laboratory services and facilities face many constraints in disasters and conflict areas including getting the affected region, deficiency in reagents and equipment, limited electrical power and utensil supplies, inadequate personnel, and ineffective supervision. In some situations, laboratories themselves may be in a state of emergency (156, 157). Security risks, damaged infrastructure, and discontinuity in supply chains due to blockades are additional burdens in laboratory systems in conflict-affected areas (158).
Disease surveillance is one of the important components of disaster assessment and monitoring the effectiveness of interventions (156). Diseases that have typical clinical presentations, such as measles, do not require laboratory investigations for diagnosis however most infectious diseases need laboratory facilities to make or confirm the diagnosis (156). Timely and accurate diagnosis is essential in conflict situations and disasters in order to realize disease clusters or even outbreaks, as well as generate data to manage public health interventions (158).
The role of laboratory services in disasters is the prevention or control of infectious diseases, by identification of the causative agent(s) of outbreaks which is especially important in effective disease/outbreak control and the management of conditions that occur secondary to the prime cause of the outbreak/disaster (21, 156). The decrease in etiologic diagnosis can cause an increase in broad-spectrum antibiotics usage which can contribute to the emergence of antimicrobial resistance (21).
Displaced persons or refugees are generally at great distance from access to laboratory facilities (156) which makes them more vulnerable to disease outbreaks. Testing for different pathogens, particularly water-borne ones is especially important in these groups with poor hygiene and overcrowding in order to manage disease outbreaks (158).
The debilitating earthquake in 2005 in Pakistan posed a unique problem with the supplies for the maintenance of laboratory services. The health management in affected areas was almost paralyzed and the existing healthcare system was completely demolished leading to total disruption of the primary and secondary healthcare system. Additionally, health staff became vulnerable due to landslides and weather conditions. The establishment of diagnostic laboratory services was a unique and special challenge. Unavailable appropriate shelters specified for laboratory equipment caused to work in uncontrolled conditions. The efficiency of sensitive equipment was affected due to drastic changes in temperature and electricity supply as the generators were limited. There was a great problem with effective logistic and technical support, and the supply of reagents and diagnostic kits (159). The weak health system including insufficient laboratory diagnostic capacity and a lack of experience in patient treatment and community awareness resulted in the further spread of Ebola in Sierra Leone in 2014 (160). Patients died due to the late laboratory confirmation, leading them to not being transported to treatment centers. Additional problems regarding inappropriate specimen delivery systems, a lack of consistent electricity which resulted in broken laboratory equipment and water supply, and the disruption of instruments owing to high temperature and humidity had been also experienced during the outbreak (160).
7.4. Breakdown in infection control
Breakdown in infection control in natural disasters and wars is generally observed. The principles of infection prevention and control (IPC) remain especially important given the disrupted health services, insufficient trained staff and personal protective equipment, and the unhygienic circumstances after disasters, which can enable the amplification of infectious diseases. Infection control is especially important in areas where there is a transmission risk of infectious diseases such as Ebola, Middle East Respiratory Syndrome (MERS) (10, 138).
Staff of healthcare centers are at high risk of contracting and transmitting the diseases to their family and the community (161, 162). Prior to infection control procedures, the relative risk of healthcare workers contracting Ebola during the outbreak in West Africa was approximately 100 times higher than in the general population (162, 163). Nosocomial transmission played a major role in two Ebola outbreaks in conflict-affected countries, the DRC in 1995 and Uganda in 2000 (10), most probably from a breakdown in infection control. Another nosocomial outbreak of Lassa fever occurred in Sierra Leone in 2004 due to a breakdown of infection control measures due to a weakened health system during war years (10, 164).
A Marburg hemorrhagic fever outbreak was recorded in long-term war affected Angola in 2004–2005. The outbreak was amplified by multiple usage of injectors and multi-dose ampoules in health facilities (10, 32, 165).
7.5. Treatment ineffectiveness and development of drug resistance
The collapse of health facilities, impeded access of the population to surviving health facilities, and insufficient quantities of treatment drugs can cause the cessation of continuing treatment regimens and usage of un-prescribed drugs or inappropriate drug regimens and outdated drugs resulting in pathogens’ increase in infectious diseases transmission and resistance to drugs in conflict situations and natural disasters (43, 85, 166).
The emergence of Antimicrobial Resistance (AMR) in microorganisms is a major health problem. It is linked to insufficient health facilities, inappropriate sanitation measures, weak health care, overuse of antimicrobials, and lack of or outdated treatment guidelines (167, 168). Evidence also indicates that increased international travel and migration including forced migration, can contribute to the spread of drug resistance. Displaced populations and migrants may have a higher burden of AMR due to specific factors such as poor living conditions, limited access to good-quality health care or interruption of treatment during their journey, and poor nutrition (167–169).
Studies show that refugees have an elevated prevalence of AMR carriage and infection and significantly higher rates of methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Enterobacteriaceae (MDRE) than the host country population (168, 170). A study which compared the AMR levels of refugees and Germans found that the refugee group carried five or more antibiotic resistance genes whereas most Germans carried three or fewer (171, 172). In a study conducted in Türkiye, it is found that MRSA prevalence and extended-spectrum β-lactams (ESBL) positivity rates of Syrian refugees were higher than that of Turkish citizens (172).
The rapid worldwide emergence and spread of Acinetobacter baumannii (A. baumannii) is one of the major causes of hospital-acquired infections in recent years (173, 174). A. baumannii strains are of an especially high concern in military field hospitals and the ones established after natural disasters (173, 175). Drug-resistant or even multi-drug-resistant (MDR) A. baumannii are a great issue of concern in patients with war injuries as war-associated wounds are prone to colonization of multidrug-resistant A. baumannii (176). Gaining attention during the 2003 and 2005 period military operations in Iraq, since then there have been several reports of warfare-associated MDR A. baumannii infections (174, 176–180).
A study indicated that drug resistance, and MDR-TB in particular, was significantly higher in patients from the refugee population than the local population in Kenya most probably due to a poorly functioning TB treatment program and an incomplete course of medication (166). Drug-resistant TB including MDR-TB has emerged in republics of the former Soviet Union after the fall of the Soviet Union due to civil war in most of the countries, leading to a large number of internally displaced persons, the collapse of public health systems and increased poverty and decline in socioeconomic status (181–184). TB remains a major public health problem in Ukraine having the second highest number of TB cases (31000), with an incidence of 71 cases per 100,000 in the World Health Organization (WHO) European Region. Additionally, the burden of multi-drug resistant tuberculosis (MDR-TB) in Ukraine is extremely high (185). Experts warn about the risk of suspension of TB and DR-TB treatment due to war could have significant results, like amplification of drug resistance, continuous transmission of infection, and death (186).
7.6. Environmental health services disruption and ecological changes
The impact of both natural disasters and wars creates several public health problems. The deterioration in environmental health services is one of the major problems (187). Alteration in ecology leads to an increase in vector proliferation and the movements of wild and domestic animals pose risks for disease outbreaks (188). Disasters alter the microbial population in the affected area which can cause new ecological interactions (118).
Vector-borne diseases represent a large group within emerging diseases and major causes of morbidity and mortality. Ecological changes and environmental conditions are very important in vector-borne agent transmission (21). The mosquito population is dramatically affected by environmental conditions such as rainfall and flooding (21).
A tularemia outbreak was reported in 1999–2000 during the civil war in Kosovo due to ecological changes, huge population movements, and deterioration of sanitation (40).
Natural disasters can cause disease outbreaks as a result of the contamination of water sources with feces and chemicals, and the living conditions of populations in overcrowded shelters (189).
8. Indirect impact through individual vulnerabilities
Malnutrition, which increases vulnerability to EIDs/REIDs, is a common situation observed both in wars and natural disasters, especially in long-term events such as droughts. Food security created by conflicts, civil strife, and extreme weather conditions—particularly repeated droughts—is the main reason for malnutrition in less developed countries (190, 191). According to World Food Programme around 258 million people were acutely food-insecure in 2022 in 58 countries (191). The Greater Horn of Africa including Djibouti, Ethiopia, Kenya, Somalia, South Sudan, Sudan, and Uganda is the worst food-insecure region for decades. A rising trend in food insecurity is being currently observed in the region due to extreme weather events such as drought and flooding, conflict and instability, high food prices, and the socioeconomic impacts of COVID-19 (192, 193).
Food insecurity can cause impairment in the immune system due to malnutrition making people more susceptible to infectious diseases and generally associated with population displacement, overcrowding, the collapse of health system measures such as vector control and vaccination programs, and poor sanitation which poses the risk of infectious diseases outbreaks (192, 194). Particularly women, children, and people living with chronic diseases like human immunodeficiency virus (HIV) and tuberculosis are vulnerable due to food insecurity (192). There are several well-established studies confirming the bidirectional link between HIV and food insecurity: Food insecurity may increase susceptibility to HIV, and HIV may lessen the HIV patient’s ability to make and/or aquire food (190).
Food insecurity can also contribute indirectly to shape, re-shape, or enhance vulnerabilities to EIDs/REIDs, especially in less developed countries. For example, the risk of contracting HIV/AIDS is much higher in women than men now even though men were more likely to contract the disease when the disease emerged (190, 195). The reason for this inequality is a mixture of several biological, cultural, economic, and social factors, especially in less-developed countries. A study assessing the data of 91 less-developed countries indicated that drought-induced food insecurity was directly and indirectly associated with women’s vulnerability to HIV. Women generally were more likely to get a lower amount of food than men, which causes nutrient deficiencies leading to increased susceptibility to infection. Additionally, food insecurity was also associated with social vulnerabilities such as a decrease in women’s access to health facilities and forced engagement in risky sexual behaviors, etc. (190).
A systematic review analyzing 111 studies even suggested an indirect relationship between drought and HIV treatment in African countries. Changes in livelihood and economic conditions due to drought-induced water and food insecurity and population movement appeared to affect HIV treatment adherence according to the review (196). Another review’s findings identified a relationship between food insecurity and initiation of antiretroviral therapy (ART) and treatment adherence (197).
9. Conclusion
The impact of wars and natural disasters on EIDs/REIDs varies depending strongly on the level of underlying fragility or vulnerability in the affected country or society. Less developed countries are significantly more vulnerable to disasters due to having a large number of vulnerable populations, limited response capacity, weak health systems, etc. (198). The inadequate health infrastructure and insufficient human and financial resources before, during, and after wars and natural disasters in less developed countries may increase the vulnerability, and EIDs/REIDs incidences such as malaria and HIV reach epidemic proportions (198). In contrast, EIDs/REIDs outbreaks can be confined within a particular area in developed countries as a result of strong health systems and their coping capacity with disasters.
However, development is not a guarantee of ‘invulnerability,” different levels of vulnerabilities within societies or populations even in developed countries can also be observed (198). The older adults and poor black populations were a significantly high proportion among victims of Hurricane Katrina in New Orleans in 2005 (199, 200). Similarly, the young, the older adults, and women were affected more than others during the earthquake in Italy in 2009 (200).
Conflicts also can generate additional vulnerabilities during natural disasters owing to weakening the respond capacity of countries. Prevailing vulnerabilities in conflict situations are generally aggravated by disasters and conflicts may worsen the impact of disasters. There are some studies suggesting that conflict has been higher in drought situations (201).
In a systematic review analyzing 132 studies, population displacement was the most frequently reported risk factor in infectious disease outbreaks followed by water, sanitation, and hygiene (WASH), housing, and vector/animal after disasters (202). In another review crowded conditions, forced displacement, poor quality shelter, poor water, sanitation and hygiene, lack of healthcare facilities, and lack of adequate surveillance were found to be the key risk factors cascades for communicable disease outbreaks in complex humanitarian emergencies (203). All these factors exacerbate the pre-existing vulnerability and increase the probability of post-disaster infectious disease outbreaks (202).
The extent (geographically, number of people affected, duration, etc.) of the disaster is also important in the context of the coping capacity of the country. For example, the devastating earthquake on 6 February 2023 with a 7.8 magnitude followed by 5,700 aftershocks directly affected 11 provinces living 15 million people in Türkiye and 11 million families in the Syrian Arab Republic (204, 205).
A Generic National Action Plan covering risk assessment, a mechanism for action, determination of roles and responsibilities of each sector, establishment of a coordination mechanism, etc. should be developed. Each province/region should prepare its own plan of action considering local conditions such as epidemiology of EIDs/REIDs, vector and reservoir species and their distribution, response capacity and additional needs, etc.
Even though there have been common risks and drivers for EIDs/REIDs in wars and natural disasters, they have idiosyncratic risks. For example, security is a great problem in wars while the continuity of a natural disaster itself, such as in an earthquake poses, additional risks in implementing control measures.
In order to increase response capacity, planning and investments including awareness raising additional to finance are needed for disaster preparedness and early warning. The establishment or adaptation of the current EIDs/REIDs Surveillance and Early Warning System covering the probable effects of wars and natural disasters on EIDs/REIDs is a prerequisite for recognizing disease clusters/outbreaks, and monitoring and evaluating control interventions. Being an important part of a strong surveillance system, enhanced laboratory services should be planned to include mobile even field laboratories which are capable of sustaining their work under inappropriate conditions in order to provide rapid diagnosis of diseases. Maintenance of surveillance and control activities, waste management services, and food security are of great importance during and after wars and natural disasters in order to reduce their impact on EIDs/REIDs. Given the risk of EIDs/REIDs is much higher in overcrowding and unsanitary conditions, priority should be given to the displaced population and refugees living in temporary shelters and regular screenings can be performed especially for vector-borne diseases such as malaria and leishmaniasis.
Free roaming animals, whether domestic/wild or stray/owned, should definitely be considered, and special control interventions should be implemented as most of the EIDs/REIDs have zoonotic origin. Planning of vector and rodent control is of great importance too. The risk of occurrence of natural disasters and, EIDs/REIDs following natural disasters and during conflict conditions are increasing due to climate change which contributes to creating environmental changes especially favorable for vector-borne diseases.
Therefore, the impact of wars and natural disasters on EIDs/REIDs should be taken seriously. Recommended actions in order to prevent and control EIDs/REIDs in wars and natural disasters are summarized in Table 4.
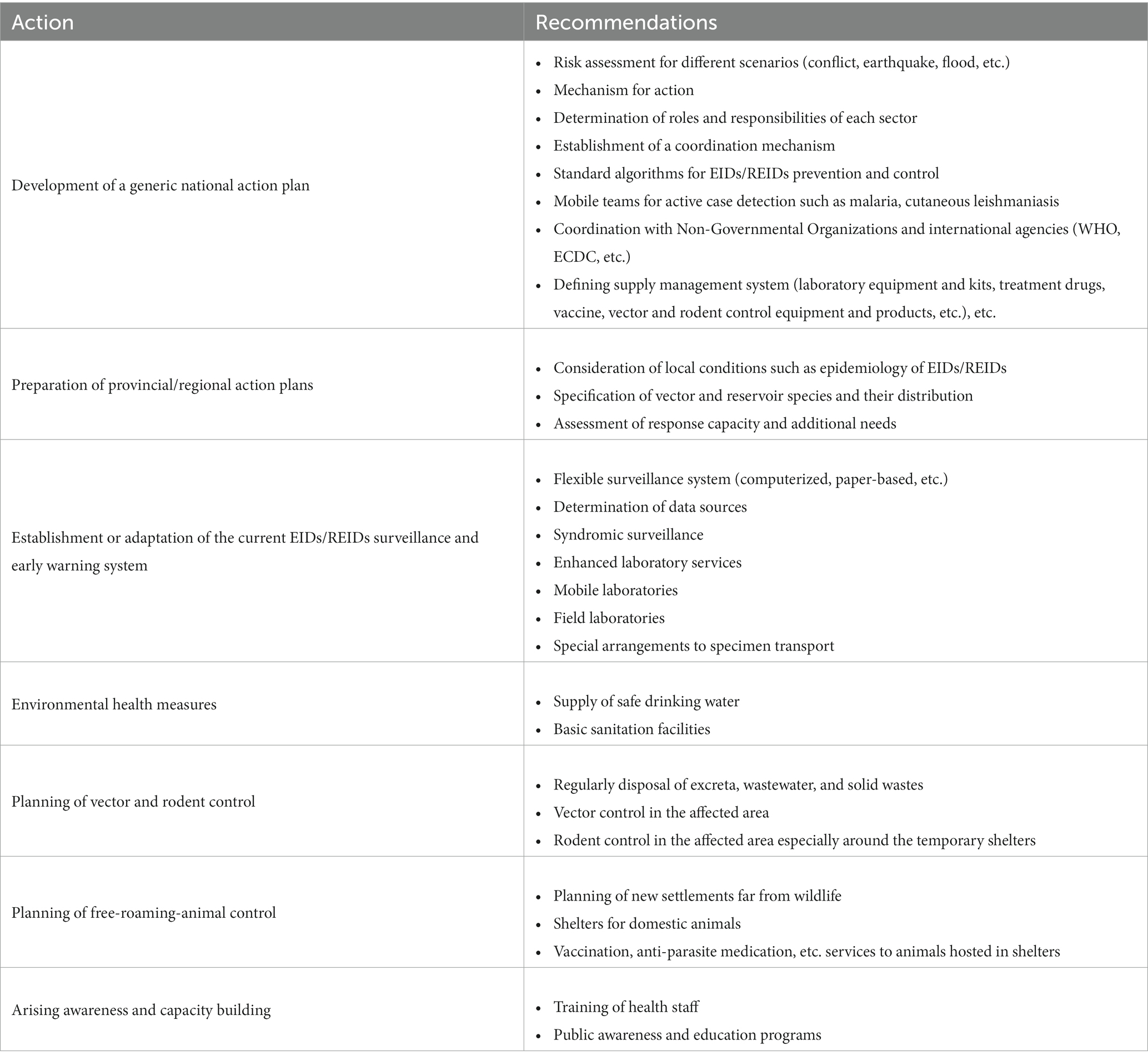
Table 4. Recommended actions in order to prevent and control EIDs/REIDs in wars and natural disasters.
Author contributions
ST, AT-O, and EA: conceptualization, writing—review and editing, and visualization. ST and AT-O: methodology. AT-O and EA: validation. ST: resources and writing—original draft preparation. EA: supervision. All authors contributed to the manuscript development and approved the final version to be published.
Funding
This study was supported by TOBB-ETU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Morse, SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. (1995) 1:7–15. doi: 10.3201/eid0101.950102
2. Sabin, NS, Calliope, AS, Simpson, SV, Arima, H, Ito, H, Nishimura, T, et al. Implications of human activities for (re)emerging infectious diseases, including COVID-19. J Physiol Anthropol. (2020) 39:29. doi: 10.1186/s40101-020-00239-5
4. Morens, D, Folkers, G, and Fauci, A. The challenge of emerging and re-emerging infectious diseases. Nature. (2004) 430:242–9. doi: 10.1038/nature02759
5. Kovats, RS, Bouma, MJ, Hajat, S, Worrall, E, and Haines, A. El Niño and health. Lancet. (2003) 362:1481–9. doi: 10.1016/S0140-6736(03)14695-8
6. WHO Regional Office for South-East Asia. A brief guide to emerging infectious diseases and zoonoses. India: WHO Regional Office for South-East Asia (2014).
7. Church, DL. Major factors affecting the emergence and re-emergence of infectious diseases. Clin Lab Med. (2004) 24:559–86. doi: 10.1016/j.cll.2004.05.008
8. Venkatesan, P. Re-emergence of infectious diseases associated with the past. Lancet Microbe. (2021) 2:e140. doi: 10.1016/S2666-5247(21)00066-5
9. World Bank Group. Maximizing the impact of the World Bank Group in fragile and conflict-affected situations. Washington, DC: World Bank Group (2018).
10. Gayer, M, Legros, D, Formenty, P, and Connolly, MA. Conflict and emerging infectious diseases. Emerg Infect Dis. (2007) 13:1625–31. doi: 10.3201/eid1311.061093
11. Garry, S, and Checchi, F. Armed conflict and public health: into the 21st century. J Public Health (Oxf). (2020) 42:e287–98. doi: 10.1093/pubmed/fdz095
13. Karagoz, E, Turhan, V, Hatipoglu, M, and Ozkuzugudenli, B. Wartime infections and tragedies at the beginning of the 20th century in the Eastern part of Turkey. Infez Med. (2017) 25:84–7.
14. Drolet, GJ. World war I and tuberculosis. A statistical summary and review. Am J Public Health Nations Health. (1945) 35:689–97. doi: 10.2105/AJPH.35.7.689
15. Murray, JF. Tuberculosis and world war I. Am J Respir Crit Care Med. (2015) 192:411–4. doi: 10.1164/rccm.201501-0135OE
16. Kimbrough, W, Saliba, V, Dahab, M, Haskew, C, and Checchi, F. The burden of tuberculosis in crisis-affected populations: a systematic review. Lancet Infect Dis. (2012) 12:950–65. doi: 10.1016/S1473-3099(12)70225-6
17. Ismail, MB, Rafei, R, Dabboussi, F, and Hamze, M. Tuberculosis, war, and refugees: spotlight on the Syrian humanitarian crisis. PLoS Pathog. (2018) 14:e1007014. doi: 10.1371/journal.ppat.1007014
18. Türkiye’de Verem Savaşı 2018 Raporu (In Turkish). Ankara, Türkiye: T.C. Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü Tüberküloz Dairesi Başkanlığı (2018).
19. Türkiye’de Verem Savaşı 2019 Raporu (In Turkish). Ankara, Türkiye: T.C. Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü Tüberküloz Dairesi Başkanlığı (2020).
20. Gustafson, P, Gomes, VF, Vieira, CS, Jensen, H, Seng, R, Norberg, R, et al. Tuberculosis mortality during a civil war in Guinea–Bissau. JAMA. (2001) 286:599–603. doi: 10.1001/jama.286.5.599
21. Institute of Medicine (US). Committee on emerging microbial threats to health in the 21st century. Microbial threats to health: Emergence, detection, and response. Washington, DC: National Academies Press (US) (2003).
22. Wells, CR, Pandey, A, Ndeffo Mbah, ML, Gaüzère, BA, Malvy, D, Singer, BH, et al. The exacerbation of Ebola outbreaks by conflict in the Democratic Republic of the Congo. Proc Natl Acad Sci U S A. (2019) 116:24366–72. doi: 10.1073/pnas.1913980116
23. Van Nieuwenhove, S, Betu-Ku-Mesu, VK, Diabakana, PM, Declercq, J, and Bilenge, CM. Sleeping sickness resurgence in the DRC: the past decade. Tropical Med Int Health. (2001) 6:335–41. doi: 10.1046/j.1365-3156.2001.00731.x
24. Makenga Bof, JC, Maketa, V, Bakajika, DK, Ntumba, F, Mpunga, D, Murdoch, ME, et al. Onchocerciasis control in the Democratic Republic of Congo (DRC): challenges in a post-war environment. Tropical Med Int Health. (2015) 20:48–62. doi: 10.1111/tmi.12397
25. World Health Organization. Plague-Democratic Republic of the Congo (2020). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/plague-democratic-republic-of-the-congo (Accessed January 06, 2023).
26. Social Sciences Analytics Cell (CASS). Community dynamics around the plague outbreak. Exploring community dynamics around the plague outbreak in Ituri province (August 2021). Available at: https://www.unicef.org/drcongo/media/6246/file/Rapport%20Ituri%20EN.pdf (Accessed January 7, 2023).
27. World Health Organization. Plague in the Democratic Republic of the Congo (2006). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2006_06_14-en (Accessed January 5, 2023).
28. Ghobarah, H, Huth, P, and Russett, B. Civil wars kill and maim people-long after the shooting stops. Am Polit Sci Rev. (2003) 97:189–202. doi: 10.1017/S0003055403000613
29. Global Polio Eradication Initiative. Reaching the hard to reach: Ending polio in conflict zones (2017). Available at: http://polioeradication.org/news-post/ending-polio-in-conflict-zones/ (Accessed February 10, 2023).
30. United Nations High Commissioner for Refugees (UNHCR). Operational Data Portal-Ukraine Refugee Situation (2023). Available at: https://data.unhcr.org/en/situations/ukraine (Accessed March 23, 2023).
31. European Centre for Disease Prevention and Control (ECDC). Operational public health considerations for the prevention and control of infectious diseases in the context of Russia’s aggression towards Ukraine. Stockholm: ECDC (2022).
32. Ndayimirije, N, and Kindhauser, MK. Marburg hemorrhagic fever in Angola-fighting fear and a lethal pathogen. N Engl J Med. (2005) 352:2155–7. doi: 10.1056/NEJMp058115
33. World Health Organization. Yellow fever-Guinea (2020). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON302 (Accessed February 10, 2023).
34. World Health Organization. Ebola virus disease-Democratic Republic of the Congo (2022). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON404 (Accessed February 10, 2023).
35. World Health Organization. Wild poliovirus type 1 (WPV1). Malawi: (2022). (Accessed January 06, 2023)https://www.who.int/emergencies/disease-outbreak-news/item/wild-poliovirus-type-1-(WPV1)-malawi.
36. World Health Organization. World malaria situation in 1994. Part III Wkly Epidemiol Rec. (1997) 72:285–90.
37. Rowland, M, Munir, A, Durrani, N, Noyes, H, and Reyburn, H. An outbreak of cutaneous leishmaniasis in an afghan refugee settlement in north-West Pakistan. Trans R Soc Trop Med Hyg. (1999) 93:133–6. doi: 10.1016/s0035-9203(99)90285-7
38. Faulde, MK, Hoffmann, R, Fazilat, KM, and Hoerauf, A. Malaria reemergence in northern Afghanistan. Emerg Infect Dis. (2007) 13:1402–4. doi: 10.3201/eid1309.061325
39. Bizri, NA, Alam, W, Khoury, M, Musharrafieh, U, Ghosn, N, Berri, A, et al. The association between the syrian crisis and cutaneous leishmaniasis in Lebanon. Acta Parasitol. (2021) 66:1240–5. doi: 10.1007/s11686-021-00395-3
40. Reintjes, R, Dedushaj, I, Gjini, A, Jorgensen, TR, Cotter, B, Lieftucht, A, et al. Tularemia outbreak investigation in Kosovo: case control and environmental studies. Emerg Infect Dis. (2002) 8:69–73. doi: 10.3201/eid0801.010131
41. Suk, JE, Vaughan, EC, Cook, RG, and Semenza, JC. Natural disasters and infectious disease in Europe: a literature review to identify cascading risk pathways. Eur J Pub Health. (2020) 30:928–35. doi: 10.1093/eurpub/ckz111
42. Donner, W, and Rodríguez, H. Disaster risk and vulnerability: the role and impact of population and society (2011). Available at: https://www.prb.org/resources/disaster-risk/ (Accessed January 20, 2023).
43. Kouadio, IK, Aljunid, S, Kamigaki, T, Hammad, K, and Oshitani, H. Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti-Infect Ther. (2012) 10:95–104. doi: 10.1586/eri.11.155
44. Centre for Research on the Epidemiology of Disasters (CRED). Disasters in numbers 2021 (2022). Available at: https://www.emdat.be/cred-crunch-66-disasters-year-review-2021 (Accessed January 20, 2023).
45. Rathore, MH. Infections after natural disasters. Pediatr Rev. (2020) 41:501–10. doi: 10.1542/pir.2018-0208
46. Disaster category classification and peril terminology for operational purposes. Brussels, Belgium: Centre for Research on the Epidemiology of Disasters (CRED) and Munich Reinsurance Company (Munich RE) (2009).
47. van Middelkoop, A, van Wyk, JE, Küstner, HG, Windsor, I, Vinsen, C, Schoub, BD, et al. Poliomyelitis outbreak in Natal/KwaZulu, South Africa, 1987-1988. 1. Epidemiology. Trans R Soc Trop Med Hyg. (1992) 86:80–2. doi: 10.1016/0035-9203(92)90451-h
48. Kondo, H, Seo, N, Yasuda, T, Hasizume, M, Koido, Y, Ninomiya, N, et al. Post-flood--infectious diseases in Mozambique. Prehosp Disaster Med. (2002) 17:126–33. doi: 10.1017/S1049023X00000340
49. Saenz, R, Bissell, RA, and Paniagua, F. Post-disaster malaria in Costa Rica. Prehosp Disaster Med. (1995) 10:154–60. doi: 10.1017/S1049023X00041935
50. Jackson, LA, Kaufmann, AF, Adams, WG, Phelps, MB, Andreasen, C, Langkop, CW, et al. Outbreak of leptospirosis associated with swimming. Pediatr Infect Dis J. (1993) 12:48–54. doi: 10.1097/00006454-199301000-00012
51. Fuortes, L, and Nettleman, M. Leptospirosis: a consequence of the Iowa flood. Iowa Med. (1994) 84:449–50.
52. Schneider, E, Hajjeh, RA, Spiegel, RA, Jibson, RW, Harp, EL, Marshall, GA, et al. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA. (1997) 277:904–8. doi: 10.1001/jama.1997.03540350054033
53. Barcellos, C, and Sabroza, PC. The place behind the case: leptospirosis risks and associated environmental conditions in a flood-related outbreak in Rio de Janeiro. Cad Saude Publica. (2001) 17:59–67. doi: 10.1590/s0102-311x2001000700014
54. Pan American Health Organization. Impact of hurricane Mitch on Central America. Epidemiol Bull. (1998) 19:1–14.
55. Shaman, J, Day, JF, and Stieglitz, M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J Med Entomol. (2005) 42:134–41. doi: 10.1093/jmedent/42.2.134
56. Johnson, BJ, and Sukhdeo, MV. Drought-induced amplification of local and regional West Nile virus infection rates in New Jersey. J Med Entomol. (2013) 50:195–204. doi: 10.1603/ME12035
57. World Health Organization. Flooding and communicable diseases fact sheet. Wkly Epidemiol Rec. (2005) 80:21–8.
58. Center for Disease Control (CDC). Transmission of malaria in resort areas – Dominican Republic, 2004. MMWR Morb Mortal Wkly Rep. (2005) 53:1195–8.
59. Centers for Disease Control and Prevention (CDC). Brief report: leptospirosis after flooding of a university campus-Hawaii, 2004. MMWR Morb Mortal Wkly Rep. (2006) 55:125–7.
60. Dechet, AM, Parsons, M, Rambaran, M, Mohamed-Rambaran, P, Florendo-Cumbermack, A, Persaud, S, et al. Leptospirosis outbreak following severe flooding: a rapid assessment and mass prophylaxis campaign; Guyana, January-February 2005. PLoS One. (2012) 7:e39672. doi: 10.1371/journal.pone.0039672
61. Liverpool, J, Francis, S, Liverpool, CE, Dean, GT, and Mendez, DD. Leptospirosis: case reports of an outbreak in Guyana. Ann Trop Med Parasitol. (2008) 102:239–45. doi: 10.1179/136485908X278784
62. Centers for Disease Control and Prevention (CDC). Malaria acquired in Haiti-2010. Morb Mortal Wkly Rep. (2010) 59:217–9.
63. Townes, D, Existe, A, Boncy, J, Magloire, R, Vely, JF, Amsalu, R, et al. Malaria survey in post-earthquake Haiti-2010. Am J Trop Med Hyg. (2012) 86:29–31. doi: 10.4269/ajtmh.2012.11-0431
64. Neblett Fanfair, R, Benedict, K, Bos, J, Bennett, SD, Lo, YC, Adebanjo, T, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. (2012) 367:2214–25. doi: 10.1056/NEJMoa1204781
65. Fakoorziba, MR, Baseri, A, Eghbal, F, Rezaee, S, Azizi, K, and Moemenbellah-Fard, MD. Post-earthquake outbreak of cutaneous leishmaniasis in a rural region of southern Iran. Ann Trop Med Parasitol. (2011) 105:217–24. doi: 10.1179/136485911X12899838683449
66. Sharifi, I, Nakhaei, N, Aflatoonian, M, Parizi, MH, Fekri, A, Safizadeh, H, et al. Cutaneous leishmaniasis in Bam: a comparative evaluation of pre- and post-earthquake years (1999–2008). Iran J Public Health. (2011) 40:49–56.
67. Sorensen, CJ, Borbor-Cordova, MJ, Calvello-Hynes, E, Diaz, A, Lemery, J, and Stewart-Ibarra, AM. Climate variability, vulnerability, and natural disasters: a case study of Zika virus in Manabi, Ecuador following the 2016 earthquake. Geohealth. (2017) 1:298–304. doi: 10.1002/2017GH000104
68. Bangs, MJ, and Subianto, DB. El Niño and associated outbreaks of severe malaria in highland populations in Irian Jaya, Indonesia: a review and epidemiological perspective. Southeast Asian J Trop Med Public Health. (1999) 30:608–19.
69. Narita, M, Fujitani, S, Haake, DA, and Paterson, DL. Leptospirosis after recreational exposure to water in the Yaeyama islands, Japan. Am J Trop Med Hyg. (2005) 73:652–6. doi: 10.4269/ajtmh.2005.73.652
70. Karande, S, Bhatt, M, Kelkar, A, Kulkarni, M, De, A, and Varaiya, A. An observational study to detect leptospirosis in Mumbai, India, 2000. Arch Dis Child. (2003) 88:1070–5. doi: 10.1136/adc.88.12.1070
71. Sharifi, I, Poursmaelian, S, Aflatoonian, MR, Ardakani, RF, Mirzaei, M, Fekri, AR, et al. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake. Iran Trop Med Int Health. (2011) 16:510–3. doi: 10.1111/j.1365-3156.2011.02729.x
72. Athan, E, Allworth, AM, Engler, C, Bastian, I, and Cheng, AC. Melioidosis in tsunami survivors. Emerg Infect Dis. (2005) 11:1638–9. doi: 10.3201/eid1110.050740
73. Ding, G, Gao, L, Li, X, Zhou, M, Liu, Q, Ren, H, et al. A mixed method to evaluate burden of malaria due to flooding and waterlogging in Mengcheng County, China: a case study. PLoS One. (2014) 9:e97520. doi: 10.1371/journal.pone.0097520
74. Su, HP, Chan, TC, and Chang, CC. Typhoon-related leptospirosis and melioidosis, Taiwan, 2009. Emerg Infect Dis. (2011) 17:1322–4. doi: 10.3201/eid1707.101050
75. Agampodi, SB, Dahanayaka, NJ, Bandaranayaka, AK, Perera, M, Priyankara, S, Weerawansa, P, et al. Regional differences of leptospirosis in Sri Lanka: observations from a flood-associated outbreak in 2011. PLoS Negl Trop Dis. (2014) 8:e2626. doi: 10.1371/journal.pntd.0002626
76. Basnyat, B. Typhoid versus typhus fever in post-earthquake Nepal. Lancet Glob Health. (2016) 4:e516–7. doi: 10.1016/S2214-109X(16)30094-8
77. Bastola, A, Marahatta, SB, Jha, S, and Pant, N. Aftermath earthquake in Nepal: burden of scrub typhus cases and their presentations. J Trop Dis. (2017) 5:236. doi: 10.4172/2329-891X.1000236
78. Dhimal, M, Dumre, SP, Sharma, GN, Khanal, P, Ranabhat, K, Shah, LP, et al. An outbreak investigation of scrub typhus in Nepal: confirmation of local transmission. BMC Infect Dis. (2021) 21:193. doi: 10.1186/s12879-021-05866-6
79. Zitek, K, and Benes, C. Longitudinal epidemiology of leptospirosis in the Czech Republic (1963-2003). Epidemiol Mikrobiol Imunol. (2005) 54:21–6.
80. Karadenizli, A, Gurcan, S, Kolayli, F, and Vahaboglu, H. Outbreak of tularaemia in Golcuk, Turkey in 2005: report of 5 cases and an overview of the literature from Turkey. Scand J Infect Dis. (2005) 37:712–6. doi: 10.1080/00365540510012125
81. Nieminen, T, and Vaara, M. Burkholderia pseudomallei infections in Finnish tourists injured by the December 2004 tsunami in Thailand. Euro Surveill. (2005) 10:10. doi: 10.2807/esw.10.09.02656-en
82. Radl, C, Müller, M, Revilla-Fernandez, S, Karner-Zuser, S, de Martin, A, Schauer, U, et al. Outbreak of leptospirosis among triathlon participants in Langau, Austria, 2010. Wien Klin Wochenschr. (2011) 123:751–5. doi: 10.1007/s00508-011-0100-2
83. Mavrouli, M, Mavroulis, S, Lekkas, E, and Tsakris, A. Infectious diseases associated with hydrometeorological hazards in Europe: disaster risk reduction in the context of the climate crisis and the ongoing COVID-19 pandemic. Int J Environ Res Public Health. (2022) 19:10206. doi: 10.3390/ijerph191610206
84. Chowell, G, Mizumoto, K, Banda, JM, Poccia, S, and Perrings, C. Assessing the potential impact of vector-borne disease transmission following heavy rainfall events: a mathematical framework. Phil Trans R Soc B. (2019) 374:20180272. doi: 10.1098/rstb.2018.0272
85. Tabbaa, D, and Seimenis, A. Population displacements as a risk factor for the emergence of epidemics. Vet Ital. (2013) 49:19–23.
86. WHO Regional Office for Europe. Floods in the WHO European Region: health effects and their prevention. Copenhagen, Denmark: WHO Regional Office for Europe (2013).
87. Reeves, WC, Hardy, JL, Reisen, WK, and Milby, MM. Potential effect of global warming on mosquito-borne arboviruses. J Med Entomol. (1994) 31:323–32. doi: 10.1093/jmedent/31.3.323
88. Zhang, F, Liu, Z, Zhang, C, and Jiang, B. Short-term effects of floods on Japanese encephalitis in Nanchong, China, 2007-2012: a time-stratified case-crossover study. Sci Total Environ. (2016) 563-564:1105–10. doi: 10.1016/j.scitotenv.2016.05.162
89. Vasquez, D, Palacio, A, Nuñez, J, Briones, W, Beier, JC, Pareja, DC, et al. Impact of the 2016 Ecuador earthquake on Zika virus cases. Am J Public Health. (2017) 107:1137–42. doi: 10.2105/AJPH.2017.303769
90. Reina Ortiz, M, Le, NK, Sharma, V, Hoare, I, Quizhpe, E, Teran, E, et al. Post-earthquake Zika virus surge: disaster and public health threat amid climatic conduciveness. Sci Rep. (2017) 7:15408. doi: 10.1038/s41598-017-15706-w
91. Aflatoonian, MR, Sharifi, I, Aflatoonian, B, Shirzadi, MR, Gouya, MM, and Kermanizadeh, A. A review of impact of bam earthquake on cutaneous leishmaniasis and status: epidemic of old foci, emergence of new foci and changes in features of the disease. J Arthropod Borne Dis. (2016) 10:271–80.
92. Centers for Disease Control and Prevention. Health implications of drought (2023). Available at: https://www.cdc.gov/nceh/drought/implications.htm (Accessed January 5, 2023).
93. Linscott, AJ. Natural disasters – a microbe’s paradise. Clin Microbiol Newsl. (2007) 29:57–62. doi: 10.1016/j.clinmicnews.2007.04.001
94. Lau, CL, Smythe, LD, Craig, SB, and Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Trans R Soc Trop Med Hyg. (2010) 104:631–8. doi: 10.1016/j.trstmh.2010.07.002
95. Ahern, M, Kovats, RS, Wilkinson, P, Few, R, and Matthies, F. Global health impacts of floods: epidemiologic evidence. Epidemiol Rev. (2005) 27:36–46. doi: 10.1093/epirev/mxi004
96. Brown, L, and Murray, V. Examining the relationship between infectious diseases and flooding in Europe: a systematic literature review and summary of possible public health interventions. Disaster Health. (2013) 1:117–27. doi: 10.4161/dish.25216
97. Socolovschi, C, Angelakis, E, Renvoisé, A, Fournier, PE, Marié, JL, Davoust, B, et al. Strikes, flooding, rats, and leptospirosis in Marseille, France. Int J Infect Dis. (2011) 15:e710–5. doi: 10.1016/j.ijid.2011.05.017
98. Centers for Disease Control and Prevention (CDC). Coccidioidomycosis following the Northridge Earthquake--California, 1994. MMWR Morb Mortal Wkly Rep. (1994) 43:194–5.
99. The Katmandu Post. Rats causing scrub typhus: WHO team (2023). Available at: https://kathmandupost.com/national/2015/10/07/rats-causing-scrub-typhus-who-team (Accessed January 30, 2023).
100. Izumikawa, K. Infection control after and during natural disaster. Acute Med Surg. (2018) 6:5–11. doi: 10.1002/ams2.367
101. Western, KA. Epidemiologic surveillance and disease control after natural disaster. Scientific Publication, No. 420. Washington DC: Pan American Health Organization (1982).
102. World Health Organization. Tropical cyclones (2023). Available at: https://www.who.int/health-topics/tropical-cyclones#tab=tab_1 (Accessed January 30, 2023).
103. Yates, T, Mills, J, Parmenter, C, Ksiazek, T, Parmenter, R, Calisher, C, et al. The ecology and evolutionary history of an emergent disease: hantavirus pulmonary syndrome. Bioscience. (2002) 52:989–98. doi: 10.1641/0006-3568(2002)052[0989:TEAEHO]2.0.CO;2
104. Gagnon, AS, Smoyer-Tomic, KE, and Bush, AB. The El Niño southern oscillation and malaria epidemics in South America. Int J Biometeorol. (2002) 46:81–9. doi: 10.1007/s00484-001-0119-6
105. Deola, C, and Patel, R. Health outcomes of crisis driven urban displacement: a conceptual framework. Disaster Health. (2014) 2:92–6. doi: 10.4161/21665044.2014.990306
106. Goniewicz, K, Burkle, F, Horne, S, Borowska-Stefańska, M, Wiśniewski, S, and Khorram-Manesh, A. The influence of war and conflict on infectious disease: a rapid review of historical lessons we have yet to learn. Sustainability. (2021) 13:10783. doi: 10.3390/su131910783
107. Aagaard-Hansen, J, Nombela, N, and Alvar, J. Population movement: a key factor in the epidemiology of neglected tropical diseases. Tropical Med Int Health. (2010) 15:1281–8. doi: 10.1111/j.1365-3156.2010.02629.x
108. Saker, L, Lee, K, Cannito, B, Gilmore, A, and Campbell-Lendrum, D. Globalization and infectious diseases: a review of the linkages. Geneva: World Health Organization (2004).
110. Sharara, SL, and Kanj, SS. War and infectious diseases: challenges of the Syrian civil war. PLoS Pathog. (2014) 10:e1004438. doi: 10.1371/journal.ppat.1004438
111. Muhjazi, G, Gabrielli, AF, Ruiz-Postigo, JA, Atta, H, Osman, M, Bashour, H, et al. Cutaneous leishmaniasis in Syria: a review of available data during the war years: 2011–2018. PLoS Negl Trop Dis. (2019) 13:e0007827. doi: 10.1371/journal.pntd.0007827
112. World Health Organization. Leishmaniasis, status of endemicity of cutenaous leishmaniasis: 2021 (2023). Available at: https://apps.who.int/neglected_diseases/ntddata/leishmaniasis/leishmaniasis.html (Accessed January 6, 2023).
113. World Health Organization. Syrian Arab Republic: Annual report 2021. Cairo: WHO Regional Office for the Eastern Mediterranean (2022).
114. El Safadi, D, Merhabi, S, Rafei, R, Mallat, H, Hamze, M, and Acosta-Serrano, A. Cutaneous leishmaniasis in North Lebanon: re-emergence of an important neglected tropical disease. Trans R Soc Trop Med Hyg. (2019) 113:471–6. doi: 10.1093/trstmh/trz030
115. Halk Sagligi Genel Mudurlugu. Sark Cibani Istatistik Verileri (2023). Available at: https://hsgm.saglik.gov.tr/tr/zoonotikvektorel-sarkcibani/istatistik (Accessed January 6, 2023).
116. Floret, N, Viel, J-F, Mauny, F, Hoen, B, and Piarroux, R. Negligible risk for epidemics after geophysical disasters. Emerg Infect Dis. (2006) 12:543–8. doi: 10.3201/eid1204.051569
117. De Bruycker, M, Greco, D, Lechat, MF, Annino, I, De Ruggiero, N, and Triassi, M. The 1980 earthquake in southern Italy: morbidity and mortality. Int J Epidemiol. (1985) 14:113–7. doi: 10.1093/ije/14.1.113
118. Smith, DFQ, and Casadevall, A. Disaster microbiology-a new field of study. MBio. (2022) 13:e0168022. doi: 10.1128/mbio.01680-22
119. Schmid, BV, Büntgen, U, Easterday, WR, Ginzler, C, Walløe, L, Bramanti, B, et al. Climate-driven introduction of the black death and successive plague reintroductions into Europe. Proc Natl Acad Sci U S A. (2015) 112:3020–5. doi: 10.1073/pnas.1412887112
120. Dalla Villa, P, Migliaccio, P, Innocenti, I, Nardoia, M, and Lafiandra, DC. Companion animals welfare in non-epidemic emergencies: the case of Central Italy, post-earthquake 2016/2017. J Appl Anim Ethics Res. (2019) 1:253–79. doi: 10.1163/25889567-12340012
121. Chomel, BB. Emerging and re-emerging Zoonoses of dogs and cats. Animals. (2014) 4:434–45. doi: 10.3390/ani4030434
122. Garde, E, Acosta-Jamett, G, and Bronsvoort, BM. Review of the risks of some canine zoonoses from free-roaming dogs in the post-disaster setting of Latin America. Animals. (2013) 3:855–65. doi: 10.3390/ani3030855
123. Reese, JF. Dogs and dog control in developing countries In: DJ Salem and AN Rowan, editors. The state of the animals III: 2005. Washington DC: Humane Society Press (2005). 55–64.
124. Levy, JK, Lappin, MR, Glaser, AL, Birkenheuer, AJ, Anderson, TC, and Edinboro, CH. Prevalence of infectious diseases in cats and dogs rescued following hurricane Katrina. J Am Vet Med Assoc. (2011) 238:311–7. doi: 10.2460/javma.238.3.311
125. Warner, GS. Increased incidence of domestic animal bites following a disaster due to natural hazards. Prehosp Disaster Med. (2010) 25:188–90. doi: 10.1017/S1049023X00007962
126. Centers for Disease Control, Prevention (CDC). Morbidity and mortality associated with hurricane Floyd-North Carolina, September-October 1999. MMWR Morb Mortal Wkly Rep. (2000) 49:369–72.
127. Spencer, HC, Campbell, CC, Romero, A, Zeissig, O, Feldman, RA, Boostrom, ER, et al. Disease-surveillance and decision-making after the 1976 Guatemala earthquake. Lancet. (1977) 2:181–4. doi: 10.1016/s0140-6736(77)90193-3
128. Mori, J, Tsubokura, M, Sugimoto, A, Tanimoto, T, Kami, M, Oikawa, T, et al. Increased incidence of dog-bite injuries after the Fukushima nuclear accident. Prev Med. (2013) 57:363–5. doi: 10.1016/j.ypmed.2013.06.013
129. Baladi, Enab. Injuries and deaths, stray dogs threaten residents of northwestern Syria (2022). (https://english.enabbaladi.net/archives/2022/12/injuries-and-deaths-stray-dogs-threaten-residents-of-northwestern-syria/).
130. Omoleke, S, Mohammed, I, and Saidu, Y. Ebola viral disease in West Africa: a threat to global health, economy and political stability. J Public Health Afr. (2016) 7:534. doi: 10.4081/jphia.2016.534
131. Kruk, ME, Freedman, LP, Anglin, GA, and Waldman, RJ. Rebuilding health systems to improve health and promote statebuilding in post-conflict countries: a theoretical framework and research agenda. Soc Sci Med. (2010) 70:89–97. doi: 10.1016/j.socscimed.2009.09.042
132. McPake, B, Witter, S, Ssali, S, Wurie, H, Namakula, J, and Ssengooba, F. Ebola in the context of conflict affected states and health systems: case studies of northern Uganda and Sierra Leone. Confl Heal. (2015) 9:23. doi: 10.1186/s13031-015-0052-7
133. Connolly, MA, Gayer, M, Ryan, MJ, Salama, P, Spiegel, P, and Heymann, DL. Communicable diseases in complex emergencies: impact and challenges. Lancet. (2004) 364:1974–83. doi: 10.1016/S0140-6736(04)17481-3
134. Gates, S, Hegre, H, Nygård, HM, and Strand, H. Consequences of civil conflict. World Development Report 2011 Background Paper. Washington, DC: World Bank (2011).
135. Noji, EK. Public health issues in disasters. Crit Care Med. (2005) 33:S29–33. doi: 10.1097/01.CCM.0000151064.98207.9C
136. Sabatinelli, G. Determinants in malaria resurgence in the former USRR. Giornme Italiano Di Medjcina Tropicale. (1999) 4:N.3-4.
137. Ejov, M, Sergiev, V, Baranova, A, Kurdova-Mintcheva, R, Emiroglu, N, Gasimov, E, et al. On the road to elimination 2000–2015. Summary. Copenhagen, Denmark: World Health Organization (2018).
138. World Health Organization. Early Rehabilitation in Conflicts and Disasters Geneva World Health Organization (2020). p. 46–47.
139. World Health Organization. Poliomyelitis (polio) (2023). Available at: https://www.who.int/health-topics/poliomyelitis#tab=tab_1 (Accessed January 6, 2023).
140. World Health Organization. Poliomyelitis, Ethiopia and Somalia. Wkly Epidemiol Rec. (2006) 81:349–56.
141. World Health Organization Regional Office for the Eastern Mediterranean Polio Eradication Initiative. Syria (2023). Available at: https://www.emro.who.int/polio-eradication/priority-countries/syria.html (Accessed January 6, 2023).
142. Polio Global Eradication Initiative. Syria takes steps to advance polio transition while strengthening essential health priorities (2023). Available at: https://polioeradication.org/news-post/syria-takes-steps-to-advance-polio-transition-while-strengthening-essential-health-priorities/ (Accessed January 06, 2023).
143. Al-Moujahed, A, Alahdab, F, Abolaban, H, and Beletsky, L. Polio in Syria: problem still not solved. Avicenna J Med. (2017) 7:64–6. doi: 10.4103/ajm.AJM_173_16
144. World Health Organization. Wild poliovirus type 1 (WPV1)–Mozambique (2022). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON395 (Accessed January 6, 2023).
145. World Health Organization. Yellow fever situation in Africa and South America in 2004. Wkly Epidemiol Rec. (2005) 80:250–6.
146. World Health Organization. Yellow fever-African Region (AFRO) (2022). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON405 (Accessed September 02, 2022).
147. World Health Organization. Yellow fever-west and Central Africa (2021). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/yellow-fever---west-and-central-africa (Accessed January 06, 2023).
148. World Health Organization. Yellow fever-African Region (AFRO) (2022). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON431 (Accessed January 03, 2023).
149. Bertherat, E, Lamine, KM, Formenty, P, Thuier, P, Mondonge, V, Mitifu, A, et al. Major pulmonary plague outbreak in a mining camp in the Democratic Republic of Congo: brutal awakening of an old scourge. Med Trop (Mars). (2005) 65:511–4.
150. International Federation of Red Cross and Red Crescent Societies (IFRC). DREF operation-final report. Democratic Republic of Congo, Plague Outbreak. (2023). Available at: https://www.ifrc.org/appeals?date_from=&date_to=&appeal_code=MDRCD035&text= (Accessed February 16, 2023).
151. World Health Organization. Outbreak news. Plague, Democratic Republic of the Congo. Wkly Epidemiol Rec. (2006) 81:241–2.
152. World Health Organization. WHO guidance on research methods for health emergency and disaster risk management, revised 2022. Geneva, Switzerland: World Health Organization (2022). Available at: https://apps.who.int/iris/handle/10665/363502
153. Cookson, ST, and Buehler, JW. Emergency and disaster health surveillance In: W Ahrens and I Pigeot, editors. Handbook of epidemiology. New York: Springer (2014)
154. Degutis, LC. Disaster epidemiology and surveillance In: JG Elmore, D Wild, HD Nelson, and DL Katz, editors. Jekel's epidemiology, biostatistics, preventive medicine, and public health. 5th edn. Missouri, USA: Elsevier (2020)
155. Goyet, C, Sarmiento, J, and Grünewald, F. Health response to the earthquake in Haiti: January 2010. Washington, DC: Pan American Health Organization (PAHO) (2011).
156. World Health Organization. Health laboratory facilities in emergency and disaster situations. 2nd ed. Geneva, Switzerland: World Health Organization (2017).
157. World Health Organization. Emergency response framework. 2nd ed. Geneva, Switzerland: World Health Organization (2017).
158. Markby, J, Gygax, M, Savoy, C, Giebens, Y, Janjanin, S, Machoka, F, et al. Assessment of laboratory capacity in conflict-affected low-resource settings using two World Health Organization laboratory assessment tools. Clin Chem Lab Med. (2023) 61:1015–24. doi: 10.1515/cclm-2022-1203
159. Khadim, MT, Wiqar, MA, Khan, A, and Gardezi, A. 8th October 2005 earthquake – an experience of diagnostic laboratory services in disaster. Pakistan Armed Forces Med J. (2006) 56:433–7.
160. Paweska, JT, Jansen van Vuren, P, Meier, GH, le Roux, C, Conteh, OS, Kemp, A, et al. South African Ebola diagnostic response in Sierra Leone: a modular high biosafety field laboratory. PLoS Negl Trop Dis. (2017) 11:e0005665. doi: 10.1371/journal.pntd.0005665
161. Coltart, CE, Lindsey, B, Ghinai, I, Johnson, AM, and Heymann, DL. The Ebola outbreak, 2013–2016: old lessons for new epidemics. Philos Trans R Soc Lond Ser B Biol Sci. (2017) 372:20160297. doi: 10.1098/rstb.2016.0297
162. Coltart, CE, Johnson, AM, and Whitty, CJ. Role of healthcare workers in early epidemic spread of Ebola: policy implications of prophylactic compared to reactive vaccination policy in outbreak prevention and control. BMC Med. (2015) 13:271. doi: 10.1186/s12916-015-0477-2
163. Kilmarx, PH, Clarke, KR, Dietz, PM, Hamel, MJ, Husain, F, McFadden, JD, et al. Centers for disease control and prevention (CDC). Ebola virus disease in health care workers--Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep. (2014) 63:1168–71.
164. World Health Organization. Update on Lassa fever in West Africa. Wkly Epidemiol Rec. (2005) 80:85–92.
165. Fisher-Hoch, SP. Lessons from nosocomial viral haemorrhagic fever outbreaks. Br Med Bull. (2005) 73-74:123–37. doi: 10.1093/bmb/ldh054
166. Githui, WA, Hawken, MP, Juma, ES, Godfrey-Faussett, P, Swai, OB, and Kibuga, DK. Surveillance of drug resistant tuberculosis and molecular evaluation of transmission of resistant strains in refugee and nonrefugee populations in North-Eastern Kenya. Int J Tuberc Lung Dis. (2000) 4:947–55.
167. Holmes, AH, Moore, LS, Sundsfjord, A, Steinbakk, M, Regmi, S, Karkey, A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. (2016) 387:176–87. doi: 10.1016/S0140-6736(15)00473-0
168. Nellums, LB, Thompson, H, Holmes, A, Castro-Sánchez, E, Otter, JA, Norredam, M, et al. Antimicrobial resistance among migrants in Europe: a systematic review and meta-analysis. Lancet Infect Dis. (2018) 18:796–811. doi: 10.1016/S1473-3099(18)30219-6
169. Lillebaek, T, Andersen, AB, Dirksen, A, Smith, E, Skovgaard, LT, and Kok-Jensen, A. Persistent high incidence of tuberculosis in immigrants in a low-incidence country. Emerg Infect Dis. (2002) 8:679–84. doi: 10.3201/eid0807.010482
170. Ravensbergen, SJ, Berends, M, Stienstra, Y, and Ott, A. High prevalence of MRSA and ESBL among asylum seekers in the Netherlands. PLoS One. (2017) 12:e0176481. doi: 10.1371/journal.pone.0176481
171. Häsler, R, Kautz, C, Rehman, A, Podschun, R, Gassling, V, Brzoska, P, et al. The antibiotic resistome and microbiota landscape of refugees from Syria, Iraq and Afghanistan in Germany. Microbiome. (2018) 6:37. doi: 10.1186/s40168-018-0414-7
172. WHO Regional Office for Europe. Community-based antimicrobial resistance screening among Syrian refugees and the host community in Turkey. Copenhagen: WHO Regional Office for Europe (2021).
173. Tokajian, S, Eisen, JA, Jospin, G, Hamze, M, Rafei, R, Salloum, T, et al. Draft genome sequences of Acinetobacter baumannii strains harboring the blaNDM-1 gene isolated in lebanon from civilians wounded during the Syrian civil war. Genome Announc. (2016) 4:e01678–15. doi: 10.1128/genomeA.01678-15
174. Salloum, T, Tannous, E, Alousi, S, Arabaghian, H, Rafei, R, Hamze, M, et al. Genomic mapping of ST85 blaNDM-1 and blaOXA-94 producing Acinetobacter baumannii isolates from Syrian civil war victims. Int J Infect Dis. (2018) 74:100–8. doi: 10.1016/j.ijid.2018.07.017
175. Weinstein, RA, Gaynes, R, and Edwards, JR, National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram negative bacilli. Clin Infect Dis. (2005) 41:848–54. doi: 10.1086/432803
176. Higgins, PG, Hagen, RM, Podbielski, A, Frickmann, H, and Warnke, P. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii isolated from war-injured patients from the eastern Ukraine. Antibiotics. (2020) 9:579. doi: 10.3390/antibiotics9090579
177. Scott, P, Deye, G, Srinivasan, A, Murray, C, Moran, K, Hulten, E, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. (2007) 44:1577–84. doi: 10.1086/518170
178. Calhoun, JH, Murray, CK, and Manring, MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. (2008) 466:1356–62. doi: 10.1007/s11999-008-0212-9
179. Granzer, H, Hagen, RM, Warnke, P, Bock, W, Baumann, T, Schwarz, NG, et al. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii complex isolates from patients that were injured during the Eastern Ukrainian conflict. Eur J Microbiol Immunol. (2016) 6:109–17. doi: 10.1556/1886.2016.00014
180. Rafei, R, Dabboussi, F, Hamze, M, Eveillard, M, Lemarié, C, Mallat, H, et al. First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Int J Infect Dis. (2014) 21:21–3. doi: 10.1016/j.ijid.2014.01.004
181. Eldholm, V, Pettersson, JH, Brynildsrud, OB, Kitchen, A, Rasmussen, EM, Lillebaek, T, et al. Armed conflict and population displacement as drivers of the evolution and dispersal of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. (2016) 113:13881–6. doi: 10.1073/pnas.1611283113
182. Lomtadze, N, Aspindzelashvili, R, Janjgava, M, Mirtskhulava, V, Wright, A, Blumberg, HM, et al. Prevalence and risk factors for multidrug-resistant tuberculosis in the republic of Georgia: a population-based study. Int J Tuberc Lung Dis. (2009) 13:68–73.
183. Schwalbe, N, and Harrington, P. HIV and tuberculosis in the former Soviet Union. Lancet. (2002) 360:s19–20. doi: 10.1016/S0140-6736(02)11805-8
184. Dean, AS, Tosas Auguet, O, Glaziou, P, Zignol, M, Ismail, N, Kasaeva, T, et al. 25 years of surveillance of drug-resistant tuberculosis: achievements, challenges, and way forward. Lancet Infect Dis. (2022) 22:e191–6. doi: 10.1016/S1473-3099(21)00808-2
185. European Centre for Disease Prevention and Control, WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2023–2021 data. European Centre for Disease Prevention and Control and Copenhagen. Stockholm, Sweden: WHO Regional Office for Europe (2023).
186. Holt, E. Tuberculosis services disrupted by war in Ukraine. Lancet Infect Dis. (2022) 22:e129. doi: 10.1016/S1473-3099(22)00214-6
187. World Health Organization. Environmental health in emergencies and disasters: a practical guide. Malta: World Health Organization (2002).
188. Pan American Health Organization. Natural disasters: protecting the public’s health. Washington, DC: PAHO (2000).
189. Howard, MJ, Brillman, JC, and Burkle, FM Jr. Infectious disease emergencies in disasters. Emerg Med Clin North Am. (1996) 14:413–28. doi: 10.1016/S0733-8627(05)70259-5
190. Austin, KF, Noble, MD, and Berndt, VK. Drying climates and gendered suffering: links between drought, food insecurity, and Women's HIV in less-developed countries. Soc Indic Res. (2021) 154:313–34. doi: 10.1007/s11205-020-02562-x
191. FSIN and Global Network Against Food Crises. The global network against food crises (GRFC), 2023. Rome (2023).
192. World Health Organization. Appeal greater horn of Africa (2023). Available at: https://www.who.int/emergencies/funding/outbreak-and-crisis-response-appeal/2023/2023-appeals/appeal-horn-of-africa (Accessed May 16, 2023).
193. World Health Organization. Drought and food insecurity in the greater horn of Africa (2023). Available at: https://www.who.int/emergencies/situations/drought-food-insecurity-greater-horn-of-africa (Accessed May 16, 2023).
194. World Health Organization. Communicable diseases and severe food shortage. Geneva, Switzerland: WHO Technical Note. WHO/HSE/GAR/DCE/2010.6 (2010).
195. UNAIDS. When women lead, change happens (2017). Available at: (https://www.unaids.org/sites/default/files/media-asset/when-women-lead-change-happens_en.pdf).
196. Orievulu, KS, Ayeb-Karlsson, S, Ngema, S, Baisley, K, Tanser, F, Ngwenya, N, et al. Exploring linkages between drought and HIV treatment adherence in Africa: a systematic review. Lancet Planet Health. (2022) 6:e359–70. doi: 10.1016/S2542-5196(22)00016-X
197. Chop, E, Duggaraju, A, Malley, A, Burke, V, Caldas, S, Yeh, PT, et al. Food insecurity, sexual risk behavior, and adherence to antiretroviral therapy among women living with HIV: a systematic review. Health Care Women Int. (2017) 38:927–44. doi: 10.1080/07399332.2017.1337774
198. Cardona, OD, Van Aalst, MK, Birkmann, J, Fordham, M, Mc Gregor, G, Perez, R, et al. Determinants of risk: exposure and vulnerability In: CB Field, V Barros, TF Stocker, D Qin, DJ Dokken, and KL Ebi, et al., editors. Managing the risks of extreme events and disasters to advance climate change adaptation, a special report of working groups I and II of the intergovernmental panel on climate change (IPCC). Cambridge and New York: Cambridge University Press (2012). 65–108.
199. Cutter, SL, Emrich, CT, Mitchell, JT, Boruff, BJ, Gall, M, Schmidtlein, MC, et al. The long road home: race, class, and recovery from Hurricane Katrina. Environ Sci Policy Sustain Dev. (2006) 48:8–20. doi: 10.3200/ENVT.48.2.8-20
200. Schneiderbauer, S, Calliari, E, Eidsvig, U, and Michael, Hagenlocher. The most recent view of vulnerability. Science for disaster risk management 2017: Knowing better and loosing less 68–82). Luxembourg: Publications Office of the European Union. K Poljanšek, M Marin Ferrer, T GroeveDe, and I Clark (2017).
201. Global Facility for Disaster Reduction and Recovery (GFDRR) Consultative Group. Disasters, conflict and fragility: a joint agenda. Available at: https://www.gfdrr.org/sites/default/files/publication/Disasters,%20Conflict%20%26%20Fragility.pdf (Accesses May 15, 2023).
202. Charnley, GEC, Kelman, I, Gaythorpe, KAM, and Murray, KA. Traits and risk factors of post-disaster infectious disease outbreaks: a systematic review. Sci Rep. (2021) 11:5616. doi: 10.1038/s41598-021-85146-0
203. Hammer, CC, Brainard, J, and Hunter, PR. Risk factors and risk factor cascades for communicable disease outbreaks in complex humanitarian emergencies: a qualitative systematic review. BMJ Glob Health. (2018) 3:e000647. doi: 10.1136/bmjgh-2017-000647
204. World Health Organization European Region. Türkiye earthquake external situation report no: 1:13–19 February 2023. Geneva: World Health Organization (2023) WHO/EURO: 2023-7145-46911-68441.
Keywords: emerging infectious diseases, re-emerging infectious diseases, wars, natural disasters, public health
Citation: Topluoglu S, Taylan-Ozkan A and Alp E (2023) Impact of wars and natural disasters on emerging and re-emerging infectious diseases. Front. Public Health. 11:1215929. doi: 10.3389/fpubh.2023.1215929
Edited by:
Fathiah Zakham, University of Helsinki, FinlandReviewed by:
Edmond Puca, Service of Infection Diseases University Hospital Center, AlbaniaXavier Vallès, Hospital Germans Trias i Pujol, Spain
Copyright © 2023 Topluoglu, Taylan-Ozkan and ALP. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aysegul Taylan-Ozkan, YXlzZWd1bHRheWxhbm96a2FuQGV0dS5lZHUudHI=
 Seher Topluoglu
Seher Topluoglu Aysegul Taylan-Ozkan2*
Aysegul Taylan-Ozkan2* Emine Alp
Emine Alp