
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Public Health , 07 August 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1211894
This article is part of the Research Topic Women in Science: Infectious Diseases: Epidemiology and Prevention 2023 View all 23 articles
Cervical cancer (CC), caused by a group of human papillomaviruses (HPV), is primarily a sexually transmitted disease. In Ethiopia, CC is the second most common cancer with estimated annual new cases and deaths of 7,450 and 5,300, respectively (1). CC and other high-risk (HR), probable-risk (PR), and low-risk (LR) carcinogenic genotypes-associated diseases are vaccine-preventable. Currently, six commercial prophylactic HPV vaccines are available: three bivalent (HPV 16 and 18) vaccines (2vHPV), two quadrivalent (HPV 6, 11, 16, and 18) vaccines (4vHPV), and a nonavalent (HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58) vaccine (9vHPV) (2). All these vaccines were originally licensed as a three-dose (3-dose) schedule over six months intervals, but a two-dose (2-dose) schedule was approved in girls younger than 15 years in 2016 after post-license efficacy evidence became available (3, 4). Subsequently, Ethiopia launched the HPV vaccination program with 2-dose Gardasil®4 (4vHPV vaccine) in December 2018 with the support of Gavi, the Global Vaccine Alliance.
Like many Gavi- eligible countries, Ethiopia had to choose a single-age cohort primarily targeting schoolgirls aged 14 years old because of the global vaccine supply constraint. The supply constraint is projected to continue for several years with many Gavi-eligible countries planning the introduction of HPV national immunization programs (5). Importantly, WHO set a goal of achieving CC elimination by 2030 worldwide. A dose-reduction vaccination approach is urgently needed if the WHO goal of CC elimination by 2030 is to be met. High-quality large-scale observational studies in Costa Rica and India demonstrated that a one-dose (1-dose) HPV vaccine regimen induces similar long-term protection to that of multidose (3, 4). On 7th April 2022, based on these efficacy data, the WHO's Strategic Advisory Group of Experts on Immunization (SAGE) recommended that each country could decide to implement alternative, off-label regimens, including a 2-dose schedule in all age groups or a 1-dose schedule for individuals aged 9–20 years (6). Before the implementation of a 1-dose HPV vaccine regime, however, the following key policy questions should be answered based on the currently available evidence in the Ethiopian context: (i) which 1-dose vaccine type regime (1-dose 4vHPV versus 1-dose 9vHPV) is optimal; (ii) does Ethiopia need locally generated evidence to make a policy switch to a 1-dose 9vHPV vaccine regime; (iii) will 1-dose 9vHPV vaccine be sufficient to offer protection to HIV-infected girls; and (iv) is the HPV vaccine uptake optimal? In this opinion paper, we provide our insights related to these key policy questions.
According to the recent systematic review, HPV 16 is the most dominant HPV genotype among HPV-positive women in Ethiopia, accounting for over 37% of HR-HPV infections while HPV18 is responsible for 4.4% of infections (Table 1) (7) with a combined HPV16/18 infection prevalence of 54%. The seven common non-HPV16/18 HR-HPV genotypes detected in Ethiopia were [HPV-31 (3.8%), 33 (1.7%), 35 (4.8%), 39 (1.9%), 45 (3.5%), 52 (6.8%), 58 (3.1%), and 68 (2.8%)] (7). The data implies that 46% of women's infections with HR-HPV genotypes in Ethiopia are related to non-HPV 16/18 genotypes. A very recent study found that HPV16 (31.8%), 31 (19.1%), 52 (11.8%), 58(10.9%), and 35 (10%) were the most frequently detected HR-HPV genotypes while HPV18 was detected in only 2.7% of HPV-positive women (Table 1) (8). These data altogether suggest that vaccinating girls with the 4vHPV vaccine would protect only 54 % of infections with HR-HPV genotypes, leaving 46% of vaccines vulnerable to infections with non-4vHPV vaccine HR-HPV genotypes. By contrast, the 9vHPV vaccine is highly efficacious in offering protection to five additional HR-HPV genotypes (9). Thus, if Ethiopia switches to the 9vHPV vaccine, over 90% of vaccinated women will likely be protected from the 9vHPV vaccine HR-HPV genotypes-related persistent infections and diseases (Table 1).
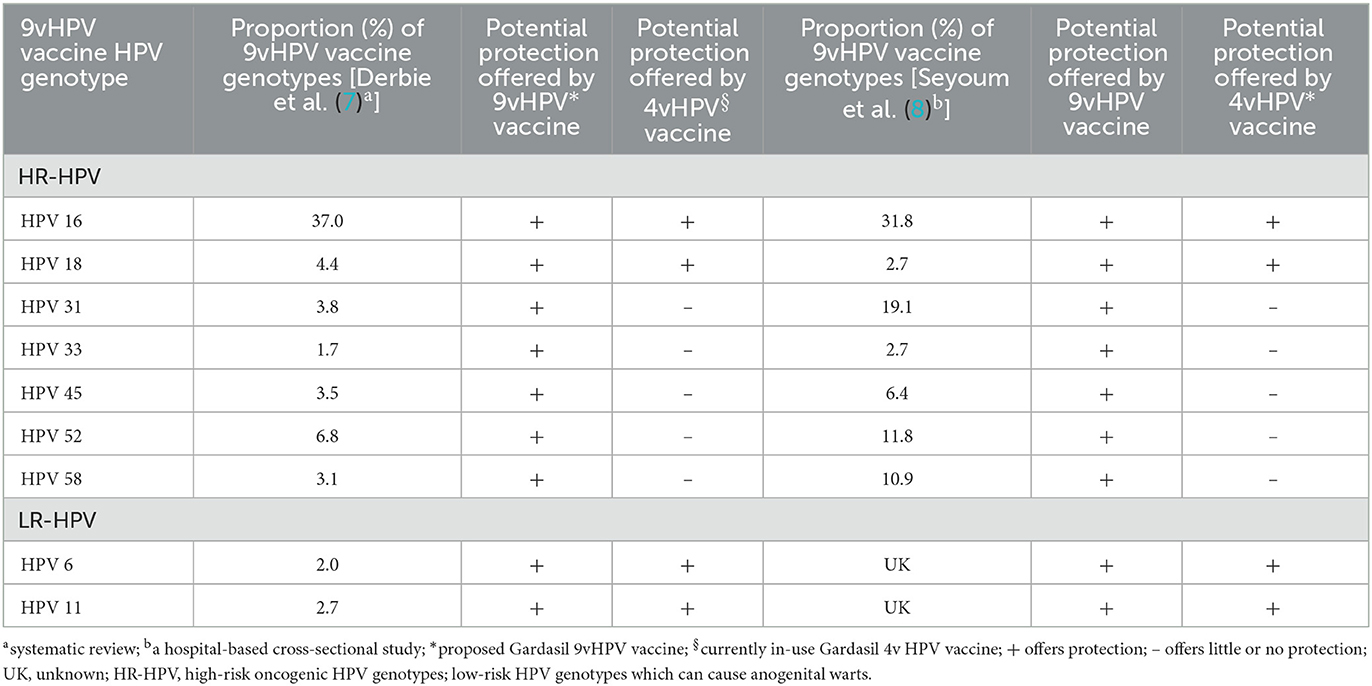
Table 1. Relative distribution of 9vHPV vaccine HPV genotypes in women with and without abnormal cervical dysplasia in Ethiopia and potential protection offered by 9vHPV and 4vHPV vaccination.
Given obtaining efficacy data based on virological or cervical dysplasia (disease) endpoints will take several years when vaccination given to girls before first sexual debut, the efficacy of the 1-dose 9vHPV vaccine can be assessed through immunobridging trials (10). An immunobridging trial is a non-inferiority comparison of geometric mean concentrations (GMCs) of anti-HPV antibodies for specific HPV genotypes in a new population group with those in a population group in whom efficacy had been established in randomized clinical trial (RCT) with virological or disease endpoints. It assumes if the GMCs of antibodies between two groups are comparable, the efficacy between the two groups is comparable too. Accordingly, immunobridging RCTs on a 1-dose 9vHPV vaccine schedule have been conducted in sub-Saharan African countries where the 90% of the global CC burden occurs and dose reduction is a critical strategy for increasing HPV vaccine coverage (10). Recent randomized muti-center, double-blind, controlled trials with clinical endpoints at 35 months in Tanzania (11) and at 18 months in Kenya (12) indicate 96.0% and 91.4% efficacies, respectively in the HPV 16/18/31/33/45/52/58, substantiating the evidence obtained from immunobridging RCT in Tanzania. These results from RCTs conducted in Tanzania and Kenya, where like Ethiopia their populations have similar additional comorbidities (e.g., HIV, parasites, and malnutrition) that may compromise the quality and durability of vaccine-induced immune responses, proved the efficacy of 1-dose 9vHPV vaccine in preventing CC associated with infections with HPV-16, 18, 31, 33, 45, 52, and 58. Based on these established efficacy results along with the diversity of HR-HPV genotypes distribution data in Ethiopia, we are advocating a policy switch to a 1-dose 9vHPV vaccine schedule. Nevertheless, if the Ministry of Health needs prior local evidence to make the in-demand decision, conducting an immunobridging RCT study that aims to compare antibody GMC specific to HPV genotypes with the Tanzanian 1-dose 9vHPV vaccine immunobridging cohort (10) can be considered instead of waiting for several years until local efficacy data based on virological or disease endpoints will be generated.
In sub-Saharan Africa, including Ethiopia, the burden of HIV infection is also high. In 2023 alone, >620,000 new HIV infections are estimated to occur in Ethiopia with women being disproportionately infected (13). Approximately 1 in 5 CCs occur in women living with HIV (WLWH). Additionally, WLWH are more likely to be infected with more HR-HPV genotypes not covered by the 4vHPV vaccine (14). Considering this, WLWH will more likely benefit from the introduction of the 9vHPV vaccine. Despite accumulated evidence on immunogenicity and tolerability of 3-dose of both 4vHPV and 9vHPV vaccines in HIV-infected children and women (15), however, there is no RCT regarding the efficacy of 1-dose 9vHPV vaccine in HIV-positive individuals so far. An immunobridging study would be the fastest strategy to get approval for the use of a 1-dose 9vHPV vaccine in WLWH. Until results from immunobridging studies are publicly available, we dither advocating the use of 1-dose 9vHPV in HIV-infected girls. Instead, we recommend at least 2-dose 9vHPV vaccination over the ongoing 2-dose 4vHPV given the 9vHPV vaccine's potential to offer protection to more non-4vHPV vaccine HR-HPV genotypes (9).
Besides the additional public health benefits, a mathematical model-based study suggests the cost-effectiveness of the 9vHPV vaccine in Ethiopia compared to the current 2-dose 4vHPV vaccination program (16). Another modeling analysis showed similar economic beneficial impact of a 1-dose of 9vHPV vaccination schedule in Tanzania (17), where like Ethiopia, high diversity of HR HPV genotypes has been detected in women with abnormal cervical lesions. The minimum price per dose for Gardasil 4vHPV and 9vHPV vaccines are $4.50 and $5.18, respectively (16). In Ethiopia, the estimated number of girls of age 14 years who are eligible to take the current 2-dose 4vHPV vaccine in 2023 alone is above 1.5 million (13). If Ethiopia adopts a 1-dose 9vHPV vaccine as its national HPV immunization schedule, it will save a minimum of $5.73 million per year and a total of $40.11 million by end of 2030, assuming that the vaccine uptake and coverage is 100% and the number of HPV vaccine eligible girls remain the same. In addition, it will save the costs required for second dose injection needle and delivery, including personnel, transportation and storage costs. And this saved money can be stretched to support vaccine delivery or building other healthcare services.
Despite the excellent safety profile of all HPV vaccines, its uptake in Ethiopia is suboptimal as low as 44.4% (18). In addition, a low disparity between uptake of 1-dose and 2-dose was observed (19), suggesting that switching to a 1-dose schedule alone may not necessarily increase HPV vaccination coverage to an optimal level. The main barriers to vaccine uptake were concerns about side effects (primarily fear of injection and pain at the site of injection), a misconception that the vaccine is given as anti-fertility, and a lack of adequate information on the benefit of the HPV vaccine before the vaccination schedule and lack of regular vaccination program (18, 19). However, barriers related to vaccine side effects can be circumvented by dose reduction as girls will prefer a 1-dose over 2-dose schedule to avoid pain caused by the second shot. Future country-wide study that involves all stakeholders is urgently needed to identify the main barriers to single-dose vaccine uptake and mitigation strategies.
Until the global vaccine shortage is circumvented and becomes affordable to low and middle-income countries (LMICs), including Ethiopia, we suggest switching to a 1-dose 9vHPV vaccination program to accelerate the elimination of CC in Ethiopia by 2030. For HIV-infected girls, however, we recommend the use of at least a 2-dose 9vHPV vaccination schedule until evidence regarding the efficacy of 1-dose 9vHPV vaccination in WLWH are available. Switching to a 1-dose vaccination schedule can also partially circumvent the current suboptimal HPV vaccine uptake in Ethiopia.
Despite its health benefits, the 9vHPV vaccine is not yet included in the Gavi support list for low-income countries (LICs). Thus, high CC burden LICs, including Ethiopia with a high prevalence of non-4vHPV vaccine HR-HPV genotypes should request Gavi to consider the 9vHPV vaccine in its support list or seek other global international support. If Gavi requires local evidence to support switching to a 1-dose 9vHPV vaccine schedule, Ethiopia may consider conducting a pilot immunobridging RCT instead of clinical efficacy data.
Conceptualization: TG and AMu. Writing of the initial draft of the manuscript: TG. Editing and reviewing the last draft: TG, LW, AMi, and AMu.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cancer Tomorrow. Available online at: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=23&single_unit=500&populations=231&sexes=2&types=1 (accessed March 17, 2023).
2. Human Papillomavirus Vaccines: WHO Position Paper, December 2022. Available online at: https://www.who.int/publications-detail-redirect/who-wer9750-645-672 (accessed March 21, 2023).
3. Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. (2020) 112:1038–46. doi: 10.1093/jnci/djaa011
4. Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. (2021) 22:1518–29. doi: 10.1016/S1470-2045(21)00453-8
5. Tsu VD, LaMontagne DS, Atuhebwe P, Bloem PN, Ndiaye C. National implementation of HPV vaccination programs in low-resource countries: Lessons, challenges, and future prospects. Prev Med. (2021) 144:106335. doi: 10.1016/j.ypmed.2020.106335
6. One-dose Human Papillomavirus (HPV) Vaccine Offers Solid Protection Against Cervical Cancer. Available online at: https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer (accessed April 15, 2023).
7. Derbie A, Mekonnen D, Nibret E, Maier M, Woldeamanuel Y, Abebe T. Human papillomavirus genotype distribution in Ethiopia: an updated systematic review. Virol J. (2022) 19:13. doi: 10.1186/s12985-022-01741-1
8. Seyoum A, Seyoum B, Gure T, Alemu A, Belachew A, Abeje D, et al. Genotype heterogeneity of high-risk human papillomavirus infection in Ethiopia. Front Microbiol. (2023) 14: 1116685. doi: 10.3389/fmicb.2023.1116685
9. Chatterjee A. The next generation of HPV vaccines: nonavalent vaccine V503 on the horizon. Expert Rev Vaccines. (2014) 13:1279–90. doi: 10.1586/14760584.2014.963561
10. Baisley K, Kemp TJ, Kreimer AR, Basu P, Changalucha J, Hildesheim A, et al. Comparing one dose of HPV vaccine in girls aged 9–14 years in Tanzania (DoRIS) with one dose of HPV vaccine in historical cohorts: an immunobridging analysis of a randomised controlled trial. Lancet Global Health. (2022) 10:e1485–93. doi: 10.1016/S2214-109X(22)00306-0
11. Watson-Jones D, Changalucha J, Whitworth H, Pinto L, Mutani P, Indangasi J, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial. Lancet Global Health. (2022) 10:e1473–84. doi: 10.1016/S2214-109X(22)00309-6
12. Barnabas RV, Brown ER, Onono MA, Bukusi EA, Njoroge B, Winer RL, et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. (2022) 1:EVIDoa2100056. doi: 10.1056/EVIDoa2100056
13. HIV Rates by Country 2023. Available online at: https://worldpopulationreview.com/country-rankings/hiv-rates-by-country (accessed April 7, 2023).
14. Bogale AL, Belay NB, Medhin G, Ali JH. Molecular epidemiology of human papillomavirus among HIV infected women in developing countries: systematic review and meta-analysis. Virol J. (2020) 17:179. doi: 10.1186/s12985-020-01448-1
15. Zizza A, Banchelli F, Guido M, Marotta C, Di Gennaro F, Mazzucco W, et al. Efficacy and safety of human papillomavirus vaccination in HIV-infected patients: a systematic review and meta-analysis. Sci Rep. (2021) 11:4954. doi: 10.1038/s41598-021-83727-7
16. Wondimu A, Postma MJ, van Hulst M. Cost-effectiveness analysis of quadrivalent and nonavalent human papillomavirus vaccines in Ethiopia. Vaccine. (2022) 40:2161–7. doi: 10.1016/j.vaccine.2022.02.080
17. Hsiao A, Struckmann V, Stephani V, Mmbando D, Changalucha J, Baisley K, et al. Costs of delivering human papillomavirus vaccination using a one- or two-dose strategy in Tanzania. Vaccine. (2023) 41:372–9. doi: 10.1016/j.vaccine.2022.11.032
18. Lakneh EA, Mersha EA, Asresie MB, Belay HG. Knowledge, attitude, and uptake of human papilloma virus vaccine and associated factors among female preparatory school students in Bahir Dar City, Amhara Region, Ethiopia. PLoS ONE. (2022) 17:e0276465. doi: 10.1371/journal.pone.0276465
Keywords: cervical cancer, HPV, genotype, girls, HIV, vaccine, dose, Ethiopia
Citation: Gelanew T, Wondwossen L, Mihret A and Mulu A (2023) A call for switching to a 1-dose 9vHPV national vaccination program in Ethiopia. Front. Public Health 11:1211894. doi: 10.3389/fpubh.2023.1211894
Received: 25 April 2023; Accepted: 25 July 2023;
Published: 07 August 2023.
Edited by:
Aleksandra Barac, University of Belgrade, SerbiaReviewed by:
Claudio Costantino, University of Palermo, ItalyCopyright © 2023 Gelanew, Wondwossen, Mihret and Mulu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tesfaye Gelanew, dGVzZmF5ZS5nZWxhbmV3QGFocmkuZ292LmV0; dGVzZmF5ZWd0YXllQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.