
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 24 January 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1203913
This article is part of the Research Topic Encouraging Health Research Productivity in Complex Humanitarian Crises: Somalia View all 8 articles
 Fartun Yasin Mohamed1*
Fartun Yasin Mohamed1* Hassan Abdullahi Dahie2
Hassan Abdullahi Dahie2 Jamal Hassan Mohamoud3
Jamal Hassan Mohamoud3 Mohamed Hussein Adam3
Mohamed Hussein Adam3 Hassan Mohamud Dirie1
Hassan Mohamud Dirie1Background: Uropathogenic Escherichia coli (UPEC) is a strain of E. coli commonly associated with urinary tract infections. In addition, antibiotic resistance in UPEC is one of the most significant health problems. This study was conducted to determine the prevalence, antimicrobial resistance, and factors linked to uropathogenic Escherichia coli (UPEC) in pregnant women.
Methods: This cross-sectional study was conducted within a hospital setting between August 2022 and December 2022. Using consecutive convenient sampling, the research enrolled 220 pregnant women. The urine samples obtained from these women were cultured on MacConkey and blood agar and incubated at 37°C overnight, followed by sub-culturing on Mueller Hinton media. Bacterial identification involved Gram staining and biochemical characterization (TSI, indole, citrate, methyl red, urea agar, and motility tests). Conversely, susceptibility tests were performed using the Kirby–Bauer disk diffusion method. A binary logistic regression model and analysis of odds ratios (ORs) were employed to evaluate the risk factors associated with E. coli infection, and statistical significance was attributed to p-values of ≤0.05.
Results: Out of the 220 urine samples examined, 42 (19%) exhibited a positive culture, indicating an E. coli infection in pregnant women. Our analysis revealed that income, gestational age, and history of UTIs were identified as risk factors associated with E. coli infection. Most E. coli isolates demonstrated sensitivity to amikacin (100%), nitrofurantoin (85.7%), amoxicillin/clavulanic acid, and meropenem (83.3%).
Conclusion: The prevalence of E. coli was remarkable. It could be recommended that pregnant women in antenatal care have routine culture and antimicrobial susceptibility tests to prevent transmission of resistant pathogens and complications in both pregnant mothers and the unborn baby.
A urinary tract infection (UTI) occurs when a pathogen invades and multiplies within the urinary system, disrupting kidney and urinary function, and may present as symptomatic or asymptomatic bacteriuria (1). Uropathogenic E. coli (UPEC) is the predominant agent leading to urinary tract infections (UTIs), and it is widely recognized that pregnant women are at a higher risk of experiencing UTIs (2). Urinary tract infections are highly prevalent in pregnancy and can pose significant risks for both the mother and the unborn child (3). Due to the anatomical, physiological, and functional changes brought on by pregnancy, the urinary system is frequently susceptible to urinary tract infections (UTIs), which are a result of bacteria entering the urinary bladder (4). Globally, 13%–33% of pregnant women have UTIs, with 1%–18% experiencing symptoms and 2%–10% asymptomatic (5). Urinary tract infections (UTIs) are commonly seen in both community and hospital settings globally. Uropathogenic Escherichia coli is the causative agent in up to 90% of UTIs acquired in the community and 50% of those acquired in healthcare facilities (1). Several factors, such as multiple pregnancies, age, a history of urinary tract infections, diabetes, anatomical abnormalities in the urinary tract, inadequate personal hygiene, and socioeconomic status, influence the occurrence of bacteriuria during pregnancy (6).
Escherichia coli is the most prevalent pathogen, causing between 75% and 90% of bacteriuria in pregnant women (7–9). Additionally, pregnant women are considered vulnerable hosts for UTIs due to the physiological changes associated with pregnancy, leading to compromised immunity (4). Long-term untreated UPEC infection has also been known to be associated with pregnancy complications such as eclampsia, low birth weight, and preterm birth (10). Therefore, early UTI diagnosis, appropriate management, and a suitable therapeutic and preventive approach are crucial to avoiding pregnancy complications (11). The bacterial population in the human body can be significantly influenced by brief antibiotic exposure, leading to the development of resistant pathogens and symbiotic organisms. Antibiotic resistance may result in heightened patient morbidity, prolonged treatment, increased hospitalization, and reliance on broad-spectrum antibiotics (12). The emergence of drug-resistant strains of UPEC increases the serious threat to global health (13). The development of antimicrobial resistance in uropathogenic Escherichia coli (UPEC) and the emergence of multi-drug resistance (MDR) UPEC in recent years pose a clinical challenge, especially in women experiencing recurrent UTIs (14). The resistance patterns of bacteria can differ based on geographical location and time, highlighting the importance of regular testing for antibiotic resistance. E. coli strains are primary contributors to severe bacterial infections in healthcare settings, and distinct antibiotic patterns have been documented depending on the source (15).
Available literature reports indicate that there are high and worrying levels of resistance to commonly used antimicrobial drugs for treating UTIs in pregnancy, which is becoming an increasing cause for concern, especially in low- or middle-income countries (LMICs) (16).
In Somalia, empirical treatment is usually used in place of routine UTI culture and antimicrobial susceptibility testing. This could encourage the excessive use of antibiotics and the emergence of microbial strains that are resistant to them (17). In Somalia, there are not many articles expressing any alarming issues, whether it is virulence, genotypes, or antimicrobials. Moreover, there are little or no published research results on the prevalence of E. coli and the antimicrobial susceptibility pattern in the country. Hence, this investigation aimed to assess the prevalence and antimicrobial susceptibility profile of uropathogenic E. coli and its associated factors among pregnant women attending the maternity department of Dr. Sumait Hospital, Mogadishu, Somalia.
A cross-sectional approach was used to study pregnant women attending the antenatal care department at Dr. Sumait Hospital in Mogadishu, Somalia, between August 2022 and December 2022.
Employing consecutive convenient sampling, a cohort of 220 pregnant women meeting the inclusion criteria willingly enrolled in the study to assess the prevalence of E. coli. The isolates examined were of community-acquired origin. Pregnant women who declined participation and those who had undergone antimicrobial therapy within the past 7 days were exempted from the study.
The number of participants was determined based on the Cochrane formula. A reported prevalence of 17.3% for E. coli was used, with the assumption of a 95% confidence level and a desired absolute precision of 5% (18).
A sample size of 220 pregnant women was achieved.
The pregnant study participants were interviewed using a structured questionnaire. The questionnaire comprises two sections: sociodemographic data of the respondents, including marital status, age, monthly income, and level of education; and questions regarding the potential risk factors for UTIs, including clinical data.
The patients voided a modest volume of urine, and midstream urine samples (220) were then collected into a sterile container. All the samples were immediately examined within an hour after collection.
Then, using a disposable loop and following conventional culture protocols, 10 μL of the urine sample was inoculated on blood and MacConkey agar (Oxoid, United Kingdom) by streaking.
Bacterial growth was then discovered after the plates had been cultured at 37°C overnight. The examination of growth and assessment was based on a growth rate of 105 CFU/mL of E. coli. The bacterial isolates were Gram-stained; biochemical characterization was conducted, including citrate, triple sugar iron, indole, methyl red, urea agar, and motility tests, which were utilized to detect E. coli.
Antimicrobial resistance was assessed using the Kirby–Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute protocol (19). Colonies of the bacteria were resuspended in a normal saline solution to obtain a turbidity of 0.5 McFarland standard before being applied to cover the surface of a Mueller–Hinton agar plate. The bacterial isolate was tested against a panel of 13 antimicrobials (amikacin (10 μg), nitrofurantoin (300 μg), meropenem (10 μg), gentamicin (10 μg), amoxicillin/clavulanic (20/10 μg), cefoxitin (30 μg), ciprofloxacin (10 μg), ceftazidime (30 μg), ceftriaxone (30 μg), nalidixic acid (30 μg), trimethoprim/sulfamethoxazole (25 μg, 1.25/23.75 μg), and ampicillin (10 μg)). After embedding the Mueller–Hinton agar plate with the antibiotic disks, the plate was then incubated at 37°C for 18–24 h. Resistance was measured based on the zone of growth inhibition according to the CLSI guidelines.
The raw data were tested for accuracy, consistency, and comprehensiveness. After that, the information was cleaned, coded, entered, and examined using SPSS version 26. A descriptive analysis was condensed using frequencies and percentages. To identify any independent variables connected to E. coli, a binary logistic regression model and odds ratio (OR) analysis were also used. Significance was determined when the p-value was ≤0.05.
This study involved a total of 220 expectant women. Most (58.6%) study participants were 21–30 years old. A total of 85.9% were married, 40.5% had no formal education, 71.4% were unemployed, and 56.8% had a monthly income below US$200 (Table 1).
With respect to the age of gestation, most of the women (62.7%) were found to be in their third trimester. Similarly, 55.9% were primigravida, 59.6% had no history of abortion, and 95% had no history of diabetes. Similarly, according to the study, 71.9% of the participants had previously experienced UTI, of whom 51.4% presented urinary tract infection symptoms (Table 2).
Concerning the occurrence of E. coli 19.1% of the urine specimen collected from the pregnant women were found to be positive for an E. coli infection, as depicted in Figure 1.
Out of the entire 220 analyzed urine specimens, 76 (34.5%) tested positive for significant bacteriuria. The bulk of the isolated 62 (28.1%) were the majority, constituting Gram-negative specimens, while the remaining 14 (6.3%) were Gram-positive. The most prevalent isolate was E. coli, 42 (19.1%) subsequent to S. aureus 14 (6.3%), K. pneumoniae 11 (5%), Proteus spp. 6 (2.7%), and Pseudomonas 3 (1.3%) (Figure 2).
The antibiotic susceptibility testing against E. coli isolates (Table 3) showed more susceptibility to amikacin (100%) followed by nitrofurantoin (85.7%), meropenem (83.3%), amoxicillin/clavulanic (83.3%), gentamicin (76.2%), cefoxitin (57.1%), ciprofloxacin (31%), ceftazidime (23.8%), ceftriaxone (21.4%), nalidixic acid (19.1%), trimethoprim + sulfamethoxazole (9.5%), and ampicillin (7.1%) (Table 3).
The study also revealed that family income, gestational age, previous history of UTIs, and presence of symptoms of UTIs demonstrated a significant association with E. coli infection among pregnant women. Regarding family income, pregnant women who were from a family with a monthly income below US$200 during the study period were 2.5 times more likely to be positive for E. coli infection in contrast to those who had a higher family income [OR = 2.5, 95%CI:1.19–5.32, p < 0.015]. Similarly, expectant mothers who were in the first and second trimesters during the study period were 6.2 and 2.6 times more probable to be positive for E. coli infection compared to those who were in the third trimester, respectively [OR = 6.2, 95% CI:2.58–14.5, p < 0.050] and [OR = 2.6, 95%CI;1.11–6.30, p < 0.050]. Concerning the history of UTIs, expectant mothers with a UTI history were 4.5 times more likely to experience an E. coli infection compared to those without a UTI history [OR = 4.5, 95%CI:1.56–13.47, p < 0.050]. Moreover, the presence of UTI symptoms shows that expectant mothers who have symptoms of UTI are 2.4 times more likely to be positive for E. coli infection compared to those who have no symptoms [OR = 2.4, 95%CI: 1.16–5.20, p < 0.050]. On the other hand, the study did not find any statistically significant association between age, educational level, occupational status, gravidity, history of abortion, history of diabetic mellitus, and E. coli infection (Table 4).
The study did not find any statistically significant differences regarding family monthly income, gestational age, and history of UTI in women with E. coli infection and in women with infection due to other uropathogens (Table 5).
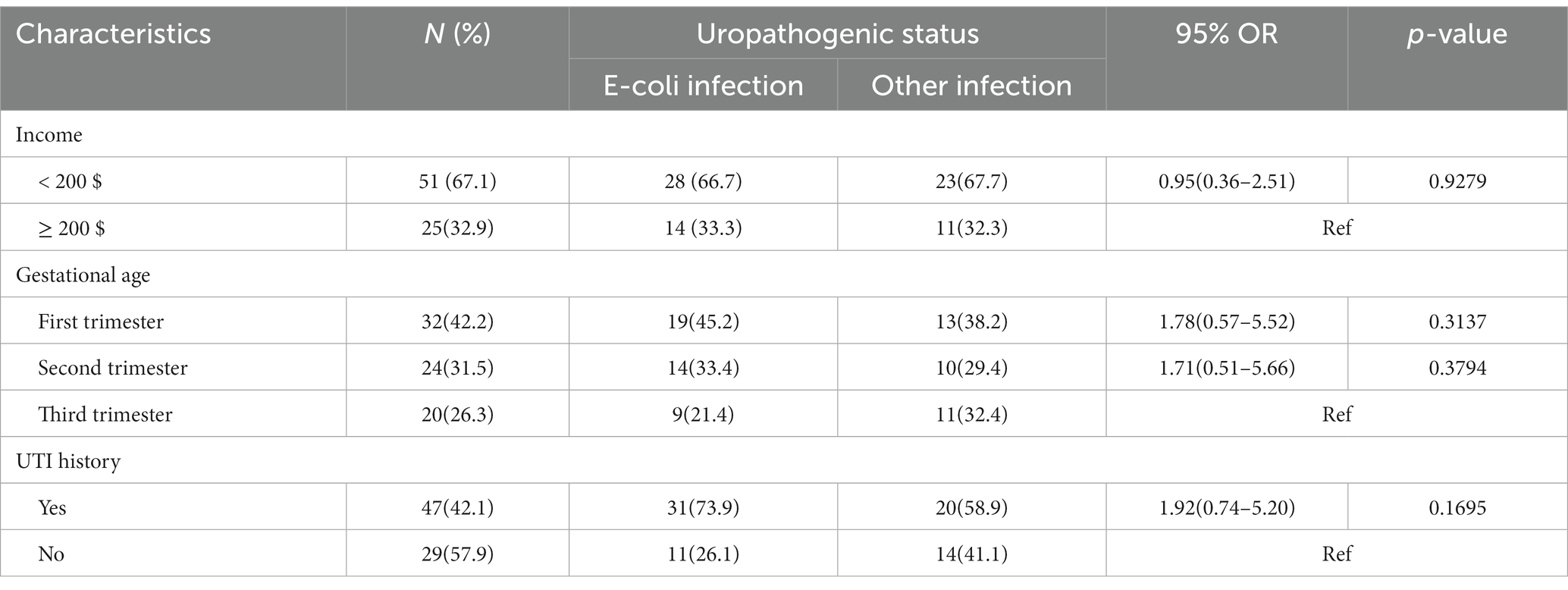
Table 5. Potential risk factors associated with uropathogenic E. coli and women with other uropathogenic infections.
As seen in Table 6, there is a statistically significant difference between women with E. coli infection and those with negative urine culture reports regarding their monthly income and history of UTI (p = 0.0014 and p = 0.007), respectively. However, there was no significant association between gestational age and E. coli infection between the groups.
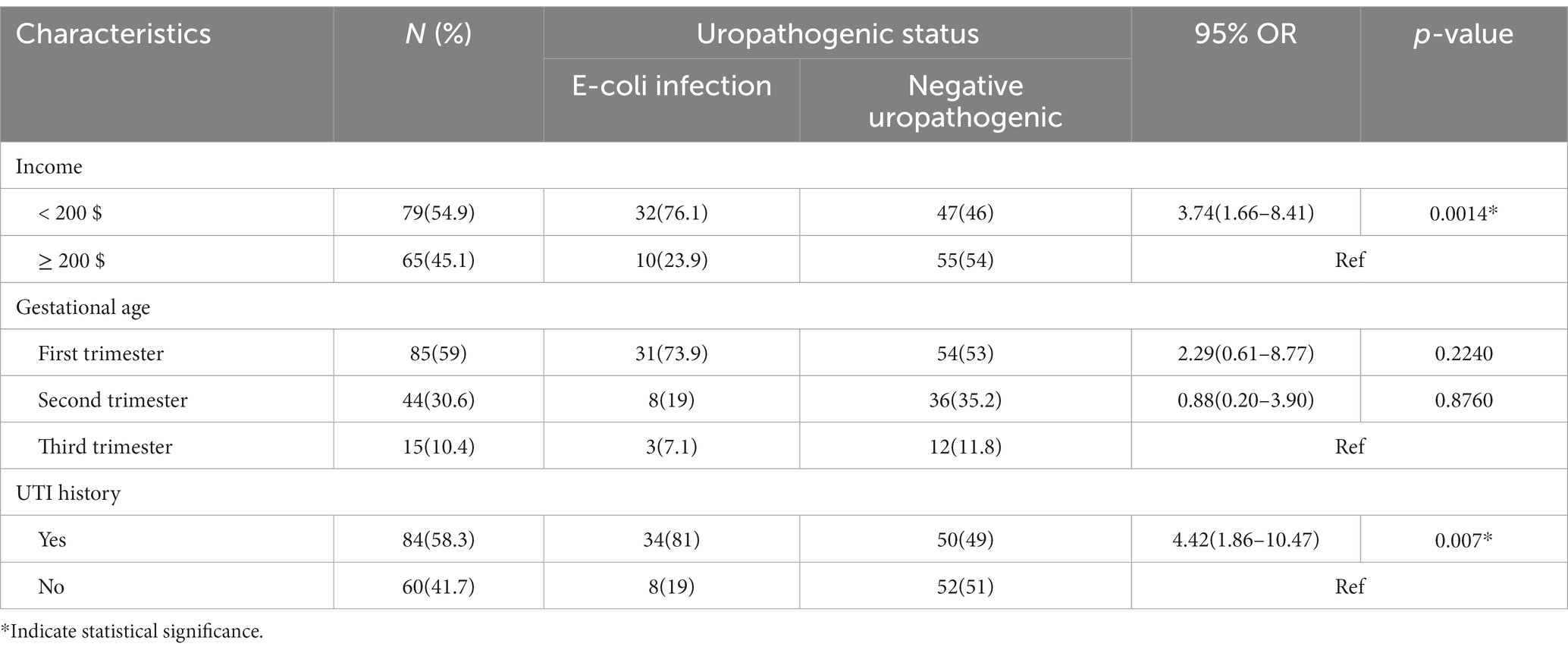
Table 6. Potential risk factors associated with uropathogenic E. coli and women without other uropathogenic infections.
Urinary tract infections are highly prevalent during pregnancy, with E. coli being the most common pathogen among expectant mothers. The anatomical and physiological alterations in the body during pregnancy can increase women’s vulnerability to developing UTIs (20). This study found that 19% (42/220) of the pregnant women were positive for Escherichia coli. A higher prevalence was reported in Kenya at 23.5% (21), southeast Ethiopia at 27.3% (22), and south-western Uganda at 28.78% (23). The variation may result from local social norms, environmental factors, personal hygiene expectations, and healthcare utilization patterns.
Regarding sociodemographic characteristics, it has been found that the monthly income of pregnant women below US$200 was notably associated with an E. coli infection. It has been revealed that expectant mothers who had a low monthly income were twice as likely to have UPEC compared to those who had a higher income. Similar study findings were reported from northern Ethiopia (24). This could result from the relationship between low socioeconomic status and poor nutrition and immunity, especially in pregnant women.
According to the gestational period, pregnant women who were first and second trimesters pregnant during the study period were 6.2 and 2.6 times more likely to get an E. coli infection, respectively compared to their third-trimester counterparts. Studies conducted in various locations yielded similar results in Saudi Arabia and Western and Northern Ethiopia (11, 24, 25). As argued by Tadesse et al. (24), this could be the result of UTIs in pregnant women, which usually initiate approximately at week six, reaching its peak between weeks 22 and 24. This is attributed to factors such as urethral dilation, heightened bladder volume, diminished bladder, and urethral tone, all of which foster bacterial proliferation in the urine.
Regarding the presence of UTI symptoms, expectant mothers with prior symptoms of UTIs exhibited a 2.4 times greater probability of developing a positive E. coli infection compared to those who had no symptoms. A similar study finding was reported in Khartoum, Sudan (26). This may be because E. coli is strongly linked to the typical symptoms of infection.
Moreover, the study revealed that pregnant women with a prior history of UTIs had a 4.5-fold higher likelihood of E. coli infection compared to individuals without a history of UTIs. This was similar to other studies from Libya, Egypt, and Nigeria (27–29). This association might be explained by the presence of strains resistant to antibiotics from previous infections.
In our study, no statistically significant correlation was observed between the prevalence of E. coli and age, educational attainment, employment status, gravidity, history of abortion, or history of diabetes mellitus. Studies conducted in Bangladesh and Nigeria revealed similar findings (30, 31). However, these studies reported that E. coli has a significant association with the mother’s gestational age. However, according to the comorbidities, previous studies identified that there is a significant association between having diabetes mellitus and chronic kidney disease and the risk of developing UTIs due to their altered immunological integrity (32).
As per the study findings, no distinctions were observed between uropathogenic E. coli and other uropathogenic infections concerning potential risk factors such as income, gestational age, and history of urinary tract infections. Comparable results were observed in studies conducted in both Eastern and Southeast Ethiopia (22, 33). On the other hand, it was observed that women from low-income families and those with a history of UTIs were 3.7 and 4.4 times more likely to contract an E. coli infection compared to their counterparts. A similar result was reported by a study conducted in Central Ethiopia (17). This might suggest that individuals with a lower income experience diminished socioeconomic status, leading to inadequate nutrition and weakened immunity, thereby elevating the likelihood of uropathogenic infections. Additionally, a prior history of UTIs during pregnancy, coupled with the persistence of antibiotic-resistant strains from the earlier infection, could contribute to the recurrence of such infections.
According to the study’s analysis of antibiotic susceptibility patterns, E. coli was most susceptible to amikacin (100%), followed by nitrofurantoin (85.7%), meropenem (83.3%), amoxicillin/clavulanic (83.3%), and gentamicin (76.2%). Similar results were observed in studies conducted in South Africa and Ethiopia (34–36). However, E. coli were resistant to nalidixic acid (80.9%), sulfamethoxazole (90.5%), and ampicillin (92.9%) This aligned with research conducted in Nairobi, Kenya (37).
According to the current study, uropathogenic E. coli bacteria were exceedingly resistant to third-generation cephalosporin antibiotics. Similarly, the World Health Organization (WHO) also observed resistance to third-generation cephalosporins in 68% of E. coli isolates and 81% of Klebsiella isolates (38, 39). Additional studies related to multi-drug resistance in uropathogenic E. coli bacteria have also been described (16, 40). The present investigation indicated that, regarding simple and complex bacteriuria, carbapenems are preferable to cephalosporins. The most prescribed antibiotics are easily accessible at nearby drugstores in Somalia, and people can buy and utilize them without needing a prescription, potentially hastening the onset of drug resistance.
The prevalence of E. coli was 19%. Monthly income, gestational period, previous experience of UTIs, and existence of symptoms of UTIs were associated with E. coli in antibiotic sensitivity patterns. The main antibiotics that proved effective against E. coli isolated from urine samples in pregnant women were amikacin, nitrofurantoin, and meropenem. It could be recommended that pregnant women in antenatal care have routine culture and antimicrobial susceptibility tests to prevent resistance and complications in both the expectant mother and the unborn child.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by SIMAD University’s Human Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
FM: conceptualization. MA: methodology and data curation. HMD: data collection. JM: formal data analysis. HAD: writing original draft preparation. FM, HAD, JM, MA, and HMD: writing—review and editing. All authors contributed to the article and approved the submitted version.
The authors express their gratitude to the expectant mothers who took part in the study, along with the staff and administration of Dr. Sumait Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
UPEC, uropathogenic Escherichia coli; LMICs, low-or middle-income countries; TSI, triple sugar iron; OR, odds ratio; E. coli, Escherichia coli; CFU, colony forming unit; MDR, multi-drug resistance.
1. Zhao, F, Yang, H, Bi, D, Khaledi, A, and Qiao, M. A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections. Microb Pathog. (2020) 144:104196. doi: 10.1016/j.micpath.2020.104196
2. Ballesteros-Monrreal, MG, Arenas-Hernández, MMP, Enciso-Martínez, Y, Martínez-De la Peña, CF, Rocha-Gracia, RDC, Lozano-Zaraín, P, et al. Virulence and resistance determinants of uropathogenic Escherichia coli strains isolated from pregnant and non-pregnant women from two states in Mexico. Infect Drug Resist. (2020) 13:295–310. doi: 10.2147/IDR.S226215
3. Ramos, NL, Sekikubo, M, Kironde, F, Mirembe, F, Sääf, M, and Brauner, A. The impact of vitamin D on the innate immune response to uropathogenic Escherichia coli during pregnancy. Clin Microbiol Infect. (2015) 21:482.e1–7. doi: 10.1016/j.cmi.2014.12.010
4. Matuszkiewicz-Rowińska, J, Małyszko, J, and Wieliczko, M. Urinary tract infections in pregnancy: old and new unresolved diagnostic and therapeutic problems. Arch Med Sci. (2015) 1:67–77. doi: 10.5114/aoms.2013.39202
5. Mugisha, A, Mujuzi, S, and Akampurira, A. A cross-sectional study to determine urinary tract infections and antibiotic susceptibility patterns among pregnant women attending antenatal Clinic at Kawempe National Referral Hospital, Uganda. Student’s J Heal Res Africa [Internet]. (2022) 3:10. doi: 10.51168/sjhrafrica.v3i3.107
6. Azami, M, Jaafari, Z, Masoumi, M, Shohani, M, Badfar, G, Mahmudi, L, et al. The etiology and prevalence of urinary tract infection and asymptomatic bacteriuria in pregnant women in Iran: a systematic review and meta-analysis. BMC Urol. (2019) 19:43. doi: 10.1186/s12894-019-0454-8
7. Awoke, N, Tekalign, T, Teshome, M, Lolaso, T, Dendir, G, and Obsa, MS. Bacterial profile and asymptomatic bacteriuria among pregnant women in Africa: a systematic review and meta analysis. EClinicalMedicine. (2021) 37:100952. doi: 10.1016/j.eclinm.2021.100952
8. Tahir, PH. Prevalence of urinary tract infections caused by some gram-negative bacteria among pregnant women in Kirkuk Province – Iraq. Microbiol Infect Dis. (2022) 6:1–4. doi: 10.33425/2639-9458.1154
9. Dube, R, Al-Zuheiri, STS, Syed, M, Harilal, L, Zuhaira, DAL, and Kar, SS. Prevalence, clinico-bacteriological profile, and antibiotic resistance of symptomatic urinary tract infections in pregnant women. Antibiotics. (2022) 12:33. doi: 10.3390/antibiotics12010033
10. Storme, O, Saucedo, JT, Garcia-Mora, A, Dehesa-Dávila, M, and Naber, KG. Risk factors and predisposing conditions for urinary tract infection. Ther Adv Urol. (2019) 11:19–28. doi: 10.1177/1756287218814382
11. AlShamlan, NA, AlOmar, RS, Aldossary, R, Alahmari, M, Alghamdi, A, AlGhamdi, M, et al. The epidemiology, associated factors and bacterial profile of asymptomatic bacteriuria in pregnant women: a retrospective chart review study in Saudi Arabia. Int J Womens Health. (2022) 14:1749–59. doi: 10.2147/IJWH.S394936
12. Critchley, IA, Cotroneo, N, Pucci, MJ, and Mendes, R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One. (2019) 14:e0220265. doi: 10.1371/journal.pone.0220265
13. Shah, C, Baral, R, Bartaula, B, and Shrestha, LB. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. Microbiology. (2019) 19:1–6. doi: 10.1186/s12866-019-1587-3
14. Kot, B. Antibiotic resistance among uropathogenic Escherichia coli. Pol J Microbiol. (2019) 68:403–15. doi: 10.33073/pjm-2019-048
15. Raeispour, M, and Ranjbar, R. Antibiotic resistance, virulence factors and genotyping of uropathogenic Escherichia coli strains. Antimicrob Resist Infect Control. (2018) 7:118. doi: 10.1186/s13756-018-0411-4
16. Lee, ACC, Mullany, LC, Koffi, AK, Rafiqullah, I, Khanam, R, Folger, LV, et al. Urinary tract infections in pregnancy in a rural population of Bangladesh: population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth. (2019) 20:1. doi: 10.1186/s12884-019-2665-0
17. Ali, AH, Reda, DY, and Ormago, MD. Prevalence and antimicrobial susceptibility pattern of urinary tract infection among pregnant women attending Hargeisa group hospital, Hargeisa, Somaliland. Sci Rep. (2022) 12:1–10. doi: 10.1038/s41598-022-05452-z
18. Polse, RF, Yousif, SY, and Assafi, MS. Prevalence and antimicrobial susceptibility patterns of uropathogenic E. coli among people in Zakho, Iraq. Int J Res Med Sci. (2016) 4:1219–23. doi: 10.18203/2320-6012.ijrms20160813
19. Tula, A, Mikru, A, Alemayehu, T, and Dobo, B. Bacterial profile and antibiotic susceptibility pattern of urinary tract infection among pregnant women attending antenatal Care at a Tertiary Care Hospital in southern Ethiopia. Can J Infect Dis Med Microbiol. (2020) 2020:1–9. doi: 10.1155/2020/5321276
20. View of prevalence of urinary tract infection during pregnancy at tertiary care hospital: a cross-sectional study [Internet]. Available at: https://pjmhsonline.com/index.php/pjmhs/article/view/1460/1446
21. Simba, SM, Omwenga, EO, and Musyoki, SK. Prevalence of E. coli as a causative agent of urinary tract infections and its drug susceptibility patterns among pregnant mothers seeking medicare at Kisii teaching and referral hospital, Kenya. Int J Community Med Public Heal [Internet]. (2022) 9:1161–9. doi: 10.18203/2394-6040.ijcmph20220671
22. Taye, S, Getachew, M, Desalegn, Z, Biratu, A, and Mubashir, K. Bacterial profile, antibiotic susceptibility pattern and associated factors among pregnant women with urinary tract infection in Goba and Sinana Woredas, bale zone, Southeast Ethiopia. Biomed Res Notes. (2018) 11:799. doi: 10.1186/s13104-018-3910-8
23. Johnson, B, Stephen, BM, Joseph, N, Asiphas, O, Musa, K, and Taseera, K. Prevalence and bacteriology of culture-positive urinary tract infection among pregnant women with suspected urinary tract infection at Mbarara regional referral hospital, South-Western Uganda. BMC Pregnancy Childbirth. (2021) 21:159. doi: 10.1186/s12884-021-03641-8
24. Tadesse, S, Kahsay, T, Adhanom, G, Kahsu, G, Legese, H, Gwahid, A, et al. Prevalence, antimicrobial susceptibility profile and predictors of asymptomatic bacteriuria among pregnant women in Adigrat general hospital, northern Ethiopia. Biomed Res Notes. (2018) 11:740. doi: 10.1186/s13104-018-3844-1
25. Abu, D, Abula, T, Zewdu, T, Berhanu, M, and Sahilu, T. Asymptomatic bacteriuria, antimicrobial susceptibility pattern and associated risk factors among pregnant women attending antenatal care in Assosa general hospital, Western Ethiopia. Microbiology. (2021) 21:348–8. doi: 10.1186/s12866-021-02417-6
26. Bayoumi, MA, Hamid, O, Hamid, MA, Hamid, OM, and Ali, M. Antibiotic resistance view project urinary tract infections: prevalence, risk factors, and antimicrobial susceptibility profile of associated bacterial pathogens among pregnant women visiting teaching hospitals, Khartoum, Sudan. Artic Merit Res J Med Med Sci [Internet]. (2022) 10:86–092.
27. Elzahaf, R, Younis, M, Ajroud, S, Elgade, LHA, Uahua, AS, and Elzahaf, RA. Prevalence of urinary tract infection among pregnant women and its risk factor in Derna City. Sch Int J Obstet Gynecol. (2019) 2:219–23. doi: 10.21276/sijog.2019.2.8.4
28. Jamiu, MO, Okesola, AO, Ogunleye, VO, and Fasulu, PE. Prevalence of and factors associated with significant bacteriuria among pregnant women attending the antenatal clinic of Adeoyo maternity hospital, Yemetu, Ibadan, Nigeria. Afr J Clin Exp Microbiol. (2021) 22:489–97. doi: 10.4314/ajcem.v22i4.9
29. Amiri, M, Lavasani, Z, Norouzirad, R, Najibpour, R, Mohamadpour, M, Nikpoor, AR, et al. Prevalence of urinary tract infection among pregnant women and its complications in their newborns during the birth in the hospitals of Dezful City, Iran, 2012 – 2013. Iran Red Crescent Med J. (2015) 17:e26946. doi: 10.5812/ircmj.26946
30. Uddin, MN, and Khan, T. Prevalence of urinary tract infection among pregnant women at Ibrahim Iqbal memorial hospital, Chandanaish, Bangladesh. Am J Clin Med Res [Internet]. (2016) 4:47–51. doi: 10.12691/ajcmr-4-3-3
31. Akpan, NG, Umoyen, AJ, Luka, TT, Esua, IS, Okon, AS, and Antia, UE. Bacterial etiologic agents, prevalence and associated risk factors of asymptomatic bacteriuria among pregnant and non-pregnant women in primary health care centers in south-South Nigeria. Int J Med Heal Res [Internet]. (2019) 5:66–76.
32. Ter, CC, Lee, SY, Wang, J, Chien, KL, and Huang, JW. Frailty increases the risk for developing urinary tract infection among 79,887 patients with diabetic mellitus and chronic kidney disease. Geriatrics. (2021) 21:349. doi: 10.1186/s12877-021-02299-3
33. Abate, D, Marami, D, and Letta, S. Prevalence, antimicrobial susceptibility pattern, and associated factors of urinary tract infections among pregnant and nonpregnant women at public health facilities, Harar, eastern Ethiopia: a comparative cross-sectional study. Can J Infect Dis Med Microbiol. (2020) 2020:1–9. doi: 10.1155/2020/9356865
34. Orji, O, Dlamini, Z, and Wise, AJ. Urinary bacterial profile and antibiotic susceptibility pattern among pregnant women in Rahima Moosa mother and child hospital, Johannesburg. S Afr J Infect Dis. (2022) 37:343. doi: 10.4102/sajid.v37i1.343
35. Wabe, YA, Reda, DY, Abreham, ET, Gobene, DB, and Ali, MM. Prevalence of asymptomatic bacteriuria, associated factors and antimicrobial susceptibility profile of bacteria among pregnant women attending Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. Ther Clin Risk Manag. (2020) 16:923–32. doi: 10.2147/TCRM.S267101
36. Zwane, T, Shuping, L, and Perovic, O. Etiology and antimicrobial susceptibility of pathogens associated with urinary tract infections among women attending antenatal care in four South African Tertiary-Level Facilities, 2015-2019. Antibiotics (Basel). 10:1–9. doi: 10.3390/antibiotics10060669
37. Onyango, HA, Ngugi, C, Maina, J, Kiiru, J, Onyango, HA, Ngugi, C, et al. Urinary tract infection among pregnant women at Pumwani maternity hospital, Nairobi, Kenya: bacterial etiologic agents, antimicrobial susceptibility profiles and associated risk factors. Adv Microbiol [Internet]. (2018) 8:175–87. doi: 10.4236/aim.2018.83012
38. The world is running out of antibiotics, WHO report confirms [Internet]. Available at: https://www.who.int/news/item/20-09-2017-the-world-is-running-out-of-antibiotics-who-report-confirms
39. Antimicrobial resistance: Global report on surveillance [Internet]. Available at: https://www.who.int/publications/i/item/9789241564748
Keywords: pregnant women, Escherichia coli , antimicrobial, prevalence of Escherichia coli, urinary tract infections
Citation: Mohamed FY, Dahie HA, Mohamoud JH, Adam MH and Dirie HM (2024) Prevalence, antimicrobial susceptibility profile, and associated risk factors of uropathogenic Escherichia coli among pregnant women attending Dr. Sumait Hospital Mogadishu, Somalia. Front. Public Health. 11:1203913. doi: 10.3389/fpubh.2023.1203913
Received: 11 April 2023; Accepted: 28 December 2023;
Published: 24 January 2024.
Edited by:
Marc Jean Struelens, Université libre de Bruxelles, BelgiumReviewed by:
Faham Khamesipour, Tehran University of Medical Sciences, IranCopyright © 2024 Mohamed, Dahie, Mohamoud, Adam and Dirie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fartun Yasin Mohamed, ZmFydHVueWFzaW4xNDFAc2ltYWQuZWR1LnNv
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.