
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Public Health, 22 June 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1197149
This article is part of the Research TopicIntegration of NGS in clinical and public health microbiology workflows: applications, compliance, quality considerationsView all 16 articles
Background: Visceral leishmaniasis (VL) is a neglected vector-borne tropical disease caused by Leishmania donovani (L. donovani) and Leishmania infantum (L. infantum). Due to the very small dimensions of the protozoa impounded within blood cells and reticuloendothelial structure, diagnosing VL remains challenging.
Case presentation: Herein, we reported a case of VL in a 17-month-old boy with acute lymphoblastic leukemia (ALL). The patient was admitted to West China Second University Hospital, Sichuan University, due to repeated fever after chemotherapy. After admission, chemotherapy-related bone marrow suppression and infection were suspected based on clinical symptoms and laboratory test results. However, there was no growth in the conventional peripheral blood culture, and the patient was unresponsive to routine antibiotics. Metagenomics next-generation sequencing (mNGS) of peripheral blood identified 196123 L. donovani reads, followed by Leishmania spp amastigotes using cytomorphology examination of the bone marrow specimen. The patient was given pentavalent antimonials as parasite-resistant therapy for 10 days. After the initial treatment, 356 L. donovani reads were still found in peripheral blood by mNGS. Subsequently, the anti-leishmanial drug amphotericin B was administrated as rescue therapy, and the patient was discharged after a clinical cure.
Conclusion: Our results indicated that leishmaniasis still exists in China. Unbiased mNGS provided a clinically actionable diagnosis of a specific infectious disease from an uncommon pathogen that eluded conventional testing.
Visceral leishmaniasis (VL) is a vector-borne protozoan neglected tropical disease (NTDs) caused by Leishmania donovani complex (L. donovani and L. infantum) and L. donovani (1–3). It is caused by an infection of blood cells in the lymphoid organs, primarily the spleen, bone marrow, and liver, and is fatal in more than 95% of untreated cases (4). In China, 3,169 cases of VL have been reported, with ~140–509 cases diagnosed per year between 2002 and 2011. VL is considered endemic in over 50 counties across 6 provinces/autonomous regions in western China, including Xinjiang, Gansu, Sichuan, Shaanxi, Shanxi, and Inner Mongolia (5–8). According to these data, leishmaniasis is not extinct and could potentially cause a public health problem in China.
Acute lymphoblastic leukemia (ALL) is the most frequent type of pediatric cancer, with an incidence of 5.4 per 100,000 cases in patients aged < 15 years old (9). In addition, cases of leishmaniasis found in patients formerly diagnosed with various cancers and treated with long-term anti-cancer chemotherapy have been previously reported, clearly suggesting an overlap between leishmaniasis transmission and malignant disease (10).
The diagnosis of VL is based on detecting Leishmania amastigote parasites in bone marrow or spleen biopsies. However, the very small dimensions of the protozoa impounded within blood cells and reticuloendothelial structure makes diagnosing leishmaniasis challenging (11). Recently developed metagenomics next-generation sequencing (mNGS) analyses forego the use of specific primers or probes. Instead, the entirety of the DNA and/or RNA (after reverse transcription to cDNA) is sequenced, thus providing a practical approach for diagnosing rare, novel, and atypical infectious etiologies (12). In the following description, we reported a VL case in an ALL infant after chemotherapy diagnosed by mNGS and parasitological microscopy.
A 17-month-old boy was admitted to West China Second University Hospital, Sichuan University, in March of 2022 for the insidious onset of fever, ecchymosis of skin, anhelation, and pancytopenia. On admission, blood routine examination results were as follows: white blood cell (WBC) 2.5 × 109/L, hemoglobin (Hb) 60 g/L, platelet (PLT) 45 × 109/L, and immature granulocytes found in peripheral blood smears. Subsequently, bone marrow morphology, immunophenotyping, cytogenetics, and molecular genetics were carried out. Based on the above testing, the boy was diagnosed with B-cell acute lymphoblastic leukemia (B-ALL, L2, ETV6-PEX5 fusion gene positive, KRAS A146V and KRAS A146T mutation, IKZF1–8 heterozygous deletion, 45, XY, der (7; 12) (q10; q10)(5)/46, XY (15). Then, according to ALL-low risk (ALL-LR) of the Chinese Children's Cancer Group study ALL 2020 (CCCG-ALL-2020), conventional and continuous therapy was administered. After the remission induction regimen of 4 weeks, complete remission (CR) was reached. Subsequently, the child was supposed to receive consolidation therapy according to CCCG-ALL-2020 with three courses after 4 weeks of achievement of CR.
On October 2022, which was also the interval between the first consolidation treatments, the patient was hospitalized again for a repeated fever of 13 days and coagulopathy after the second cycle of consolidation chemotherapy. Laboratory tests are shown in Table 1. Color Doppler ultrasonic examination showed swollen liver and spleen. The above results suggested that the infant had chemotherapy-related bone marrow suppression, infection, and coagulation dysfunction. Vancomycin, imipenem, and voriconazole were used for empirical antibiotic therapy. Fresh frozen plasma, fibrinogen, and prothrombin complex were used for improving coagulation function. Seven days after therapy, the patient still had a fever (38.4–39.4°C), and his liver and spleen were enlarged. CRP and PCT levels were 125.5 mg/L and 3.99 mg/L, respectively (Table 1). No microorganisms were detected by blood culture.
Then, mNGS was carried out in peripheral blood. After DNA was extracted from 200 μl of peripheral blood, the DNA library was built and sequenced on Nextseq 550 platform (Illumina, USA). All human host DNA was filtered out, and the valuable reads were aligned to Microbial Genome Databases (ftp://ftp.ncbi.nlm.nih.gov/genomes/) using BWA. Finally, a number of 196123 special reads of L. donovani were detected, and the coverage of the genome and relative abundance of L. donovani was 39.43 and 97.9%, respectively (Figure 1), which was indicative of L. donovani infection. In addition, 1 special read of Klebsiella pneumoniae was detected and defined as a background bacteria. Examination of patients' demographic information revealed the following: ever since birth, he resided in Jiuzhaigou county of Sichuan province, which is known as an endemic leishmaniasis region. Subsequently, microscopy of the bone marrow showed a larger number of Leishmania spp amastigotes, while phagocytic phenomena of histiocytes were found in all smears (Figure 2). All bone marrow smears from confirmed leukemia were reviewed, revealing no Leishmania spp amastigote in microscopy. Furthermore, RK39 antigens were also positive by immunochromatography. Thus, VL was diagnosed, and sodium stibogluconates were used as an anti-leishmanial drug. Nevertheless, after 10 days of anti-leishmanial therapy, 356 special reads of L. donovani were detected in peripheral blood by mNGS (Figure 3), and Leishmania spp amastigotes were also observed in the bone marrow. Subsequently, the anti-leishmanial drug Amphotericin B was used as rescue therapy. After completion of therapy, there was no Leishmania spp amastigote in the bone marrow. Finally, the child was discharged 56 days after admission. He was subsequently referred to the hematological clinic for leukemia and follow-up. On February 2023, he was readmitted to the hospital for chemotherapy, when mNGS test for peripheral blood was performed, and no reads of L. donovani were detected.
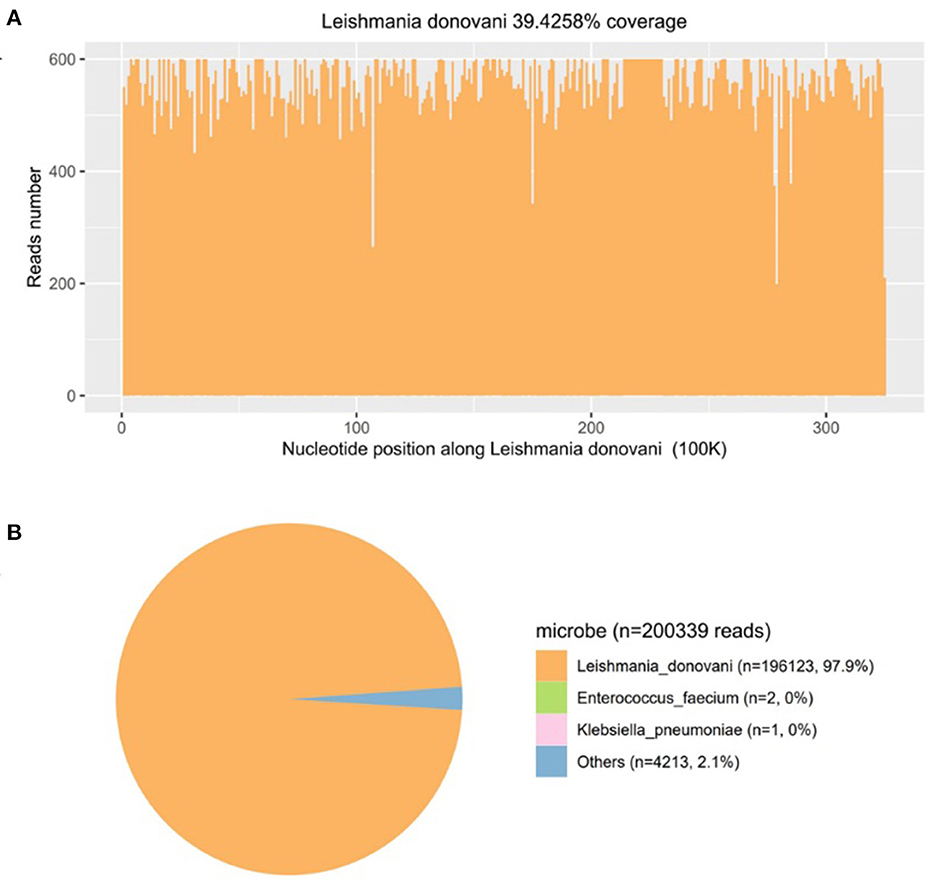
Figure 1. The diagnosis of Leishmania infection by metagenomics next-generation sequencing (mNGS). (A) Mapping of Leishmania donovani reads on the genome. (B) Distribution of pathogenic microorganisms reads in the absence of human, others, and unclassified reads.
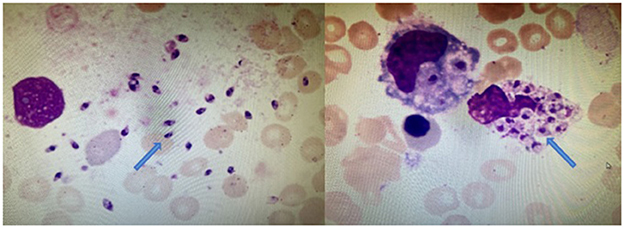
Figure 2. Bone marrow cytology of this patient. Arrowheads show the Leishmania spp amastigotes in extracellular and phagocyte, which are oval and 2.9–5.7 × 1.8–4.0 μm in size (Wright's stain, ×1,000).
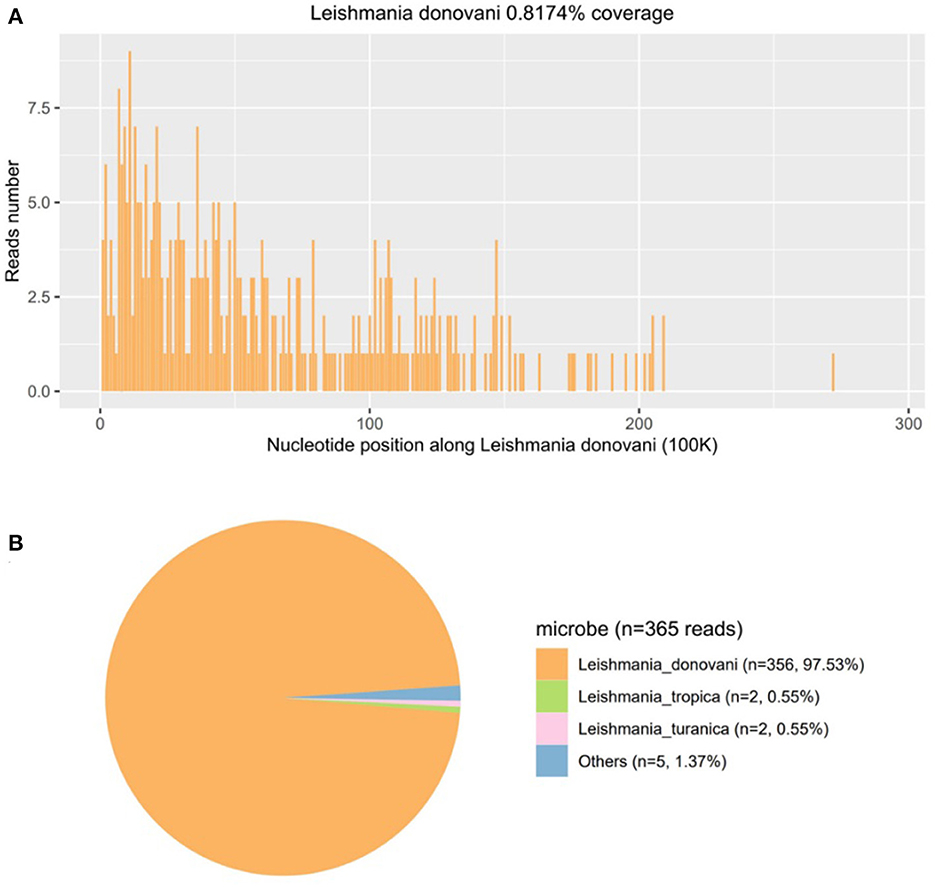
Figure 3. The follow-up diagnosis of Leishmania infection after using pentavalent antimonials 10 days by mNGS. (A) Mapping of Leishmania donovani reads on the genome. (B) Distribution of pathogenic microorganisms reads in the absence of human, others, and unclassified reads.
In the present study, we described a case of an infant diagnosed with ALL on admission to the hospital with repeated fever and coagulopathy after chemotherapy. A specific pathogen infection was suspected after collecting demographic information and learning about the history of lifelong residence in the forest region in Sichuan province, clinical symptoms, laboratory test results, and treatment history. Subsequently, the diagnosis of VL was definitely confirmed by mNGS. The patient was treated with pentavalent-Sb with adequate dosage and duration, and mNGS detected 356 L. donovani reads from the patient's plasma sample. Anti-leishmanial drug amphotericin B was subsequently administrated as rescue therapy. To the best of our knowledge, this is a rare report of leishmaniasis diagnosed by mNGS in leukemia, which provides a valuable reference for VL diagnosis and therapy follow-up.
Due to its wide geographic distribution, leishmaniosis constitutes a major public health problem. It is the second most prevalent pathogen among parasitic diseases. Hepatosplenomegaly, anemia, fever, cachexia, and leucopenia are all symptoms of this kind of VL, which can be significantly more dangerous (13). Factors that negatively impair the immune response, such as malnutrition or AIDS, are known to increase the risk of acquiring the infection and result in more severe manifestations (14). Previous studies have reported that VL is a frequent opportunistic infection in HIV-infected immunodeficient individuals that is very rarely found in cancer patients (10, 13). Although immune suppression by treatments or diseases has been rarely described as a risk factor for VL, the most common underlying cause of immunodeficiency in patients with VL are hematological malignancies apart from HIV infection (15). Nonspecific manifestations such as fever, fatigue, hepatosplenomegaly, hepatosplenomegaly, and weight loss may be attributed to malignancy or related treatment, which is difficult to diagnose in patients with tumors (13). Considering the risk of infection, there is a semiquantitative interaction of 2 factors, i.e., epidemiological exposures and the net state of immunosuppression. In this study, the boy resided since birth in an endemic leishmaniasis region of Sichuan province, and anti-leukemia chemotherapy resulted in immunosuppression. Several studies reported a possible association between Leishmania infection and cancer (16). Although local immune suppression induced by malignant disorders may promote leishmaniasis development, it is more likely that immunosuppression induced by long-term anti-cancer chemotherapy is responsible for parasite expansion (16).
As L. donovani is a specific pathogen that is not commonly present in the environment, patient's epidemiological history and nonspecific manifestations may be easily overlooked. Also, serological or polymerase chain reaction (PCR) reagents for this pathogen are not routinely prepared in the laboratory, which may delay the diagnosis of this infection. Some VL cases have been misdiagnosed as autoimmune hepatitis, ALL, and malignant lymphoma. They can also be asymptomatic, occur in unusual locations, or be clinically or microbiologically refractory (10, 15, 17, 18). In the present case, the symptoms of fever, fatigue, and hepatosplenomegaly were attributed to the ALL or related treatment, and the absence of amastigotes on repeat bone marrow smears to leukemia diagnosis and surveillance, which is why the infection of L. donovani was initially ignored. Finally, the infection was definitely confirmed by mNGS as an unknown and refractory infection. The diagnosis of Leishmania infection is based on detecting Leishmania amastigote, and various diagnostic techniques were used in making the diagnosis. Most studies used combined immunological methods, while others used plot molecular and parasitological tests. Some cases are also challenging to diagnose due to the low parasite load and low levels of antibodies (19). As an unbiased approach to the detection of pathogens, mNGS has allowed crossing the divide from microbial research to diagnostic microbiology, overcoming limitations of current diagnostic tests and allowing for hypothesis-free, culture-independent pathogen detection directly from clinical specimens (20, 21). In addition, for infectious diseases, collecting patient medical history, especially epidemiological history, is very important for diagnosis, as it can help us quickly adopt appropriate detection methods to identify the pathogen. However, in this case, the history of leukemia has its specificity, which has led to the neglect of typical bone marrow microscopy and medical history collection. Nonetheless, future clinical studies are needed to further confirm the value of mNGS in diagnosing Leishmania infection.
In summary, VL should be considered a potential opportunistic infection in patients with hematologic malignancies, especially in immunosuppressed patients living in or having visited areas where the disease is endemic. Unbiased mNGS may provide a clinically actionable diagnosis of a specific infectious disease from an uncommon pathogen, eluding conventional testing for weeks after the initial presentation.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Medicine Ethics Committee of West China Second University Hospital, Sichuan University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Conception and design: FL. Provision of study materials or patients: LC. Collection and assembly of data: LC, GC, and QY. Data analysis and interpretation: SL, JT, and JD. Manuscript writing and final approval of manuscript: all authors. All authors contributed to the article and approved the submitted version.
This research received funding support by Key R & D Projects of Science and Technology Department of Sichuan Province, 2020YFS0106 and 2023YFS0186.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. (2018) 15:951–70. doi: 10.1016/S0140-6736(18)31204-2
2. Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. (2006) 22:552–7. doi: 10.1016/j.pt.2006.09.004
3. Leta S, Dao THT, Mesele F, Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Trop Dis. (2014) 4:8. doi: 10.1371/journal.pntd.0003131
5. Lun ZR, Wu MS, Chen YF, Wang JY, Zhou XN, Liao LF, et al. Visceral leishmaniasis in china: an endemic disease under control. Clin Microbiol Rev. (2015) 28:987–1004. doi: 10.1128/CMR.00080-14
6. Etebari K, Hegde S, Saldaña MA, Widen SG, Wood TG, Asgari S, et al. Global transcriptome analysis of Aedes aegypti mosquitoes in response to Zika Virus infection. mSphere. (2017) 22:2. doi: 10.1128/mSphere.00456-17
7. Wang JY, Feng Y, Gao CH, Jin CF, Chen SB, Zhang CJ, et al. Asymptomatic Leishmania infection in human population of Wenxian County, Gansu Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. (2007) 25:62–4.
8. Guan LR, Zuo XP, Yimamu. Reemergence of visceral leishmaniasis in Kashi Prefecture. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. (2003) 21:285.
9. Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer. (2018) 18:471–84. doi: 10.1038/s41568-018-0015-6
10. Kopterides P, Mourtzoukou EG, Skopelitis E, Tsavaris N, Falagas ME. Aspects of the association between leishmaniasis and malignant disorders. Trans R Soc Trop Med Hyg. (2007) 101:1181–9. doi: 10.1016/j.trstmh.2007.08.003
11. Sreedharan V, Rao KVB. Protease inhibitors as a potential agent against visceral leishmaniasis: a review to inspire future study. Braz J Infect Dis. (2023) 27:1. doi: 10.1016/j.bjid.2022.102739
12. Charles Y, Chiu SA. Miller clinical metagenomics. Nat Rev Genet. (2019) 20:341–55. doi: 10.1038/s41576-019-0113-7
13. Albrecht H, Sobottka I, Emminger C, Jablonowski H, Just G. Stoehr A, et al.Visceral leishmaniasis emerging as an important opportunistic infection in HIV-infected persons living in areas nonendemic for Leishmania donovani. Arch Pathol Lab Med. (1996) 120:189–98.
14. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. (2004) 27:305–18. doi: 10.1016/j.cimid.2004.03.004
15. Fernández-Guerrero ML, Aguado JM, Buzón L, Barros C, Montalbán C, Martín T, et al. Visceral leishmaniasis in immunocompromised hosts. Am J Med. (1987) 83:1098–102. doi: 10.1016/0002-9343(87)90948-X
16. Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. (2011) 17:3. doi: 10.1002/lt.22378
17. Dalgic B, Dursun I, Akyol G. A case of visceral leishmaniasis misdiagnosed as autoimmune hepatitis. Turk J Gastroenterol. (2005) 16:52–3.
18. Jones SG, Forman KM, Clark D, Myers B. Visceral leishmaniasis misdiagnosed as probable acute lymphoblastic leukaemia. Hosp Med. (2003) 64:308–9. doi: 10.12968/hosp.2003.64.5.1767
19. Neitzke-Abreu HC, Venazzi MS, Bernal MV, Reinhold-Castro KR, Vagetti F, Mota CA, et al. Detection of DNA from Leishmania (Viannia): accuracy of polymerase chain reaction for the diagnosis of cutaneous leishmaniasis. PLoS ONE. (2013) 5:e0062473. doi: 10.1371/journal.pone.0062473
20. Schlaberg R Chiu CY Miller S Procop GW Weinstock G Professional Professional Practice Committee and Committee on Laboratory Practices of the American Society for Microbiology . Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch Pathol Lab Med. (2017) 141:776–86. doi: 10.5858/arpa.2016-0539-RA
Keywords: visceral leishmaniasis, Leishmania donovani, metagenomic next-generation sequencing, acute lymphoblastic leukemia, rapid diagnosis, case report
Citation: Chang L, Che G, Yang Q, Lai S, Teng J, Duan J, Liu T and Liu F (2023) Leishmania donovani visceral leishmaniasis diagnosed by metagenomics next-generation sequencing in an infant with acute lymphoblastic leukemia: a case report. Front. Public Health 11:1197149. doi: 10.3389/fpubh.2023.1197149
Received: 30 March 2023; Accepted: 30 May 2023;
Published: 22 June 2023.
Edited by:
Shangxin Yang, University of California, Los Angeles, United StatesReviewed by:
Evann Hilt, University of Minnesota Twin Cities, United StatesCopyright © 2023 Chang, Che, Yang, Lai, Teng, Duan, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu, TGl1ZmFuZ19jZDQyNEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.