- 1Department of Nursing, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2School of Nursing, Peking University, Beijing, China
- 3School of Nursing, Fudan University, Shanghai, China
- 4Department of Cardiology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
- 5School of Public Health, Wuhan University, Wuhan, China
Background: A shift in research interest from separate care problem to care problem clusters among caregivers of people living with dementia may contribute to a better understanding of dementia care. However, the care problems network among caregivers of people living with dementia are still unknown. This study aimed to identify care problem clusters and core care problems, and explore demographic variables associated with these care problem clusters among caregivers of people living with dementia.
Methods: Participants were recruited through memory clinics and WeChat groups. The principal component analysis was applied to identify care problem clusters. The network analysis was conducted to describe the relationships among care problems and clusters. Multiple linear models were used to explore the associated factors for the occurrence of the overall care problems and top three central care problem clusters.
Results: A total of 1,012 carer-patient pairs were included in the analysis. Nine care problem clusters were identified. In the entire care problem network, “deterioration in activities of daily living” was the most core care problem cluster across the three centrality indices, followed by “verbal and nonverbal aggression” and “loss of activities of daily living.” Variables including marital status, years of dementia diagnosis, number of dementia medication type, and caregiver’s educational attainment were associated with the prevalence of these three care problem clusters.
Conclusion: Our study suggests that there is a need to evaluate care problem clusters for the improvement of care problem management among people living with dementia. It is particularly important to include assessment and treatment of core care problem as an essential component of the dementia care.
Introduction
Dementia affects more than 55 million people worldwide, with a new case of dementia occurring around the world every 3 s; this number is expected to increase to 78 million by 2030 and 139 million by 2050 (1). China is one of the countries with the fastest growth in the older population. It is estimated that 15.07 million people aged 60 years or older in China living with dementia (2), accounting for about 25% of the global dementia population (3). The number of dementia cases in China is expected to reach 45.54 million by 2050 (4). The incidence, morbidity and mortality rates of dementia have steadily increased to make it presently the fifth leading cause of death among urban and rural residents in China and magnify the resulting burdens on individuals, families and society. The lack of effective treatment for dementia promotes the transition from disease treatment to health maintenance for this expanding population with dementia. In the long-term health maintenance, caregivers are faced with diversified and complicated care problems. Care problems refer to various difficulties that caregivers encounter in the process of taking care of people living with dementia (5), involving various aspects of managing daily living (6, 7), behavioral and psychological symptoms (8, 9), and safety risks (10, 11). Compared with activities of daily living (ADL) or behavioral and psychological symptoms of dementia (BPSD), care problems include a wider range and more detailed items. Effective management of these care problems is crucial because they are associated with accelerated cognitive decline (12), poorer quality of life (13), impaired daily functioning (14), and increased risk of institutionalization (15). However, the variety of care problems makes it difficult for caregivers to cover all aspects. It is of great significance to reduce the dimension of care problems and identify the core care problems.
Exploring symptom clusters is a classic analytical paradigm for reducing the dimension to simplify complex scenarios in real-world clinical practice. The defining characteristics of a symptom cluster are described as two or more symptoms co-occurring, which may have shared underlying mechanisms or shared outcomes (16–18). In the field of dementia care, most current studies have focused on isolated care problems and thus may fail to represent the real-world situation where caregivers of people living with dementia usually have experienced more than one care problem (19, 20). Symptom clusters provide an idea for reducing the dimension of care problems for caregivers of people living with dementia. A recent study (21) conducted cluster analysis of care problems based on the minimum spanning tree algorithm on caregivers of people living with dementia. But the minimum spanning tree can only focus on the strength between care problems, and the association network among care problem clusters cannot be identified. There are a variety of analytical approaches to identify symptom clusters (22, 23), among which network analysis is a novel statistical approach that models the relationship between symptomatic constructs at the component level. The network nodes represent variables and network edges represent relationships between variables. Network analysis has many advantages in the identification of symptom clusters: (1) can not only focus on the strength, but also on the betweenness and closeness between symptoms; (2) can not only identify symptom clusters, but also the association network and centrality indices among symptom clusters; and (3) can focus on the microlevel interactions among symptoms. Currently, network analysis has been applied to patients with mental illness (24–26), cancers (27, 28), and acquired immune deficiency syndrome (29–31).
With the application of network analysis in symptom cluster identification, symptom network paradigm was proposed (32). Symptom networks not only have the function of reducing dimensionality similar to symptom clusters, but also can guide researchers and healthcare providers to develop precise personalized health interventions. The main functions of the symptom network paradigm include clustering symptoms, identifying core symptoms, determining the density of symptom network, and focusing on micro-level interactions among symptoms (32–34). However, the care problems network among caregivers of people living with dementia are still unknown. Under the guidance of symptom network paradigm, this study aimed to achieve the following goals through network analysis: (1) cluster care problems among caregivers of people living with dementia; (2) identify core care problems among caregivers of people living with dementia; and (3) explore demographic and health-related factors associated with these care problem clusters.
Methods
Setting and study participants
This study was approved by the Peking University Biomedical Ethics Committee (IRB00001052-21095). We recruited dementia caregivers in memory clinics in Beijing and WeChat groups established by memory clinic physicians in Beijing, Tianjin, Hangzhou, and Guangzhou between September 2019 and October 2021. Data collectors in each study setting received training and collected data through paper-based questionnaires or online questionnaires. They explained the study objectives and procedures to the participants and obtained the informed consent from them. Participants usually spent 15–20 min completing the questionnaires. Participants were eligible if they: (1) aged 18 years or older; (2) had care recipients who were diagnosed with dementia; (3) undertook the main care task for people with dementia for more than 3 months; (4) were able to provide people with dementia’s personal information; (5) signed the informed consent form.
Measures
Demographic and clinical characteristics
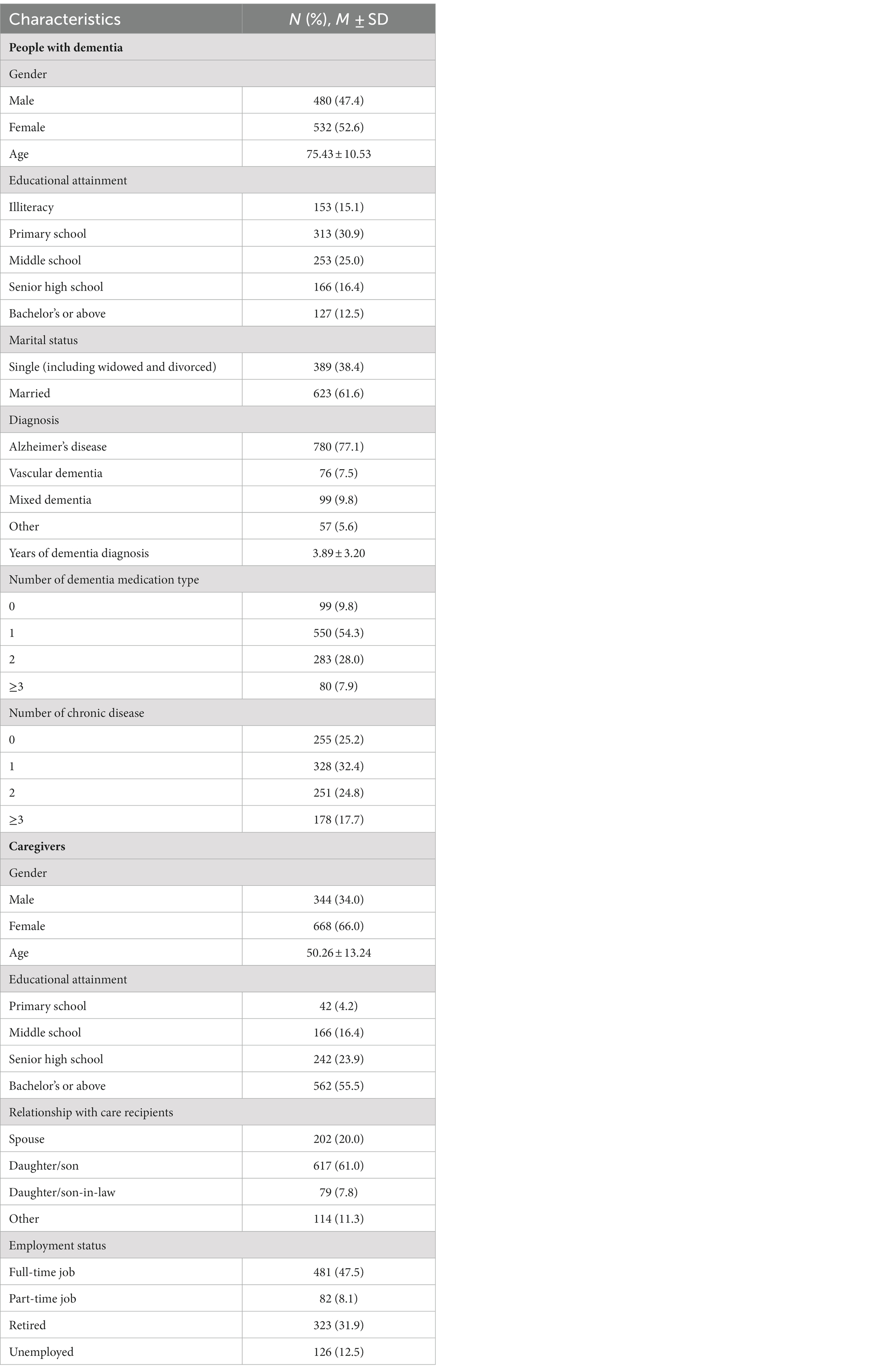
A standard questionnaire was used to collect data of people with dementia’s demographic and clinical characteristics, and caregivers’ demographic characteristics. People with dementia’s demographic variables included gender, age, educational attainment, and marital status. Their clinical variables included diagnosis, years of dementia diagnosis, number of dementia medication type, and number of chronic diseases. Data of gender, age, educational attainment, relationship with care recipients, and employment status were collected to describe caregivers’ demographic characteristics.
Care problem
A dementia caregiver’s care problem checklist that designed by our research team was applied to assess participant’s care problems (5). Our research team combined the specific performance of people living with dementia, based on literature review, and conducted caregiving experience interviews with dementia caregivers to form the first draft of a dementia caregiver’s care problem checklist. Then, 32 dementia care experts were selected for two rounds of expert letter consultation. The importance of each item was rated, and each item could be modified and supplemented to form the final draft of the dementia caregiver’s care problem checklist. This checklist included three dimensions: daily living care problems (30 items), behavioral and psychological problems (15 items), and safety risk problems (13 items). Each item responded 0 (no) or 1 (yes). Daily living care problems and behavioral and psychological problems had a recall period of 2 weeks. For safety risk problems, participants were asked to report if they had experienced these care problems in the past 3 months. This assessment tool showed satisfying content validity (CVI = 0.879) and internal consistency (Cronbach’s α = 0.857). Detailed information of dementia caregiver’s care problem checklist is available in the Supplementary material.
Data analysis
We applied SPSS 24.0 and Python 3.6.0 for statistical analysis. The mean and standard deviation (S.D.) was estimated for continuous variables and the frequencies and percentages for categorical variables. The principal component analysis (PCA) using the orthogonal transformation (varimax rotation) was performed to identify care problem clusters. Kaiser–Meyer–Olkin test was first conducted to judge if the dataset was suitable for factor analysis. Factors with eigen values greater than 1.0 were included. The number of factors was also determined by a scree plot. We defined factor loading that greater than 0.40 were eligible for clusters. The results of care problem clusters were discussed about clinical relevance within our research group.
The network analysis was performed to describe the relationships among care problems and clusters. The Spearman correlation was applied to estimate the correlation relationships between the nodes in the networks; the Fruchterman–Reingold algorithm was applied to place nodes with the strongest correlations at the center of the network (35). Centrality indices, including strength, closeness, and betweenness were used to identify the most central care problem and clusters in the networks (32). All the centrality indices were standardized (reporting r between 0 and 1) to make all the nodes more comparable.
Multiple linear models were used to explore factors associated with the overall care problem and top three central care problem clusters in the cluster network. Independent variables included demographic and clinical variables of people with dementia, and demographic variables of caregivers. Multicollinearity is diagnosed when the variance inflation factor (VIF) is higher than 5 (36). A p-value <0.05 was considered to be statistically significant for all the data analysis.
Results
Descriptive analysis
A total of 1,105 carer-patient pairs participated in our study, 93 (8.4%) of were excluded because of missing data or invalid data logic. Participants’ and their care recipients’ characteristics are shown in Table 1. The majority of people with dementia were female (52.6%), married (61.4%), and diagnosed with Alzheimer’s disease (77.1%). Most of them did not have a high education level, which only 12.5% of them had a bachelor’s degree or above. Their average age was 75.43 years old. The average years of dementia diagnosis was 3.89 years. More than 90% of the people with dementia took more than one type dementia medication, and approximately 75% of the them had at least one kind of chronic disease. The majority of participants were female (66.0%) and were offspring of the people with dementia (61.0%). Their average age was about 50 years old. More than half of the participants had a bachelor’s degree or above (55.5%), and had a full-time job or part-time job (55.6%).
Factor analysis
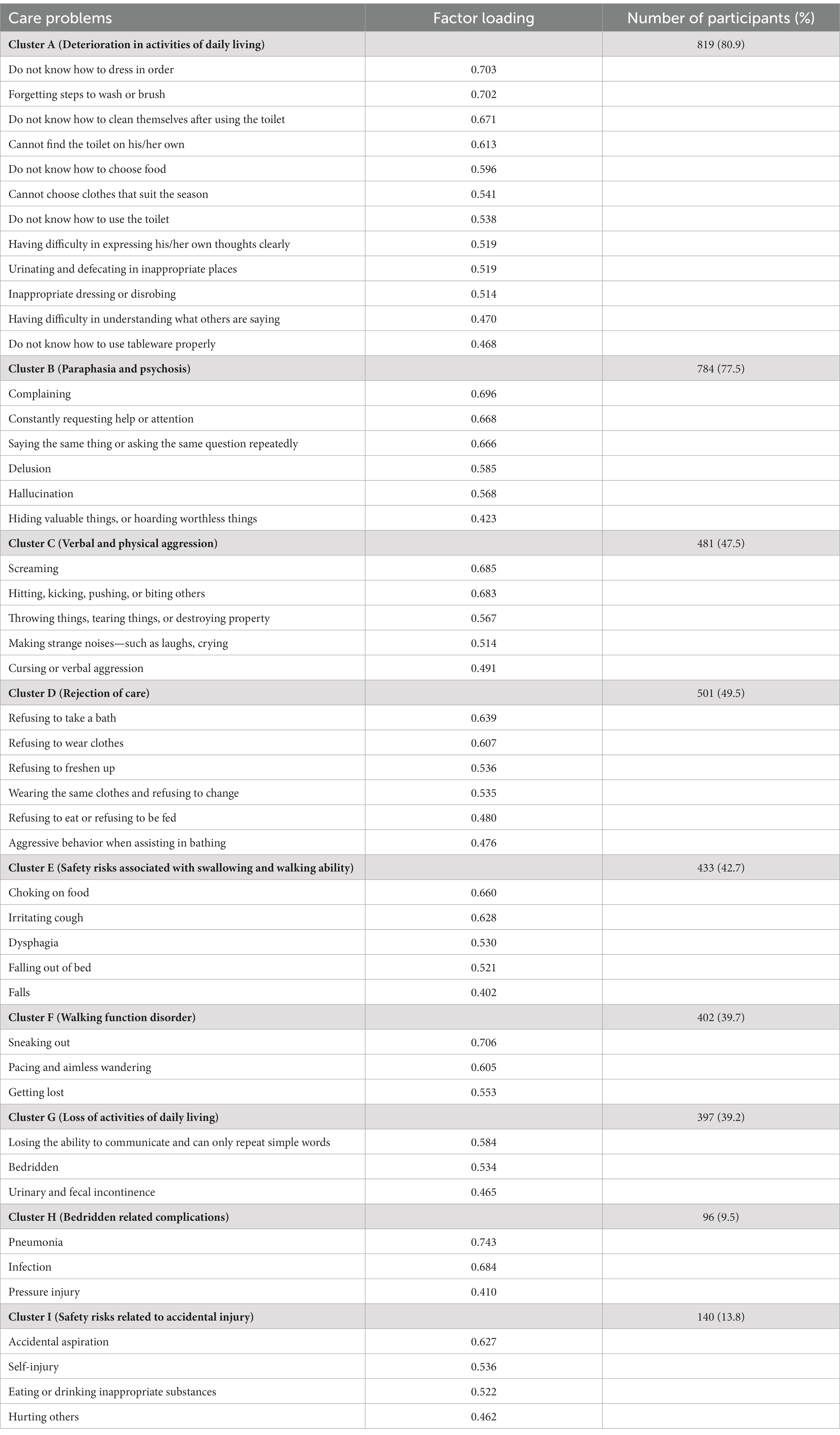
The Kaiser–Meyer–Olkin value in our study was 0.845, and Bartlett’s test of sphericity was significant (p < 0.01), which indicated that our dataset was suitable for PCA. As presented in Table 2, nine care problem clusters were identified. Eleven care problems, including eating or drinking inappropriate substances, performing repeated action, forgetting that he/she had eaten and wanted to eat again, making verbal or physical sexual advances, dysphagia, day and night reversed, getting up multiple times during the night, having difficulty in falling asleep, feeding through a nasogastric tube, apathy, and constipation, had low loading on all factors. The most common care problem cluster was Cluster A (80.9%), followed by Cluster B (77.5%), and Cluster D (49.5%).
Network analysis
Figure 1 shows the association network and centrality indices among 58 care problems. The five strongest edges were between “cannot choose clothes that suit the season” and “forgetting steps to wash or brush” (r = 0.52), “hiding valuable things, or hoarding worthless things” and “constantly requesting help or attention” (r = 0.48), “hitting, kicking, pushing, or biting others” and “throwing things, tearing things, or destroying property” (r = 0.48), “hitting, kicking, pushing, or biting others” and “making strange noises–such as laughs, crying” (r = 0.47), and “do not know how to dress in order” and “do not know how to clean themselves after using the toilet” (r = 0.47). In the entire network, “performing repeated action” (rS = 0.30, rC = 0.47, rB = 0.16) was the most central care problem cluster across the three centrality indices, followed by “do not know how to clean themselves after using the toilet” (rS = 0.30, rC = 0.49, rB = 0.10), “urinating and defecating in inappropriate places” (rS = 0.30, rC = 0.47, rB = 0.08), “hitting, kicking, pushing, or biting others” (rS = 0.26, rC = 0.47, rB = 0.07), and “cursing or verbal aggression” (rS = 0.28, rC = 0.43, rB = 0.06).
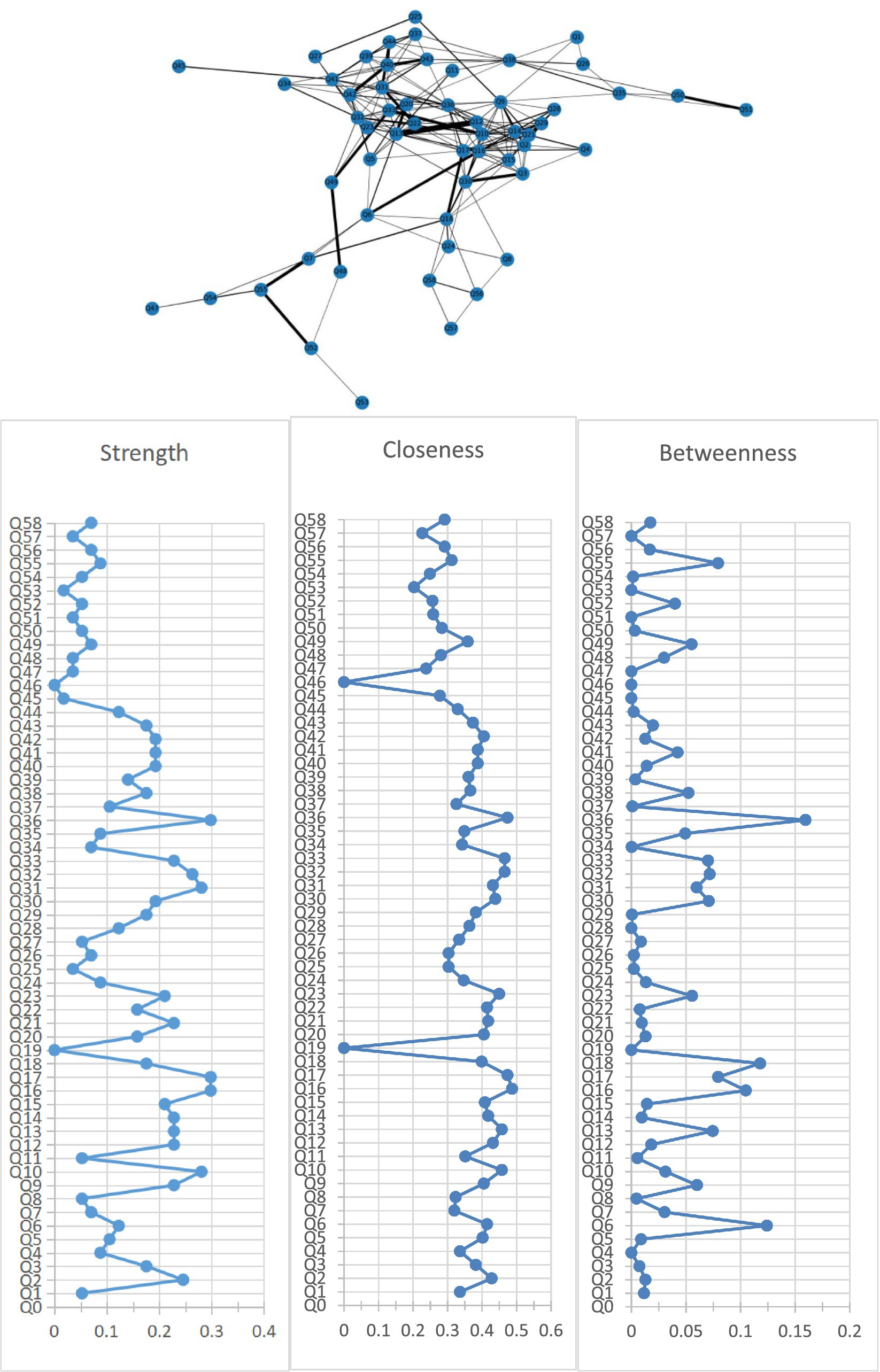
Figure 1. Network of care problems and centrality indices. Q1. Forgetting that he/she had eaten and wanted to eat again; Q2. Do not know how to choose food. Q3. Do not know how to use tableware properly; Q4. Eating or drinking inappropriate substances; Q5. Refusing to eat or refusing to be fed; Q6. Difficulty chewing; Q7. Dysphagia; Q8. Feeding through a nasogastric tube; Q9. Cannot choose clothes that suit the season; Q10. Do not know how to dress in order; Q11. Wearing the same clothes and refusing to change; Q12. Inappropriate dressing or disrobing; Q13. Refusing to wear clothes; Q14. Cannot find the toilet on his/her own; Q15. Do not know how to use the toilet; Q16. Do not know how to clean themselves after using the toilet; Q17. Urinating and defecating in inappropriate places; Q18. Urinary and fecal incontinence; Q19. Constipation; Q20. Refusing to take a bath; Q21. Forgetting steps to wash or brush; Q22. Refusing to freshen up; Q23. Aggressive behavior when assisting in bathing; Q24. Bedridden; Q25. Having difficulty in falling asleep; Q26. Getting up multiple times during the night; Q27. Day and night reversed; Q28. Having difficulty in understanding what others are saying; Q29. Having difficulty in expressing his/her own thoughts clearly; Q30. Losing the ability to communicate and can only repeat simple words; Q31. Cursing or verbal aggression; Q32. Hitting, kicking, pushing, or biting others; Q33. Throwing things, tearing things, or destroying property; Q34. Making verbal or physical sexual advances; Q35. Pacing and aimless wandering; Q36. Performing repeated action; Q37. Saying the same thing or asking the same question repeatedly; Q38. Hiding valuable things, or hoarding worthless things; Q39. Constantly requesting help or attention; Q40. Complaining; Q41. Making strange noises—such as laughs, crying; Q42. Screaming; Q43. Hallucination; Q44. Delusion; Q45. Apathy; Q46. Falls; Q47. Falling out of bed; Q48. Self-injury; Q49. Hurting others; Q50. Sneaking out; Q51. Getting lost; Q52. Accidental aspiration; Q53. Eating or drinking inappropriate substances; Q54. Irritating cough; Q55. Choking on food; Q56. Pneumonia; Q57. Infection; Q58. Pressure injury.
Figure 2 shows the association network and centrality indices among the nine care problem clusters and eleven care problems. The three strongest edges were between Cluster C and Cluster D (r = 0.44), Cluster B and Cluster C (r = 0.42), and Cluster C and “performing repeated action” (r = 0.38). In the entire network, Cluster A (rS = 0.47, rC = 0.61, rB = 0.37) was the most central care problem cluster across the three centrality indices, followed by Cluster C (rS = 0.42, rC = 0.52, rB = 0.28), and Cluster G (rS = 0.26, rC = 0.46, rB = 0.26).
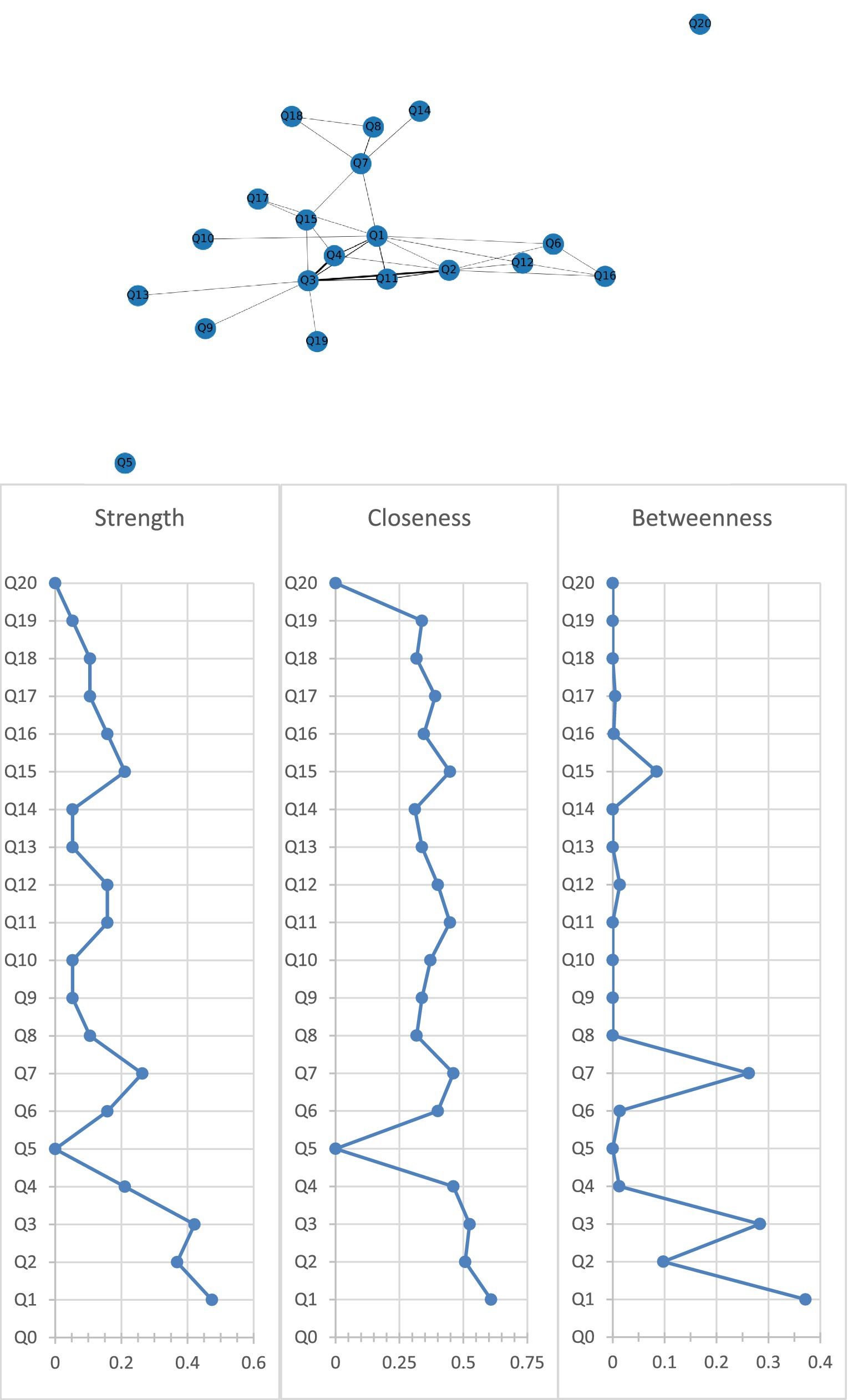
Figure 2. Network of care problem clusters and centrality indices. Q1. Cluster A (Deterioration in activities of daily living); Q2. Cluster B (Paraphasia and psychosis); Q3. Cluster C (Verbal and physical aggression); Q4. Cluster D (Rejection of care); Q5. Cluster E (Safety risks associated with swallowing and walking ability); Q6. Cluster F (Walking function disorder); Q7. Cluster G (Loss of activities of daily living); Q8. Cluster H (Bedridden related complications); Q9. Cluster I (Safety risks related to accidental injury); Q10. Eating or drinking inappropriate substances; Q11. Performing repeated action; Q12. Forgetting that he/she had eaten and wanted to eat again; Q13. Making verbal or physical sexual advances; Q14. Difficulty chewing; Q15. Day and night reversed; Q16. Getting up multiple times during the night; Q17. Having difficulty in falling asleep; Q18. Feeding through a nasogastric tube; Q19. Apathy; Q20. Constipation.
Regression analysis
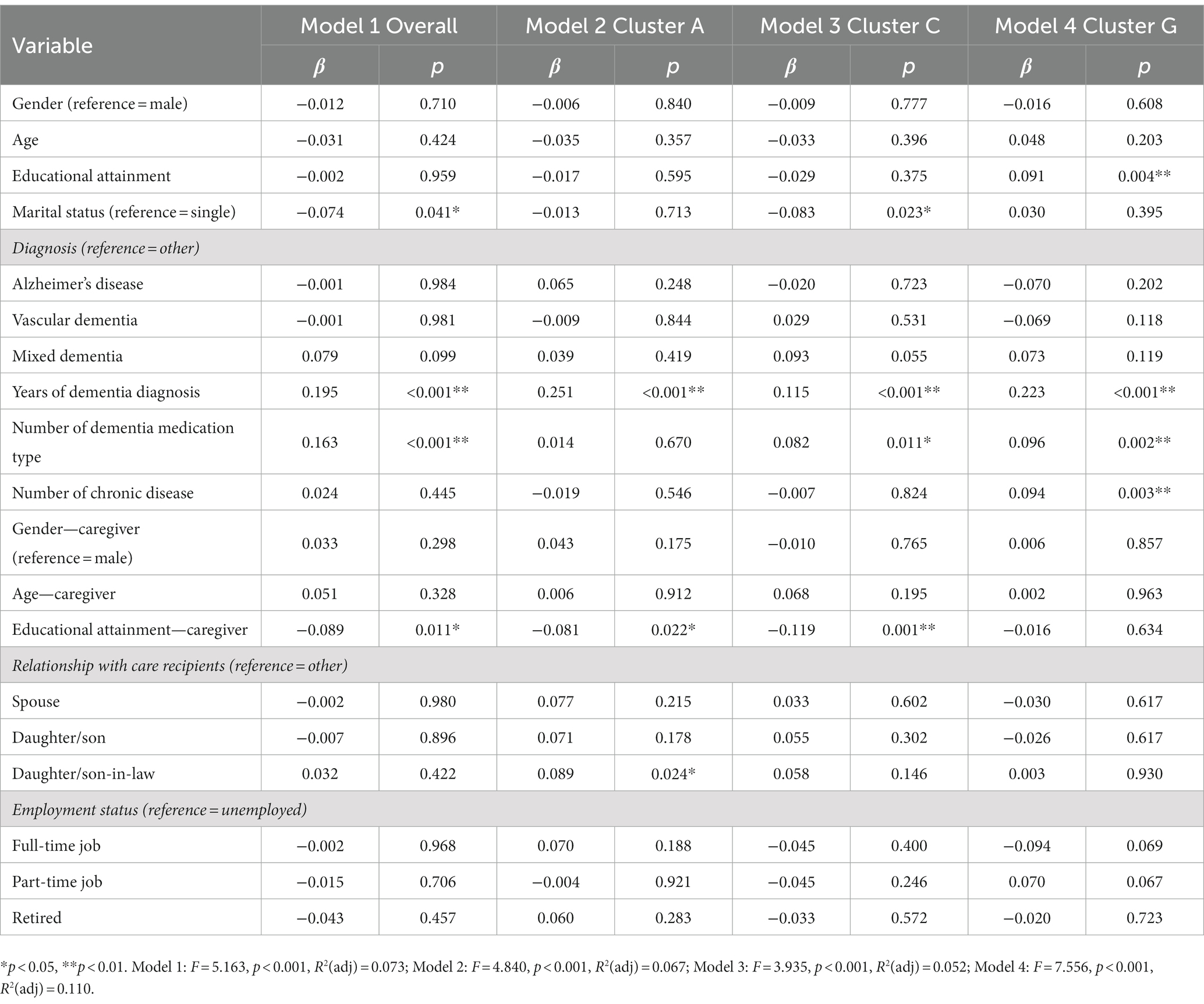
The results of the exploratory multiple linear regression models of overall care problems and top three central care problem clusters are shown in Table 3. People with dementia who were single (β = −0.074, p = 0.041), had longer years of dementia diagnosis (β = 0.195, p < 0.01), took more types of dementia medication (β = 0.163, p < 0.01), and dementia caregivers who had lower education level (β = −0.089, p = 0.011) were significantly associated with more care problems. Variables including people with dementia’s educational attainment, marital status, years of dementia diagnosis, number of dementia medication type, number of chronic diseases, dementia caregivers’ educational attainment, and the relationship between people with dementia and caregivers were significantly associated with the prevalence of these care problem clusters.
Discussion
This study aimed to identify care problem clusters and core care problems, and explore demographic variables associated with these care problem clusters among caregivers of people living with dementia. Nine care problem clusters were identified, including “Deterioration in activities of daily living,” “Paraphasia and psychosis,” “Verbal and physical aggression,” “Rejection of care,” “Safety risks associated with swallowing and walking ability,” “Walking function disorder,” “Loss of activities of daily living,” “Bedridden related complications,” and “Safety risks related to accidental injury.” In the entire care problem network, “Deterioration in activities of daily living” was the most core care problem cluster across the three centrality indices, followed by “Verbal and nonverbal aggression” and “Loss of activities of daily living.” Variables including marital status, years of dementia diagnosis, number of dementia medication type, and caregiver’s educational attainment were associated with the prevalence of these three care problem clusters.
A shift in research interest from separate care problem to care problem clusters among caregivers of people living with dementia may contribute to a better understanding of dementia care, as related care problem clusters may respond to the same nursing or treatment interventions (37–39). Nine care problem clusters were derived from the data in this study. A previous study (38) showed that BPSD were not independent, but consist of a group or cluster of related symptoms, which was consistent with our findings. Another previous study revealed the co-occurrence of symptoms between hallucinations and delusions, and hinted at a common etiology and treatment for these symptoms (40). In our study, the “Paraphasia and psychosis” cluster (Cluster B) also contained hallucinations and delusions, suggesting that the care problems in the cluster may be treated as a whole to optimize care and treatment plan. Some researchers proposed that BPSD was not a unitary concept, instead it should be divided into several symptom groups, each of which may reflect different psychosocial determinants, biological correlates, and disease duration (41), which was in line with our research philosophy. However, the above studies only focused on the presence of BPSD, while our study focused not only on BPSD, but also on a series of care problems related to daily life and safety risks. A recent study (21) conducted cluster analysis based on the minimum spanning tree algorithm on 687 dementia samples and obtained 7 care problem clusters, which was not completely consistent with our research results. The reasons for the difference in results may be different sample sizes and analysis methods. In terms of sample size, our study was based on a large sample of 1,012 cases; in terms of analysis methods, the minimum spanning tree only focused on the strength between care problems, while the network analysis used in our study focused not only on the strength, but also on the betweenness and closeness between care problems.
Our study demonstrated that “Deterioration in activities of daily living” cluster (Cluster A) was the most core care problem cluster across the three centrality indices in the entire care problem network. In this cluster, there are mainly some manifestations of functional degradation, such as “Do not know how to dress in order,” “Do not know how to clean themselves after using the toilet,” and “Do not know how to use tableware properly.” Studies (42, 43) showed that functional decline in activities of daily living appeared more pronounced and disrupted more aspects of life activities for individuals with dementia versus individuals without dementia. Our study also found that “Loss of activities of daily living” cluster (Cluster G) was the third core care problem cluster across the three centrality indices. This cluster includes “Losing the ability to communicate,” “Bedridden,” and “Urinary and fecal incontinence.” Dementia is a progressive disease. As the disease progresses, it may eventually lead to loss of multiple functions, which will bring greater burden to caregivers. The deterioration of activities of daily living is common in people living with dementia, which suggests that nursing interventions aimed at delaying the functional deterioration may need to be prioritized in order to optimize the care plan and improve the quality of life of people living with dementia.
Similar to “Deterioration in activities of daily living” cluster (Cluster A), we found high coefficients of the three centrality indices in “Verbal and physical aggression” cluster (Cluster C) among all included care problems. In this cluster, verbal aggression includes “Cursing,” “Screaming,” and “Making strange noises—such as laughs, crying,” and physical aggression includes “Hitting, kicking, pushing, or biting others” and “Throwing things, tearing things, or destroying property.” Verbal and physical aggressive behaviors in people living with dementia have been shown to be highly prevalent in many previous studies (44–46). In addition, some studies demonstrated that verbal aggression and physical aggression often coexist (47, 48). Our results were in line with the findings from these studies. Mechanisms behind the co-occurrence of verbal aggression and physical aggression are multifactorial, including different neurobiological factors as well as social, psychological, and environmental factors. The onset of physical aggression in dementia patients can be very dangerous for caregivers because it is often an unexpected attack. The above information suggests that for dementia patients with verbal aggression, caregivers should strengthen observation, raise vigilance, find out the potential causes of verbal aggression and actively carry out nursing intervention, so as to prevent the occurrence of physical aggression and avoid harm to caregivers.
Our study revealed that the strongest edge was between “Verbal and physical aggression” cluster (Cluster C) and “Rejection of care” cluster (Cluster D) (r = 0.44), followed by “Paraphasia and psychosis” cluster (Cluster B) and “Verbal and physical aggression” cluster (Cluster C) (r = 0.42) among nine care problem clusters. A higher strong edge means that the symptom cluster is more likely to occur in conjunction with other symptom clusters. “Rejection of care” cluster (Cluster D) covers care problems such as “Refusing to take a bath,” “Refusing to wear clothes,” “Refusing to eat or refusing to be fed” and other manifestations of refusal to care. In the practice of caring for people living with dementia, we often find that if the caregiver forces the people living with dementia to do something he does not want to do, such as taking a shower, it is likely to induce verbal aggression or physical aggression (49, 50). This practical experience also indirectly corresponds to the strong correlation between “Rejection of care” cluster (Cluster D) and “Verbal and physical aggression” cluster (Cluster C). In “Paraphasia and psychosis” cluster (Cluster B), the main care problems included hallucinations, delusions, etc. Dementia patients with these care problems are prone to delusions that distort facts, such as firmly believing that others have stolen their money, and easily triggering verbal or physical aggression when others argue with them. From this perspective, the strong correlation between “Paraphasia and psychosis” cluster (Cluster B) and “Verbal and physical aggression” cluster (Cluster C) is well explained. The above reminds us that in the process of caring for people living with dementia, we should follow his will and recognize his ideas, even if his ideas are wrong, so as to avoid inducing his aggressive behavior.
The sociodemographic characteristics of dementia patients and their caregivers may have an impact on the occurrence of care problems. This study revealed that marital status, years of dementia diagnosis, number of dementia medication type, and caregiver’s educational attainment were associated with the prevalence of overall care problem clusters. In terms of marital status, being married was a protective factor against having “Verbal and physical aggression” cluster (Cluster C). The possible reason is that the familiarity and intimacy between dementia patients and their spouse caregivers can give them enough sense of security, avoid intergenerational conflicts, and therefore reduce the occurrence of aggressive behavior (51, 52). In terms of disease course, the longer the dementia was diagnosed, the more likely to have “Deterioration in activities of daily living” (Cluster A), “Verbal and physical aggression” cluster (Cluster C), and “Loss of activities of daily living” cluster (Cluster G). In other words, as the disease progresses, people with dementia gradually deteriorate and lose various functions, and are more likely to develop BPSD. Rockwood et al. (53) used two analytical methods (connectivity graph analysis and multiple correspondence analysis) to identify psychotic symptom clusters and found that moderate/severe dementia was associated with more psychotic symptoms, which was consistent with our results. Mouriz-Corbelle et al. (54) and Umesh et al. (55) also found similar results that high level of aggression was associated with low level of cognitive function.
In terms of number of dementia medication type, the more types of medication taken by dementia patients, the more likely “Verbal and physical aggression” cluster (Cluster C) and “Loss of activities of daily living” cluster (Cluster G) were induced. This finding was consistent with the results of a recent study that showed that exacerbated BPSD were associated with patients taking psychotropic drugs (56). In addition to dementia medications, we should also consider that this population may be taking medications for other age-related diseases (such as hypertension, diabetes, hypercholesterolemia), which may increase the risk of adverse interactions with their dementia medications. This finding indicates that excessive use of dementia medications may cause serious side effects or adverse interactions with medications for other age-related diseases, so it is important to weigh the benefits against the risks before taking them. In addition, our study found that higher education level of caregivers had a protective effect on the occurrence of “Deterioration in activities of daily living” (Cluster A) and “Verbal and physical aggression” cluster (Cluster C), which may be that caregivers with higher education level had stronger ability to acquire dementia care knowledge, so the quality of care for patients is higher (57, 58). This result suggests that we should strengthen guidance and support for caregivers with low education level to enhance their knowledge reserve and improve care outcomes.
Strengths and limitations
This is the first time that the network analysis has been applied to explore the care problem clusters and core care problems among caregivers of people living with dementia. Network analysis has sufficient statistical power to identify care problem clusters and core care problems through three centrality indicators, namely, strength, closeness, and betweenness. In addition, compared with other studies, the care problems that we focused on are more comprehensive. Our study focused not only on BPSD, but also on a series of care problems related to daily life and safety risks. What’s more, our study not only identified the core care problem cluster, but also explored the influencing factors, which can provide guidance for dementia care. This study also has several limitations that should be recognized. First, we used convenient sampling method, and the survey data were collected mainly from caregivers of dementia patients in Beijing, Tianjin, Guangzhou, and Hangzhou. Due to the limited sample representativeness, our findings cannot be generalized to the entire Chinese dementia population. Second, only a cross-sectional design was used, and care problems were not tracked longitudinally, which may be useful to examine changes in the prevalence of care problems over time. Last, additional subsample analysis by disease severity and type was not performed. With the accumulation of sample size, care problem clusters with different dementia severity and dementia types can be further identified in the future.
Conclusion
Our study generated new knowledge of symptom network among people living with dementia by identifying nine care problem clusters. In the entire care problem network, “Deterioration in activities of daily living” was the most core care problem cluster, followed by “Verbal and nonverbal aggression” cluster. Our study suggests that there is a need to evaluate care problem clusters for the improvement of care problem management among people living with dementia. Caregivers need to consider each care problem in its own right and also to be aware of the interrelations between them when assessing patients and developing strategies for care. It is particularly important to include assessment and treatment of core care problem as an essential component of the dementia care.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Peking University Biomedical Ethics Committee (IRB00001052-21095). The patients/participants provided their informed consent to participate in this study.
Author contributions
SH and ZZ participated in the research design. ML and SH drafted the manuscript. ML, YaZ, and YS contributed the acquisition of data. YiZ and XY performed the data analysis. ZW reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 72274007) and the China Postdoctoral Science Foundation (grant number 2023M732119).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1195637/full#supplementary-material
References
1. Alzheimer’s Disease International. (2021). Dementia statistics. E. coli. Available at: https://www.alz.co.uk/research/statistics (Accessed February 10, 2023).
2. Jia, L, du, Y, Chu, L, Zhang, Z, Li, F, Lyu, D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/s2468-2667(20)30185-7
3. Ren, R, Qi, J, Lin, S, Liu, X, Yin, P, Wang, Z, et al. The China Alzheimer report 2022. Gen Psychiatr. (2022) 35:e100751. doi: 10.1136/gpsych-2022-100751
4. Collaborators, GDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/s2468-2667(21)00249-8
5. Hao, W, Wang, Z, Zhang, H, and Zou, B. Development of care problems assessment indicator system for community-dwelling patients with dementia. Chin J Nurs. (2016) 51:1504–8. doi: 10.3761/J.Issn.0254-1769.2016.12.021
6. Kazawa, K, Kubo, T, Akishita, M, and Ishii, S. Long-term impact of the COVID-19 pandemic on facility- and home-dwelling people with dementia: perspectives from professionals involved in dementia care. Geriatr Gerontol Int. (2022) 22:832–8. doi: 10.1111/ggi.14465
7. Spigelmyer, PC, and Schreiber, JB. A pilot study: resistive behavior in the context of informal caregiver-assisted activities of daily living. Geriatr Nurs. (2019) 40:399–404. doi: 10.1016/j.gerinurse.2019.01.005
8. Kim, D, Choi, YR, Lee, YN, Park, WH, and Chang, SO. How about an educational framework for nursing staff in long-term care facilities to improve the care of behavioral and psychological symptoms of dementia? Int J Environ Res Public Health. (2022) 19:10493. doi: 10.3390/ijerph191710493
9. Kai, A, Ishii, S, Ishii, T, Fuchino, K, and Okamura, H. Factors associated with caregiver burden and depressive states among family caregivers of patients admitted due to behavioral and psychological symptoms of dementia. Dement Geriatr Cogn Disord. (2022) 51:262–70. doi: 10.1159/000525354
10. Dong, X, Liu, G, Yin, X, Min, R, and Hu, Y. Fall risks and the related factors for the homebound older people with dementia: evidence from east China. Front Public Health. (2022) 10:946097. doi: 10.3389/fpubh.2022.946097
11. Neubauer, NA, and Liu, L. Dissemination and implementation of strategy adoption guidelines for persons with dementia at risk of getting lost. Aging Ment Health. (2021) 25:528–34. doi: 10.1080/13607863.2019.1699017
12. Peters, ME, Schwartz, S, Han, D, Rabins, PV, Steinberg, M, Tschanz, JT, et al. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: the Cache County dementia progression study. Am J Psychiatry. (2015) 172:460–5. doi: 10.1176/appi.ajp.2014.14040480
13. Dijk, MT, Tabak, S, Hertogh, C, Kok, RM, van Marum, RJ, Zuidema, SU, et al. Psychotropic drug treatment for agitated behaviour in dementia: what if the guideline prescribing recommendations are not sufficient? A qualitative study. Age Ageing. (2022) 51:afac189. doi: 10.1093/ageing/afac189
14. Zhang, W, Wang, X, Lü, Y, and Yu, W. Relations of neuropsychiatric symptoms with disease stage, sex, and daily function in mild cognitive impairment and dementia due to Alzheimer's disease: a cross-sectional study. J Psychosom Res. (2022) 161:110994. doi: 10.1016/j.jpsychores.2022.110994
15. Tampi, RR, Bhattacharya, G, and Marpuri, P. Managing behavioral and psychological symptoms of dementia (BPSD) in the era of boxed warnings. Curr Psychiatry Rep. (2022) 24:431–40. doi: 10.1007/s11920-022-01347-y
16. Brant, JM, Beck, S, and Miaskowski, C. Building dynamic models and theories to advance the science of symptom management research. J Adv Nurs. (2010) 66:228–40. doi: 10.1111/j.1365-2648.2009.05179.x
17. Miaskowski, C, Barsevick, A, Berger, A, Casagrande, R, Grady, PA, Jacobsen, P, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst. (2017) 109:djw253. doi: 10.1093/jnci/djw253
18. Barsevick, A. Defining the symptom cluster: how far have we come? Semin Oncol Nurs. (2016) 32:334–50. doi: 10.1016/j.soncn.2016.08.001
19. Chang, CYM, Baber, W, Dening, T, and Yates, J. “He Just Doesn't Want to Get Out of the Chair and Do It”: the impact of apathy in people with dementia on their carers. Int J Environ Res Public Health. (2021) 18:6317. doi: 10.3390/ijerph18126317
20. Schein, J, Houle, CR, Urganus, AL, Jones, E, Pike, J, Husbands, J, et al. The impact of agitation in dementia on caregivers: a real-world survey. J Alzheimers Dis. (2022) 88:663–77. doi: 10.3233/jad-215670
21. Leng, M, Sun, Y, Chang, H, and Wang, Z. Clustering analysis of the care problems of people with dementia based on the minimum spanning tree algorithm: a cross-sectional study. J Alzheimers Dis. (2022) 87:1637–46. doi: 10.3233/jad-215682
22. Kim, HJ, Abraham, I, and Malone, PS. Analytical methods and issues for symptom cluster research in oncology. Curr Opin Support Palliat Care. (2013) 7:45–53. doi: 10.1097/SPC.0b013e32835bf28b
23. Sun, Y, Chen, Q, Li, Y, Wang, J, Bazzano, AN, and Cao, F. Prenatal symptom cluster of psychopathology and associations with mindfulness and rumination: a network analysis. J Nerv Ment Dis. (2022) 210:515–24. doi: 10.1097/nmd.0000000000001485
24. Demyttenaere, K, Anthonis, E, Acsai, K, and Correll, CU. Depressive symptoms and PANSS symptom dimensions in patients with predominant negative symptom schizophrenia: a network analysis. Front Psychiatry. (2022) 13:795866. doi: 10.3389/fpsyt.2022.795866
25. Chen, C, Li, F, Liu, C, Li, K, Yang, Q, and Ren, L. The relations between mental well-being and burnout in medical staff during the COVID-19 pandemic: a network analysis. Front Public Health. (2022) 10:919692. doi: 10.3389/fpubh.2022.919692
26. Jin, Y, Xu, S, Hu, Z, Li, J, Li, H, Wang, X, et al. Co-occurrence of PTSD and affective symptoms in a large sample with childhood trauma subtypes: a network analysis. Front Public Health. (2023) 11:1093687. doi: 10.3389/fpubh.2023.1093687
27. Lin, Y, Bruner, DW, Paul, S, Miller, AH, Saba, NF, Higgins, KA, et al. A network analysis of self-reported psychoneurological symptoms in patients with head and neck cancer undergoing intensity-modulated radiotherapy. Cancer. (2022) 128:3734–43. doi: 10.1002/cncr.34424
28. Rha, SY, and Lee, J. Stable symptom clusters and evolving symptom networks in relation to chemotherapy cycles. J Pain Symptom Manag. (2021) 61:544–54. doi: 10.1016/j.jpainsymman.2020.08.008
29. Zhu, Z, Hu, Y, Xing, W, Guo, M, Zhao, R, Han, S, et al. Identifying symptom clusters among people living with HIV on antiretroviral therapy in China: a network analysis. J Pain Symptom Manag. (2019) 57:617–26. doi: 10.1016/j.jpainsymman.2018.11.011
30. Zhu, Z, Wen, H, Yang, Z, Han, S, Fu, Y, Zhang, L, et al. Evolving symptom networks in relation to HIV-positive duration among people living with HIV: a network analysis. Int J Infect Dis. (2021) 108:503–9. doi: 10.1016/j.ijid.2021.05.084
31. Wen, H, Zhu, Z, Hu, T, Li, C, Jiang, T, Li, L, et al. Unraveling the central and bridge psychological symptoms of people living with HIV: a network analysis. Front Public Health. (2023) 10:1024436. doi: 10.3389/fpubh.2022.1024436
32. Zhu, Z, Xing, W, Hu, Y, Wu, B, and So, WKW. Paradigm shift: moving from symptom clusters to symptom networks. Asia Pac J Oncol Nurs. (2022) 9:5–6. doi: 10.1016/j.apjon.2021.12.001
33. Epskamp, S, and Fried, EI. A tutorial on regularized partial correlation networks. Psychol Methods. (2018) 23:617–34. doi: 10.1037/met0000167
34. Jones, PJ, Ma, R, and McNally, RJ. Bridge centrality: a network approach to understanding comorbidity. Multivariate Behav Res. (2021) 56:353–67. doi: 10.1080/00273171.2019.1614898
35. Gajdoš, P, Ježowicz, T, Uher, V, and Dohnálek, P. A parallel Fruchterman–Reingold algorithm optimized for fast visualization of large graphs and swarms of data. Swarm Evol Comput. (2016):26. doi: 10.1016/J.Swevo.2015.07.006
36. Kim, JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. (2019) 72:558–69. doi: 10.4097/kja.19087
37. Bessey, LJ, and Walaszek, A. Management of behavioral and psychological symptoms of dementia. Curr Psychiatry Rep. (2019) 21:66. doi: 10.1007/s11920-019-1049-5
38. Vilalta-Franch, J, López-Pousa, S, Turon-Estrada, A, Lozano-Gallego, M, Hernàndez-Ferràndiz, M, Pericot-Nierga, I, et al. Syndromic association of behavioral and psychological symptoms of dementia in Alzheimer disease and patient classification. Am J Geriatr Psychiatry. (2010) 18:421–32. doi: 10.1097/JGP.0b013e3181c6532f
39. Kales, HC, Gitlin, LN, and Lyketsos, CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. (2015) 350:h369. doi: 10.1136/bmj.h369
40. van der Linde, RM, Dening, T, Matthews, FE, and Brayne, C. Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry. (2014) 29:562–8. doi: 10.1002/gps.4037
41. Robert, PH, Verhey, FR, Byrne, EJ, Hurt, C, de Deyn, PP, Nobili, F, et al. Grouping for behavioral and psychological symptoms in dementia: clinical and biological aspects. Consensus paper of the European Alzheimer disease consortium. Eur Psychiatry. (2005) 20:490–6. doi: 10.1016/j.eurpsy.2004.09.031
42. Lima-Silva, TB, Bahia, VS, Carvalho, VA, Guimarães, HC, Caramelli, P, Balthazar, ML, et al. Direct and indirect assessments of activities of daily living in behavioral variant frontotemporal dementia and Alzheimer disease. J Geriatr Psychiatry Neurol. (2015) 28:19–26. doi: 10.1177/0891988714541874
43. Moeller, S, Sridhar, J, Martersteck, A, Coventry, C, Kuang, A, Zhang, H, et al. Functional decline in the aphasic variant of Alzheimer’s disease. Alzheimers Dement. (2021) 17:1641–8. doi: 10.1002/alz.12331
44. Veldwijk-Rouwenhorst, AE, Zuidema, SU, Smalbrugge, M, Bor, H, Wetzels, R, Gerritsen, DL, et al. Very frequent physical aggression and vocalizations in nursing home residents with dementia. Aging Ment Health. (2021) 25:1442–51. doi: 10.1080/13607863.2020.1786799
45. Liljegren, M, Landqvist Waldö, M, and Englund, E. Physical aggression among patients with dementia, neuropathologically confirmed post-mortem. Int J Geriatr Psychiatry. (2018) 33:e242–8. doi: 10.1002/gps.4777
46. Kwon, CY, and Lee, B. Prevalence of Behavioral and psychological symptoms of dementia in community-dwelling dementia patients: a systematic review. Front Psychiatry. (2021) 12:741059. doi: 10.3389/fpsyt.2021.741059
47. Choi, SSW, Budhathoki, C, and Gitlin, LN. Co-occurrence and predictors of three commonly occurring behavioral symptoms in dementia: agitation, aggression, and rejection of care. Am J Geriatr Psychiatry. (2017) 25:459–68. doi: 10.1016/j.jagp.2016.10.013
48. Wetzels, RB, Zuidema, SU, de Jonghe, JF, Verhey, FR, and Koopmans, RT. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. Am J Geriatr Psychiatry. (2010) 18:1054–65. doi: 10.1097/jgp.0b013e3181f60fa1
49. Nguyen, H, Eccleston, CE, Doherty, KV, Jang, S, and McInerney, F. Communication in dementia care: experiences and needs of carers. Dementia. (2022) 21:1381–98. doi: 10.1177/14713012221080003
50. Ashrafizadeh, H, Gheibizadeh, M, Rassouli, M, Hajibabaee, F, and Rostami, S. Explain the experience of family caregivers regarding care of Alzheimer’s patients: a qualitative study. Front Psychol. (2021) 12:699959. doi: 10.3389/fpsyg.2021.699959
51. Wong, OL, Kwong, PS, Ho, CK, Chow, SM, Kwok, T, Wong, B, et al. Living with dementia: an exploratory study of caregiving in a Chinese family context. Soc Work Health Care. (2015) 54:758–76. doi: 10.1080/00981389.2015.1057355
52. Liu, J. Spouse and adult-child dementia caregivers in Chinese American families: who are more stressed out? J Am Med Dir Assoc. (2021) 22:1512–7. doi: 10.1016/j.jamda.2020.12.012
53. Rockwood, K, Mitnitski, A, Richard, M, Kurth, M, Kesslak, P, and Abushakra, S. Neuropsychiatric symptom clusters targeted for treatment at earlier versus later stages of dementia. Int J Geriatr Psychiatry. (2015) 30:357–67. doi: 10.1002/gps.4136
54. Mouriz-Corbelle, R, Caamaño-Ponte, J, Dosil, C, Picón, E, and Facal, D. Apathy and agitation in institutionalized older adults: an empirically derived classification. Psychogeriatrics. (2021) 21:272–8. doi: 10.1111/psyg.12659
55. Umesh, S, Goyal, N, Grover, S, Bhattacharyya, R, Menon, V, Mohapatra, D, et al. A multicentric exploratory study of behavioral and psychological symptom characteristics of dementia. Indian J Psychiatry. (2022) 64:370–6. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_117_21
56. Wan, Z, Dong, W, Sun, D, Ma, D, Zhao, Y, Li, H, et al. Modifiable factors associated with behavioural and psychological symptoms of dementia among patients residing at home: the impacts of patient, caregiver and environmental variables. Geriatr Nurs. (2021) 42:358–65. doi: 10.1016/j.gerinurse.2021.01.008
57. Chen, R, Lang, L, Clifford, A, Chen, Y, Hu, Z, and Han, TS. Demographic and socio-economic influences on community-based care and caregivers of people with dementia in China. JRSM Cardiovasc Dis. (2016) 5:2048004016652314. doi: 10.1177/2048004016652314
Keywords: dementia, caregivers, care problem, cluster, symptom network, network analysis
Citation: Leng M, Han S, Sun Y, Zhu Z, Zhao Y, Zhang Y, Yang X and Wang Z (2023) Identifying care problem clusters and core care problems of older adults with dementia for caregivers: a network analysis. Front. Public Health. 11:1195637. doi: 10.3389/fpubh.2023.1195637
Edited by:
Carlo Custodero, University of Bari Medical School, ItalyReviewed by:
Daiki Ishimaru, Osaka University, JapanKevin Nicholas Hascup, Southern Illinois University Carbondale, United States
Copyright © 2023 Leng, Han, Sun, Zhu, Zhao, Zhang, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwen Wang, d3p3amluZ0BzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Minmin Leng
Minmin Leng Shuyu Han
Shuyu Han Yue Sun2
Yue Sun2 Zheng Zhu
Zheng Zhu Yajie Zhao
Yajie Zhao Yizhu Zhang
Yizhu Zhang Xianxia Yang
Xianxia Yang Zhiwen Wang
Zhiwen Wang