- 1Reproductive Medicine Center, Gansu Maternal and Child Health Care Hospital, Lanzhou, Gansu, China
- 2Reproductive Medicine Center, Gansu Provincial Central Hospital, Lanzhou, Gansu, China
- 3School of Nursing, Gansu University of Chinese Medicine, Lanzhou, Gansu, China
Introduction: Isolation strategies have been implemented in numerous countries worldwide during the ongoing community transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, various countries and organizations have implemented their isolation measures at varying intensities, even during the same period. Therefore, we systematically reviewed the key information contained in currently available guidelines regarding the isolation of the general population, aiming to better identify the heterogeneity of the current isolation strategies.
Methods: We conducted searches in four evidence-based medicine (EBM) databases and five guideline websites to identify guidelines, guidance, protocols, and policy documents published by authoritative advisory bodies or healthcare organizations, which provided information on the implementation of isolation for general populations with COVID-19. One author extracted data using a standardized data extraction checklist, and a second author double-checked all extractions for completeness and correctness. Discrepancies were resolved through discussion. The information extracted from the included articles was summarized both narratively and using tables.
Results: We included 15 articles that provided information on isolation measures recommended by nine different countries and organizations. The included articles consistently recommended isolating individuals with a positive COVID-19 test, regardless of the presence of symptoms. However, there were variations in the duration of isolation, and substantial differences also existed in the criteria for ending the isolation of COVID-19 patients.
Conclusion: Different countries and organizations have substantial differences in their isolation policies. This reminds us that scientifically sound guidelines on isolation that balance the risk of prematurely ending isolation with the burden of prolonged isolation are a crucial topic of discussion when faced with a pandemic.
1. Introduction
It has been nearly 4 years since the first coronavirus disease 2019 (COVID-19) case was reported in Wuhan, China (1). According to the WHO dashboard, millions of COVID-19 cases are diagnosed worldwide every week (2). Among these newly diagnosed cases, many are laboratory-confirmed with no symptoms (3). For patients who develop symptoms, the majority experience mild or moderate disease (4–6). This situation is mainly attributed to vaccine-induced and infection-induced immunity, as well as the emergence of new variants (7–10).
While severe COVID-19, hospitalization, and death decreased in infected individuals, the Omicron variant showcased increased transmissibility as its main characteristic (11–16). The Omicron virus has caused waves of infections worldwide as the Omicron variant of concern (VOC), suggesting that the pandemic may persist for a longer duration. Although most patients recovered either spontaneously or with acute-phase management, the global healthcare and economies suffered a significant consequence. Moreover, a proportion of individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) experienced long-term COVID-19 complications, regardless of the initial severity of infection, exhibiting a diverse range of symptoms (17). Guidelines for managing long-term COVID-19 recommend that the prevention of SARS-CoV-2 is the most effective approach to preventing the post-COVID state (18, 19). Consequently, it remains a global priority to prevent COVID-19 transmission and the subsequent impact on associated illnesses and deaths.
Early in the pandemic, isolation was pivotal in controlling the outbreak (20, 21). Similarly, in the context of the ongoing community transmission of SARS-CoV-2, isolation strategies continue to be crucial and effective non-pharmaceutical interventions that have been implemented in many countries worldwide. However, it is important to note that different countries and organizations have implemented these strategies with varying degrees of intensity.
Therefore, in order to better identify inconsistent isolation measures, we have summarized the key information regarding the isolation of the general population as outlined in current guidelines or guidance documents.
2. Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22).
2.1. Inclusion criteria
We included guidelines, policy statements, protocol documents, and interim guidance documents published by authoritative advising bodies or healthcare organizations, which provide information on implementing isolation for general populations with COVID-19. We identified the latest version of each eligible article. For feasibility reasons, articles had to be published in English or Chinese.
2.2. Information sources
An experienced team member specializing in systematic reviews conducted electronic searches on four evidence-based medicine (EBM) databases, namely Clinicalkey, UpToDate, Best Practices, and DynaMed Plus. In addition, we searched other sources of guidelines, including the Guidelines International Network (GIN), the National Institute for Health and Clinical Excellence (NICE), the Scottish Intercollegiate Guidelines Network (SIGN), the Chinese Medlive Guidelines Network, and the website of WHO. Articles were identified using the keywords “COVID-19” and “novel coronavirus pneumonia”. Furthermore, the reference lists of topic-related reviews were hand-searched to supplement the electronic database searches. The initial search was performed in September 2022, and an update was conducted in December 2022.
2.3. Selection of studies
An experienced review author screened the literature searches based on the title or descriptors, excluding the articles that clearly did not meet the inclusion criteria of this review. The full texts of all included titles were retrieved. Two review authors independently screened all full-text articles to assess their eligibility for inclusion. Any disagreements were resolved through discussion or by seeking an independent third opinion.
2.4. Quality assessment
Producing COVID-19-related guidelines may not follow the standards for developing guidelines compared with usual times because COVID-19 is an urgent global health threat that needs prompt responses. Additionally, this review aimed to provide a comprehensive overview of the isolation measures adopted by different countries. Our intention was not to select the optimal isolation policy. Therefore, the methodological and reporting quality of included articles was not assessed.
2.5. Data extraction
One experienced review author used a standardized data extraction checklist to extract data from the included articles. A second reviewer double-checked all extractions to ensure completeness and correctness. Any disagreements were documented and resolved through discussion, if necessary. The data items included (1) characteristics of articles (e.g., name, source, and date); (2) population to be isolated—the definitions of different populations (e.g., asymptomatic, mild, and moderate cases) were defined as reported by the authors; (3) setting of isolation (e.g., personal residence, community facility, and health facility); (4) when to discontinue isolation; and (5) criteria for releasing individuals from isolation.
2.6. Data synthesis
We presented the information extracted from the included articles in narrative format and summary tables.
3. Results
3.1. Study selection and characteristics of included articles
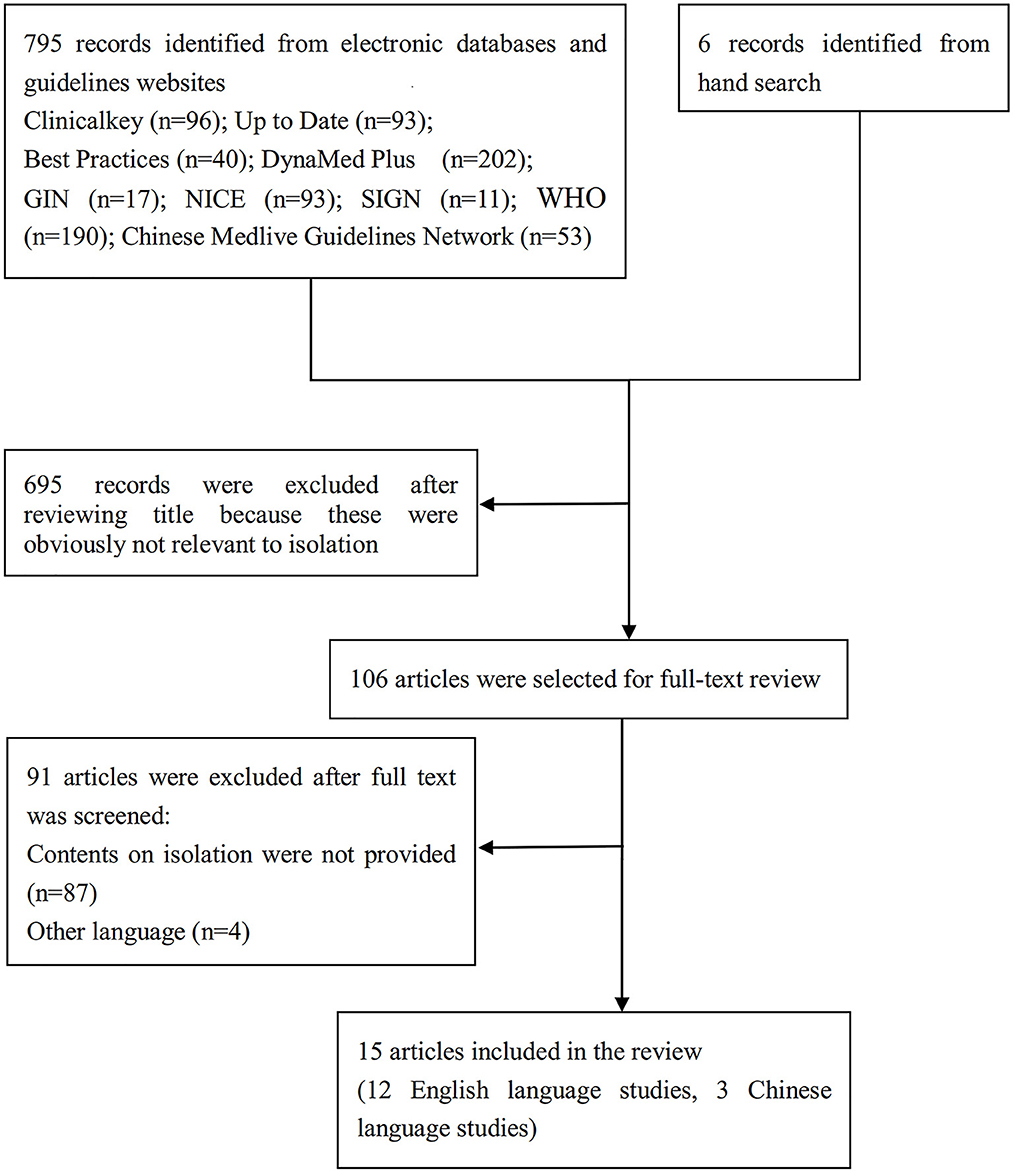
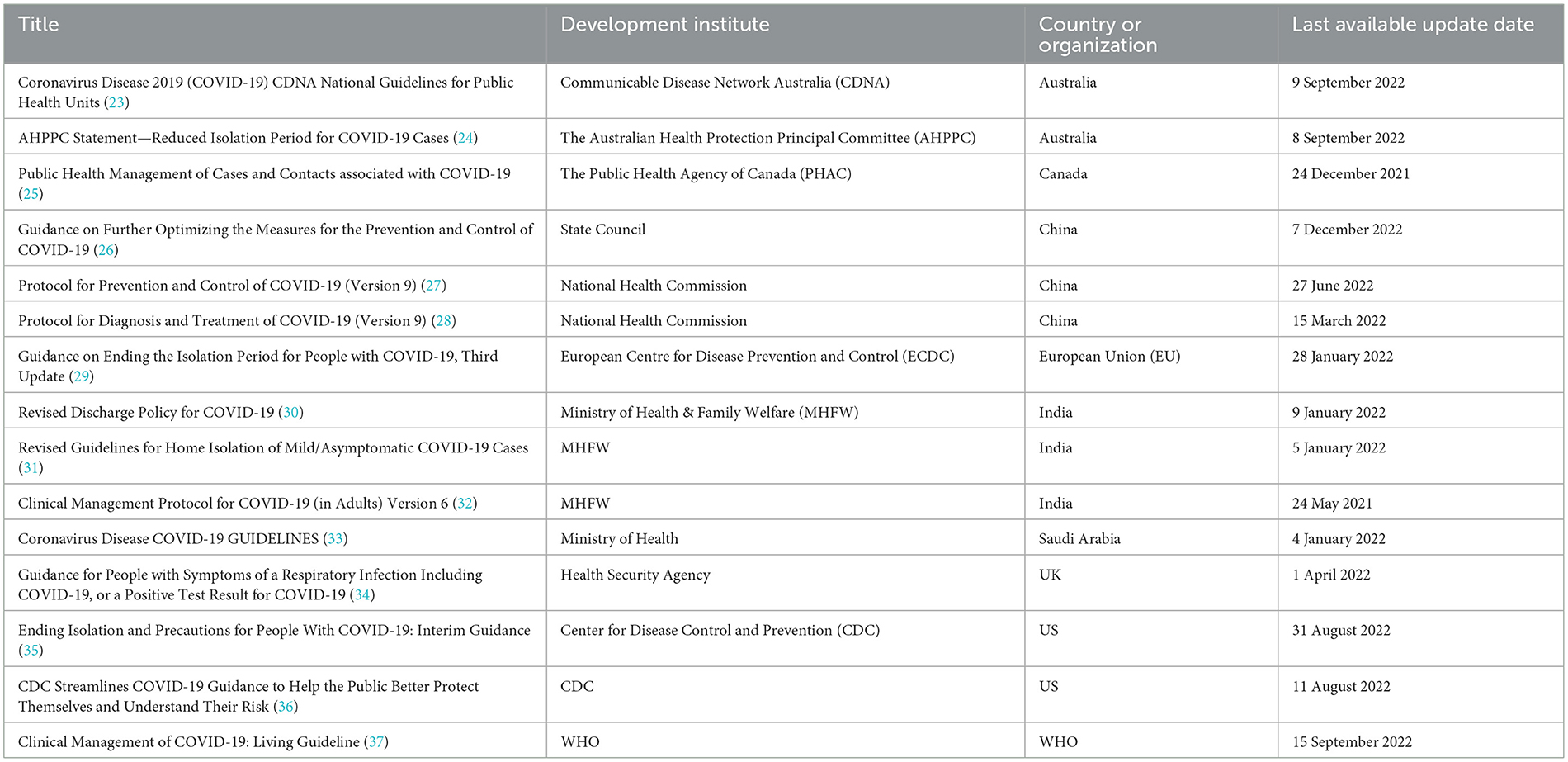
We screened 801 titles retrieved from our electronic search of databases, guideline websites, and hand search. Out of the 106 full texts that were retrieved for further assessment, 15 relevant articles (23–37) fulfilled our eligibility criteria. We also examined the references cited in published relevant review articles until no additional articles were found. Figure 1 presents a flow diagram illustrating the study selection process, and detailed characteristics of the included articles are presented in Table 1. The included articles (23–37) provided information on isolation measures recommended by nine different countries and organizations, namely Australia, Canada, China, India, the European Union (EU), Saudi Arabia, the United Kingdom (UK), the United States (US), and the World Health Organization (WHO). Most of the articles were interim guidance, while others included guidelines, protocols, and statements. For convenience reasons, we will refer to all these articles as “guidance” henceforth.
3.2. People who need isolation
All included guidance recommended isolating individuals with a positive COVID-19 test result, regardless of whether they have symptoms or not.
3.3. Locations of isolation
Most of the included guidance recommended home isolation for patients with asymptomatic or mild COVID-19, including moderate cases. However, WHO highlighted that the decision to monitor a symptomatic case in a health facility, community facility, or home should depend on the clinical presentation, need for supportive care, risk factors, and conditions at the private residence (37). Patients with one or more risk factors for rapid COVID-19 deterioration, severe disease, and increased mortality should preferably be referred to a health facility for monitoring and treatment. It is worth mentioning that several countries and regions, such as China (26, 27), India (31, 32), Saudi Arabia (33), and the EU (29), have fully or partly acknowledged these factors in their policies.
3.4. Duration of isolation
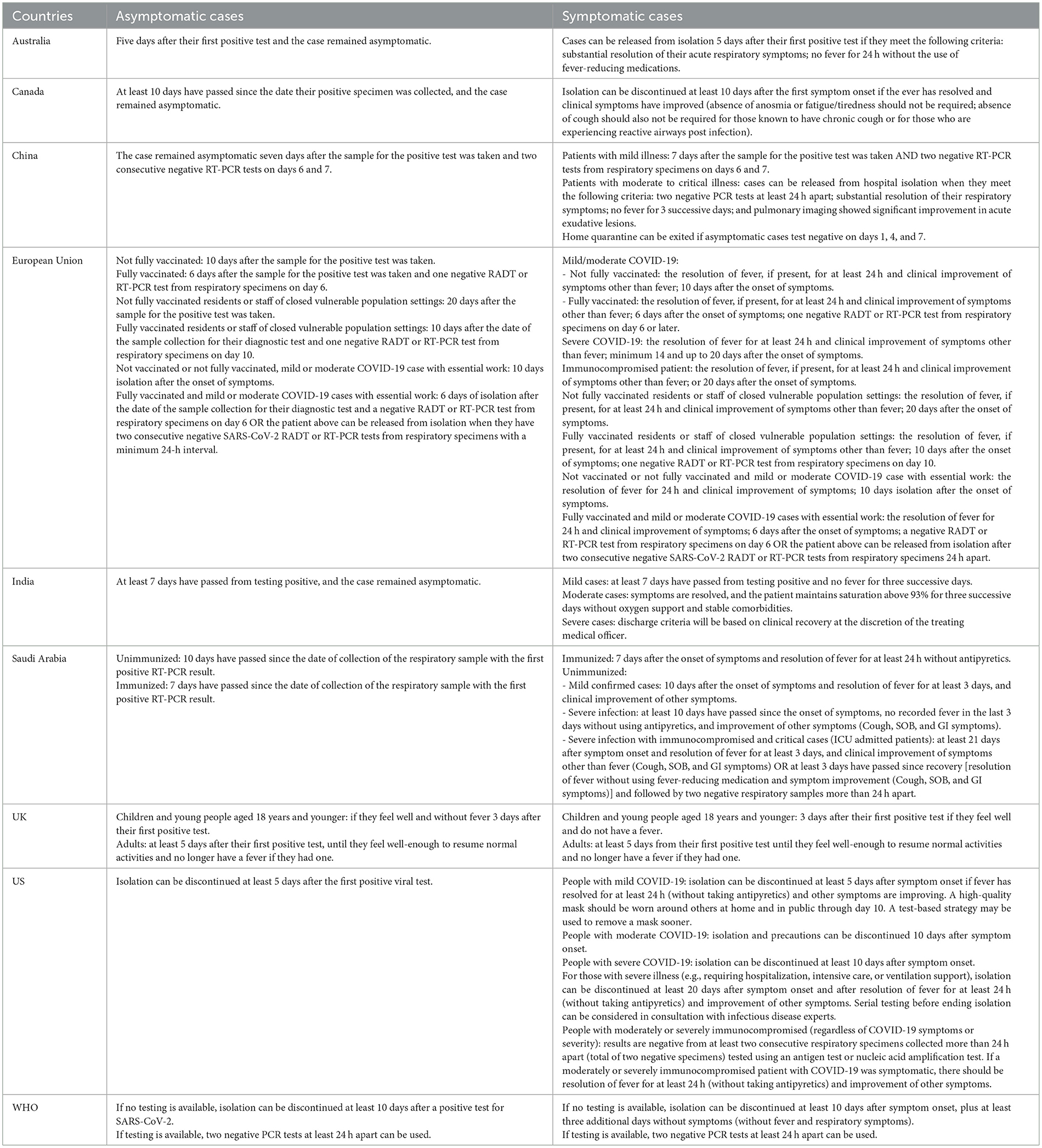
As presented in Table 2, the recommendations for isolation duration were influenced by factors such as the severity of the disease, the vaccination status of the person exposed, and their immunosuppression status.
Generally, in most included guidance, the isolation period for asymptomatic and mild cases was reduced to 5–7 days. However, individuals with more severe disease and weakened immunity were advised to have a longer isolation duration. Of note, we found revealed significant variation in these recommendations across different countries for patients with similar conditions. For example, an 18-year-old asymptomatic patient would be required to have an isolation period of only 3 days in the UK (34), whereas, in Saudi Arabia, the recommended isolation period would be 10 days (33).
3.5. Criteria for releasing individuals from isolation
As shown in Table 2, asymptomatic cases could be released from isolation once they complete the necessary duration of isolation and remain asymptomatic, except for guidance from China and the EU (26, 29). China and the EU recommended implementing an antigen detection rapid diagnostic test (Ag-RAT) or reverse transcription polymerase chain reaction test (RT-PCR) to confirm the absence of contagious virus when ending isolation.
For symptomatic cases, in addition to the recommended duration of isolation, the improvement of symptoms was the most common requirement for ending isolation, usually including the resolution of fever without using antipyretics and substantial improvement in respiratory symptoms. Moreover, the guidance from India and China also includes some physiological indicators (28, 30). Nevertheless, there is disagreement among different countries regarding the necessity of testing. While some countries did not recommend repeat testing for SARS-CoV-2 as the basis for discontinuing isolation, WHO still suggested that countries may continue using testing as part of the release criteria (37), and several countries have adopted this suggestion.
4. Discussion
This systematic review provides a comprehensive summary of available guidance documents that report information on isolation measures for the general population with COVID-19. The results of the review indicate significant heterogeneity in isolation policies across different counties and regions, particularly pertaining to the duration of isolation and the criteria for ending isolation. Moreover, this review offers some insights into relevant implementation strategies.
4.1. Who needs isolation?
As we know, controlling the spread of infectious sources is crucial for mitigating diseases. In the case of the current SARS-CoV-2, evidence has demonstrated that individuals who are asymptomatic at the time of testing, as well as those who are pre-symptomatic, can shed replicating virus to their close contacts (38, 39). Additionally, studies have shown that the viral load in the upper respiratory tract and the probability of detecting viable viruses are comparable between asymptomatic individuals and those with symptomatic SARS-CoV-2 infection (40, 41). This indicates that asymptomatic patients can serve as a source of SARS-CoV-2 transmission. Consequently, all the guidance analyzed in this review recommended isolating COVID-19 patients, regardless of whether they exhibit symptoms.
4.2. Where to isolate?
During the period dominated by the Omicron variant, home isolation has been widely adopted by many countries in comparison to centralized isolation. The preference for home isolation can be explained by the fact that isolating in a community or health facility incurs high economic, societal, and psychological costs. In addition, most cases do not require hospital-level care to recover. Of note, a high household secondary attack rate has been reported in many countries (42–45), including 31.8% in Japan (43), 50% in Korea (44), 52.7% in the US (45), and 80.9% in Spain (42). These elevated attack rates may indicate a low level of compliance with home isolation measures among index-case patients and their households. This suggests that the effectiveness of isolation would significantly decrease without adequate implementation of infection prevention and control measures recommended for isolated individuals and their households.
In addition to that, the mental health of individuals who are isolated at home should not be overlooked. While psychological issues are significantly higher in individuals in centralized isolation than in those isolated at home, it does not mean that people who isolate at home will not experience psychological problems. Many studies have indicated that prolonged periods of home isolation can still lead to feelings of loneliness, anxiety, depression, and other negative emotions (46–48). Therefore, when implementing home isolation measures, it is important to consider the negative impact of home isolation and take steps to address and support people's mental wellbeing by providing resources such as psychological counseling and support services to alleviate the psychological distress they may be facing.
4.3. How long to isolate?
Scientifically sound guidelines on isolation that strike a balance between the risk of prematurely ending isolation and the burden of prolonged isolation are crucial to discuss. The ideal duration of isolation after infection should be determined based on the transmissibility of the current VOC. Regarding individuals infected with the SARS-CoV-2 Omicron variant, Jang et al. analyzed the duration of the infectious stage using viral culture of respiratory samples. The result found that all samples taken 9 days after symptom onset showed negative viral cultures (49). Similarly, a comparative analysis of the transmissibility period reported that Omicron infections featured a mean duration of 9.87 days inferred from the viral load (50). While viral load or cell culture infectivity cannot be directly translated to transmission probability, they are commonly used as proxies to estimate infectiousness and hence transmission.
We learned from the guidance included that many countries have shortened the recommended duration of isolation from 10 to 5 days. However, this recommendation was received with skepticism, as many people think that there was insufficient fully elaborated scientific evidence to support this decision. Recent data from the US indicate that 35% of symptomatic non-severe individuals infected with the Omicron variant, despite having received a booster vaccine, continued to shed culture-able virus more than 5 days after the onset of symptoms or an initial positive test (51). Similarly, a study conducted in Turkey showed that among symptomatic non-severe SARS-CoV-2 Omicron variant infected patients, 83% shed infectious viral particles on day 5, 52% on day 7, 13.5% on day 10, and 8.5% on day 14 (52). This suggests that some cases may still be infectious at the end of the recommended isolation period.
Although it is advised for these individuals to wear a high-quality mask when around others at home and in public after the cessation of isolation on day 5 (35), it is impossible to follow up on the cases on whether they maintain isolation precautions while working. Therefore, the potential increase in virus transmission due to infectious residual viral load related to non-adherence to the recommended mitigation measures is a dramatic challenge to the global response to COVID-19.
Additionally, it is worth considering whether isolation measures should be relaxed for vaccinated individuals. Preliminary evidence suggests that the duration of viral shedding may be shorter and clearance more rapid in vaccinated patients who are infected with recently emerged VOCs (53). As a result, guidance has recommended a shorter isolation period for vaccinated individuals. However, recently published studies have indicated conflicting results regarding the viral infection dynamics of the Omicron variant. One study by Selvavinayagam et al. reported that the viral load was generally lower among vaccinated individuals than in non-vaccinated infected individuals (54). Conversely, Puhach et al. found that reduced infectious viral load was observed only in boosted individuals, not in fully vaccinated individuals, when compared to unvaccinated individuals (55). However, a study published in the New England Journal of Medicine found no significant differences in the median duration of viral shedding among unvaccinated participants, participants who were vaccinated but not boosted, and participants who were both vaccinated and boosted (51). Possible reasons for this conflicting result may include characteristics of the study population, type of vaccine received, history of SARS-CoV-2 infection, underlying comorbidities, and type of VOC. Of course, conducting a systematic review that synthesizes multiple studies regarding this topic is an optimal way to address the inconsistency.
4.4. How to release from isolation?
The most current guidance recommends a symptom-based strategy as an additional criterion for ending the isolation of symptomatic COVID-19 patients, after a necessary time interval has elapsed since the onset of symptoms. This approach avoids the use of SARS-CoV-2 testing, particularly for immunocompetent patients with mild-to-moderate COVID-19. Nevertheless, recent evidence suggests that this policy may not be reliable. According to Jang et al. there is a weak correlation between the duration of fever and the time taken for viral culture conversion in patients infected with the Omicron variant (49). Keske et al. reported that among SARS-CoV-2-confirmed patients who stated that their symptoms had resolved, the rate of detected viral shedding was 58% on day 7, 11% on day 10, and 5% on day 14 (52). These findings suggest that the resolution of symptoms does not guarantee the absence of viral shedding.
Furthermore, several studies have compared different criteria for determining when to end isolation using mathematical models. The findings of these studies indicate that implementing testing protocols for isolated individuals can help minimize unnecessary isolation while still effectively controlling the risk of further transmission (56–58). Additionally, some studies have evaluated the use of antigen tests to guide the end of isolation and consistently concluded that using these tests may reduce redundant isolations or prevent forward transmission (58–60). However, it is worth noting that many sets of guidance do not advocate for repeating laboratory testing as the sole basis for discontinuing isolation, primarily due to resources and cost considerations. Nonetheless, laboratory testing does provide a more accurate assessment of ongoing risk. Thus, when preparing for future pandemics, such as the COVID-19 outbreak, governments should prioritize the development of effective and cost-efficient testing methods.
4.5. Limitations
There are several limitations in retrieving and reviewing the guidance. First, our search was limited to the EBM databases and guideline websites, potentially missing out on guidance published by other countries and the latest version of the included guidance. Second, we only included guidance documents in English or Chinese, potentially overlooking those published in other languages. Third, we utilized descriptive analysis alone to address the research question, without incorporating additional evidence from data analysis. Finally, our review was not registered in PROSPERO as it did not meet the inclusion criteria. These limitations may potentially impact the reliability of the results. However, we believe that the included sets of guidance are representative, indicating that the contentiousness on isolation reflected by the included guidance may likely exist in other guidance as well.
5. Conclusion
Different countries and organizations have substantial differences in their isolation policies for COVID-19. The findings of this study remind us that, in dealing with similar pandemics in the future, decision-makers should take into account the negative impacts of isolation on individuals and society while effectively curbing virus transmission. Additionally, it is imperative to prioritize the development of cost-effective laboratory tests that can inform scientifically accurate isolation policies, thereby avoiding the risk of prematurely ending isolation and the burden of prolonged isolation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JZ and GX participated in the conception and design of the study. GX and LW contributed to data collection and analysis. All authors drafted and critically reviewed this manuscript and approved the final version.
Funding
This research was funded by the Nature Science Foundation of Gansu Province (grant 21JR7RA659).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang CL, Wang YM, Li XW, Ren LL, Zhao JP, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. (2022). Available online at: https://covid19.who.int/ (accessed December 20, 2022).
3. Shang WJ, Kang LY, Cao GY, Wang YP, Gao P, Liu J, et al. Percentage of asymptomatic infections among SARS-CoV-2 omicron variant-positive individuals: a systematic review and meta-analysis. Vaccines. (2022) 10:1049. doi: 10.3390/vaccines10071049
4. Zeng QL, Lv YJ, Liu XJ, Jiang ZY, Huang S, Li WZ, et al. Clinical characteristics of omicron SARS-CoV-2 variant infection after non-mRNA-based vaccination in China. Front Microbiol. (2022) 13:901826. doi: 10.3389/fmicb.2022.901826
5. Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. (2021) 25:8012–8. doi: 10.26355/eurrev_202112_27652
6. Kirca F, Aydogan S, Gözalan A, Kayipmaz AE, Özdemir FAE, Tekçe YT, et al. Comparison of clinical characteristics of wild-type SARS-CoV-2 and Omicron. Rev Assoc Med Bras (1992). (2022) 68:1476–80. doi: 10.1590/1806-9282.20220880
7. Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Protection from previous natural infection compared with mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar: a retrospective cohort study. Lancet Microbe. (2022) 3:e944–55. doi: 10.1016/S2666-5247(22)00287-7
8. Feikin DR, Abu-Raddad LJ, Andrews N, Davies MA, Higdon MM, Orenstein WA, et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. (2022) 40:3516–27. doi: 10.1016/j.vaccine.2022.04.069
9. World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. (2021). Available online at: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed December 12, 2022).
10. World Health Organization. Severity of Disease Associated With Omicron Variant as Compared With Delta Variant in Hospitalized Patients With Suspected or Confirmed SARS-CoV-2 Infection. (2022). Available online at: https://apps.who.int/iris/bitstream/handle/10665/354794/9789240051829-eng.pdf (accessed December 12, 2022).
11. Torjesen I. Covid-19: omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. (2021) 375:n2943. doi: 10.1136/bmj.n2943
12. Sun C, Xie C, Bu GL, Zhong LY, Zeng MS. Molecular characteristics, immune evasion, and impact of SARS-CoV-2 variants. Signal Transduct Target Ther. (2022) 7:202. doi: 10.1038/s41392-022-01039-2
13. Hirabara SM, Serdan TDA, Gorjao R, Masi LN, Pithon-Curi TC, Covas DT, et al. SARS-CoV-2 Variants: differences and potential of immune evasion. Front Cell Infect Microbiol. (2022) 11:781429. doi: 10.3389/fcimb.2021.781429
14. Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. (2022) 20:200. doi: 10.1186/s12916-022-02397-y
15. Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. (2022) 376:eabn4947. doi: 10.1126/science.abn4947
16. Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. (2022) 602:657–63. doi: 10.1038/s41586-021-04385-3
17. Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection. (2021) 49:1163–86. doi: 10.1007/s15010-021-01666-x
18. National Institute for Health Care Excellence Scottish Intercollegiate Guidelines Network Royal Royal College of General Practitioners. COVID-19 Rapid Guideline: Managing the Long Term Effects of COVID-19. (2021). Available online at: www.nice.org.uk/guidance/ng188 (accessed November 7, 2022).
19. Nurek M, Rayner C, Freyer A, Taylor S, Järte L, MacDermott N, et al. Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. Br J Gen Pract. (2021) 71:e815–25. doi: 10.3399/BJGP.2021.0265
20. Nussbaumer-Streit B, Mayr V, Dobrescu AI. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochr Database Syst Rev. (2020) 9:CD013574. doi: 10.1002/14651858.CD013574.pub2
21. Girum T, Lentiro K, Geremew M, Migora B, Shewamare S. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Trop Med Health. (2020) 48:91. doi: 10.1186/s41182-020-00285-w
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
23. Communicable Disease Network Australia,. Coronavirus Disease 2019 (COVID-19) CDNA National Guidelines for Public Health Units. (2022). Available online at: https://www.health.gov.au/resources/publications/coronavirus-covid-19-cdna-national-guidelines-for-public-health-units (accessed October 8, 2022).
24. The Australian Health Protection Principal Committee. AHPPC Statement – Reduced Isolation Period for COVID-19 Cases. (2022). Available online at: https://www.health.gov.au/news/ahppc-statement-reduced-isolation-period-for-covid-19-cases (accessed October 8, 2022).
25. The Public Health Agency of Canada. Public Health Management of Cases and Contacts Associated With COVID-19. (2021). Available online at: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-cases-contacts.html#a8 (accessed October 1, 2022).
26. State Council,. Guidance on Further Optimizing the Measures for the Prevention Control of COVID-19. (2022). Available online at: http://www.gov.cn/xinwen/2022-12/07/content_5730443.htm (accessed December 6, 2022).
27. State Council,. Protocol for Prevention Control of COVID-19 (Version 9). (2022). Available online at: http://www.gov.cn/xinwen/2022-06/28/content_5698168.htm (accessed September 28, 2022).
28. State Council,. Protocol for Diagnosis Treatment of COVID-19 (Version 9). (2022). Available online at: http://www.nhc.gov.cn/cms-search/downFiles/ef09aa4070244620b010951b088b8a27.pdf (accessed September 28, 2022).
29. European Centre for Disease Prevention Control. Guidance on Ending the Isolation Period for People with COVID-19, Third Update. (2022). Available online at: https://www.ecdc.europa.eu/en/publications-data/covid-19-guidance-discharge-and-ending-isolation (accessed September 28, 2022).
30. Government of India Ministry of Health & Family Welfare. Revised Discharge Policy for COVID-19. (2022). Available online at: https://www.mohfw.gov.in/pdf/RevisedDischargePolicyforCOVID19updatedon9thJanuary2022.pdf (accessed September 29, 2022).
31. Government of India Ministry of Health & Family Welfare. Revised Guidelines for Home Isolation of Mild/Asymptomatic COVID-19 Cases. (2022). Available online at: https://www.mohfw.gov.in/pdf/RevisedIllustratedGuidelinesforHomeIsolationofMildAsymptomaticCOVID19Cases.pdf (accessed September 29, 2022).
32. Government of India Ministry of Health & Family Welfare. Clinical Management Protocol for COVID-19 (in Adults) Version 6. (2021). Available online at: https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf (accessed September 29, 2022).
33. Ministry of Health. Coronavirus Disease COVID-19 Guidelines. (2022). Available online at: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/COVID_19_Coronavirus_Disease_Guidelines_v2.0.pdf (accessed September 30, 2022).
34. Health Security Agency. Guidance for People with Symptoms of a Respiratory Infection Including COVID-19, or a Positive Test Result for COVID-19. (2022). Available online at: https://www.gov.uk/guidance/people-with-symptoms-of-a-respiratory-infection-including-covid-19#Children (accessed September 30, 2022).
35. Centers for Disease Control Prevention. Ending Isolation and Precautions for People with COVID-19: Interim Guidance. (2022). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html (accessed September 30, 2022).
36. Centers for Disease Control Prevention. CDC Streamlines COVID-19 Guidance to Help the Public Better Protect Themselves and Understand Their Risk. (2022). Available online at: https://stacks.cdc.gov/view/cdc/120166 (accessed September 30, 2022).
37. World Health Organization. Clinical Management of COVID-19: Living Guideline. (2022). Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical-2022.2 (accessed September 30, 2022).
38. Jefferson T, Spencer EA, Brassey J, Onakpoya IJ, Rosca EC, Plüddemann A, et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) from pre and asymptomatic infected individuals: a systematic review. Clin Microbiol Infect. (2022) 28:178–89. doi: 10.1016/j.cmi.2021.10.015
39. Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. (2021) 4:e2035057. doi: 10.1001/jamanetworkopen.2020.35057
40. Lee S, Kim T, Lee E, Lee C, Kim H, Rheeet H, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med. (2020) 180:1447–52. doi: 10.1001/jamainternmed.2020.3862
41. Ra SH, Lim JS, Kim GU, Kim MJ, Jung J, Kim SH. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. (2021) 76:61–3. doi: 10.1136/thoraxjnl-2020-215042
42. Águila-Mejía JD, Wallmann R, Calvo-Montes J, Rodríguez-Lozano J, Valle-Madrazo T, Aginagalde-Llorente A. Secondary attack rate, transmission and incubation periods, and serial interval of SARS-CoV-2 Omicron variant, Spain. Emerg Infect Dis. (2022) 28:1224–28. doi: 10.3201/eid2806.220158
43. Ogata T, Tanaka H, Tanaka E, Osaki N, Noguchi E, Osaki Y, et al. Increased secondary attack rates among the household contacts of patients with the Omicron variant of the coronavirus disease 2019 in Japan. Int J Environ Res Public Health. (2022) 19:8068. doi: 10.3390/ijerph19138068
44. Song JS, Lee J, Kim M, Jeong HS, Kim MS, Kim SG, et al. Serial intervals and household transmission of SARS-CoV-2 Omicron variant, South Korea, 2021. Emerg Infect Dis. (2022) 28:756–9. doi: 10.3201/eid2803.212607
45. Baker JM, Nakayama JY, O'Hegarty M, McGowan A, Teran RA, Bart SM, et al. SARS-CoV-2 B.1.1.529 (Omicron) variant transmission within households - four U.S. Jurisdictions, November 2021-February 2022. Morb Mortal Wkly Rep. (2022) 71:341–6. doi: 10.15585/mmwr.mm7109e1
46. Zhang MM, Niu N, Zhi XX, Zhu P, Wu B, Wu BN, et al. Nurses' psychological changes and coping strategies during home isolation for the 2019 novel coronavirus in China: a qualitative study. J Adv Nurs. (2021) 77:308–17. doi: 10.1111/jan.14572
47. Slone M, Pe'er A, Mor F. Previous trauma exposure and self-mastery as moderators of psychiatric effects of home isolation during the Covid-19 pandemic: a field study. BMC Psychiatry. (2022) 22:450. doi: 10.1186/s12888-022-04087-8
48. Parisi S, Lehner N, Schrader H, Kierer L, Fleischer A, Miljukov O, et al. Experiencing COVID-19, home isolation and primary health care: a mixed-methods study. Front Public Health. (2023) 10:1023431. doi: 10.3389/fpubh.2022.1023431
49. Jang YR, Kim JM, Rhee JE, Kim D, Lee NJ, Lee H, et al. Clinical features and duration of viral shedding in individuals with SARS-CoV-2 Omicron variant infection. Open Forum Infect Dis. (2022) 9:ofac237. doi: 10.1093/ofid/ofac237
50. Hay JA, Kissler SM, Fauver JR, Mack C, Tai G, Samant RM, et al. Viral dynamics and duration of PCR positivity of the SARS-CoV-2 Omicron variant. medRxiv. (2022). doi: 10.1101/2022.01.13.22269257
51. Boucau J, Marino C, Regan J, Uddin R, Choudhary MC, Flynnet JP, et al. Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA1) infection. N Engl J Med. (2022) 387:275–7. doi: 10.1056/NEJMc2202092
52. Keske S, Güney-Esken G, Vatansever C, Beşli Y, Kuloglu ZE, Nergiz Z, et al. Duration of infectious shedding of SARS-CoV-2 Omicron variant and its relation with symptoms. Clin Microbiol Infect. (2023) 29:221–4. doi: 10.1016/j.cmi.2022.07.009
53. Singanayagam A, Hakki S, Dunning J, Madon KJ, Crone MA, Koycheva A, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B16172) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. (2022) 22:183–95. doi: 10.1016/S1473-3099(21)00648-4
54. Selvavinayagam ST, Yong YK, Joseph N, Hemashree K, Tan HY, Zhang Y, et al. Low SARS-CoV-2 viral load among vaccinated individuals infected with Delta B16172 and Omicron BA11529 but not with Omicron BA11 and BA2 variants. Front Public Health. (2022) 10:1018399. doi: 10.3389/fpubh.2022.1018399
55. Puhach O, Adea K, Hulo N, Sattonnet P, Genecand C, Iten A, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. (2022) 28:1491–500. doi: 10.1038/s41591-022-01816-0
56. Deckert A, Anders S, de Allegri M, Nguyen HT, Souares A, McMahon S, et al. Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial. Trials. (2021) 22:39. doi: 10.1186/s13063-020-04982-z
57. Maya S, Kahn JG. COVID-19 testing protocols to guide duration of isolation: a cost-effectiveness analysis. BMC Public Health. (2023) 23:864. doi: 10.1186/s12889-023-15762-0
58. Jeong YD, Ejima K, Kim KS, Joohyeon W, Iwanami S, Fujita Y, et al. Designing isolation guidelines for COVID-19 patients with rapid antigen tests. Nat Commun. (2022) 13:4910. doi: 10.1038/s41467-022-32663-9
59. Zigo L, Wilkinson A, Landry M, Castel AD, Vyas A, McDonnell K, et al. Use of rapid antigen tests to end isolation in a university setting: observational study. JMIR Form Res. (2023) 7:e45003. doi: 10.2196/45003
Keywords: COVID-19, isolation, guidance, guidelines, systematic review
Citation: Xie G, Wang L and Zhang J (2023) How are countries responding differently to COVID-19: a systematic review of guidelines on isolation measures. Front. Public Health 11:1190519. doi: 10.3389/fpubh.2023.1190519
Received: 21 March 2023; Accepted: 08 August 2023;
Published: 30 August 2023.
Edited by:
Reza Lashgari, Shahid Beheshti University, IranReviewed by:
Joseph Ntayi, Makerere University, UgandaBardia Karim, Babol University of Medical Sciences, Iran
Copyright © 2023 Xie, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhang, anVuejg3MDEyNEAxNjMuY29t
†These authors have contributed equally to this work
 Guangmei Xie1,2†
Guangmei Xie1,2† Li Wang
Li Wang Jun Zhang
Jun Zhang