
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 15 June 2023
Sec. Children and Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1190285
Objective: To investigate the prevalence of anisometropia and associated parameters among school-aged children in Nantong, China.
Methods: This school-based, cross-sectional study examined students from primary schools, junior high schools, and senior high schools in an urban area of Nantong, China. Univariate and multivariate logistic regression analyses were used to investigate the specific correlations between anisometropia and related parameters. Non-cycloplegic autorefraction was assessed for each student. Anisometropia was defined as the spherical equivalent refraction (SE) difference ≥ 1.0 D between eyes.
Results: A total of 9,501 participants were validated for analyses, of which 53.2% (n = 5,054) were male, and 46.8% (n = 4,447) were female. The mean of age was 13.32 ± 3.49 years, ranging from 7–19 years. The overall prevalence of anisometropia was 25.6%. Factors such as myopia, scoliosis screening positive, hyperopia, female sex, older age, and higher weight had a significantly higher risk of anisometropia (p < 0.05).
Conclusion: There was a high prevalence of anisometropia in school-age children. Some physical examination parameters are closely related to children’s anisometropia, especially myopia and scoliosis. Preventing myopia and controlling its progression may be the most important ways to reduce the prevalence of anisometropia. Correcting scoliosis may be an important factor in controlling the prevalence of anisometropia, and maintaining good reading and writing posture may be helpful in controlling the prevalence of anisometropia.
Anisometropia involves asymmetry between eyes in the refractive state (1), it affects around 10% of the population in early adulthood (2). This phenomenon is manifested in eyes of individuals who may have similar sociodemographic, environmental, and genetic effects, and showing asymmetric eye growth (3). Anisometropia can be associated with strabismus, amblyopia, hyperopia, intolerance to glasses, and deepening of myopia (4). A previous study reported that the presence of anisometropia increased the risk of poor stereoscopic acuity by 6.73 times (5). It is one of the main causes of monocular vision loss, and unilateral amblyopia increases the risk of vision loss during a patient’s life (6, 7). The asymmetry of visual experience in childhood may change the growth of the ocular axis. Considering that the baseline spherical equivalent refraction (SE) of the two eyes may be different, and that differences between the two eyes may be caused by various reasons (8–11), the length of the eye axis may be asymmetrically prolonged, which may lead to anisometropia.
At present, research on children’s refractive errors and physical development status mainly focuses on the correlation between myopia and anthropometric measures (12–17). Although studies have described the refractive status, eye structure, demography, and lifestyle of patients with anisometropia (5, 18–23), there is still a lack of epidemiological research on the relationship between anisometropia of school age children and related parameters of children’s growth and development.
The specific cause of anisometropia is not clear. In China, scoliosis is the main type of spinal curvature abnormality (24, 25). In recent years, some regions in China have included scoliosis screening in students’ routine physical examinations. Considering that it has been confirmed that scoliosis is associated with poor reading and writing posture (26, 27), and that poor reading and writing posture may cause anisometropia (28), we also included scoliosis in the study of anisometropia.
Knowing the status of children’s’ anisometropia and identifying the causes will help in preventing permanent damage to binocular and stereoscopic vision. In the present study, we therefore analyzed the baseline data of routine physical examinations of students in 2022, to report the prevalence of refractive anisometropia and evaluate the association between other physical examination parameters and refractive anisometropia among school-age children.
This school-based study was designed to investigate the refractive status in schoolchildren in 2022 in Nantong, a moderately sized city on the east coast of China. According to data from the census of 2020, the total population of Nantong was 77,266,000. This study was approved by the ethics committee of the Second Affiliated Hospital of Nantong University, China (approval number: 2020KT068). All protocols used in this study followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from parents of the participants before being enrolled.
Based on previous research, a stratified cluster sampling method was used (29). The cluster was stratified by grade and age to ensure that all age groups from 7 to 19 years of age were included in the study. Classes in each grade were selected by simple random sampling, and all students in these classes were required to participate in the study. The sampling framework was based on the statistics of classes of specific grades in schools at all levels. Previous studies have shown that the prevalence of anisometropia was stable at about 7% from 1 year of age to teenage years (5). To achieve a power of 80%, the sample size was calculated using the formula, n = t2pq/d2, assuming a design effect of 1.5 due to cluster sampling and a nonresponse rate of 5% [t = 2 for a 95% confidence interval (CI), q = 1−P, d = 0.1 P]. The total sample size was at least 8,391. To ensure better multifactor analyses (including some factors that previously were rarely studied), more samples were included in the protocol. This research included primary school, junior high school, and senior high school students in Nantong City as research subjects in the urban area of Nantong, from 27 schools (nine primary schools, nine middle schools, and nine high schools), which participated in the survey. At least two classes were randomly selected from each grade of each school to ensure that no less than 80 students were selected at a time. This sample size was sufficient to detect risk factors using multivariate analyses.
Before the study began, researchers visited and arranged each venue to standardize the lighting and test distance. To minimize interference and limit the number of students examined, closed classrooms were used to facilitate testing. Autorefractors were calibrated every day. Students with current corneal refractive therapy were asked to wear spectacle glasses on the day of testing. The proportion of children who volunteered to participate in the invitation was 96.3%.
During the visual examination, well-trained investigators performed eye examinations, and non-cycloplegic refraction was measured using three repeated measurements using an autorefractor (WSRMK-8000; Biobase, Shandong, China). The average data of three repeated measurements were used for analysis. Refractive error was measured three times starting with the right eye; if any two of the three results were greater than 0.50 D (diopters), additional examinations were conducted at the same visit. Vision measurement started with the right eye, and uncorrected visual acuity was measured by a standard logarithmic liquid crystal tumbling E chart (WSVC-100; Qingda Optometry, Berkeley, CA, United States) at 5 m. The best-corrected visual acuity was corrected according to the autorefractor results. Refinement of the sphere, cylinder, and axis was done to achieve the best-corrected visual acuity. Spherical equivalent refraction (SE) was calculated using the cylindrical degree and spherical degree as follows: SE = cylindrical degree × 0.5 + spherical degree. Similar to previous epidemiological studies, the present study used the Refractive Error Study in Children surveys (30, 31). Anisometropia was defined as the spherical equivalent refraction (SE) difference ≥ 1.0 D between eyes. To minimize the potential impact of spurious associations between anisometropia and ametropia, subjects were categorized according to the SE in less ametropic eyes. Myopia was defined as a spherical equivalent of ≤−0.5 D. Hyperopia was defined as an SE > 0.5 D. Emmetropia was defined as −0.5 D < SE ≤ 0.5 D. Low myopia was defined as −3.0 D < SE ≤ −0.5 D. Moderate myopia was defined as −6.0 D < SE ≤ −3.0 D. High myopia was defined as a SE < −6.0 D. The degree of anisometropia was categorized into mild (SE difference ≥ 1.0 D and < 2.0 D), moderate (SE difference ≥ 2.0 D and < 3.0 D), and severe (SE difference ≥ 3.0 D). Those students who were suspected of ocular abnormality were referred to subspecialists for further investigation.
An experienced screening team, comprising of 10 spine surgeons, rehabilitation physicians, therapists, and nurses, from the Second Affiliated Hospital of Nantong University performed the school-based screening. Boys and girls received relevant examinations. Inspectors could only assume their posts after receiving unified training and passing the necessary examinations. Participants with spine and chest deformities (including treatment with a brace), musculoskeletal anomalies, neurological disorders, and operation histories were questioned and excluded from the study. According to the national standard “Screening of Children and Adolescents with Abnormal Spinal Curvature” (GB/T 16133–2014), they had passed the general examination, forward flexion test, spine movement test, and prone test, and the scoliosis measuring instrument was used to screen scoliosis in schools. The results were divided into no side bending, side bending degree 1, side bending degree 2, and side bending degree 3. Students with positive scoliosis screening were registered in this study and recommended to go to a specialized hospital for further examinations.
Other conventional physical examinations were conducted by physicians from tertiary hospitals, including height, weight, and blood pressure. In addition, basic information such as sex and age were recorded. The height was determined to the nearest 0.1 cm in a standardized manner without shoes. Weight was measured to the nearest 0.1 kg without thick clothes.
Data from school-age children enrolled in 2022 who had completed the study were analyzed. Data were analyzed using SPSS statistical software for Windows, version 22 (SPSS, Chicago, IL, United States). Correlations between anisometropia and various parameters considered in this study were then determined. The differences between groups in terms of refractive status and physical examination parameters were compared using chi-square or independent t-tests, as appropriate. The chi-squared test was used for disordered enumeration data, the rank-sum test was used for orderly enumeration data, and the independent samples t-tests were used for measurement data. The polynomial linear correlation in one-way ANOVA was used for the trend test (Ptrend). Univariate and multivariate logistic regression analyses were used to investigate the specific correlation between anisometropia and related parameters. The odds ratio (OR) and 95% confidence interval (CI) for the associated factors were then calculated. Factors with an OR < 1 were regarded to be protective against anisometropia, whereas those with an OR > 1 were considered to be risk factors for anisometropia. Continuous variables are expressed as the mean ± standard deviation, and categorical variables are expressed as percentages. A value of p < 0.05 was considered statistically significant.
A total of 9,864 students were invited to participate in the study. The completion percentage of students out of all schools was 6.5%. A total of 9,501 students were finally recruited into the statistical analysis. The distributions of basic demographic and ocular parameters are shown in Table 1. Of 9,501 students, 53.2% (n = 5,054) were male, and 46.8% (n = 4,447) were female. The mean of age was 13.31 ± 3.47 years, ranging from 7–19 years. The prevalence of anisometropia was not related to sex (x2: 3.14, p = 0.077). The prevalence of anisometropia in students with scoliosis screening positive (45.6%) was significantly higher than that in students without scoliosis screening positive (24.7%) (x2: 83.64, p < 0.001). In addition, Table 1 shows that anisometropia was related to refractive state, age, height, weight, systolic blood pressure, and diastolic blood pressure (all, p < 0.001).
As illustrated in Table 1 and Figure 1, anisometropia was more prevalent in the myopic and hyperopic groups (30.4 and 17.7%, respectively) than in the emmetropic group (4.6%). In the population with myopia, as the degree of myopia deepened, the proportion of anisometropia also increased. The prevalence of anisometropia in the low myopia group was 24.7%, in the moderate myopia group it was 34.9%, and even reached 42.4% in the high myopia group. The proportion of anisometropia ≥2.0 D in the emmetropia group was 1.3%, in the low myopia group it was 5.4%, in the moderate myopia group it was 12.1%, and reached 14.8% in the high myopia group.
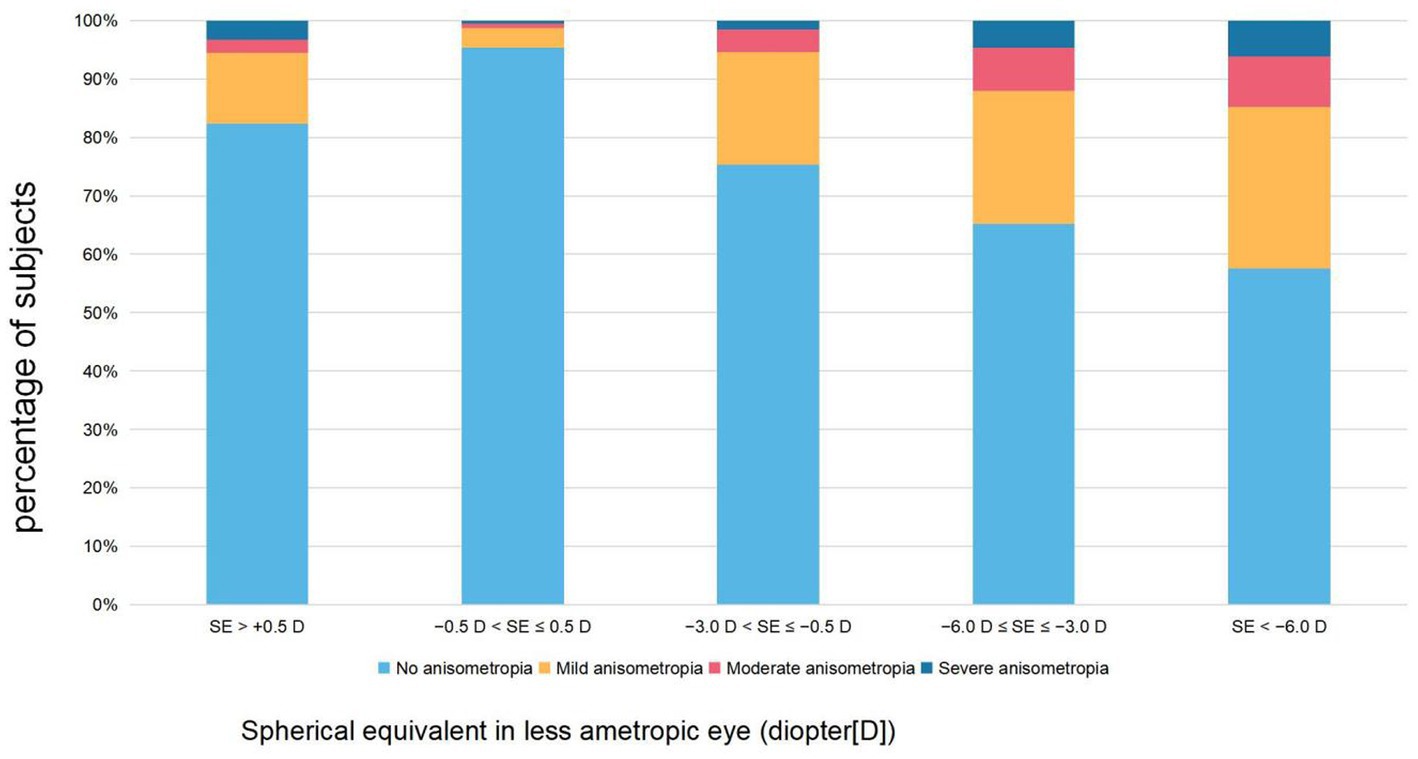
Figure 1. The proportion of different severity levels of refractive anisometropia by refractive groups.
As illustrated in Table 2 and Figure 2, the overall prevalence of anisometropia was 25.6% (n = 2,428), and the mean SE difference between eyes was 0.72 ± 0.83 D. The overall prevalence of myopia was 79.8%. The prevalence of anisometropia gradually increased with age (Ptrend < 0.001). The prevalence of anisometropia at the age of 7 years was 7.8%, reaching 39.0% at the age of 19 years. The prevalence of myopia tended to increase with age (Ptrend < 0.001). The prevalence of myopia at the age of 7 years was 10.1%, reaching 89.7% at the age of 19 years. With increasing age, the difference in inter-eye SE gradually widened (Ptrend < 0.001).In addition, the prevalence of hyperopia tended to decrease with age (Ptrend < 0.001), and the prevalence of scoliosis screening positive did not change with age (Ptrend = 0.911).
Table 3 lists the results of univariate and multiple logistic regression analyses. After adjustment for other characteristics, the results suggested that females were more likely to suffer from anisometropia than males (OR: 1.25, 95% CI: 1.13–1.39, p < 0.001). Compared with students with emmetropia, students with hyperopia were 2.25 times more likely to suffer from anisometropia (OR: 2.25, 95% CI: 1.45–3.47, p < 0.001) and students with myopia were 6.40 times more likely to suffer from anisometropia (OR: 6.40, 95% CI: 5.01–8.18, p < 0.001). Compared with students with screening negative, students with scoliosis screening positive were 4.08 times more likely to suffer from anisometropia (OR: 4.08, 95% CI: 3.14–5.30, p < 0.001). In addition, anisometropia was independently associated with students with older age (OR: 1.05, 95% CI: 1.02–1.08, p < 0.05) and higher weight (OR: 1.01, 95% CI: 1.01–1.02, p < 0.001).
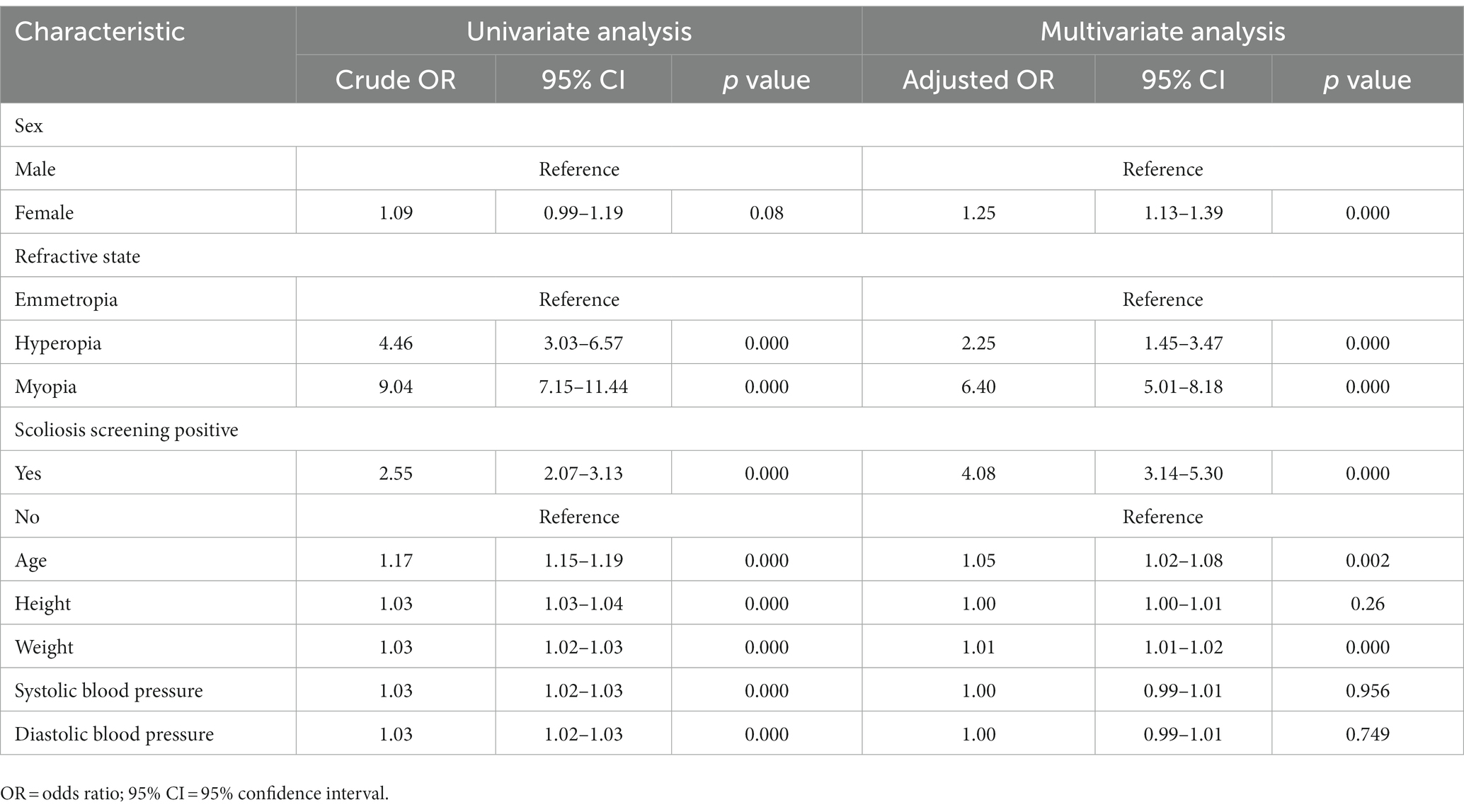
Table 3. Univariate and multivariate logistic regression analysis of anisometropia among all participants (n = 9,501).
The current study evaluated the prevalence and associated factors of anisometropia related to growth and development. This was a unique study involving anisometropia and growth and development of school-age children between 7 and 19 years of age. We found that anisometropia was related to myopia, scoliosis screening positive, hyperopia, female sex, older age, and higher weight.
Considering that most studies (32–34) focused on ≥1.00 D as the standard of anisometropia, we also used this critical point to assess its correlation with other parameters. Table 4 summarizes the main results of epidemiological studies on the prevalence of anisometropia in school aged children in recent years. There were differences in the prevalence of anisometropia in studies of different countries, races, and ages. In an epidemiological study of 2,090, 6–72 months-old children in Australia (23), the prevalence of anisometropia was 2.7%. In another epidemiological study of 1,765, 6-year-old children in Australia, the prevalence of anisometropia was only 1.6% (20). In Northern Ireland (35), the prevalence of anisometropia in an epidemiological study of 389 Caucasian children aged 6–7 years was 8.5%, and in 661 Caucasian children 12–13 years of age it was 9.4%. In Portugal (33), the prevalence of anisometropia varied from 2.9% in pre-school children to 9.4% in their 3rd study cycle. Deng & Gwiazda (36) found that when using a cutoff of 1.00 D SER for anisometropia, the prevalence were 2.0, 1.3, and 5.8% at 6 months, 5 years, and 12–15 years, respectively. In a large-scale school study in Taipei (19), 5.3% of 23,114 8-year-old children had anisometropia. In 2016, a school-based study conducted in Shandong, China (5) found that 7.0% of 6,025 school children aged 4–17 years had refractive anisometropia. In 2022, in another epidemiological survey of students 4–17 years of age in Shandong, China (37), the prevalence rose to 13.2%. In the current study, we found that the prevalence of anisometropia was relatively low in early school age children (7–9 years of age) (7.8–9.0%), but it increased to 16.0% at 10 years of age, and even to 39.0% at 19 years of age. In addition, the prevalence of anisometropia from 15 years of age was even higher than that of previous relevant studies on adults (1, 38). Compared with previous studies, such a high prevalence of anisometropia is rare. In fact, compared to previous studies (5, 35, 37), there is not much difference in the prevalence of anisometropia between 7 to 9 years of age. The sharp increase in the prevalence of anisometropia is worth noting.
An interesting finding of this study was that scoliosis screening positive was one of the important factors of anisometropia. This has not been reported in previous studies. Scoliosis refers to deformity of the spine, with one or more segments of the spine bending to the side or accompanied by vertebral rotation. Recent studies (39, 40) reported that there may be millions of children with idiopathic scoliosis in China. To detect, diagnose, and treat early, and improve long-term prognoses, it is necessary to conduct relevant screening. Scoliosis can be divided into idiopathic scoliosis and non-idiopathic scoliosis, of which idiopathic scoliosis accounts for about 80% of patients. The prevalence of idiopathic scoliosis ranges from 2–16% (41–44). Diagnosis of scoliosis requires radiographic examination, and students with a Cobb angle of at least 10° are diagnosed as positive (45–47). In the current study, children with scoliosis screening positive were referred for further appointments, so we could not obtain additional relevant data. Although X-ray examinations were not used for final diagnoses in this study, the positive predictive value of idiopathic scoliosis has reached 78.4% in previous similar three-stage design examinations (24, 41). Furthermore, the main objective of this study was not to diagnose scoliosis, but to study the prevalence of anisometropia and its related parameters. It was therefore appropriate to use the parameters of suspected scoliosis for relevant studies. In the present study, the overall prevalence of positive screening for scoliosis in children and adolescents was 4.0%, similar to the study conducted in Zhejiang Province, China in 2019 (24). The results of this study showed that the prevalence of anisometropia in children with scoliosis screening positive was 4.08 times higher than that in children without scoliosis (OR: 4.08, 95% CI: 3.14–5.30, p < 0.001). Spinal scoliosis inevitably leads to poor reading and writing posture (26, 27). Pärssinen et al. (48) found that in school-aged children, the trend of myopia progression was closely related to steeper reading angle. When poor reading and writing posture persists, the refractive stimulation to both eyes vary. Assuming adaptation to fixed points is maintained, much of the peripheral field must be greatly out-of-focus. Hence, myopia and anisometropia would result (28). In previous studies on near work habits (18, 49, 50), including the age at which close working began, the distance between eyes and objects, the use of computers or mobile devices, and the average daily number near working activities, no correlation was found between near working and anisometropia. But the impact of poor reading and writing posture on refractive error has not been fully studied. Therefore, correcting scoliosis may be an important factor in controlling the prevalence of anisometropia, and maintaining good reading and writing posture may be helpful in controlling the prevalence of anisometropia.
However, the prevalence of scoliosis screening positive was only 4%, and it did not increase with age (Ptrend = 0.911), which could not explain the high prevalence of anisometropia and the characteristics of anisometropia with age. Similarly, although students with hyperopia were 2.25 times more likely to suffer from anisometropia (OR: 2.25, 95% CI: 1.45–3.47, p < 0.001), considering the trend of decreasing prevalence of hyperopia with age (Ptrend<0.001), it could not explain the high prevalence of anisometropia and the characteristics of anisometropia with age. According to current research, myopia is the most important factor associated with anisometropia. Students with myopia were 6.40 times more likely to suffer from anisometropia (OR: 6.40, 95% CI: 5.01–8.18, p < 0.001). And as shown in Table 2, both the prevalence of anisometropia and myopia showed a significant increasing trend with age (Ptrend<0.001).
In experimental anisometropia (34, 51, 52), anisometropia is highly correlated with the difference in axial length, mainly the growth of the posterior segment of the eye, which means that the induced anisometropia is essentially axial, and abnormal visual input of one eye may cause uneven axial elongation of both eyes. Similarly, significant correlation between anisometropia and axial length difference between eyes has also been found in human studies, and with an increase in myopia, both eyes gradually lose a balance of refractive errors (37, 38, 53, 54). In Sweden (55), the prevalence of myopia in 10-year-old children was 7.8; the prevalence of anisometropia was only 1%. In Dutch school children 11–13 years of age (56), the prevalence of myopia was 28% and the prevalence of anisometropia was 4.60%. In children aged 4 to 17 in Shandong, China (37), although there was no detailed list of the prevalence of myopia, the SE of the worse eye gradually decreased from 1.31 ± 0.77 to −3.92 ± 2.37 (Ptrend < 0.001), indicating the deepening of myopia. At this time, the prevalence of anisometropia increased from 1.1 to 28.4% (Ptrend < 0.001). In the present study, with increased age, the refractive status of many school-aged children shifted to myopia, and the prevalence of myopia increased from 10.1% at the age of 7 to 89.7% at the age of 19 (Ptrend < 0.001), which may have led to expansion of the range of refractive errors and significant differences between eyes. The prevalence of anisometropia in the low myopia group was 24.71%, in the moderate myopia group it was 34.87%, and even reached 42.41% in the high myopia group, which also indicated that the higher the degree of myopia, the greater the possibility of anisometropia. Therefore, preventing myopia and controlling its progression may be the most important ways to reduce the prevalence of anisometropia.
The impact of sex on anisometropia is controversial (57–60). A study conducted among high school students in Singapore (57) showed a higher prevalence of anisometropia among female participants. A study of participants aged ≥30 years in Bangladesh also showed similar results (58). In the present study, it was also found that females were more likely to suffer from anisometropia than males (OR: 1.25, 95% CI: 1.13–1.39, p < 0.001). In addition, some studies have reported the relationships between dominant eyes and anisometropic myopia, but there is still controversy (61, 62). Because no information about ocular advantages was collected in our study, we could not confirm a similar association.
There were some potential limitations in the present study. First, the diopters used in this study were measured without cycloplegia, which ensured a high participation percentage. The prevalence of myopia may be overestimated in the absence of ciliary muscle paralysis (63). Second, we did not measure axial lengths to differentiate mechanisms related to anisometropia development during the growth of children’s’ eyes. Third, in this study, there was no final imaging diagnosis during the measurement of scoliosis. In addition, the use of autorefractometer may also lead to an overestimate of the prevalence of anisometropia. In the one hand, the autorefractometer measures the refraction close to the eye, which activates accommodation and tends to register measurements that overestimate myopia (64). On the other hand, when measuring each eye separately, that effect can be different between eyes, which overestimates anisometropia.
There was a high prevalence of anisometropia in school-age children in Nantong, China. The present study showed that parameters such as myopia, scoliosis, hyperopia, female sex, older age, and higher weight were significantly associated with a higher risk of anisometropia, especially myopia and scoliosis. Preventing myopia and controlling its progression may be the most important ways to reduce the prevalence of anisometropia. Correcting scoliosis may be an important factor in controlling the prevalence of anisometropia, and maintaining good reading and writing posture may helpful in controlling the prevalence of anisometropia.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the ethics committee of the Second Affiliated Hospital of Nantong University, China. The patients/participants provided their written informed consent to participate in this study.
YZ, XZ, YS, and ZS were involved in the design of the study. XC, MW, JC, and YX were involved in data collection and data analysis. All authors contributed to the conception of the work by writing sections of the manuscript and drafting and revising it critically as well as final approval of the published version.
This work was supported by Nantong Science and Technology Program (project number: MS2020035).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Parssinen, O, and Kauppinen, M. Anisometropia of spherical equivalent and astigmatism among myopes: a 23-year follow-up study of prevalence and changes from childhood to adulthood. Acta Ophthalmol. (2017) 95:518–24. doi: 10.1111/aos.13405
2. Liang, YB, Wong, TY, Sun, LP, Tao, QS, Wang, JJ, Yang, XH, et al. Refractive errors in a rural Chinese adult population the Handan eye study. Ophthalmology. (2009) 116:2119–27. doi: 10.1016/j.ophtha.2009.04.040
3. Weale, RA. On the age-related prevalence of anisometropia. Ophthalmic Res. (2002) 34:389–92. doi: 10.1159/000067040
4. Tong, L, Chan, YH, Gazzard, G, Tan, D, and Saw, SM. Longitudinal study of anisometropia in Singaporean school children. Invest Ophthalmol Vis Sci. (2006) 47:3247–52. doi: 10.1167/iovs.05-0906
5. Hu, YY, Wu, JF, Lu, TL, Wu, H, Sun, W, Guo, DD, et al. Prevalence and associations of Anisometropia in children. Invest Ophthalmol Vis Sci. (2016) 57:979–88. doi: 10.1167/iovs.15-18647
6. Simons, K. Amblyopia characterization, treatment, and prophylaxis. Surv Ophthalmol. (2005) 50:123–66. doi: 10.1016/j.survophthal.2004.12.005
7. Rahi, J, Logan, S, Timms, C, and Taylor, D. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet. (2002) 360:597–602. doi: 10.1016/S0140-6736(02)09782-9
8. Gee, S, and Tabbara, KF. Increase in ocular axial length in patients with corneal opacification. Ophthalmology. (1988) 95:1276–8. doi: 10.1016/S0161-6420(88)33035-6
9. von Noorden, G, and Klewis, RA. Ocular axial length in unilateral congenital cataracts and blepharoptosis. Invest Ophthalmol Vis Sci. (1987) 28:750–2. Available at: https://iovs.arvojournals.org/article.aspx?articleid=2177806
10. Swarbrick, HA, Alharbi, A, Watt, K, Lum, E, and Kang, P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology. (2015) 122:620–30. doi: 10.1016/j.ophtha.2014.09.028
11. Woodman, EC, Read, SA, Collins, MJ, Hegarty, KJ, Priddle, SB, Smith, JM, et al. Axial elongation following prolonged near work in myopes and emmetropes. Br J Ophthalmol. (2011) 95:652–6. doi: 10.1136/bjo.2010.180323
12. Wang, SK, Guo, Y, Liao, C, Chen, Y, Su, G, Zhang, G, et al. Incidence of and factors associated with myopia and high myopia in Chinese children, based on refraction without Cycloplegia. JAMA Ophthalmol. (2018) 136:1017–24. doi: 10.1001/jamaophthalmol.2018.2658
13. Lai, LJ, Hsu, W, and Tung, TH. Prevalence and associated factors of myopia among rural school students in Chia-Yi, Taiwan. BMC Ophthalmol. (2020) 20:320. doi: 10.1186/s12886-020-01590-y
14. Chen, J, Chen, Z, Lin, S, Zhang, J, Wang, Q, Zhong, H, et al. Correlation analysis for school-age children's height and refractive errors. Adv Clin Exp Med. (2018) 27:1125–30. doi: 10.17219/acem/78773
15. Yue, Y, Liu, X, Yi, S, Liu, B, Yi, H, and Li, H. High prevalence of myopia and low hyperopia reserve in 4411 Chinese primary school students and associated risk factors. BMC Ophthalmol. (2022) 22:212. doi: 10.1186/s12886-022-02436-5
16. Huang, CY, Hou, CH, Lin, KK, Lee, JS, and Yang, ML. Relationship of lifestyle and body stature growth with the development of myopia and axial length elongation in Taiwanese elementary school children. Indian J Ophthalmol. (2014) 62:865–9. doi: 10.4103/0301-4738.141047
17. Rim, TH, Kim, SH, Lim, KH, Kim, HY, and Baek, SH. Body stature as an age-dependent risk factor for myopia in a south Korean population. Semin Ophthalmol. (2017) 32:326–36. doi: 10.3109/08820538.2015.1088554
18. Lee, CW, Fang, SY, Tsai, DC, Huang, N, Hsu, CC, Chen, SY, et al. Prevalence and association of refractive anisometropia with near work habits among young schoolchildren: the evidence from a population-based study. PLoS One. (2017) 12:e0173519. doi: 10.1371/journal.pone.0173519
19. Yamashita, T, and Watanabe Sohba, N. A longitudinal study of cycloplegic refraction in a cohort of 350 Japanese schoolchildren. Cycloplegic refraction. Ophthalmic Physiol Opt. (1999) 19:30–3. doi: 10.1046/j.1475-1313.1998.00407.x
20. Huynh, SC, Wang, XY, Ip, J, Robaei, D, Kifley, A, Rose, KA, et al. Prevalence and associations of anisometropia and aniso-astigmatism in a population based sample of 6 year old children. Br J Ophthalmol. (2006) 90:597–601. doi: 10.1136/bjo.2005.083154
21. Giordano, L, Friedman, DS, Repka, MX, Katz, J, Ibironke, J, Hawes, P, et al. Prevalence of refractive error among preschool children in an urban population: the Baltimore pediatric eye disease study. Ophthalmology. (2009) 116:739–746.e4. doi: 10.1016/j.ophtha.2008.12.030
22. Yekta, A, Fotouhi, A, Hashemi, H, Dehghani, C, Ostadimoghaddam, H, Heravian, J, et al. Prevalence of refractive errors among schoolchildren in shiraz, Iran. Clin Exp Ophthalmol. (2010) 38:242–8. doi: 10.1111/j.1442-9071.2010.02247.x
23. Afsari, S, Rose, KA, Gole, GA, Philip, K, Leone, JF, French, A, et al. Prevalence of anisometropia and its association with refractive error and amblyopia in preschool children. Br J Ophthalmol. (2013) 97:1095–9. doi: 10.1136/bjophthalmol-2012-302637
24. Zou, Y, Lin, Y, Meng, J, Li, J, Gu, F, and Zhang, R. The prevalence of scoliosis screening positive and its influencing factors: a school-based cross-sectional study in Zhejiang Province, China. Front Public Health. (2022) 10:773594. doi: 10.3389/fpubh.2022.773594
25. Huang, F, Liu, Y, Wu, J, Yang, J, Huang, S, Zhang, Z, et al. Incidence of scoliosis among junior high school students in Zhongshan city, Guangdong and the possible importance of decreased miR-30e expression. J Int Med Res. (2020) 48:300060519889438. doi: 10.1177/0300060519889438
26. Dou, Q, Zhu, Z, Zhu, L, Wang, W, Guo, L, Ru, S, et al. Academic-related factors and daily lifestyle habits associated with adolescent idiopathic scoliosis: a case-control study. Environ Health Prev Med. (2023) 28:23. doi: 10.1265/ehpm.22-00243
27. Yang, J, Huang, S, Cheng, M, Tan, W, and Yang, J. Postural habits and lifestyle factors associated with adolescent idiopathic scoliosis (AIS) in China: results from a big case-control study. J Orthop Surg Res. (2022) 17:472. doi: 10.1186/s13018-022-03366-0
28. Charman, WN. Myopia, posture and the visual environment. Ophthalmic Physiol Opt. (2011) 31:494–501. doi: 10.1111/j.1475-1313.2011.00825.x
29. Wu, JF, Bi, HS, Wang, SM, Hu, YY, Wu, H, Sun, W, et al. Refractive error, visual acuity and causes of vision loss in children in Shandong, China. The Shandong children eye study. PLoS One. (2013) 8:e82763. doi: 10.1371/journal.pone.0082763
30. McKean-Cowdin, R, Cotter, SA, Tarczy-Hornoch, K, Wen, G, Kim, J, Borchert, M, et al. Prevalence of amblyopia or strabismus in asian and non-Hispanic white preschool children: multi-ethnic pediatric eye disease study. Ophthalmology. (2013) 120:2117–24. doi: 10.1016/j.ophtha.2013.03.001
31. Tarczy-Hornoch, K, Cotter, SA, Borchert, M, McKean-Cowdin, R, Lin, J, Wen, G, et al. Prevalence and causes of visual impairment in Asian and non-Hispanic white preschool children: multi-ethnic pediatric eye disease study. Ophthalmology. (2013) 120:1220–6. doi: 10.1016/j.ophtha.2012.12.029
32. Mohammadi, E, Hashemi, H, Khabazkhoob, M, Emamian, MH, Shariati, M, and Fotouhi, A. The prevalence of anisometropia and its associated factors in an adult population from Shahroud, Iran. Clin Exp Optom. (2013) 96:455–9. doi: 10.1111/cxo.12045
33. Nunes, AF, Batista, M, and Monteiro, P. Prevalence of anisometropia in children and adolescents. F1000Res. (2021) 10:1101. doi: 10.12688/f1000research.73657.4
34. Vincent, SJ, Collins, MJ, Read, SA, and Carney, LG. Myopic anisometropia: ocular characteristics and aetiological considerations. Clin Exp Optom. (2014) 97:291–307. doi: 10.1111/cxo.12171
35. O'Donoghue, L, McClelland, JF, Logan, NS, Rudnicka, AR, Owen, CG, and Saunders, KJ. Profile of anisometropia and aniso-astigmatism in children: prevalence and association with age, ocular biometric measures, and refractive status. Invest Ophthalmol Vis Sci. (2013) 54:602–8. doi: 10.1167/iovs.12-11066
36. Deng, L, and Gwiazda, JE. Anisometropia in children from infancy to 15 years. Invest Ophthalmol Vis Sci. (2012) 53:3782–7. doi: 10.1167/iovs.11-8727
37. Xu, Z, Wu, Z, Wen, Y, Ding, M, Sun, W, Wang, Y, et al. Prevalence of anisometropia and associated factors in Shandong school-aged children. Front Public Health. (2022) 10:1072574. doi: 10.3389/fpubh.2022.1072574
38. Wang, X, Pan, J, Zhang, Y, Lan, Y, Zuo, J, and Jiang, Z. Prevalence and associations of myopic Anisometropia in Chinese adults. Eye Contact Lens. (2020) 46:147–53. doi: 10.1097/ICL.0000000000000627
39. Fong, DY, Lee, CF, Cheung, KM, Ng, BKW, Lam, TP, Mak, KH, et al. A meta-analysis of the clinical effectiveness of school scoliosis screening. Spine (Phila Pa 1976). (2010) 35:1061–71. doi: 10.1097/BRS.0b013e3181bcc835
40. Plaszewski, M, and Bettany-Saltikov, J. Are current scoliosis school screening recommendations evidence-based and up to date? A best evidence synthesis umbrella review. Eur Spine J. (2014) 23:2572–85. doi: 10.1007/s00586-014-3307-x
41. Hengwei, F, Zifang, H, Qifei, W, Weiqing, T, Nali, D, Ping, Y, et al. Prevalence of idiopathic scoliosis in Chinese schoolchildren: a large, population-based study. Spine (Phila Pa 1976). (2016) 41:259–64. doi: 10.1097/BRS.0000000000001197
42. Heemskerk, JL, Kruyt, MC, Colo, D, Castelein, RM, and Kempen, DHR. Prevalence and risk factors for neural axis anomalies in idiopathic scoliosis: a systematic review. Spine J. (2018) 18:1261–71. doi: 10.1016/j.spinee.2018.02.013
43. Moalej, S, Asadabadi, M, Hashemi, R, Khedmat, L, Tavacolizadeh, R, Vahabi, Z, et al. Screening of scoliosis in school children in Tehran: the prevalence rate of idiopathic scoliosis. J Back Musculoskelet Rehabil. (2018) 31:767–74. doi: 10.3233/BMR-171078
44. Penha, PJ, Ramos, N, de Carvalho, BKG, Andrade, RM, Schmitt, ACB, and João, SMA. Prevalence of adolescent idiopathic scoliosis in the state of São Paulo, Brazil. Spine (Phila Pa 1976). (2018) 43:1710–8. doi: 10.1097/BRS.0000000000002725
45. Yan, B, Lu, X, Nie, G, and Huang, Y. China urgently needs a nationwide scoliosis screening system. Acta Paediatr. (2020) 109:2416–7. doi: 10.1111/apa.15326
46. Kuznia, AL, Hernandez, A, and KLee, LU. Adolescent idiopathic scoliosis: common questions and answers. Am Fam Physician. (2020) 101:19–23. Available at: https://www.aafp.org/pubs/afp/issues/2020/0101/p19.html
47. Ceballos Laita, L, Tejedor Cubillo, C, Mingo Gomez, T, and Del Barrio, SJ. Effects of corrective, therapeutic exercise techniques on adolescent idiopathic scoliosis. a systematic review. Arch Argent Pediatr. (2018) 116:e582–9. doi: 10.5546/aap.2018.eng.e582
48. Parssinen, O, and Kauppinen, M. Associations of reading posture, gaze angle and reading distance with myopia and myopic progression. Acta Ophthalmol. (2016) 94:775–9. doi: 10.1111/aos.13148
49. Mutti, DO, Mitchell, GL, Moeschberger, ML, Jones, LA, and Zadnik, K. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. (2002) 43:3633–40. Available at: https://iovs.arvojournals.org/article.aspx?articleid=2162292
50. Saw, SM, Chua, WH, Hong, CY, Wu, HM, Chan, WY, Chia, KS, et al. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci. (2002) 43:332–9. Available at: https://iovs.arvojournals.org/article.aspx?articleid=2162578
51. Zhong, X, Ge, J, Nie, H, and Smith, EL III. Compensation for experimentally induced hyperopic anisometropia in adolescent monkeys. Invest Ophthalmol Vis Sci. (2004) 45:3373–9. doi: 10.1167/iovs.04-0226
52. Siegwart, JT, and Jr Norton, TT. Binocular lens treatment in tree shrews: effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. (2010) 91:660–9. doi: 10.1016/j.exer.2010.08.010
53. Tian, Y, Tarrant, J, and Wildsoet, CF. Optical and biometric characteristics of anisomyopia in human adults. Ophthalmic Physiol Opt. (2011) 31:540–9. doi: 10.1111/j.1475-1313.2011.00858.x
54. Zaka-Ur-Rab, S. Evaluation of relationship of ocular parameters and depth of anisometropic amblyopia with the degree of anisometropia. Indian J Ophthalmol. (2006) 54:99–103. doi: 10.4103/0301-4738.25830
55. Larsson, E, Holmstrom, G, and Rydberg, A. Ophthalmological findings in 10-year-old full-term children - a population-based study. Acta Ophthalmol. (2015) 93:192–8. doi: 10.1111/aos.12476
56. Hendricks, TJ, de Brabander, J, Vankan-Hendricks, MH, van der Horst, FG, Hendrikse, F, and Knottnerus, JA. Prevalence of habitual refractive errors and anisometropia among Dutch schoolchildren and hospital employees. Acta Ophthalmol. (2009) 87:538–43. doi: 10.1111/j.1755-3768.2008.01251.x
57. Quek, TP, Chua, CG, Chong, CS, Chong, JH, Hey, HW, Lee, J, et al. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic Physiol Opt. (2004) 24:47–55. doi: 10.1046/j.1475-1313.2003.00166.x
58. Bourne, RR, Dineen, BP, Ali, SM, Noorul Huq, DM, and Johnson, GJ. Prevalence of refractive error in Bangladeshi adults: results of the National Blindness and low vision survey of Bangladesh. Ophthalmology. (2004) 111:1150–60. doi: 10.1016/j.ophtha.2003.09.046
59. Wu, HM, Casson, RJ, Newland, HS, Muecke, J, Selva, D, and Aung, T. Anisometropia in an adult population in rural Myanmar: the Meiktila eye study. Ophthalmic Epidemiol. (2008) 15:162–6. doi: 10.1080/09286580701843796
60. Hashemi, H, Khabazkhoob, M, Yekta, A, Mohammad, K, and Fotouhi, A. Prevalence and risk factors for anisometropia in the Tehran eye study, Iran. Ophthalmic Epidemiol. (2011) 18:122–8. doi: 10.3109/09286586.2011.574333
61. Vincent, SJ, Collins, MJ, Read, SA, Carney, LG, and Yap, MKH. Interocular symmetry in myopic anisometropia. Optom Vis Sci. (2011) 88:1454–62. doi: 10.1097/OPX.0b013e318233ee5f
62. Linke, SJ, Baviera, J, Munzer, G, Steinberg, J, Richard, G, and Katz, T. Association between ocular dominance and spherical/astigmatic anisometropia, age, and sex: analysis of 10,264 myopic individuals. Invest Ophthalmol Vis Sci. (2011) 52:9166–73. doi: 10.1167/iovs.11-8131
63. Flitcroft, DI, He, M, Jonas, JB, Jong, M, Naidoo, K, Ohno-Matsui, K, et al. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. (2019) 60:M20–30. doi: 10.1167/iovs.18-25957
Keywords: Anisometropia, prevalence, school age children, risk factors, scoliosis
Citation: Zhou Y, Zhang XF, Chen XJ, Wang M, Cai JR, Xiong YJ, Song Y and Sun ZM (2023) Prevalence of anisometropia and influencing factors among school-age children in Nantong, China: a cross-sectional study. Front. Public Health. 11:1190285. doi: 10.3389/fpubh.2023.1190285
Received: 21 March 2023; Accepted: 25 May 2023;
Published: 15 June 2023.
Edited by:
Kevin Duffy, Dalhousie University, CanadaReviewed by:
Amelia Nunes, Universidade da Beira Interior, PortugalCopyright © 2023 Zhou, Zhang, Chen, Wang, Cai, Xiong, Song and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Song, U29uZ3l1ZXllQG50dS5lZHUuY24=; Zhi Min Sun, c3Vuem1leWVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.