
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 08 August 2023
Sec. Children and Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1182386
 Angelica E. Miranda1,2*
Angelica E. Miranda1,2* Patricia C. Santos2
Patricia C. Santos2 Ronaldo A. Coelho2
Ronaldo A. Coelho2 Ana Roberta P. Pascom2
Ana Roberta P. Pascom2 Leonor Henriette de Lannoy1,2
Leonor Henriette de Lannoy1,2 Ana Cristina Garcia Ferreira2
Ana Cristina Garcia Ferreira2 Pamela Cristina Gaspar2,3
Pamela Cristina Gaspar2,3 Ethel Leonor Maciel1,2
Ethel Leonor Maciel1,2 Draurio Barreira2
Draurio Barreira2 Gerson Fernando Mendes Pereira2
Gerson Fernando Mendes Pereira2Background: Eliminating mother-to-child transmission (MTCT) of HIV, hepatitis B, and syphilis is a challenge in Brazil. Many policies have been implemented since 1986, but important gaps remain. This study aimed to describe the trends of MTCT in Brazil and evaluate the gaps and perspectives in this scenario.
Methods: This is a descriptive study conducted with secondary data publicly available in the information systems of the Brazilian Ministry of Health regarding data on HIV, syphilis, and hepatitis B in pregnant women and children from 2011 to 2021.
Results: HIV and hepatitis B have had constant rates over the years in pregnant women, with the detection rates around 2.5/1,000 live birth (LB) and 0.5/1.000LB, respectively. The same did not happen with syphilis, which has shown an increasing line in the last decade. In 2011, the detection rate of syphilis in pregnancy was 4.7/1,000LB, and in 2021 it reached 27.1/1,000LB. Regarding the trends in children, an important decrease was observed in HIV/AIDS (incidence rate from 0.18/1,000 in 2011 to 0.04/1,000 in 2021) and Hepatitis B (incidence rate from 0.9/1,000LB in 2011 to 0.5/1,000LB in 2021). For congenital syphilis, there is a continuous increase, being 3.3/1,000LB in 2011 and 9.9/1,000LB in 2021. Data from the HIV clinical monitoring showed that antiretroviral treatment coverage among pregnant women identified increased slightly between 2011 and 2021, in Brazil, from 92.3% to 94.3%. For syphilis, 82.5% of pregnant women were treated with benzathine penicillin, and 88.7% in 2011. The historical series of hepatitis B vaccination coverage in children has decreased over the years; it was 96% in 2013 and 76% in 2021.
Conclusion: These data show many gaps and some perspectives in the MTCT program in Brazil. The country is close to reaching MTCT HIV elimination, but there are many challenges regarding HBV and syphilis. These data can be used to organize the strategies to improve the Brazilian response to MTCT elimination of HIV, hepatitis B, and syphilis.
The elimination of mother-to-child transmission (MTCT) of HIV, hepatitis B, and syphilis is an important challenge for the integrality of care, that is a result of the care offered to the user by multiple professionals, and expresses an indicator of the quality of services provided in the health care network (1). Prevention is possible with adequate diagnosis and treatment during prenatal care, childbirth, puerperium, or neonatal period (2). Achieving the goal of eliminating these conditions is a priority for the countries committed to the implementation of the 2030 Agenda to reach the Sustainable Development Goals (SDG), which was proposed by the United Nations (UN) for the elimination of important infections to public health by 2030 (3, 4).
Actions to prevent MTCT of HIV, syphilis, and viral hepatitis need to include a broad and comprehensive approach to women's health. Governments should think not only about the line of care during pregnancy but also about offering intervention opportunities throughout the life cycle of women (5, 6). Expanding access to diagnosis of these infections during prenatal care and adequate treatment to prevent MTCT should be priority measures in public health. Also, it is essential to be attentive to women's health beyond childbearing, which encompasses notions of empowerment, gender equality, and protection of human rights, including maternal health (2, 7).
Achieving the SDG goals is a priority for the Brazilian Ministry of Health (MoH) because MTCT of HIV, hepatitis B, and syphilis are public health problems in the country and are included in national policies (8, 9). Since 2017, Brazil has started to plan specific actions to approach the MTCT. A first national guide based on the WHO guidelines and focused on eliminating HIV was published in 2017. The document established indicators based on the WHO proposal and focused on a subnational elimination, so municipalities with 100,000 or more inhabitants could apply to be certified for the elimination of MTCT of HIV. In 2021, this document was updated to include syphilis and also criteria for tiers of good practices in health care to eliminate these infections. The tiers were proposed to recognize the achievements of countries as they progress along the path to elimination, and consider a set of criteria of good practice that include access to tests and treatment for the infections during antenatal care (10–12). The inclusion of hepatitis B MTCT elimination is planned for the 2023 process in Brazil.
Brazil presents inequalities and faces complex economic, social, and environmental transformations. Social, economic, and regional differences are still severe and extensive in the country; basic living conditions must be improved for a large part of the population; the Southeast and South regions are more populous and more developed, while the North and Northeast regions have a lower rate of access to health and a lower rate of urban development. Health problems are often a result of social and environmental variations and have remained persistent over the years (13). In recent years, the COVID-19 pandemic has put pressure on the public health system, evidenced its vulnerabilities, and contributed to the disruption of adequate antenatal and hospitalization care. The impact was heterogeneous across Brazil, depending on how local responses to COVID-19 and other health conditions (14).
Despite all the challenges, since 1989 the country has implemented many policies to control the MTCT of HIV, syphilis, and hepatitis B. The policies were focused on improving case surveillance, providing accurate tests to diagnose these infections, offering drugs and vaccines, and increasing access for the most vulnerable populations, among others (8). Box 1 summarizes the main Brazilian policies by year of implementation. Our goal in this study was to assess the trends of MTCT in Brazil in the last decade.
This is a descriptive study conducted with secondary data publicly available in the information systems of the Brazilian Ministry of Health regarding data on HIV, syphilis, and hepatitis B among pregnant women and children from 2011 to 2021.
The indicators analyzed were obtained from the data available in the panel of indicators of the Department of Sexually Transmitted Infections, Tuberculosis, HIV/AIDS and Viral Hepatitis at the Health Surveillance Secretary of the Ministry of Health of Brazil (DVIAHV/HHS/MoH). The panel of indicators generates data from the national notifiable diseases information system, as known as SINAN. The information of this system is originated from the Individual Notification Form (INF), which is completed electronically by primary care unit professionals for each patient when there is suspicion of a health problem that requires compulsory notification. The INF is forwarded to the services responsible for epidemiological surveillance in the Municipal Health Secretariats, which must weekly transfer the files to the State Health Secretariats. After review in the states, data are sent electronically to the Ministry of Health, which is responsible for consolidating and publishing national data.
The following indicators were analyzed: (1) detection rates of HIV in pregnancy and children identified in one of the HIV databases (treatment and CD4 or viral load exams) (http://indicadores.aids.gov.br); (2) antiretroviral (ART) coverage among pregnant women (http://indicadoresgestantes.aids.gov.br/); (3) syphilis in pregnancy and congenital syphilis (http://indicadoressifilis.aids.gov.br); (4) detection rates of hepatitis B in pregnancy and in children (http://indicadoreshepatites.aids.gov.br/); and (5) Hepatitis B vaccination within 30 days of birth and the pentavalent (Diphtheria, Pertussis, Tetanus, Hepatitis B and Hib) coverage (http://tabnet.datasus.gov.br/cgi/dhdat.exe?bd_pni/cpnibr.def).
The detection rates of HIV and hepatitis B in children were presented by year of birth; the incidence of congenital syphilis was presented by year of diagnosis. The detection rates of pregnant women with syphilis, HIV, and HBV and the incidence of congenital syphilis and the detection rate of HBV in children under 1 year and AIDS in children under 5 years of age, and HIV infection in children under 1 year of age were calculated over the historical series from 2011 to 2021. The vertical HIV transmission rate, which is the ratio between exposed and infected children, was calculated from the number of children infected with HIV per year of birth divided by the number of pregnant women notified according to the year of delivery, taking this information as a proxy for the total number of exposed children.
Data collected from the panel of indicators were included in a spreadsheet in the Statistical Package for the Social Sciences - SPSS 20.0 software, where they were verified to exclude duplication and prepared for analyzing the variables included in the study. These data were analyzed descriptively using the SPSS 20.0 software tools. Descriptive analysis was performed, including frequency distribution for qualitative variables, and appropriate graphics were developed.
As the analyzed data were publicly available, the study was dispensed by the evaluation by the ethics committee. Still, it was conducted in agreement with the National Ethics Committee #466 from December 12, 2012.
During the study period, 85,470 pregnant women were notified with HIV, 466.584 with syphilis, and 15,411 with HBV. Regarding children, 3,781 were notified with HIV, 221,600 with congenital syphilis, and 1,196 with HBV.
Figure 1 shows the rates of HIV, syphilis, and HBV notifications among pregnant women, in Brazil, from 2011 to 2021. It can be observed that trends of HIV and hepatitis B have remained constant over the years. The same did not happen with syphilis, which has shown an increasing line in the last decade. Regarding hepatitis B, available information indicated that 10.2% from all 150,761 notified cases occurred in pregnant women from 2011 to 2021.
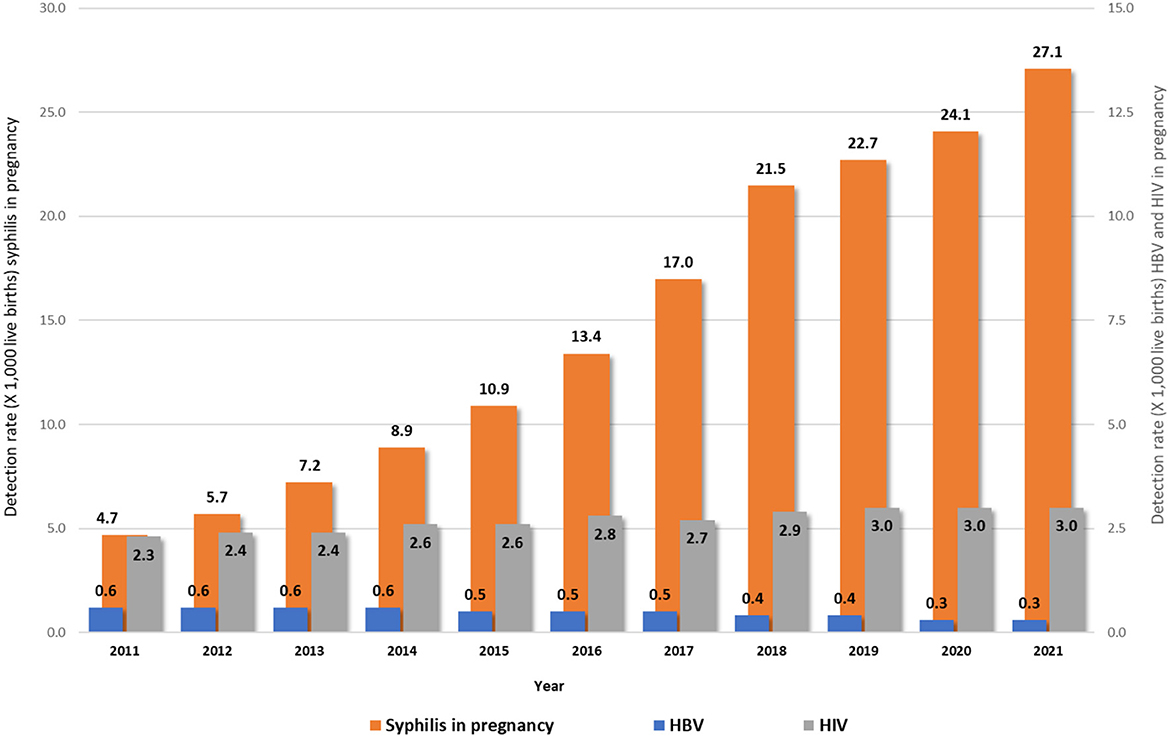
Figure 1. Rates of HIV1, syphilis1, and HBV2 notifications in pregnant women in Brazil, 2011–2021. Source: DVIAHV/HHS/MoH. (1) Cases reported on Sinan by 06/30/2022. (2) Cases reported on Sinan by 12/31/2021.
The rates of HIV, syphilis, and HBV notifications in children in Brazil from 2011 to 2021 are presented in Figure 2. Hepatitis rates in children remained low during the analyzed periods, and HIV infections have been falling over the years. However, syphilis rates have remained high in the last decade and kept increasing.

Figure 2. Rates of HIV/AIDS1, syphilis1, and HBV2 notifications in children, by year of birth. Brazil, 2011–2021. Source: DVIAHV/HHS/MoH. (1) Cases reported on Sinan by 06/30/2022. (2) Cases reported on Sinan by 12/31/2021.
Figure 3 shows that the antiretroviral treatment coverage among pregnant women identified increased slightly between 2011 and 2021, in Brazil, from 92% to 94%. It can be observed that the proportion is stable over the years.

Figure 3. Proportion of antiretroviral treatment coverage among pregnant women by year. Brazil, 2011–2021. Source: DVIAHV/HHS/MoH.
The historical series of the proportion of cases of pregnant women with syphilis according to the prescribed treatment regimen in Brazil from 2011 to 2021 are described in Figure 4. Almost 90% are adequality treated using Benzathine Penicillin, but there is still concern about the fact that 6.2% did not undergo treatment during pregnancy in 2021.

Figure 4. Distribution of cases proportion1 of pregnant women with syphilis according to the prescribed treatment regimen2 by year of diagnosis. Brazil, 2011 to 2021. Source: DVIAHV/HHS/MoH. (1) Cases reported on Sinan by 06/30/2022. (2) Prescribed treatment with at least one dose of benzathine penicillin, regardless of the clinical form.
The historical series of the proportion of hepatitis B and pentavalent vaccination coverage in children are described in Figure 5. The universal offer and notification of these data started in 2013 and 2014. A drop in the proportion of vaccinated children over the years can be observed, despite the availability of vaccines in the national immunization program.
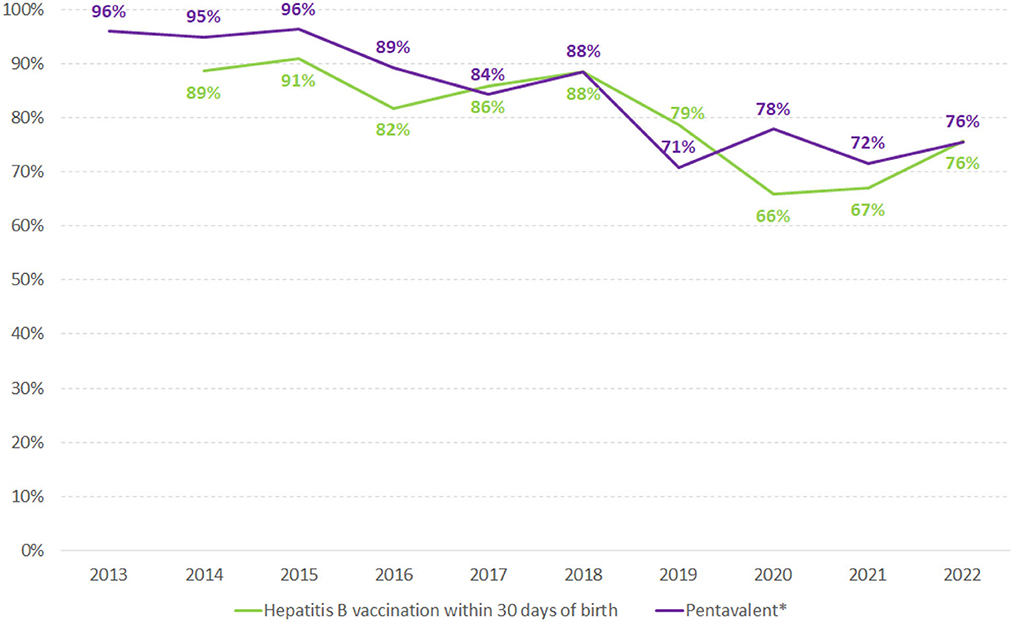
Figure 5. Proportion of hepatitis B vaccination within 30 days of birth and pentavalent* vaccination in children in Brazil, 2013–2021. Source: National Immunization Program/HSS/MoH. (*) Pentavalent vaccine includes Diphtheria, Pertussis, Tetanus, Hepatitis B, and Hib.
These data can help assess the aspects related to MTCT prevention of HIV, syphilis, and hepatitis B in Brazil, and will facilitate the strategic planning to enable the progress of the Brazilian response to MTCT elimination of these infections. The results point out that Brazil is approaching the WHO rate of HIV MTCT elimination as a public health problem and still needs to improve strategies for eliminating syphilis and viral hepatitis MTCT. As the strengths of these policies, Brazil has a universal public health system, the SUS, and a strong STI/AIDS program that includes free counseling, vaccines for HBV, diagnostic tests and treatment for HIV, hepatitis B, and syphilis, lactation inhibitors and infant milk formula, MTCT guidelines, and national health information systems (12, 15–17).
However, to achieve the SDG, health surveillance processes must be integrated with current strategies to strengthen Primary Health Care (PHC). A permanent effort is needed at the tripartite government level, involving federal, state, and municipal management for the success of the strategies, the incorporation of technologies, the permanent education of health workers, and the qualification of care and health surveillance actions. From a broad perspective, it also seeks to articulate health workers, teaching and research institutions, public and private health services, and civil society, mobilizing engagement in the implementation, monitoring, and dissemination of these actions (17–19).
The country has faced many challenges regarding the prevention of MTCT of HIV and syphilis and is now approaching a new one, which is to include HBV in the process (20–22). The identified weaknesses are the large geographic dimensions, demography, regional sociocultural diversity, overload of the public health system, the volatility of the technical team attending at the primary health care units, and clinical monitoring for the exposed and infected children (17, 23). These situations can be considered threats to the process. Also, it can be cited the completeness of the information data systems, such as inconsistencies, duplicities, and missing cases that happens because of the enormous amount of data and three management instances (municipal, state, and federal), data verification, which is a complex process (24, 25).
It is also worth mentioning that HBV notification is unique and occurs at the time of diagnosis of the infection. It is essential to point out that there is no specific form for notifying pregnant women with hepatitis B in Brazil, which causes difficulties in knowing the real number. It is known that from all notified cases, around 10% occurred in pregnant women from 2000 to 2021. Children exposed to hepatitis B are also not notified, as there is still no complete implementation of surveillance of vertical transmission of hepatitis B in the country (26). Notification data on HBV vaccination show a decrease in the country, mainly regarding the vaccination in the first 30 days of birth. Unpublish data reported that this data could be underestimated because the maternity hospitals give the vaccine but do not register it because of problematic communication with the national vaccination information system. A more detailed investigation will be important to elucidate this information.
Although, different opportunities can be identified: the Pan American Health Organization supports Member States to commit to the elimination of MTCT of HIV, syphilis, hepatitis B, and Chagas disease in the Region (10); the national visibility of the problem in Brazil, multisectoral approach, and availability of political interest on MTCT control (12, 20). Among the opportunities to succeed in this process, the SUS makes available the necessary inputs to prevent vertical transmissions free of charge. There are official documents that regulate the quality of care, develop the training of multidisciplinary teams in maternity hospitals and improve the quality of care for pregnant women, women in labor, and newborns, to reduce vertical transmission of HIV and control congenital syphilis (9). The proposal is to share responsibilities with states and municipalities and targets for the staggered and regionalized reduction of vertical transmission rates.
This process has many threats, such as the quality of prenatal care, which sometimes misses opportunities to test and treat the pregnant. Access to prenatal care needs to be improved to increase the inclusion of the most vulnerable people and reduce stigma and discrimination, complicating access. In addition, the training processes of the health professional team still need to be improved and often show regional differences in a country of continental dimensions (27, 28).
Among the limitations of this study, it can be mentioned the use of secondary data without independent validation, data coming from the health administration, and subject to information bias. Another limitation comes from the design of an ecological study, which does not allow direct interpretation of the results at the individual level. The descriptive approach is limited to univariate analyses, not adjusting for different risk factors and their interactions, nor for the spatial structure of data, which would be possible in a more complex analytical approach. However, these infections are of compulsory notification, and the information systems are the most comprehensive data sources available to carry out this evaluation. Therefore, the carried-out analyses allow for a distribution of possible underreporting of cases to be diluted in a less heterogeneous way. In addition, these diseases participate in national agreements, which involve the rendering of accounts and financing for states and municipalities. Good data coverage is expected, allowing extrapolation to the total population.
These data indicate that there is still a considerable distance to cover in order to eliminate MTCT in Brazil. Nevertheless, the collaborative efforts across various sectors represent a significant step forward in this regard. Moreover, since the implementation of the Sanitary Reform in Brazil, the country has made substantial progress toward achieving universal, equitable, and comprehensive access to healthcare services. There has also been a commitment to aligning with global recommendations to promote the surveillance, prevention, and control of MTCT since the 1990s. These initiatives offer invaluable tools for a collective national endeavor.
The certification of subnational elimination of MTCT of HIV and syphilis has emerged as a strategic approach recommended by the Ministry of Health to streamline the healthcare network. It has played a crucial role in enhancing preventive measures, diagnostics, care, and treatment for pregnant women and their infants, while also improving the quality of epidemiological surveillance. This continuous certification process has been developed in collaboration with state and municipal authorities. In addition to the certification itself, this process has strengthened the management and care network for maternal and child health in the Brazilian public health system and improved the quality of care.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
AM: conceptualization, formal analysis, investigation, methodology, supervision, validation, writing original draft, and final writing (review and editing). PS, RC, and AP: conceptualization, data curation, formal analysis, methodology, software analysis, validation, and final writing (review and editing). LL: conceptualization, data curation, formal analysis, methodology, validation, and final writing (review and editing). AF and PG: investigation, methodology, validation, writing original draft, and final writing (review and editing). EM and DB: investigation, methodology, validation, and final writing (review and editing). GP: investigation, methodology, validation, writing original draft, and final writing (review and editing). All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Paim JS, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. Lancet. (2011) 377:1778–97. doi: 10.1016/S0140-6736(11)60054-8
2. WHO. World Health Organization? Governance for the Validation of Elimination of mother-to-child Transmission of HIV, Syphilis and Hepatitis B virus: An Overview of Validation Structures and Responsibilities at National, Regional and Global Levels. Geneva: World Health Organization (2022).
3. WHO. World Health Organization. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022-2030. Geneva: World Health Organization (2022).
4. Taylor MM, Wi T, Gerbase A, Thwin SS, Gottlieb S, Babovic MT, et al. Assessment of country implementation of the WHO global health sector strategy on sexually transmitted infections (2016-2021). PLoS ONE. (2022) 17:e0263550. doi: 10.1371/journal.pone.0263550
5. Narasimhan M, Yeh PT, Haberlen S, Warren CE, Kennedy CE. Integration of HIV testing services into family planning services: a systematic review. Reprod Health. (2019) 16:61. doi: 10.1186/s12978-019-0714-9
6. Taylor MM, Wi TE. Transforming and integrating STI surveillance to enhance global advocacy and investment in STI control. J Int AIDS Soc. (2019) 52:e25361. doi: 10.1002/jia2.25361
7. Warren CE, Hopkins J, Narasimhan M, Collins L, Askew I, Mayhew SH. Health systems and the SDGs: lessons from a joint HIV and sexual and reproductive health and rights response. Health Policy Plan. (2017) 32:iv102–7. doi: 10.1093/heapol/czx052
8. Miranda AE, Freitas FLS, Passos MRL, Lopez MAA, Pereira GFM. Public policies on sexually transmitted infections in Brazil. Rev Soc Bras Med Trop. (2021) 54:e2020611. doi: 10.1590/0037-8682-611-2020
9. Brasil. Ministério da Saúde. Secretaria de Ciência, Tecnologia, Inovação e Insumos Estratégicos em Saúde. Secretaria de Vigilância em Saúde. Protocolo Clínico e Diretrizes Terapêuticas para Prevenção da Transmissão Vertical do HIV, Sífilis e Hepatites Virais [recurso eletrônico] / Ministério da Saúde, Secretaria de Ciência, Tecnologia, Inovação e Insumos Estratégicos em Saúde, Secretaria de Vigilância em Saúde, 2nd Edn. Brasília: Ministério da Saúde (2022), 224.
10. Pan American Health Organization. EMTCT. Plus. Framework for elimination of mother-to-child transmission of HIV, Syphilis, Hepatitis B, and Chagas. Washington, DC: PAHO. (2017).
11. World Health Organization. Global Guidance on Criteria and Processes for Validation: Elimination of mother-to-child Transmission of HIV, Syphilis and Hepatitis B Virus. Geneva: WHO (2021).
12. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Guia para Certificação da Eliminação da Transmissão Vertical de HIV e/ou Sífilis. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis, 2nd Edn. Brasília : Ministério da Saúde (2021), 48.
13. Victora CG, Barreto ML, do Carmo Leal M, Monteiro CA, Schmidt MI, Paim J, et al. Lancet Brazil Series Working Group. Health conditions and health-policy innovations in Brazil: the way forward. Lancet. (2011) 377:2042–53. doi: 10.1016/S0140-6736(11)60055-X
14. Rocha R, Atun R, Massuda A, Rache B, Spinola P, Nunes L, et al. Effect of socioeconomic inequalities and vulnerabilities on health-system preparedness and response to COVID-19 in Brazil: a comprehensive analysis. Lancet Glob Health. (2021) 9:e782–92. doi: 10.1016/S2214-109X(21)00081-4
15. Brasil. Ministério da Saúde. Portaria N° 1.984, de 12 de setemBro de 2014. Define a Lista Nacional de Doenças e Agravos de Notificação Compulsória, na Forma do Anexo, a serEm Monitorados Por Meio da Estratégia de Vigilância em Unidades Sentinelas e suas diretrizes. Diário Oficial da União, Brasília (DF). (2020). Available online at: http://www.acm.org.br/acm/acamt/documentos/emfoco/portaria-n-1984-12-09-2014.pdf (accessed May 31, 2020).
16. Massuda A, Hone T, Leles FAG, de Castro MC, Atun R. The Brazilian health system at crossroads: progress, crisis and resilience. BMJ Glob Health. (2018) 3:e000829. doi: 10.1136/bmjgh-2018-000829
17. Castro MC, Massuda A, Almeida G, Menezes-Filho NA, Andrade MV, de Souza Noronha KVM, et al. Brazil's unified health system: the first 30 years and prospects for the future. Lancet. (2019) 394:345–56. doi: 10.1016/S0140-6736(19)31243-7
18. Atun R, de Andrade LOM, Almeida G, Cotlear D, Dmytraczenko T, Frenz P, et al. Health-system reform and universal health coverage in Latin America. Lancet. (2015) 385:1230–47. doi: 10.1016/S0140-6736(14)61646-9
19. CONASS -DESAFIOS DO SUS. The Challenges Faced by the National Health Services. (2023). Available online at: https://www.conass.org.br/biblioteca/desafios-do-sus/ (accessed January 17, 2023).
20. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Pacto Nacional para a Eliminação da Transmissão Vertical de HIV, Sífilis, Hepatite B e Doença de Chagas como Problema de Saúde Pública / Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Brasília: Ministério da Saúde (2022), p. 29.
21. Domingues RMSM, Saraceni V, Leal MDC. Mother to child transmission of HIV in Brazil: data from the “birth in Brazil study”, a national hospital-based study. PLoS ONE. (2018) 13:e0192985. doi: 10.1371/journal.pone.0192985
22. Pinto R, Valentim R, Fernandes da, Silva L, Fontoura de, Souza G, Góis Farias, de Moura Santos Lima T. Use of interrupted time series analysis in understanding the course of the congenital syphilis epidemic in Brazil. Lancet Reg Health Am. (2021) 7:100163. doi: 10.1016/j.lana.2021.100163
23. Machado CV, Silva GAE. Political struggles for a universal health system in Brazil: successes and limits in the reduction of inequalities. Global Health. (2019) 15:77. doi: 10.1186/s12992-019-0523-5
24. European Centre for Disease Prevention and Control. Data Quality Monitoring and Surveillance System Evaluation – A Handbook of Methods and Applications. Stockholm: ECDC (2014).
25. Oliveira CM, Cruz MM. Sistema de Vigilância em Saúde no Brasil: avanços e desafios. Saúde Debate. (2015) 39:255–67. Available online at: https://www.scielo.br/j/sdeb/a/nYmJZ63cRJWnts4SDG7wN5C/?format=pdf&lang=pt
26. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Epidemiological Report - Viral Hepatitis. (2022). Available at: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2022/boletim-epidemiologico-de-hepatites-virais-2022-numero-especial (accessed May 16, 2022).
27. Ramos NA. Persistência da sífilis como desafio para a saúde pública no Brasil: o caminho é fortalecer o SUS, em defesa da democracia e da vida. Cad Saúde Pública. (2022) 38:22. doi: 10.1590/0102-311XPT069022
Keywords: MTCT, HIV, syphilis, hepatitis B, pregnancy
Citation: Miranda AE, Santos PC, Coelho RA, Pascom ARP, de Lannoy LH, Ferreira ACG, Gaspar PC, Maciel EL, Barreira D and Pereira GFM (2023) Perspectives and challenges for mother-to-child transmission of HIV, hepatitis B, and syphilis in Brazil. Front. Public Health 11:1182386. doi: 10.3389/fpubh.2023.1182386
Received: 08 March 2023; Accepted: 21 July 2023;
Published: 08 August 2023.
Edited by:
Carla S. Farinha, New University of Lisbon, PortugalReviewed by:
Jean-Paul Dossou, Institute of Tropical Medicine Antwerp, BelgiumCopyright © 2023 Miranda, Santos, Coelho, Pascom, de Lannoy, Ferreira, Gaspar, Maciel, Barreira and Pereira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelica E. Miranda, YW1pcmFuZGEudWZlc0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.