
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 09 May 2023
Sec. Aging and Public Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1159119
Background: In April 2009, the Chinese government launched Zero Markup Drug Policy (ZMDP) to adjust medical institutions' revenue and expenditure structures.
Objective: This study evaluated the impact of implementing ZMDP (as an intervention) on the drug costs for managing Parkinson's disease (PD) and its complications from the healthcare providers' perspective.
Methods: The drug costs for managing PD and its complications per outpatient visit or inpatient stay were estimated using electronic health data from a tertiary hospital in China from January 2016 to August 2018. An interrupted time series analysis was conducted to evaluate the immediate change following the intervention (step change, β1) and the change in slope, comparing post-intervention with the pre-intervention period (trend change, β2). Subgroup analyses were conducted in outpatients within the strata of age, patients with or without health insurance, and whether drugs were listed in the national Essential Medicine List (EML).
Results: Overall, 18,158 outpatient visits and 366 inpatient stays were included. Outpatient (β1 = −201.7, 95%CI: −285.4, −117.9) and inpatient (β1 = −372.1, 95% CI: −643.6, −100.6) drug costs for managing PD significantly decreased when implementing ZMDP. However, for outpatients without health insurance, the trend change in drug costs for managing PD (β2 = 16.8, 95% CI: 8.0, 25.6) or PD complications (β2 = 12.6, 95% CI: 5.5, 19.7) significantly increased. Trend changes in outpatient drug costs for managing PD differed when stratifying drugs listed in EML (β2 = −1.4, 95% CI: −2.6, −0.2) or not (β2 = 6.3, 95%CI: 2.0, 10.7). Trend changes of outpatient drug costs for managing PD complications significantly increased in drugs listed in EML (β2 = 14.7, 95% CI 9.2, 20.3), patients without health insurance (β2 = 12.6, 95% CI 5.5, 19.7), and age under 65 (β2 = 24.3, 95% CI 17.3, 31.4).
Conclusions: Drug costs for managing PD and its complications significantly decreased when implementing ZMDP. However, the trend in drug costs increased significantly in several subgroups, which may offset the decrease at the implementation.
To mitigate the economic incentives of prescriptions, the Chinese government launched the Zero Markup Drug Policy (ZMDP) in 2017 to ban the markup on drug procurement in Health Care Institutions (HCIs). Since the 1980s, Chinese Public HCIs have been allowed to charge over 15% of the drug price as a service fee. This revenue led to supplier-induced demand (SID) for increasing drug expenditure in public hospitals (1, 2). In light of this, the ZMDP were implemented in public tertiary hospitals in Beijing in April 2017 as one of the various measures to reform the revenue and expenditure structures in public hospitals, such as increasing fees for labor-intensive services and reducing fees for diagnostic tests, which significantly impacted the composition of total direct medical costs (3–5).
Notably, to promote the availability and affordability of drugs, the National Essential Medicine Policy (NEMP) was launched in 2009, which may also impact drug costs (6). The purpose of essential medicines is to ensure that low- and middle-income countries have access to medicines for priority medical problems (7, 8). Therefore, the National Essential Medicines List (NEML) is considered to be the basis for public procurement or reimbursement to provide all citizens with equal access to basic health care with reasonable quality and financial risk protection. In addition, the government keeps expanding social health insurance coverage, but many cross-region encounters still could not be reimbursed due to the separate administration and operation nationally and locally (9, 10).
Parkinson's disease (PD) is a progressive neurodegenerative disease with an incidence rate of 1.7% in the older population in China. It requires long-term medication to manage the symptoms (11). The drug cost of managing PD and its complications is a heavy economic burden on healthcare systems and patients (12–14). Non-persistent medication use may impair disease control and incur complications of PD (e.g., depression, insomnia, and dementia), leading to increased disease burden and overall healthcare expenditures (15–17). Previous studies on antibiotics, anticancer, chronic obstructive pulmonary disease and other non-communicable diseases have found that implementing ZMDP reduced the drug cost and its weight in the direct medical cost (18–23). However, little is known about the impact of the ZMDP on the drug cost for patients with PD. Our previous research on healthcare costs for patients with PD found that direct medical and drug costs decreased gradually from 2016 to 2018. Nevertheless, the influencing factors need further exploration (24).
Theoretically, drug costs in Chinese HCIs are expected to decrease immediately after the implementation of the ZMDP in April 2017. However, the ZMDP's impact on the cost of drugs for managing PD and its complications may vary by the price elasticity of demand between drugs (25). Moreover, the impact of ZMDP on drug cost composition in different patient groups has not been comprehensively evaluated. The long-term impact of ZMDP on changes in drug costs and medication-taking behaviors is also unclear. Consequently, whether the reduction in drug cost benefits patients with PD in the long term remains unknown. Therefore, we conduct a longitudinal study to investigate the ZMDP's impact on the drug costs of PD and its complication from a medical Center with a neurological speciality, where movement disorders specialists formulate and adjust drug regimens of PD.
In addition, aging and other policy-related factors may influence drug costs for patients with PD (26). Previous studies have found that patients' age, patients with or without health insurance (27, 28), and whether the drug is listed in the Essential Medicines List (EML) (29–31) influenced Chinese physicians' prescribing behaviors (20, 27–29). The impact of these factors and the implementation of ZMDP on the drug cost in patients with PD is also unknown, and the policy effect on different patient groups might be various. Therefore, we also conducted subgroup analyses to explore the impact of implementing ZMDP on drug costs of patients in different age groups, with or without health insurance and whether drugs are listed in the national EML.
A quasi-experimental design was adopted to evaluate the impact of the ZMDP on drug costs using the electronic medical record (EMR) from January 1st, 2016, to August 15th, 2018, at Peking University Third Hospital (PUTH), a tertiary general medical center and teaching institution in Beijing, China. The PUTH EMR database contains individual patients' outpatient visits and inpatient admission datasets covering the patient ID, number of visits, visiting date (outpatient), admission and discharge date (inpatient), gender, age, type of medical insurance, diagnosis and costs of patients, and the name, quantity and price of drugs at PUTH.
Patients were included if they had a PD diagnosis in EMR identified using the International Classification of Diseases, Tenth Revision, Clinical Modification code (ICD-10 code: G20) and relevant disease terms (e.g., Parkinson). Patient characteristics and prescriptions of the study cohort were extracted for analysis. This study was nested within a protocol approved by the Institutional Ethics Board of Peking University Third Hospital (IRB00006761-M2018228) in 2018.
In this study, the complications of PD included in our analysis were depression, insomnia, and dementia, which have the highest incidence (22). Each month, total drug costs for managing PD and its three complications were summed for outpatient and inpatient departments and divided by the number of outpatient visits, or inpatient stays to generate four series of the average drug cost. Costs were measured in the Chinese yuan (¥) after inflation adjustment using the medical care component of the Consumer Price Index (32, 33).
Due to the limited sample size of the inpatient stays, only the outpatient drug costs were stratified into subgroups to explore factors that might be associated with ZMDP's impact. The drug cost per outpatient visit was further stratified by patients' health insurance status (all sample drugs were in the National Reimbursement Drug List) and age (Age < 65, 65 ≤ age < 75 or age ≥ 75) for subgroup analysis. In the study, patients without health insurance mainly included self-pay patients and those whose health insurance did not cover health services in PUTH. Moreover, the drug cost for PD treatment per outpatient visit was further stratified into EML (levodopa/benserazide, amantadine and trihexyphenidyl) and non-EML drugs (selegiline, pramipexole, piribedil, carbidopa/levodopa and entacapone) according to the 2012 edition of the EML in China (34–36). Likewise, the drug cost for PD-related complications per outpatient visit was classified by drugs listed in EML (fluoxetine, paroxetine, mirtazapine, estazolam and zopiclone) and not listed in EML (sertraline, eszopiclone, zolpidem, donepezil, memantine and rivastigmine).
Interrupted time series (ITS) analysis was conducted to evaluate the effect of the ZMDP on the drug cost per outpatient visit or inpatient stay. The monthly time-series data from 2016 to 2018 were divided into the pre-intervention period (January 1st, 2016, to March 31th, 2017) and post-intervention (April 1st, 2017, to August 15th, 2018) periods by the implementation of ZMDP in April 2017, the following 3 months were tested for the possible policy lag effect. The interrupted time-series regression model is presented as the following equation. Step change (β1) and trend change (β2) were estimated and reported for each time series. The step change (β1) is an immediate change following the policy intervention. The trend change (β2) is a change in slope comparing the post-intervention with the pre-intervention period. The model was set up according to Schaffer et al. (37).
Dickey-Fuller and Durbin-Watson tests were used to test the stationarity and serial autocorrelation. If the non-stationarity or autocorrelation exists, then the autoregressive integrated moving average (ARIMA) model (38) was applied. Autoregressive (AR) refers to a model that uses the past values of a time series to predict its future values. Integrated (I) refers to the use of differencing to make the time series stationary, which means that its statistical properties, such as the mean and variance, remain constant over time. Moving Average (MA) is a model that uses past forecast errors to predict future values. ARIMA combines these three components (AR, I, and MA) to create a model that can handle non-stationary time series data. The parameters of an ARIMA model include the number of autoregressive terms (p), the degree of differencing (d), and the number of moving average terms (q).
As depicted in the following equation, in the ARIMA model, φ is the magnitude of the autocorrelation, θ is the value of the autocorrelation of the errors, p is the number of lags of the autoregressive model, q is the number of lags of the moving-average model, d is the degree of non-seasonal differencing, L is the lag operator, and εt is the error term (37, 39, 40).
The autocorrelation function (ACF) and partial autocorrelation function (PACF) were used to determine the appropriate time series model (41). The goodness-of-fit between different models was assessed by the Ljung-Box test (42), the Akaike (AIC) and Schwarz or Bayesian (BIC) information criteria (43, 44).
The coefficient and coefficient and 95% confidence interval of the step change (β1) and trend change (β2) derived from the ARIMA model were summarized in tables. Besides, results from the linear regression (data before and after intervention), the counterfactual trend (predicting results in the absence of the intervention), and the ARIMA model (controlling for autocorrelation, seasonality, or non-stationarity) were presented in graphs. Moreover, based on the step and trend change parameter estimates, the absolute changes were calculated by the difference between the predicted pre-intervention trend of the outcomes and the estimated trend at the end of the study period. The absolute change assessed the relative changes as a relative proportion.
Furthermore, seven subgroup analyses were conducted on outpatients, including drugs listed in the EML, drugs not listed in the EML, patients with health insurance, patients without health insurance, patients < 65 years old (age < 65), patients between 65 and 75 years old (65 ≤ age < 75), and patients no < 75 years old (age ≥ 75).
All statistical analyses were performed with the software Microsoft Office Excel 2019 (Microsoft Corp.) and Stata version 15 (Stata Corp. LP) (45).
In total, 18,158 outpatient visits and 366 inpatient stays were included in this study, with 2,640 outpatients and 330 inpatients. Outpatient visits and inpatient stays of medications for PD, and PD complications before and after the ZMDP were presented, respectively (Table 1). Due to the duplication between outpatient visits or inpatient stays with medications for PD and PD complications, there are differences between the summation results of numbers in Table 1 and the total number of outpatient visits or inpatient stays during the study period. There were 1,493 and 2,083 outpatients before and after the implementation of the ZMDP, respectively (Table 2). The drug costs for managing PD significantly decreased when the ZMDP was implemented (outpatients: β1 = −201.7; 95% CI: −285.4, −117.9; inpatients: β1 = −372.1; 95% CI: −643.6, −100.6). After implementing ZMDP, the drug costs for managing PD non-significantly changed (outpatient: β2 = −4.9; 95% CI: −14.6, 4.9, inpatient: β2 = 17.2; 95% CI: −10.3, 44.8) (Table 3; Figure 1; Supplementary Figure 1).
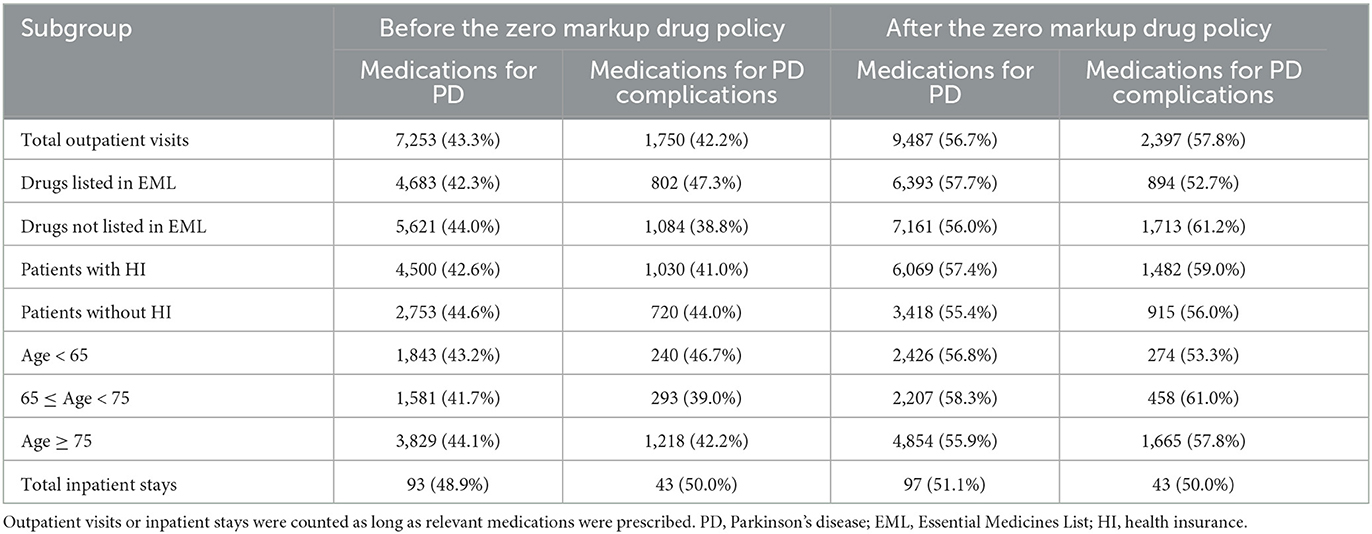
Table 1. Outpatient visits of subgroups and inpatient stays before and after the implementation of the Zero Markup Drug Policy.
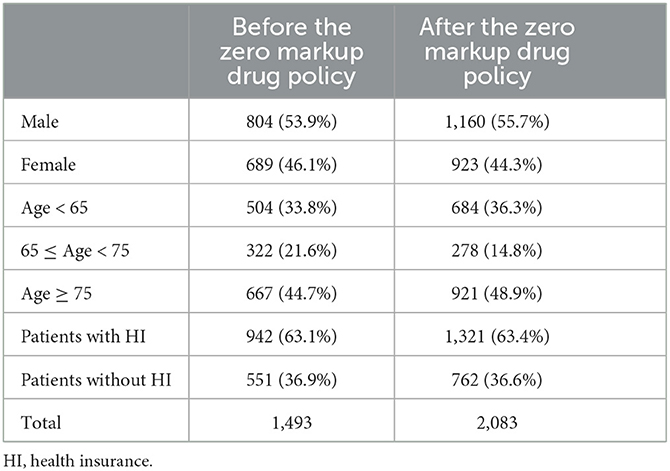
Table 2. Characteristics of outpatients before and after the implementation of the Zero Markup Drug Policy.
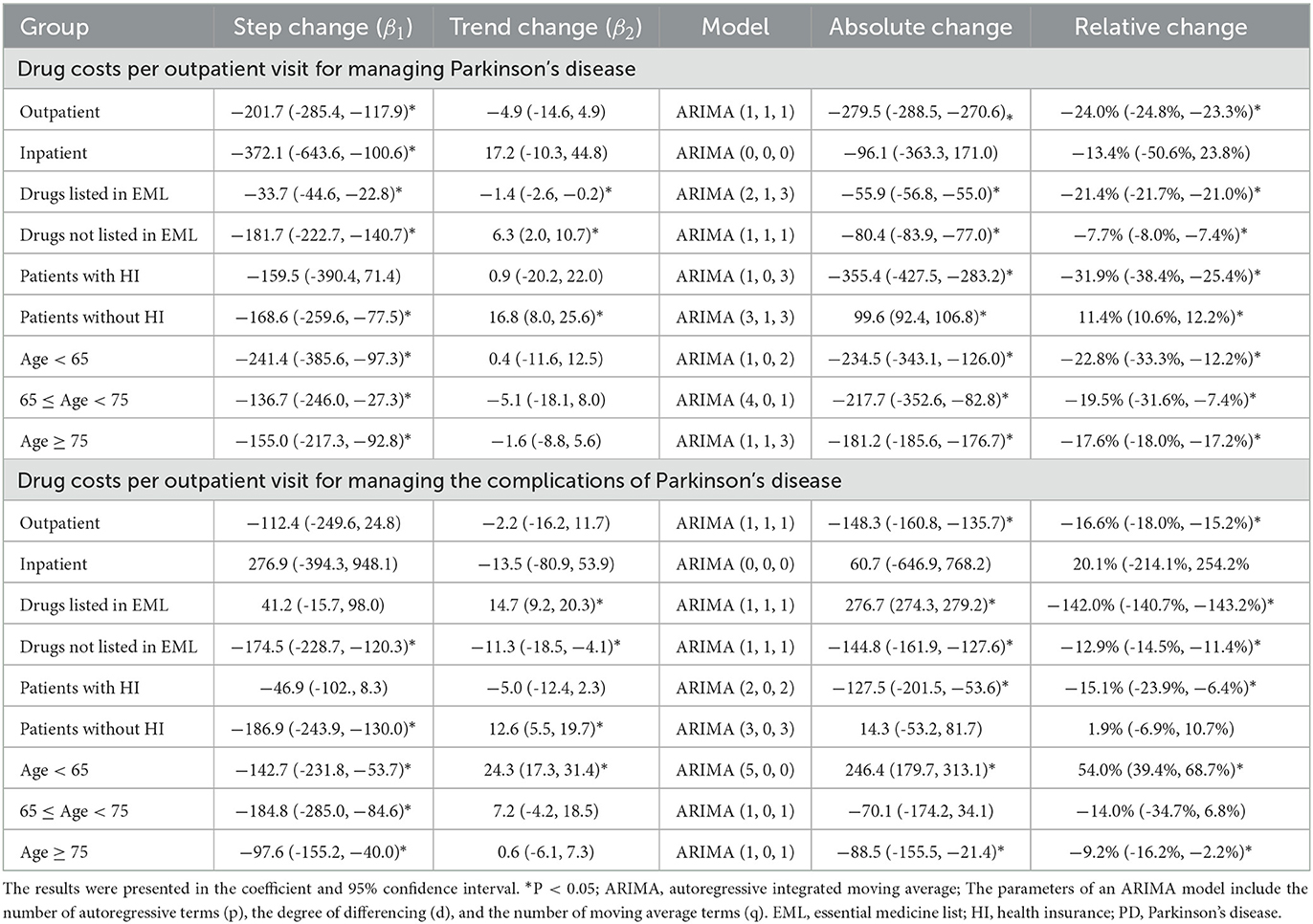
Table 3. The impact of the Zero markup drug policy on the drug costs per outpatient visit or inpatient stay.
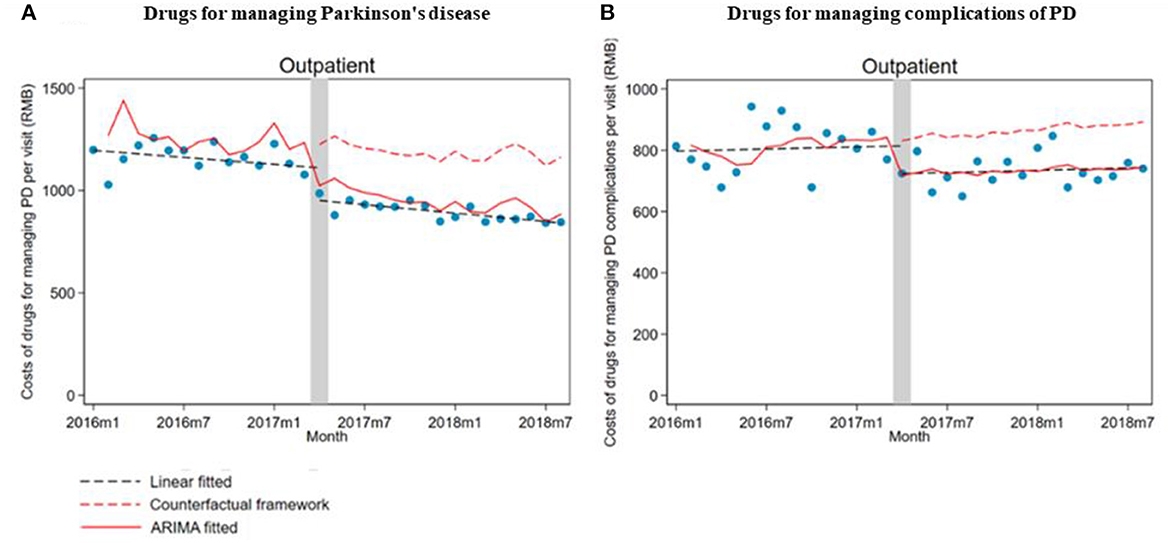
Figure 1. The impact of the Zero Mark-up Drug Policy on drug costs per outpatient visit for managing Parkinson's disease and its complications. (A) Drug costs for managing Parkinson's disease. (B) Drug costs for managing complications of Parkinson's disease.
When stratifying by drug subgroups, the monthly drug cost per outpatient visit significantly decreased at implementing the ZMDP regardless of whether drugs are listed in EML (listed, β1 = −33.7; 95% CI: −44.6, −22.8; not listed, β1 = −181.7; 95% CI: −222.7, −140.7). Moreover, for drugs listed in EML for managing PD, the monthly trend significantly decreased (β2 = −1.4; 95% CI: −2.6, −0.2) comparing post-intervention with the pre-intervention period. However, the trend of drug costs significantly increased in post-intervention compared with the pre-intervention period for drugs listed in EML for managing PD complications (β2 = 14.7; 95% CI: 9.2, 20.3) and not listed in EML for managing PD (β2 = 6.3; 95% CI: 2.0, 10.7) (Table 3; Figure 2).
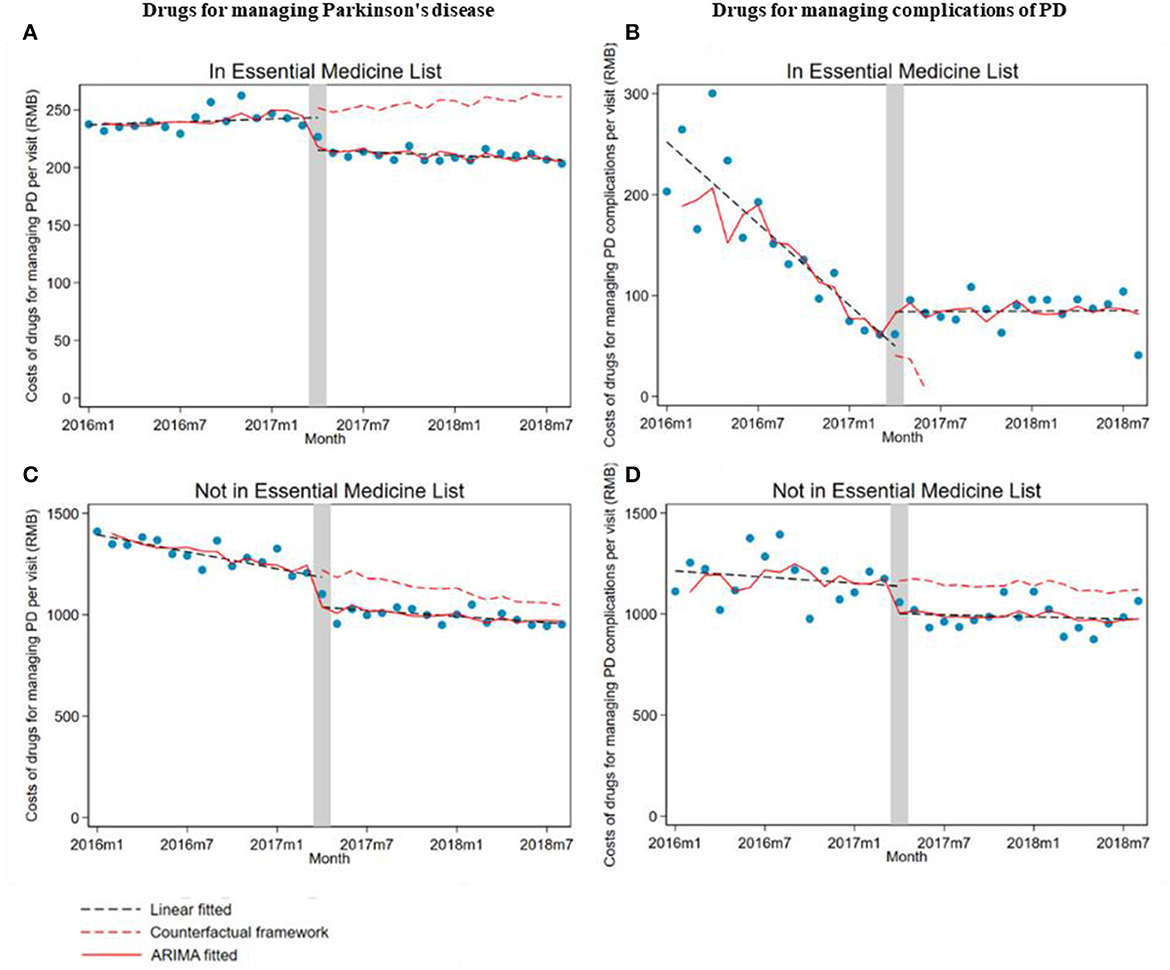
Figure 2. The impact of the Zero Mark-up Drug Policy on drug costs per outpatient visit of drugs, whether listed in EML for managing Parkinson's disease and its complications. (A) Drug listed in EML for managing Parkinson's disease. (B) Drug listed in EML for managing complications of Parkinson's disease. (C) Drugs not listed in EML for managing Parkinson's disease. (D) Drugs not listed in EML for managing complications of Parkinson's disease. EML, essential medicine list.
Monthly drug costs per visit for managing PD significantly (β1 = −168.6; 95% CI: −259.6, −77.5) and PD complications (β1 = −186.9; 95% CI: −243.9, −130) decreased in outpatients without health insurance. However, the trend changes in drug costs for managing PD (β2 = 16.8; 95% CI: 8.0, 25.6) and PD complications (β2 = 12.6; 95% CI: 5.5 to 19.7) increased in outpatients without health insurance compared with the pre-intervention period (Table 3; Figure 3).
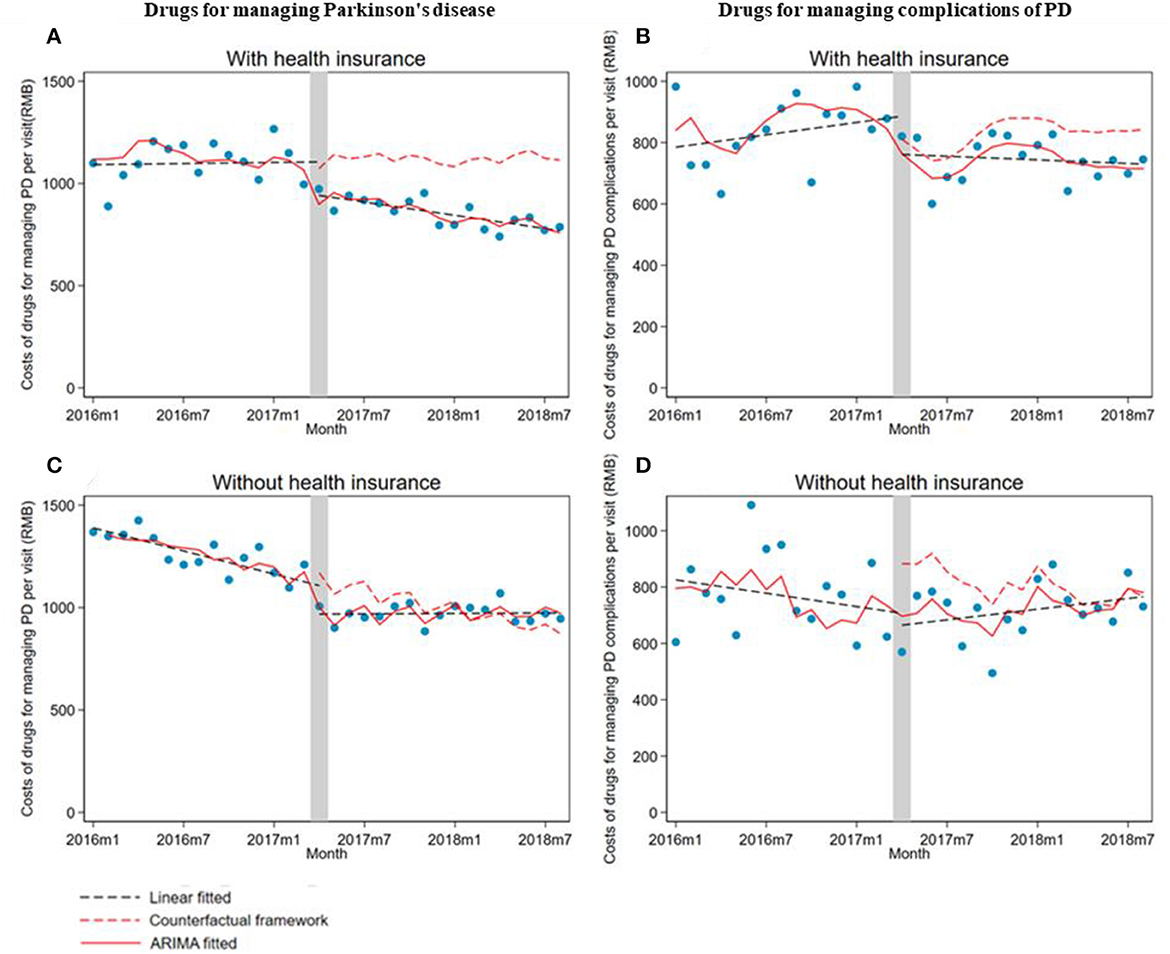
Figure 3. The impact of the Zero Mark-up Drug Policy on drug costs per outpatient visit of patients with or without health insurance for managing Parkinson's disease and its complications. (A) Drugs for managing Parkinson's disease in patients with health insurance. (B) Drugs for managing complications of Parkinson's disease in patients with health insurance. (C) Drugs for managing Parkinson's disease in patients without health insurance. (D) Drugs for managing complications of Parkinson's disease in patients without health insurance. HI, health insurance.
There was a significant decrease in drug costs (β1) for all age groups of outpatients when the ZMDP was implemented. Moreover, compared with the pre-intervention period, a significantly increasing trend change in drug costs for managing PD complications in outpatients under 65 years old was observed (β2 = 24.3; 95% CI: 17.3, 31.4) (Table 3; Figure 4).
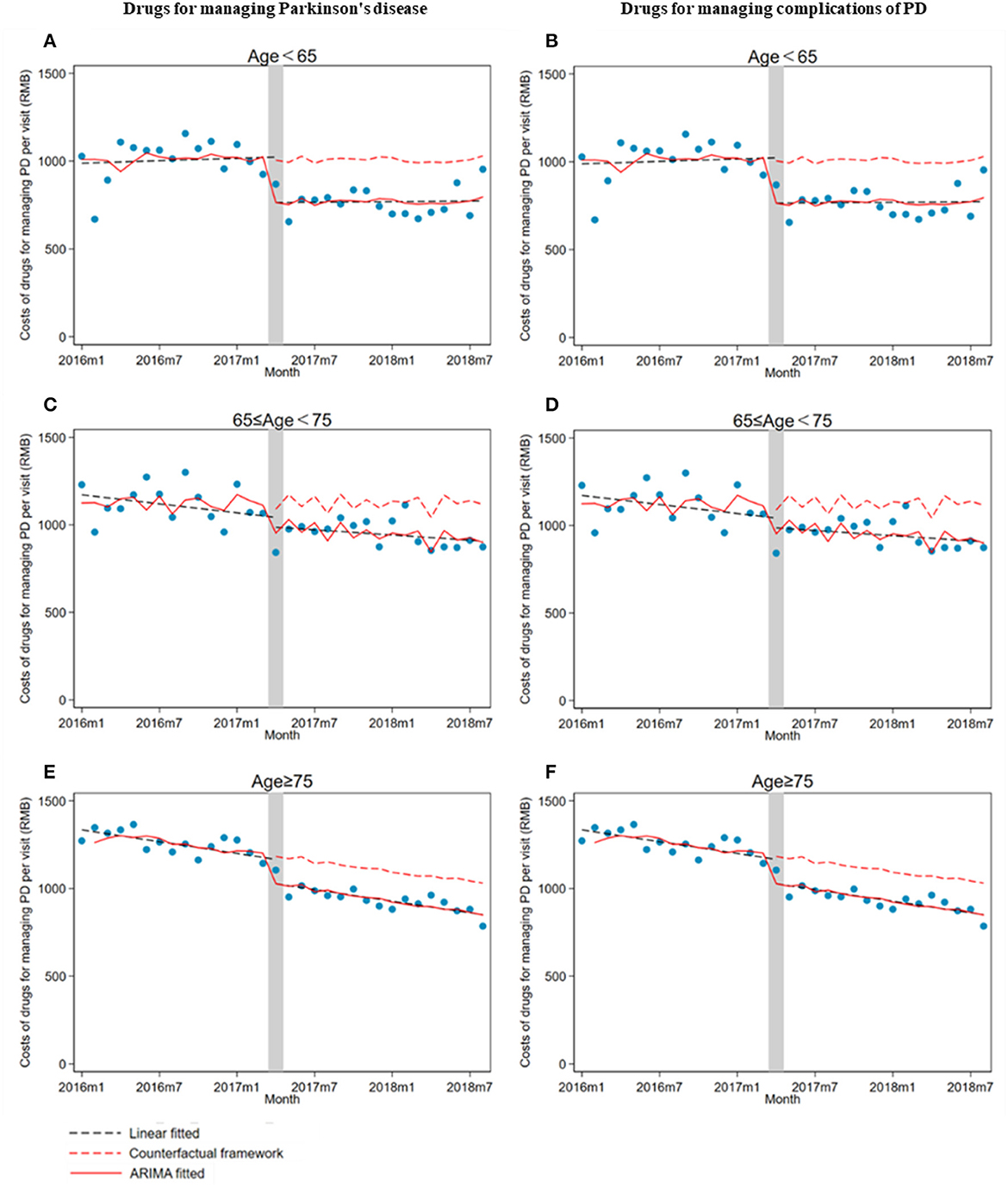
Figure 4. The impact of the Zero Mark-up Drug Policy on drug costs per outpatient visit for managing Parkinson's disease and its complications by age groups. (A) Drugs for managing Parkinson's disease in patients less than 65 years old. (B) Drugs for managing complications of Parkinson's disease in patients less than 65 years old. (C) Drugs for managing Parkinson's disease in patients greater than or equal to 65 years old and less than 75 years. (D) Drugs for managing complications of Parkinson's disease in patients greater than or equal to 65 years old and less than 75 years old. (E) Drugs for managing Parkinson's disease in patients greater than or equal to 75 years old. (F) Drugs for managing complications of Parkinson's disease in patients greater than or equal to 75 years old.
This study found that drug costs for managing PD significantly decreased with implementing the ZMDP. The impact of the ZMDP on the cost of drugs for managing PD or PD complications is in line with other studies, which demonstrated the universal and significant effects of policy on reducing the step and trend changes after policy implementation (20, 21). For example, Wang et al. (23) reported the implementation of the ZMDP was associated with a steep decline in drug costs for outpatients in 24 tertiary hospitals in Shanghai. However, in our study, compared with the pre-intervention period, the trend changes significantly increased in several subgroups when stratifying the cost of drugs for managing PD (drugs not listed in EML and patients without health insurance) or PD complications (drugs listed in EML, patients without health insurance and under 65 years old). The increase in trend change may likely offset the decrease in step-change compared with the pre-intervention period (46).
For those listed in EML, the trend change of drug costs per visit for managing PD significantly decreased after the ZMDP implementation. In contrast, opposite trend changes were shown for those not listed in EML, which may reflect the impact of China's national essential medicine list enabling patients to access appropriate, available, affordable, and quality essential medicines, especially in PD management. The World Health Organization Essential Medicines List (WHO-EML) was established to strengthen access to drugs of utmost importance, fundamental, indispensable and necessary for the health and needs of the population (47). In 2009, the Chinese government released the National Essential Medicines List, updated in 2012 and 2018. Besides, measures were implemented to strengthen the accessibility of these drugs, such as monitoring drug utilization, establishing the drug shortage list, and taking corresponding measures to ensure the supply of medicines (34–36). All these measures promote the use of essential medicines considered well-affordable and high-quality, which might contribute to the decrease in trend change compared with the pre-intervention period (48, 49).
Drug costs for PD and its complications in patients without health insurance significantly decreased in the step while increasing significantly in the trend compared with the pre-intervention period. This may be related to the change in physicians' prescribing behaviors to a certain extent (50–52). Since the drug cost decreased after the policy implementation, physicians might gradually prescribe more medication for managing PD and its complications because those medicines became more affordable after the price decreased. Once the decrease in drug costs is offset by the long-term upward trend for those without health insurance, the financial burden might be even heavier compared to the pre-international period (53). Therefore, policymakers should continue to expand health insurance coverage for patients and break through the regional barriers to health insurance coverage. Besides, for those subgroups with an increasing trend of outpatient drug costs, other policies are still needed to reduce the financial burden on PD patients, such as promoting the centralized purchasing policy and using generics (27).
It was found that while the cost of drugs for managing PD decreased, there was a long-term upward trend in the cost of drugs for managing PD among patients under 65 years old compared with the pre-intervention period. Consequently, the step decrease might be offset by the long-term upward trend, resulting in an increased financial burden to younger patients with PD. Patients with early-onset PD, usually under 65, have fewer complications than later-onset PD (54). Also, patients in different age groups vary in treatment preferences and medication compliance (55, 56). It is still unclear whether the increased drug costs for managing PD complications were due to the nature of disease progression or the irrational over-prescription motived by the reduced unit cost of drugs after the ZMDP. Further research is needed to evaluate the optimal medicine use associated with healthcare policies.
Furthermore, physicians' prescribing behaviors could be further optimized by actively promoting pharmacist services (57). After the implementation of ZMDP, the prices of some drugs, such as pramipexole, entacapone, and selegiline, decreased significantly, which might lead to more prescriptions for these drugs. In this process, clinical pharmacists specializing in neurology provided suggestions for drug therapy regimens, which reduced potential events of irrational drug use to some extent. Moreover, to promote the rational use of drugs, it is recommended to educate physicians on the value of relevant policies (28).
To the best of our knowledge, the study is the first to evaluate the effect of the ZMDP on drug costs for managing PD and its complications. We used the ARIMA model, a more flexible and powerful tool for modeling time-series data than simple linear regression, especially when the data exhibit autocorrelation, seasonality, or non-stationarity. The graphical presentation comparing results from linear regression, counterfactual frameworks and the ARIMA model gave a better insight into the policy's impact. The findings indicated the policy's effects varied in drugs for therapeutic and complication management and patients in different subgroups of patient age and health insurance. The subgroup analysis is valuable for investigating whether the policy disadvantaged vulnerable subgroups. Moreover, these insights can help policy decision-makers to adjust the policy further to ensure equitable access to health services. Besides, this study was conducted at a representative tertiary medical center in Beijing with a well-reputed neurological department. In this setting, neurologists' prescribing practices largely follow the clinical guideline. Therefore, this study's results represent the standard practice in medical centers with neurological specialities in China.
There are several worthy noting limitations to this study. Firstly, the single-center retrospective data may not fully represent the policy implementation's impact. The tertiary hospital could not represent all HCIs, especially the primary healthcare facilities. Therefore, further study on other medical settings is needed. Secondly, the retrospective data collected from EMR may introduce selection bias.
Nevertheless, in this study, patients were included according to the clinical diagnosis rather than prescribed drugs, thus ensuring drugs were likely prescribed for the intended purposes of interest to this study. Thirdly, changes in direct medical costs besides drug costs were not assessed due to the limited access to information. Although the final month (August 2018) did not include a whole month of data, we used the cost per visit as our outcome measure. Hence the impact is considered to be minimal. Finally, the impact of policy on appropriate drug use has not been thoroughly investigated. Further research is needed to investigate the optimal use of medicines by applying individual patient-level healthcare data.
We found that the ZMDP would reduce the financial burden on PD patients quickly to some extent. Still, in several subgroups, the significantly increasing trend in drug costs compared with the pre-intervention period might offset the decrease in drug costs at the implementing time. Drug prices may induce additional care needs for patients with chronic diseases, impacting the composition of patients' medical costs. Healthcare providers and policymakers must focus on the heterogeneity of policy's impacts and ensure care equity for different patient groups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Institutional Ethics Board of Peking University Third Hospital (IRB00006761-M2018228) in 2018.
Z-MY and L-CC conceptualized the study and revised for the finalization of the manuscript. Z-MY, L-CC, RW, and XL designed the study. Z-MY acquired the funding and collected and assembled the data. RW, XL, XG, QC, and YW conducted data analysis and interpretation. RW and XL drafted the manuscript. All authors approved the final manuscript.
This study was funded by the China National Natural Science Foundation (72104003).
We thank Dr. Andrea Schaffer from the University of Oxford for clarifications on the ARIMA model. This manuscript was invited to be presented as an ePoster at the 14th Asian Conference on Pharmacoepidemiology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1159119/full#supplementary-material
1. Shi X, Zhu D, Man X, Wang W, Zhu K, Nicholas S, et al. “The biggest reform to China's health system”: did the zero-markup drug policy achieve its goal at traditional Chinese medicines county hospitals? Health Policy Plan. (2019) 34:483–91. doi: 10.1093/heapol/czz053
2. Fu H, Li L, Yip W. Intended and unintended impacts of price changes for drugs and medical services: evidence from China. Soc Sci Med. (2018) 211:114–22. doi: 10.1016/j.socscimed.2018.06.007
3. Cheng H, Zhang Y, Sun J, Liu Y. Impact of zero-mark-up medicines policy on hospital revenue structure: a panel data analysis of 136 public tertiary hospitals in China, 2012-2020. BMJ Glob Health. (2021) 6:e007089. doi: 10.1136/bmjgh-2021-007089
4. Xiong W, Deng Y, Yang Y, Zhang Y, Pan J. Assessment of medical service pricing in China's healthcare system: challenges, constraints, and policy recommendations. Front Public Health. (2021) 9:787865. doi: 10.3389/fpubh.2021.787865
5. Yip W, Fu H, Chen AT, Zhai T, Jian W, Xu R, et al. 10 years of healthcare reform in China: progress and gaps in universal health coverage. Lancet. (2019) 394:1192–204. doi: 10.1016/S0140-6736(19)32136-1
6. Ding L, Wu J. The impact of China's national essential medicine policy and its implications for urban outpatients: a multivariate difference-in-differences study. Value Health. (2017) 20:412–9. doi: 10.1016/j.jval.2016.10.018
7. Bazargani YT, de Boer A, Schellens JH, Leufkens HG, Mantel-Teeuwisse AK. Selection of oncology medicines in low- and middle-income countries. Ann Oncol. (2014) 25:270–6. doi: 10.1093/annonc/mdt514
8. Hwang TJ, Kesselheim AS, Vokinger KN. Reforming the world health organization's essential medicines list: essential but unaffordable. JAMA. (2022) 328:1807–8. doi: 10.1001/jama.2022.19459
9. Meng Q, Fang H, Liu X, Yuan B, Xu J. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet. (2015) 386:1484–92. doi: 10.1016/S0140-6736(15)00342-6
10. Dong W, Zwi AB, Bai R, Shen C, Gao J. Benefit of China's social health insurance schemes: trend analysis and associated factors since health reform. Int J Environ Res Public Health. (2021) 18:5672. doi: 10.3390/ijerph18115672
11. Parkinson's Disease and Movement Disorders Group Neurology Neurology Branch of Chinese Medical Association Parkinson's Disease and Movement Disorders Group Neurologist Neurologist Branch of Chinese Medical Doctor Association. Guidelines for the treatment of Parkinson's Disease in China (Fourth Edition). Chin J Neurol. (2020) 53:973–86. doi: 10.3760/cma.j.cn113694-20200331-00233
12. Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, et al. Current and projected future economic burden of Parkinson's disease in the US. NPJ Parkinsons Dis. (2020) 6:15. doi: 10.1038/s41531-020-0117-1
13. Li X, Jiang D, Chen J, Wang Y, Guo W, Zhao K. Estimation on direct medical cost of patients with Parkinson's disease in China from the perspective of suppliers. Health Econ Res. (2021) 38:26–8. doi: 10.14055/j.cnki.33-1056/f.2021.01.027
14. Dahodwala N, Li P, Jahnke J, Ladage VP, Pettit AR, Kandukuri PL, et al. Burden of Parkinson's disease by severity: health care costs in the U. S. medicare population. Mov Disord. (2021) 36:133–42. doi: 10.1002/mds.28265
15. Wei YJ, Palumbo FB, Simoni-Wastila L, Shulman LM, Stuart B, Beardsley R, et al. Antiparkinson drug adherence and its association with health care utilization and economic outcomes in a medicare part D population. Value Health. (2014) 17:196–204. doi: 10.1016/j.jval.2013.12.003
16. Malek N, Grosset DG. Medication adherence in patients with Parkinson's disease. CNS Drugs. (2015) 29:47–53. doi: 10.1007/s40263-014-0220-0
17. Aggarwal S, Paul G, Paul BS, Mahendru D, Goyal S. Factors affecting adherence to pharmacotherapy in Parkinson's disease. Ann Indian Acad Neurol. (2021) 24:879–84. doi: 10.4103/aian.AIAN_143_21
18. Chen C, Feng Z, Ding Y, Yan Z, Wang J, Wang R, et al. What factors hindered the access to essential anticancer medicine in public hospitals for the local population in Hubei province, China. Front Pharmacol. (2021) 12:734637. doi: 10.3389/fphar.2021.734637
19. Yin J, Wu C, Wei X, Sun Q. Antibiotic expenditure by public healthcare institutions in Shandong province in China, 2012-2016. Front Pharmacol. (2018) 9:1396. doi: 10.3389/fphar.2018.01396
20. Du J, Xie J, Qian Y, Wu M, Huang W, Yin J, et al. Impact of China's zero mark-up drug policy on drug cost of NCDs' outpatients: an interrupted time series analysis. BMC Health Serv Res. (2021) 21:404. doi: 10.1186/s12913-021-06414-3
21. Wang J, Li P, Wen J. Impacts of the zero mark-up drug policy on hospitalization expenses of COPD inpatients in Sichuan province, western China: an interrupted time series analysis. BMC Health Serv Res. (2020) 20:519. doi: 10.1186/s12913-020-05378-0
22. Cheng W, Fang Y, Fan D, Sun J, Shi X, Li J. The effect of implementing “medicines zero mark-up policy” in Beijing community health facilities. South Med Rev. (2012) 5:53–6.
23. Wang X, Li F, Wang X, Zhang X, Liu C, Wang D, et al. Effects of different mark-up drug policies on drug-related expenditures in tertiary public hospitals: an interrupted time series study in Shanghai, China, 2015-2018. Biosci Trends. (2020) 14:16–22. doi: 10.5582/bst.2019.01350
24. Yi Z, Li X, Wang Y, Wang R, Ma Q, Zhao R, et al. Evaluating the direct medical cost, drug utilization and expenditure for managing Parkinson's disease: a costing study at a medical center in China. Ann Transl Med. (2022) 10:330. doi: 10.21037/atm-22-1014
25. Zhao M, Nie P, Wu J. Heterogeneity in price elasticity of medicine demand in China: moderate effect from economic incentive and quality difference. Front Pharmacol. (2021) 12:688069. doi: 10.3389/fphar.2021.688069
26. Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet. (2021) 397:2284–303. doi: 10.1016/S0140-6736(21)00218-X
27. Hu J, Mossialos E. Pharmaceutical pricing and reimbursement in China: when the whole is less than the sum of its parts. Health Policy. (2016) 120:519–34. doi: 10.1016/j.healthpol.2016.03.014
28. Zhou X, Zhang X, Yang L, Hu X, Shen A, Huang X, et al. Influencing factors of physicians' prescription behavior in selecting essential medicines: a cross-sectional survey in Chinese county hospitals. BMC Health Serv Res. (2019) 19:980. doi: 10.1186/s12913-019-4831-5
29. Vassal G, Kozhaeva O, Griskjane S, Arnold F, Nysom K, Basset L, et al. Access to essential anticancer medicines for children and adolescents in Europe. Ann Oncol. (2021) 32:560–8. doi: 10.1016/j.annonc.2020.12.015
30. Chen W, Tang S, Sun J, Ross-Degnan D, Wagner AK. Availability and use of essential medicines in China: manufacturing, supply, and prescribing in Shandong and Gansu provinces. BMC Health Serv Res. (2010) 10:211. doi: 10.1186/1472-6963-10-211
31. Shi Y, Yang P, Li X, Lv J, Yin T, Gong Z, et al. Combating drug shortages in China: surveillance warning and practice standardization. Int J Clin Pharm. (2020) 42:309–14. doi: 10.1007/s11096-020-00987-5
32. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. (2016) 316:858–71. doi: 10.1001/jama.2016.11237
33. Acuña AJ, Jella TK, Samuel LT, Schwarzkopf R, Fehring TK, Kamath AF. Inflation-adjusted medicare reimbursement for revision hip arthroplasty: study showing significant decrease from 2002 to 2019. J Bone Joint Surg Am. (2021) 103:1212–9. doi: 10.2106/JBJS.20.01643
34. Zuo W, Mei D, Sun W, Tang X, Niu Z, Gao D, et al. The interpretation of China national essential medicines list 2018. Expert Rev Clin Pharmacol. (2020) 13:191–200. doi: 10.1080/17512433.2020.1713749
35. Liu D, Cheng J, Zhang LL Li YP, Zeng LN, Zhang C, et al. Essential medicines lists for children of WHO, India, South Africa, and EML of China: a comparative study. J Evid Based Med. (2017) 10:271–80. doi: 10.1111/jebm.12240
36. The Ministry of Health released the 2012 version of the National Essential Medicines List. In: China MoHotPsRo, editor (2013).
37. Schaffer AL, Dobbins TA, Pearson SA. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med Res Methodol. (2021) 21:58. doi: 10.1186/s12874-021-01235-8
38. Feng L, Tian Y, He M, Tang J, Peng Y, Dong C, et al. Impact of DRGs-based inpatient service management on the performance of regional inpatient services in Shanghai, China: an interrupted time series study, 2013-2019. BMC Health Serv Res. (2020) 20:942. doi: 10.1186/s12913-020-05790-6
39. Gondwe T, Yang Y, Yosefe S, Kasanga M, Mulula G, Luwemba MP, et al. Epidemiological Trends of malaria in five years and under children of Nsanje district in Malawi, 2015-2019. Int J Environ Res Public Health. (2021) 18:12784. doi: 10.3390/ijerph182312784
40. Campitelli MA, Bronskill SE, Maclagan LC, Harris DA, Cotton CA, Tadrous M, et al. Comparison of medication prescribing before and after the COVID-19 pandemic among nursing home residents in Ontario, Canada. JAMA Netw Open. (2021) 4:e2118441. doi: 10.1001/jamanetworkopen.2021.18441
41. Ben S, Xin J, Chen S, Jiang Y, Yuan Q, Su L, et al. Global internet search trends related to gastrointestinal symptoms predict regional COVID-19 outbreaks. J Infect. (2022) 84:56–63. doi: 10.1016/j.jinf.2021.11.003
42. Zhang X, Pang Y, Cui M, Stallones L, Xiang H. Forecasting mortality of road traffic injuries in China using seasonal autoregressive integrated moving average model. Ann Epidemiol. (2015) 25:101–6. doi: 10.1016/j.annepidem.2014.10.015
43. Dziak JJ, Coffman DL, Lanza ST, Li R, Jermiin LS. Sensitivity and specificity of information criteria. Brief Bioinform. (2020) 21:553–65. doi: 10.1093/bib/bbz016
44. ArunKumar KE, Kalaga DV, Sai Kumar CM, Chilkoor G, Kawaji M, Brenza TM. Forecasting the dynamics of cumulative COVID-19 cases (confirmed, recovered and deaths) for top-16 countries using statistical machine learning models: auto-regressive integrated moving average (ARIMA) and seasonal auto-regressive integrated moving average (SARIMA). Appl Soft Comput. (2021) 103:107161. doi: 10.1016/j.asoc.2021.107161
46. He Y, Dou G, Huang Q, Zhang X, Ye Y, Qian M, et al. Does the leading pharmaceutical reform in China really solve the issue of overly expensive healthcare services? evidence from an empirical study. PLoS ONE. (2018) 13:e0190320. doi: 10.1371/journal.pone.0190320
47. Peacocke EF, Myhre SL, Foss HS, Gopinathan U. National adaptation and implementation of WHO model list of essential medicines: a qualitative evidence synthesis. PLoS Med. (2022) 19:e1003944. doi: 10.1371/journal.pmed.1003944
48. The National Drug Use Monitoring Platform Goes. Available online at: http://www.nhc.gov.cn/mohwsbwstjxxzx/s8561/201912/a642af0b6418473a94dc668d53a4f3fd.shtml
49. Notice on Printing and Distributing the Administrative Measures for the National Drug Shortage List. Available online at: http://www.gov.cn/zhengce/zhengceku/2020-04/24/content_5505943.htm
50. Hu H, Jian W, Fu H, Zhang H, Pan J, Yip W. Health service underutilization and its associated factors for chronic diseases patients in poverty-stricken areas in China: a multilevel analysis. BMC Health Serv Res. (2021) 21:707. doi: 10.1186/s12913-021-06725-5
51. Zeng J, Chen X, Fu H, Lu M, Jian W. Short-term and long-term unintended impacts of a pilot reform on Beijing's zero markup drug policy: a propensity score-matched study. BMC Health Serv Res. (2019) 19:916. doi: 10.1186/s12913-019-4764-z
52. Yi H, Miller G, Zhang L, Li S, Rozelle S. Intended and unintended consequences of China's zero markup drug policy. Health Aff. (2015) 34:1391–8. doi: 10.1377/hlthaff.2014.1114
53. Zhang Y, Ma Q, Chen Y, Gao H. Effects of public hospital reform on inpatient expenditures in rural China. Health Econ. (2017) 26:421–30. doi: 10.1002/hec.3320
54. Camerucci E, Stang CD, Hajeb M, Turcano P, Mullan AF, Martin P, et al. Early-onset parkinsonism and early-onset Parkinson's disease: a population-based study (2010-2015). J Parkinsons Dis. (2021) 11:1197–207. doi: 10.3233/JPD-202464
55. Fernández O, Lázaro-Quintela M, Crespo G, Soto de Prado D, Pinto Á, Basterretxea L, et al. Preferences for renal cell carcinoma pharmacological treatment: a discrete choice experiment in patients and oncologists. Front Oncol. (2021) 11:773366. doi: 10.3389/fonc.2021.773366
56. Wakui N, Ozawa M, Yanagiya T, Endo S, Togawa C, Matsuoka R, et al. Factors associated with medication compliance in elderly patients with type 2 diabetes mellitus: a cross-sectional study. Front Public Health. (2021) 9:771593. doi: 10.3389/fpubh.2021.771593
Keywords: interrupted time series, zero markup drug policy, Parkinson's disease, drug costs, disease burden study
Citation: Wang R, Li X, Gu X, Cai Q, Wang Y, Yi Z-M and Chen L-C (2023) The impact of China's zero markup drug policy on drug costs for managing Parkinson's disease and its complications: an interrupted time series analysis. Front. Public Health 11:1159119. doi: 10.3389/fpubh.2023.1159119
Received: 05 February 2023; Accepted: 17 April 2023;
Published: 09 May 2023.
Edited by:
Narelle Warren, Monash University, AustraliaReviewed by:
Ryoung Choi, Dongshin University, Republic of KoreaCopyright © 2023 Wang, Li, Gu, Cai, Wang, Yi and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhan-Miao Yi, eXptQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡ORCID: Zhan-Miao Yi orcid.org/0000-0001-8575-3770
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.