- 1Department of Pharmacy, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Evidence-Based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, China
- 3National Medical Products Administration (NMPA) Key Laboratory for Technical Research on Drug Products In Vitro and In Vivo Correlation, Chengdu, China
- 4Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
- 5West China School of Pharmacy, Sichuan University, Chengdu, China
- 6West China School of Medicine, Sichuan University, Chengdu, China
- 7Healthcare Evaluation and Organizational Analysis (HEOA) Group, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China
- 8School of Economics, Sichuan University, Chengdu, China
- 9Academic Division of Child Health, University of Nottingham, Derbyshire Children's Hospital, Derby, United Kingdom
- 10Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, Chengdu, China
Background: Essential medicines are the backbone of healthcare and meet the priority healthcare needs of the population. However, approximately one-third of the global population does not have access to essential medicines. Although China formulated essential medicine policies in 2009, the progress of availability of essential medicines and regional variations remains unknown. Therefore, this study was conducted to evaluate the availability of essential medicines, their progress, and regional distribution in China in the last decade.
Methods: We searched eight databases from their inception to February 2022, relevant websites, and reference lists of included studies. Two reviewers selected studies, extracted data, and evaluated the risk of bias independently. Meta-analyses were performed to quantify the availability of essential medicines, their progress, and regional distribution.
Results: Overall 36 cross-sectional studies conducted from 2009 to 2019 were included, with regional data for 14 provinces. The availability of essential medicines in 2015–2019 [28.1%, 95% confidence interval (CI): 26.4–29.9%] was similar to that in 2009–2014 (29.4%, 95% CI: 27.5–31.3%); lower in the Western region (19.8%, 95% CI: 18.1–21.5%) than Eastern (33.8%, 95% CI: 31.6–36.1%) and Central region (34.5%, 95% CI: 30.6–38.5%); very low for 8 Anatomical Therapeutic Chemical (ATC) categories (57.1%), and low for 5 categories (35.7%) among all ATC groups.
Conclusion: The availability of essential medicines in China is low compared with the World Health Organization goal, has not changed much in the last decade, is unequal across regions, and lacks data for half of provinces. For policy-making, the monitoring system of the availability of essential medicines is to be strengthened to enable long-term surveillance, especially in provinces where the data has been missing. Meanwhile, Joint efforts from all stakeholders are warranted to improve the availability of essential medicines in China toward the universal health coverage target.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=315267, identifier: PROSPERO CRD42022315267.
1. Introduction
Access to essential medicines is a vital component of the millennium development goals (MDGs), sustainable development goals (SDGs) and universal health coverage (UHC) (1–3). The World Health Organization (WHO) defined essential medicines as those can meet basic medical and healthcare needs, have appropriate dosage forms, guaranteed supply, can be equipped at the grass-roots level, and can be equitably accessed by the people (4). Since 1977, WHO has been updating essential medicines list every 2 years, with the latest 22nd version in 2021 (5). Currently, the list forms an integral part of national essential medicines policies in 146 countries guiding the selection of drugs based on public health relevance, efficacy, safety, and cost (6). However, studies have shown that millions of people around the world face illness, disability, and death every year because of poor access to medicines (7, 8). Approximately one-third of the global population does not have access to essential medicines. In 2012, a survey performed by WHO estimated that more than 10 million deaths around the world could be avoided every year by providing essential medicines through the effective National Essential Medicines Policy (NEMP) (9). To facilitate and promote monitoring the progress of the availability of essential medicines and the national essential medicine system (NEMS), the WHO and Health Action International (HAI) jointly developed the WHO/HAI standardized method, which provided a unified method for countries and organizations to investigate the availability, price, and affordability of essential medicines (10).
In 2009, China initialized the national essential medicine system, as one of the five key components of the “new health reform” to improve the medicine supply system and ensure the safe use of medicines in the population (11). Since the new health reform in China in 2009, several studies have been conducted to examine the availability of essential medicines in China (12–19). However, several key questions have not been fully addressed. Firstly, nationwide studies of the availability of essential medicines were lacking, as most of these studies were conducted in single or several provinces only (20). Secondly, studies of the findings on its secular trend have been inconsistent (17–19, 21–23). For example, Zhu et al. (18) and Wei et al. (19) showed that the availability of essential medicines in 2016 was lower than that in 2012. In contrast, a study found that the availability of essential medicines increased from 2010 to 2014 (17). A thorough evaluation on the secular progress and geographical distribution of the availability of essential medicines in China could enable benchmarking and provide vital evidence for policy-making on the NEMS for years to come. However, to our knowledge, no such study has been conducted in China since its 2009 health reform.
Therefore, this study was conducted to systematically evaluate the availability of essential medicines, their secular progress, and regional distribution in China in the last decade, to provide evidence and support policy-making of the NEMS to improve the availability of essential medicines toward universal health coverage.
2. Methods
This systematic review and meta-analysis was reported in accordance with the preferred reporting items for systematic review and meta-analysis (PRISMA) (24), and was registered in PROSPERO (CRD42022315267).
2.1. Search strategy
We searched literature databases of PubMed, Embase (Ovid), the Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang database, VIP database, and Chinese Biomedical Literature database (CBM) from their inceptions to February 2022. We also searched the websites the of WHO and International Pharmaceutical Federation (FIP) and manually checked reference lists of included studies and relevant published reviews. The search terms included: essential drug, essential medicine, China, Chinese, etc. The search strategy in PubMed is presented in Supplementary Table S1.
2.2. Eligibility criteria
Primary studies were included if they had participants of medical institutions or pharmacies, public or private, that may provide essential medicines in mainland China; outcome of availability of essential medicines (frequency and percentage); study design of the cross-sectional study, interrupted times series study, uncontrolled before-after study, and controlled before-after study. Publications in English or Chinese were included. Studies were excluded if they were duplicate publications or their full texts were not available.
2.3. Study selection and data extraction
Two reviewers (MZ and ZL) selected studies and extracted data independently. The following data were extracted: (1) general information of the study: title, first author, year of publication, and study design; (2) characteristic of studies: study area, survey time, survey method, number of essential medicines investigated, number of medical organizations, and names of investigated medicines; (3) outcome: availability rate of essential medicines. Reviewers resolved disagreements by discussion to consensus, and if necessary, by consulting a third reviewer (KZ).
2.4. Risk of bias assessment
Two reviewers (MZ and ZL) evaluated the risk of bias of cross-sectional studies independently using the Joanna Briggs Institute (JBI) Critical Appraisal Tools (25). The tool consists of nine items in terms of sampling methods, research objects, data collection, and analysis methods. Each item was determined by yes, no, unclear, and not applicable. For the overall quality rating of a study, more than 6 scores were considered as high, 4–6 scores as moderate, and < 4 scores as low quality (25). The risk of bias of interrupted times series study, uncontrolled before-after study, and controlled before-after study was assessed using the Cochrane Effective Practice and Organization of Care (EPOC) criteria (26). Disagreements were resolved by discussion to consensus, and if necessary, by consulting a third reviewer (KZ).
2.5. Outcome measurement
The availability of essential medicines was calculated as the percentage (%) of the number of institutions equipped with essential medicines to the number of institutions surveyed. The availability of essential medicines was classified as very low if the percentage was < 30%, low if it was 30–49%, fairly high if it was 50–80%, and high if it was more than 80% (27).
2.6. Statistical analysis
The pooled availability of essential medicines was estimated using percentage and its 95% CI. Heterogeneity was evaluated using I2 and Chi-square (χ2) test. If heterogeneity was significant (I2 > 50%), the random effect model was used, otherwise, the fixed effect model was used. The overall availability rates of essential medicines in China, and that from 2009 to 2014 and from 2015 to 2019 were estimated. Subgroup analyses were conducted by regions in mainland China (Eastern region including 11 provinces or municipalities, Central region including 8 provinces or autonomous regions, and Western region including 12 provinces, municipality or autonomous regions) (28), and provinces to examine its geographic distribution across China, and by Anatomical Therapeutic Chemical (ATC) Classification of medicines.
3. Results
3.1. Literature search and study selection
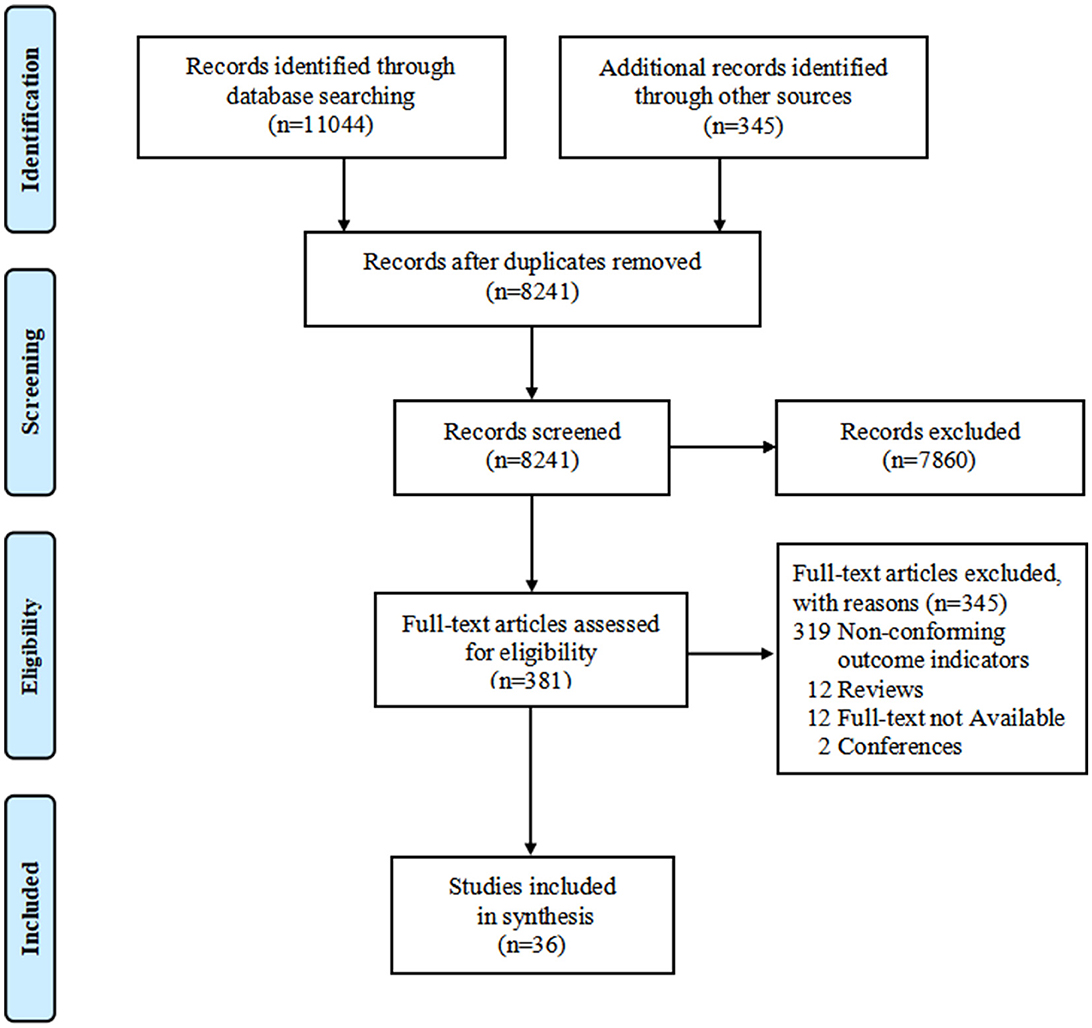
A total of 11,389 records were identified by the initial search. After removing duplicates and irrelevant records by screening for titles and abstracts, 381 studies were assessed for eligibility at full-text screening. Eventually, 36 studies were included in this systematic review (13, 16–23, 29–55). The study selection process is shown in Figure 1.
3.2. Characteristics of included studies
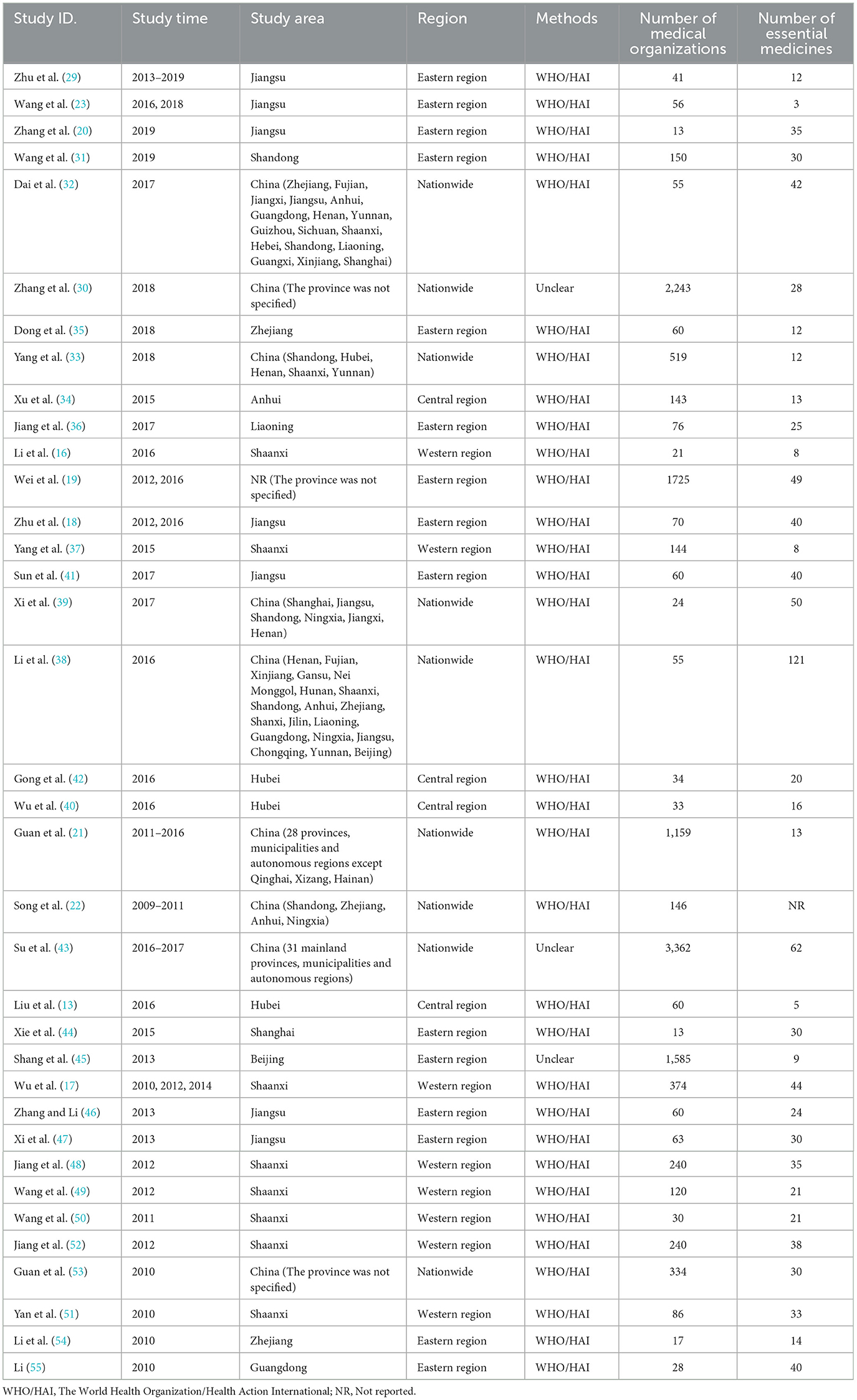
The characteristics of the included studies are presented in Table 1. The included were all cross-sectional studies, which were conducted from 2009 to 2019. Among them, 15 studies (41.7%) were conducted in the Eastern region, 8 studies (22%) in the Western region, 4 studies (11%) in the Central region, and the remaining 9 studies (25%) were conducted across several provinces or nationwide. Among them, 33 studies (92%) adopted the WHO/HAI standardized methodology, while the investigation method was unclear in 3 studies. However, they all adopted the definition of availability of essential medicines according to the WHO/HAI methods, and therefore, were also included in the analyses. All studies selected investigated medicines based on the WHO Model List of Essential Medicines (22nd List) and China National Essential Medicines List (2018) (56, 57). The median number of investigated essential medicines was 28 (ranging from 3 to 121). The list of essential medicines investigated in included studies is presented in Supplementary Table S2.
3.3. Risk of bias assessment
Among the 36 studies, 26 studies were assessed as high quality, and the remaining 10 studies were of moderate quality (Supplementary Table S3).
3.4. Availability of essential medicines in China from 2009 to 2019
A total of 36 studies reported the availability of essential medicines in China. Overall, the availability of essential medicines was 28.8% (95% CI: 27.5–30.1%) from 2009 to 2019. Detailed availability of the essential medicines by ATC is presented in Supplementary Table S4.
3.4.1. Secular trend of availability of essential medicines in China between 2009–2014 and 2015–2019
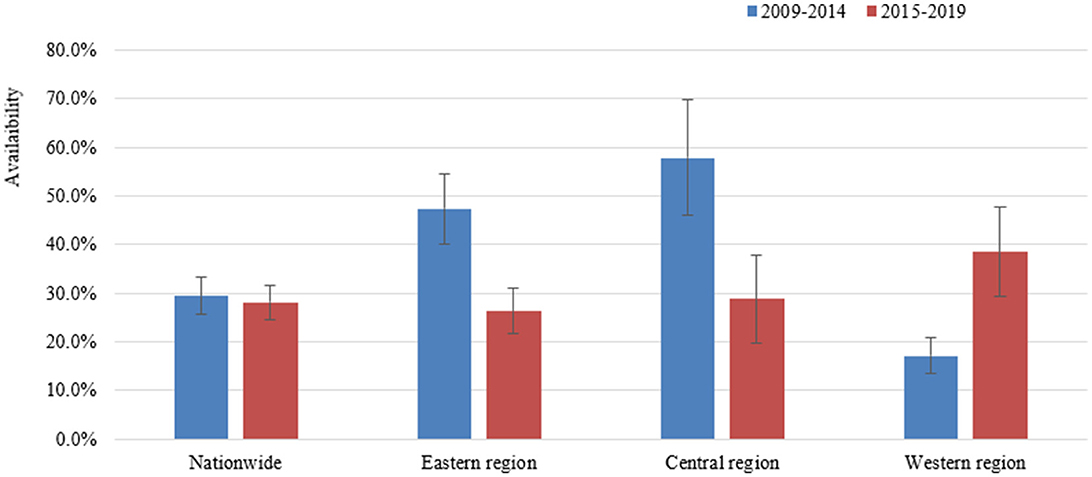
The changes of the availability of essential medicines in China during the two periods are shown in Figure 2. A total of 17 studies reported the availability from 2009 to 2014, and 23 studies from 2015 to 2019. The overall availability of essential medicines in China during the two periods was similar, which was 29.4% (95% CI: 27.5–31.3%) from 2009 to 2014 and 28.1% (95% CI: 26.4–29.9%) from 2015 to 2019. Detailed availability of the essential medicines by ATC between the two periods is presented in Supplementary Tables S5, S6.
3.4.2. Availability of essential medicines by regions
The number of studies reported the availability of essential medicines for the Eastern, Central and Western regions was 20, 9, and 13, respectively. From 2009 to 2019, the overall availability of essential medicines in the Western regions (19.8%, 95% CI: 18.1–21.5%) was lower than that in the Eastern (33.8%, 95% CI: 31.6–36.1%) and Central regions (34.5%, 95% CI: 30.6–38.5%) (Supplementary Table S3). As regards to secular trend, the availability of essential medicines in the Eastern region and the Central region reduced notably from 2015 to 2019 to that from 2009 to 2014, while increasing substantially in the Western region from 17% during 2009–2014 to 38% from 2015 to 2020 (Figure 2; Supplementary Tables S5, S6).
3.4.3. Availability of essential medicines by provinces
The variations of availability of essential medicines across provinces during the two time periods are shown in Figures 3A, B. Provincial data of the availability of essential medicines were only reported for 14 provinces, while the other 17 provinces neither conducted a survey nor reported the provincial data. Among them, 5 provinces (16.1%) had provincial data of availability of essential medicines from 2009 to 2014, and 12 provinces (38.7%) had provincial data from 2015 to 2019.
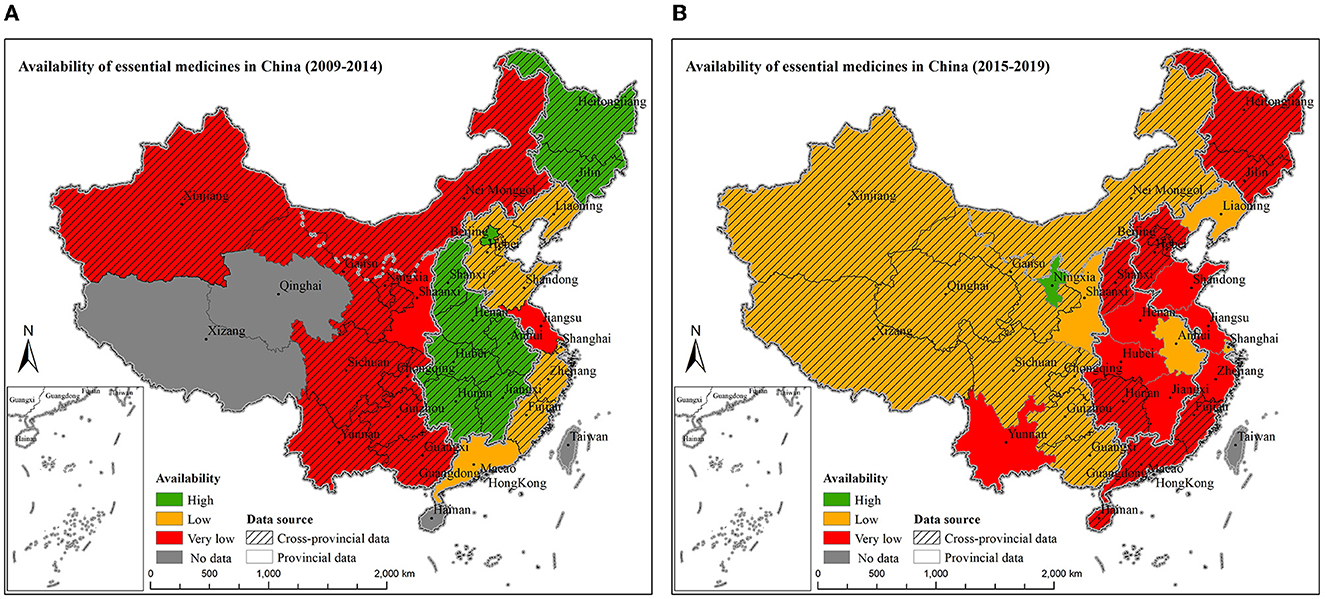
Figure 3. The availability of essential medicines by provinces from 2009 to 2014 (A) and from 2015 to 2019 (B). Availability of essential medicine considered as: high > 80%, fairly high 50–80%, low 30–49%, very low < 30%. Methods for data manipulation for provinces with missing data in the map: if the province has been surveyed but the provincial data were not available, regional data (pooled estimate using meta-analysis) were used to fill in the data; if no survey has been conducted in the province at all, then it was labeled as no data. (From 2009 to 2014, provincial data were available for Beijing, Jiangsu, Guangdong, and Shaanxi; whereas, from 2015 to 2019, provincial data were available for Jiangsu, Liaoning, Shanghai, Zhejiang, Shandong, Anhui, Jiangxi, Henan, Hubei, Shaanxi, Yunnan, and Ningxia).
From 2009 to 2019, the three provinces with the lowest overall availability of essential medicines were Yunnan (19.4%, 95% CI: 6.3–3.2%), Shaanxi (19.8%, 95% CI: 18.0–21.6%), and Jiangsu (22.3%, 95% CI: 19.4–25.1%). More detailed availability of essential medicines in different provinces from 2009 to 2014 and from 2015 to 2019 were presented in Supplementary Tables S5, S6.
3.4.4. Availability of essential medicines by ATC categories
Thirteen categories of essential medicines were investigated from 2009 to 2014, and 14 categories were investigated from 2015 to 2019. Changes in the availability of essential medicines by ATC categories in the two periods are presented in Figure 4.
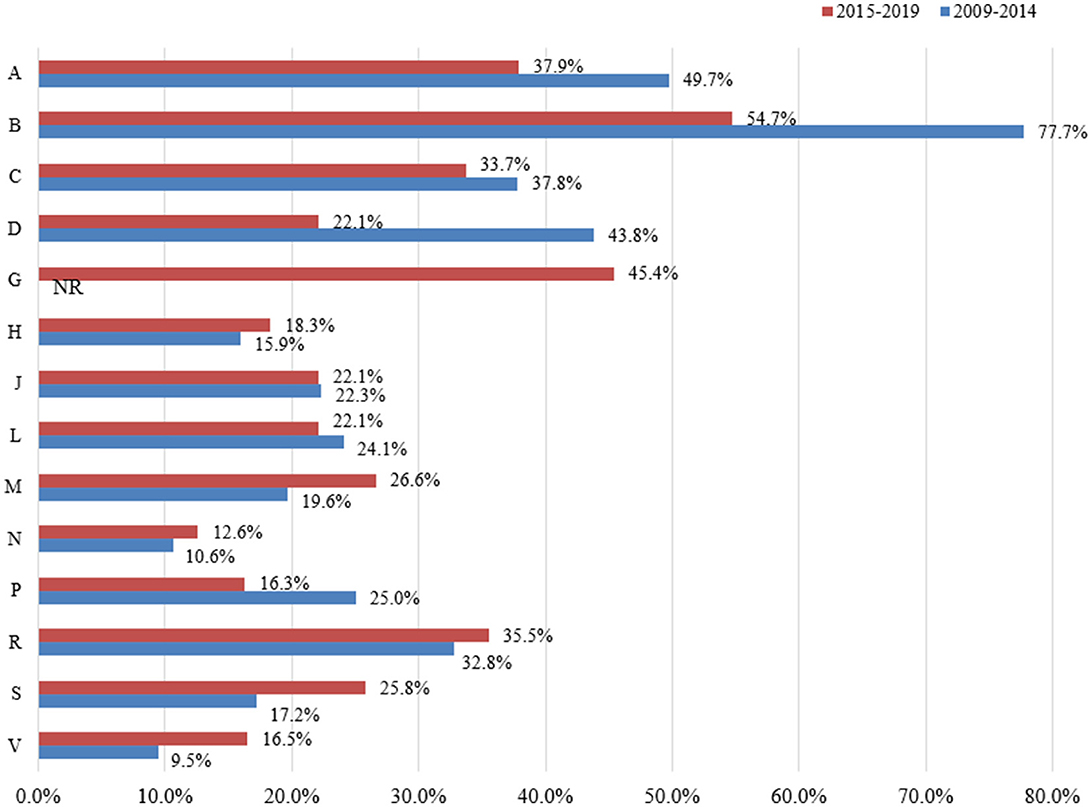
Figure 4. Changes in the availability of essential medicines between different categories based on ATC (2009–2014 vs. 2015–2019). G: Not reported the availability of Genito urinary system and sex hormones from 2009 to 2014. A: Alimentary tract and metabolism; B: Blood and blood forming organs; C: Cardiovascular system; D: Dermatologicals; G: Genito urinary system and sex hormones; H: Systemic hormonal preparations, excl. sex hormones and insulins; J: Antiinfectives for systemic use; L: Antineoplastic and immunomodulating agents; M: Musculo-skeletal system; N: Nervous system; P: Antiparasitic products, insecticides and repellents; R: Respiratory system; S: Sensory organs: V: Various.
From 2009 to 2014, the number of ATC categories of essential medicines with fairly high, low, and very low availability were 1, 4, and 8, respectively, while no data was available for the genito urinary system and sex hormones. From 2015 to 2019, the number of ATC categories of essential medicines with fairly high, low, and very low availability were 1, 4, and 9, respectively. From 2009 to 2014, the three ATC categories of essential medicines with the lowest availability were nervous system (10.6%, 95% CI: 8.1–13.4%), systemic hormonal preparations excluding sex hormones and insulins (15.9%, 95% CI: 2.8–34.6%), and sensory organs (17.2%, 95% CI: 7.0–30.5%). From 2015 to 2019, the three ATC categories of essential medicines with the lowest availability were nervous system (12.6%, 95% CI: 8.4–17.5%), antiparasitic products, insecticides and repellents (16.3%, 95% CI: 6.7–28.2%), and systemic hormonal preparations excluding sex hormones and insulins (18.3%, 95% CI: 6.6–33.5%). The three medicines with the lowest availability were similar to the national level, especially nervous system, which was very low (< 30%) in all regions (Supplementary Tables S3–S5).
4. Discussion
To our knowledge, this is the first systematic review and meta-analysis that has comprehensively evaluated the secular trend, regional and provincial distribution of the availability of essential medicines in China in the last decade. There are four important findings in this study. First, the availability of essential medicines was low in China from 2009 to 2019 with little overall change between 2009–2014 and 2015–2019, and much is to be done to achieve the goal of 80% availability suggested by the WHO. Second, the availability of essential medicines in the Western region was lower than that in the Eastern and Central regions. As regards to secular trend, the availability of essential medicines in the Eastern region and the Central region reduced slightly from 2015 to 2019 compared with that from 2009 to 2014, while increasing moderately in the Western region. Third, among 14 ATC categories of essential medicines, the availability was very low for 8 categories (57.1%), and low for 5 categories (35.7%) in most recent studies. Finally, the provincial data are only available for less than half provinces (14 provinces) while lacking for the others, as they were not surveyed at all or not reported, indicating substantial research gaps and needs.
Overall, our results revealed that the availability of essential medicines was still low in China according to the WHO availability goal, which was consistent with the findings of published studies. A national study found that the mean availability of essential medicines in China was low (4.29–43.75%) (33). Another national survey found that the overall availability of essential medicines for children in China was low (< 35%) (38). Internationally, Mahmić-Kaknjo et al. found that the availability of essential medicines is still suboptimal from 2003 to 2011 in low- and middle-income countries (58). It suggested that the availability of essential medicines in China is at the middle level among all low- and middle-income countries. There may be several reasons for the low availability of essential medicines in China. First, on the supply side, the low price and meager profit of essential medicines may lower the motivation of the production and distribution enterprises to produce and supply essential medicines (12, 59). Second, on the demand side, patients may be influenced by misunderstood beliefs that cheap medicines may not be as effective as those with high prices (60), which may also lead to a decrease in demand, production and supply of these cheap price essential medicines. Last, the WHO/HAI method has strict restrictions on dosage forms and specifications which may be different from that in the China market, and therefore, may underestimate the actual availability of those medicines (17).
We found the availability of essential medicine in China has changed little in the last decade. This finding was supported by several other studies. Guan et al. found that the nationwide availability was steady from 2011 to 2016 (21), and Song et al. showed that the availability of essential medicines did not change radically (22). The possible reason may be that the availability of essential medicines decreased in the Central and Eastern regions, while increasing in the Western region from 2009–2014 to 2015–2019. In addition, in this study, based on ATC categories, we found that the availability of some essential medicines has increased and that of others has decreased during the two periods. Since our study included all available studies of essential medicines across China from 2009 to 2019, it is more likely to reflect the overall trend of China. However, research on causes of the changes is lacking, as most studies on the availability of essential medicines only reflected its status quo but not examined its trend and reasons. Therefore, more studies are needed to understand why there has been little change in essential medicines over the past decade.
In our study, it is shown that the availability of essential medicines in the Western region was lower than in the Eastern region, which was consistent with findings from previous studies. Guan et al. and Su et al. both found that compared with the Eastern region, the Western region had lower availability of essential medicines (21, 43, 55). This may be related to the different economic levels of the Eastern, Central, and Western regions. Existing literature showed that, compared with the developed Eastern region, the Western region has insufficient health resources and a lack of high-quality essential medicines (61), which indicated the inequality in the allocation of health resources and the utilization of health services in China (62). Notably, we found that the availability of essential medicines increased moderately in the Western region, but reduced slightly in the Eastern region and the Central region from 2015 to 2019 compared with that from 2009 to 2014. The investigation is needed to shed light on the reasons for the unwanted changes, and tailored measures are warranted to reverse the downward trend of the availability of essential medicines in more developed regions.
Finally, none ATC categories except one reached the fairly high (>50%) availability rate in most recent years, indicating systematic challenges for the NEMS. And the three categories of essential medicines with the lowest availability were similar during two periods, which were nervous system, systemic hormonal preparations, excl. sex hormones and insulins, and antiparasitic products, insecticides and repellents. There may be several reasons for this. First, it may be related to the imperfect pricing mechanism and procurement and distribution system of these medicines (51, 52). Second, pharmacies may unable to sell some medicines due to their small sale volumes, such as antiparasitic products, insecticides and repellents, which may be related to the reduced prevalence of such diseases in the Chinese population (53). Third, some medicines for the nervous system, such as diazepam, are not available in private pharmacies, because it is under special management and patients must purchase them with psychiatrist specialists' prescriptions (17).
Our study has some strengths. First, this is the first systematic review and meta-analysis to comprehensively evaluate the availability of essential medicines in China, which is one of the critical pillars of the healthcare system. Second, methodologically, this systematic review was conducted following consolidated standards including the extensiveness of search strategies, rigor in study selection criteria, extraction of relevant information, and data analysis, which ensured the comprehensiveness and robustness of research findings. Third, the research findings shed lights on the availability of essential medicines in China in aspects of its secular trend, regional distribution, and that by ATC categories in the last decade, which may have important implications for future research and pharmaceutical policy-making toward the goal of universal access to medicines.
Our study has several limitations. Firstly, due to the limited number of included studies, we were unable to perform more detailed analysis to estimate the annual availability of essential medicines. Secondly, the accuracy of pooled estimates of availability of essential medicines may be influenced by varied types of investigated essential medicines involved in primary studies, though we have used pooled estimates based on ATC categories. Thirdly, the WHO/HAI survey methodology required medicines with a specific dosage and form, which may result in underestimates of the availability of some medicines, for other forms and dosages might be available in the pharmaceutical market in China. Finally, though we have systematically searched important databases, websites, and published reviews, there may still be gray literature that has not been included.
5. Conclusion
The availability of essential medicines in China is still low compared with the WHO's availability goal, has not changed much in the last decade, is unequal across regions, and lacks data for half of provinces. For research, more studies are warranted, to reveal the reasons and mechanisms of the low availability of essential medicines and facilitate targeted policy-making. A unified investigation method and a standardized list of investigated essential medicines should be formulated according to the healthcare need of Chinese population to promote comparison between studies. For policy-making, the monitoring system of the availability of essential medicines is to be strengthened to enable long-term surveillance, especially in provinces where the data has been missing, as a research and policy priority to enable benchmarking and dedicated efforts for improvement. Meanwhile, joint efforts from all stakeholders including health commission, regulators on drugs, health insurance, pharmaceutical industry, and hospitals are warranted to improve the availability of essential medicines in China toward the universal health coverage target.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LZh, KZ, IC, and DL contributed to the conception and designate the study. MZ and KZ participated in drafting and writing the review. MZ, ZL, KZ, DL, YS, ZC, XC, and BL participated in the formulation of retrieval strategies. MZ, ZL, and KZ participated in study selection, data acquisition, and quality assessment. MZ, XW, HL, YJ, YT, SZ, and LZe participated in the data analysis and drawing of tables and figures. All authors contributed to the critical revision of the manuscript and approved the final manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central University (SCU2022D006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1149838/full#supplementary-material
References
1. World Health Organization. Equitable Access to Essential Medicines: A Framework for Collective Action. (2004). Available online at: https://apps.who.int/iris/handle/10665/68571 (accessed October 5, 2022).
2. Mendis S, Fukino K, Cameron A, Laing R, Filipe A Jr, Khatib O, et al. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ. (2007) 85:279–88. doi: 10.2471/blt.06.033647
3. Leisinger KM, Garabedian LF, Wagner AK. Improving access to medicines in low and middle income countries: corporate responsibilities in context. South Med Rev. (2012) 5:3–8. Available online at: https://dash.harvard.edu/bitstream/handle/1/10623010/3606933.pdf;sequence=1
4. World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. (2009). Available online at: https://apps.who.int/iris/handle/10665/68571 (accessed October 5, 2022).
5. World Health Organization. WHO Model Lists of Essential Medicines. (2021). Available online at: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists (accessed May 28, 2022).
6. Smith MK, Tickell S. The essential drugs concept is needed now more than ever. Trans R Soc Trop Med Hyg. (2003) 97:2–5. doi: 10.1016/S0035-9203(03)90001-0
7. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
8. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61689-4
9. World Health Organization. Regional Office for the Eastern M. Price, Availability and Affordability: An International Comparison of Chronic Disease Medicines. (2006). Available online at: https://apps.who.int/iris/handle/10665/116493?show=full (accessed March 20, 2023).
10. World Health Organization Health Action International. Measuring Medicine Prices, Availability, Affordability and Price Components. 2nd ed. (2008). Available online at: https://apps.who.int/iris/handle/10665/70013 (accessed November 11, 2022).
11. The Central People's Government of the People's Republic of China. Opinions of the CPC Central Committee and The State Council on Deepening the Reform of the Medical and Health Care System. (2009). Available online at: http://zqb.cyol.com/content/2009-04/07/content_2611061.htm (accessed August 7, 2022).
12. Li Y, Ying C, Sufang G, Brant P, Bin L, Hipgrave D. Evaluation in three provinces of the introduction and impact of China's National Essential Medicines Scheme. Bull World Health Organ. (2013) 91:184–94. doi: 10.2471/BLT.11.097998
13. Liu C, Zhang X, Liu C, Ewen M, Zhang Z, Liu G. Insulin prices, availability and affordability: a cross-sectional survey of pharmacies in Hubei Province, China. BMC Health Serv Res. (2017) 17:597. doi: 10.1186/s12913-017-2553-0
14. Washington DC: World Bank. Financing, Pricing, and Utilization of Pharmaceuticals in China: The Road to Reform Policy. (2018). Available online at: https://openknowledge.worldbank.org/handle/10986/27598 (accessed October 13, 2022).
15. Fang Y, Wagner AK, Yang S, Jiang M, Zhang F, Ross-Degnan D. Access to affordable medicines after health reform: evidence from two cross-sectional surveys in Shaanxi Province, western China. Lancet Glob Health. (2013) 1:e227–37. doi: 10.1016/S2214-109X(13)70072-X
16. Li Z, Feng Q, Kabba JA, Yang C, Chang J, Jiang M, et al. Prices, availability and affordability of insulin products: a cross-sectional survey in Shaanxi Province, western China. Trop Med Int Health. (2019) 24:43–52. doi: 10.1111/tmi.13167
17. Wu LN, Yang CJ, Shen Q, Chang J, Zhu WW, Ye D, et al. Comparative study on the availability of drugs in public hospitals and retail pharmacies in Shaanxi Province. Chin Pharm Aff. (2016) 30:8–16. doi: 10.16153/j.1002-7777
18. Zhu Y, Wang Y, Sun X, Li X. Availability, price and affordability of anticancer medicines: evidence from two cross-sectional surveys in the Jiangsu Province, China. Int J Environ Res Public Health. (2019) 16:3728. doi: 10.3390/ijerph16193728
19. Wei GX, Wang XL, Li X, Li LY, Chen J, Shi LW. A survey on the availability, price and affordability of essential medicine for children in Eastern China. Chin J Health Policy. (2019) 12:72–8. doi: 10.3969/j.issn.1674-2982.2019.10.011
20. Zhang L, Zhou QY, Zhang XM, Yu Y, Wang SS, Shao R. Investigation and study on the accessibility of antibiotics in essential medicine list in medical institutions of Nanjing area. Chin Pharm. (2020) 31:1281–7. doi: 10.6039/j.issn.1001-0408.2020.11.01
21. Guan X, Hu H, Man C, Shi L. A survey of availability, price and affordability of essential medicines from 2011 to 2016 in Chinese secondary and tertiary hospitals. Int J Equity Health. (2018) 17:158. doi: 10.1186/s12939-018-0870-5
22. Song Y, Bian Y, Zhen T. Making medicines more accessible in China: an empirical study investigating the early progress of essential medicine system. PLoS ONE. (2018) 13:e0201582. doi: 10.1371/journal.pone.0201582
23. Wang L, Dai L, Liu H, Dai H, Li X, Ge W. Availability, affordability and price components of insulin products in different-level hospital pharmacies: evidence from two cross-sectional surveys in Nanjing, China. PLoS ONE. (2021) 16:e0255742. doi: 10.1371/journal.pone.0255742
24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
25. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, et al. Chapter 7: systematic reviews of etiology and risk. JBI Manual Evid Synth. (2020). doi: 10.46658/JBIRM-17-06
26. Cochrane Effective Practice Organisation of Care (EPOC). Suggested Risk of Bias Criteria for EPOC Reviews. (2017). Available online at: epoc.cochrane.org/resources/epoc-resources-review-authors (accessed December 12, 2022).
27. Gelders S, Ewen M, Noguchi N, Laing R. Price, Availability Affordability: An International Comparison of Chronic Disease Medicines. (2006). Available online at: http://apps.who.int/iris/bitstream/10665/116493/1/dsa560.pdf
28. National Bureau of Statistics. Li Yin, the Chief statistician of the Social Science and Culture Department of the National Bureau of Statistics, Interpreted the 2021 Statistical Bulletin on Investment in Science and Technology Funds. (2022). Available online at: http://www.stats.gov.cn/tjsj/sjjd/202208/t20220831_1887755.html (accessed July 20, 2022).
29. Zhu Y, Xu X, Fang W, Wang Y, Dai H, Li X. Availability, cost and affordability of selected antibiotics and antiviral medicines against infectious diseases from 2013 to 2019 in Nanjing, China. Trop Med Int Health. (2021) 26:518–29. doi: 10.1111/tmi.13559
30. Zhang XJ, Jiang XT, Zheng JL, Peng B, Li YZ. Analysis on the availability and allocation of essential drugs for hypertension and diabetes in primary health institutions. Chin J Health Policy. (2020) 13:58–65. doi: 10.3969/j.issn.1674-2982.2020.07.010
31. Wang X, Zhang AC, Wang H, Xv DL. Evaluation study on the availability and prices of paediatric essential drugs in Weifang. Chin Pharm Aff. (2020) 34:1085–92. doi: 10.16153/j.1002-7777.2020.09.013
32. Dai Y, Li ZP, Xv H, Zhu L, Zhu YQ, Chen H, et al. A multicenter survey of the accessibility of essential medicines for children in China. Chin J Pediatrics. (2020) 58:301–7. doi: 10.3760/cma.j.cn112140-20190820-00527
33. Yang C, Hu S, Ye D, Jiang M, Babar ZU, Fang Y. Evaluating price and availability of essential medicines in china: a mixed cross-sectional and longitudinal study. Front Pharmacol. (2020) 11:602421. doi: 10.3389/fphar.2020.602421
34. Xu R, Li S, Lv X, Xie X. Prices, availability, and affordability of national essential medicines in public primary hospitals: a cross-sectional survey in poverty-stricken rural areas in China. Int J Health Plann Manage. (2020) 35:545–57. doi: 10.1002/hpm.2963
35. Dong Z, Tao Q, Yan B, Sun G. Availability, prices and affordability of essential medicines in Zhejiang Province, China. PLoS ONE. (2020) 15:e0241761. doi: 10.1371/journal.pone.0241761
36. Jiang XT, Wang YQ, Jia SH, Gao YB, Sun LH. Evaluation of essential medicines accessibility in Liaoning Province. Chin Pharmacol J. (2019) 54:501–5. doi: 10.11669/cpj.2019.06.013
37. Yang C, Hu S, Zhu Y, Zhu W, Li Z, Fang Y. Evaluating access to oral anti-diabetic medicines: a cross-sectional survey of prices, availability and affordability in Shaanxi Province, Western China. PLoS ONE. (2019) 14:e0223769. doi: 10.1371/journal.pone.0223769
38. Li SS, Xv W, Du WW, Fu YB. Study on the availability of pediatric essential medicines in China: based on the surveys in 19 provinces. Chin J Health Policy. (2018) 11:12–8. doi: 10.3969/j.issn.1674-2982.2018.12.003
39. Xi X, Chen P, Yang F, Yang Y, Chen L, Mao N. Evaluating the accessibility of essential medicines in China. J Med Econ. (2018) 21:784–92. doi: 10.1080/13696998.2018.1474744
40. Wu G, Gong S, Cai H, Ding Y. The availability, price and affordability of essential antibacterials in Hubei province, China. BMC Health Serv Res. (2018) 18:1013. doi: 10.1186/s12913-018-3835-x
41. Sun X, Wei J, Yao Y, Chen Q, You D, Xu X, et al. Availability, prices and affordability of essential medicines for children: a cross-sectional survey in Jiangsu Province, China. BMJ Open. (2018) 8:e023646. doi: 10.1136/bmjopen-2018-023646
42. Gong S, Cai H, Ding Y, Li W, Juan X, Peng J, et al. The availability, price and affordability of antidiabetic drugs in Hubei province, China. China Health Policy Plan. (2018) 33:937–47. doi: 10.1093/heapol/czy076
43. Su M, Zhang Q, Bai X, Wu C, Li Y, Mossialos E, et al. Availability, cost, and prescription patterns of antihypertensive medications in primary health care in China: a nationwide cross-sectional survey. Lancet. (2017) 390:2559–68. doi: 10.1016/S0140-6736(17)32476-5
44. Xie N, Shen Y, Ren JM, Tang KM. Empirical study on the availability of essential medicines in public health and medical institutions of Shanghai Qingpu district. Chin Pharm. (2016) 27:3331–4. doi: 10.6039/j.issn.1001-0408.2016.24.05
45. Shang JX, Guo ZG, Lin QM, Li L, Chen CX, Feng L, et al. Analysis of accessibility of essential medicine in Beijing. Chin J Health Policy. (2016) 9:52–8. doi: 10.3969/j.issn.1674-2982.2016 02. 010
46. Zhang Y, Li X. Investigation and analysis of availability and affordability of essential medicine in Nanjing based on WHO/HAI standard survey method. Chin Pharm. (2015) 26:4188–92. doi: 10.6039/j.issn.1001-0408.2015.30.04
47. Xi X, Li W, Li J, Zhu X, Fu C, Wei X, et al. A survey of the availability, prices and affordability of essential medicines in Jiangsu Province, China. BMC Health Serv Res. (2015) 15:345. doi: 10.1186/s12913-015-1008-8
48. Jiang M, Zhou Z, Wu L, Shen Q, Lv B, Wang X, et al. Medicine prices, availability, and affordability in the Shaanxi Province in China: implications for the future. Int J Clin Pharm. (2015) 37:12–7. doi: 10.1007/s11096-014-0037-4
49. Wang X, Yang SM, Fang Y, Jiang MH, Wu LN. Study on the availability and price pediatric essential medicines in retail pharmacies in Shaanxi Province using WHO/HAI methodology. Chin Pharm. (2014) 25:678–81. doi: 10.6039/j.issn.1001-0408.2014.08.02
50. Wang X, Fang Y, Yang S, Jiang M, Yan K, Wu L, et al. Access to paediatric essential medicines: a survey of prices, availability, affordability and price components in Shaanxi Province, China. PLoS ONE. (2014) 9:e90365. doi: 10.1371/journal.pone.0090365
51. Yan KK, Yang SM, Fang Y, Zhao J, Liu J. Study on prices and availability of 47 kinds of drugs in Shaanxi Province. Chin Pharm. (2013) 24:1072–5. doi: 10.6039/j.issn.1001-0408.2013.12.06
52. Jiang MH, Wang L, Wang WJ, Wang X, Fang Y, Yang SM, et al. Comparative study on the price and availability of essential drugs in public hospital and retail pharmacy of Shaanxi Province. Chin Pharma. (2013) 24:308–13. doi: 10.6039/j.issn.1001-0408.2013.04.08
53. Guan XD, Xin XX, Liu Y, Wang TS, Shi LW. Empirical study on availability of essential medicine in China. Chin Pharm. (2013) 24:2216–9. doi: 10.6039/j.issn.1001-0408.2013.24.03
54. Li XW, Liang HJ, Zhang YL, Wu SL, Xv Y. Survey on accessibility of the essential drugs in grassroots medical facilities. Health Econ Res. (2012) 6:24–6. doi: 10.14055/j.cnki.33-1056/f.2012.06.023
55. Li F. Availability of Essential Medicines Research of Community Health Servicces in Guangzhou. Guangzhou: Guangzhou University of Chinese Medicine (2011).
56. World Health Organization. WHO Model List of Essential Medicines (22nd List). (2021). Available online at: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed June 25, 2022).
57. National Health Commission of the People's Republic of China. National Essential Medicines List. (2018). Available online at: http://www.gov.cn/fuwu/2018-10/30/5335721/files/e7473e46d9b24aadad3eb25127ffd986.pdf (accessed June 25, 2022).
58. Mahmić-Kaknjo M, Jeličić-Kadić A, Utrobičić A, Chan K, Bero L, Marušić A. Essential medicines availability is still suboptimal in many countries: a scoping review. J Clin Epidemiol. (2018) 98:41–52. doi: 10.1016/j.jclinepi.2018.02.006
59. Hogerzeil HV, Liberman J, Wirtz VJ, Kishore SP, Selvaraj S, Kiddell-Monroe R, et al. Promotion of access to essential medicines for non-communicable diseases: practical implications of the UN political declaration. Lancet. (2013) 381:680–9. doi: 10.1016/S0140-6736(12)62128-X
60. Mani H. Patient education is crucial for the access to essential medicine. Lancet. (2015) 385:230. doi: 10.1016/S0140-6736(15)60057-5
61. McLeod L, Bereza BG, Shim M, Grootendorst P. Financial burden of household out-of-pocket expenditures for prescription drugs: cross-sectional analysis based on national survey data. Open Med. (2011) 5:e1–9. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3205811/pdf/OpenMed-05-e1.pdf
Keywords: essential medicines, availability, China, regional distribution, systematic review
Citation: Zhang M, Zou K, Liu Z, Liu D, Wang X, Shi Y, Chen Z, Cheng X, Lang B, Li H, Zeng L, Tang Y, Zhao S, Jiang Y, Choonara I and Zhang L (2023) Availability of essential medicines, progress and regional distribution in China: a systematic review and meta-analysis. Front. Public Health 11:1149838. doi: 10.3389/fpubh.2023.1149838
Received: 23 January 2023; Accepted: 06 April 2023;
Published: 25 April 2023.
Edited by:
Carla Sofia e Sá Farinha, New University of Lisbon, PortugalReviewed by:
Mojtaba Vaismoradi, Nord University, NorwayKirti Sundar Sahu, Canadian Red Cross, Canada
Bhavna Bharati, KIIT University, India, in collaboration with reviewer KS
Copyright © 2023 Zhang, Zou, Liu, Liu, Wang, Shi, Chen, Cheng, Lang, Li, Zeng, Tang, Zhao, Jiang, Choonara and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingli Zhang, emhhbmdsaW5nbGlAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Miao Zhang
Miao Zhang Kun Zou
Kun Zou Zheng Liu
Zheng Liu Dan Liu1,2,3,4,5
Dan Liu1,2,3,4,5 Zhe Chen
Zhe Chen Xiao Cheng
Xiao Cheng Hailong Li
Hailong Li Shaoyang Zhao
Shaoyang Zhao Imti Choonara
Imti Choonara Lingli Zhang
Lingli Zhang