
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 10 May 2023
Sec. Aging and Public Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1146456
Background: The muscle quality index (MQI), as an important component of sarcopenia, is defined as the ratio of muscle strength to muscle mass. Lung function, is a clinical indicator to assess ventilation and air exchange function. This study investigated the relationship between lung function indices and MQI in the NHANES database from 2011 to 2012.
Methods: This study included 1,558 adults from the National Health and Nutrition Examination Survey from 2011 to 2012. Muscle mass and muscle strength were assessed using DXA and handgrip strength, and all participants underwent pulmonary function measurements. Multiple linear regression and multivariable logistic regression were used to assess the correlation between the MQI and lung function indices.
Results: In the adjusted model, MQI was significantly correlated with FVC% and PEF%. And, after quartiles of MQI in Q3, where FEV1%, FVC%, and PEF% were all associated with MQI, in Q4, a lower relative risk of a restrictive spirometry pattern was linked to increased MQI. Compared to the lower age group, the relationship between the MQI and lung function indices was more significant in the higher age group.
Conclusion: There was an association between the MQI and lung function indices. Furthermore, in the middle-aged and older adult populations, lung function indicators and restrictive ventilation impairment were significantly associated with MQI. This implies that improving lung function through muscle training may be beneficial to this group.
To our knowledge, sarcopenia is a condition that causes gradual loss of muscle strength and muscle mass. It always affects older adults and those suffering from chronic disease (1). Additionally, it can lead to falls, weakness, disability, functional impairment and even death (2). Recently, some research has shown that expiratory and inspiratory muscle strength is known to decline with age and may result from the decrease of elastic retraction and thorax compliance, which leads to the decline of some lung functions, such as forced expiratory volume in the first 1.0 s (FEV1), forced vital capacity (FVC) and peak expiratory flow rate (PEF) (3, 4). Furthermore, another study has presented that the decrease in muscle mass and strength is related to the rapid decline in lung functions (5). The components of lung function tests are very important tools in the clinical evaluation of respiratory health and disease. The main indicators of pulmonary ventilation include FEV1/FVC, FEV1, FVC, and PEF. An obstruction in airflow is defined as a ratio of less than 0.70 of forced expiratory volume in the 1.0 s (FEV1) to FVC, with FVC < 80% of predicted representing restrictive ventilatory dysfunction. FEV1 determines the severity of ventilatory function, whether it is obstructive, restrictive or mixed (6). PEF reflects respiratory muscle strength and changes in large airway caliber (7). Also, FENO, nitric oxide (NO) in the exhaled breath of humans, is important as a potentially useful non-invasive indicator of airway inflammation for the diagnosis and treatment of different airway inflammatory diseases (8, 9). Sarcopenia is an important syndrome of respiratory disease and is closely associated with poor outcomes. Early assessment of systemic body muscle strength and mass can be used to identify early lung function decline and high-risk healthy individuals developing chronic respiratory disease. For example, chronic obstructive pulmonary disease (COPD), which is a disease involving airflow limitation and persistent respiratory. In addition, COPD, a systemic disease, that can lead to muscle atrophy and dysfunction (10, 11).
The muscle quality index (MQI), defined as the ratio of muscle strength to muscle mass, is calculated by the handgrip strength (HGS) and the appendicular skeletal muscle mass (ASM) (12). ASM is usually assessed by dual-energy X-ray absorptiometry (DXA), a non-invasive instrument widely used in clinical practice to determine muscle mass (13). HGS is used to evaluate muscle strength and can be easily assessed by handgrip dynamometer, Moreover, HGS has been shown to correlate with whole-body muscle strength and can be combined with body composition to determine MQI (14, 15). Since ASM and HGS are easily available in clinical situations, we chose to use muscle quality (MQI) to access muscle condition. From what is known, no one has studied the relationship between the MQI and lung functions, and this is our initial attempt.
In the current study, we examined into how the MQI and lung function indices from the NHANES 2011–2012 correlated. We focused on exploring the correlation between MQI and different lung function indices (FEV1/FVC, FEV1%, FCV%, PEF%, FENO and obstructive or restrictive spirometry pattern) and performed restricted cubic splines, as well as exploring the differences in the associations between MQI and lung function indices in different age groups.
Using a sophisticated, stratified, multistage sampling strategy, the National Health and Nutrition Examination Survey (NHANES) is a population-based, national cross-sectional survey. The primary target population is the noninstitutionalized civilian residing in the US who underwent laboratory assessment, physical examination and questionnaire survey related to nutrition and health. The conduct of NHANES was approved by the Ethics Review Board of the National Centre for Health Statistics and written informed consent was given by each participant.
In this study, we used data from the NHANES collected from 2011 to 2012. The flow chart for inclusion and exclusion is shown in Figure 1. Among the 9,756 participants in the NHANES 2011–2012, individuals were excluded if (1) they were aged less than 20 years (n = 4,196), (2) they lost data for basic demographic data, including gender, age, race, etc. (n = 1,086), (3) they lost data for any spirometry data, or the quality of spirometry was unacceptable (n = 1,334), (4) they lost data for DXA and handgrip tests (n = 1,582). Finally, a total of 1,588 individuals were recruited for this study.
Participants aged between 6 and 79 years were eligible for the spirometry test. The main exclusion criteria included recent chest pain and dyspnea, using supplemental oxygen, current surgery of the chest, abdomen and eye, and a recent stroke or heart attack. The test procedure complies with the recommendations of the American Thoracic Society (ATS). Each health technician received formal training. Expert consultants from the NIOSH Quality Control Center reviewed all the spirometry data collected. The lung function indices required for this study include FEV1, FVC, PEF, and FENO. We only use data with a quality grade of A and B to ensure the accuracy of the data. The Hankinson equation was used to translate the initial numbers to predicted percentages (16). The FENO data was calculated by averaging two reproducible FENO measurements. FEV1/FVC < 0.70 is considered an obstructive spirometry pattern and a restrictive spirometry pattern is defined by an FVC < 80% of predicted with FEV1/FVC ≥ 0.70. Further details can be accessed on the NHANES website.
Using dual-energy X-ray absorptiometry (DXA) data and handgrip strength (HGS) data from 2011 to 2012 in the NHANES database. Body composition data obtained from DXA scans included arm lean mass (kg) and leg lean mass (kg). It’s worth noting that lean mass from DXA includes both non-bone and non-fat tissue. ASM is defined as the sum of the lean mass of arms and legs (17). HGS was measured by a handgrip dynamometer. Participants who had no surgery and pain on either hand or wrist in the last 3 months can be eligible for the test. The grip test was performed in the standing position and participants were requested to squeeze the dynamometer as hard as possible. Each hand was tested three times, with 60secs between each test. The highest measurement of the hand was used. MQI (kg/kg) was defined as the ration between combined HGS divided by ASM (12). Information about DXA and HGS can be found on the NHANES website.
The potential covariates included age, sex, BMI, ethnicity, education level, the ration of family income to poverty (PIR) and cotinine. BMI was calculated as weight divided by height squared (kg/m2). We categorized education level as “no high school,” “high school graduate,” and “college or above.” The ration of family income to poverty (PIR) is an index of poverty status and it was classified into <1, 1–3, >3. Cotinine, a metabolite of nicotine, was used to assess the smoking status.
We used the recommended weighting scheme to analyze the data. All continuous variables were tested by the Shapiro–Wilk test. The continuous variables were expressed as a weighted mean and standard deviation or median and interquartile (IQR). Categorical variables were represented by the unweighted frequencies (weighted percentages). To compare characteristic differences between different MQI groups (quartiles), the weighted chi-squared and Kruskal-Wallis tests were used for categorical and continuous variables, respectively.
Multiple linear regression analyses were performed to analyze the relationship between the MQI and lung function indices between the four groups, and the lowest quartile group was the reference group. Besides, we treated the quartiles as continuous variables and performed a trend test (p for trend). Multivariable logistic regression was performed to analyze the relative risk ratio between MQI and different spirometry patterns, using the normal pattern as the reference group. We next used the restricted cubic splines to further show the association between MQI and lung function indices. Furthermore, we performed subgroup analysis based on whether the age was greater than 40 years. Age, sex, ethnicity, BMI, PIR, and cotinine were adjusted for in the model because we considered these covariates to be confounding factors associated with the study results. Lung function may be influenced by age, sex and ethnicity, etc. Cotinine represents an indicator of active and passive smoking and may better represent individual smoking status. In addition, BMI and PIR may influence MQI. All regressions are considered survey weights. All analyses were performed in R4.2.1 and two-tailed p < 0.05 was accounted for statistical significant.
A total of 1,588 participants were included in this study. The basic characteristics of the study population can be shown in Table 1. Among the 1,588 individuals, 839 were males and 719 were females. The weighted average age of the participants was 38 years. Most participants were non-Hispanic white, which accounted for 65%. 70% of individuals had a college degree or above. The media BMI was 27.1 (kg/m2), and the first quartile had the highest BMI relative to the other quartiles. There was no significant difference in cotinine intake among the four groups. Moreover, the media of FEV1/FVC, FVC%, FEV1%, PEF%, FENO were 80, 99, 97, 105, and 12 (ppb), respectively. A small number of participants (4.6%) had a restrictive pattern for spirometry.
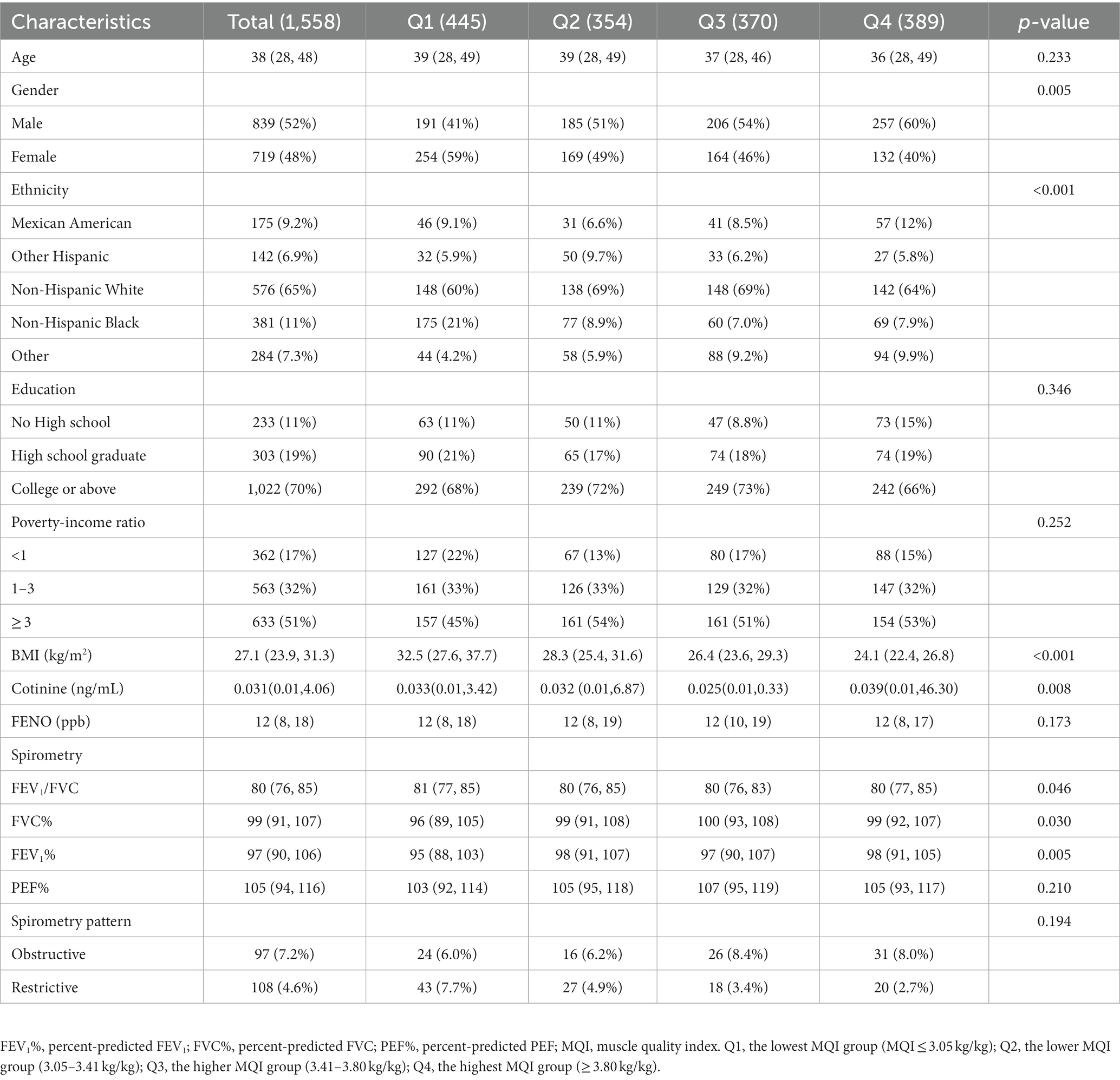
Table 1. Baseline characteristics of study participants by muscle quality index quartiles (N = 1,588).
Table 2 showed the association between the total MQI and lung function indices. In the unadjusted model, we did not find a correlation between PEF% and MQI; however, in the adjusted model, we were surprised to find a correlation between PEF% and MQI, and FVC% was always correlated with MQI in both the unadjusted and adjusted models. In addition, restrictive spirometry pattern lost its association with MQI in the adjusted model, which may be due to an inadequate sample size as patients with restrictive spirometry pattern only account for 1/15 of the total sample size in this study, which may have produced some error. A one-unit increase in MQI was related to higher percent predicted FVC (adjusted mean difference, 1.35; 95%CI, 0.10–2.59) and percent predicted PEF (adjusted mean difference, 1.92; 95%CI, 0.03–3.81).
Table 3 showed the relationship between MQI quartiles and lung function indices. Using MQI as a categorical variable and the first quartile as a reference, after model adjustment, FEV1% increased in Q2 and Q3 as MQI increased. We also found that after adjusting for confounding factors, FVC% increased only in Q3, with a mean difference of 4.22 (1.20, 7.23). It was obvious that PEF% increased in all quartiles and the value of p for the trend was 0.022. Frustratingly, neither FEV1/FVC nor FENO were found to be associated with MQI in each quartile. Moreover, in Q4, compared to normal individuals, higher MQI was related with a lower relative risk of a restrictive spirometry pattern in both the crude and adjusted models. (adjusted relative risk ratio, 0.34; 95% CI, 0.14–0.80).
The results from the restricted cubic splines analysis were shown in Figure 2. Lung function indices increased significantly with increasing MQI and the relationships were in a non-linear trend. When the MQI was less than about 3.5 (kg/kg), the changes of lung function indices tended to increase in a linear trend and then gradually flat.
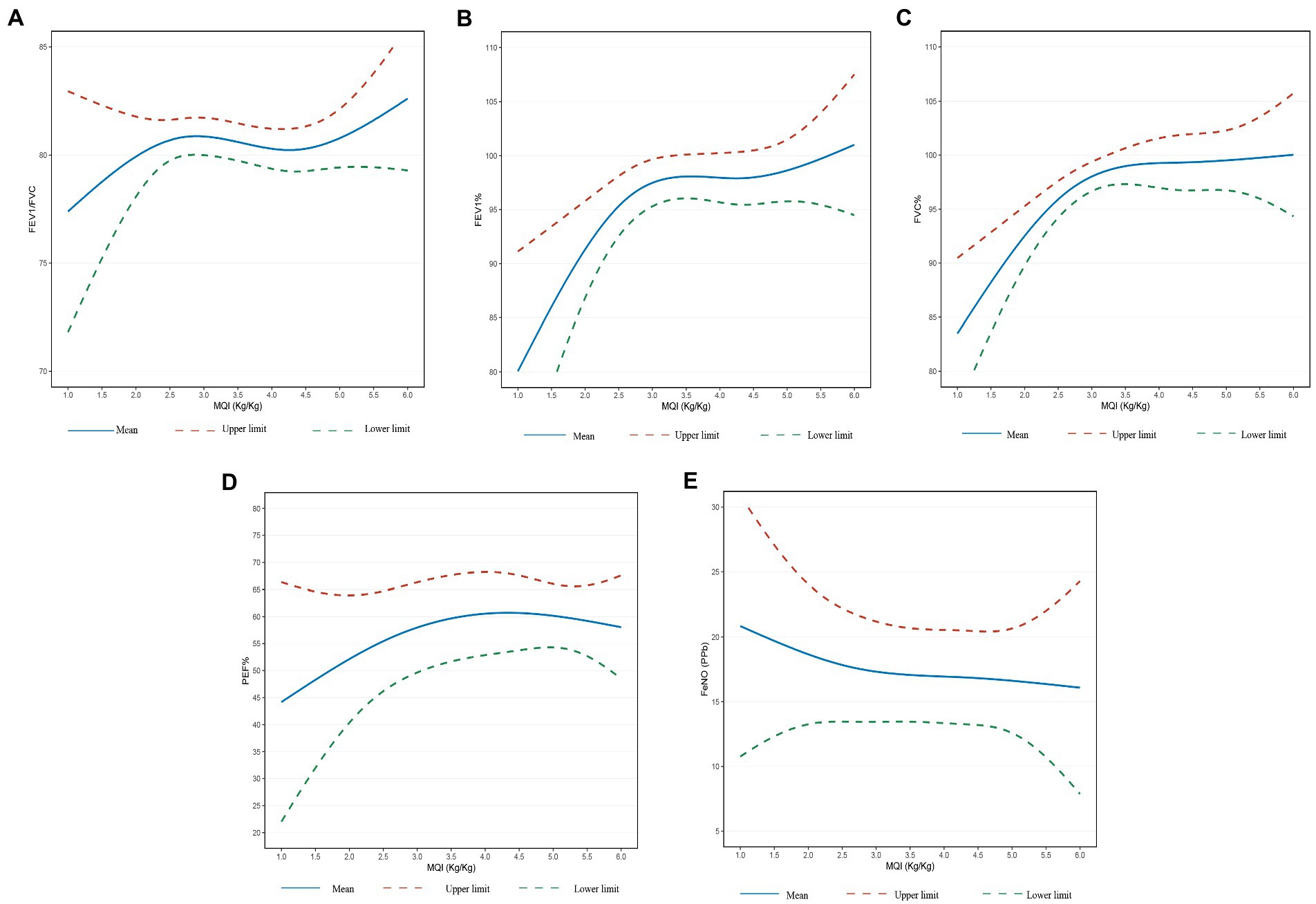
Figure 2. (A) Relationship between MQI and FEV1/FVC. (B) Relationship between MQI and FEV1% predicted. (C) Relationship between MQI and FVC% predicted. (D) Relationship between MQI and PEF% predicted. (E) Relationship between MQI and FENO. Adjusted for age, sex, BMI, ethnicity, poverty-income ratio and cotinine.
Table 4 displayed the results of stratified analyses according to age. In the group of participants aged less than 40 years, we found no consistent evidence between MQI and lung function indices. In the group of participants aged over 40 years, in the unadjusted model, the three lung function indices (FEV1%, FVC%, and PEF%) increased with the increase of the MQI. And in the adjusted model, although FVC% reduced the relationship with MQI, the relationship between FEV1%, PEF% and MQI remained significant. The mean differences and 95% CI are 4.39 (1.09–7.69) and 7.02 (3.04–11.01), respectively. FEV1/FVC and FENO were not significantly associated with MQI in either of the two age groups. In addition, a lower MQI was associated with a higher relative risk of restrictive spirometry pattern (adjusted relative risk ratio, 0. 4; 95% CI, 0.17–0.96).
As far as we know, this was the first study to explore the connection among MQI and lung function indices in a sizeable and complex cohort of American adults. The results showed that as MQI rose, there seemed to be an increase in FEV1%, FVC%, and PEF%. Besides that, compared to individuals who have normal lung ventilation, the relative risk of a restrictive spirometry pattern was raised by a lower MQI. In addition, we found that the relationship between MQI and lung function indices was more significant in the higher age group compared to the lower age groups. Lung function is an important indicator of lung health, and when scores on a lung function test are below average, it is usually considered a lung disease. The reduced lung function in this study may reflect a loss of muscle mass and strength, which may be associated with sarcopenia.
MQI, a prominent component of sarcopenia, is regarded as a predictor of mortality and the probability of disability. In a study by Hu et al., it was demonstrated that asthma patients with sarcopenia, especially severe sarcopenia, had a much increased risk of airway obstruction and a significantly lower PEF (18). Our study also found that as MQI decreased, PEF% decreased significantly, however, we were unable to detect a higher risk of airway obstruction, which may be due to the varied populations that were selected for our investigation. Park et al. found that it is possible to identify healthy middle-aged people at high risk for rapid FEV1 reduction using the changes in body composition over time (19). This is basically consistent with our research results. We discovered that the correlation between MQI and lung function indices was better in Q3 compared to Q4, but their reference groups were all Q1, so we calculated P for trend and found p > 0.05, indicating that the effect of MQI on lung function was not linearly increasing, and also in the restricted cubic spline curve, which more intuitively proved to be non-linear, indicating MQI and lung function were in a non-linear relationship. We speculated that the reason for this non-linear trend may be that lung volume does not increase indefinitely with MQI in the population and there is a maximum limit to lung function, therefore the curve gradually flattens. BMI was highest in Q1 and no association was found between MQI and lung function in Q1, indicating that calculating height and weight alone is not representative of muscle mass. Additionally, several studies have demonstrated a connection between sarcopenia and deteriorated lung function and negative outcomes in chronic obstructive pulmonary disease (COPD) patients (5, 20).
In our research, we discovered a significant association between lung function decline and muscle loss in the middle-aged and older age groups. Such opinions have also been confirmed by a lot of research. Kera et al. concluded that PEF may be a reliable indication of sarcopenia using data from 427 older adults in the local community (4). Ridwan et al. further supported the view that PEF was independently associated with sarcopenia emergence in older people in a nationwide study (21). Also, in an older community-dwelling Korean population, Jeon et al. investigated a relationship between reduced muscle mass and low lung function, as indicated by lower FEV1 or FVC (22). All of these studies were consistent with our views that, after controlling for confounding variables, there was no significant relationship between MQI and lung function indices in the lower age groups. The higher age groups, however, showed the opposite result. Sarcopenia in older individuals has been used to categorize risk, predict negative outcomes, and trigger intervention targeted at avoiding deterioration in those most at risk (5). This is mainly because there is a gradual loss of muscle mass with age due to the progressive loss of motor neurons combined with a reduction in the quantity and size of muscle fibers. In some experimental models, specific intervention techniques have demonstrated positive outcomes for muscle loss and functional degeneration. In the clinic, if these or comparable therapies could benefit older people with sarcopenia by preserving muscle and enhancing mobility, there would be significant humanitarian advantages as well as financial savings for health care systems (23).
Some studies pointed out that in older adults, type 2 diabetes, chronic liver diseases (CLD), chronic kidney diseases (CKD), and cardiovascular diseases (CVD) are associated with accelerated loss of muscle strength and quality (24–26). Although some studies have not shown such a link, prevention of muscle loss remains important in middle-aged and older adults (21). This serves as a caution that muscle loss prevention is crucial for a variety of chronic conditions. Related studies have shown that resistance exercise (RE) can improve muscle mass, strength and physical performance (27, 28). Intake of protein, amino acids and vitamin D can also increase muscle mass and muscle strength (29, 30). In addition, a number of drugs have entered clinical trials to improve muscle mass through the administration of testosterone, selective androgen receptor modulators, myostatin inhibitors and growth hormone (31). For example, higher protein intakes at baseline were associated with less muscle strength loss over the period of the studies in the Women’s Health Initiative and the Framingham Offspring Cohort follow-up studies (32, 33). Visser et al. showed that the older adults with lower serum 25 (OH) D were twice as likely to have sarcopenia as those with higher serum 25 (OH) D (34). Another study proved resistance training can improve muscle mass and performance in older men (35). Even though some muscle deterioration is a natural part of aging, there are measures we can take to prevent the negative effects.
Our choice of predicted percentages instead of actual spirometry allowed us to reduce the interference of factors such as age, sex and height and is more suitable for the analysis of complex populations, which is one of our advantages over other studies. We chose cotinine instead of self-reported smoking status to represent smoking status and it reflects the overall biological effect of total smoking exposure. In addition, we integrated muscle mass as well as muscle strength to assess the muscle condition of the population, which is superior to those studies that just focused on muscle mass. We concur that MQI might not accurately represent sarcopenia as a whole because muscle function is implicated in sarcopenia. Our study did not, however, evaluate muscle function, which is an area we need to improve in the future. Although, according to the EWGSOP2, sarcopenia will be diagnosed in those who have low muscle mass or quality and low muscle strength, some published consensus by expert groups now include muscle function in the concept of sarcopenia (36). Also, our study could have missed some additional significant factors that might have affected the outcomes. Because of the small sample size in this study, we did not investigate whether other chronic diseases have an effect on lung function and muscle loss. A prospective study with a larger sample size and an all-age group may be done in the future. Finally, as this was a cross-sectional investigation, additional longitudinal cohort studies would be required to strengthen the temporal or causal correlation between MQI and lung function indices. Future multi-center, large-sample research will be necessary to confirm the findings presented above.
In conclusion, we found that MQI may associated with changes in lung function. Furthermore, in the middle-aged and older adult populations, lung function indicators and restrictive ventilation impairment were significantly associated with MQI, which seems to suggest that we should pay attention to muscle exercise in this group to improve lung function and avoid the development of chronic lung disease. This finding needs to be further confirmed by prospective future studies.
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
The NCHS Research Ethics Review Board examined and approved all research involving human subjects before they were used to gather data for the NHANES. Written informed permission was acquired by each subject. We conducted a study that was exempt from institutional review since it involves secondary data analysis from the NHANES.
CC designed the study and revised the manuscript. LW and ZX was responsible for data collection, analysis, and manuscript writing. YC collected the data. All authors reviewed and approved the article’s submission.
This study was financially supported by the Key Laboratory of Interventional Pulmonology of Zhejiang Province (2019E10014), the Zhejiang Provincial Key Research and Development Program (2020C03067), and the National Nature Science Foundation of China (82170017).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sayer, AA, and Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing. (2022) 51:afac220. doi: 10.1093/ageing/afac220
2. Ali, S, and Garcia, JM. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. (2014) 60:294–305. doi: 10.1159/000356760
3. Janssens, JP, Pache, JC, and Nicod, LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. (1999) 13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x
4. Kera, T, Kawai, H, Hirano, H, Kojima, M, Fujiwara, Y, Ihara, K, et al. Relationships among peak expiratory flow rate, body composition, physical function, and sarcopenia in community-dwelling older adults. Aging Clin Exp Res. (2018) 30:331–40. doi: 10.1007/s40520-017-0777-9
5. Bone, AE, Hepgul, N, Kon, S, and Maddocks, M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. (2017) 14:85–99. doi: 10.1177/1479972316679664
6. Liang, BM, Lam, DC, and Feng, YL. Clinical applications of lung function tests: A revisit. Respirology. (2012) 17:611–9. doi: 10.1111/j.1440-1843.2012.02149.x
7. Lebowitz, MD. The use of peak expiratory flow rate measurements in respiratory disease. Pediatr Pulmonol. (1991) 11:166–74. doi: 10.1002/ppul.1950110215
8. Kharitonov, SA, and Barnes, PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. (2001) 163:1693–722. doi: 10.1164/ajrccm.163.7.2009041
9. Gustafsson, LE, Leone, AM, Persson, MG, Wiklund, NP, and Moncada, S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. (1991) 181:852–7. doi: 10.1016/0006-291x(91)91268-h
10. Feizi, H, Alizadeh, M, Nejadghaderi, SA, Noori, M, Sullman, MJM, Ahmadian Heris, J, et al. The burden of chronic obstructive pulmonary disease and its attributable risk factors in the Middle East and North Africa region, 1990-2019. Respir Res. (2022) 23:319. doi: 10.1186/s12931-022-02242-z
11. Limpawattana, P, Inthasuwan, P, Putraveephong, S, Boonsawat, W, Theerakulpisut, D, and Sawanyawisuth, K. Sarcopenia in chronic obstructive pulmonary disease: A study of prevalence and associated factors in the Southeast Asian population. Chron Respir Dis. (2018) 15:250–7. doi: 10.1177/1479972317743759
12. Lopes, LCC, Vaz-Gonçalves, L, Schincaglia, RM, Gonzalez, MC, Prado, CM, de Oliveira, EP, et al. Sex and population-specific cutoff values of muscle quality index: Results from NHANES 2011-2014. Clin Nutr. (2022) 41:1328–34. doi: 10.1016/j.clnu.2022.04.026
13. Minetto, MA, Ballatore, MG, Botter, A, Busso, C, Pietrobelli, A, and Tabacco, A. DXA-based detection of low muscle mass using the total body muscularity assessment index (TB-MAXI): A new index with cutoff values from the NHANES 1999-2004. J Clin Med. (2022) 11:603. doi: 10.3390/jcm11030603
14. Soysal, P, Hurst, C, Demurtas, J, Firth, J, Howden, R, Yang, L, et al. Handgrip strength and health outcomes: Umbrella review of systematic reviews with meta-analyses of observational studies. J Sport Health Sci. (2021) 10:290–5. doi: 10.1016/j.jshs.2020.06.009
15. Hairi, NN, Cumming, RG, Naganathan, V, Handelsman, DJ, Le Couteur, DG, Creasey, H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: The Concord health and ageing in men project. J Am Geriatr Soc. (2010) 58:2055–62. doi: 10.1111/j.1532-5415.2010.03145.x
16. Hankinson, JL, Odencrantz, JR, and Fedan, KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. (1999) 159:179–87. doi: 10.1164/ajrccm.159.1.9712108
17. Heymsfield, SB, Stanley, A, Pietrobelli, A, and Heo, M. Simple skeletal muscle mass estimation formulas: What we can learn from them. Front Endocrinol. (2020) 11:31. doi: 10.3389/fendo.2020.00031
18. Hu, Z, Tian, Y, Song, X, Zeng, F, and Yang, A. Associations between sarcopenia with asthmatic prevalence, lung function and comorbidity. BMC Geriatr. (2022) 22:703. doi: 10.1186/s12877-022-03394-9
19. Park, HK, Lee, SH, Lee, SY, Kim, SS, and Park, HW. Relationships between lung function decline and skeletal muscle and fat mass changes: A longitudinal study in healthy individuals. J Cachexia Sarcopenia Muscle. (2021) 12:2145–53. doi: 10.1002/jcsm.12821
20. Chua, JR, Albay, AB Jr, and Tee, ML. Body composition of Filipino chronic obstructive pulmonary disease (COPD) patients in relation to their lung function, exercise capacity and quality of life. Int J Chron Obstruct Pulmon Dis. (2019) 14:2759–65. doi: 10.2147/copd.S222809
21. Ridwan, ES, Wiratama, BS, Lin, MY, Hou, WH, Liu, MF, Chen, CM, et al. Peak expiratory flow rate and sarcopenia risk in older Indonesian people: A nationwide survey. PLoS One. (2021) 16:e0246179. doi: 10.1371/journal.pone.0246179
22. Jeon, YK, Shin, MJ, Kim, MH, Mok, JH, Kim, SS, Kim, BH, et al. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: The Korea National Health and nutrition examination survey (KNHANES) 2008-2011. Osteoporos Int. (2015) 26:2423–9. doi: 10.1007/s00198-015-3152-8
23. Larsson, L, Degens, H, Li, M, Salviati, L, Lee, YI, Thompson, W, et al. Sarcopenia: Aging-related loss of muscle mass and function. Physiol Rev. (2019) 99:427–511. doi: 10.1152/physrev.00061.2017
24. Gungor, O, Sevinc, M, Ulu, S, and Kocyigit, I. Sarcopenia and cardiovascular disease in patients with and without kidney disease: What do we know? Int Urol Nephrol. (2022). doi: 10.1007/s11255-022-03393-0
25. Hsu, CS, and Kao, JH. Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol. (2018) 12:1229–44. doi: 10.1080/17474124.2018.1534586
26. Park, SW, Goodpaster, BH, Strotmeyer, ES, Kuller, LH, Broudeau, R, Kammerer, C, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care. (2007) 30:1507–12. doi: 10.2337/dc06-2537
27. Beaudart, C, Dawson, A, Shaw, SC, Harvey, NC, Kanis, JA, Binkley, N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos Int. (2017) 28:1817–33. doi: 10.1007/s00198-017-3980-9
28. Gielen, E, Beckwée, D, Delaere, A, De Breucker, S, Vandewoude, M, and Bautmans, I. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr Rev. (2021) 79:121–47. doi: 10.1093/nutrit/nuaa011
29. Coll, PP, Phu, S, Hajjar, SH, Kirk, B, Duque, G, and Taxel, P. The prevention of osteoporosis and sarcopenia in older adults. J Am Geriatr Soc. (2021) 69:1388–98. doi: 10.1111/jgs.17043
30. Uchitomi, R, Oyabu, M, and Kamei, Y. Vitamin D and sarcopenia: Potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. (2020) 12:3189. doi: 10.3390/nu12103189
31. Morley, JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int. (2016) 98:319–33. doi: 10.1007/s00223-015-0022-5
32. McLean, RR, Mangano, KM, Hannan, MT, Kiel, DP, and Sahni, S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci. (2016) 71:356–61. doi: 10.1093/gerona/glv184
33. Beasley, JM, Wertheim, BC, LaCroix, AZ, Prentice, RL, Neuhouser, ML, Tinker, LF, et al. Biomarker-calibrated protein intake and physical function in the Women’s Health Initiative. J Am Geriatr Soc. (2013) 61:1863–71. doi: 10.1111/jgs.12503
34. Visser, M, Deeg, DJ, and Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The longitudinal aging study Amsterdam. J Clin Endocrinol Metab. (2003) 88:5766–72. doi: 10.1210/jc.2003-030604
35. Villanueva, MG, He, J, and Schroeder, ET. Periodized resistance training with and without supplementation improve body composition and performance in older men. Eur J Appl Physiol. (2014) 114:891–905. doi: 10.1007/s00421-014-2821-1
Keywords: muscle quality index, lung function, NHANES, public health, cross-sectional research
Citation: Weng L, Xu Z, Chen Y and Chen C (2023) Associations between the muscle quality index and adult lung functions from NHANES 2011–2012. Front. Public Health. 11:1146456. doi: 10.3389/fpubh.2023.1146456
Received: 17 January 2023; Accepted: 28 March 2023;
Published: 10 May 2023.
Edited by:
Alessandro Vittori, Department of Anesthesia, Intensive Care and Operating Sections, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Lingling Xu, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2023 Weng, Xu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengshui Chen, Y2hlbmNoZW5nc2h1aUB3bXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.