- 1Central Laboratory of Liaocheng Peoples' Hospital, Liaocheng, Shandong, China
- 2Department of Clinical Laboratory Liaocheng City Dongchangfu District Maternal and Child Health Hospital, Liaocheng, Shandong, China
- 3State Key Laboratory of Microbial Technology, and Marine Biotechnology Research Center, Shandong University, Qingdao, China
Human papilloma virus (HPV) infection and its associated disease are major problems affecting millions of individuals around the world. The distribution of HPV genotypes is specific to different areas and different populations. Therefore, understanding the prevalence and genotype distribution of HPV in different populations in different geographical regions is essential to optimize HPV vaccination strategies and to maximize vaccine effects. In this study, 34,076 women from January 2016 to July 2022 were retrospectively analyzed at Liaocheng People's Hospital. Of these, 7540 women were high-risk HPV positive and the infection rate was 22.13%. The top ten genotypes were as follows in descending order: HPV16, HPV52, HPV58, HPV53, HPV39, HPV59, HPV66, HPV51, HPV18, and HPV56 and the least frequent genotypes were, in order, HPV 26, HPV45, and HPV82. The HPV16 positive infection rate was 25.37% and was reduced with the increase in the number of individuals who had undergone HPV screening. The HPV52 infection rate increased with increasing numbers of individuals undergoing HPV screening, and then remained unchanged. The proportion of 20–29-year-olds among all positive women began to decrease since the vaccine was available in 2018. The 30–39-year-old group accounted for the highest percentage of positive women, and the 50–59-year-old group of HPV-positive women with cervical cancer accounted for most infections. This study confirmed that HPV16, HPV52, HPV 58, and HPV53 is widely distributed in this population and the total HR-HPV infection rate remains high in this region. Our findings indicate that prevention of HPV infection in this region still faces important challenges.
Introduction
Human papilloma virus (HPV) infection and its related diseases remain a major public health concern throughout the world, with 690,000 cases of HPV-induced cancer in 2018, including 604,127 cases of cervical cancer (CC) and 341,831 deaths (1). HPV infection places an enormous burden on both individuals and the health care system. Cervical cancer caused by HPV is the fourth most common cancer among women worldwide (2). In 2020 alone, China recorded ~109,741 new cases of cervical cancer (18.2%) and 60,590 deaths (17.3%) (3).
HPV has many genotypes, and its infection is widespread in the population (4). Until now, ~450 HPV genotypes have been isolated and sequenced (5). HPV genotypes are clinically classified based on the L1 gene sequence alignment and their oncogenic risk by the World Health Organization (WHO). The most frequent high-risk HPV (HR-HPV) genotypes of cancer include HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59; HPV68 is the second most frequent genotype associated with potential carcinogenicity (2a), and several other HPV types (26, 53, 66, 67, 70, 73, 82, 30, 34, 69, 85, and 97) are also considered HR-HPV and are thought to be potentially carcinogenic (2b) (6).
Persistent HR-HPV infection is the main pathogenic factor (7). HR-HPV plays a key role in both cervical invasive squamous cell carcinoma (SCC) and most cervical adenocarcinoma (EAC) lesions (8–13). Viral oncogenes play the pivotal role in the occurrence of these pathological processes, such as E5–E7. E5 is known to play an important role in early cervical carcinogenesis by upregulate VEGF through EGFR, MEK/ERK1 and 2 along with PI3K/Akt, which induces cell invasion and metastasis (14, 15), while E6 and E7 are the key genes in driving the cells toward oncogenesis (16, 17). E6 protein binds to the cellular tumor suppressor protein p53 and induces degradation of p53 protein (18). E7 protein forms a complex with retinoblastoma protein (pRb) and degrades pRb through the ubiquitin-proteasome pathway (19, 20). The development of HPV-mediated cervical carcinogenesis is a multistep process, and cervical intraepithelial neoplasia (CIN) is a cervical precancerous lesion. In November 2020, the WHO announced a global strategy to accelerate cervical cancer elimination, setting HPV vaccination coverage to 90% by 2030, screening coverage of 70% for cervical lesions, and accessibility to cervical cancer treatment of 90% (21). Together with the European Beating Cancer Plan (22), this approach has greatly facilitated the elimination of all cancer causes by HPV. Cervical cancer is expected to be the first cancer that can be prevented and eliminated. However, the prevalence of common HPV genotypes varies in different regions and across different populations (23, 24); understanding the ecological diversity of HPV prevalence and genotype distribution between various populations in different geographical regions and at different times is essential for optimizing HPV vaccination strategies and to maximize vaccination effects.
The city of Liaocheng is located on the border of three provinces with a dense population and has a relatively poorly advanced economy in Shandong province. The Liaocheng People's Hospital is a typical regional medical center in Shandong Province and is well-known in the border area of Hebei-Shandong-Henan Province. Thus, HPV prevalence and genotype distribution in this region have important significance for Chinese epidemiological data and can provide an important rationale or reference for Chinese public health measures on cervical cancer prevention.
Materials and methods
Data sources
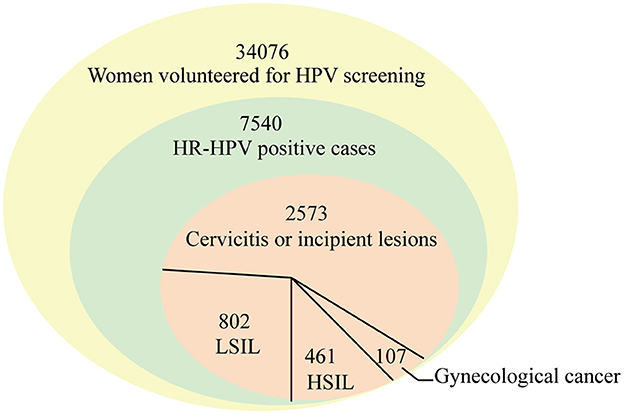
In this study, the data of 34,076 female from the Liaocheng People's Hospital between January 2016 and July 2022 were retrospectively analyzed. Samples were collected using a sterile disposable cervical sampler and stored in a cell preservation solution (Tellgen, China). The HR-HPV positive cases were 7,540, of which 3,943 women aged 16–87 years were voluntary simultaneously screened for cervical cytology. The studies were reviewed and approved by Medical Ethics Committee of Liaocheng People's Hospital.
HPV positivity was identified by PCR, and the HPV PCR-Flow fluorescence assay was used for HPV genotyping. Seventeen HR-HPV genotypes were tested using the Nucleic Acid genotyping Kit for Human Papillomavirus (Tellgen, China), including those closely related to cervical cancer (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66) and those associated with cervical cancer (HPV26, 53, 68, 82).
Statistical analysis
The prevalence of HPV infection, HPV genotypes of different grade, and the presence of single or multiple HPV infections, as well as their corresponding 95% confidence intervals (CIs), were estimated using binomial distribution analysis. The chi-square test was performed to compare differences in infection rates over time. A P-value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 17.0 (SPSS, IBM, USA).
Results and discussion
HR-HPV prevalence among 34,076 women
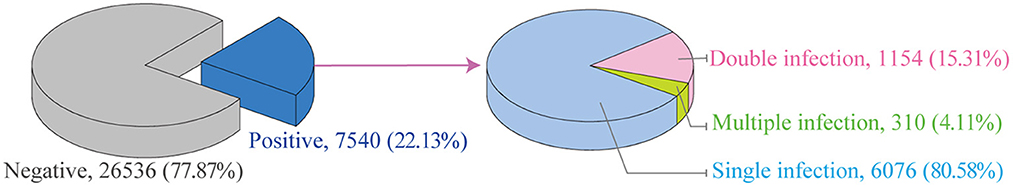
Between January 2016 and July 2022, the HR-HPV infection rates in 34,076 women were determined (Figure 1). A total of 7,540 HR-HPV positive cases were identified, and the total infection rate of 17 genotypes was 22.13% (7,540/34,076, 95% CI 21.69–22.57). The HR-HPV positive rate in this study was higher than the overall prevalence of HR-HPV positive (19.1%, range 15.3–24.4%) in China from July 2018 to June 2019 (25) and in Latin America (16%) in 2012 (23), which were similar to those in rural North China (22.2%) in 2017 (26) and in sub-Saharan Africa (24%) and in eastern Europe (21%) in 2012.
HPV DNA screening showed that single genotype infections were significantly more prominent than double (15.30%) or multiple infections (4.11%) (Figure 1). In this study, the infection rate for single genotype infections was 80.58%, which was comparable to those reported in Sichuan (77.2%) in November 2015 and August 2021 (27). The multiple infections in this study were similar to those observed in Yunnan Province (4.2%) (28, 29), but were lower than those reported in atypical squamous cells of women of undefined significance in Sichuan (22.8%) (27). The high rate of multiple infections may be associated with higher incidence of degree of cervical lesions, which was in agreements with the report that multiple infections could increase the risk of cervical cancer (30). Therefore, HPV screening is crucial for all women in this region.
Genotype distribution in 7,540 women
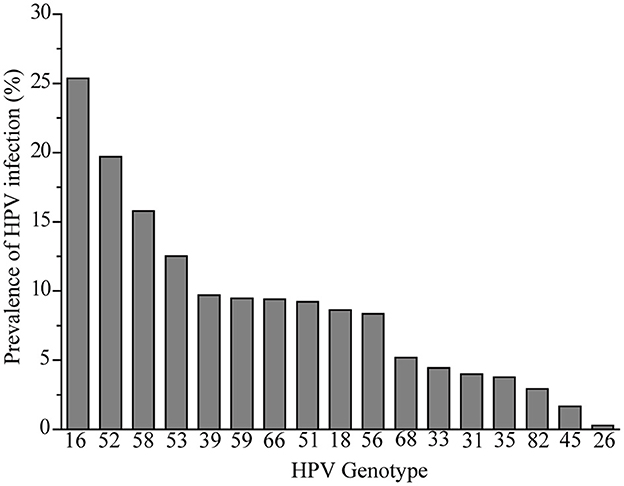
The infection rate of common HR-HPV genotypes was retrospectively analyzed. Seventeen different HPV genotypes were identified among the 7,540 HR-HPV positive women. As shown in Figure 2, the most common genotype was HPV16, and the top ten most prevalent genotypes were as follows in descending order: HPV16 (25.37%), HPV52 (19.71%), HPV58 (15.78%), HPV53 (12.52%), HPV39 (9.71%), HPV59 (9.46%), HPV66 (9.40), HPV51 (9.23%), HPV18 (8.63%), and HPV56 (8.36%); the least prevalent genotypes in order were HPV 26 (0.28%) and HPV45 (1.67%).
The prevalence of HPV prevalence in women around the world had significant regional variations. In western countries or in continents of Europe/ America, such as Guatemala, HPV18 is the second most prevalent (31, 32). However, in west Africa Lomé, Togo, the most common genotypes are HPV56 and HPV51 (33). In Asian countries, HPV58 and HPV52 are becoming more prevalent than HPV18 (34, 35). The HR-HPV genotypes detected the most frequently were HPV52, HPV16, HPV53, HPV58, HPV51, and HPV68 in China (26). Similar with our study, the top HPV genotype was HPV16 in Hengyang and Shanxi, while HPV58 was the second most prevalent in Hengyang (36, 37). Thus, popular HPV types are not identical in different countries or in different areas of China. The differences in prevalence of HPV in Liaocheng populations may be the women investigated in this study were women with gynecological diseases, of different ethnicity, lifestyle, and urbanization.
HPV prevalence of vaccine-targeted genotypes in 7 years
The currently approved 9-valent HPV vaccine has the highest valency of all vaccine types, targeting HR-HPV16, 18, 31, 33, 45, 52, and 58. As shown in Table 1, the prevalence of genotypes targeted by the HR-HPV16 vaccine (5.61%), HPV52 (4.36%), HPV58 (3.49%), and HPV 18 (1.91%) was higher in this study. Other vaccine-targeted genotypes HPV45 (0.37%), HPV31 (0.88%), and HPV33 (0.98%) were rarely identified in women (Table 1), with positivity rates <10%. However, the prevalence of genotypes HR-HPV53 (2.77%), HPV39 (2.15%), HPV59 (2.09%), HPV 66 (2.08%), HPV51 (2.04%), HPV56 (1.85%), and HPV 68 (1.15%), which were genotypes not targeted by the effective vaccine, was also high in this study.
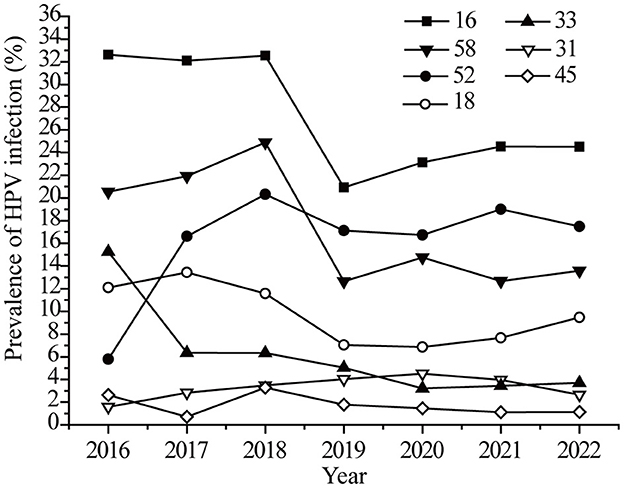
The infection rates of targeted vaccine genotypes in the last seven years was analyzed. As shown in Figure 3, the HPV16 infection rate showed a sudden drop (32.53 to 20.92%) in 2019, and then the declining rate slowed. The changing trend for HPV 18 and 58 were similar to that of HPV 16. The HPV52 infection rate showed an obvious growth trend (5.79 to 19.34%) from 2016 to 2017, and then leveled off (Figure 3). There were no significant changes in the HPV 32, 33, and 45 infection rates. It should be noted that HPV screening was added to female medical physical examination protocols in 2019, with more healthy women involved in screening. Therefore, the trends observed for HPV 16, 18, and 58 were possibly not only due to the effects of the vaccine but also to the increased screening.
The percent of vaccine genotypes in different age groups was further analyzed, as shown in Figure 4A, the proportion of vaccine genotypes in 20–29-year-old females markedly decreased from 27.86% in 2016 to 12.41% in 2021 (P = 0.01) (Table 2). The proportion of vaccine genotypes in the 15–20-year-old women increased in 2018, peaked in 2019 (4.23%), and then declined (P = 0.56), while no obvious changes were observed in the 30+ year-old groups (P = 0.92) (Figure 4A, Table 2). The HPV vaccine has been available in China since 2018, which led to increased awareness of HPV screening among young people, and the change in HPV infection in individuals aged 20–29 years may be due to the efficacy of the vaccines. Furthermore, the prevalence of HPV genotypes targeted by vaccines was different in individuals aged 20–29 years (Figure 4B). The ratio in positivity of HPV16 in 20–29-year-olds decreased slightly from 2018 to 2022, but the change was not significant (P = 0.88).
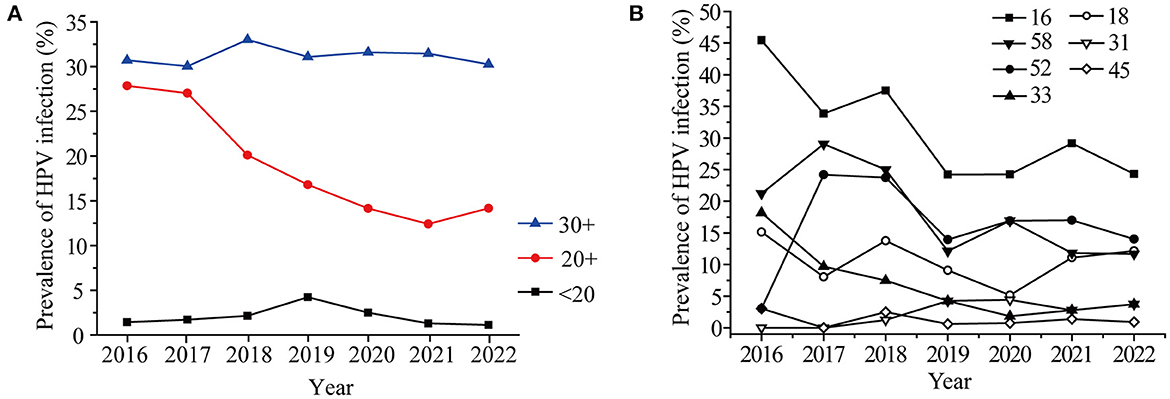
Figure 4. Prevalence of HR-HPV in women under 40 years old (A) and Prevalence of seven HR-HPV genotypes in women aged 20-29 years old in different years (B).
Different vaccines achieve cross-protection to potential precancerous lesions, in the case of effective vaccines, a decline in the incidence of global CIN 3 of 25.8% has been achieved in 6 years (38). In Denmark, Norway, and Sweden, the HPV infection rate decreased from 36.5% in 2006–2008 to 34.5% in 2012–2013 after the vaccine was introduced (39). In early 2018, the vaccine was already available in China. The HPV16, 18, and 58 infection rates showed a slow drop after 2019 in this study, and the vaccine may play important roles. However, the other vaccine genotypes except for HPV16 did not achieved a reduction in prevalence among 20–29-years-old individuals in our study. Furthermore, the prevalence of HR-HPV, especially HPV52 and HPV58, is higher in black rural women (40). HPV52 (21.7%) is the most common HR-HPV genotype in rural North China (26). The increasing prevalence of HPV52 from 2016–2017 in this study may be associated with rural women. Therefore, the HPV vaccine promotes the public's understanding of HPV, although it is not yet very common in this region. Meanwhile, it is worth noting that HPV 53, 39, 59, 66, 51, 56 were also the most commonly observed in the present study. However, they are still not covered in the latest 9-valent HPV vaccine, these HR-HPV types should attract the attention of HPV vaccines researchers.
Distribution of cervical lesions in 3943 HR-HPV positive women
Among 3,943 women receiving cervical cytology examination, as seen in Table 3 and Figure 5, women with cervicitis or incipient lesions were 2,573 (65.25%), women with low-grade squamous intraepithelial lesion (LISL) were 802 (20.34%), and women with high-grade squamous intraepithelial lesion (HISL) were 461 (11.69%). Furthermore, 107 cases (2.71%) of gynecological cancer were identified, including cervical squamous cell carcinomas (2.16%), adenocarcinoma (0.25%), endometrial cancer (0.25%), teratoma (0.03%), and ovarian cancer (0.03%). The most frequently HR-HPV genotypes were HPV16, HPV58 and HPV52 in women with gynecological cancer (43.97, 22.43, and 16.82%), HISL (46.20, 17.79, and 13.88%), LISL (24.44, 17.21, and 15.46%) and cervicitis or incipient lesions (20.05, 18.77, and 13.76%). HPV16 was the highest HR-HPV genotype in women with gynecological cancer and HISL compared with other two groups (P = 8.5E-05), while no obvious changes of other two genotypes HPV52 and HPV58 were observed in each group (HPV52: P = 0.90; HPV58: P = 0.97). It can be seen that HPV16 plays a key role in cervical lesions.
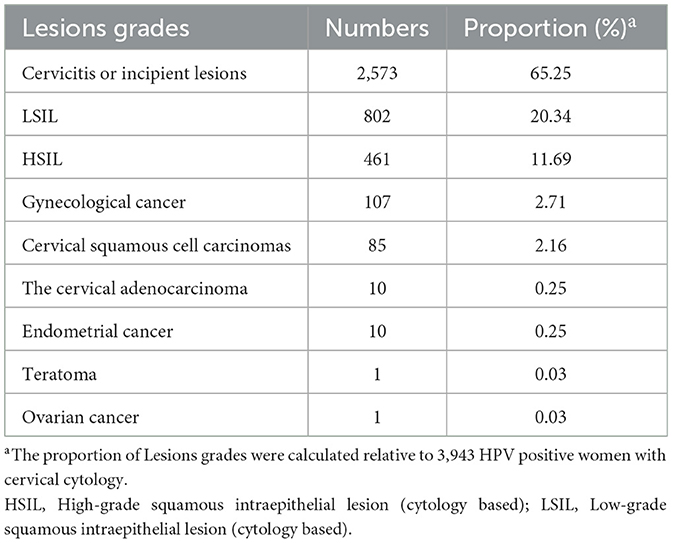
Table 3. Proportion of different lesion grades in 3,943 HR-HPV positive women with cervical cytology.
Our findings were similar to those of previous studies, in which HPV infection was closely associated with cervical squamous carcinoma and adenocarcinoma (9–14). HR-HPV infection may also be associated with the appearance of primary ovarian squamous cell carcinoma (41). The estimated incidence rate of cervical cancer was 6.7–11.2 per 100,000 in 2008 in Central Arkansas (24). The rate of cervical cancer showed a higher incidence (85/34,076) in this study (Tables 1, 3). Furthermore, the prevalence of cytologic cervical pathology is directly proportional to the severity of the lesion. Globally, the HR-HPV positive rate in women with normal cytology was around 11–12%, reaching around 90% in grade 3 CIN or invasive cervical cancer, and 100% in cervical cancer (23). The results suggest that cervical cancer screening is necessary in the local region.
Age distribution of HR-HPV prevalence
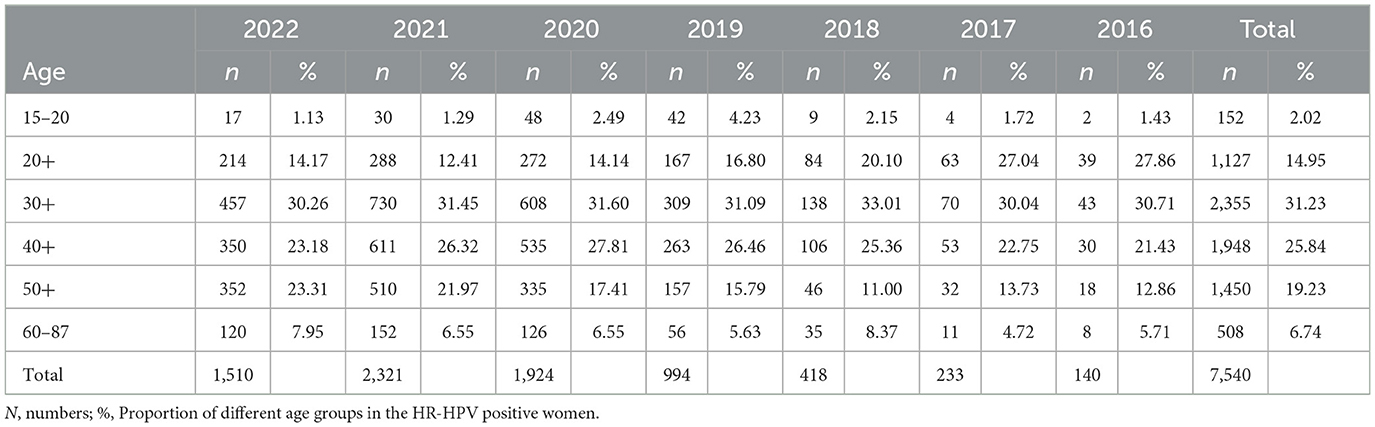
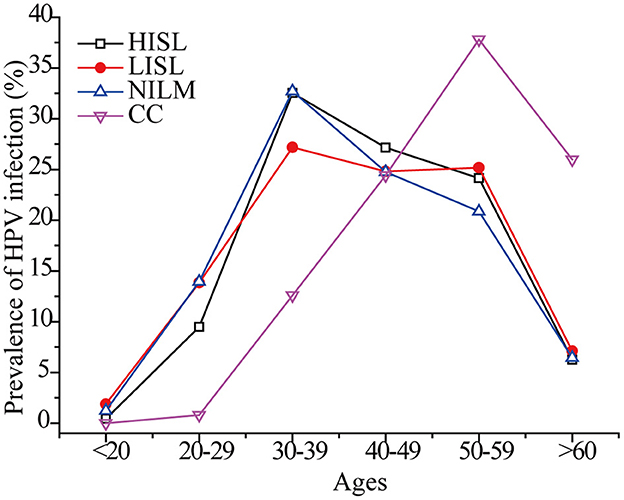
As shown in Table 2, the ages of 7,540 HR-HPV positive women ranged from 15 to 87 years, of which 91.25% (6,880/7,540) were 20–59 years old. HR-HPV infection rates of the women in the 30–39-year group were 31.23% (2,355/7,540) and were the highest (P = 0.04), followed by that of the 40–49-year-old group (25.84%, 1,948/7,540) and the 50–59-year group (19.23%, 1,450/7,540). It should be noted that the youngest HR-HPV positive patient was 15 years old, and the youngest cervical cancer patient was 28 years old. A case in young women as young as 19 years old (42) has also been reported. Thus, HR-HPV infection rates of women decreased with increasing age, and the risk of cervical cancer in the younger age groups should also be taken much more seriously.
As shown in Figure 6, the peak incidence of cervical cancer was among 50–59-year-old females. In other age groups, the maximum HR-HPV prevalence rate was observed in women 30–39 years of age, and slowly decreased in older ages, while a second small peak appeared in women 50–59 years of age. The global prevalence of HPV infection in women with normal cytology presents two peaks in women < 25 years and perimenopausal or early menopausal women (3). Similar dual peaks indicating the highest HR-HPV infection rates were observed in women younger than 30 years of age (22.0%) and in women 50 years of age and older (21.8%) (26). The proportion of postmenopausal women with persistent HPV infection is high (43), and middle-aged women may be infected with new HPV genotypes (44). The time from CIN 3 to the progression of invasive cervical cancer is estimated to be 10–20 years or more (45). This may explain the highest infection rates in 30–39 and the highest cervical cancer rates in women aged 50–59 years. Therefore, it is also necessary to detect pathological changes in the genital tract and perform continuous HPV screening in perimenopausal, menopausal, and postmenopausal women.
Conclusion
This study revealed HPV prevalence and genotype distribution in Liaocheng city, Shandong province, which were mainly consistent with the prevalence of HPV in China. In addition to HPV16, HPV52, and HPV 58, the prevalence of HPV53, HPV39, HPV59, HPV66, HPV51, HPV56, and HPV68 was also high in this region, and this has important guiding role in the optimization and development of vaccine research. This study also indicated the urgent need to improve HPV prevention and control measures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Review Committees of Liaocheng People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LZ analyzed and interpreted the patient data and was a major contributor in writing the manuscript. LZ also analyzed and interpreted the patient data collaboratively. CX, SC, FY, and WW collected data. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HPV, Human papilloma virus; HR-HPV, high-risk human papilloma virus; Cis, confidence intervals; CIN, cervical intraepithelial neoplasia; LISL, low-grade squamous intraepithelial lesion; HISL, high-grade squamous intraepithelial lesion.
References
1. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. (2020) 8:e180–90. doi: 10.1016/S2214-109X(19)30488-7
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–E86. doi: 10.1002/ijc.29210
3. World Health Organization. Human Papillomavirus and Related Diseases Report. (2021). Available online at: https://hpvcentre.net/statistics/reports/X-WX.pdf (accessed June 6, 2021).
4. de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology. (2013) 445:2–10. doi: 10.1016/j.virol.2013.04.023
5. MCBRIDE AA. Human papilloma viruses: diversity, infection and host interactions. Nat Rev Microbiol. (2022) 20:95–108. doi: 10.1038/s41579-021-00617-5
6. World Health Organization. Human Papillomaviruses. World Health Organization: Lyon, France, (2006).
7. Kjaer S, Høgdall E, Frederiksen K, Munk C, van den Brule A, Svare E, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal woman over a 10-year period. Cancer Res. (2006) 66:10630–6. doi: 10.1158/0008-5472.CAN-06-1057
8. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. (1999) 189:12–9.
9. José FXB, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. (2010) 11:1048–56. doi: 10.1016/S1470-2045(10)70230-8
10. Pirog EC, Lloveras B, Molijn A, Tous S, Guimerà N, Alejo M, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Modern Pathol. (2014) 27:1559–67. doi: 10.1038/modpathol.2014.55
11. Molijn A, Jenkins D, Chen W, Zhang X, Pirog E, Enqi W, et al. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int J Cancer. (2016) 138:409–16. doi: 10.1002/ijc.29722
12. Pulkkinen J, Kares S, Huhtala H, Kholová I. Detection and outcome of endocervical atypia in cytology in primary HPV screening programme. Diagnostics. (2021) 11:2402. doi: 10.3390/diagnostics11122402
13. Wang WP, An JS, Yao H, Li N, Zhang YY, Ge L, et al. High-risk type HPV subtypes in the infection status of different pathological type of cervical cancer and attribution analysis. Zhonghua Fu Chan Ke Za Zhi. (2019) 54:293–300. doi: 10.3760/cma.j.issn.0529-567x.2019.05.002
14. Kim SH, Juhnn YS, Kang S, Park SW, Sung MW, Bang YJ, et al. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ ERK1,2 and PI3K/Akt. Cell Mol Life Sci. (2006) 63:930–8. doi: 10.1007/s00018-005-5561-x
15. Venuti A, Paolini F, Nasir L, Corteggio A, Roperto S, Campo MS, et al. Papillomavirus E5: the smallest oncoprotein with many functions. Mol Cancer. (2011) 10:140. doi: 10.1186/1476-4598-10-140
16. Jabbar SF, Abrams L, Glick A, Lambert PF. Persistence of high-grade cervical dysplasia and cervical cancer requires the continuous expression of the human papillomavirus type 16 E7 oncogene. Cancer Res. (2009) 69:4407–14. doi: 10.1158/0008-5472.CAN-09-0023
17. Yamato K, Yamada T, Kizaki M, Ui-Tei K, Natori Y, Fujino M, et al. New highly potent and specific E6 and E7 siRNAs for treatment of HPV16 positive cervical cancer. Cancer Gene Ther. (2008) 15:140–53. doi: 10.1038/sj.cgt.7701118
18. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. (1990) 63:1129–36. doi: 10.1016/0092-8674(90)90409-8
19. Dyson N, Howley PM, Münger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. (1989) 243:934–7. doi: 10.1126/science.2537532
20. Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. (1996) 56:4620–4.
21. WHO. Global Strategy to Accelerate the elimination of Cervical Cancer as a Public Health Problem. (2020). Available online at: https://apps.who.int/iris/rest/bitstreams/1315304/retrieve (accessed August 29, 2022).
22. European Commission. Communication from the Commission to the European Parliament the Council. Europe's Beating Cancer plan (2021). Available online at: https://ec.europa.eu/health/sites/health/files/non_communicable_diseases/docs/eu_cancer-plan_en.pdf (accessed August 29, 2022).
23. Formana D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. (2012) 30:F12–23. doi: 10.1016/j.vaccine.2012.07.055
24. Nakagawa M, Spencer HJ, Coleman HN, Greenfield WW. Distribution of human papillomavirus (HPV) types and anti-HPV T-cell immune responses among different racial/ethnic groups in Central Arkansas. J Ark Med Soc. (2013) 109:160–3.
25. Zeng Z, Austin RM, Wang L, Guo X, Zeng Q, Zhao C, et al. Nationwide prevalence and genotype distribution of high-risk human papillomavirus infection in China. Am J Clin Pathol. (2022) 157:718–23. doi: 10.1093/ajcp/aqab181
26. Zhao S, Zhao X, Hu S, Lu J, Duan X, Zhao F, et al. Distribution of high-risk human papillomavirus genotype prevalence and attribution to cervical precancerous lesions in rural North China. Chinese J Cancer Res. (2019) 31:663–72. doi: 10.21147/j.issn.1000-9604
27. Jiang W, Austin RM, Zhang H, He Y, Xu L., Zhao C, et al. The clinical utility of extended high-risk HPV genotyping in women with ASC-US cytology. Am J Clin Pathol. (2022) 158:472–9. doi: 10.1093/ajcp/aqac073
28. Baloch Z, Yuan T, Wang B, Tai W, Feng Y, Liu Y, et al. Ethnic and geographic variations in HPV prevalence and genotype distribution in North–Western Yunnan, China. J Med Virol. (2016) 88:532–40. doi: 10.1002/jmv.24352
29. Sun LL, Jin Q, Li H, Zhou XR, Song ZQ, Cheng XM, et al. Population-based study on the prevalence of and risk factors for human papillomavirus infection in Qujing of Yunnan province, Southwest China. Virol J. (2012) 9:153. doi: 10.1186/1743-422X-9-153
30. Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. (2005) 191:1796–1707. doi: 10.1086/428850
31. Clifford, G. M., Tully S., Franceschi S. Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: A meta-analysis from HPV infection to cervical cancer. Clin Infect Dis. (2017) 64:1228–1235. doi: 10.1093/cid/cix135
32. Lou H, Gharzouzi E, Guerra SP, Domgue JF, Sawitzke J, Villagran G, et al. Low-cost HPV testing and the prevalence of cervical infection in asymptomatic populations in Guatemala. BMC Cancer. (2018) 18:562. doi: 10.1186/s12885-018-4438-y
33. Kuassi-Kpede AP, Dolou E, Zohoncon TM, Traore IMA, Katawa G, Ouedraogo RA, et al. Molecular characterization of high-risk human papillomavirus (HR-HPV) in women in Lomé, Togo. BMC Infect Dis. (2021) 21:278. doi: 10.1186/s12879-021-05956-5
34. Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. (2007) 7:453–59. doi: 10.1016/S1473-3099(07)70158-5
35. Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. (2008) 26:K1–16. doi: 10.1016/j.vaccine.2008.05.064
36. Tang SY, Liao YQ, Hu Y, Shen HY, Wan YP, Wu YM, et al. HPV Prevalence and genotype distribution among women from Hengyang District of Hunan Province, China. Front Public Health. (2021) 9:710209. doi: 10.3389/fpubh.2021.710209
37. Zhao XL, Hu SY, Zhang Q, Dong L, Feng RM, Zhao FH, et al. High-risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. J Gynecol Oncol. (2017) 28:e30. doi: 10.3802/jgo.2017.28.e30
38. Neis F, Holleczek B, Henes M, Juhasz-Böss I, Wallwiener D, Neis KJ. Proposal for a descriptive and differentiated presentation of the longitudinal impact of the new organized cancer screening guideline and HPV vaccination in Germany. Arch Gynecol Obstet. (2022) 1–2. doi: 10.1007/s00404-022-06747-2
39. Dillner J, Nygård M, Munk C, Hortlund M, Hansen BT, Lagheden C, et al. Decline of HPV infections in Scandinavian cervical screening populations after introduction of HPV vaccination programs. Vaccine. (2018) 36:3820–9. doi: 10.1016/j.vaccine.2018.05.019
40. Volpini LPB, Dias JA, de Freitas LB, Silva MCLF, Miranda AE, Spano LC. Viral load and high prevalence of HR-HPV52 and 58 types in black women from rural communities. BMC Infect Dis. (2021) 2:362. doi: 10.1186/s12879-021-06042-6
41. Xi Y, Zhang ML, He C, Cheng GP, Jin JY, Su D, et al. Primary ovarian squamous cell carcinoma: clinicopathological features and prognostic analysis of fifteen cases. Zhonghua Bing Li Xue Za Zhi. (2022) 51:332–7. doi: 10.3760/cma.j.cn112151-20210719-00516
42. Zhang J, Liu Z, Zhang QY, Gong TT. Successful treatment of a 19-year-old patient with locally advanced clear cell adenocarcinoma of the uterine cervix using recombinant human adenovirus type 5 (Oncorine) combined with chemoradiotherapy: a case report. Ann Transl Med. (2021) 9:1747. doi: 10.21037/atm-21-5963
43. Smith EM, Johnson SR, Ritchie JM, Feddersen D, Wang D, Haugen TH, et al. Persistent HPV infection in postmenopausal age women. Int J Gynecol Obstet. (2004) 87:131–7. doi: 10.1016/j.ijgo.2004.07.013
44. Ferris DG, Brown DR, Giuliano AR, Myers E, Joura EA, Velicer C, et al. Prevalence, incidence, and natural history of HPV infection in adult women ages 24 to 45 participating in a vaccine trial. Papillomavirus Res. (2020) 10:100202. doi: 10.1016/j.pvr.2020.100202
Keywords: human papillomavirus, prevalence, genotype, cervical cancer, vaccine
Citation: Zheng L-l, Chen S-f, Yang F, Wang W-h, Xu C and Zheng L-y (2023) High-risk HPV prevalence and genotype distribution among women in Liaocheng, Shandong Province, China from 2016 to 2022. Front. Public Health 11:1145396. doi: 10.3389/fpubh.2023.1145396
Received: 16 January 2023; Accepted: 01 March 2023;
Published: 30 March 2023.
Edited by:
Senthil Kumaran Satyanarayanan, China Medical University Hospital, TaiwanReviewed by:
Basem Fares, Cannabotech Ltd., IsraelZhihua Ou, Beijing Genomics Institute (BGI), China
Copyright © 2023 Zheng, Chen, Yang, Wang, Xu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-yuan Zheng, MjAxODIwMjkyQG1haWwuc2R1LmVkdS5jbg==
 Li-li Zheng1
Li-li Zheng1 Li-yuan Zheng
Li-yuan Zheng