- 1School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
- 2Department of Pharmacy, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, Sichuan, China
- 3Department of Pharmacy, Chengdu Second People's Hospital, Chengdu, Sichuan, China
- 4Department of Pharmacy, The Third People's Hospital of Chengdu, Chengdu, Sichuan, China
Background: Whether the high cost of cancer drugs is commensurate with their value to patients, which has become the focus of public concern. We aimed to assess the value of new cancer drugs approved for solid cancer in China and to explore the association between price and value of drugs.
Methods: We identified all new drugs for solid tumor that approved by the China's National Medical Products Administration (NMPA) between 2016 and 2020. The value of these drugs was assessed according to the American Society of Clinical Oncology Value Framework (ASCO-VF) and the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). We calculated Cohen's κ statistic to describe agreement between the two frameworks. Spearman's correlation coefficient was used to evaluate the correlation between price and value of drugs.
Results: Between 2016 and 2020, 37 new drugs were approved by the NMPA for solid tumor and we could evaluate the value of 28 drugs (76%). Eight (29%) of drugs were approved for non-small-cell lung cancer and 6 (21%) for breast cancer. ASCO-VF scores had a range of −20 to 110.1, and the median score was 43.3 (inter-quartile range 27.1–58.35). Only seven drugs (25%) met the ASCO-VF cutoff score. By the ESMO-MCBS, 13 drugs showed a meaningful value. Agreement between these two frameworks thresholds was only fair (κ = 0.515, P < 0.05). We found no statistically significant correlation between launch price of drugs and clinical benefit according to both frameworks.
Conclusions: Not all NMPA-approved new cancer drugs had meaningful value as measured by ASCO-VF or ESMO-MCBS. There was no significant correlation between drug price and the level of clinical benefit.
Introduction
Innovations in cancer therapy, particularly the influx of new drugs have yielded high expectations of transform treatment of the disease from all healthcare stakeholders (1, 2). Nevertheless, dramatic rise in drug costs has recently highlighted a vigorous debate over whether cancer drugs prices, especially for that of targeted drugs and immunotherapies, commensurate with their value to patients, within reach of who need them not only in developed country, but also in developing country like China with scarce resources and rising demand for health services (3, 4).
To our knowledge, not all people know the price of everything but the value of nothing. It has never been more important to assess the value of new cancer drugs, and several organizations including the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), Institute for Clinical and Economic Review (ICER), National Comprehensive Cancer Network (NCCN), and the pan-Canadian Oncology Drug Review Expert Review Committee (pCODRERC) have recently taken a step forward in this endeavor, developing tools for value assessment (5–11). All these tools have been designed for value assessment with the aim of weighing up the balance between efficacy, toxicity, quality of life and costs. Despite of their different conceptual definition of “value,” Bentley et al. (12) reported that the ASCO and ESMO tools demonstrated convergent validity and inter-rater reliability for value assessment for new cancer drugs.
In recent years, regulatory reforms have led to the introduction of a series of expedited programs to accelerate development, review, and approval of new drugs in China (13). Here, we overview the landscape of new cancer drugs approved by NMPA for solid cancer between 2016 and 2020 in China, describe the value of these drugs and further explore whether value is related to the drug price.
Methods
Data sources and extraction
We used the publicly available data to identify all new drugs (new molecular entities and novel biologic agents) approved by the China's National Medical Products Administration (NMPA) between January 1, 2016 and December 31, 2020, with initial indications for solid tumor. Meanwhile, we assessed whether the drug was granted with one of expedited programs in NMPA pathways and designations to accelerate drug approval (special review, priority review, conditional approval, urgently needed overseas drugs, and breakthrough therapy). Notably, drugs that were later approved for additional indications were not considered in this study.
The launch price and postlaunch price of drugs were extracted from the trade name and generic name recorded in the Hospital Information System (HIS). To estimate monthly treatment cost of a drug, we used the prescription and dosing information from the NMPA-approved label. Monthly treatment costs were calculated over an average of 30 days on the basis of the dosage schedule for an adult patient weighing 60 kg with a body surface area of 1.70 m2. The cost of all regimes was adjusted to provide the price per 4-week period (33.3% increase for 3-week treatment cycles and 100% increase for 2-week treatment cycles). Drug prices were converted to US dollars at the exchange rate as of August 29, 2022.
To quantify the clinical benefit from the pivotal clinical trials supporting regulatory approval, we applied two value frameworks developed by ASCO and ESMO, namely the American Society of Clinical Oncology Value Framework (ASCO-VF) version 2 (6), and European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) version 1.1 (8). Scores were assessed by one reviewer and checked by a second one, with any discrepancies resolved by a senior reviewer. In contrast to ESMO-MCBS, ASCO-VF was not planned to score single-arm studies and was therefore only suitable for phase II or III randomized clinical trials. In cases in which multiple pivotal clinical trials have been done and yield different clinical benefit scores for a given drug, the highest score was considered. Consistent with the developer of the value frameworks, meaningful clinical benefit was defined as a grade of A or B (for the curative setting) or 4, 5 (for the palliative setting) using ESMO-MCBS, whereas ASCO-VF did not clearly define what score was deemed “meaningful value.” Cherny et al. (14) recommended that the optimal threshold score of 45 or higher was proposed for recognizing substantial benefit for ASCO-VF by generating receiver operating characteristic (ROC) curves. Nevertheless, given the differences in construction and goals of ASCO-VF and ESMO-MCBS, they might yield some discordance in a cohort of studies. Thus, we split scores at the 75th percentile of ASCO-VF scores as the cutoff score for subsequent analyses, referring to the meaningful value achieved of ESMO-MCBS as a grade of 4, 5, B, or A (15).
Statistical analysis
All data were collected in an Excel file designed for this study. Statistical analysis was conducted in IBM SPSS 25.0. Continuous data were graphed and analyzed to assess the normality of the underlying distribution. Spearman's correlation coefficient was used to describe the association between launch prices and clinical benefit according to ESMO-MCBS and ASCO-VF. We generated a ROC curve to establish a discrimination threshold of ASCO-VF scores to meet ESMO-MCBS criteria. P < 0.05 was deemed statistically significant.
Results
Number and characteristics of new drugs
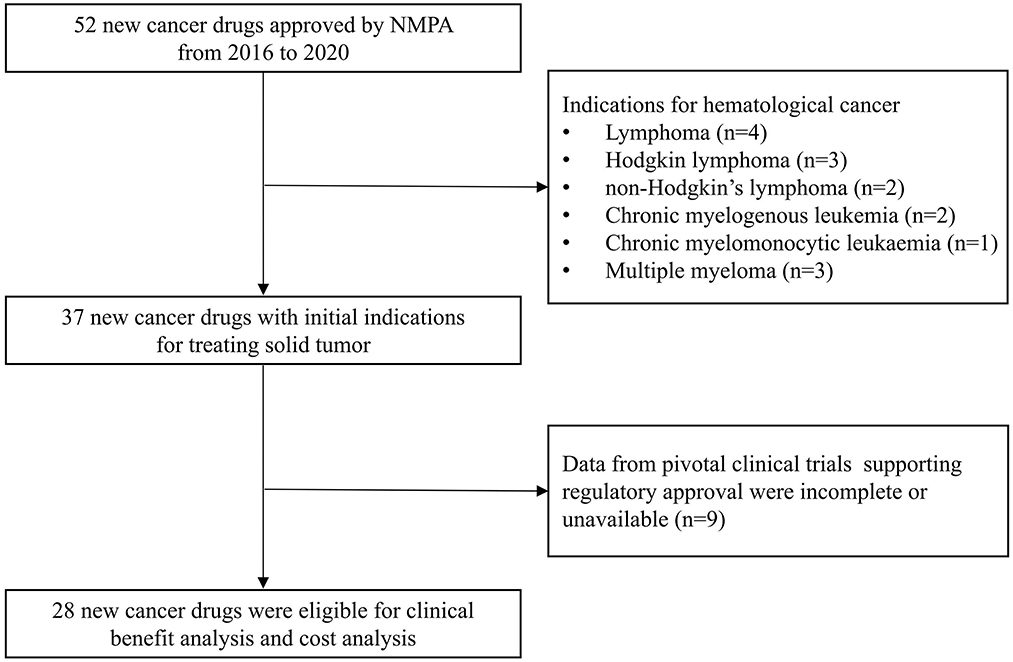
From 2016 to 2020, 52 new cancer drugs received initial regulatory approval by NMPA, 37 (71%) of which were approved for treating solid tumors and 15 (29%) were approved for treatment of hematologic cancers (Figure 1). Because data from pivotal clinical trials of nine drugs were incomplete or unavailable, only 28 drugs with prices and pivotal trials data were analyzed for subsequent analyses. The most common indications were non-small-cell lung cancer (N = 8, 29%) and breast cancer (N = 6, 21%) (Table 1). Of these, 23 drugs were imported from abroad and five drugs were domestic. Furthermore, 24 of which have benefited from at least one expedited program and most received priority review and special review.
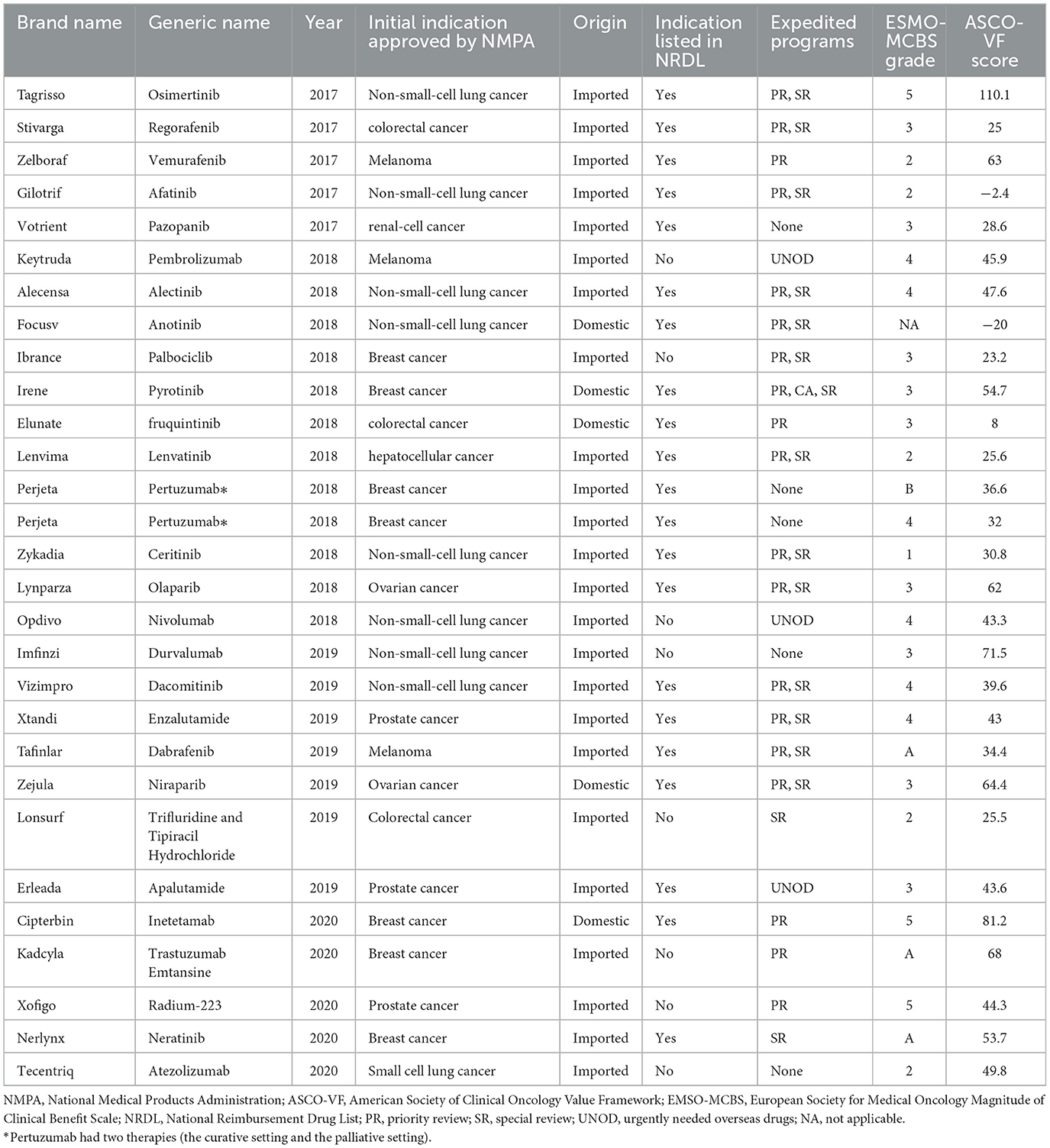
Table 1. Characteristics and clinical benefit of NMPA-approved new drugs for treating solid tumor from 2016 to 2020 using the ASCO-VF and the ESMO-MCBS.
Clinical benefit of new drugs
For new drugs used for treating solid tumors, the median ASCO-VF score was 43.3 (interquartile range, 27.1–58.35; range −20 to 110.1), and the scores were normally distributed (Supplementary Figure S1). 14 drugs fell below, 14 drugs were above. We split scores at the 75th percentile of ASCO-VF scores-−58.35 as the cutoff score that deemed “meaningful clinical benefit”. Seven drugs were above the threshold whereas 21 (75%) fell below. By the ESMO-MCBS, 13 drugs met the criteria for meaningful benefit. Three (27%) of the 13 drugs meeting ESMO-MCBS thresholds were above the 75th percentile of ASCO-VF scores −58.35. For drugs in the palliative setting, Of the 19 drugs that did not meet the ASCO-VF cutoff score, only 12 fell below the ESMO-MCBS criteria. For drugs in curative setting, four (100%) of four drugs met ESMO-MCBS thresholds, only one were above the ASCO-VF cutoff score. The clinical benefit was shown in Table 1.
Association between ASCO-VF and ESMO-MCBS
ROC curve was used to establish a discrimination threshold for ASCO-VF score to meet the ESMO-MCBS criteria of meaningful clinical benefit, and the threshold was determined to be approximately 31. However, the area under the curve was 0.662, suggesting only fair predictive value (Supplementary Figure S2). Agreement between ASCO-VF and ESMO-MCBS thresholds was only fair (κ = 0.515, P < 0.05).
Correlation between price and value of drugs
In China, the median monthly treatment costs per patient at launch for the included cancer drugs were $4,381. As of August 25, 2022, the median monthly treatment costs were $1,408, indicating that the postlaunch price changes for most NMPA approved cancer drugs were roughly three times less than the launch prices (Figure 2). We found no statistically significant associations between launch prices of drugs approved for solid tumors and clinical benefit were observed according to both frameworks (Figure 3). For ASCO-VF, launch prices had weak correlation with clinical benefit (Spearman's ρ < 0.30; P > 0.05). The launch price of new cancer drugs and ESMO-MCBS grades had weak correlation (Spearman's ρ < 0.30; P > 0.05).
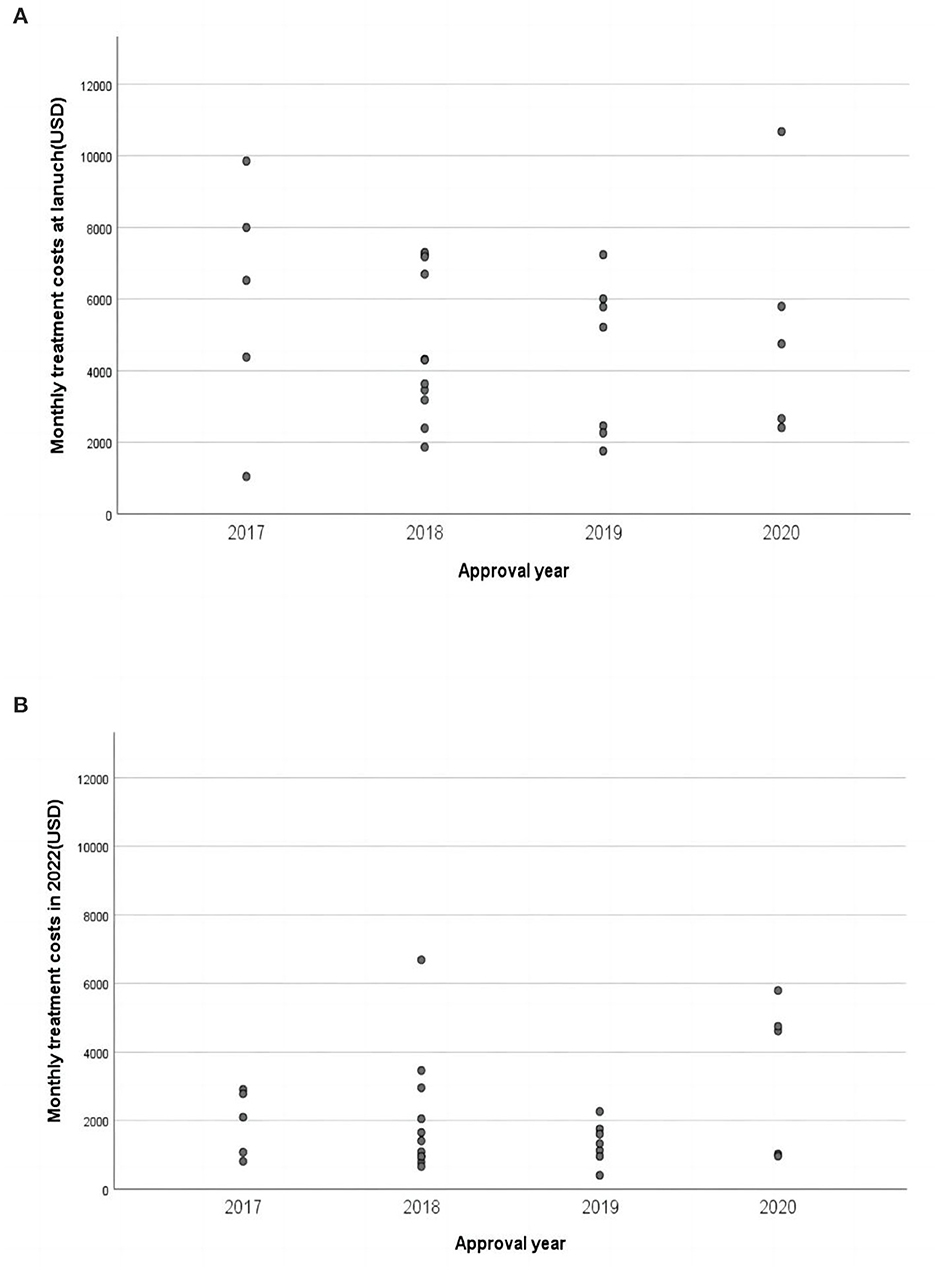
Figure 2. Monthly treatment costs of approved cancer drugs in China. (A) Monthly treatment costs of approved cancer drugs at launch. (B) Monthly treatment costs of approved cancer drugs in 2022.
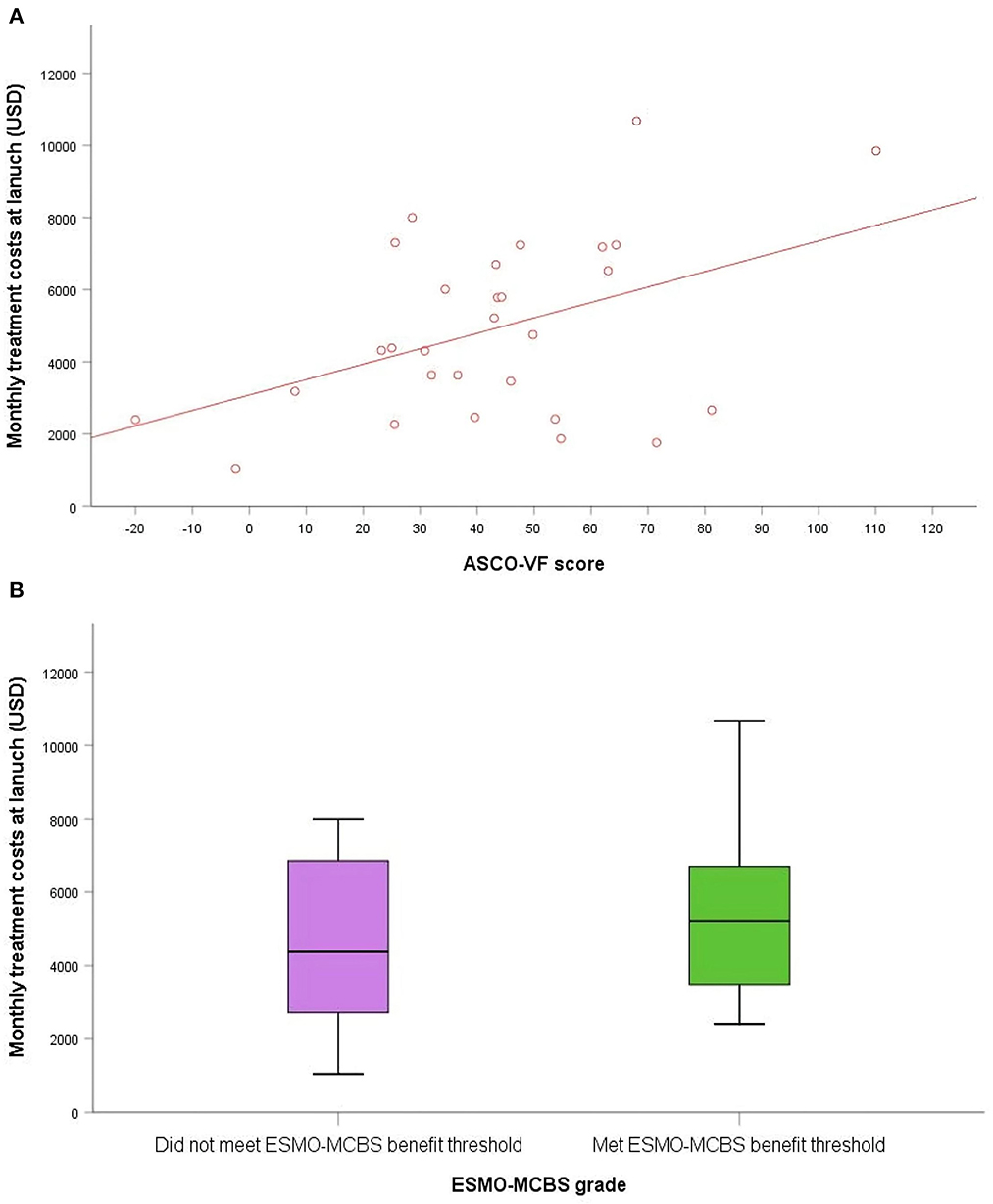
Figure 3. The association between clinical benefit and costs of approved cancer drugs in China. (A) Scatterplot of association between ASCO-VF scores and monthly treatment costs. (B) Box plot of association between ESMO-MCBS grades and monthly treatment costs.
Discussion
To the best of our knowledge, this is the first study in China to comprehensively evaluate the value of new cancer drugs using ASCO-VF and ESMO-MCBS, and to investigate the correlation between price of new drugs and their clinical benefits. In our review of all new cancer drugs approved by NMPA for solid cancer between 2016 and 2020, approximately half of new drugs achieved meaningful clinical benefit according to ESMO-MCBS. We found that all new drugs had a wide range of ASCO-VF scores and only fair association between ASCO-VF and ESMO-MCBS, which was consistent with previous studies (15, 16).
About three-quarters of new drugs were listed in the National Reimbursement Drug List (NRDL). In contrast to the increasing prices of cancer drugs in the years after approval in the US, the daily treatment cost of cancer agents has fallen in China, especially for targeted therapies and branded products (17). However, we found no significant correlation between price and clinical benefit according to the two frameworks. With rising demand for health services in China, prices should be better aligned with value, especially for expensive cancer drugs. Nearly half of cancer drug indications approved in China had shown OS benefit (18). Lack of a clear association between price and clinical benefit indicates that value frameworks can help not only identify drugs with low or uncertain clinical benefit that should be targeted for price negotiations, but also therapies with evidence of higher clinical benefit to improve access to benefit drugs (19, 20).
In 2015, China's government proposed to establish an open and transparent price negotiation mechanism with multi-party participation for some patented, high-priced drugs and exclusively produced drugs. In the same year, the first round of national-level drug pricing negotiations was launched (21). The dimensions of national price-negotiation cancer drugs include the value of drugs and the affordability of China's healthcare system funds. China's government has sufficient bargaining power. Since 2017, the government has started annual centralized price negotiation, which has sharply fallen drug prices compared with launch prices, resulting in increased affordability of expensive cancer drugs (22). However, as new clinical trials were conducted, results of post-approval clinical trials might lead to dynamic changes in value frameworks scores. Moreover, the qualifications of medical insurance payment tend to be strict. For new indications of cancer drugs, the out-of-pocket spending of patients have not been reduced, leading to increase the financial toxicity of patients. Therefore, the government needs to routinely monitor the impacts of shifts in medicine on resource utilization.
This study has several limitations. Most breakthrough-designated drugs were approved based on single-arm or non-randomized trials, resulting in the level of evidence for breakthrough therapies was inferior to that for non-breakthrough therapies when assessing the value through value frameworks (23). Thence, it is highly unfair to assess clinical benefit in this situation. Furthermore, treatment effects are known to be heterogeneous, and some patients can benefit greatly from drugs with a low value score (24). This was one of the limitations of the study, which did not evaluate the value of all new drugs. It is complicated to precisely define value of a drug, and our assessment relied entirely on data reported of clinical trial, not taking into account other factors that may influence value of a drug. Secondly, the association between ASCO-VF and ESMO-MCBS was only fair based on our findings. The ASCO-VF and the ESMO-MCBS have shared the goal of assisting clinicians and patients to measure the relative benefits of new cancer drugs (23). Nevertheless, due to the differences in the frameworks' inherent designs, especially the method and indicators of the frameworks differ greatly, resulting in greatly divergences in scores and grades (14, 16). In addition, we used scores at the 75th percentile of ASCO-VF scores as a threshold for comparisons. Nevertheless, changing this cutoff score will influence the degree of correlation between the two value frameworks. Meanwhile, we did not consider the duration of treatment when calculating the costs of a drug. But most of drugs in the palliative setting, so as long as response to treatment continues, monthly drug costs could be used. Furthermore, we did not investigate whether all cancer drugs that were approved by the NMPA were also approved in other countries. Therefore, our study needs to be followed by further study to assess the value in other countries especially emerging or developing countries comprehensively, promoting the situation of other countries in terms of access to oncology medicines with value assessment.
Conclusion
In summary, ASCO-VF and ESMO-MCBS are important tools for assessing value of cancer drugs, although the correlation between these frameworks is fair. Based on the available evidence, not all new drugs met the meaningful threshold according to ASCO-VF or ESMO-MCBS. The price of a drug was not significantly related to the level of clinical benefit, and the cost could not justify its value. Policy makers requires to improve the alignment between drug prices and clinical benefits in order to provide optimal cancer treatments for patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization and writing—review and editing: JL and QJ. Methodology: JL and SO. Software and writing—original draft preparation: JL. Data curation: HW, XQ, and RP. Formal analysis: JL, SO, and SW. Visualization: HW and XQ. Supervision and funding acquisition: QJ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Youth of the Natural Science Foundation of China (Grant No. 72204039), the Fundamental Research Funds for the Central Universities of China (Grant No. ZYGX2021J033), and the Medical Science and Technology Project of the Sichuan Provincial Health Commission (Grant No. 21PJ115).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1109668/full#supplementary-material
References
1. Cohen D. Cancer drugs: high price, uncertain value. BMJ. (2017) 359:j4543. doi: 10.1136/bmj.j4543
2. Li G, Qin Y, Xie C, Wu Y-L, Chen X. Trends in oncology drug innovation in China. Nat Rev Drug Discov. (2021) 20:15–16. doi: 10.1038/d41573-020-00195-w
3. Kelly RJ, Smith TJ. Delivering maximum clinical benefit at an affordable price: engaging stakeholders in cancer care. Lancet Oncol. (2014) 15:e112–8. doi: 10.1016/S1470-2045(13)70578-3
4. Promoting Value Affordability and Innovation in Cancer Drug Treatment. A Report to the President of the United States from the President's Cancer Panel. Bethesda, MD: President's Cancer Panel (2018).
5. Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. (2015) 33:2563–77. doi: 10.1200/JCO.2015.61.6706
6. Schnipper LE, Davidson NE, Wollins DS, Blayney DW, Dicker AP, Ganz PA, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol. (2016) 34:2925–34. doi: 10.1200/JCO.2016.68.2518
7. Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. (2015) 26:1547–73. doi: 10.1093/annonc/mdv249
8. Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. (2017) 28:2901–5. doi: 10.1093/annonc/mdw258
9. Institute for Clinical Economic Review. Overview of the ICER Assessment Framework and Update for 2017–2019. Available online at: https://icer-review.org/wp-content/uploads/2017/06/ICER-value-assessment-framework-update-FINAL-062217.pdf (accessed January 25, 2019).
10. Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee Deliberative Framework. (2011). Available online at: https://cadth.ca/pcodr (accessed February 28, 2019).
11. Jiang Q, Li Y, Yu J, Hu Z, Liu H. Evidence-based evaluation of global value assessment tools for antineoplastic agents. J Chinese Evid Based Med. (2019) 19:856–62. doi: 10.7507/1672-2531.201903126
12. Bentley TGK, Cohen JT, Elkin EB, Huynh J, Mukherjea A, Neville TH, et al. Measuring the value of new drugs: validity and reliability of 4 value assessment frameworks in the oncology setting. J Manag Care Spec Pharm. (2017) 23: S34–48. doi: 10.18553/jmcp.2017.23.6-a.s34
13. Li G, Liu Y, Xie C, Zhou Q, Chen X. Characteristics of expedited programmes for cancer drug approval in China. Nat Rev Drug Discov. (2021) 20:416. doi: 10.1038/d41573-021-00080-0
14. Cherny NI, de Vries EGE, Dafni U, Garrett-Mayer E, McKernin SE, Piccart M, et al. Comparative assessment of clinical benefit using the ESMO-magnitude of clinical benefit scale version 1.1 and the ASCO value framework net health benefit score. J Clin Oncol. (2019) 37:336–49. doi: 10.1200/JCO.18.00729
15. Jiang Q, Feng M, Li Y, Lang J, Wei H, Yu T. Choosing PD-1 inhibitors in oncology setting, left or right?-Lessons from value assessment with ASCO-VF and ESMO-MCBS. Front Pharmacol. (2020) 11:574511. doi: 10.3389/fphar.2020.574511
16. Paggio JCD, Sullivan R, Schrag D, Hopman WM, Azariah B, Pramesh CS, et al. Delivery of meaningful cancer care: a retrospective cohort study assessing cost and benefit with the ASCO and ESMO frameworks. Lancet Oncol. (2017) 18:887–94. doi: 10.1016/S1470-2045(17)30415-1
17. Huang T, Wagner AK, Bai L, Huang C, Guan X, Shi L. Anticancer medicines in China: trends in daily therapy cost and relative procurement volume and spending. Cancer Commun. (2021) 41:345–8. doi: 10.1002/cac2.12144
18. Zhang Y, Naci H, Wagner AK, Xu Z, Yang Y, Zhu J, et al. Overall survival benefits of cancer drugs approved in China from 2005 to 2020. JAMA Netw Open. (2022) 5:e2225973. doi: 10.1001/jamanetworkopen.2022.25973
19. Vokinger KN, Hwang TJ, Grischott T, Reichert S, Tibau A, Rosemann T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost-benefit analysis. Lancet Oncol. (2020) 21:664–70. doi: 10.1016/S1470-2045(20)30139-X
20. Vokinger KN, Hwang TJ, Daniore P, Lee CC, Tibau A, Grischott T, et al. Analysis of launch and postapproval cancer drug pricing, clinical benefit, and policy implications in the US and Europe. JAMA Oncol. (2021) 7:e212026. doi: 10.1001/jamaoncol.2021.2026
21. Tang M, Song P, He J. Progress on drug pricing negotiations in China. Biosci Trends. (2020) 13:464–8. doi: 10.5582/bst.2019.01339
22. Zhang Y, Wei Y, Li H, Chen Y, Guo Y, Han S, et al. Prices and clinical benefit of national price-negotiated anticancer medicines in China. Pharmacoeconomics. (2022) 40:715–24. doi: 10.1007/s40273-022-01161-7
23. Molto C, Hwang TJ, Borrell M, Andres M, Gich I, Barnadas A, et al. Clinical benefit and cost of breakthrough cancer drugs approved by the US Food and Drug Administration. Cancer. (2020) 126:4390–9. doi: 10.1002/cncr.33095
Keywords: cancer drugs, clinical benefit, cost, ASCO-VF, ESMO-MCBS
Citation: Luo J, Ou S, Wei H, Qin X, Peng R, Wang S and Jiang Q (2023) Value assessment of NMPA-approved new cancer drugs for solid cancer in China, 2016–2020. Front. Public Health 11:1109668. doi: 10.3389/fpubh.2023.1109668
Received: 28 November 2022; Accepted: 09 February 2023;
Published: 24 February 2023.
Edited by:
Haibo Li, Army Medical University, ChinaReviewed by:
Guvenc Kockaya, ECONiX Research, Analysis and Consultancy Plc., TürkiyeZhe Zhang, Third Military Medical University, China
Copyright © 2023 Luo, Ou, Wei, Qin, Peng, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Jiang,  amlhbmdxaWFuXzM4MDUuc3R1ZGVudEBzaW5hLmNvbQ==
amlhbmdxaWFuXzM4MDUuc3R1ZGVudEBzaW5hLmNvbQ==
 Jing Luo
Jing Luo Shunlong Ou
Shunlong Ou Hua Wei
Hua Wei Xiaoli Qin
Xiaoli Qin Rui Peng2
Rui Peng2 Song Wang
Song Wang Qian Jiang
Qian Jiang