
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 02 February 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1105847
This article is part of the Research Topic Neurosyphilis: Epidemiology, Clinical Manifestations, Diagnosis, Immunology and Treatment View all 7 articles
Background: Nontreponemal and treponemal tests for analyzing cerebrospinal fluid to confirm the existence of neurosyphilis have been widely used, so we aim to evaluate and compare their performance on the cerebrospinal fluid in the diagnosis of neurosyphilis.
Methods: We conducted a systematic literature search on five databases and utilized a bivariate random-effects model to perform the quantitative synthesis.
Results: Nontreponemal tests demonstrated a pooled sensitivity of 0.77 (95% CI: 0.68–0.83), a pooled specificity of 0.99 (95% CI: 0.97–1.00), and a summary AUC of 0.97 (95% CI: 0.95–0.98). The pooled sensitivity, pooled specificity, and summary AUC of treponemal tests were 0.95 (95% CI: 0.90–0.98), 0.85 (95% CI: 0.67–0.94), and 0.97 (95% CI: 0.95–0.98), respectively. The pooled specificity of all nontreponemal tests varied minimally (ranging from 0.97 to 0.99), with TRUST (0.83) having a higher pooled sensitivity than VDRL (0.77) and RPR (0.73). Among all treponemal tests, EIA has outstanding diagnostic performance with a pooled sensitivity of 0.99 and a pooled specificity of 0.98.
Conclusion: Nontreponemal tests exhibited a higher pooled specificity, and treponemal tests exhibited a higher pooled sensitivity in diagnosing neurosyphilis on cerebrospinal fluid. TRUST may be a satisfactory substitute for VDRL. EIA is a prospective diagnostic tool that deserves further study in the future. Our study may be useful to clinical laboratories in selecting appropriate serological tests on the cerebrospinal fluid for the diagnosis of neurosyphilis.
Syphilis, caused by Treponema pallidum, is a chronic bacterial infection. At any stage during the process of the illness, neurosyphilis might develop, it is a frightening complication of syphilis. Analysis of cerebrospinal fluid is often useful to confirm the existence of neurosyphilis because the disease can be asymptomatic or manifest in various ways (1). Numerous approaches for analyzing cerebrospinal fluid have been developed, with serological assays being the most commonly used diagnostic tests. There are two serologic tests for syphilis: nontreponemal and treponemal tests, the former measuring antibodies against cardiolipin and are not specific to Treponema pallidum, and the latter detecting specific antibodies to Treponema pallidum (2, 3).
Venereal disease research laboratory test (VDRL), a nontreponemal test, is often considered the standard test for confirming the diagnosis of neurosyphilis, but a light microscope is required for detection, and the reagent needs to be produced and utilized within 2 h. Rapid plasma reagin (RPR) and Toluidine red unheated serum test (TRUST), which are accessible as commercial kits, share a similar test principle as VDRL but are much easier to perform (4). Whether RPR and TRUST are promising alternatives to VDRL in the detection of cerebrospinal fluid neurosyphilis needs to be fully evaluated.
When applying treponemal tests to diagnose neurosyphilis, more and more laboratories are adopting Enzyme immunosorbent assays (EIA)-based treponemal tests instead of conventional treponemal tests, such as Fluorescent treponemal antibody absorption (FTA-ABS) or Treponema pallidum particle agglutination (TPPA), since it allows interfacing with the electronic medical record and achieving higher test throughput. The agreement between EIA and FTA-ABS exceeds 95% when using serum (5), however, it is not known whether the diagnostic performance of contemporary treponemal tests and conventional treponemal tests is comparable when applied to cerebrospinal fluid.
Currently, the diagnosis of neurosyphilis remains challenging, and recognizing the performance and limitations of the presently available tests is crucial. In this meta-analysis, we aim to evaluate and compare the performance of nontreponemal tests and treponemal tests on the cerebrospinal fluid in diagnosing neurosyphilis.
This systematic review and meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and the protocol of this study was registered in the PROSPERO database (CRD42022371321).
Two independent reviewers searched studies published up to 1 November 2022 in the PubMed, Web of Science, CNKI, BioRxiv, and MedRxiv databases. To find relevant papers, we employed a combination of free text and MeSH terms, the following were the main search terms: “Cerebrospinal fluid,” “Neurosyphilis,” “Sensitivity,” and “Specificity.” There are no restrictions on language. Supplementary Table S1 provides the detailed search strategy.
Papers reporting the clinical accuracy of nontreponemal tests or treponemal tests on cerebrospinal fluid for the diagnosis of neurosyphilis could be included in this meta-analysis, the sensitivity and specificity of the test should be available through article review or calculation in the full text or the Supplementary material. Reviews, editorials, letters, and case reports were excluded.
Two reviewers extracted the following data independently from all qualified papers: (1) first author and publication year; (2) the country of residence of the study participants; (3) definition of neurosyphilis or guidelines followed for the diagnosis of neurosyphilis; (4) assays evaluated in the article and true positive (TP), false positive (FP), false negative (FN), and true negative (TN) values of each assay; (5) status of the study population (HIV positive or HIV negative, symptomatic or asymptomatic), if available. Any disagreements were settled by consensus together with a third reviewer.
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool was applied to evaluate the risk of bias and applicability of each included study (6). Patient selection, index test, reference standard, and flow and timing are the four domains that the tool assesses.
The quantitative synthesis was performed by using a bivariate random-effects model. We utilize bivariate boxplots, qualitative Q tests (p < 0.05 indicating statistical significance), and quantitative I2 tests (ranging from 0 to 100%, with a lower value suggesting less heterogeneity) to access interstudy heterogeneity. The sensitivity analysis was used to determine if the meta-analysis's pooled effects were reliable. We calculated the pooled sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) and created forest plots to display the overall effects of all studies based on effect size and 95% confidence intervals. The summary receiver operating characteristic (SROC) curve was drawn to obtain the area under the curve (AUC). The relationship between the pre-test probability and the post-test probability is illustrated by the Probability Modifying Plot and Fagan Plot. Statistical significance was set at P < 0.05. All statistical analyses were conducted using STATA software (Stata Corporation, College Station, TX, USA).
Figure 1 provided the PRISMA flow diagram, 2,279 publications were initially retrieved. The screening process resulted in the exclusion of 190 reviews, 39 editorials, 50 letters, 371 case reports, and 489 duplicate studies. A total of 1,140 full-text publications were evaluated for eligibility, 489 were deemed irrelevant to the objective, and 622 lacked sufficient data therefore they were excluded. Ultimately, our meta-analysis was limited to 29 publications (2, 4, 7–33) to perform the quantitative synthesis.
Of the included 29 articles (Table 1), 22 articles evaluated two or more serological assays. One dataset describes the clinical accuracy data of an independent serological assay, nontreponemal tests, and treponemal tests each accounting for 33 datasets, therefore generating 66 datasets (Supplementary Table S2) containing 17,733 samples. In total, data on the diagnostic accuracy of 10 serological tests were included, including three nontreponemal tests (VDRL, RPR, and TRUST) and seven treponemal tests (FTA-ABS, TPPA, Treponema pallidum hemagglutination assay [TPHA], Microhemagglutination Assay for Treponema pallidum [MHA-TP], Enzyme-linked immunosorbent assay [ELISA], EIA, and line immunoassay [INNO-LIA]). According to the provided acknowledgments, all articles were manufacturer-independent. Nearly half of the studies were conducted in China (48.3%; n = 14). Nine articles reported whether the cases were co-infected with HIV (4, 10, 11, 22, 26, 27, 30–32). Information on the presence or absence of symptoms in patients with neurosyphilis can be found in 13 studies (2, 4, 7, 8, 11, 13, 20–22, 27, 28, 30, 32).
Based on the QUADAS-2 tool, Supplementary Table S3 presents the quality of the papers included in our meta-analysis. Regarding the patient selection domain, 7 (24.1%) publications were considered at high risk of bias as patients were not selected randomly, and case-control designs were performed. Twenty (68.9%) papers provided detailed explanations of the index test and were thus resulting in a low risk of bias. All studies were judged to have a low risk of bias in the reference standard domain. Given that all selected patients were enrolled in the analysis and received the same reference standard, 89.6% (26/29) of the articles were deemed to have a low risk of bias in flow and timing domains. In terms of applicability concerns, all domains were thought to meet the objectives of this meta-analysis.
According to the statistical analysis of Stata, the proportion of heterogeneity likely due to the threshold effect for nontreponemal tests and treponemal tests was 0.08 and 0.15, respectively, suggesting that the heterogeneity of this meta-analysis was unrelated to the threshold effect. I2 for sensitivity and specificity in nontreponemal tests was 93.95 and 94.36, and in treponemal tests was 97.45 and 98.87. Furthermore, as shown in Supplementary Figure S1, the bivariate boxplot of nontreponemal tests and treponemal tests demonstrated three and five outliers, respectively, indicating the existence of interstudy heterogeneity. Therefore, the traditional fixed effect model was inapplicable to our analysis, hence the bivariate random-effects model was employed in the following quantitative synthesis.
For both nontreponemal tests and treponemal tests, it can be seen visually from their Goodness-Of-Fit (Supplementary Figures S2A, E) and Bivariate Normality analyses (Supplementary Figures S2B, F) to conclude that the statistical findings we obtained were relatively robust because the data were primarily focused on the diagonal. In nontreponemal tests, influence analysis (Supplementary Figure S2C) identified three influential observations, while outlier detection (Supplementary Figure S2D) depicted no outlier studies. When removing the three datasets, only the pooled specificity decreased minimally in the overall effect (Sensitivity: 0.77 vs. 0.77; Specificity: 0.99 vs. 0.98; AUC: 0.97 vs. 0.97). In treponemal tests, influence analysis (Supplementary Figure S2G) and outlier detection (Supplementary Figure S2H) demonstrated two and one influencing points, respectively, where the influencing point of outlier detection overlaps with one of that in influence analysis. The overall pooled effects dropped slightly after the two datasets were excluded (Sensitivity: 0.95 vs. 0.95; Specificity: 0.85 vs. 0.82; AUC: 0.97 vs. 0.96). The results indicated that these outliers did not influence our findings.
We summarized the datasets of independent assays belonging to the nontreponemal tests or treponemal tests separately to calculate their corresponding diagnostic performance parameters. Nontreponemal tests demonstrated a pooled sensitivity of 0.77 (95% CI: 0.68–0.83), a pooled specificity of 0.99 (95% CI: 0.97–1.00) and a summary AUC of 0.97 (95% CI: 0.95–0.98) (Figure 2). The pooled sensitivity, pooled specificity, and summary AUC of treponemal tests were 0.95 (95% CI: 0.90–0.98), 0.85 (95% CI: 0.67–0.94), and 0.97 (95% CI: 0.95–0.98), respectively (Figure 3).
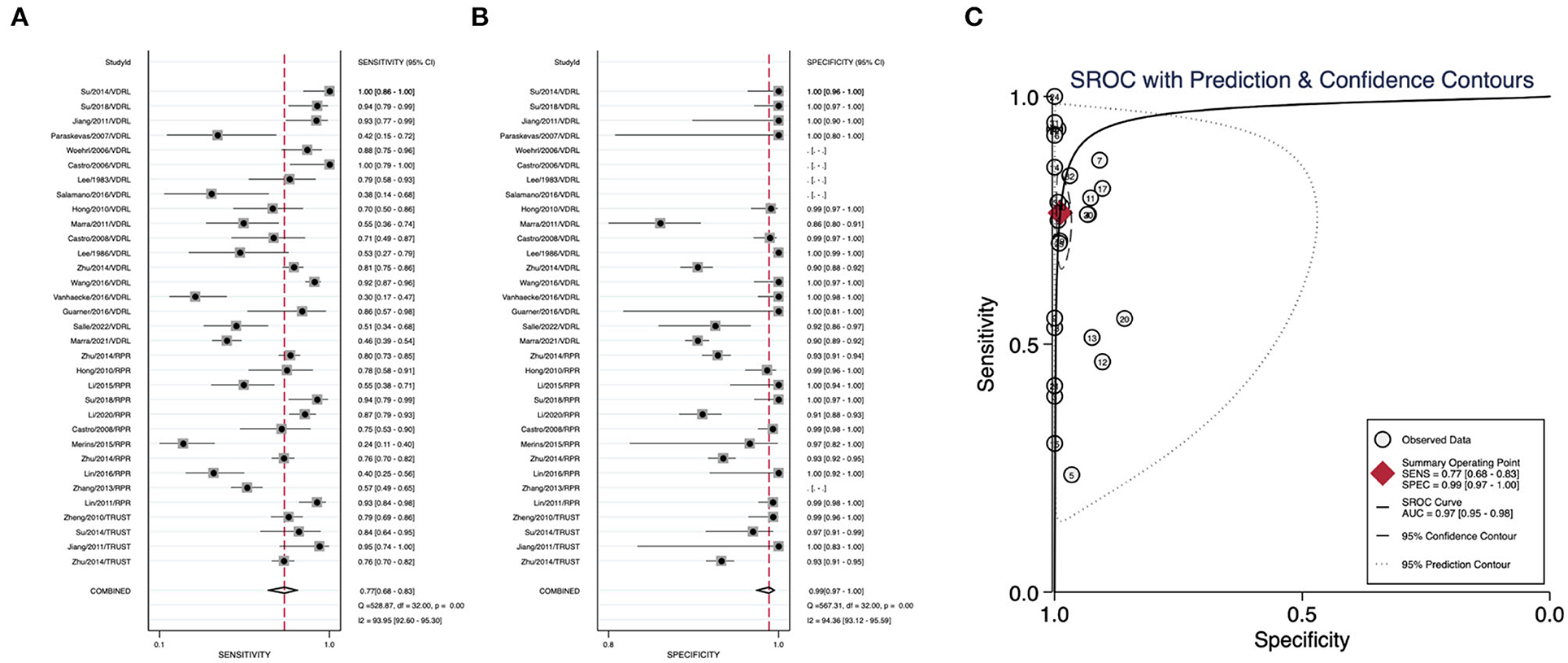
Figure 2. Pooled sensitivity, pooled specificity, and summary ROC curve of nontreponemal tests. (A) Forest plots of pooled sensitivity. (B) Forest plots of pooled specificity. (C) Summary ROC curve and its area under the curve.
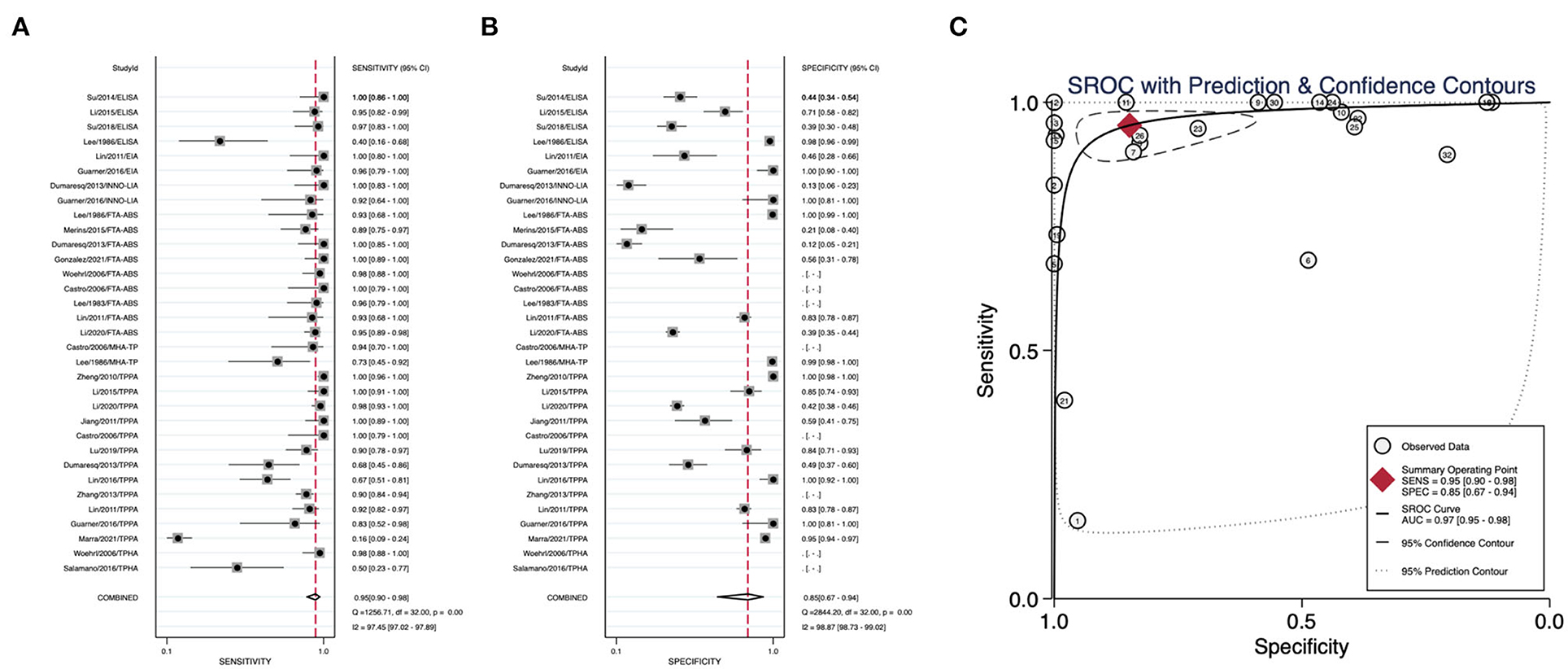
Figure 3. Pooled sensitivity, pooled specificity, and summary ROC curve of treponemal tests. (A) Forest plots of pooled sensitivity. (B) Forest plots of pooled specificity. (C) Summary ROC curve and its area under the curve.
As shown in Figure 4, the pooled sensitivity of all treponemal tests, including ELISA, EIA, INNO-LIA, FTA-ABS, MHA-TP, TPPA, and TPHA (ranging from 0.84 to 0.99) is higher than that of all nontreponemal tests, including VDRL, RPR, and TRUST (ranging from 0.73 to 0.83). EIA obtained the highest pooled sensitivity of 0.99, followed by INNO-LIA with a pooled sensitivity of 0.98. The pooled specificity of the three nontreponemal tests ranged from 0.97 to 0.99. No relevant data were available for TPHA to calculate the pooled specificity, therefore six of the seven treponemal tests yielded data for pooled specificity with a range from 0.62 to 0.99, the pooled specificity for VDRL, RPR, and MHA-TP all achieved 0.99, followed by EIA (0.98) and TRUST (0.97). Among these 10 serological assays, only EIA had pooled sensitivity (0.99) and pooled specificity (0.98) both exceeding 0.95.
Figure 5 plotted the relationship between pre-test and post-test probability based on positive likelihood ratios and negative likelihood ratios. In contrast to tests with more informative negative results, which create curves tending toward the (1, 0) location, tests with more informative positive results have curves that tend toward the (0, 1) location. Nontreponemal tests yield a pooled positive likelihood ratio of 67.55 (95% CI: 28.43–160.45) and a pooled negative likelihood ratio of 0.24 (95% CI: 0.17–0.33) (Figure 5A), and treponemal tests generated a pooled positive likelihood ratio of 6.27 (95% CI: 2.65–14.85) and a pooled negative likelihood ratio of 0.05 (95% CI: 0.03–0.11) (Figure 5B). Based on the statistical analysis of all the data we included, the prevalence of neurosyphilis in this meta-analysis, which is the pretest probability, was 20.00%. According to the Fagan Plot in Figures 5B, D, the positive post-test probabilities of nontreponemal tests and treponemal tests were 94.00 and 61.00%, respectively, and the negative post-test probabilities were 6.00 and 1.00%, respectively. In comparison, nontreponemal tests produced more informative positive results, while treponemal tests produced more informative negative results.
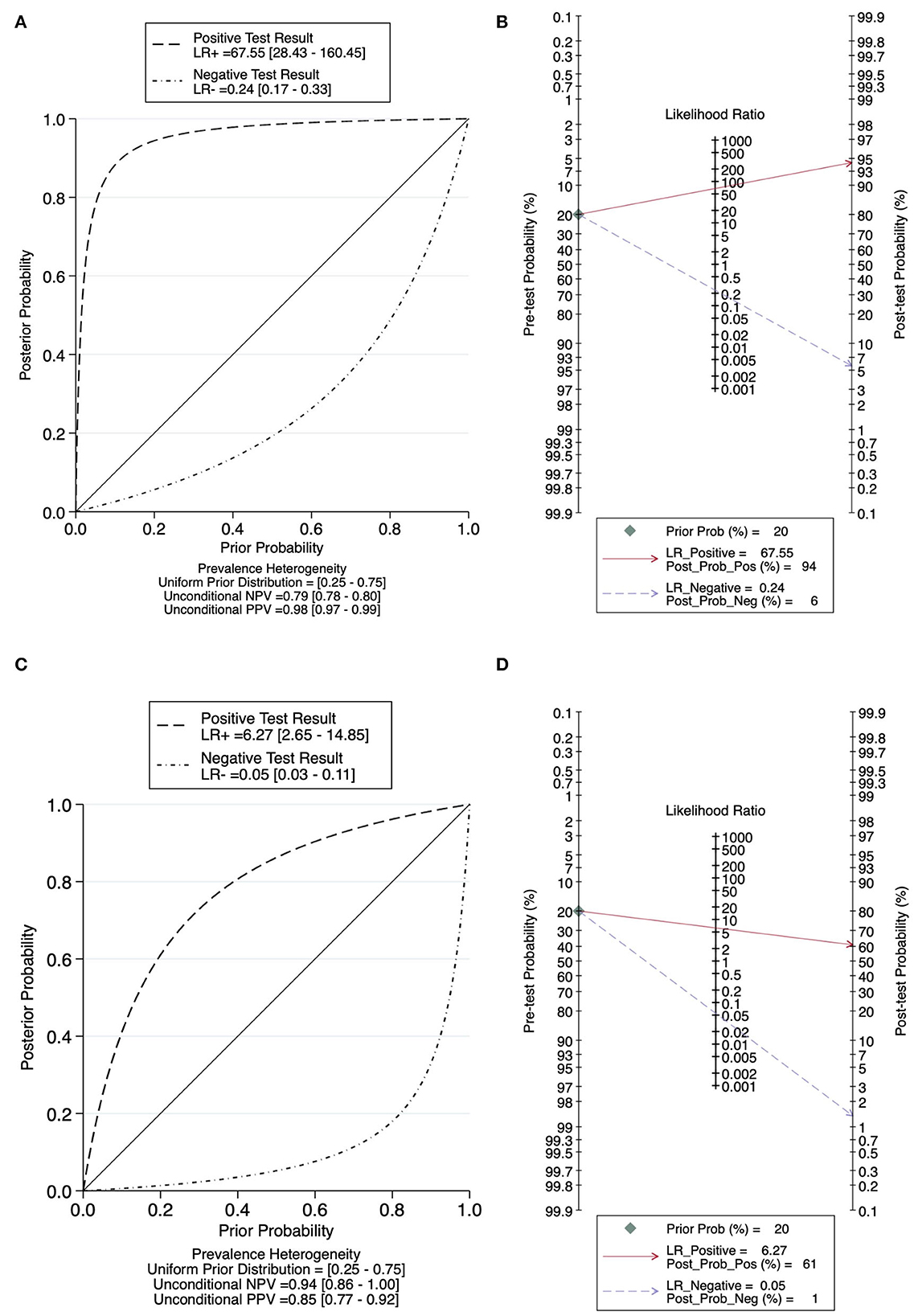
Figure 5. Probability Modifying Plot and Fagan Plot for evaluating the diagnostic value. (A) Probability Modifying Plot of nontreponemal tests. (B) Fagan Plot of nontreponemal tests. (C) Probability Modifying Plot of treponemal tests. (D) Fagan Plot of treponemal tests.
We subdivided the included datasets and performed subgroup analysis according to the following four characteristics: asymptomatic, symptomatic, HIV-negative, and HIV-positive (Table 2). In all subgroups, when we compared nontreponemal tests with treponemal tests, nontreponemal tests always had a higher pooled specificity, whereas treponemal tests always had a higher pooled sensitivity, regardless of the asymptomatic or symptomatic subgroup, HIV-negative or HIV-positive subgroup.
When comparing the asymptomatic and symptomatic subgroups, the same situation occurred for nontreponemal tests and treponemal tests, whose pooled sensitivity in the asymptomatic subgroup was lower than that in the symptomatic subgroup (nontreponemal tests: 0.63 vs. 0.86, treponemal tests: 0.90 vs. 0.99), while the pooled specificity was higher in the asymptomatic subgroup than in the symptomatic subgroup (nontreponemal tests: 0.92 vs. 0.89, treponemal tests: 0.67 vs. 0.58).
In the HIV-negative subgroup, the pooled sensitivity of both nontreponemal tests and treponemal tests was higher than that of the HIV-positive subgroup (nontreponemal tests: 0.77 vs. 0.47, treponemal tests: 0.92 vs. 0.91), and the pooled specificity was also higher than that of the HIV-positive subgroup (nontreponemal tests: 0.95 vs. 0.69, treponemal tests: 0.72 vs. 0.58).
It is the first meta-analysis to summarize data from all relevant publications to evaluate the diagnostic accuracy of nontreponemal and treponemal tests in diagnosing neurosyphilis. We found that nearly half of the studies were conducted in China, we speculate that this may be attributed to the Chinese government's emphasis on the prevention and control of neurosyphilis, which then led to an increase in independent validations of neurosyphilis testing in China. In recent years, the incidence of neurosyphilis has been increasing in China (34, 35). A 10-year syphilis control plan was launched by the Chinese government in 2010, meanwhile, due to the irreversibility of neurosyphilis, the Chinese Center for Disease Control has set up a neurosyphilis-specific consortium and established sentinel surveillance for neurosyphilis nationwide (36). To present, no VDRL kits in China have received SFDA (State Food and Drug Administration) approval, and the “China National Guidelines for the Diagnosis and Treatment of Syphilis, Gonorrhea and Chlamydia Trachomatis Infection (2020)” suggested the RPR and TRUST for substitute tests (34). The results of the CSF-FTA-ABS test can be utilized as a diagnostic indicator of neurosyphilis, in accordance with the guidelines for the diagnosis and treatment of syphilis in China, and in the absence of these conditions, the CSF-FTA-ABS test could be substituted with the CSF-TPPA test (37).
The pooled sensitivities of nontreponemal tests and treponemal tests were 0.77 (95% CI: 0.68–0.83) and 0.95 (95% CI: 0.90–0.98), respectively, and the pooled specificities were 0.99 (95% CI: 0.97–1.00) and 0.85 (95% CI: 0.67–0.94), respectively. Comparatively, nontreponemal tests exhibited a higher pooled specificity, and treponemal tests exhibited a higher pooled sensitivity, this conclusion also stood in the subgroup analysis. The diagnosis of a disease can be confirmed by a positive likelihood ratio >10, whereas a negative likelihood ratio <0.1 eliminates the probability of disease (38). Nontreponemal tests and treponemal tests demonstrated a pooled positive likelihood ratio of 67.55 (95% CI: 28.43–160.45) and 6.27 (95% CI: 2.65–14.85) and a pooled negative likelihood ratio of 0.24 (95% CI: 0.17–0.33) and 0.05 (95% CI: 0.03–0.11), respectively. In the diagnosis of neurosyphilis, nontreponemal tests exhibited a higher pooled specificity and produced more informative positive results, while treponemal tests exhibited a higher pooled sensitivity and produced more informative negative results. According to the Guideline for performance characteristics of immunological qualitative tests (39), the sensitivity of the screening test should be higher than 95%, and the specificity of the confirmatory test should be higher than 98%. We speculated that maybe treponemal tests were more suitable for screening tests, and nontreponemal tests might be better suited for confirmation tests.
For diagnostic purposes, Gonzalez et al. found that comparing the false-negative results of both the Treponemal test and the Nontreponemal test with the true-positive results, the false-negative results had lower CSF-VDRL titers and fewer cerebrospinal fluid white blood cells, which may be related to cerebrospinal fluid dilution (33).
Among the three nontreponemal tests, TRUST had the highest pooled sensitivity (0.83), although the pooled specificity (0.97) was slightly lower than that of VDRL (0.99) and RPR (0.99). TRUST is cost-saving, simple to manufacture, commercially available to be used in common hospitals, and could be carried out quantitatively to help with the follow-up of treatment plans (4, 11). VDRL has long been regarded as the best nontreponemal test for the diagnosing of neurosyphilis, our findings suggest that TRUST may be a satisfactory substitute for VDRL.
According to the antigens used in the assays, the treponemal tests can be divided into three categories: whole protein (FTA-ABS), soluble protein (MHA-TP, TPPA, and TPHA), and recombinant protein (ELISA, EIA, and INNO-LIA). The highest pooled sensitivity in each type was FTA-ABS (0.84), TPPA (0.94), and EIA (0.99), respectively. Park et al. evaluated the sensitivity and specificity of treponemal tests for the diagnosis of syphilis and suggested that more data on the comparative performance of FTA-ABS, TPPA, and chemiluminescence immunoassay (CIA) for diagnosing neurosyphilis on the cerebrospinal fluid are needed in the future (40), and we discovered that EIA had the highest pooled sensitivity and pooled specificity of these three tests. Compared to FTA-ABS and TPPA, which are manual tests, automated EIA reduces the burden on healthcare workers, furthermore, it requires less specimen volume and quicker turnaround time (18), which may meet the growing need for neurosyphilis screening using cerebrospinal fluid.
Among all serological assays, only EIA, with a pooled sensitivity of 0.99 and a pooled specificity of 0.98, achieved the required sensitivity of 0.95 and specificity of 0.95 for diagnostic tests (39). EIA may be a promising serological test for the diagnosis of neurosyphilis, and more studies are needed in the future to further evaluate the diagnostic performance of EIA for diagnosing neurosyphilis on cerebrospinal fluid.
In the symptomatic subgroup, whether employing the nontreponemal tests or the treponemal tests for the diagnosis of neurosyphilis, the pooled sensitivity was higher than that of the asymptomatic subgroup, however, the pooled specificity was lower than that of the asymptomatic subgroup. Previous studies have demonstrated that patients with symptomatic neurosyphilis are more likely to have a serologic reaction to cerebrospinal fluid syphilis testing than patients with asymptomatic neurosyphilis (41, 42), and our study further confirms this finding. This is probably attributable to the fact that abnormal cerebrospinal fluid white blood cell counts and cerebrospinal fluid protein concentrations are more common in patients with symptomatic neurosyphilis, suggesting that symptomatic neurosyphilis is related to a more severe blood-brain barrier damage and a larger inflammatory response (43). Clinical manifestations of symptomatic neurosyphilis vary and closely resemble the signs and symptoms of other neurological diseases. It is challenging to differentiate symptomatic neurosyphilis from other central nervous system disorders (44). Our study also revealed that the pooled specificity of symptomatic neurosyphilis is much lower, which means that patients with other neurological disorders may be misdiagnosed as neurosyphilis.
In the HIV-positive cohort, the pooled sensitivity and pooled specificity of nontreponemal tests were lower than in the HIV-negative cohort, and treponemal tests displayed the same results. Neurosyphilis co-infection with HIV has been proven to increase HIV viral load in cerebrospinal fluid, Treponema pallidum may interact with HIV since the two pathogens share the same antigen-presenting cells (45). So we suspect that HIV may have impaired antibody responses to the antigen used in nontreponemal tests and treponemal tests on CSF, however, this speculation requires subsequent experiments to verify.
There were some limitations existed in our article. CIA has been shown to be highly discriminative in the diagnosis of syphilis (46), but we did not evaluate the diagnostic performance of CIA in neurosyphilis because no literature was found on the use of CIA on cerebrospinal fluid for the diagnosis of neurosyphilis. Although we performed the subgroup analysis of symptomatic and asymptomatic neurosyphilis, we were unable to perform further subgroup analysis of neurosyphilis with different symptoms because data on the sensitivity and/or specificity of specific symptoms of neurosyphilis were not available. Meanwhile, we detected a high level of heterogeneity since there is no gold standard for the diagnosis of neurosyphilis, most of the articles we included had different definitions of neurosyphilis, which was likely to be the source of heterogeneity in our analysis. In the meantime, we initially identified 2,279 articles, but many were excluded for reasons such as not matching the topic or not providing data of sensitivity and specificity, so we ended up including only 29 articles, which could cause some degree of bias. However, the bivariate random-effects model we employed provided a relatively stable result, and the sensitivity analysis proved that our results were robust to a certain extent. Our work could serve as a starting point for future multicenter studies to help better understand how well cerebrospinal fluid serological testing performs in diagnosing neurosyphilis.
Nontreponemal tests yielded higher specificity than treponemal tests and might be better suited for confirmation tests, while treponemal tests were of higher sensitivity than nontreponemal tests and might be more suitable for screening tests. TRUST may be a satisfactory substitute for VDRL. EIA is a potential diagnostic tool for neurosyphilis that deserves further study in the future. Our study may be useful to clinical laboratories in selecting appropriate serological tests on the cerebrospinal fluid for the diagnosis of neurosyphilis in patients.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
J-WX: methodology and writing–original draft. YH: software and investigation. Y-WZ: formal analysis and investigation. MW: validation and data curation. YL: visualization and supervision. L-RL: conceptualization and writing–review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China [grant numbers 82172331, 81972028, and 81672094] and the Key Projects for Province Science and Technology Program of Fujian Province, China [grant number 2020D017]. The funders played no role in the study design, data collection, analyses, the decision to publish, or manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1105847/full#supplementary-material
Supplementary Figure S1. Bivariate boxplot for evaluating heterogeneity. (A) Nontreponemal tests. (B) Treponemal tests.
Supplementary Figure S2. Graphs for sensitivity analyses. (A) Goodness-Of-Fit of nontreponemal tests. (B) Bivariate normality of nontreponemal tests. (C) Influence analysis of nontreponemal tests. (D) Outlier detection of nontreponemal tests. (E) Goodness-Of-Fit of treponemal tests. (F) Bivariate normality of treponemal tests. (G) Influence analysis of treponemal tests. (H) Outlier detection of treponemal tests.
Supplementary Table S1. Literature search strategy.
Supplementary Table S2. Clinical accuracy data of nontreponemal and treponemal tests.
Supplementary Table S3. Quality of studies.
2. Salle R, Grange PA, Ollagnier G, Benhaddou N, Heller U, Dupin N. Comparison of molecular and serological assays on cerebrospinal fluid for the diagnosis of neurosyphilis. J Eur Acad Dermatol Venereol. (2023) 37:390–4. doi: 10.1111/jdv.18604
3. Binnicker MJ. Which algorithm should be used to screen for syphilis? Curr Opin Infect Dis. (2012) 25:79–85. doi: 10.1097/QCO.0b013e32834e9a3c
4. Zhu L, Gu X, Peng RR, Wang C, Gao Z, Zhou P, et al. Comparison of the Cerebrospinal Fluid (CSF) Toluidine Red Unheated Serum Test and the CSF Rapid Plasma Reagin Test with the CSF Venereal Disease Research Laboratory Test for Diagnosis of Neurosyphilis among HIV-Negative Syphilis Patients in China. J Clin Microbiol. (2014) 52:736–40. doi: 10.1128/JCM.02522-13
5. Binnicker MJ, Jespersen DJ, Rollins LO. Treponema-specific tests for serodiagnosis of syphilis: comparative evaluation of seven assays. J Clin Microbiol. (2011) 49:1313–7. doi: 10.1128/JCM.02555-10
6. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
7. Castro R, Prieto ES, João Águas M, José Manata M, Botas J, Araújo C, et al. Evaluation of the Treponema pallidum particle agglutination technique (TPPA) in the diagnosis of neurosyphilis. J Clin Lab Anal. (2006) 20:233–8. doi: 10.1002/jcla.20147
8. Su ZY, Gong AH, Sui Q, Zhang ZG, Le HY, Li CH, et al. Value of TP-ELISA, TRUST and VDRL on cerebrospinal fluid to diagnosis of neurosyphilis. Chin J Microecol. (2014) 26:1411−3+7. doi: 10.13381/j.cnki.cjm.201412013
9. Chan Y, Yeung KH, Ho HF, Ho KM, Lam ETK, Leung WL, et al. Use of cerebrospinal fluid enzyme immunoassay for diagnosis of neurosyphilis. Int J STD AIDS. (2014) 25:571–8. doi: 10.1177/0956462413515452
10. Li Y, Lou JL, Feng X, Liu Y, Wei HJ, Tang DS. The value of TP-ELISA, TPPA and RPR detection in serum and cerebrospinal fluid for the diagnosis of neurosyphilis. Beijing Med J. (2015) 37:1149–51. doi: 10.15932/j.0253-9713.2015.12.006
11. Jiang Y, Chen X, Ma X, Yang Y, Peng F, Hu X. The usefulness of toluidine red unheated serum test in the diagnosis of HIV-negative neurosyphilis. Sex Transm Dis. (2011) 38:244. doi: 10.1097/OLQ.0b013e3181f42093
12. Merins V, Hahn K. Syphilis and neurosyphilis: HIV-coinfection and value of diagnostic parameters in cerebrospinal fluid. Eur J Med Res. (2015) 20:81. doi: 10.1186/s40001-015-0175-8
13. Lee JB, Kim SC, Lee S, Whang KH, Choi IS. Symptomatic Neurosyphilis. Int J Dermatol. (1983) 22:577–80. doi: 10.1111/j.1365-4362.1983.tb02129.x
14. Hong J, Rao Y. Sensitivity and specificity of the venereal disease research laboratory (VDRL)and the rapid plasma reagin (PR) tests in the diagnosis of neurosyphilis:a comparative study. Sichuan Med J. (2010) 31:1686−8. doi: 10.16252/j.cnki.issn1004-0501-2010.11.069
15. Castro R, Prieto ES, da Luz Martins Pereira F. Nontreponemal tests in the diagnosis of neurosyphilis: an evaluation of the Venereal Disease Research Laboratory (VDRL) and the Rapid Plasma Reagin (RPR) tests. J Clin Lab Anal. (2008) 22:257–61. doi: 10.1002/jcla.20254
16. Woehrl S, Geusau A. Neurosyphilis is unlikely in patients with late latent syphilis and a negative blood VDRL test. Acta Derm-Venereol. (2006) 86:335–9. doi: 10.2340/00015555-0092
17. Lin LR, Lin DH, Tong ML, Liu LL, Fan JY, Zhu XZ, et al. Macrophage migration inhibitory factor as a novel cerebrospinal fluid marker for neurosyphilis among HIV-negative patients. Clinica Chimica Acta. (2016) 463:103–8. doi: 10.1016/j.cca.2016.10.018
18. Guarner J, Jost H, Pillay A, Sun Y, Cox D, Notenboom R, et al. Evaluation of treponemal serum tests performed on cerebrospinal fluid for diagnosis of neurosyphilis. Am J Clin Pathol. (2015) 143:479–84. doi: 10.1309/AJCPWSL3G8RXMCQR
19. Zheng D, Peng Y, Zhang L, Liu X, Zhou L. Diagnostic value of detection of TRUST and TPPA in CSF on neurosyphilis. Chin J Neuromed. (2010) 841–3. doi: 10.3760/cma.j.issn.1671-8925.2010.08.023
20. Lee J, Farshy C, Hunter E, Hambie E, Wobig G, Larsen S. Detection of immunoglobulin-M in cerebrospinal-fluid from syphilis patients by enzyme-linked-immunosorbent-assay. J Clin Microbiol. (1986) 24:736–40. doi: 10.1128/jcm.24.5.736-740.1986
21. Wang C, Wu K, Yu Q, Zhang S, Gao Z, Liu Y, et al. CXCL13, CXCL10 and CXCL8 as potential biomarkers for the diagnosis of neurosyphilis patients. Sci Rep. (2016) 6:33569. doi: 10.1038/srep33569
22. Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. CXCL13 as a cerebrospinal fluid marker for neurosyphilis in HIV-infected patients with syphilis. Sex Transm Dis. (2010) 37:283–7. doi: 10.1097/OLQ.0b013e3181d877a1
23. Lin L, Yang R, Zhang X, Xu L, Song W, Bi C, et al. Comparisons of several laboratory tests in the diagnosis of neurosyphilis. Chin J Dermatol. (2011) 127–9. doi: 10.3760/cma.j.issn.0412-4030.2011.02.019
24. Su ZY, Sui Q, Gong AH, Liu XY. Comparison of the application of VDRL and RPR in the detection of neurosyphilis. Med Forum. (2018) 22:3928–30. doi: 10.19435/j.1672-1721.2018.28.002
25. Zhang HL, Lin LR, Liu GL, Zeng YL, Wu JY, Zheng WH, et al. Clinical spectrum of neurosyphilis among HIV-negative patients in the modern era. Dermatology. (2013) 226:148–56. doi: 10.1159/000347109
26. Lu Y, Ke W, Yang L, Wang Z, Lv P, Gu J, et al. Clinical prediction and diagnosis of neurosyphilis in HIV-negative patients: a case-control study. BMC Infect Dis. (2019) 19:1017. doi: 10.1186/s12879-019-4582-2
27. Dumaresq J, Langevin S, Gagnon S, Serhir B, Deligne B, Tremblay C, et al. Clinical prediction and diagnosis of neurosyphilis in HIV-infected patients with early syphilis. J Clin Microbiol. (2013) 51:4060–6. doi: 10.1128/JCM.01989-13
28. Vanhaecke C, Grange P, Benhaddou N, Blanche P, Salmon D, Parize P, et al. Clinical and biological characteristics of 40 patients with neurosyphilis and evaluation of treponema pallidum nested polymerase chain reaction in cerebrospinal fluid samples. Clin Infect Dis. (2016) 63:1180–6. doi: 10.1093/cid/ciw499
29. Paraskevas GP, Kapaki E, Kararizou E, Mitsonis C, Sfagos C, Vassilopoulos D. Cerebrospinal fluid tau protein is increased in neurosyphilis: A discrimination from syphilis without nervous system involvement? Sex Transm Dis. (2007) 34:220. doi: 10.1097/01.olq.0000233738.23278.4e
30. Salamano R, Ballesté R, Perna A, Rodriguez N, Lombardo D, García N, et al. Cerebrospinal fluid examination may be useful in diagnosing neurosyphilis in asymptomatic HIV+ patients with syphilis. Arq Neuropsiquiatr. (2016) 74:128–32. doi: 10.1590/0004-282X20160016
31. Li Y, Lou J, Liu Y, Feng X, Wei H, Sun L. Analysis of laboratory examination results of cerebrospinal fluid in suspected neurosyphilis patients. Beijing Med J. (2020) 42:500–3. doi: 10.15932/j.0253-9713.2020.06.006
32. Marra CM. Alternatives to the cerebrospinal fluid venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. (2021) 48:S54–7. doi: 10.1097/OLQ.0000000000001450
33. Gonzalez H, Koralnik IJ, Huhn GD, Tantalo LC, Ritz EM, Orban Z, et al. A Dual platform point of care test for neurosyphilis diagnosis. Sex Transm Dis. (2021) 48:353–6. doi: 10.1097/OLQ.0000000000001308
34. Du FZ, Zhang HN, Li JJ, Zheng ZJ, Zhang X, Zhang RL, et al. Neurosyphilis in China: a systematic review of cases from 2009–2021. Front Med. (2022) 9:894841. doi: 10.3389/fmed.2022.894841
35. Tao Y, Chen MY, Tucker JD, Ong JJ, Tang W, Wong NS, et al. A nationwide spatiotemporal analysis of syphilis over 21 years and implications for prevention and control in China. Clin Infect Dis. (2020) 70:136–9. doi: 10.1093/cid/ciz331
36. Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. (2011) 24:50. doi: 10.1097/QCO.0b013e32834204bf
37. Gao ZX, Gou Y, Liu XQ, Peng LW. Advances in laboratory diagnostic methods for cerebrospinal fluid testing for neurosyphilis. Front Public Health. (2022) 10:1030480. doi: 10.3389/fpubh.2022.1030480
38. Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. (2004) 329:168–9. doi: 10.1136/bmj.329.7458.168
39. Guideline for Performance Characteristics of Immunological Qualitative Test. (2017). Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=SCHF&dbname=SCHF&filename=SCHF201806139&uniplatform=NZKPT&v=TpT7QtC4sWjuHhxNYBEPm8ok5CQSeCzQWUavOpc3lW_aANWp-aPCwzWdqO-dCqCx (accessed November 15, 2022).
40. Park IU, Tran A, Pereira L, Fakile Y. Sensitivity and specificity of treponemal-specific tests for the diagnosis of syphilis. Clin Infect Dis. (2020) 71:S13–20. doi: 10.1093/cid/ciaa349
41. Wang C, Zhu L, Gao Z, Guan Z, Lu H, Shi M, et al. Increased interleukin-17 in peripheral blood and cerebrospinal fluid of neurosyphilis patients. PLoS Negl Trop Dis. (2014) 8:e3004. doi: 10.1371/journal.pntd.0003004
42. Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS. (2008) 22:1145–51. doi: 10.1097/QAD.0b013e32830184df
43. He C, Kong Q, Shang X, Duan Y, Cui Y, Wang J, et al. Clinical, laboratory and brain Magnetic Resonance Imaging (MRI) characteristics of asymptomatic and symptomatic HIV-negative neurosyphilis patients. J Infect Chemother. (2021) 27:1596–601. doi: 10.1016/j.jiac.2021.07.004
44. Xiao Y, Tong ML, Liu LL, Lin LR, Chen MJ, Zhang HL, et al. Novel predictors of neurosyphilis among HIV-negative syphilis patients with neurological symptoms: an observational study. BMC Infect Dis. (2017) 17:310. doi: 10.1186/s12879-017-2453-2
45. de Almeida SM, Bhatt A, Riggs PK, Durelle J, Lazzaretto D, Marquie-Beck J, et al. Cerebrospinal fluid human immunodeficiency virus viral load in patients with neurosyphilis. J Neurovirol. (2010) 16:6–12. doi: 10.3109/13550280903514776
46. Qiu XH, Zhang YF, Chen YY, Zhang Q, Chen FY, Liu L, et al. Evaluation of the boson chemiluminescence immunoassay as a first-line screening test in the ECDC algorithm for syphilis serodiagnosis in a population with a high prevalence of syphilis. J Clin Microbiol. (2015) 53:1371–4. doi: 10.1128/JCM.00069-15
Keywords: neurosyphilis, nontreponemal tests, treponemal tests, serological assays, diagnostic performance
Citation: Xie J-W, Wang M, Zheng Y-W, Lin Y, He Y and Lin L-R (2023) Performance of the nontreponemal tests and treponemal tests on cerebrospinal fluid for the diagnosis of neurosyphilis: A meta-analysis. Front. Public Health 11:1105847. doi: 10.3389/fpubh.2023.1105847
Received: 23 November 2022; Accepted: 16 January 2023;
Published: 02 February 2023.
Edited by:
Dongdong Li, Sichuan University, ChinaReviewed by:
Edmond Puca, Service of Infection Diseases University Hospital Center, AlbaniaCopyright © 2023 Xie, Wang, Zheng, Lin, He and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Rong Lin,  bGlubGlyb25nQHhtdS5lZHUuY24=
bGlubGlyb25nQHhtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.