
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 30 January 2023
Sec. Environmental Health and Exposome
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1104195
This article is part of the Research Topic Perinatal Environmental Exposure and Reproductive Health: Epidemiology and Biomechanism View all 5 articles
Introduction: Environmental pollutants, such as rare earth elements, affect human health and particularly induce reproductive system injury. Yttrium (Y), one of the most widely used heavy rare earth elements, has been reported the cytotoxicity. However, the biological effects of Y3+ in the human body are largely unknown.
Methods: To further investigate the effects of Y on the reproductive system, in vivo (rat models) and in vitro studies were performed. Histopathological and immunohistochemical examination were conducted, and western blotting assays were performed to detect the protein expression. TUNEL/DAPI staining were used to detect cell apoptosis, and the intracellular calcium concentrations were also determined.
Results: Long-term exposure to YCl3 in rats produced significant pathological changes. YCl3 treatment could induce cell apoptosis in vivo and in vitro. In addition, YCl3 enhanced the concentration of cytosolic Ca2+ and up regulated the expression of IP3R1/CaMKII axis in Leydig cells. However, inhibition of IP3R1 and CaMKII with 2-APB and KN93, respectively, could reverse these effects.
Conclusion: Long-term exposure to yttrium could induce testicular injury by stimulating cell apoptosis, which might be associated with activation of Ca2+/IP3R1/CaMKII axis in Leydig cells.
Rare earth elements have been comprehensively employed in agricultural, industrial, and medicinal fields. Yttrium (Y) is one of the most widely used heavy rare earth elements. Most studies on Y in the medical field are focusing on its beneficial characteristics for the improvement of laser system, which is employed for insemination in vitro (1). Chemically, Y may irritate to eyes, skin, and respiratory system. Inhalation of dust containing Y can lead to occupational pneumoconiosis, and YCl3 may detrimentally stimulate the mucous membrane of the eyes. The safety and efficacy of Y90 in patients with lung shunt fraction >15% have been discussed. Some patients may have non-specific pulmonary symptoms, such as cough, shortness of breath, wheezing in the 1-year post-Y90 (2). Y has been demonstrated to potentially produce liver damage with inflammation, necrosis, and portal fibrosis (3). The ion Y3+ can be accessible in the south of Ganzhou city in China. Our previous study indicated that Y3+ can induce neuronal cell death by triggering apoptotic pathway in rats (4). However, the biological effects of Y3+ in the human body are largely unknown.
Yttrium oxide nanoparticles have been recently to shown to alleviate the reproductive toxicity induced by silver nanoparticle (5). However, it has been reported that the rare earth elements can be accumulated in the human body and produce detrimental effects in a dose-dependent manner (6). The rare earth elements can be accumulated in the liver, bone, and lungs (7). It has been demonstrated that the rare earth elements show reproductive toxicity through multiple interventions, including agricultural and industrial pathways (8). The interaction between the Tb (IV)-NR complex and sperm DNA gene information has been verified (9). Cerium oxide nanoparticles can be accumulated in the testis, inducing sperm DNA damage and reproductive toxicity (10). The biological effects of Y3+ on the reproductive system still need to be clearly elucidated.
Ca2+, a second messenger, has been involved in various cell functions. The critical roles of Ca2+ in the testis have been demonstrated (11). The imbalanced homeostasis of Ca2+ may contribute to cell death. Particularly, Ca2+ can sensitize the signal in the pro-apoptotic transition of the mitochondria and stimulate cell apoptosis. Ca2+ signaling includes reactive proteins, such as calmodulin (CaM) and Ca2+/CaM-dependent protein kinase II (CaMKII) (12). Inositol 1,4,5-triphosphate receptor 1 (IP3R1) maintains intracellular Ca2+ homeostasis (13). Increased activity of the Ca2+-mediated IP3R1/CaMKII pathway has been associated with cell apoptosis (14). Many rare earth elements, such as Eu3+ (15) and La3+ (16), may have similar biological activities by interacting with Ca2+-related channels. Whether Y3+ exhibits reproductive toxicity and induces cell apoptosis by activating the IP3R1/CaMKII pathway is still unclear. In this article, we will mainly investigate the biological roles of Y3+ in the testis.
This project (GMU2017012) was approved by the Ethics Committee of Gannan Medical University, according to the Declaration of Helsinki Principles. The study was conducted in accordance with the internationally accepted principles for laboratory animal use and care in the European Community guidelines (EEC Directive of 1986; 86/609/EEC). Thirty-six-week-old male rats were kept in an adaptive circumstance in an SPF-grade room with a 12 h light/dark cycle (Temperature: 21 −23°C; humidity: 45%−55%) for 1 week before further experiments. All rats were free to access water and food. Yttrium chloride (YCl3, purity ≥ 99.99%) was purchased from Sigma-Aldrich (St. Louis, USA).
Rats were randomly divided into three groups of 10 rats each, including the negative control (NC) group (free access to blank water) and YCl3-treated groups (free access to water with 12 and 24 mmol/L of YCl3, respectively) (4). After 6 months, all rats were sacrificed. The testicular tissues were obtained for weight measurement, histopathological and immunohistochemical examination, and protein extraction.
The testicular weight and the body weight of each rat were determined. The gonadosomatic index was reached by the formula: gonadosomatic index = the testicular weight /the body weight × 100% (17).
The cauda epididymis was harvested and minced in the physiological saline at 36°C to prepare a sperm suspension. The sperms (100 sperms) moving in a straight line were counted using a blood cell counting plate, which was preheated at 36°C. To detect the concentration of sperm, the suspension (100 μl) was killed by a preheated water bath at 60°C. The sperm was counted using a blood cell counting plate. To examine the sperm deformity rate, the suspension (500 μl) was smeared and fixed with methanol. The sperm deformity was observed using a microscope.
A testosterone ELISA kit (Cat. no. KB3117, BOYAO, Shanghai, China) was employed to detect the concentrations of serum testosterone in rats. The procedures were conducted according to the instructions recommended by the kit manufacturers.
The collected testicular tissues were fixed with 4% paraformaldehyde. Then, they were embedded in the paraffin. The 5-μm thickness of sections was sliced and then stained with hematoxylin-eosin (H&E). Light microscopy was used to observe the pathological changes. For the immunohistochemical examination, the testicular tissues embedded in paraffin were sectioned for 5-μm thickness, deparaffinized in xylene, and rehydrated with different graded concentrations of ethanol on glass slides. The tissues were then interacted with by hydrogen peroxide for 10 min and incubated the primary antibodies (1:100) for 6 min and horseradish peroxidase (HRP)-conjugated secondary antibody for 8 min, respectively. After further incubation with 3,3-diaminobenzidine (DAB) for 10 min. Light microscopy was used to observe the immunized proteins (18, 19).
The CCK8 kit (Cat.no.C0038) (Beyotime, Shanghai, China) was used for testing the viability of Leydig cells (purchased from Procell Life Science&Technology Co. Ltd, Wuhan, China), according to the manufacturer's instructions. Leydig cells were grown in 96-well plates at a density of 5,000 cells/well for 24 h. Different concentrations of YCl3 (0, 3, 10, 30, 100, and 300 μM) were used to pretreat the Leydig cells for 24 h. Then, CCK-8 solution (10 μl) was added into each well and incubated at 37°C for 4 h. After that, the absorbance was detected at the wavelength of 450 nm by using a microplate system (Leica microsystems, Germany).
TUNEL and DAPI staining kits were purchased from Abcam (Cambridge, MA, USA). Leydig cells were cultured and treated with YCl3 for 48 h. Cells were harvested and then fixed with 4% paraformaldehyde. The apoptosis was analysis according to the instructions recommended by the manufacturer. The fluorescent images were observed using a Leica microsystem, and the florescence intensity was determined using ImageJ (an open-source image processing package).
The total protein was harvested, and the protein concentrations were detected by BCA protein assay kit (Beyotime). Each sample (30 μg) was subjected to SDS-PAGE and then transferred onto PVDF membranes. After being blocked, the membranes were co-incubated with the primary antibodies at 4°C overnight against IP3R1 (1: 1,000 dilution; Sigma), CaMKII (1: 1,000 dilution; Sigma), p-CaMKII (1: 1,000 dilution; Sigma), Bcl-2 (1: 1,000 dilution; Sigma), caspase-3 (1: 1,000 dilution; Sigma), cleaved-caspase-3 (1: 1,000 dilution; Sigma), and β-actin (1: 1,000 dilution; Sigma). Then, the secondary antibody conjugated with peroxidase (1: 5,000 dilution; Sigma) was employed. Protein bands were detected using the enhanced chemiluminescence detection system.
The experimental procedures were conducted according to the instructions of the kit's manufacturer (HL10153.1, Shanghai Haling Biological Technology, Shanghai, China). Leydig cells were incubated with Rhod-2/AM for 1 h at 37°C. Analysis was conducted by a fluorescence microplate reader (Varioskanlux, Thermo Fisher Scientific, Waltham, MA, USA) with the parameters, including the wavelength of 550 nm (excitation) and 590 nm (emission).
All experiments were performed three times independently and data are expressed as the mean ± standard deviation (SD). SPSS 20.0 software was employed for statistical analysis. One-way ANOVA and Turkey's post hoc test were used to analyze the differences between multiple groups. P < 0.05 indicated a significant difference statistically.
Rats were free to access water containing 12 and 24 mmol/L of YCl3, respectively, for 6 months, and the chronic effects of YCl3 on the testis in rats were investigated. The results showed that YCl3 could dose-dependently reduce the semen quality in rats, as indicated by decreased sperm motility (Figure 1A) and concentrations (Figure 1B) and increased sperm deformity (Figure 1C). In addition, the gonadosomatic index (Figure 1D) and the levels of serum testosterone (Figure 1E) were also decreased by YCl3 treatment in a dose-dependent manner. Histopathological examination by HE staining (Figure 1F) in the YCl3-treated group indicated that the arrangement of spermatogenic cells at all levels was loose and disordered and the number of mature spermatozoa was reduced, compared with those in the negative control group. Furthermore, the anatomic structure of seminiferous tubules was damaged and the distance among the seminiferous tubules was enlarged. These suggested that YCl3 treatment induced significant toxicity and damage to the testis.
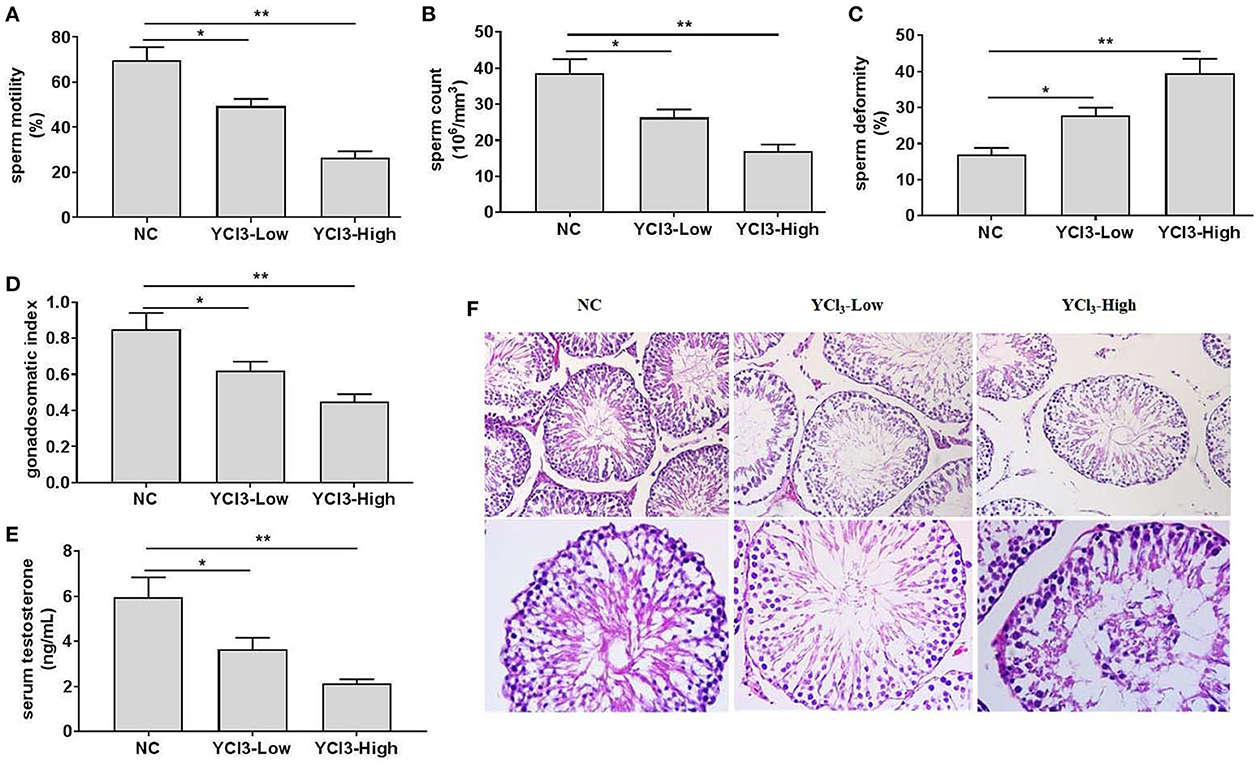
Figure 1. The effects of chronic exposure to YCl3 on the rat testis (n = 10) were investigated. The sperm quality, including sperm motility (A), sperm count (B), and sperm deformity (C) was detected. The gonadosomatic index (D) and the serum testosterone level (E) were measured. The histopathological examination (F) of testicular tissues [magnification ×200 (up) and ×400 (down)] by HE staining was conducted. *P < 0.05, **P < 0.01. NC, negative control; YCl3-Low, 12 mmol/L YCl3; YCl3-High, 24 mmol/L YCl3.
To further investigate how YCl3 treatment induced testis damage, the protein expression of Bcl-2 and caspase-3 in the testis organs was detected. YCl3 treatment could down regulate Bcl-2 expression (Figures 2A, B) and up regulate caspase-3 expression (Figures 2A, C), indicating induction of apoptosis. In addition, the in vitro study on the cytotoxicity of YCl3 on the cultured Leydig cells was detected. The results from the cellular viability detection showed that YCl3 at the doses of 300 μM indicated almost the greatest cytotoxicity (Figure 2D). Additionally, the expression of cleaved caspase-3 was up regulated, and the expression of Bcl-2 was down regulated (Figures 2E–G). In the TUNEL/DAPI staining assays, YCl3 significantly increased cell apoptosis (Figure 2H). Collectively, the induction of testis damage by YCl3 was associated with increased apoptosis.
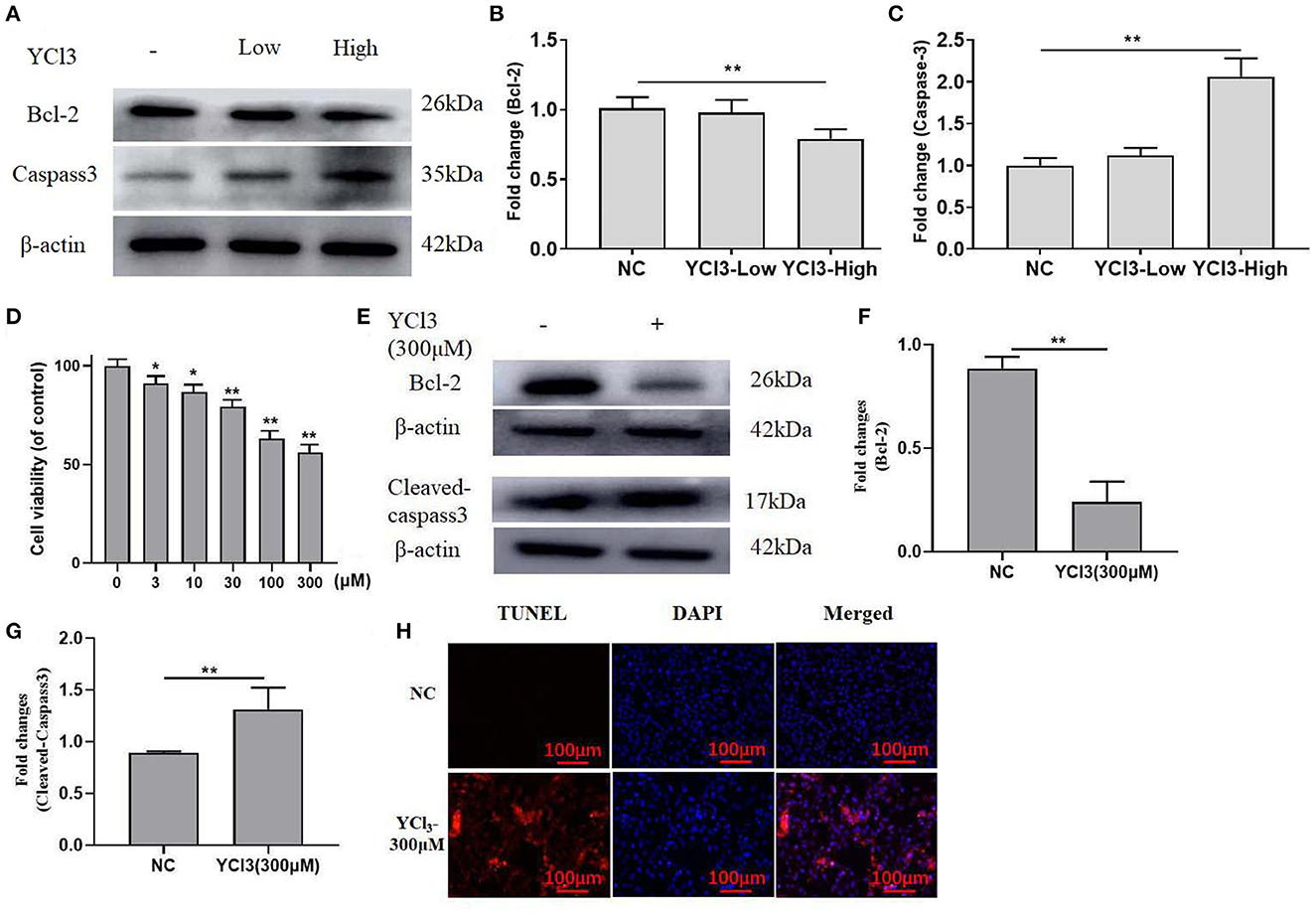
Figure 2. The effects of YCl3 on cell apoptosis. The protein expression of Bcl-2 (A, B) and caspase-3 (A, C) in the testis was detected by Western blot. (D) CCK8 assays were detected for the effects of YCl3 on the cell viability, the statistical difference was compared to the NC group (0 μM). Western blot assays were conducted for determination of Bcl-2 (E, F), and cleaved-caspase-3 (E, G) protein expression. TUNEL/DAPI assays were conducted for apoptosis detection (H). *P < 0.05, **P < 0.01. NC, negative control; YCl3-Low, 12 mmol/L YCl3; YCl3-High, 24 mmol/L YCl3.
To further explore the possible mechanism of YCl3 in the regulation of apoptosis, the expression of IP3R1 was detected. The in vivo immunohistochemical study (Figures 3A, B) showed that the expression of IP3R1 in YCl3-treated rats was increased. In YCl3-treated Leydig cells in vitro, the protein expression of IP3R1, p-CaMKII, and CaMKII (Figures 3C–F) was up regulated. The protein expression of Bcl-2 (Figures 3C, G) was increased, and the expression of cleaved caspase-3 (Figures 3C, H) was decreased. In addition, the concentration of cytosolic Ca2+ (Figure 3I) was significantly enhanced. The apoptosis rate (Figure 3J) was elevated. The stimulating effects of YCl3 were blocked by co-treatment with IP3R1 inhibitor 2-APB (1 μM). These suggested that YCl3 might induce cell apoptosis by up regulating the expression of Ca2+/IP3R1 signaling in Leydig cells.
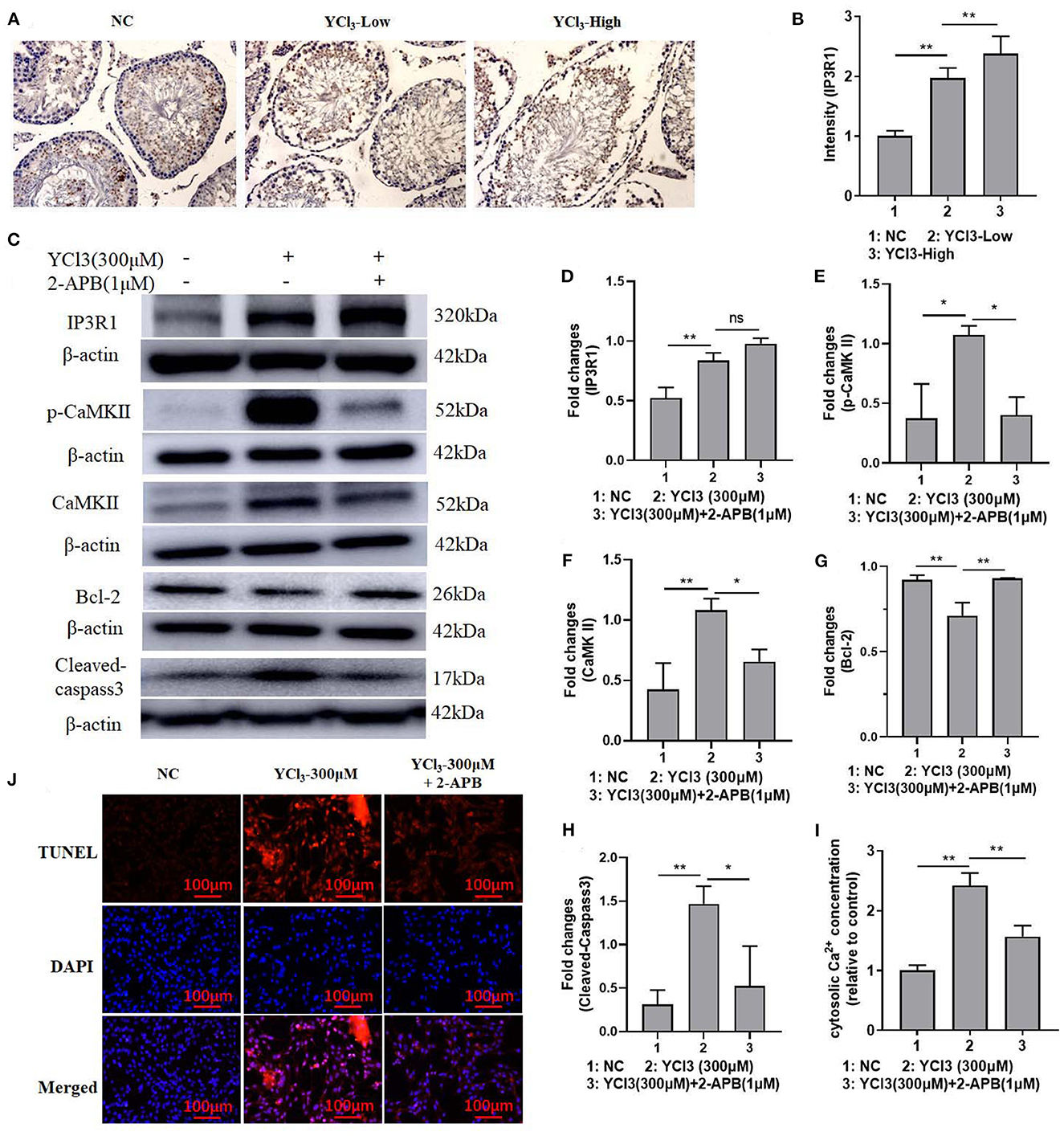
Figure 3. The roles of IP3R1 in the induction of cell apoptosis by YCl3. The immunohistochemical study (magnification ×200) of IP3R1 in YCl3-treated rat testicular tissue was tested (A), and it is quantified by intensity measurement (B). Western blot assays were conducted for determination of IP3R1 (C, D), p-CaMKII (C, E), CaMKII (C, F), Bcl-2 (C, G), and cleaved-caspase-3 (C, H) protein expression. The cytosolic Ca2+ concentration was detected by the kits (I). TUNEL/DAPI assays were conducted for apoptosis detection (J). *P < 0.05, **P < 0.01. NC, negative control; YCl3-Low, 12 mmol/L YCl3; YCl3-High, 24 mmol/L YCl3.
To further explore the possible mechanism of YCl3 in the regulation of cell apoptosis, the activity of CaMKII was determined. The expression of CaMKII (Figures 4A, B) in YCl3-treated rats was investigated by an in vivo immunohistochemical study, and it was significantly increased. The expression of CaMKII and p-CaMKII in YCl3-treated Leydig cells in vitro was also up regulated. After co-treatment with CaMKII inhibitor KN93 (1.48 μM), the increased expression of p-CaMKII and cleaved caspase-3 and the decreased expression of Bcl-2 were reversed (Figures 4C–G). Similarly, the changes in cell apoptosis (Figure 4H) were rescued. Collectively, YCl3 induced cell apoptosis by up regulating the activity of CaMKII in Leydig cells.
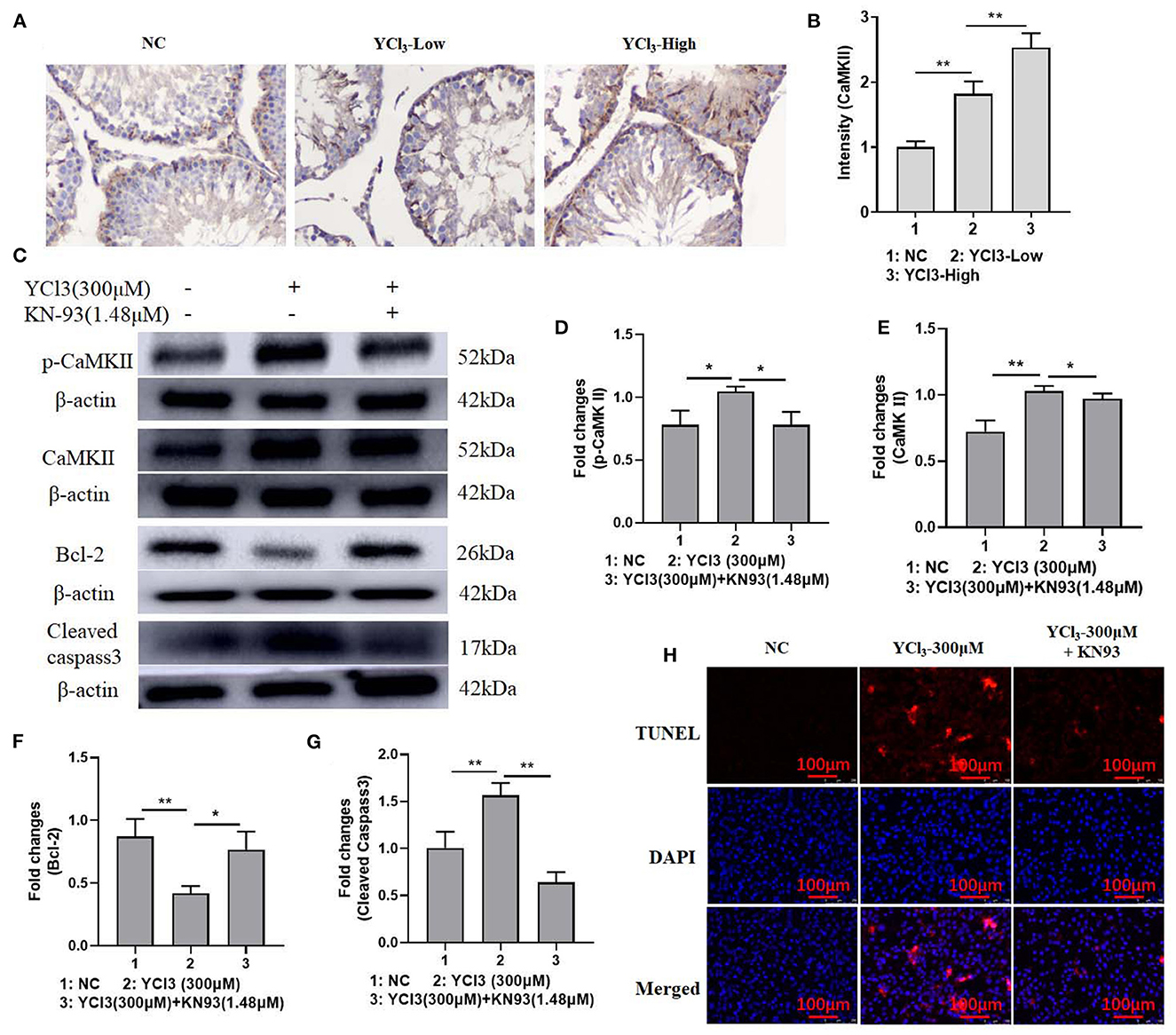
Figure 4. The effects of YCl3 on the activity of Ca2+/CaMKII axis. (A) The immunohistochemical study (magnification ×200) (A) of CaMKII in YCl3-treated rat testicular tissue was tested, and it is quantified by intensity measurement (B). Western blot assays were conducted for determination of p-CaMKII (C, D), CaMKII (C, E), Bcl-2 (C, F), and cleaved-caspase-3 (C, G) protein expression. TUNEL/DAPI assays were conducted for apoptosis detection (H). *P < 0.05, **P < 0.01. NC, negative control; YCl3-Low, 12 mmol/L YCl3; YCl3-High, 24 mmol/L YCl3.
Many rare earth elements in the drinking water and foods have been reported to significantly affect our health (8, 20). Long-term exposure to the rare earth elements leading to accumulation in the body has caused negative outcomes (21). In this study, we found that long-term exposure to the rare earth element yttrium produced reproductive toxicity by damaging the testis. The pathological changes might be association with increased cell apoptosis induced by yttrium treatment, which could activate the Ca2+/IP3R1/CaMKII axis in Leydig cells.
Recently, male infertility increases dramatically. Exposure to environmental pollutants might be the attributing factor (22). It has been reported that cadmium (Cd) has become a serious environmental pollutant to induce testis injury by inducing cell apoptosis (23). Lanthanum, one of the important rare earth elements, has been reported to induce severe pathological changes in testicular tissues and apoptosis in spermatogenic cells by induction of oxidative stress and reduction of anti-oxidant enzyme expression, such as NRF2/HO-1 and PI3K/AKT signaling pathways (24). Gadolinium has been demonstrated to be accumulated in the testis organ and leads to its malfunctions, as shown by the increased loss of spermatozoa and accumulation of immature germinal cells in the seminiferous tubule lumen (25). Consistently, our study showed that long-term exposure to yttrium resulted in increased cell apoptosis and testicular malfunctions.
The underlying mechanism of the rare earth elements in inducing pathological changes may be complex. Cadmium has been assumed to pass through the cellular membrane by the transporters for Ca, Fe, Mn, and Zn, due to their similar chemical and physical properties (26). Many channels, such as voltage-dependent cation channels (VDCCs) and depolarization-activated calcium channels (DACCs), responsible for Ca2+ transportation have been reported to transport Cd2+. These channels are featured as non-selective cation channels (27). Cadmium induces neurological apoptosis by promoting mitochondrial distribution and mitochondrial fission, which might be associated with overload of calcium and excessive activation Ca2+ signaling pathway (28). Long-term exposure to the trivalent lanthanum and gadolinium rare earth elements promotes their internalization and activation of intracellular Ca2+/calmodulin signaling (29). Consistently, our study also found that treatment with YCl3 induced increased cytosolic Ca2+ concentration. In addition, the expression of IP3R1/CaMKII was also increased. Inhibition of IP3R1 and CaMKII, respectively, might reverse the detrimental effects of YCl3 on Leydig cells, indicating that Yttrium induced cell apoptosis by activating Ca2+/IP3R1/CaMKII signaling pathway.
However, there are limitations to this study. In the rat models, the administration doses are not scientific strict because rats were not ensured to receive their supposed amount of YCl3. Fortunately, the study in vivo was designed for 6 months, and this makes it possible to diminish the discrepancy. In addition, free access to water containing YCl3 can alleviate the discomfort induced by gavage or injection. More efforts should be made in exploring the mechanism of yttrium in passing through the blood testicular barrier. In addition, the effects of YCl3 after inhibition of calcium channels on Leydig cells should be investigated.
Long-term exposure to the rare earth element yttrium induced testicular injury. Specifically, Y could reduce the semen quality, gonadosomatic index, and serum testosterone levels. In addition, Y might increase cell apoptosis in vivo and in vitro and enhance the concentration of cytosolic Ca2+. The underlying mechanism of Y in inducing testicular injury might be associated with activation of Ca2+/IP3R1/CaMKII signaling pathway. After inhibition of IP3R1 and CaMKII activities, the negative effects of Y on Leydig cells might be ameliorated. This might provide a potential therapeutic strategy for treating those patients with Y-induced testis damage.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Ethics Committee of Gannan Medical University.
Conceptualization: XF. Investigation: ZL and YD. Writing—original draft preparation: SX and YH. Review and editing: HX and XL. All authors have read and agreed to the published version of the manuscript.
This study was financially supported by National Natural Science Foundation of China, grant number 21767002, Scientific Research Fund of Jiangxi Provincial Education Department, grant number GJJ160966, and Research Fund of Key Laboratory Project of Prevention and Treatment Cardiovascular and Cerebrovascular Disease of Ministry of Education of Gannan Medical University, grant number XN201902.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1104195/full#supplementary-material
1. Aung NPS, Watanabe S, Okiji T. Er:YAG laser-activated irrigation in comparison with different irrigation systems for cleaning the apical root canal area beyond ledge. Photobiomodul Photomed Laser Surg. (2021) 39:759–65. doi: 10.1089/photob.2021.0044
2. Das A, Riaz A, Gabr A, Ali R, Mora R, Al Asadi A, et al. Safety and efficacy of radioembolization with glass microspheres in hepatocellular carcinoma patients with elevated lung shunt fraction: analysis of a 103-patient cohort. Eur J Nucl Med Mol Imaging. (2020) 47:807–15. doi: 10.1007/s00259-019-04517-y
3. Páramo M, Santamaría E, Idoate MA, Rodríguez-Fraile M, Benito A, Collantes M, et al. A new animal model of atrophy-hypertrophy complex and liver damage following Yttrium-90 lobar selective internal radiation therapy in rabbits. Sci Rep. (2022) 12:1777. doi: 10.1038/s41598-022-05672-3
4. Ding Y, Tian Y, Zeng Z, Shuai P, Lan H, Zhu X, et al. YCl promotes neuronal cell death by inducing apoptotic pathways in rats. Biomed Res Int. (2017) 2017:2183658. doi: 10.1155/2017/2183658
5. Abu-Taweel GM, Albetran HM, Al-Mutary MG, Ahmad M, Low IM. Alleviation of silver nanoparticle-induced sexual behavior and testicular parameters dysfunction in male mice by yttrium oxide nanoparticles. Toxicol Rep. (2021) 8:1121–30. doi: 10.1016/j.toxrep.2021.05.014
6. Zielińska-Dawidziak M, Czlapka-Matyasik M, Wojciechowska Z, Proch J, Kowalski R, Niedzielski P. Rare earth elements accumulation in the hair of malagasy children and adolescents in relation to their age and nutritional status. Int J Environ Res Public Health. (2022) 19:455. doi: 10.3390/ijerph19010455
7. Rim KT, Koo KH, Park JS. Toxicological evaluations of rare earths and their health impacts to workers: a literature review. Saf Health Work. (2013) 4:12–26. doi: 10.5491/SHAW.2013.4.1.12
8. Du X, Graedel TE. Uncovering the end uses of the rare earth elements. Sci Total Environ 461-462. (2013) 781–4. doi: 10.1016/j.scitotenv.2013.02.099
9. Zhao W, Xiong M, Liu M, Wang S, Xian X, Lin B, et al. Evaluation of the effect of Tb(IV)-NR complex on herring sperm DNA genetic information by mean of spectroscopic. Nucleosides Nucleotides Nucleic Acids. (2020) 39:964–78. doi: 10.1080/15257770.2020.1725042
10. Qin F, Shen T, Li J, Qian J, Zhang J, Zhou G, et al. SF-1 mediates reproductive toxicity induced by Cerium oxide nanoparticles in male mice. J Nanobiotechnology. (2019) 17:41. doi: 10.1186/s12951-019-0474-2
11. Limanjaya I, Hsu TI, Chuang JY, Kao TJ. L-selectin activation regulates Rho GTPase activity via Ca(+2) influx in Sertoli cell line, ASC-17D cells. Biochem Biophys Res Commun. (2020) 525:1011–7. doi: 10.1016/j.bbrc.2020.03.011
12. Qian W, Zhu J, Mao C, Liu J, Wang Y, Wang Q, et al. Involvement of CaM-CaMKII-ERK in bisphenol A-induced Sertoli cell apoptosis. Toxicology. (2014) 324:27–34. doi: 10.1016/j.tox.2014.06.001
13. Cai R, Wang P, Zhao X, Lu X, Deng R, Wang X, et al. Reticulocalbin3: a Ca(2+) homeostasis regulator that promotes esophageal squamous cell carcinoma progression and cisplatin resistance. Cancer Sci. (2022) 113:3593–607. doi: 10.1111/cas.15487
14. Huang W, Dai M, Qiu T, Liang T, Xie J, Mi C, et al. Novel lncRNA-HZ04 promotes BPDE-induced human trophoblast cell apoptosis and miscarriage by upregulating IP R/CaMKII/SGCB pathway by competitively binding with miR-hz04. FASEB J. (2021) 35:e21789. doi: 10.1096/fj.202100376RR
15. Zeng F, Tian HE, Wang Z, An Y, Gao F, Zhang L, et al. Effect of rare earth element europium on amaranthin synthesis in Amarathus caudatus seedlings. Biol Trace Elem Res. (2003) 93:271–82. doi: 10.1385/BTER:93:1-3:271
16. Lin C, Kadono T, Yoshizuka K, Furuichi T, Kawano T. Effects of fifteen rare-earth metals on Ca2+ influx in tobacco cells. Z Naturforsch C J Biosci. (2006) 61:74–80. doi: 10.1515/znc-2006-1-214
17. Ekhoye EI, Olerimi SE, Ehebha SE. Comparison of the deleterious effects of yaji and cadmium chloride on testicular physiomorphological and oxidative stress status: the gonadoprotective effects of an omega-3 fatty acid. Clin Exp Reprod Med. (2020) 47:168–79. doi: 10.5653/cerm.2019.03517
18. Khodavirdipour A, Mehregan M, Rajabi A, Shiri Y. Microscopy and its application in microbiology and medicine from light to quantum microscopy: a mini-review. Avicenna J Clin Microbiol Infect. (2019) 6:133–7. doi: 10.34172/ajcmi.2019.24
19. Khodavirdipour A, Piri M, Jabbari S, Keshavarzi S, Safaralizadeh R, Alikhani MY. Apoptosis detection methods in diagnosis of cancer and their potential role in treatment: advantages and disadvantages: a review. J Gastrointest Cancer. (2021) 52:422–30. doi: 10.1007/s12029-020-00576-9
20. Khodavirdipour A, Haddadi F, Keshavarzi S. Chromium supplementation; negotiation with diabetes mellitus, hyperlipidemia and depression. J Diabetes Metab Disord. (2020) 19:585–95. doi: 10.1007/s40200-020-00501-8
21. Wang B, Yan L, Huo W, Lu Q, Cheng Z, Zhang J, et al. Rare earth elements and hypertension risk among housewives: a pilot study in Shanxi Province, China. Environ Pollut. (2017) 220(Pt B):837–42. doi: 10.1016/j.envpol.2016.10.066
22. Abdel-Wahab A, Hassanin KMA, Mahmoud AA, Abdel-Badeea WIE, Abdel-Razik AH, Attia EZ, et al. Physiological roles of red carrot methanolic extract and vitamin E to abrogate cadmium-induced oxidative challenge and apoptosis in rat testes: involvement of the Bax/Bcl-2 ratio. Antioxidants. (2021) 10:1653. doi: 10.3390/antiox10111653
23. Montaño-González RI, Gutiérrez-Salmeán G, Mojica-Villegas MA, Cristóbal-Luna JM, Briseño-Bugarín J, Chamorro-Cevallos G. Phycobiliproteins extract from Spirulina protects against single-dose cadmium-induced reproductive toxicity in male mice. Environ Sci Pollut Res Int. (2022) 29:17441–55. doi: 10.1007/s11356-021-16668-3
24. Ji J, Hong F, Zhou Y, Liu T, Fan D, Zhang X, et al. Molecular mechanisms associated with oxidative damage in the mouse testis induced by LaCl. Environ Toxicol. (2021) 36:408–16. doi: 10.1002/tox.23046
25. Beyazal Celiker F, Tumkaya L, Mercantepe T, Turan G, Yilmaz A, Beyazal M, et al. The effect of gadolinium-based contrast agents on rat testis. Andrologia. (2018) 50:e13031. doi: 10.1111/and.13031
26. Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. (2006) 88:1707–19. doi: 10.1016/j.biochi.2006.07.003
27. Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I. Calcium transport across plant membranes: mechanisms and functions. New Phytol. (2018) 220:49–69. doi: 10.1111/nph.15266
28. Tang J, Duan W, Deng P, Li H, Liu C, Duan Y, et al. Cadmium disrupts mitochondrial distribution and activates excessive mitochondrial fission by elevating cytosolic calcium independent of MCU-mediated mitochondrial calcium uptake in its neurotoxicity. Toxicology. (2021) 453:152726. doi: 10.1016/j.tox.2021.152726
29. Lee E, Santana BVN, Samuels E, Benitez-Fuente F, Corsi E, Botella MA, et al. Rare earth elements induce cytoskeleton-dependent and PI4P-associated rearrangement of SYT1/SYT5 endoplasmic reticulum-plasma membrane contact site complexes in Arabidopsis. J Exp Bot. (2020) 71:3986–98. doi: 10.1093/jxb/eraa138
Keywords: rare earth element, testis, YCl3, IP3R1, CaMKII
Citation: Liu Z, Ding Y, Xie S, Hu Y, Xiao H, Liu X and Fan X (2023) Chronic exposure to yttrium induced cell apoptosis in the testis by mediating Ca2+/IP3R1/CaMKII signaling. Front. Public Health 11:1104195. doi: 10.3389/fpubh.2023.1104195
Received: 21 November 2022; Accepted: 13 January 2023;
Published: 30 January 2023.
Edited by:
Nan Zhao, Peking Union Medical College Hospital, ChinaReviewed by:
Amir Khodavirdipour, University of Brighton, United KingdomCopyright © 2023 Liu, Ding, Xie, Hu, Xiao, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaona Fan,  ZnhuOTE4QGdtdS5lZHUuY24=
ZnhuOTE4QGdtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.