- 1Department of Pathology, Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan
- 2Department of Parasitology, Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan
- 3Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraidah, Qassim, Saudi Arabia
Crimean–Congo Hemorrhagic Fever (CCHF) is one of the most important vector-borne diseases of zoonotic potential that can be acquired following the bite of the Hyalomma species of ticks. It is a highly prevalent disease in Asia and the Middle East. The risk factors of this disease are contact with infected tissue, blood, patient, or livestock in the acute viremic phase, infected tick bites, or the manual removal of ticks. The disease is clinically described as progressive hemorrhages, fever, and pain in musculature. Biochemical tests reveal elevated levels of creatinine phosphokinase, alanine transaminase, aspartate aminotransferase, and lactate dehydrogenase. Clotting time is prolonged in pro-thrombin tests, and pathogenesis is mostly related to the disruption of the epithelium during viral replication and indirectly by secreting cytotoxic molecules. These molecules cause endothelial activation and result in the loss of function. Supportive therapy is given through blood or plasma infusions to treat or manage the patients. According to the most advanced studies, CCHF can be treated by Ribavirin, which is an antiviral drug that shows excellent results in preventing the disease. Health-care staff are more prone to infection. The hemorrhagic phase represents a high risk for accidental exposures. This literature review presents a comprehensive overview of the viral epidemiology, zoonotic perspectives, and significant risk factors of CCHF in various Middle East and Asian countries. Furthermore, the pathophysiology and preventive strategies of CCHF have also been discussed as well as legislation and policies regarding public outreach programs, research, and development aimed at infection prevention and control that are required at a global level.
1. Introduction
Ticks and tick-borne diseases have been a threat to humans and animals for many years (1–4). Ticks play an important role as a vector for the transmission of several diseases (5–9). Tick-borne viruses belong to the Bunyavirales and Mononegavirales orders. These orders contain nine families that cause tick-borne diseases (10). Southeast Asian countries are more vulnerable because of the increasing population and the developing nature of healthcare infrastructure and communities. Crimean-Congo Hemorrhagic Fever (CCHF) is a life-threatening zoonotic disease that affects a vast geographical area (11). CCHF is caused by a virus that belongs to the genus Nairo virus, and its family is Nairoviridae. It is a negative sense RNA virus containing a segmented genome that is further divided into small (S), medium (M), and large (L) segments as illustrated in Figure 1. The small segment is responsible for the diversity among the viral isolates of different regions (12). The Hyalomma tick is responsible for its spread to animals and humans by salivary pathways. CCHF is virulent and potentially hazardous, with the ability to be used as a bioterrorism weapon. CCHF causes 3 to 30% mortality in humans, and becomes disastrous when occurs above the endemic level (13). Tick-bites, animals in the viremic phase, and contact with the blood of an infected patient in the acute phase of infection are all sources of transmission of infection (14). Clinically, the disease is characterized by fever, extensive hemorrhages, and myalgia. Some signs and symptoms, such as hepatomegaly and splenomegaly, are mostly observed in different regions where the disease is prevalent, and these typical signs also vary according to the geographical area and the types of vectors (15). Ribavirin is used as a treatment for CCHF. Effectiveness of the medicine is based only on observational studies, thus it is tentative (16). A few studies suggest that certain beneficial effects are associated with the use of ribavirin (17). In high-risk exposures, treatment is done via the use of ribavirin (16). However, there is controversy over the effectiveness of ribavirin as a treatment of CCHF (18).
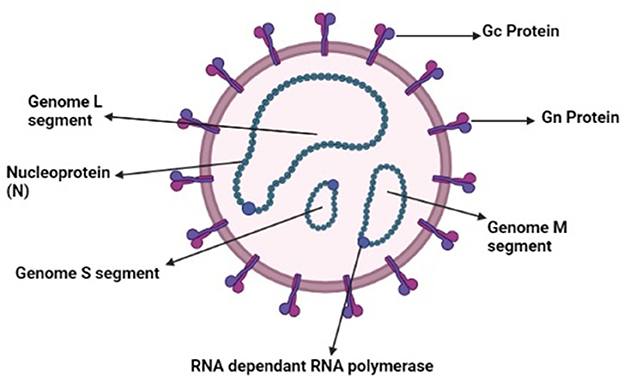
Figure 1. Schematic diagram of Nairo virus (Drawn by BioRender app).
The fatality rate of the disease warrants the adoption of preventive measures against the CCHF virus (19). In geographical areas where the disease is endemic due to the abundance of tick vectors, one should use protective clothing when these ticks are biologically active and are transmitting the virus to humans (20). The use of repellents and examining the skin and clothing for the removal of ticks could spare tick bite incidents (21). People who live in urban areas are at higher risk of exposing themselves to viremic animals, and, therefore, are advised to wear gloves while handling animal tissue or blood to avoid infection (19). The use of protective aprons or clothing is necessary while treating viremic animal herds. Medical staff around CCHF-infected patients are advised to keep barriers while providing medical care (22). The use of goggles, face shields, gowns, and gloves is mandatory when treating patients or soiled surfaces (23). Health-care staff who experience needle stick injury are administered ribavirin by parenteral route to spare the chances of contracting the viral infection (24).
Further research studies are required to reveal risk factors and transmission patterns of the virus among various hosts (25). The biological roles of previously described vectors need to be discussed in detail while risk factors, such asclimatic changes, reservoir hosts, and other contributory factors, need more investigation. This sort of work requires a consortium that takes on board multidisciplinary professionals for infection prevention and control. Epidemiologists, microbiologists, entomologists, and veterinarians should work together under the theme of One Health to devise ways to curtail the occurrence of the disease. There must be rapid risk communication between these disciplines and ecologists in order to avoid the disease in a particular geographical area. Certain drug trials are needed to develop a drug of choice for CCHF, e.g., heparin and other anti-coagulants can be tested to treat disseminated intravascular coagulation. The prevalence of the CCHF virus in Asian and Middle Eastern countries, its association with humans, pathogenesis, pathophysiology, and treatment strategies of the disease have been discussed in the following review.
2. Viral clades
The genomic clades of CCHF are Clade I, which involves the region of West Africa, Clade II has been found in Central Africa, while Clade III has been found in South and West Africa. Clade IV exists in the Middle East and Asia, while Clade V and VI belong to Europe and Greece, respectively. The asian Clade is further divided into two distinct clades, Asia 1 and Asia 2, respectively (12). This phylogenetic classification of the virus is done based on S segments of the CCHF virus (26).
3. Pattern of distribution in Asian and Middle Eastern countries
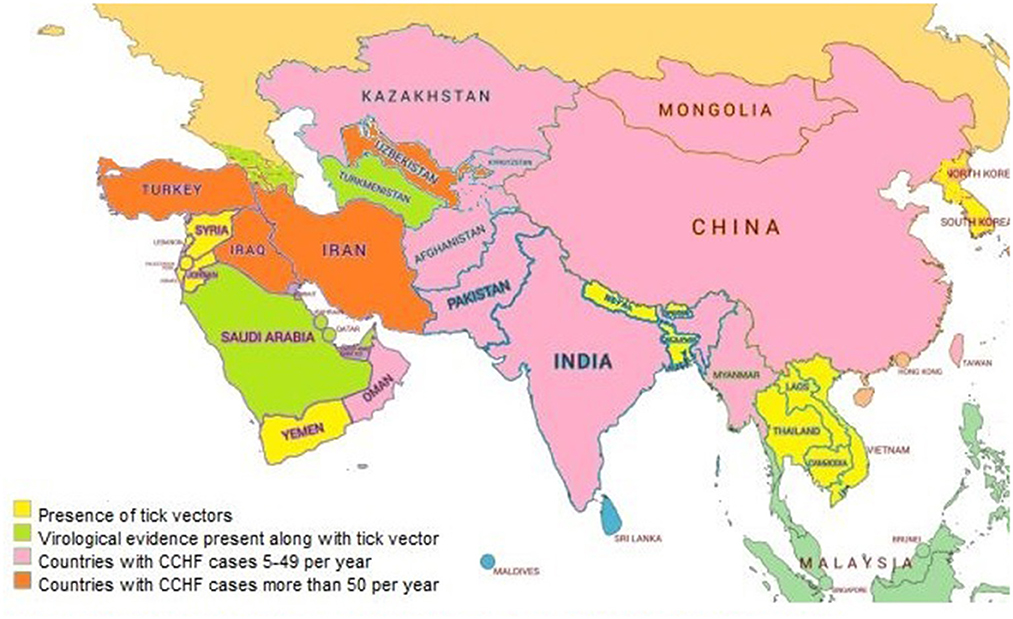
CCHF is a highly prevalent disease involving different countries of Asia and other continents (27). The prevalence of CCHF in Asian and Middle Eastern countries is described country-wise in Figure 2.
3.1. Pakistan
CCHF cases in the country are increasing with each passing year (28). In the 1960's, the virus was first identified in ticks infesting local livestock (29). In 1976, CCHF was observed in Pakistan for the first time as a human case. Up to 2010, only 14 cases were reported (30). After 2010, CCHF cases began to increase at a rapid rate. From 2014 to 2020, more than 350 cases of CCHF were confirmed by the National Institute of Health, Islamabad (31). The mortality rate was proposed to be more than 25% (32). Among these cases of CCHF, only 38% were reported from the Balochistan province, 23% were from the Punjab province, 19% from Khyber Pakhtunkhwa, 14% from Sindh, and 6% were reported from the capital city Islamabad (33). In another research study, the prevalence of CCHF was recorded to be 24.7% in Punjab, 16.2% in Sindh, 52.4% in Khyber-Pakhtunkhwa, and 59.3% in Baluchistan (34). The disease was more prevalent in rural areas due to the close interaction of people with the animals. People who live in urban areas tend to be more infected and at a higher rate on the eve of Eid Ul Adha, when sacrificial animals are sold and then consumed without proper inspection of animal/animal by-products (35).
Prevalent strains from neighboring countries like Iran and India tend to spread and circulate in Pakistan, and vice versa (36). In 2004, 248 cases were positive for CCHF and, among those cases, only 68% were reported from Baluchistan (Pakistan) and Sistan of Iran (37). In the period from 2004 to 2006, there were annual increases in cases of up to 300 patients. On average, 6% of ticks were positive for harboring the virus, and the Asia 1 and Asia 2 were prevalent strains (38). They are considered clade IV among the genomic sequence.
There are certain risk factors that are playing a crucial role in the spread of CCHF. Rapid climatic changes result in biannual peaks between March to May and August to October. Poor sanitation, unhygienic slaughterhouses, transport of animals within cities, nomadic lifestyle, and lack of trained animals and medical care staff contribute to the spread of CCHF (39).
3.2. China
In 1965, there were reports of hemorrhagic fever in western China. The samples collected from ticks, animals, and humans yielded the CCHF virus upon diagnosis. From 1965 to 1994, 260 farmers were reported to be infected with CCHF. The mortality rate was 80% (40). One imported case was reported in 2013 in the city of Beijing, China. Confirmed reports of the CCHF virus were also received from Xinjiang, Yunnan, and Qinghai provinces in China (41, 42). The CCHF virus was detected among ticks of sheep and camels in the Inner Mongolia region of China (43). From 1951 to 2021, only 447,848 cases were reported to be infected from bunyavirales viruses, and CCHFV, along with three other viruses, was reported to cause the most disease burden (44).
3.3. Kazakhstan
In 1944, CCHF was reported for the first time in Kazakhstan (45). Most of the prevalence was reported in Zhambyl and Kyzylorda, regions of the south of Kazakhstan (46). The first reported case of CCHF was in the Turkestan region and was termed Central Asian Fever (47). Later, in 1963 and 1982, cases were reported from Kyzylorda (48) and Zhambyl (49). These regions show a high prevalence of CCHF every year. The mortality rate from CCHF was 14.8% (50) and about 16 cases were reported annually in Kazakhstan (51). In the Zhambyl region, cattle and sheep were tested for the virus and the CCHF virus was found in ~2.4% of sheep and 3.8% of the cattle population (52).
3.4. India
The first case of CCHF was identified in the Gujrat state of India, and it was the result of a nosocomial infection that was related to Pakistan across the border (53). A local survey of livestock revealed that serum and tissue were analyzed to check the prevalence of H. anatolicum (54). From 2010 to 2019, 34 outbreaks were reported from the region of Uttar Pradesh, Rajasthan, and Gujarat (55). Eight secondary cases of CCHF were reported out of the 34 cases (56). In another study, CCHF cases were detected in four states of India (57).
3.5. Afghanistan
In 2009, there was an outbreak of CCHF in the region of Herat, Afghanistan. Only 60 positive cases were detected. It was revealed that native breeds of cattle and sheep were harboring high levels of IgG in their blood in the surrounding area, indicative of probable pre-exposure (58). In a study, 51 positive cases of CCHF were detected by ELISA and, of these, 11 patients died. These were butchers and shepherds. Patients with CCHF increased significantly between June to September of the endemic year. This proved that lifestyle and climatic conditions are the risk factors for the spread of the disease (59). Vector ticks of CCHF were identified on the border of Afghanistan and Iran, giving rise to the potential risk of CCHF in humans (60).
3.6. Malaysia
In a research study, the seroprevalence of CCHF was determined in 2015 within the Orang Asli, a minority population. The titer was too low in them for detection, and it was negative in other populations (61).
3.7. Iran
In the 1970's, the first case of CCHF was reported in Iran, based on the presence of antibodies against CCHF in the serum of cattle, sheep, and humans (62). In Tehran, a sheep abattoir was found to be harboring viral antigens (63). The source of the virus was the ticks of the Ixodes genus (64). Human disease reports were reported in 1999 and since then CCHF outbreaks are reported from different regions of the country (65). The mortality rate was 20% in 2000 and it reduced to 6% in 2007 (66). In a study, 203 ticks were checked for the presence of CCHFV and this was absent in the Kerman province although it was an endemic region (67). In 2011, seven butchers from 104 slaughterhouses were detected to be seropositive for CCHF (68).
3.8. United Arab Emirates
In 1979, CCHF was reported for the first time in Dubai, in the Arabian Peninsula, in a health facility outbreak (69) and, thereafter, no other case was observed until 1994 when an epidemic was reported in the United Arab Emirates among abattoir workers (70). A serum investigation was done in native and imported breeds of livestock to determine the antibody titer, and results showed that native breeds were positive for CCHF antibodies while the imported breeds lacked the CCHF antibody titer. This further confirmed the presence of the disease in that region. Livestock import was reported to be the major cause of the disease (71). It also led to 35 confirmed cases of CCHF with clinical pictures (70). In 2010, two cases of CCHF were reported in Dubai (72). Five cases and two deaths were reported between the time frame from 1998 to 2013 (73). Hyalomma tick presence was proven in native livestock giving rise to an alarming situation for the risk of spread of CCHF (74, 75).
3.9. Oman
In the mid-1990's, human-infected cases of CCHF were reported from the region of Oman, and the serum analysis of local animals confirmed the presence of the CCHF virus in the region (76). The first case of CCHF was observed in 2011, which was the first case after 15 years (77). In 2014, one death, along with a further 18 human-infected cases, were reported in Oman. Only 16 confirmed cases of CCHF were reported in 2015. The mode of disease transmission was either slaughtered animals or livestock (78). CCHF was transferred via occupation in most of the cases occurring in the period from 2011 to 2017 (79). In 2019, from the Northern region of Oman, four patients with CCHF were reported during the festival of Eid Ul Adha (80).
3.10. Iraq
Due to war and civil unrest, data collection for CCHF was not efficiently done in Iraq, but certain reports claimed the presence of CCHF in the country (81). Six cases of CCHF were reported between 1989 and 2009. In 2010, 11 cases of CCHF were reported. Three fatal cases were reported in 2018, while 33 cases were reported in 2021, with 13 fatal cases (82). The World Health Organization (WHO) reported 1085 suspected cases of CCHF in 2022, and laboratory confirmation revealed 287 positive cases, with 83 deaths being of suspected CCHF patients and 52 patients confirmed patients of CCHF (18.1% case fatality rate) (83).
3.11. Kuwait
From 1979 to 1982, a serological analysis of 502 patients was done to confirm the disease in two hospitals. Only 18 cases were reported to harbor the disease. According to a research study, only 17 patients who had a close association with livestock had pathognomonic signs of CCHF. About 38% of patients were from rural backgrounds. They were located on the borders of Kuwait so there were chances of imported cases of CCHF (84).
3.12. Egypt
Egypt is a transcontinental country and it showed infections of CCHF in 1978 in many wild and domestic animals. According to a serological study, camels had 8.8% titer while sheep demonstrated 23.1% titer (85). From 1986 to 1987, camel import was the reason for the spread of the disease (86). Antibodies against the CCHF virus were detected again in a 2004 to 2005 serological survey, which confirmed the role of ruminants as a maintenance host (87). Human cases were also observed, mostly in health-care workers and people of rural backgrounds. In 1981 and 2012, a total of four cases were detected, with one death (88, 89).
3.13. Saudi Arabia
In 1990, a case of CCHF was first reported in the country. According to this report, seven people were infected with the virus in Makkah (90). From 1989 to 1990, only 40 workers of slaughterhouses showed suspected signs of CCHF in Makkah, and twelve patients died due to these suspected signs. Imported sheep were considered to be the source of the disease (91). Imported animals and humans who were working on the seaport were also tested for titer of antibodies. Animals along with the staff of the seaport of Jeddah also tested positive for the presence of antibodies against CCHF (92).
3.14. Turkey
It has been observed that Turkey is the hub of CCHF, with reports of about 1,000 confirmed cases per year. In the past few decades, the country was CCHF-free, but with time, Turkey became the lodestone of the disease. It may be possible that there was underreporting or that CCHF was not differentiated from other diseases or was misdiagnosed as some other disease, but it might have been present in the region. Moreover, the ecological and environmental conditions in the country are very favorable for the successful completion of tick life cycles (93). In 2002, the first case of CCHF was reported in patients in the region of the eastern Black Sea in Turkey (93). In 2009, the fatality rate among 500 patients was reported to be 5% (94). This rate was high because initially the disease was misdiagnosed in 66% of patients, as the early symptoms of the disease were not pathognomonic (95). According to a serosurvey conducted in the region of eastern Turkey and Anatolia, specific antibodies against the CCHF virus were about 80%, indicating that the region was most prone to the disease among all regions of Turkey (96). Hyalomma ticks were used for viral isolation, and about 20% of them harbored the virus (97). In a study, conducted at a secondary care hospital in Kastamonu, patients with CCHF were evaluated in the period of 2014–2017. A total of 76 suspected cases appeared, and CCHF was confirmed in 46.1% of cases. During those 4 years, the case fatality rate was of only 9.6% (98). A woman suffering from both SARS-CoV-2 and CCHF was treated against both infections and she was lucky enough to beat both viral variants (99).
4. The CCHF virus and its association with humans
The life cycle of the CCHF virus depends upon a vector (ticks) and an amplifying host. There are many species of ticks that are proven to harbor the CCHF virus. The Ixodes genus of ticks is the most efficient among all other genera (100), while the Hyalomma marginatum is the most efficient vector (101). Other tick species that are reported to transmit the CCHF virus are H. aegyptium, H. schulzei, H. onatoli, H. dromedarii, H. rufipes, H. excavatum, H. anatolicum, R. sanguineus, R. turanicus, R. annulatus, Ha. punctata, A. variegatum, H. truncatum, H. turanicum, I. ricinus, A. lepidum, and H. impeltatum (74). Hyalomma marginatum has certain characteristics that make it a triumphant vector over others. It harbors the virus in its saliva and when the virus reproduces sufficiently in the intestinal tract of ticks then it replicates significantly and tends to spread to the other organs (102). Some organs have a low titer of the virus and few other organs possess a greater number of populations of the virus-like salivary gland and reproductive tract (103). The virus transmits vertically in different stages of life, other than adult stages, i.e., larvae and nymph (104). Female ticks have a tendency to lay thousands of eggs and the transovarian route is also present (105), so even a very low viral titer in females transfers to offspring and also circulates in the environment. These infected ticks also infect the non-infected animals in the vicinity, on the other hand, the infected animals also spread the CCHF virus to ticks that feed blood from infected animals (27). True natural CCHF reservoirs are those ticks that remain infective for a lifetime and never become free from the virus. The Argasid genus is not able to transfer virus vertically or horizontally (106).
An amplifying host in the case of the CCHF virus is a vertebrate host, which amplifies the virus and transfers it to humans (107). The CCHF virus tends to multiply at a faster rate in blood and develops a high viral titer within or <14 days. Clinical symptoms are not observed in animals. Large herbivore animals are reported to be seropositive (108). Ostriches are the only birds that harbor the virus, and no other bird species has yet been reported to harbor the virus (109). Birds have a role in outspreading the disease during migration from one area to another as they carry infected ticks in their fur (110). Some small vertebrates, such as hares and hedgehogs, act as a reservoir, maintain the viral load in society, and tend to spread the disease, as illustrated in Figure 3 (111). They are usually infested with larval stages of ticks that transfer the virus in their life stages by vertical mode. On the other hand, large animals are infested with a great number of ticks at the same time (112). The condition can be worsened by the additive effect of horizontal transmission. Favorable conditions such as the hot and arid season help in the molting of tick larvae into the adult tick. Vegetation and humidity also help in the propagation of the ticks. Furthermore, the presence of large and small mammals in a particular area helps in the maintenance of the disease in a particular region. Researchers claim that the CCHF may spread to unaffected regions and countries of the Mediterranean region because they have a feasible environment for ticks (78). Some authors claim that in a few years, Western Europe will be facing a devastating outbreak of the disease because of its climatic adaptation and consistent distribution pattern of Hyalomma ticks (113).
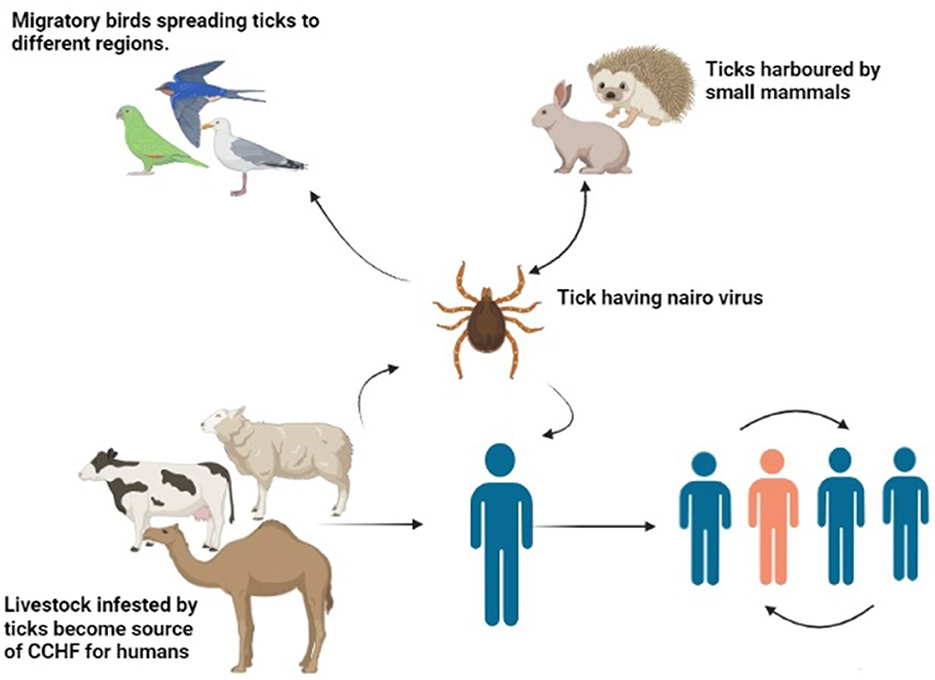
Figure 3. Transmission of CCHF virus (Drawn by BioRender app).
5. Transmission and zoonotic impact of CCHF
Humans usually get bitten by an infected tick and develop the infection when they are rearing livestock and handling animals,. In some cases, humans remove ticks from animals and squash them by bare hand, ultimately developing the disease. Tick bites are responsible for 60 to 69% of patients (114). Adult ticks of the Hyalomma species complete their lifecycle in spring and summer whenthey feed on amplifying hosts (115). If the accompanied winter was not too harsh, the chances of CCHF cases increase as the tick population does not reduce (116). The ecosystem is also responsible for the spread of the disease in particular areas where a higher number of mammals, large or small, roam, with chances of a spillover to the human population reducing significantly because the virus tends to roam silently in those mammals, causing only sporadic cases of the disease in humans. On the other hand, in 1944 in the era of the Germans, livestock and farm animals were reduced significantly in Crimea, but the cases of CCHF in humans increased (27). Wild hares harbored the ticks rather than livestock and these hares transmitted infections to humans by the bite of Hyalomma ticks (27).
Infected tissues and blood of infected animals can also be the source of infection in the human population. An extensive study was done in Turkey from 2002 to 2007 that claimed that out of 1,820 patients, only 62% were in close association with animals (117). After the death of an animal, acidification of the internal environment of the body tends to reduce the viral load, but certain reports showed that tissue and blood infected with the virus can be problematic (108). Only 90% of cases were reported from patients that were professionally in contact with animals or animal tissue, such as farmers, butchers, and people working in slaughterhouses. A serological study revealed that older people have a high titer of anti-CCHF IgG antibodies and so do people who are in close contact with livestock or do not have a good socio-economic status. These people have a higher tendency to be bitten by ticks while handling livestock (118). Most human cases are observed in men because they are more likely to handle livestock and, in Middle Eastern countries where men are mostly performing outdoor jobs, are professionally adapted to animal-associated environments. Sexual transmission has been reported, but there are few such cases, so it has not been prioritized enough (119). In some cases, the virus was transferred vertically and the child showed symptoms of severe hemorrhage and ultimately died (120). On the other hand, infected mothers gave birth to non-infected babies, which suggests that vertical transmission from mother to child does not going happen in all cases of infected mothers (121).
Health workers usually get infected by accidental needlestick injuries while dealing with CCHF-positive patients. In Turkey, nine sites, where 4,869 patients of CCHF were admitted, were examined to check for the prevalence of the disease in health-care staff. Accidental exposure to CCHF was observed in 51 cases, and 25 of those developed CCHF. Among these accidentally exposed medical staff, 16% died due to the exposure. Among these 16%, needlestick injury-associated deaths were about 62.7%. Transmission due to body fluids was about 23.5% (115). Ribavirin was recommended to health workers who were accidentally exposed and, out of 32 patients, 19 were given prophylactic ribavirin and did not develop the disease. On the other hand, eight out of 13 developed clinical diseases and did not receive prophylactic treatment (122). On these bases, ribavirin is recommended as prophylaxis, but in this regard, no document has been formulated yet (122). The hemorrhagic phase of the disease is the riskiest in terms of transmission of the disease to health workers. No case has been reported during the incubation period of the disease (108). The absence of protective measures and the occurrence of needlestick injuries make health workers prone to the disease (108).
6. Pathogenesis of the disease
The exact mechanism by which viruses produce pathogenic effects is not fully known. All the viruses that cause hemorrhagic fever have a common characteristic in which they disable the host's immune function and make it prone to the disease (123). They do so by attacking the antiviral cells. The virus starts to replicate speedily, as shown in Figure 4, and alters the normal functioning of the vascular system and lymphatic organs (124). CCHF pathogenesis is mainly dependent on the infection associated with the epithelium (125). The epithelium is damaged by the continuous replication of viral particles. The second method is indirect damage by the virus, in which the virus releases tissue-toxic factors or produces host-derived soluble factors that result in endothelial activation and loss of proper cellular functions. Damaged endothelium attracts the platelets to aggregate, and the intrinsic pathway of coagulation is activated. It is an early symptom that is obvious and ends as a hemostatic failure.
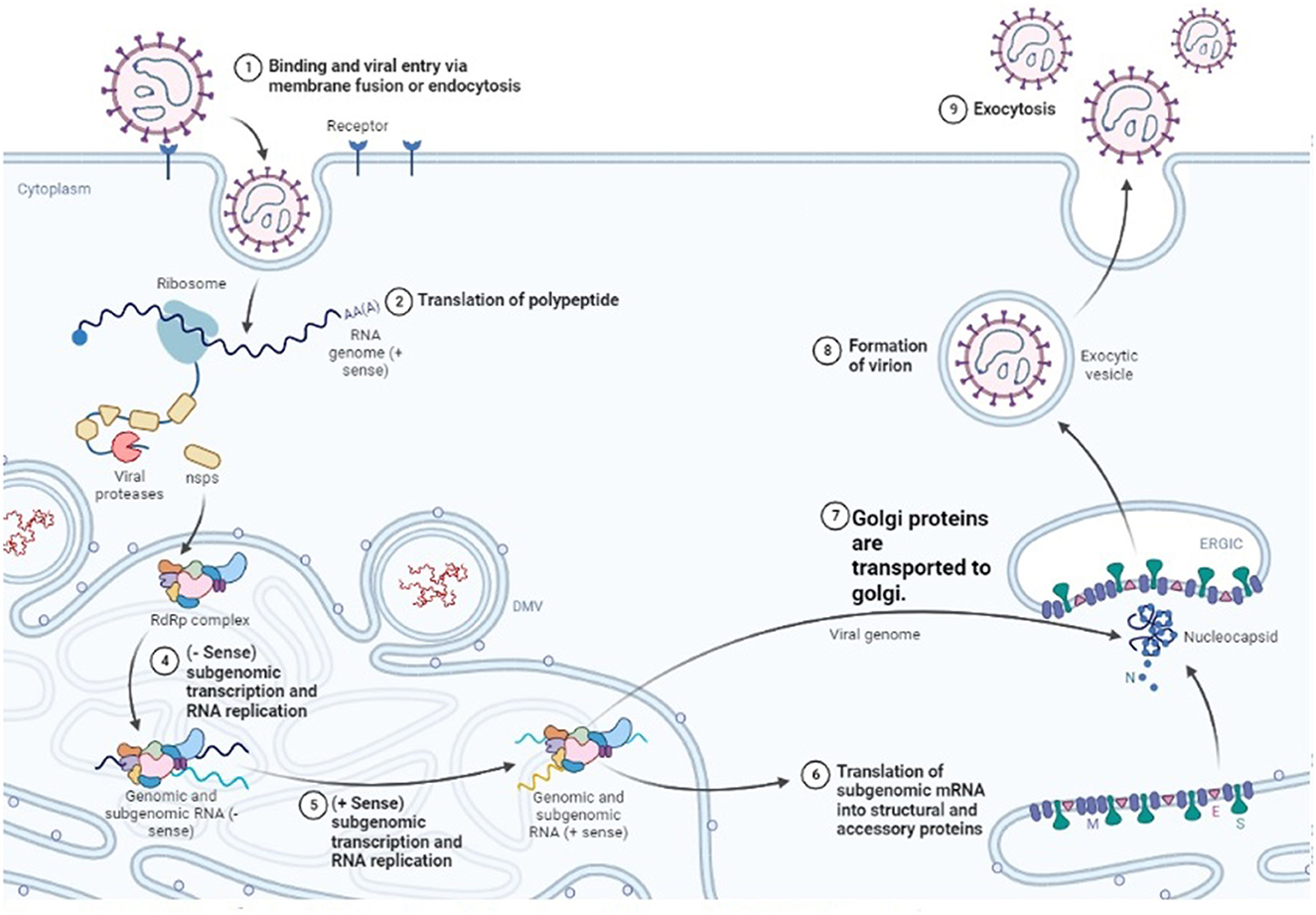
Figure 4. Replication cycle of Nairo virus within a host cell (Drawn by BioRender app).
Cytopenia was associated with haemophagocytosis because it was a consistent finding in half of the patients in Turkey (93). High levels of Type 1 T helper cytokine, such as Tumor Necrosis Factor α, Interferon γ, Interleukin 6, and Interleukin 1, give rise to increased activation of monocytes that lead to haemophagocytic lymphohistiocytosis (126). In this study, it was also revealed that cytokines have roles in the pathogenesis of CCHF (93). It was further confirmed by another study that the level of Type 1 T helper cell cytokines was detected in patients who died and in those who survived. It was noted that the level of all these cytokines was lower in the patients who survived and higher in those who died because of that infection. In grave cases, Interleukin 6 and Tumor Necrosis Factor α levels were higher, along with disseminated intravascular coagulation. While Interleukin10 level was inversely related to them (127).
It was noted that cellular pathology was usually associated with the viral division among the cell, but the receptor that allows the virus to move into the cell was not identified yet. Protein domains that are present outside the cell, i.e., GC and GN glycoproteins, play an important role in the binding of the virus to the host cell. The nucleolin present in the host cell also plays a vital role in permitting the virus to cause cellular injury (128). Clathrin-dependent endocytosis enables the virus to enter the host cell (129). Upon entry into the cell, positive strand intermediates are made by viral RNA-dependent RNA polymerase (RdRp) when it interacts with encapsulated genome segments present in the cytoplasm of the host cell. With the help of these positive strands, their complementary negative strands are prepared. A model is drawn in which primer-independent manufacturing of both positive and negative sense genomes is claimed (130).
Microtubules of the host are the main players on which viral internalization, assembly, and egression are dependent (131). PreGN and PreGC, which are immature forms of GN and GC, are synthesized in the rough endoplasmic reticulum and upon their production, the synthesis of viral surface glycoprotein initiates. Both PreGC and PreGn are transferred to the Golgi body as a heterodimer. They are cleaved further, then the process of glycosylation occurs, and the heterodimer is folded and converted into a viral membrane (132). The Golgi body releases mature viruses into the neighboring environment, and this process is known as budding. In this way, the virus replicates and causes different degenerative changes.
7. Pathophysiology of the disease
Humans are the only known host that show clinical symptoms associated with the disease (133). According to a study, the chances of the development of the clinical disease in people harboring the virus were 0.215 to 1 among every five infected people (134). The development of the disease has four phases, which include an incubatory phase, in which replication of the virus happens in the body, the pre-hemorrhagic phase, the hemorrhagic phase, and the convalescent phase (135). The incubation period starts right after the infected tick bite and usually lasts between 3 to 7 days (136). The incubation period depends upon the amount of viral load injected during the bite and the route of exposure (137). Incubation period is shorter when tick directly feeds on blood rather than other transmission routes. Blood and tissue of infected animals take ~5 days to develop the infection. The human-to-human transmission also takes 5 to 7 days on average (138). The mean duration before presenting patients to the hospital is reported to be 3–5 days in UAE and 5 to 6 days in Turkey (13).
The second phase of the infection is the pre-hemorrhagic phase, in which the person with the infection shows signs of a fever that ranges from 39 to 41°C (139). There is severe headache, dizziness, and muscular pain (140). The patient remains with the fever for 4 to 5 days and then the fever subsides (63). In some cases, additional symptoms, such as diarrhea, vomiting, and nausea, are observed (141). This phase lasts for about 3 days and different parts of the body, such as face and neck, become hyperemic (142). The sclera becomes congested and conjunctivitis is usually observed (143).
The third phase of CCHF is the hemorrhagic phase, which is shorter and tends to be more prominent in terms of clinical symptoms because of hemorrhages. It usually appears on the 3rd to 5th day of the disease (144). No association is generally observed between fever and hemorrhages in patients (133). The shape of hemorrhages ranges from smaller ecchymotic to petechial hemorrhages. Large hematomas are present on the skin and the mucous membranes (145). Clotting time increases in patients suffering from CCHF and a stage comes when blood is thin enough to ooze out of natural orifices, such as the vagina, gingival tissues, and nose (15). Blood is also seen in urine (hematuria) and feces (melena), and bloody discharge also occurs from the uterus (Menometorrhagia) (146). Hemoptysis is also observed in the hemorrhagic phase (147). This phase is often confused with appendicitis if there is only internal bleeding and there is no sign of external bleeding (148). Persistent pain was thought to be caused due to inflammation in the appendix, but with further investigation, it was claimed that there were internal hemorrhages and bleeding in the cecum, and internal and external oblique muscle with no pathology related to the appendix (149). Hepatomegaly and splenomegaly were also observed in some patients suffering from CCHF, but it was not a consistent finding (150). These were the clinical features of CCHF patients who either recovered from that phase or died due to extensive bleeding and hemorrhages (151).
The last phase is the convalescent phase for those who survived the infection, and it starts about 10 to 20 days after the infection (152). Patients recovering from CCHF have a weak pulse, often accompanied by tachycardia, partial or complete alopecia, dyspnea, polyneuritis, xerostomia, deafness, memory loss, blindness, or weak eyesight (32). Some patients may have bradycardia and a drop in blood pressure (153).
8. Lab investigations in CCHF patients
The basic marker in the diagnosis of CCHF lab reports is a decreased level of platelets and leukocytes (Figure 5). Enzymes, such as aspartate aminotransferase, alanine aminotransaminase, creatinine phosphokinase, and lactate dehydrogenase, tend to increase. Prolonged clotting time is checked with a prothrombin test and an activated partial thromboplastin test. Fibrinogen is reduced, which tends to make a meshwork to bind platelets and protein to make a clot. An increase in the degradation products of fibrin could be observed (154). Within 5 to 9 days, the surviving patients' lab results tend to become normal.
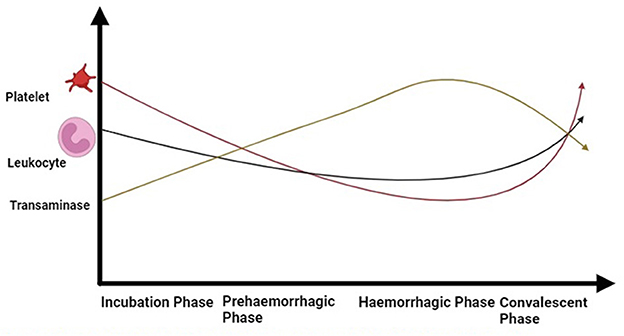
Figure 5. Dynamic of blood cells during different phases of CCHF in patients (Drawn by BioRender app).
9. Treatment
The treatment strategy of CCHF involves two aspects, one is to give symptomatic treatment to cover up the deficiencies that occur because of the extensive loss of blood cells, such as blood transfusion, platelets, or plasma is given to the patients (115). Hypovolemic patients are given electrolytes. Secondary infections are also addressed as there is immune suppression and the person becomes prone to other diseases (155). Any ongoing infection is also treated. For example, in some cases, malaria occurs along with CCHF in some patients (156).
Ribavirin is also used as treatment and, in some instances, it is given as prophylaxis. The WHO recommends the dose rate of ribavirin for patients. Firstly, it is recommended to give two grams of ribavirin per oral route. Then, until the 4th day, it should be given after an interval of 6 h and by a dose rate of 1 g. From day 5 to 10, it should be increased up to 500 g, with the same hour interval. Parental use has poor bioavailability, but it is used in some instances. The dose of ribavirin recommended is 17 mg/kg, which should not exceed 1 g until the 4th day and with an interval of 6 h. Then it is reduced to half (8 mg per kg) from day 5th to the 10th day. Ribavirin is considered safe with limited side effects and is used efficiently for the treatment of CCHF. Favipiravir, an antiviral drug, was tested in mice and reported to be better as compared to ribavirin, displaying results even when it was given to patients who exhibited symptoms of CCHF (157). However, due to a lack of enough evidence, it cannot be recommended for daily use for the treatment of CCHF.
10. Future perspectives
The exact manner of pathogenesis other than replication needs to be discovered. Research is needed to reveal the various mechanisms of disease production. Once identified, they can be used for the development of certain drugs or candidate vaccine virus that can block the pathway of development of infection by the virus. The impact of the enzootic environment needs to be examined in further detail. The transmission cycle of the CCHF virus and its vectors needs to be analyzed so that there may be a step from where we can break their cycle, ultimately resulting in the downregulation of the disease in a specific region. The field of pharmacology needs to excel to produce such antiviral drugs that can reduce the number of viruses in patients, either by killing the viruses or blocking their replication pathways. Death by CCHF is mostly attributed to disseminated intravascular coagulation. Anticoagulation factors, such as heparin and certain oxalates, can be tested to prevent Disseminated Intravascular Coagulation in patients and clinical symptoms of the disease. The mechanism by which the virus develops Disseminated Intravascular Coagulation in patients needs to be studied, and further studies on CCHF will result in the discovery of thisexact mechanism and how bacterial sepsis develops along with Disseminated Intravascular Coagulation. This understanding will lead to the development of drug molecules that will help to eliminate the disease around the globe. For this, all medical fields, including pharmacologists, pathologists, parasitologists, microbiologists, and clinicians have to work hand in hand to 1 day conquer the disease.
11. Conclusion
The presence of ticks and a suitable environment make CCHF an alarming disease in Asian and Middle Eastern countries. The prevalence of CCHF is noted to be increased per annum almost in all countries, including Pakistan, India, China, Iran, Kazakhstan, Egypt, Iraq, U.A.E, Saudi Arabia, and Turkey. Humans in close contact with livestock are at a greater risk than those in urban areas. Virus pathogenesis is attributed either by directly damaging cells by proliferation or indirectly by releasing cytotoxic compounds. Further investigations are required to discover the exact mechanism of the disease and to provide better healthcare to patients in different clinical phases of CCHF. To prevent zoonosis and transfer to medical health workers, certain measures should be taken to avoid the infection. Drugs with higher efficiency can be prepared once hidden mechanisms of disease are known. Prevention can be the key to success. Treatment can be undertaken using ribavirin and the medication can be given as prophylaxis. Vaccination development needs to be considered in the future for the advancement of better immunity in individuals.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research, Qassim University, Saudi Arabia, for funding the publication of this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ali S, Ijaz M, Ahmed A, Aziz M, Naveed M, Javed M, et al. Prevalence and associated risk factors of bovine babesiosis in Lahore, Pakistan. Agrobiol Records. (2020) 2:17–23. doi: 10.47278/journal.abr/2020.007
2. AbouLaila M, AbdEl-Aziz AR, Menshawy S, Yokoyama N, Igarashi I, Al-Wabel M, et al. Evaluation of the inhibitory effects of coumermycin a1 on the growth of theileria and babesia parasites in vitro and in vivo. Pak Vet J. (2021) 41:469–74. doi: 10.29261/pakvetj/2021.064
3. Selim A, Abdelhady A, Alahadeb J. Prevalence and first molecular characterization of Ehrlichia canis in Egyptian dogs. Pak Vet J. (2020) 41:117–21. doi: 10.29261/pakvetj/2020.061
4. Ceylan O, Uslu A, Ozturk O, Sevinc F. Serological investigation of some vector-borne parasitic and rickettsial agents in dogs in the Western Part of Turkey. Pak Vet J. (2021) 41:386–92.
5. Ceylan O, Uslu A, Ceylan C, Sevinc F. Predominancy of Rhipicephalus turanicus in tick-infested sheep from Turkey: a large-scale survey. Pak Vet J. (2021) 41:429–33. doi: 10.29261/pakvetj/2021.036
6. Zaman MA, Rafique A, Mehreen U, Mehnaz S, Atif FA, Abbas A, et al. Epidemiological investigation and development of loop mediated isothermal amplification for the diagnosis of ovine theileriosis. Pakistan Vet J. (2022) 42:370–75. doi: 10.29261/pakvetj/2022.039
7. Basit MA, Ijaz M, Abbas RZ, Khan JA, Ashraf K. First Molecular Evidence of Ehrlichia Infection: An Emerging Pathogen of Small Ruminants in Pakistan. Pak Vet J. (2022) 42:208–14. doi: 10.29261/pakvetj/2022.012
8. Zaman MA, Mehreen U, Qamar W, Qamar MF, Kashif M, Shahid Z, et al. Brief account of bovine theileriosis prevalence in some South Asian countries. Agrobiol Rec. (2020) 2:38–48. doi: 10.47278/journal.abr/2020.010
9. Jia Y, Wang S, Yang M, Ulzhan N, Omarova K, Liu Z, et al. First Detection of Tacheng Tick Virus 2 in Hard Ticks from Southeastern Kazakhstan. Kafkas Universitesi Veteriner Fakultesi Dergisi (2021).
10. Maqbool M, Sajid MS, Saqib M, Anjum FR, Tayyab MH, Rizwan HM, et al. Potential mechanisms of transmission of tick-borne viruses at the virus-tick interface. Front Microbiol. (2022) 13:846884. doi: 10.3389/fmicb.2022.846884
11. Appannanavar SB, Mishra B. An update on Crimean–Congo Hemorrhagic Fever. J Glob Infect Dis. (2011) 3:285–92. doi: 10.4103/0974-777X.83537
12. Chinikar S, Shah-Hosseini N, Bouzari S, Jalali T, Shokrgozar MA, Mostafavi E. New circulating genomic variant of Crimean-Congo hemorrhagic fever virus in Iran. Arch virol. (2013) 158:1085–88. doi: 10.1007/s00705-012-1588-0
13. Ergönül Ö. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. (2006) 6:203–14. doi: 10.1016/S1473-3099(06)70435-2
14. Ergonul O. Crimean–Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol. (2012) 2:215–20. doi: 10.1016/j.coviro.2012.03.001
15. Mostafavi E, Pourhossein B, Chinikar S. Clinical symptoms and laboratory findings supporting early diagnosis of Crimean-Congo hemorrhagic fever in Iran. J Med Virol. (2014) 86:1188–92. doi: 10.1002/jmv.23922
16. de la Calle-Prieto F, Martín-Quirós A, Trigo E, Mora-Rillo M, Arsuaga M, Díaz-Menéndez M, et al. Therapeutic management of Crimean-Congo haemorrhagic fever. Enfermedades infecciosas y microbiologia clinica (English ed). (2018) 36:517–22. doi: 10.1016/j.eimce.2017.04.016
17. Keshtkar-Jahromi M, Kuhn JH, Christova I, Bradfute SB, Jahrling PB, Bavari S. Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antivir Res. (2011) 90:85–92. doi: 10.1016/j.antiviral.2011.02.010
18. Gholizadeh O, Jafari MM, Zoobinparan R, Yasamineh S, Tabatabaie R, Akbarzadeh S, et al. Recent advances in treatment Crimean–Congo hemorrhagic fever virus: a concise overview. Microb Pathog. (2022) 6:105657. doi: 10.1016/j.micpath.2022.105657
19. Leblebicioglu H, Sunbul M, Memish ZA, Al-Tawfiq JA, Bodur H, Ozkul A, et al. Consensus report: preventive measures for Crimean-Congo hemorrhagic fever during Eid-al-Adha festival. Int J Infect Dis. (2015) 38:9–15. doi: 10.1016/j.ijid.2015.06.029
20. Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. The Lancet Infect Dis. (2015) 15:721–30. doi: 10.1016/S1473-3099(15)70091-5
21. Kalal MN. Crimean-congo haemorrhagic fever: a global perspective. Int J Res Med Sci. (2019) 7:4812. doi: 10.18203/2320-6012.ijrms20195562
22. Tarantola A, Ergonul O, Tattevin P. Estimates and prevention of Crimean-Congo hemorrhagic fever risks for health-care workers. Crimean-Congo hemorrhagic fever: Springer Sci Rev. (2007) 3:281–94. doi: 10.1007/978-1-4020-6106-6_21
23. Conger NG, Paolino KM, Osborn EC, Rusnak JM, Günther S, Pool J, et al. Health care response to CCHF in US soldier and nosocomial transmission to health care providers, Germany, 2009. Emerg Infectious Dis. (2015) 21:23–31. doi: 10.3201/eid2101.141413
24. Gönen I, Geyik MF. Percutaneous injuries among healthcare workers at a general hospital. J Microbiol Infect Dis. (2011) 1:26–30. doi: 10.5799/ahinjs.02.2011.01.0007
25. Randolph SE, Rogers DJ. Ecology of tick-borne disease and the role of climate. Crimean-Congo hemorrhagic fever: Springer Sci Rev. (2007) 3:167–86. doi: 10.1007/978-1-4020-6106-6_14
26. Biglari P, Chinikar S, Belqeiszadeh H, Telmadarraiy Z, Mostafavi E, Ghaffari M, et al. Phylogeny of tick-derived Crimean-Congo hemorrhagic fever virus strains in Iran. Ticks Tick Borne Dis. (2016) 7:1216–21. doi: 10.1016/j.ttbdis.2016.07.012
27. Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir Res. (2013) 100:159–89. doi: 10.1016/j.antiviral.2013.07.006
28. Ahmed A, Saqlain M, Tanveer M, Tahir AH, Ud-Din F, Shinwari MI, et al. Knowledge, attitude and perceptions about Crimean Congo Haemorrhagic Fever (CCHF) among occupationally high-risk healthcare professionals of Pakistan. BMC Infect Dis. (2021) 21:1–9. doi: 10.1186/s12879-020-05714-z
29. BEGUM F, Wisseman Jr C, Casals J. Tick-borne viruses of West Pakistan: IV. Viruses similar to, or identical with, crimean hemorrhagic fever (congo-semunya), wad medani and pak argas 461 isolated from ticks of the changa manga forest, lahore district, and of hunza, gilgit agency, w. Pakistan. Am J Epidemiol. (1970) 92:197–202. doi: 10.1093/oxfordjournals.aje.a121199
30. Shahid MF, Yaqub T, Ali M, Ul-Rahman A, Bente DA. Prevalence and phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in ticks collected from Punjab province of Pakistan. Acta Trop. (2021) 218:105892. doi: 10.1016/j.actatropica.2021.105892
31. Jabeen A, Ansari J, Ikram A, Khan M, Safdar M. Impact of climate change on the epidemiology of vector-borne diseases in Pakistan. Global Biosec. (2022) 4:163. doi: 10.31646/gbio.163
32. Nasirian H. New aspects about Crimean-Congo hemorrhagic fever (CCHF) cases and associated fatality trends: a global systematic review and meta-analysis. Comp Immunol Microbiol Infect Dis. (2020) 69:101429. doi: 10.1016/j.cimid.2020.101429
33. Alam MM, Khurshid A, Sharif S, Shaukat S, Rana MS, Angez M, et al. Genetic analysis and epidemiology of Crimean–Congo Hemorrhagic Fever viruses in Baluchistan province of Pakistan. BMC Infect Dis. (2013) 13:1–8. doi: 10.1186/1471-2334-13-201
34. Zohaib A, Saqib M, Athar MA, Hussain MH, Tayyab MH, Batool M, et al. Crimean-Congo hemorrhagic fever virus in humans and livestock, Pakistan, 2015–2017. Emerg Infect Dis. (2020) 26:773–77. doi: 10.3201/eid2604.191154
35. Butt MH, Ahmad A, Misbah S, Mallhi TH, Khan YH. Crimean-Congo hemorrhagic fever and Eid-Ul-Adha: a potential threat during the COVID-19 pandemic. J Med Virol. (2021) 93:618–19. doi: 10.1002/jmv.26388
36. Di Nardo A, Knowles N, Paton D. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Revue Scientifique et Technique-OIE. (2011) 30:63–85. doi: 10.20506/rst.30.1.2022
37. Shahhosseini N, Jafarbekloo A, Telmadarraiy Z, Chinikar S, Haeri A, Nowotny N, et al. Co-circulation of Crimean-Congo Hemorrhagic Fever virus strains Asia 1 and 2 between the border of Iran and Pakistan. Heliyon. (2017) 3:e00439. doi: 10.1016/j.heliyon.2017.e00439
38. Mehravaran A, Moradi M, Telmadarraiy Z, Mostafavi E, Moradi AR, Khakifirouz S, et al. Molecular detection of Crimean-Congo haemorrhagic fever (CCHF) virus in ticks from southeastern Iran. Ticks Tick Borne Dis. (2013) 4:35–8. doi: 10.1016/j.ttbdis.2012.06.006
39. Yousaf MZ, Ashfaq UA, Anjum KM, Fatima S. Crimean-Congo hemorrhagic fever (CCHF) in Pakistan: the “Bell” is ringing silently. Crit Rev Eukaryot. (2018) 28:93–100. doi: 10.1615/CritRevEukaryotGeneExpr.2018020593
40. Papa A, Ma B, Kouidou S, Tang Q, Hang C, Antoniadis A. Genetic characterization of the M RNA segment of Crimean–Congo Hemorrhagic Fever virus strains, China. Emerg Infect Dis. (2002) 8:50–3. doi: 10.3201/eid0801.010087
41. Wu XB, Na RH, Wei SS, Zhu JS, Peng HJ. Distribution of tick-borne diseases in China. Parasites Vectors. (2013) 6:1–8. doi: 10.1186/1756-3305-6-119
42. Gao X, Nasci R, Liang G. The neglected arboviral infections in mainland China. PLoS Negl Trop Dis. (2010) 4:e624. doi: 10.1371/journal.pntd.0000624
43. Kong Y, Yan C, Liu D, Jiang L, Zhang G, He B, et al. Phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in inner Mongolia, China. Ticks Tick Borne Dis. (2022) 13:101856. doi: 10.1016/j.ttbdis.2021.101856
44. Teng AY, Che TL, Zhang AR, Zhang YY, Xu Q, Wang T, et al. Mapping the viruses belonging to the order Bunyavirales in China. Infect Dis Poverty. (2022) 11:43–61. doi: 10.1186/s40249-022-00993-x
45. Chumakov M. A New Tick-Borne Virus Disease—Crimean Hemorrhagic Fever. Crimean Hemorrhagic Fever (Acute Infectious Capillary Toxicosis) Simferopol. Moscow: Izd Otd Primorskoi Armii. 1945:13-45.
46. Perfilyeva YV, Shapiyeva ZZ, Ostapchuk YO, Berdygulova ZA, Bissenbay AO, Kulemin MV, et al. Tick-borne pathogens and their vectors in Kazakhstan—A review. Ticks Tick Borne Dis. (2020) 11:101498. doi: 10.1016/j.ttbdis.2020.101498
47. Temirbekov ZT, Dobritsa P, Kontaruk V, Vainshtein E, Marushchak O, Dobritsa N. Investigation of Crimean hemorrhagic fever in Chimkent Oblast of Kazakh SSR. Viral hemorrhagic fevers Crimean hemorrhagic fever, Omsk hemorrhagic fever, and hemorrhagic fever with renal syndrorme. Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. (1971) 3:160–6.
48. Chun-Syun F, Genis D. Natural tickborne hemorrhagic fever focus in the semidesert zone of Southern Kazakhstan. Sborn Tr Inst Polio Virus Encef Akad Med Nauk USSR. (1965) 45:312–4.
49. Temirbekov Z, Kiryushchenko T. A New Focus of Crimean Hemorrhagic Fever in the Moiynkum District of the Dzhambul Region. Almaty, Kazakhstan: Regional Features of the Epidemiology of Infectious Diseases in Kazakhstan) Research Institute of Epidemiology, Microbiology and Infectious Diseases (1984):122–5.
50. Nurmakhanov T, Sansyzbaev E, Atshabar B, Kazakov S, Deryabin P. Comparative assessment of the distribution of CCHF and TBE viruses in South Kazakhstan Province. Environ Public Health. (2013) 2:31–2.
51. Turliyev Z, Usataeva G. Epidemiological situation in the Republic of Kazakhstan on Congo-Crimean hemorrhagic fever. Vestnik KazNMU. (2019) 4:20–3.
52. Bryant-Genevier J, Bumburidi Y, Kazazian L, Seffren V, Head JR, Berezovskiy D, et al. Prevalence of Crimean-Congo hemorrhagic fever virus among Livestock and Ticks in Zhambyl Region, Kazakhstan, 2017. Am J Trop Med. (2022) 106:1478–85. doi: 10.4269/ajtmh.21-1092
53. Mishra AC, Mehta M, Mourya DT, Gandhi S. Crimean-Congo haemorrhagic fever in India. Lancet. (2011) 378:372. doi: 10.1016/S0140-6736(11)60680-6
54. Mourya DT, Yadav PD, Shete AM, Gurav YK, Raut CG, Jadi RS, et al. Detection, isolation and confirmation of Crimean-Congo hemorrhagic fever virus in human, ticks and animals in Ahmadabad, India, 2010–2011. PLoS Negl Trop Dis. (2012) 6:e1653. doi: 10.1371/journal.pntd.0001653
55. Sowmiya V. Crimean-Congo haemorrhagic fever–A ticking bomb: an overview of the current situation in India. J Entomol Zoology Stud. (2020) 8:1652–55.
56. Sahay RR, Shete AM, Yadav PD, Patil S, Majumdar T, Jain R, et al. Sequential determination of viral load, humoral responses and phylogenetic analysis in fatal and non-fatal cases of Crimean-Congo hemorrhagic fever patients from Gujarat, India, 2019. PLoS Negl Trop Dis. (2021) 15:e0009718. doi: 10.1371/journal.pntd.0009718
57. Basker P, Tiwari S, Shewale A, Nale T, Singh SK. Vector-Borne Zoonoses in India: Systematic gap analysis and Implications for policy-level interventions. CDES. (2022) 9:96–126. doi: 10.53422/JDMS.2022.91110
58. Mustafa ML, Ayazi E, Mohareb E, Yingst S, Zayed A, Rossi CA, et al. Crimean-congo hemorrhagic fever, Afghanistan, 2009. Emerg Infect Dis. (2011) 17:1940–41. doi: 10.3201/eid1710.110061
59. Qaderi S, Mardani M, Shah A, Shah J, Bazgir N, Sayad J, et al. Crimean-Congo hemorrhagic fever (CCHF) in Afghanistan: a retrospective single center study. Int J Infect Dis. (2021) 103:323–8. doi: 10.1016/j.ijid.2020.11.208
60. Jafari A, Asadolahi S, Rasekh M, Saadati D, Faghihi F, Fazlalipour M, et al. Distribution and biodiversity components of hard ticks as potential vectors of Crimean-Congo haemorrhagic fever virus (CCHFV) in borderline of Iran-Afghanistan. Int J Acarol. (2021) 47:510–9. doi: 10.1080/01647954.2021.1954085
61. Lani R, Rahim NM, Hassan H, Yaghoobi R, Chang L, AbuBakar S, et al. First report on the seroprevalence of the Crimean-Congo haemorrhagic fever virus, a tick-borne virus, in Malaysia's Orang Asli population. Eur Rev Med Pharmacol Sci. (2015) 19:461–6.
62. Saidi S, Casals J, Faghih M. Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man, and in domestic and small mammals, in Iran. Am J Trop Med. (1975) 24:353–7. doi: 10.4269/ajtmh.1975.24.353
63. Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. (1979) 15:307–417. doi: 10.1093/jmedent/15.4.307
64. Sureau P, Klein J. Arboviruses in Iran (author's transl). Medecine tropicale. Revue du Corps de sante colonial. (1980) 40:549–54.
65. Chinikar S, Ghiasi S, Hewson R, Moradi M, Haeri A. Crimean-Congo hemorrhagic fever in Iran and neighboring countries. J Clin Virol. (2010) 47:110–4. doi: 10.1016/j.jcv.2009.10.014
66. Chinikar S, Ghiasi S, Ghalyanchi-Langeroudi A, Goya M, Shirzadi M, Zeinali M, et al. An overview of Crimean-Congo hemorrhagic fever in Iran. Iran J Microbiol. (2009) 1:7–12. doi: 10.1016/j.ijid.2008.05.868
67. Khakifirouz S, Mowla SJ, Baniasadi V, Fazlalipour M, Jalali T, Mirghiasi SM, et al. No detection of Crimean–Congo Hemorrhagic Fever (CCHF) virus in ticks from Kerman Province of Iran. JoMMID. (2018) 6:108–11. doi: 10.29252/JoMMID.6.4.108
68. Salmanzadeh S, Aliakbarian Z, Mostafavi E, Salehi-Vaziri M, Moogahi S. A cross-sectional study of sero-prevalence and risk factors of brucellosis and haemorrhagic fever in slaughterhouse staff in Ahvaz City, Iran 2020. Med Stud. (2020) 37:7–15. doi: 10.5114/ms.2021.104995
69. Suleiman MNEH, Muscat-Baron J, Harries J, Satti AGO, Platt G, Bowen E, et al. Congo/Crimean haemorrhagic fever in Dubai: an outbreak at the Rashid Hospital. Lancet. (1980) 316:939–41. doi: 10.1016/S0140-6736(80)92103-0
70. Khan AS, Maupin GO, Rollin PE, Noor AM, Shurie H, Shalabi A, et al. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. Am J Trop Med. (1997) 57:519–25. doi: 10.4269/ajtmh.1997.57.519
71. Rodriguez LL, Maupin GO, Ksiazek TG, Rollin PE, Khan AS, Schwarz TF, et al. Molecular investigation of a multisource outbreak of Crimeancongo Hemorrhagic fever in the United Arab Emirates. Am J Trop Med. (1997) 57:512–8. doi: 10.4269/ajtmh.1997.57.512
72. Mohamed AL Dabal L, MR RS, Baderldin S, Abro A, Zaki A, Dessi Z, et al. Crimean-Congo Hemorrhagic Fever in Dubai, United Arab Emirates, 2010: case report. Iran Red Crescent Med J. (2016) 18:e38374. doi: 10.5812/ircmj.38374
73. Ince Y, Yasa C, Metin M, Sonmez M, Meram E, Benkli B, et al. Crimean-Congo hemorrhagic fever infections reported by ProMED. Int J Infect Dis. (2014) 26:44–6. doi: 10.1016/j.ijid.2014.04.005
74. Perveen N, Khan G. Crimean–Congo hemorrhagic fever in the Arab world: a systematic review. Front Vet Sci. (2022) 9:938601. doi: 10.3389/fvets.2022.938601
75. Perveen N, Muzaffar SB, Al-Deeb MA. Ticks and tick-borne diseases of livestock in the Middle East and North Africa: a review. Insects. (2021) 12:83. doi: 10.3390/insects12010083
76. Williams R, Al-Busaidy S, Mehta F, Maupin G, Wagoner K, Al-Awaidy S, et al. Crimean-Congo haemorrhagic fever: a seroepidemiological and tick survey in the Sultanate of Oman. Trop Med Int Health. (2000) 5:99–106. doi: 10.1046/j.1365-3156.2000.00524.x
77. Al-Zadjali M, Al-Hashim H, Al-Ghailani M, Balkhair A. A case of Crimean-Congo hemorrhagic fever in Oman. Oman Med J. (2013) 28:210–2. doi: 10.5001/omj.2013.57
78. Al-Abri SS, Al Abaidani I, Fazlalipour M, Mostafavi E, Leblebicioglu H, Pshenichnaya N, et al. Current status of Crimean-Congo haemorrhagic fever in the world health organization Eastern Mediterranean Region: issues, challenges, and future directions. Int J Infect Dis. (2017) 58:82–9. doi: 10.1016/j.ijid.2017.02.018
79. Al-Abri SS, Hewson R, Al-Kindi H, Al-Abaidani I, Al-Jardani A, Al-Maani A, et al. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in Oman. PLoS Negl Trop Dis. (2019) 13:e0007100. doi: 10.1371/journal.pntd.0007100
80. Alsaadi KS, Abd-Ellatif E, Alhashmi F, Almoqbali A, Vaidya V. Crimean-Congo hemorrhagic fever outbreak in the North Region of Oman in August 2019: case series study. Iproceedings. (2022) 8:e36495. doi: 10.2196/36495
81. Majeed B, Dicker R, Nawar A, Badri S, Noah A, Muslem H. Morbidity and mortality of Crimean-Congo hemorrhagic fever in Iraq: cases reported to the national surveillance system, 1990–2010. Trans R Soc Trop. (2012) 106:480–3. doi: 10.1016/j.trstmh.2012.04.006
82. Mardani M, Aghazadeh Sarhangipour K, Nikpour S, Hakamifard A. Crimean-Congo hemorrhagic fever in the COVID-19 pandemic: a case study. Clinical Case Reports. (2022) 10:e05518. doi: 10.1002/ccr3.5518
83. Jafar U, Usman M, Ehsan M, Naveed A, Ayyan M, Cheema HA. The outbreak of Crimean-Congo hemorrhagic fever in Iraq-Challenges and way forward. Ann Med Sur. (2022) 81:104382. doi: 10.1016/j.amsu.2022.104382
84. Al-Nakib W, Lloyd G, El-Mekki A, Platt G, Beeson A, Southee T. Preliminary report on arbovirus-antibody prevalence among patients in Kuwait: evidence of Congo/Crimean virus infection. Trans R Soc Trop. (1984) 78:474–6. doi: 10.1016/0035-9203(84)90065-8
85. Darwish M, Imam I, Omar F, Hoogstraal H. Results of a preliminary seroepidemiological survey for Crimean-Congo hemorrhagic fever virus in Egypt. Acta Virol. (1978) 22:77.
86. Morrill J, Soliman A, Imam I, Botros B, Moussa M, Watts D. Serological evidence of Crimean-Congo haemorrhagic fever viral infection among camels imported into Egypt. Trop Med Health. (1990) 93:201–4.
87. Mohamed M, Said AR, Murad A, Graham R. A serological survey of Crimean-Congo haemorrhagic fever in animals in the Sharkia Governorate of Egypt. Vet Ital. (2008) 44:513–7.
88. El-Bahnasawy M, Sabah AA, Saleh H, Morsy TA. The tick-borne Crimean-Congo hemorrhagic fever in Africa, Asia, Europe, and America: what about Egypt. J Egypt Soc Parasitol. (2012) 42:373–84. doi: 10.12816/0006324
89. Weidmann M, Avsic-Zupanc T, Bino S, Bouloy M, Burt F, Chinikar S, et al. Biosafety standards for working with Crimean-Congo hemorrhagic fever virus. J Gen Virol. (2016) 97:2799–808. doi: 10.1099/jgv.0.000610
90. El-Azazy O, Scrimgeour E. Crimean-Congo haemorrhagic fever virus infection in the western province of Saudi Arabia. Trans R Soc Trop. (1997) 91:275–8. doi: 10.1016/S0035-9203(97)90072-9
91. Memish ZA. Infection control in Saudi Arabia: meeting the challenge. Am J Infect Control. (2002) 30:57–65. doi: 10.1067/mic.2002.120905
92. Hassanein K, El-Azazy O, Yousef H. Detection of Crimean-Congo haemorrhagic fever virus antibodies in humans and imported livestock in Saudi Arabia. Trans R Soc Trop. (1997) 91:536–7. doi: 10.1016/S0035-9203(97)90014-6
93. Karti SS, Odabasi Z, Korten V, Yilmaz M, Sonmez M, Caylan R, et al. Crimean-Congo hemorrhagic fever in Turkey. Emerg Infect Dis. (2004) 10:1379–84. doi: 10.3201/eid1008.030928
94. Ozkaya E, Dincer E, Carhan A, Uyar Y, Ertek M, Whitehouse CA, et al. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus in Turkey: occurrence of local topotype. Virus Res. (2010) 149:64–70. doi: 10.1016/j.virusres.2009.12.014
95. Fisgin NT, Doganci L, Tanyel E, Tulek N. Initial high rate of misdiagnosis in Crimean Congo haemorrhagic fever patients in an endemic region of Turkey. Epidemiol Infect. (2010) 138:139–44. doi: 10.1017/S0950268809990318
96. Gunes T, Engin A, Poyraz O, Elaldi N, Kaya S, Dokmetas I, et al. Crimean-Congo hemorrhagic fever virus in high-risk population, Turkey. Emerg Infect Dis. (2009) 15:461–64. doi: 10.3201/eid1503.080687
97. Gunes T, Poyraz O, Vatansever Z. Crimean-Congo hemorrhagic fever virus in ticks collected from humans, livestock, and picnic sites in the hyperendemic region of Turkey. Vector Borne Zoonotic Dis. (2011) 11:1411–6. doi: 10.1089/vbz.2011.0651
98. Gozdas HT. Evaluation of Crimean-Congo hemorrhagic fever suspected cases admitted to a secondary care hospital in Kastamonu, Turkey between 2014–2017. Afr Health Sci. (2019) 19:1433–40. doi: 10.4314/ahs.v19i1.16
99. Mehmood Q, Tahir MJ, Jabbar A, Siddiqi AR, Ullah I. Crimean–Congo hemorrhagic fever outbreak in Turkey amid the coronavirus disease 2019 (COVID-19) pandemic) a debacle for the healthcare system of Turkey. Infect Control Hosp Epidemiol. (2022) 43:1726–7. doi: 10.1017/ice.2021.343
100. Dennis DT, Piesman JF. Overview of tick-borne infections of humans. Tick-borne. Dis Humans. (2005) 3:1–11. doi: 10.1128/9781555816490.ch1
101. Maltezou H, Andonova L, Andraghetti R, Bouloy M, Ergonul O, Jongejan F, et al. Crimean-Congo hemorrhagic fever in Europe: current situation calls for preparedness. Euro Surveill. (2010) 15:19504. doi: 10.2807/ese.15.10.19504-en
102. Estrada-Peña A, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir Res. (2014) 108:104–28. doi: 10.1016/j.antiviral.2014.05.016
103. Cleri DJ, Ricketti AJ, Porwancher RB, Ramos-Bonner LS, Vernaleo JR. Viral hemorrhagic fevers: current status of endemic disease and strategies for control. Infect Dis Clin. (2006) 20:359–93. doi: 10.1016/j.idc.2006.02.001
104. Turell MJ. Horizontal and vertical transmission of viruses by insect and tick vectors. The arboviruses: epidemiology and ecology. CRC Press. (2020) 3:127–52. doi: 10.1201/9780429280221-5
105. Bhowmick S, Kasi KK, Gethmann J, Fischer S, Conraths FJ, Sokolov IM, et al. Ticks on the run: a mathematical model of Crimean-Congo haemorrhagic fever (CCHF)—Key factors for transmission. Epidemiologia. (2022) 3:116–34. doi: 10.3390/epidemiologia3010010
106. Papa A, Mirazimi A, Köksal I, Estrada-Pena A, Feldmann H. Recent advances in research on Crimean-Congo hemorrhagic fever. J Clin Virol. (2015) 64:137–43. doi: 10.1016/j.jcv.2014.08.029
107. Kuehnert PA, Stefan CP, Badger CV, Ricks KM. Crimean-Congo hemorrhagic fever virus (CCHFV): a silent but widespread threat. Curr Trop Med Rep. (2021) 8:141–7. doi: 10.1007/s40475-021-00235-4
108. Fillâtre P, Revest M, Tattevin P. Crimean-Congo hemorrhagic fever: an update. Med Mal Infect. (2019) 49:574–85. doi: 10.1016/j.medmal.2019.09.005
109. Spengler JR, Estrada-Peña A, Garrison AR, Schmaljohn C, Spiropoulou CF, Bergeron É, et al. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antivir Res. (2016) 135:31–47. doi: 10.1016/j.antiviral.2016.09.013
110. Palomar AM, Portillo A, Santibáñez P, Mazuelas D, Arizaga J, Crespo A, et al. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg Infect Dis. (2013) 19:260–63. doi: 10.3201/eid1902.121193
111. Gingrich JB, Harlan HJ, Perkins PV, Trosper JH. Regional Disease Vector Ecology Profile East Asia. Washington Dc: Armed Forces Pest Management Board (2002).
112. Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antivir Res. (2017) 144:93–119. doi: 10.1016/j.antiviral.2017.05.010
113. Dreshaj S, Ahmeti S, Ramadani N, Dreshaj G, Humolli I, Dedushaj I. Current situation of Crimean-Congo hemorrhagic fever in Southeastern Europe and neighboring countries: a public health risk for the European Union? Travel Med Infect Dis. (2016) 14:81–91. doi: 10.1016/j.tmaid.2016.03.012
114. Bakir M, Ugurlu M, Dokuzoguz B, Bodur H, Tasyaran MA, Vahaboglu H, et al. Crimean-Congo haemorrhagic fever outbreak in Middle Anatolia: a multicentre study of clinical features and outcome measures. J Med Microbiol. (2005) 54:385–9. doi: 10.1099/jmm.0.45865-0
115. Leblebicioglu H, Ozaras R, Irmak H, Sencan I. Crimean-Congo hemorrhagic fever in Turkey: current status and future challenges. Antivir Res. (2016) 126:21–34. doi: 10.1016/j.antiviral.2015.12.003
116. Estrada-Peña A, Palomar AM, Santibáñez P, Sánchez N, Habela MA, Portillo A, et al. Crimean-Congo hemorrhagic fever virus in ticks, Southwestern Europe, 2010. Emerg Infect Dis. (2012) 18:179. doi: 10.3201/eid1801.111040
117. Orhan S, Gülsoy KY. Epidemiological, clinical, and laboratory characteristics of patients with Crimean-Congo hemorrhagic fever. Türkiye Klinikleri Tip Bilimleri Dergisi. (2021) 41:138–44. doi: 10.5336/medsci.2020-77964
118. Lwande OW, Irura Z, Tigoi C, Chepkorir E, Orindi B, Musila L, et al. Seroprevalence of Crimean–Congo Hemorrhagic Fever virus in Ijara district, Kenya. Vector Borne Zoonotic Dis. (2012) 12:727–32. doi: 10.1089/vbz.2011.0914
119. Pshenichnaya NY, Sydenko IS, Klinovaya EP, Romanova EB, Zhuravlev AS. Possible sexual transmission of Crimean-Congo hemorrhagic fever. Int J Infect Dis. (2016) 45:109–11. doi: 10.1016/j.ijid.2016.02.1008
120. Ergonul O, Celikbas A, Yildirim U, Zenciroglu A, Erdogan D, Ziraman I, et al. Pregnancy and Crimean-Congo haemorrhagic fever. Clin Microbiol Infect. (2010) 16:647–50. doi: 10.1111/j.1469-0691.2009.02905.x
121. Aydemir O, Erdeve O, Oguz SS, Dilmen U, A. healthy newborn born to a mother with Crimean-Congo hemorrhagic fever: is there protection from transplacental transmission? Int J Infect Dis. (2010) 14:e450. doi: 10.1016/j.ijid.2009.07.001
122. Ergonul O. Evidence supports ribavirin use in Crimean-Congo hemorrhagic fever. Int J Infect Dis. (2014) 29:296. doi: 10.1016/j.ijid.2014.08.016
123. Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. (2004) 10:S110–S21. doi: 10.1038/nm1142
124. Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nat Rev Immunol. (2003) 3:677–85. doi: 10.1038/nri1154
125. Schnittler HJ, Feldmann H. Viral hemorrhagic fever—A vascular disease? Thromb Haemost. (2003) 89:967–72. doi: 10.1055/s-0037-1613397
126. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. (2000) 6:601. doi: 10.3201/eid0606.000608
127. Ergonul O, Tuncbilek S, Baykam N, Celikbas A, Dokuzoguz B. Evaluation of serum levels of interleukin (IL)−6, IL-10, and tumor necrosis factor–α in patients with Crimean-Congo hemorrhagic fever. J Infect Dis. (2006) 193:941–4. doi: 10.1086/500836
128. Xiao X, Feng Y, Zhu Z, Dimitrov DS. Identification of a putative Crimean-Congo hemorrhagic fever virus entry factor. Biochem Biophys Res Commun. (2011) 411:253–8. doi: 10.1016/j.bbrc.2011.06.109
129. Simon M, Johansson C, Lundkvist Å, Mirazimi A. Microtubule-dependent and microtubule-independent steps in Crimean-Congo hemorrhagic fever virus replication cycle. Virol. (2009) 385:313–22. doi: 10.1016/j.virol.2008.11.020
130. Schmaljohn C, Nichol ST. Bunyaviridae, Fields Virology. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, RB, and SSE (Eds). Philadelphia, PA: Lippincott Williams & Wilkins (2007) 2:1741–89.
131. Simon M, Johansson C, Mirazimi A. Crimean-Congo hemorrhagic fever virus entry and replication is clathrin-, pH-and cholesterol-dependent. J Gen Virol. (2009) 90:210–5. doi: 10.1099/vir.0.006387-0
132. Shi X, van Mierlo JT, French A, Elliott RM. Visualizing the replication cycle of bunyamwera orthobunyavirus expressing fluorescent protein-tagged Gc glycoprotein. Virol J. (2010) 84:8460–9. doi: 10.1128/JVI.00902-10
133. Ergonul O. Clinical and pathologic features of Crimean-Congo hemorrhagic fever. Crimean-Congo hemorrhagic fever. Springer. (2007) 3:207–20. doi: 10.1007/978-1-4020-6106-6_16
134. Goldfarb L, Chumakov M, Myskin A, Kondratenko V, Reznikova OY. An epidemiological model of Crimean hemorrhagic fever. Am J Trop Med. (1980) 29:260–4. doi: 10.4269/ajtmh.1980.29.260
135. Tanir G, Tuygun N, Balaban I, Doksöz O. A case of Crimean-Congo hemorrhagic fever with pleural effusion. Jpn J Infect Dis. (2009) 62:70–2.
136. Naderi H, Sheybani F, Bojdi A, Khosravi N, Mostafavi I. Fatal nosocomial spread of Crimean-Congo hemorrhagic fever with very short incubation period. Am J Trop Med. (2013) 88:469. doi: 10.4269/ajtmh.2012.12-0337
137. Kaya A, Engin A, Güven AS, Içagasioglu FD, Cevit Ö, Elaldi N, et al. Crimean-Congo hemorrhagic fever disease due to tick bite with very long incubation periods. Int J Infect Dis. (2011) 15:e449–e52. doi: 10.1016/j.ijid.2011.03.007
138. Swanepoel R, Shepherd A, Leman P, Shepherd S, McGillivray G, Erasmus M, et al. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am J Trop Med. (1987) 36:120–32. doi: 10.4269/ajtmh.1987.36.120
139. Sahin I, Kaya A, Cevit O, Arslan M, A. child with an unusual complication of Crimean-Congo hemorrhagic fever: hemorrhagic pleural effusion. J Vector Borne Dis. (2016) 53:87.
140. Ahmeti S, Ajazaj-Berisha L, Halili B, Shala A. Acute arthritis in Crimean-Congo hemorrhagic fever. J Glob Infect Dis. (2014) 6:79–81. doi: 10.4103/0974-777X.132052
141. Whitehouse CA. Crimean–Congo hemorrhagic fever. Antivir Res. (2004) 64:145–60. doi: 10.1016/S0166-3542(04)00163-9
142. Saleem M, Shah SZ, Haidari A, Idrees F. Prevalence of Crimean-Congo hemorrhagic fever in Pakistan and its new research progress. J Coast Life Med. (2016) 4:259–62. doi: 10.12980/jclm.4.2016J5-239
143. Papa A, Weber F, Hewson R, Weidmann M, Koksal I, Korukluoglu G, et al. Meeting report: first international conference on Crimean-Congo hemorrhagic fever. Antivir Res. (2015) 120:57–65. doi: 10.1016/j.antiviral.2015.05.005
144. Pshenichnaya NY, Leblebicioglu H, Bozkurt I, Sannikova IV, Abuova GN, Zhuravlev AS, et al. Crimean-Congo hemorrhagic fever in pregnancy: a systematic review and case series from Russia, Kazakhstan, and Turkey. Int J Infect Dis. (2017) 58:58–64. doi: 10.1016/j.ijid.2017.02.019
145. Garrison AR, Smith DR, Golden JW. Animal models for Crimean-Congo hemorrhagic fever human disease. Viruses. (2019) 11:590. doi: 10.3390/v11070590
146. Yilmaz G, Koksal I, Topbas M, Yilmaz H, Aksoy F. The effectiveness of routine laboratory findings in determining disease severity in patients with Crimean-Congo hemorrhagic fever: severity prediction criteria. J Clin Virol. (2010) 47:361–5. doi: 10.1016/j.jcv.2010.01.010
147. Dogan OT, Engin A, Salk I, Epozturk K, Eren SH, Elaldi N, et al. Evaluation of respiratory findings in Crimean-Congo hemorrhagic fever. Southeast Asian J Trop Med Public Health. (2011) 42:1100.
148. Çelikbaş A, Ergönül Ö, Dokuzoguz B, Eren S, Baykam N, Polat-Düzgün A. Crimean–Congo Hemorrhagic Fever infection simulating acute appendicitis. J Infect. (2005) 50:363–5. doi: 10.1016/j.jinf.2004.05.020
149. Coetzee MJ, Blumberg LH, Paweska JT, Leman P, Swanepoel R, De Kock A. Crimean-Congo haemorrhagic fever presenting with undiagnosed chronic myeloid leukaemia. S Afr J Infect Dis. (2017) 32:142–4. doi: 10.4102/sajid.v32i4.41
150. Mendoza E, Warner B, Safronetz D, Ranadheera C. Crimean–Congo haemorrhagic fever virus: Past, present and future insights for animal modelling and medical countermeasures. Zoonoses Public Health. (2018) 65:465–80. doi: 10.1111/zph.12469
151. van Eeden P, Joubert JR, van de Wal, BW, King JB, de Kock A, Groenewald J. A nosocomial outbreak of Crimean-Congo haemorrhagic fever at Tygerberg Hospital-Part I. Clinical features. S Afr Med J. (1985) 68:711–7.
152. Al-Rubaye D, Al-Rubaye TS, Shaker M, Naif HM. Recent outbreaks of Crimean–Congo Hemorrhagic Fever (CCHF) In Iraq. Sci Arch. (2022) 3:109–112. doi: 10.47587/SA.2022.3205
153. Erol S, Özkurt Z, Özden K, Parlak M, Erol MK. Transient bradycardia in patients with Crimean-Congo hemorrhagic fever. Turk J of Med Sci. (2012) 42:753–6. doi: 10.3906/sag-1106-38
154. Ergonul O, Celikbas A, Baykam N, Eren S, Esener H, Dokuzoguz B. Analysis of the mortality among the patients with Crimean–Congo Hemorrhagic Fever virus infection. Clin Microbiol Infect. (2006) 12:55154. doi: 10.1111/j.1469-0691.2006.01445.x
155. Sunbul M, Leblebicioglu H, Fletcher TE, Elaldi N, Ozkurt Z, Bastug A, et al. Crimean–Congo haemorrhagic fever and secondary bacteraemia in Turkey. J Infect. (2015) 71:597–9. doi: 10.1016/j.jinf.2015.07.003
156. Sharifi-Mood B, Metanat M, Rakhshani F, Shakeri A. Co-infection of malaria and Crimean-Congo hemorrhagic fever. Iran J Parasito. (2011) 6:113–5.
Keywords: CCHF, prevalence, geographical distribution, pathophysiology, risk factors, zoonosis, ribavirin, prevention
Citation: Aslam M, Abbas RZ and Alsayeqh A (2023) Distribution pattern of Crimean–Congo Hemorrhagic Fever in Asia and the Middle East. Front. Public Health 11:1093817. doi: 10.3389/fpubh.2023.1093817
Received: 09 November 2022; Accepted: 02 January 2023;
Published: 26 January 2023.
Edited by:
Sandul Yasobant, Indian Institute of Public Health Gandhinagar (IIPHG), IndiaReviewed by:
Wen-Ping Guo, Chengde Medical University, ChinaWaqas Ahmad, University of Veterinary and Animal Sciences, Pakistan
Md. Masudur Rahman, Sylhet Agricultural University, Bangladesh
Copyright © 2023 Aslam, Abbas and Alsayeqh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah Alsayeqh,  YS5hbHNheWVxaEBxdS5lZHUuc2E=
YS5hbHNheWVxaEBxdS5lZHUuc2E=
 Munazza Aslam
Munazza Aslam Rao Zahid Abbas
Rao Zahid Abbas Abdullah Alsayeqh
Abdullah Alsayeqh