- School of Medicine, Federal University of Uberlândia, Uberlândia, Minas Gerais, Brazil
Objective: The aim of this study was to identify patterns related to health and their association with chronic kidney disease (CKD) in the Brazilian population.
Methods: We used data from the National Health Survey (PNS), 2019. Participants were interviewed and answered questions related to socioeconomic and demographic information (gender, age, education, race/color), health conditions (presence of hypertension, diabetes mellitus, hyperlipidemia, cardiovascular disease, overweight and CKD) and lifestyle (smoking, alcohol consumption, physical activity and food consumption). To identify patterns, we used exploratory factor analysis. We performed logistic regression models to describe the association of CKD with each pattern in crude models and adjusted for gender, age group, education level and race/color.
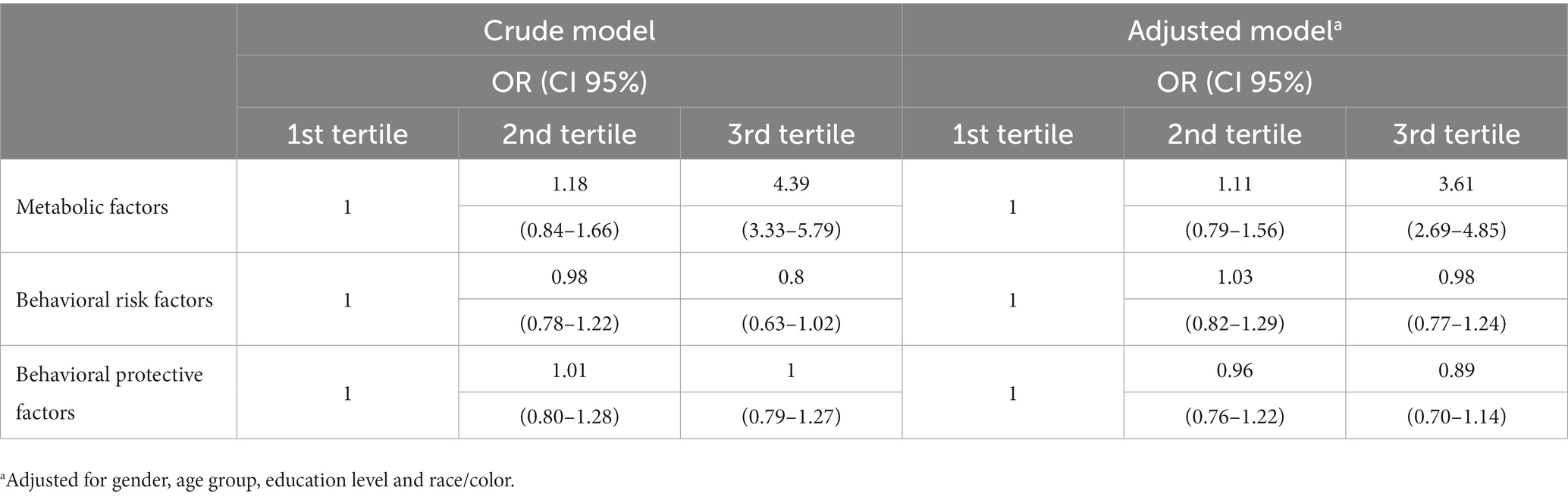
Results: A total of 90,846 individuals were evaluated. The prevalence of CKD was 1.49% (95% CI: 1.3–1.6). Three health-related patterns – metabolic factors, behavioral risk factors and behavioral protective factors – were identified by factor analysis. Metabolic factors were determined by the presence of hypertension, diabetes mellitus, hyperlipidemia and cardiovascular diseases. Behavioral risk factors were determined by smoking, alcohol consumption, regular consumption of soft drinks, sweets and artificial juices, and high salt consumption. The protective behavioral factors were established by the practice of physical activity and regular consumption of vegetables and fruits. Participants of the highest tertile for metabolic factors were more likely to have CKD in the adjusted model (OR = 3.61, 95% CI: 2.69–4.85), when compared to those of the lower tertile.
Conclusion: The pattern referring to metabolic factors was associated with a higher chance of presenting CKD.
1. Introduction
Chronic kidney disease (CKD) is a significant global health problem. According to the Global Burden of Disease Study, in 2017, 697.5 million cases of all-stage CKD were recorded, for a global prevalence of 9.1% (8.5–9.8) (1). In a systematic review and meta-analysis of observational studies that estimated the prevalence of CKD in general populations, the global prevalence was even higher, 11–13% (2). In 2017, the CKD ranked 12th in the ranking of the cause of mortality with 1.2 million deaths (1) and is expected to jump to 5th place in the ranking of the main causes of early death (3). In Brazil, it is estimated that 3–6 million people have CKD and this disease has as aggravating the fact that it is unknown by many people affected (4, 5).
The rate of CKD may increase in future, not only due to the growth and aging of the population, but mainly due to the increasing prevalence of arterial hypertension (AH), diabetes mellitus (DM), obesity and dyslipidemia (4, 6, 7), which are traditional risk factors for CKD (2, 8–10). Other lifestyle factors, such as smoking, sedentary lifestyle, alcohol abuse, excessive salt consumption and inadequate eating habits have been recognized as important predictors of CKD (9).
In addition to being prevalent, health risk factors are highly interrelated (11). Considering that the simultaneous occurrence of risk factors increases the chance of developing negative health conditions, studies have been concerned with determining how much factors and behaviors are aggregated in individuals. Evidence suggests that the concurrency of different risk behaviors may present a synergistic effect, thus resulting in a multiplicative deleterious effect, rather than an additive effect of each behavior (11, 12).
In this sense, some studies investigated multimorbidity patterns including CKD (13–15), other studies have identified dietary patterns related to CKD (16–19), but none of them included behavioral factors such as smoking, alcohol consumption and physical inactivity, which are also known to be more sensitive to interventions than clinical outcomes. In Brazil, some studies have researched the concurrency of risk behaviors for chronic non-communicable diseases (NCDs) in adults (20–22), however, only three studies identified patterns of risk behavior in a Brazilian national sample (12, 23, 24) and none of them associated these standards with CKD.
Besides, most of these studies performed co-occurrence analyses focusing on competing but independent behaviors. Analyses investigating underlying associations between competing behaviors, with clustering identified by divergences in observed and expected prevalence of combinations or by identifying latent or unobservable patterns (25), may be more relevant from the point of view of monitoring and planning of more effective multifactorial interventions (11), which should take into account substitute and complementary relationships between grouped health behaviors.
Thus, the aim of the study was to identify patterns related to health and their association with CKD in the Brazilian population.
2. Methods
2.1. Study population and sampling
The present study used data from the National Health Survey (PNS), a Brazilian household survey conducted in 2019 by the Brazilian Institute of Geography and Statistics (IBGE) in partnership with the Health Surveillance Secretariat of the Ministry of Health and the Oswaldo Cruz Foundation.
The sampling plan was defined by conglomerate in three stages of selection. In the first stage, the primary sampling units were stratified, consisting of sectors or groups of census tracts. The second stage consisted of households, and residents 15 years of age or older corresponded to the units of the third stage. The selection of a subsample of the primary sampling units was made by simple random sampling (26).
The estimated sample size was based on 108,525 households, and 94,114 individuals aged 15 years or older were interviewed. More details about the research are available in the publication of STOPA et al. (26)
2.2. Data collection and studied variables
Participants were interviewed and answered questions related to socioeconomic, demographic information, health conditions and lifestyle. The socioeconomic and demographic variables evaluated were: gender (male and female); age categorized into groups (15–29; 30–44; 45–59; 60 or more); education (without incomplete education/elementary school; complete elementary school/incomplete high school; complete high school/incomplete higher education; complete higher education) and race/color [white; black; brown and others (yellow and indigenous)].
Information on AH, DM, CKD, hypercholesterolemia and cardiovascular disease was based on self-reported previous medical diagnosis. In the identification of overweight, body mass index (BMI) was calculated according to self-reported weight and height. The cutoff point for overweight was BMI ≥ 25.0 kg/m2 for adults (27) and BMI ≥ 27.0 kg/m2 for the elderly (28).
Among the risk and protection factors related to lifestyle, we analyzed smoking (non-smoker, former smoker and smoker), alcohol consumption (heavy episodic drinking), physical activity and food consumption. Alcohol consumption, considering heavy episodic drinking, was defined as the intake of 60 g or more of alcohol (five or more doses of alcohol) on at least one occasion in the last 30 days (29). Two questions were used: “How often do you usually consume an alcoholic beverage? (I never drink, less than once a month and once or more a month)” and “In the last 30 days, did you consume five or more doses of alcoholic beverages on a single occasion? (yes and no).” A dose of drink was defined as the equivalent of a dose of cachaça, a glass of wine, a can of beer, a dose of whiskey or any other distilled alcoholic beverage. Heavy episodic drinking was considered when individuals answered “once or more a month” in the first question and “yes” in the last question. For physical activity, individuals who practiced at least 150 min of physical activity per week were considered active, considering four domains: leisure, work, commuting and domestic activities (30). Regarding food consumption, the frequency of weekly consumption of soft drinks, artificial juices, sweets (such as biscuit/stuffed cookie, chocolate, gelatin, candies and others), fruits or vegetables (such as lettuce, tomato, cabbage, carrot, chayote, eggplant, zucchini, excluding potatoes, cassava or yams) and beans were analyzed. Food consumption was considered regular when it occurred five or more times a week (6).
The perception of salt consumption was obtained considering the following question: “Considering freshly prepared food and processed foods, you think your salt intake is”: very high, high, adequate, low and very low. Salt intake was categorized as adequate (adequate, low and very low) and high (high and very high).
2.3. Statistical analysis
For descriptive analysis, the following variables were considered, according to the presence of CKD: gender, age group, education level, race/color, AH, DM, hypercholesterolemia, cardiovascular disease, overweight, physical activity, smoking, alcohol consumption, regular consumption of beans, regular consumption of vegetables, regular consumption of fruits, regular consumption of soft drinks, regular consumption of artificial juices, regular consumption of sweets and high salt consumption. The chi-square test was used to verify whether there is a statistically significant difference between individuals with and without CKD.
To identify patterns, we used exploratory factor analysis to reduce the initial number of variables in a smaller set of factors that represent, in a synthetic way, the information contained in the larger set of variables (31).
The adequacy of the data for factor analysis was initially evaluated with the Kaiser-Meyer-Olkin (KMO) sample adequacy measure. KMO assumes values between 0 and 1, and the lower values indicate that the variables have very little in common. A value of KMO = 0.62 was obtained, which means good adequacy (32).
The principal component analysis was used to extract the factors and the oblique promax rotation was performed to facilitate the interpretation of the factors. The determination of the number of factors to be retained considered the following criteria: the evaluation of the scree plot, the factor structure with loads of items above 0.30 (33), the lowest number of cross-loads of possible items, no factor with less than three items and a reasonable interpretation of the emerging factors.
Then, we identified three health-related patterns, named metabolic, risk behavior and protective behavior. Each individual had values assigned to each of the patterns, through regression models, according to their higher or lower adhering to the pattern. The values of each pattern were categorized into tertiles (the lowest category and the highest category represent the lowest and highest adhesion to a specific pattern, respectively). The first tertile of each pattern was used as a reference group.
Subsequently, we performed logistic regression models to describe the association of CKD with each pattern in crude models and adjusted for gender, age group, education level and race/color.
The analyses were performed in Stata software version 14.2, considering significance level of 5% and the effects of complex PNS sampling.
2.4. Ethical aspects
The PNS project was approved by the National Research Ethics Commission (CONEP) of the National Health Council (CNS) (opinion n. 3529376). The invited individuals who agreed to participate in the research signed the Free and Informed Consent Form.
3. Results
A total of 90,846 individuals were evaluated. The prevalence of CKD was 1.49% (CI 95%: 1.3–1.6). Regarding the characterization of the population studied, it is noteworthy that the majority of the study participants were women, aged between 30 and 44 years, brown and had completed high school education. A higher prevalence of CKD was found in older individuals (p < 0.001), with lower education level (p < 0.001) and white (p = 0.015; Table 1).
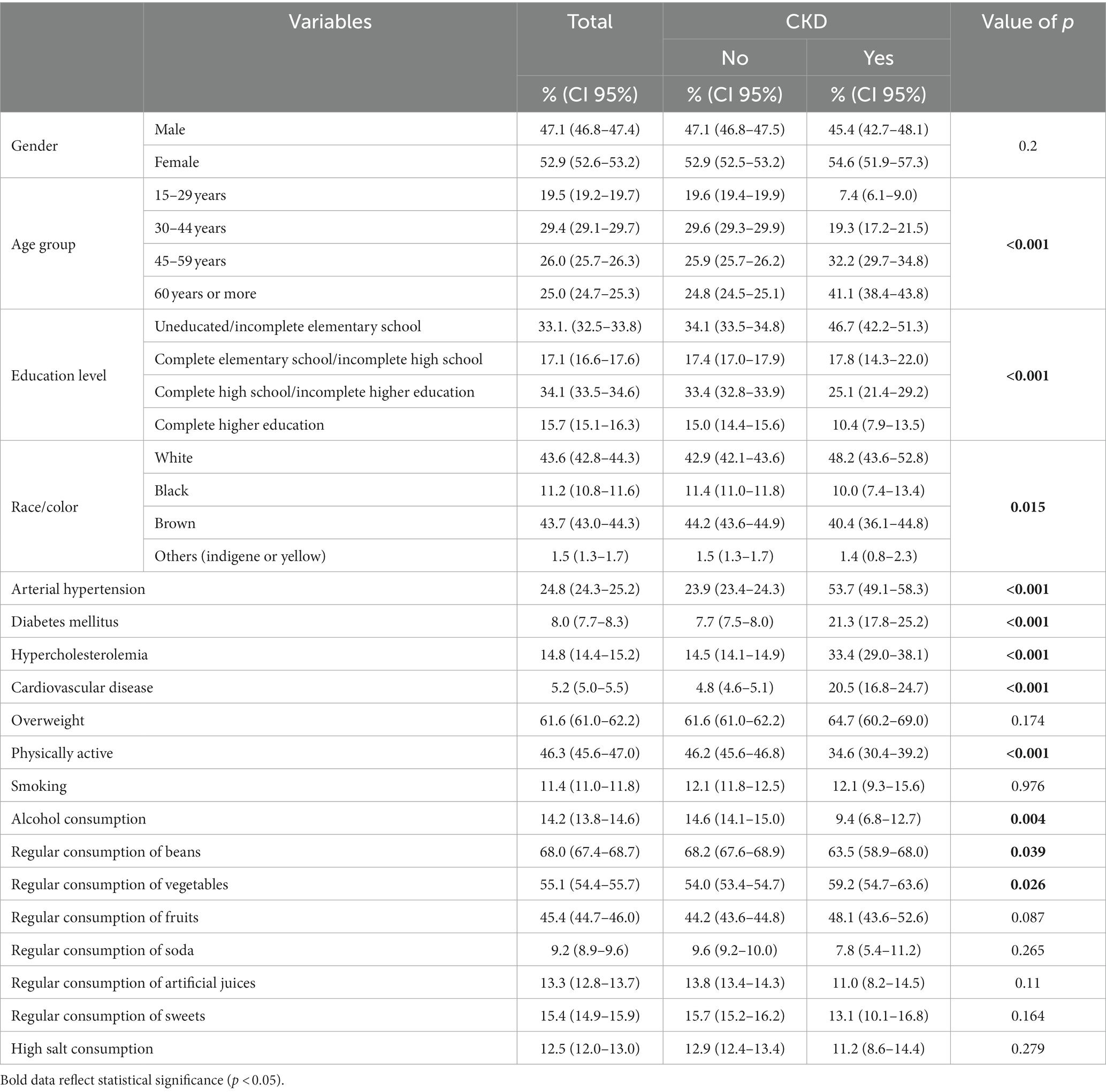
Table 1. Sociodemographic characteristics and prevalence of metabolic and behavioral factors, according to the presence of CKD. PNS, 2019.
Among individuals with CKD, it was possible to observe a higher prevalence of AH (53.7%), DM (21.3%), hypercholesterolemia (33.4%), cardiovascular disease (20.5%) and lower physical activity (34.6%; Table 1).
Alcohol consumption and regular consumption of beans were more prevalent in the group of participants without CKD, while regular consumption of vegetables was higher in the group of people with CKD (Table 1).
Three health-related patterns – metabolic factors, behavioral risk factors and behavioral protective factors – were identified by factor analysis. These patterns explained 11.25, 9.34 and 9.33% of variance, respectively (Table 2). Together, they explained ~30% of the variance.
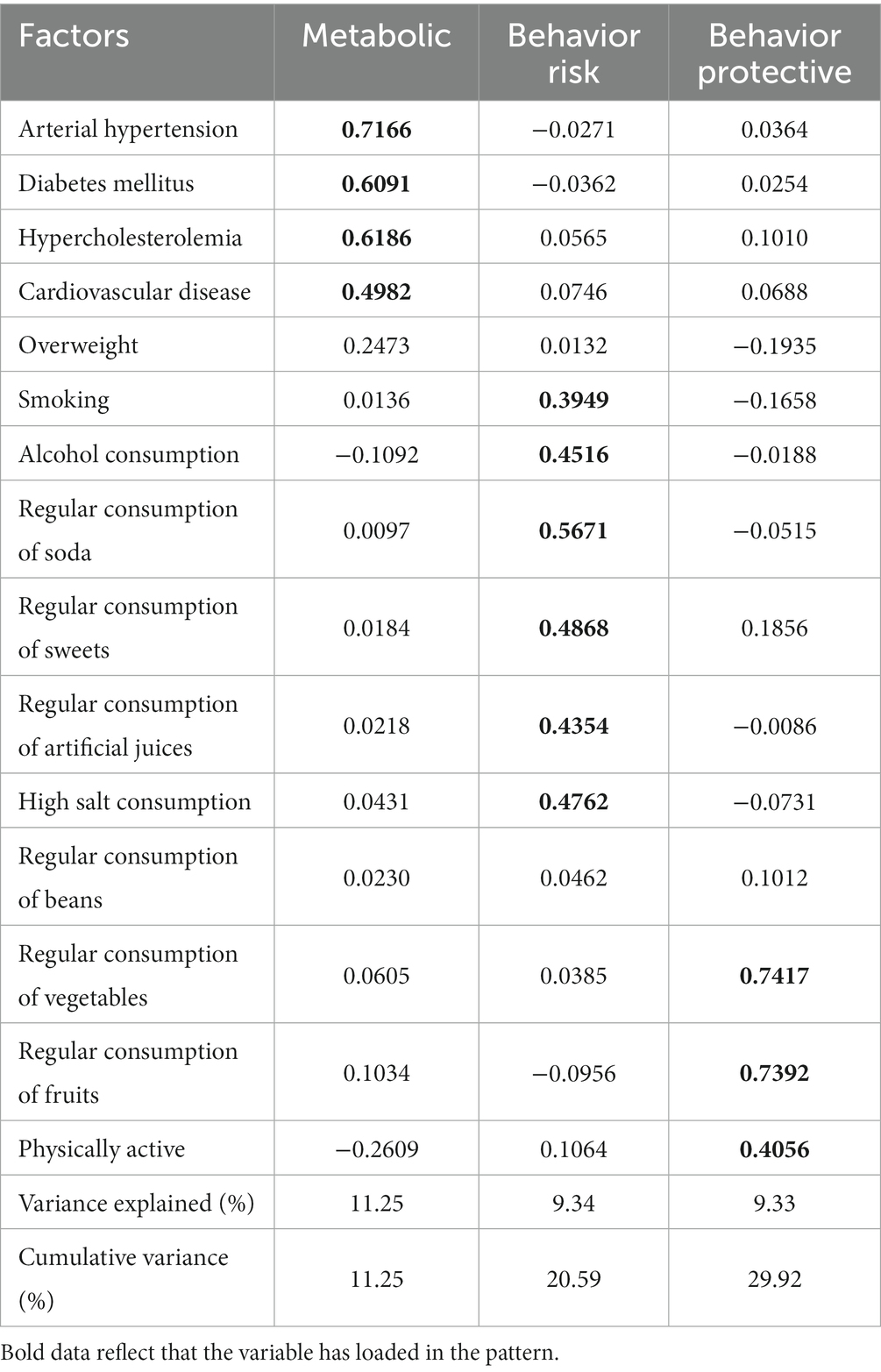
Table 2. Factor structure of metabolic, behavior risk and behavior protective in the Brazilian population. PNS, 2019.
Regarding the characterization of these patterns, metabolic factors were determined by the presence of AH, DM, hyperlipidemia and cardiovascular diseases. Behavioral risk factors were determined by smoking, alcohol consumption, regular consumption of soda, regular consumption of sweets, regular consumption of artificial juices and high salt consumption. The behavioral factors of protection were established by the practice of physical activity, regular consumption of vegetables and regular consumption of fruits. The highest factorial loads were found in the metabolic pattern for AH, DM and hypercholesterolemia and in the behavior protective pattern for regular consumption of vegetables and fruits (Table 2). Higher factor loadings indicate greater adherence to the respective pattern.
The association of the patterns identified with CKD, stratified by tertiles, were presented in Table 3. Participants of the highest tertile for metabolic factors were more likely to have CKD in the crude model (OR = 4.39, 95% CI 3.33–5.79) and adjusted (OR = 3.61, 95% CI 2.69–4.85), when compared to those of the lower tertile. No significant association was observed between behavioral risk and protection factors and the presence of CKD.
4. Discussion
The findings of the present study showed a prevalence of CKD of 1.49%. In the factor analysis, three health-related patterns were identified, labeled as: metabolic factors, behavioral risk factors and behavioral protective factors. Only metabolic factors were associated with the chance of presenting CKD.
The prevalence of CKD in the Brazilian population is still uncertain (5). According to PNS data, the prevalence of self-reported CKD was 1.4% (34), as described in the present study. In a subsample of the PNS, in which laboratory tests were performed, the prevalence of CKD was 6.48% (35). The prevalence of CKD among participants of the Longitudinal Study of Adult Health (ELSA), in six research institutions in Brazilian capitals, was 8.9% (36). The difference in self-reported prevalence and that assessed by laboratory tests shows the high percentage of unknown cases of the disease. The hidden cases of the disease can be explained by the lack of screening for the disease and by the insidious and asymptomatic loss of renal function, being a major public health problem.
About the health-related patterns, Corsonello et al. (13) reported that CKD is associated with multimorbidity and was rarely observed without any concomitant disease, and AH and DM were among the co-ocurrent pairs of greater significance involving CKD. In addition, it is known that these factors are the main causes of CKD worldwide (37). In agreement, this study showed the importance of metabolic factors, which include AH and DM, in the development of CKD.
AH may be the cause or consequence of CKD. In hypertensive patients, chronically increased systemic arterial pressures cause remodeling of the aferent arteriola and reduce its capacity for contraction and dilation. Over time, increased blood pressure and pressure transmitted to the kidney lead to nephrosclerosis and progressive loss of renal function (38).
With regard to DM, chronic hyperglycemia leads to metabolic dysregulation due to increased glycolysis, which regulates several distinct pathways and leads to glomerular hyperfiltration and proteinuria. In addition, hyperglycemia causes hemodynamic changes in the kidney, oxidative stress, inflammation, hypoxia and deregulation of the Renin-Angiotensin-Aldosterone system (RAAS), which causes adverse changes in the kidney vessels, such as thickening of the glomerular basement membrane (39–42).
In the study by Liu et al. (43), the relationship between cardiovascular diseases and CKD was demonstrated, evidencing a deep association between them, and the disease of one organ causes dysfunction in the other. Thus, it is assumed that two main mechanisms can explain this association. The kidney can release hormones (44, 45), enzymes and cytokines (44, 46) in response to kidney injury, which leads to changes in blood vessels. In addition, CKD mediators and hemodynamic changes contribute to heart damage (44, 47).
Likewise, in the study by Chang et al. (48) it has been reported that older adults with hyperlipidemia and cardiovascular diseases were at higher risk of developing CKD. This association is due to the fact that patients with CKD tend to present physiological and biochemical alterations that lead to imbalance in lipid profile. In addition, triglyceride levels are increased by 30–50%, which is related to the reduction of hepatic lipase and lipoprotein lipase activity. There is a decrease in HDL-cholesterol, increased lipoprotein A and the accumulation of LDL-cholesterol. Thus, there is a prevalence of oxidized LDL molecules, which are captured by immune system cells, with consequent contribution in the formation of atherosclerotic plaque. Another mechanism related to dyslipidemia is its ability to damage mesangial and endothelial cells, which facilitates the progression of kidney injury. The mechanisms involving dyslipidemia and CKD are not yet fully understood, but it is possible to highlight some more relevant factors such as insulin resistance and increased oxidative stress (49, 50).
The findings of this study allowed the identification of a higher chance of having CKD for those individuals in the highest tertile of the pattern called metabolic factors. Therefore, the importance of evaluating these factors simultaneously is highlighted, because the individual hardly has only one isolated factor and this demonstrates the role of multimorbidity in this disease (51, 52).
In relation to the other patterns analyzed, it is noteworthy that this study did not observe a significant association of behavioral risk factors and behavioral factors of protection with CKD, similar to the results presented by Foster et al. (53), who found no association between CKD and physical activity, smoking and alcohol consumption. In the Brazilian context, study with a representative sample of the population, in univariate analysis, found an association between CKD and some behavioral factors, such as smoking, excessive alcohol consumption and physical activity, but also found no association between CKD and regular intake of fruits and vegetables, consumption of red meat with fat and excessive consumption of salt. In an adjusted analysis, considering behavioral factors, only smoking remained associated with CKD (35).
However, other studies have shown that behavioral factors may influence the presence and development of CKD (13, 16, 18, 24, 48, 54). Regarding the association of dietary patterns with CKD, studies conducted with the populations of Iran (16), United Kingdom (55) and China (19, 56) found that dietary patterns high in fat and sugar were associated with higher chances of incidence of CKD, while plant-rich dietary patterns were associated with reduced risk of CKD. However, due to the diversity of eating habits in the world, the results found in these studies cannot be extrapolated to other populations (17). In addition, the absence of association of behavioral factors with CKD in the present study may be due to cross-sectional design and reverse causality, as lifestyle risk and protection behaviors may be altered due to the diagnosis and guidance given by health professionals to delay the progression of CKD.
Although patterns related to risk and protective factors have not been associated with CKD, it can be inferred as a practical application the encouragement of protective behaviors and the reduction of risk behaviors. Thus, investing in NCD prevention actions can be a good strategy to prevent the development and progression of CKD. In this sense, another practical application is the strengthening and implementation public policies aimed at CKD with the objective of encouraging the promotion of healthy habits, which makes it possible to prevent the occurrence of NCDs, such as AH and DM, and, consequently, CKD.
In Brazil, the implementation of public policies for the prevention and management of kidney diseases is recent and incipient (57). In 2004, the National Policy for Attention to Patients with Kidney Disease was instituted by Ordinance n° 1.168/2004. In 2006, the Ministry of Health published guidelines for the Clinical Prevention of Cardiovascular, Cerebrovascular and Chronic Kidney Disease, which recommended early screening in primary care in risk groups, such as AH, DM and family history of CKD. In 2014, the Ministry of Health published Ordinance n° 389/2014, which defined the criteria for the organization of the line of care for people with CKD and published the Clinical Guidelines for the Care of Patients with Chronic Kidney Disease in the Sistema Único de Saúde (SUS). In addition, reinforcing that the main action in the prevention of CKD cases is the reduction and treatment of the main risk factors for the development of kidney disease, in 2011, the Federal Government prepared the Strategic Action Plan to Combat Chronic Noncommunicable Diseases in Brazil 2011–2022, which was recently updated, considering the period 2021–2030 (57).
Despite all the efforts made toward the reduction of chronic conditions, it is observed that challenges still need to be overcome to ensure improved care for people with CKD. A recent study shows flaws in the screening of people at risk for CKD in primary health care in Brazil (58) and worldwide (59). It also highlights the need for actions to improve the control of the most prevalent conditions in the adult population, that is, AH, DM and dyslipidemia (58, 59). Therefore, it is necessary to implement CKD control and prevention strategies, which consist of the quality and effectiveness of existing programs in primary care, as well as the degree of motivation, training and continuing education of health professionals (57).
This study has strengths and limitations. Regarding the strengths, the sample size of the study stands out, being representative of the Brazilian population. In addition, different factors were analyzed: metabolic, risk and protective behavior. Moreover, this study used factor analysis, a statistical analysis technique that allows grouping the variables, according to the correlations between them, that is, with this analysis the pattern of the variables and the adhering to this pattern by the individuals is better represented, surpassing the analysis of the occurrence of isolated factors or the co-occurrence of factors.
As for limitations, it is found that the design of the cross-sectional study does not allow establishing a causal relationship. Consequently, the possibility of reverse causality also exists. Future studies using prospective cohorts are needed to explain and confirm a causal relationship between health-related patterns and CKD. In addition, there may have been interviewer bias and participants’ memory bias, since the data were self-reported. Also noteworthy is the possibility of underdiagnosis, especially of metabolic factors, leading to underestimated prevalence. Regarding the excessive salt consumption variable, the results of the present study cannot be seen as an approximation of the real salt consumption by the Brazilian adult population, since the agreement between the perceived and actual level of salt consumption is distorted (Brazilian consume, on average, almost twice the World Health Organization recommendations, yet a small fraction acknowledge their excessive intake) (60). Finally, it was not possible to stratify the severity of the disease.
Accordingly, our study made it possible to identify that the pattern referring to metabolic factors was associated with a higher chance of presenting CKD, while patterns related to behavioral risk factors and behavioral protective factors were not significantly associated. This suggests that underlying diseases, such as AH and DM, may be more strongly linked to the chance of CKD than behavioral factors such as diet and physical activity.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ibge.gov.br/estatisticas/sociais/saude/9160-pesquisa-nacional-de-saude.html?=&t=downloads.
Ethics statement
The 2019 National Health Survey project was forwarded to the National Research Ethics Committee (CONEP)/National Health Council (CNS) and approved under Opinion No. 3,529,376, issued on 23 August 2019. The participants provided their written informed consent to participate in this study.
Author contributions
LCMS and NRS contributed to the formal analysis of the data. CMA, AEMR, and LSS contributed to conceptualization and visualization, formal analysis of the data. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
2. Hill, NR, Fatoba, ST, Oke, JL, Hirst, JA, O’Callaghan, CA, Lasserson, DS, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One. (2016) 11:e0158765–18. doi: 10.1371/journal.pone.0158765
3. Foreman, KJ, Marquez, N, Dolgert, A, Fukutaki, K, Fullman, N, McGaughey, M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. (2018) 392:2052–90. doi: 10.1016/S0140-6736(18)31694-5
4. Instituto Brasileiro de Geografia e Estatística (IBGE) . Pesquisa Nacional de Saúde 2019: Informações sobre domicílios, acesso e utilização dos serviços de saúde: Brasil, grandes regiões e unidades de federação. Rio de Janeiro: IBGE (2020) Available at: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101748.pdf.
5. Marinho, AWGB, da Penha, AP, Silva, MT, and Galvão, TF. Prevalência de doença renal crônica em adultos no Brasil: revisão sistemática da literatura. Cad Saúde Col. (2017) 25:379–88. doi: 10.1590/1414-462X201700030134
6. Brasil . Vigitel Brasil 2019: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico: estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no Distrito Federal em 2019. Brasília. (2020). Available at: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2019_vigilancia_fatores_risco
7. Xie, Y, Bowe, B, Mokdad, AH, Xian, H, Yan, Y, Li, T, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. (2018) 94:567–81. doi: 10.1016/j.kint.2018.04.011
8. Kazancioğlu, R . Risk factors for chronic kidney disease: an update. Kidney Int Suppl. (2013) 3:368–71. doi: 10.1038/kisup.2013.79
9. Kidney Disease: Improving Global Outcomes, (KDIGO), Group CW . KDIGO 2012 Clinical practice guideline for the evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013:1–150. Available at: https://kdigo.org/wpcontent/uploads/2017/02/KDIGO_2012_CKD-GL.pdf
10. Luyckx, VA, Tuttle, KR, Garcia-Garcia, G, Gharbi, MB, Heerspink, HJL, Johnson, DW, et al. Reducing major risk factors for chronic kidney disease. Kidney Int Suppl. (2017) 7:71–87. doi: 10.1016/j.kisu.2017.07.003
11. Spring, B, Moller, AC, and Coons, MJ. Multiple health behaviours: overview and implications. J Public Health (Oxford). (2012) 34:i3–i10. doi: 10.1093/pubmed/fdr111
12. Steele, EM, Claro, RM, and Monteiro, CA. Behavioural patterns of protective and risk factors for non-communicable diseases in Brazil. Public Health Nutr. (2014) 17:369–75. doi: 10.1017/S1368980012005472
13. Corsonello, A, Fabbietti, P, Formiga, F, Moreno-Gonzalez, R, Tap, L, Mattace-Raso, F, et al. Chronic kidney disease in the context of multimorbidity patterns: the role of physical performance. BMC Geriatr. (2020) 20:350–12. doi: 10.1186/s12877-020-01696-4
14. Formiga, F, Ferrer, A, Sanz, H, Marengoni, A, Alburquerque, J, Pujol, R, et al. Patterns of comorbidity and multimorbidity in the oldest old: the Octabaix study. Eur J Intern Med. (2013) 24:40–4. doi: 10.1016/j.ejim.2012.11.003
15. Zemedikun, DT, Gray, LJ, Khunti, K, Davies, MJ, and Dhalwani, NN. Patterns of multimorbidity in middle-aged and older adults: an analysis of the UK biobank data. Mayo Clin Proc. (2018) 93:857–66. doi: 10.1016/j.mayocp.2018.02.012
16. Asghari, G, Momenan, M, Yuzbashian, E, Mirmiran, P, and Azizi, F. Dietary pattern and incidence of chronic kidney disease among adults: a population-based study. Nutr Metab. (2018) 15:88–11. doi: 10.1186/s12986-018-0322-7
17. He, LQ, Wu, XH, Huang, YQ, Zhang, XY, and Shu, L. Dietary patterns and chronic kidney disease risk: a systematic review and updated meta-analysis of observational studies. BMC Nutr. (2021) 20:4–11. doi: 10.1186/s12937-020-00661-6
18. Santin, F, Canella, D, Borges, C, Lindholm, B, and Avesani, CM. Dietary patterns of patients with chronic kidney disease: the influence of treatment modality. Nutrients. (1920) 11:1–12. doi: 10.3390/nu11081920
19. Xu, S-S, Hua, J, Huang, YQ, and Shu, L. Association between dietary patterns and chronic kidney disease in a middle-aged Chinese population. Public Health Nutr. (2020) 23:1058–66. doi: 10.1017/S1368980019002805
20. da Costa, FF, Benedet, J, Leal, DB, and de Assis, MMA. Agregação de fatores de risco para doenças e agravos crônicos não transmissíveis em adultos de Florianópolis. SC Rev Bras Epidemiol. (2013) 16:398–408. doi: 10.1590/S1415-790X2013000200015
21. Loch, MR, Bortoletto, MSS, de Souza, RKT, and Mesas, AE. Simultaneidade de comportamentos de risco para a saúde e fatores associados em estudo de base populacional. Cad Saúde Col (Rio de Janeiro). (2015) 23:180–7. doi: 10.1590/1414-462X201500020045
22. Muniz, LC, Schneider, BC, da Silva, ICM, Matijasevich, A, and Santos, IS. Accumulated behavioral risk factors for cardiovascular diseases in southern Brazil. Rev Saúde Pública. (2012) 46:534–42. doi: 10.1590/S0034-89102012005000021
23. de Barros, MBA, Lima, MG, LDPB, M, Szwarcwald, CL, and Malta, DC. Social inequalities in health behaviors among Brazilian adults: National Health Survey, 2013. Int J Equity Heal. (2016) 15:148–10. doi: 10.1186/s12939-016-0439-0
24. Duarte, APP, Rodrigues, PRM, Ferreira, MG, Cunha, DB, Moreira, NF, Sichieri, R, et al. Socio-economic and demographic characteristics associated with risk behaviour patterns for chronic non-communicable diseases in Brazil: data from the National Health Survey, 2013. Public Health Nutr. (2019) 22:2083–91. doi: 10.1017/S136898001900034X
25. McAloney, K, Graham, H, Law, C, and Platt, L. A scoping review of statistical approaches to the analysis of multiple health-related behaviours. Prev Med. (2013) 56:365–71. doi: 10.1016/j.ypmed.2013.03.002
26. Stopa, SR, Szwarcwald, CL, de Oliveira, MM, Oliveira, MM, Gouvea, ECDP, Vieira, MLFP, et al. Pesquisa Nacional de Saúde 2019: histórico, métodos e perspectivas. Epidemiol Serv Saude. (2020) 29:e2020315–2. doi: 10.1590/S1679-49742020000500004
27. World Health Organization . WHO Consultation on Obesity (1997: Geneva, Switzerland), World Health Organization. Division of Noncommunicable Diseases & World Health Organization. Programme of nutrition, family and reproductive health. (1998). Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity, Geneva, 3-5 June 1997. Available at: https://apps.who.int/iris/handle/10665/63854
28. Lipschitz, DA . Screening for nutritional status in the elderly. Prim Care (Philadelphia). (1994) 21:55–67. doi: 10.1016/S0095-4543(21)00452-8
29. World Health Organization . Global status report on alcohol and health 2018 [internet]. Geneva: World Health Organization (2018). Available at: https://www.who.int/publications/i/item/9789241565639) (Accessed January 12, 2021).
30. Matsudo, S, Araújo, T, Matsudo, V, Andrade, D, Andrade, E, Oliveira, LC, et al. Questionário Internacional de Atividade Física (IPAQ): estudo de validade e reprodutibilidade no Brasil. Rev Bras Ativ Fis Saúde. (2001) 6:5–18. Available at: https://rbafs.org.br/RBAFS/article/view/931/1222
31. Matos, DAS, and Rodrigues, EC. Análise fatorial (online). Brasília (DF): Enap, (2019) 74. Available at: https://repositorio.enap.gov.br/handle/1/4790
32. Hutcheson, GD, and Sofroniou, N. The multivariate social scientist: introductory statistics using generalized linear models. London: Sage Publications (1999).
33. Costello, AB, and Osborne, JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pratical Assess Res Eval. (2005) 10:1–9.
34. de Gouvêa, ECDP, Szwarcwald, CL, Damacena, GN, and de Moura, L. Autorrelato de diagnóstico médico de doença renal crônica: prevalência e características na população adulta brasileira, Pesquisa Nacional de Saúde 2013 e 2019. Epidemiol Serv Saúde. (2022):e2021385:31. doi: 10.1590/SS2237-9622202200017
35. de Aguiar, LK, Ladeira, RM, Machado, ÍE, Bernal, RTI, de Moura, L, and Malta, DC. Fatores associados à doença renal crônica segundo critérios laboratoriais da Pesquisa Nacional de Saúde. Rev Bras Epidemiol. (2020) 23:e200101. doi: 10.1590/1980-549720200101
36. Barreto, SM, Ladeira, RM, Duncan, BB, Schmidt, MI, Lopes, AA, Benseñor, IM, et al. Chronic kidney disease among adult participants of the ELSA-Brasil cohort: association with race and socioeconomic position. J Epidemiol Community Health. (2016) 70:380–9. doi: 10.1136/jech-2015-205834
37. Kovesdy, CP . Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. (2022) 12:7–11. doi: 10.1016/j.kisu.2021.11.003
38. Ku, E, Lee, BJ, Wei, J, and Weir, MR. Hypertension in CKD: Core curriculum 2019. Am J Kidney Dis. (2019) 74:120–31. doi: 10.1053/j.ajkd.2018.12.044
39. Forbes, J, Fukami, K, and Cooper, M. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. (2007) 115:69–84. doi: 10.1055/s-2007-949721
40. Lin, YC, Chang, YH, Yang, SY, Wu, KD, and Chu, TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc (Taipei). (2018) 117:662–75. doi: 10.1016/j.jfma.2018.02.007
41. Magee, C, Grieve, DJ, Watson, CJ, and Brazil, DP. Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther. (2017) 31:579–92. doi: 10.1007/s10557-017-6755-9
42. Toth-Manikowski, S, and Atta, MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. (2015) 2015:1–16. doi: 10.1155/2015/697010
43. Liu, M, Li, X-C, Lu, L, Cao, Y, Sun, RR, Chen, S, et al. Cardiovascular disease and its relationship with chronic kidney disease. Eur Rev Med Pharmacol Sci. (2014) 18:2918–26. Available at: https://www.europeanreview.org/article/7900
44. Jankowski, J, Floege, J, Fliser, D, Böhm, M, and Marx, N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. (2021) 143:1157–72. doi: 10.1161/CIRCULATIONAHA.120.050686
45. Onal, EM, Sag, AA, Sal, O, Yerlikaya, A, Afsar, B, and Kanbay, M. Erythropoietin mediates brain-vascular-kidney crosstalk and may be a treatment target for pulmonary and resistant essential hypertension. Clin Exp Hypertens. (2017) 39:197–209. doi: 10.1080/10641963.2016.1246565
46. Agharazii, M, St-Louis, R, and Gautier-Bastien, A. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am J Hypertens (United Kingdom). (2015) 28:746–55. doi: 10.1093/ajh/hpu225
47. Fujii, H, Goto, S, and Fukagawa, M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins (Basel). (2018) 10:1–18. doi: 10.3390/toxins10050202
48. Chang, H-J, Lin, K-R, Chang, J-L, and Lin, M-T. Risk factors for chronic kidney disease in older adults with hyperlipidemia and/or cardiovascular diseases in Taipei City, Taiwan: a community-based cross-sectional analysis. Int J Environ Res Public Health. (2020) 17:1–13. doi: 10.3390/ijerph17238763
49. Batista, M, and de Rodrigues, CJO. Diretrizes clínicas: Alterações Metabólicas. Braz J Nephrol. (2004) 26:15–9.
50. Peres, LAB, and Bettin, TE. Dislipidemia em pacientes com doença renal crônica. Rev Soc Bras Clin Med. (2015) 13:10–3. Available at: https://pesquisa.bvsalud.org/portal/resource/pt/lil-749212
51. Afkarian, M, Katz, R, Bansal, N, Correa, A, Kestenbaum, B, Himmelfarb, J, et al. Diabetes, kidney disease, and cardiovascular outcomes in the Jackson heart study. Clin J Am Soc Nephrol. (2016) 11:1384–91. doi: 10.2215/CJN.13111215
52. Hubbard, D, Colantonio, LD, Rosenson, RS, Brown, TM, Jackson, EA, Huang, L, et al. Risk for recurrent cardiovascular disease events among patients with diabetes and chronic kidney disease. Cardiovasc Diabetol. (2021) 20:58–9. doi: 10.1186/s12933-021-01247-0
53. Foster, MC, Hwang, S-J, Massaro, JM, Jacques, PF, Fox, CS, and Chu, AY. Lifestyle factors and indices of kidney function in the Framingham heart study. Am J Nephrol. (2015) 41:267–74. doi: 10.1159/000430868
54. Kurniawan, AL, Hsu, C-Y, Rau, H-H, Lin, L-Y, and Chao, JC-J. Association of kidney function-related dietary pattern, weight status, and cardiovascular risk factors with severity of impaired kidney function in middle-aged and older adults with chronic kidney disease: a cross-sectional population study. BMC Nutr. (2019) 18:27–11. doi: 10.1186/s12937-019-0452-4
55. Paterson, EN, Neville, CE, Silvestri, G, Montgomery, S, Moore, E, Silvestri, V, et al. Dietary patterns and chronic kidney disease: a cross-sectional association in the Irish nun eye study. Sci Rep. (2018) 8:6654–8. doi: 10.1038/s41598-018-25067-7
56. Shi, Z, Taylor, AW, Riley, M, Byles, J, Liu, J, and Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin Nutr. (2018) 37:276–84. doi: 10.1016/j.clnu.2016.12.025
57. Silva, PAB, Silva, LB, Santos, JFG, and Soares, SM. Brazilian public policy for chronic kidney disease prevention: challenges and perspectives. Rev Saúde Pública. (2020) 54:86. doi: 10.11606/s1518-8787.2020054001708
58. Samaan, F, Fernandes, DE, Kirsztajn, GM, de Sesso, RCC, and Malik, AM. Quality indicators for primary health care in chronic kidney disease in the public service of a city in the state of São Paulo, Brazil. Cad Saúde Pública. (2022) 38:e00090821. doi: 10.1590/0102-311X00090821
59. Htay, H, Alrukhaimi, M, Ashuntantang, GE, Bello, AK, Bellorin-Font, E, Benghanem-Gharbi, M, et al. Global access of patients with kidney disease to health technologies and medications: findings from the global kidney health atlas project. Kidney Int Suppl (2011). (2018) 8:64–73. doi: 10.1016/j.kisu.2017.10.010
Keywords: chronic kidney disease, health behavior, risk factors, factor analysis, public health
Citation: de Sousa LCM, Silva NR, Azeredo CM, Rinaldi AEM and da Silva LS (2023) Health-related patterns and chronic kidney disease in the Brazilian population: National Health Survey, 2019. Front. Public Health 11:1090196. doi: 10.3389/fpubh.2023.1090196
Edited by:
Konstantinos Giannakou, European University Cyprus, CyprusReviewed by:
Rifqah Abeeda Roomaney, South African Medical Research Council, South AfricaEduardo Augusto Fernandes Nilson, Center for Epidemiological Research in Nutrition and Health, Faculty of Public Health, University of São Paulo, Brazil
Copyright © 2023 de Sousa, Silva, Azeredo, Rinaldi and da Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luciana Saraiva da Silva, bHVjaWFuYS5zYXJhaXZhQHVmdS5icg==
 Letícia Cristina Machado de Sousa
Letícia Cristina Machado de Sousa Catarina Machado Azeredo
Catarina Machado Azeredo Luciana Saraiva da Silva
Luciana Saraiva da Silva