
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 06 September 2023
Sec. Children and Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1075946
Background: According to reports, maternal rheumatoid arthritis (RA) has been suggested as a possible adverse factor for developing small for gestational age (SGA) in offspring. However, some studies have also indicated a need for a more statistically significant association between the two. Understanding the relationship between maternal RA and the risk of SGA is crucial for identifying potential adverse outcomes and implementing appropriate interventions. Therefore, this study aims to elucidate the association between maternal RA and the risk of offspring developing SGA.
Methods: This study was registered on the International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42022357590). A systematic literature search was conducted to identify eligible studies up to August 2022. Quality assessment was performed according to the Newcastle-Ottawa scale. The Q test and I2 test tested and estimated heterogeneity among studies. Odds ratios (ORs) with 95% CI were calculated using random or fixed effects models depending on the heterogeneity. Subgroup analyses, sensitivity analyses, and publication bias assessments were also performed.
Results: Seven studies, including 12,323,918 participants, were included in the analysis. The results showed a statistically significant association between maternal RA and SGA (OR = 1.70, 95% CI = 1.29–2.23, p < 0.001). Sensitivity analysis showed stable results. The funnel plot of the symmetric distribution and the results of Begg’s and Egger’s tests showed no publication bias.
Conclusion: Maternal RA is associated with an increased risk of SGA in offspring. However, more studies are still needed to explore the potential mechanisms underlying maternal RA and SGA association.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier [CRD42022357590].
Rheumatoid arthritis (RA)is defined as a systemic autoimmune disorder associated with a chronic inflammatory process that can damage joints and extra-articular organs, including the heart, kidneys, lungs, digestive system, eyes, skin, and nervous system (1, 2). The pathophysiological process of RA is closely related to cytokines such as interleukin 6 (IL-6) (3). Studies have shown that RA is a global disease irrespective of race, sex, ethnicity, nationality, and age, but prevalence and incidence measurements vary according to population characteristics and over time (2). Notably, gender differences have been observed in the prevalence of RA. Women are two to three times more likely to suffer from this disease than men, with most cases appearing during the childbearing years (4, 5). The global prevalence of RA is approximately 1%, fluctuates less consistently, and increases significantly from south to north, rural to metropolitan areas (6).
Small for gestational age (SGA) is defined as birth weight below the 10th percentile for gestational age (GA) (7). Perinatal mortality is higher in infants with SGA compared to newborns of appropriate gestational age at birth. This association exists for both full-term and preterm infants (8–12). SGA accounts for about 22% of neonatal deaths in low- and middle-income countries (13). It is also a cause of multiple short- and long-term complications (14). Infants with SGA are at higher risk of morbidity and developmental delay in childhood. They are susceptible to chronic diseases such as obesity, type 2 diabetes, coronary artery disease, and stroke into adulthood (15). Cytokines such as IL-6 have been reported to be higher in mothers of newborns who gave birth to SGA (16). As mentioned above, maternal RA potentially impacts SGA in offspring, but the risk of maternal RA increasing SGA in offspring is controversial. Five cohort studies of 936,030 participants found a significant association between maternal RA and risk of SGA (17–21), compared to two studies that showed no statistically significant association between maternal RA and risk of SGA (22, 23). Given the increasing incidence of RA in women over the years and the inconsistent association between maternal and risk of SGA in offspring, a Meta-analysis to assess this association would be of great value.
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Furthermore, it was registered on the International Prospective Register of Systematic Reviews (PROSPERO). The register number was CRD42022357590.
The literature was searched from inception to August 2022 in Embase, PubMed, Web of Science, Cochrane Library, and Scopus databases. A comprehensive search strategy was conducted for articles examining maternal RA and SGA in offspring. The search strategy was based on previous systematic reviews on RA and SGA. The complete retrieval strategy was finalized in discussions among three authors (Lv Tian, Zhiyuan Zhang, and Yuting Mao). The search strategy for this study consisted of three components: rheumatoid arthritis, pregnant mothers, and small for gestational age. Each component was searched using a combination of free and subject terms, and the components were combined using specific database terms. The search strategy for PubMed is shown below. It was adapted for the other databases (((((((Arthritis) OR (Rheumatoid)) OR (Rheumatoid Arthritis)) OR (Rheumatoid Nodule)) OR (Rheumatoid Vasculitis)) OR (“Arthritis, Rheumatoid”[Mesh])) AND ((“Infant, Small for Gestational Age”[Mesh]) OR (“small for gestational age”))) AND (Maternal).
The inclusion criteria developed for screening eligible publications were as follows:
1. The type of study was a case–control study or cohort study.
2. The outcome of interest was the risk in SGA.
3. Estimates such as ratio ratios (ORs) or relative risks (RRs) with the corresponding 95% confidence intervals (CIs) were reported, or sufficient data were provided to perform the calculation.
4. Pregnant women with no clear indication of special treatment (biologic therapy).
Exclusion criteria were as follows:
1. The study was not in humans, e.g., in vitro and in vivo studies.
2. The article was in the review category.
3. Risk estimates could not be calculated.
4. Duplication.
Two participants (Lv Tian and Zhiyuan Zhang)extracted the data independently according to a predefined data form and then assessed the quality of the articles. If disagreements were encountered, discussions were held to reach an agreement, or a third person (Yuting Mao) was consulted regarding recommendations. Data extracted included authors’ names, year, country, study design, comparisons, total participators, OR or RR with 95% CI, quality, adjustment factors, assessment method, and age of the study participators. The quality of observational studies was assessed by the Newcastle-Ottawa scale, which has three columns: selection, comparability, and outcome/exposure. The total score is nine stars, with six or more stars for high-quality literature and 4–5 stars for moderate quality (24).
The association between maternal RA and SGA in offspring was assessed by pooling the ORs with the corresponding 95% CIs. RRs were transformed into odds ratios which yielded similar estimates as ORs. the formula, RR = OR/[(1 − P0) + (P0 × OR)], was used to perform RR to OR conversion (P0 represents the incidence of the outcome in the non-exposed group) (25). When multiple ORs were provided in a single study, the one that controlled the most confounders was selected. Heterogeneity between studies was tested by Q-test at the p = 0.1 level and then based on I2 statistics: I2 < 50% of respondents indicated no significant statistical heterogeneity and therefore a fixed effects model was selected, I2 > 50% indicated statistical heterogeneity, and a random effects model was used (26, 27). Sensitivity analyses were achieved by the leave-one-out method (28). Publication bias was assessed by observing whether the funnel plot was symmetrical and calculating Begg’s and Egger’s test values. Data were processed using the statistical software Stata 15.0 (Stata Corp., College Station, TX, United States) (29, 30). p values less than 0.05 were considered statistically significant.
According to the search strategy, the initial electronic search yielded 1872 articles, and 1,164 articles were obtained after eliminating duplicates. Seventy-eight articles were retained after reading the titles and abstracts. Finally, the full text was read through to filter the literature, and finally, seven articles were obtained for inclusion (17–23). The detailed literature screening process is shown in Figure 1.
This meta-analysis included seven articles involving a total of 12,323,918 participants, published between 2006 and 2019. Of these, three were conducted in the United States, two in Canada; one was conducted in Norway, and one in Switzerland. Six of them were adjusted for confounding factors. All included studies were of high or moderate quality. Table 1 presents more detailed information on the included studies.
Seven studies related to the association between maternal RA and the risk of SGA in offspring were included in the overall meta-analysis (17–23). Heterogeneity was observed, and a random effects model was used to combine the studies. Pooled results showed that maternal RA was statistically significantly associated with increased risk of SGA in offspring (OR = 1.70, 95% CI = 1.29–2.23, p < 0.001; I2 = 89.6%; Figure 2).
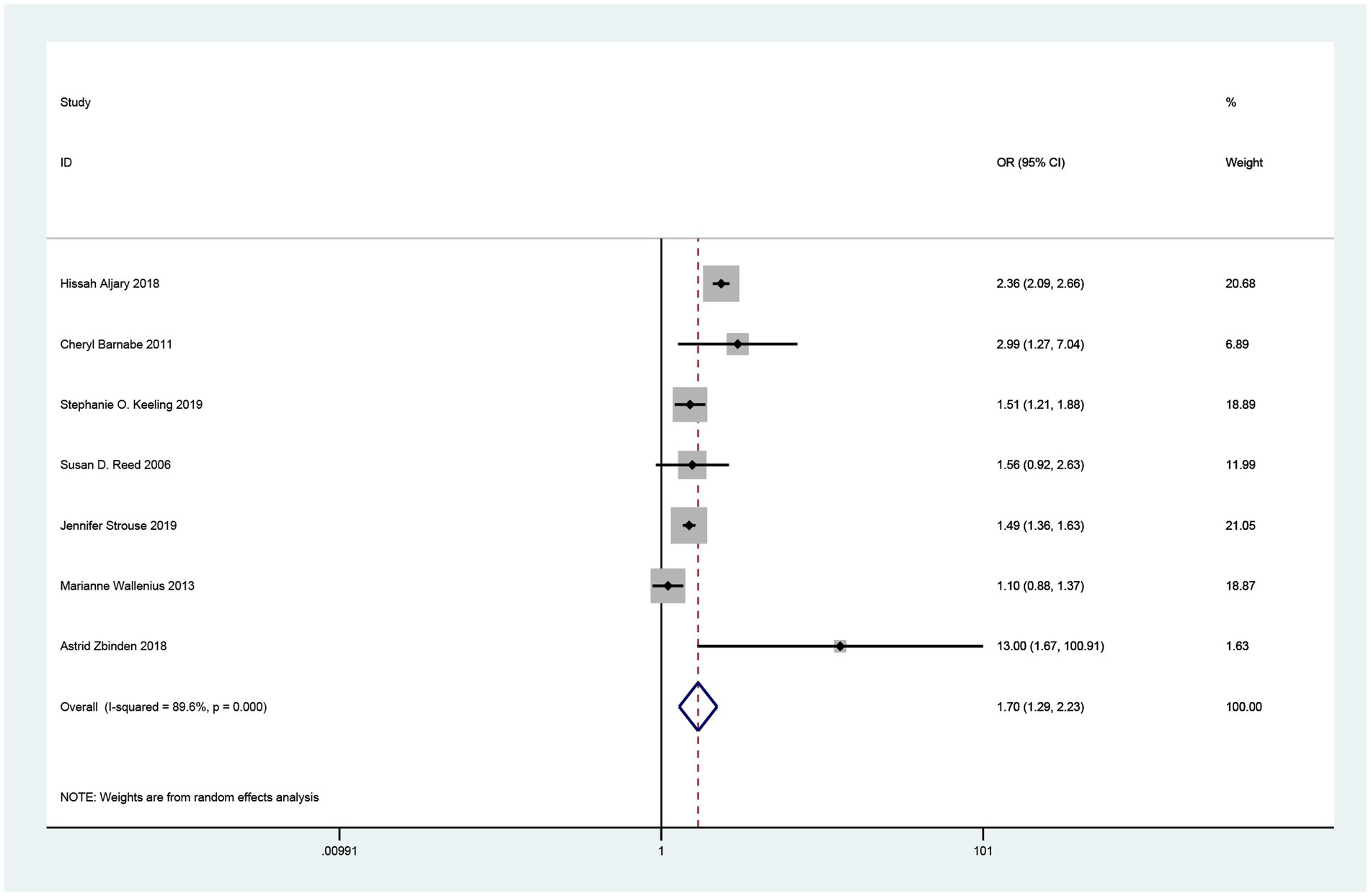
Figure 2. Forest plot of the association between maternal rheumatoid arthritis and small for gestational age neonates.
In subgroup analyses, according to the country of study, three studies were conducted in the USA (17, 20, 22) (OR = 1.80, 95% CI =1.23–2.63, p = 0.003). Two studies conducted in Canada (18, 19) (OR = 1.87, 95% CI =1.01,3.46, p = 0.048) showed a statistically significant association between maternal RA and increased risk of SGA in offspring. A study from Norway reported an OR of 1.1 (95% CI = 0.88–1.37), and a study from Switzerland reported an OR of 13.0 (95% CI =1.68–100.91). For adjustment factors, a significant association was observed in adjusted studies (OR = 1.63, 95% CI =1.23–2.16, p = 0.001). For subgroup analyses with different study qualities, the results remained unchanged [high quality (18–23): OR = 1.47 95% CI =1.20–1.80, p < 0.001]. For the age of women with RA, the results remained unchanged in the two age-restricted studies and the five studies with unrestricted age [unrestricted (17, 20, 22, 23) OR = 1.67, 95% CI =1.78–2.35, p = 0.004, restricted (18, 19) OR = 1.87,95% CI = 1.01–3.46, p = 0.048]. All these results are shown in Table 2.
Sensitivity analyses were performed using the leave-one-out method, which indicated that the result was stable (Figure 3). The funnel plot was symmetrically distributed, and Begg’s test and Egger’s test showed no publication bias (Begg’s test: Z = 0.90; p = 0.386; Egger’s test: t = 0.27; p = 0.795; Figure 4).
The association between maternal RA and the risk of SGA in offspring is a controversial topic. Based on previous studies, this meta-analysis of seven cohort studies of patients from different backgrounds found an association between maternal RA and increased risk of SGA in offspring. The results show that newborns of women with RA are more likely to be SGA compared to women with normal pregnancies. In addition, subgroup analyses confirmed the such association. The pooled estimated effects of the included subgroups were almost similar to the overall results. Statistical significance was found for subgroups grouped by country, study quality, age with or without restrictions, sample size, and adjustment factors. Sensitivity analysis showed no substantial change in the results when we used the retention method, suggesting that the results of this meta-analysis are robust. Considering the progressive increase in the prevalence of RA among women, this article will make much sense.
The potential mechanisms between maternal RA and SGA of the newborn remain obscure. It has been shown that oxidative stress markers are significantly higher in neonates with SGA than in infants of appropriate gestational age (AGA) and that prolonged excessive oxidative stress and intrauterine malnutrition can lead to SGA in offspring (31). Oxidative stress plays a crucial role in the pathophysiology of RA. In RA patients, the antioxidant system is altered, and serum and synovial fluid lipid peroxidation levels are elevated (32). Since the last decade, studies have shown a positive correlation between the level of ROS and the severity of RA (33). The findings suggest that maternal RA can affect SGA in offspring through reactive oxygen species.
In another study, IL-10 was found to be detectable in 23.4% of RA patients and IL-6 in 76.6% of patients in late pregnancy. When IL-6 levels were high, the risk of neonatal SGA was increased, suggesting that increased levels of IL-6 are associated with neonatal SGA (16). In addition, vitamin D is also involved. Vitamin D deficiency increases levels of pro-inflammatory cytokines and leads to oxidative stress. Lower 25(OH)D status is associated with increased vascular endothelial cell expression of interleukin six and reduced expression of vitamin D receptors and 1-alpha hydroxylase (34). One study reported significantly higher levels of pro-inflammatory cytokines in the cord blood of SGA infants than in the cord blood of non-SGA infants (35). Maternal rheumatoid can increase the risk of SGA in newborns through cytokines. In clinical studies, patients with RA were found to have low sleep quality and severe sleep disturbances (36). A short duration of pregnancy and poor sleep quality were associated with an increased risk of preterm birth and SGA (37). This result supports the effect of maternal rheumatoid on neonatal SGA. Our study confirms the results, but more profound and more comprehensive mechanisms remain to be explored further.
This meta-analysis has several limitations. First, heterogeneity existed between included studies, so a random effects model was applied to combine ORs and RRs. Although subgroup analysis could explain some of the heterogeneity, sufficient data from the included original studies, such as different ethnicities of female RA patients and different rheumatoid disease characteristics (duration, severity, with or without treatment), were lacking to investigate other potential sources. Second, although the sensitivity analysis results were robust and ensured the accuracy and persuasiveness of the results, the limited number of studies conducted outside of Europe and the Americas suggests the need for more data on populations of different backgrounds. The substantial heterogeneity (I2 = 89.6%) of the pooled effects may reflect the significant variation in sample size. Considering the limited number of studies in our review and the high heterogeneity, more and higher quality studies are needed. Our results should be interpreted cautiously due to the limited number of included studies. Finally, our study is the absence of correlation analysis between maternal RA disease activity and the risk of neonatal SGA. Unfortunately, we could not investigate this potential association due to the need for more sufficient data in the included studies. However, we acknowledge this limitation and encourage future research to explore the relationship between maternal RA disease activity and the risk of SGA. Despite these limitations, the following strengths of this study should be acknowledged. This is the first meta-analysis to elucidate the relationship between maternal RA and the risk of SGA in offspring. In addition, sensitivity analyses showed that the meta-analysis results did not change substantially using the leave-one-out method. The results were robust, and there was no publication bias.
Accurate knowledge of maternal RA and neonatal SGA risk is valuable for early intervention to reduce the frequency of these adverse events. Further studies are needed to investigate the underlying causes of RA and SGA, which may improve clinical care and lead to a more informed prognosis for future pregnant mothers with RA.
In summary, maternal RA increases the risk of SGA in offspring. Healthcare providers should be aware of the high risk of adverse maternal and neonatal outcomes. Medical staff should develop an individualized and continuous pregnancy screening program for pregnant women with RA, record their family history, medical history, and physical examination data in detail, and provide health education on daily precautions for pregnant women. Before pregnancy, women with RA should be encouraged to maintain optimal health and manage their RA and other chronic diseases. Pregnant women with RA should schedule detailed and regular pregnancy tests according to the actual situation. By increasing the number of pregnancy tests, we can dynamically monitor the mother and fetus’s physiological changes and effectively reduce the risk of SGA.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
LT: data curation, formal analysis, and writing original draft. ZZ: investigation. YM: resources and supervision. MZ: project administration, visualization, writing-review, and editing. All authors contributed to the article and approved the submitted version.
This study was supported by the Natural Science Foundation of Jilin Province (grant number: YDZJ202201ZYTS062, 20200201127JC).
The authors thank all the authors of the original articles.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Conforti, A, Di Cola, I, Pavlych, V, Ruscitti, P, Berardicurti, O, Ursini, F, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. (2021) 20:102735. doi: 10.1016/j.autrev.2020.102735
2. Cojocaru, M, Cojocaru, IM, Silosi, I, Vrabie, CD, and Tanasescu, R. Extra-articular manifestations in rheumatoid arthritis. Maedica (Bucur). (2010) 5:286–91.
3. Smolen, JS, Aletaha, D, and McInnes, IB. Rheumatoid arthritis. Lancet. (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
4. Crane, MM, Juneja, M, Allen, J, Kurrasch, RH, Chu, ME, Quattrocchi, E, et al. Epidemiology and treatment of new-onset and established rheumatoid arthritis in an insured US population. Arthritis Care Res (Hoboken). (2015) 67:1646–55. doi: 10.1002/acr.22646
5. Pósfai, É, Bánhidy, F, Urbán, R, and Czeizel, AE. Birth outcomes of children born to women with rheumatoid arthritis. Cent Eur J Public Health. (2015) 23:128–34. doi: 10.21101/cejph.a3968
6. Safiri, S, Kolahi, AA, Hoy, D, Smith, E, Bettampadi, D, Mansournia, MA, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. (2019) 78:1463–71. doi: 10.1136/annrheumdis-2019-215920
7. Williams, RL, Creasy, RK, Cunningham, GC, Hawes, WE, Norris, FD, and Tashiro, M. Fetal growth and perinatal viability in California. Obstet Gynecol. (1982) 59:624–32.
8. Griffin, IJ, Lee, HC, Profit, J, and Tancedi, DJ. The smallest of the small: short-term outcomes of profoundly growth restricted and profoundly low birth weight preterm infants. J Perinatol. (2015) 35:503–10. doi: 10.1038/jp.2014.233
9. Baer, RJ, Rogers, EE, Partridge, JC, Anderson, JG, Morris, M, Kuppermann, M, et al. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J Perinatol. (2016) 36:1008–13. doi: 10.1038/jp.2016.118
10. Ray, JG, Park, AL, and Fell, DB. Mortality in infants affected by preterm birth and severe small-for-gestational age birth weight. Pediatrics. (2017) 140:e20171881. doi: 10.1542/peds.2017-1881
11. Katz, J, Lee, AC, Kozuki, N, Lawn, JE, Cousens, S, Blencowe, H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. (2013) 382:417–25. doi: 10.1016/S0140-6736(13)60993-9
12. Lee, AC, Kozuki, N, Cousens, S, Stevens, GA, Blencowe, H, Silveira, MF, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ. (2017) 358:j3677. doi: 10.1136/bmj.j3677
13. Wacker-Gussmann, A, Engelhard, J, Oberhoffer-Fritz, R, Schopen, J, Ewert, P, Ortiz, JU, et al. Cardiovascular outcome of former late-onset small-for-gestational-age children at 1 year of age: CURIOSA study. Arch Gynecol Obstet. (2022) 306:1455–61. doi: 10.1007/s00404-022-06404-8
14. Barker, DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. (2006) 49:270–83. doi: 10.1097/00003081-200606000-00009
15. Parikh, NI, Gonzalez, JM, Anderson, CAM, Judd, SE, Rexrode, KM, Hlatky, MA, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. (2021) 143:e902–16. doi: 10.1161/CIR.0000000000000961
16. Atta, DS, Girbash, EF, Abdelwahab, SM, Abdeldayem, HM, Tharwat, I, and Ghonaim, R. Maternal cytokines and disease severity influence pregnancy outcomes in women with rheumatoid arthritis. J Matern Fetal Neonatal Med. (2016) 29:1–6. doi: 10.3109/14767058.2015.1127342
17. Aljary, H, Czuzoj-Shulman, N, Spence, AR, and Abenhaim, HA. Pregnancy outcomes in women with rheumatoid arthritis: a retrospective population-based cohort study. J Matern Fetal Neonatal Med. (2020) 33:618–24. doi: 10.1080/14767058.2018.1498835
18. Barnabe, C, Faris, PD, and Quan, H. Canadian pregnancy outcomes in rheumatoid arthritis and systemic lupus erythematosus. Int J Rheumatol. (2011) 2011:345727:1–6. doi: 10.1155/2011/345727
19. Keeling, SO, Bowker, SL, Savu, A, and Kaul, P. A population-level analysis of the differing effects of rheumatoid arthritis and Spondyloarthritis on Peripartum outcomes. J Rheumatol. (2020) 47:197–203. doi: 10.3899/jrheum.181320
20. Strouse, J, Donovan, BM, Fatima, M, Fernandez-Ruiz, R, Baer, RJ, Nidey, N, et al. Impact of autoimmune rheumatic diseases on birth outcomes: a population-based study. RMD Open. (2019) 5:e000878. doi: 10.1136/rmdopen-2018-000878
21. Zbinden, A, van den Brandt, S, Østensen, M, Villiger, PM, and Förger, F. Risk for adverse pregnancy outcome in axial spondyloarthritis and rheumatoid arthritis: disease activity matters. Rheumatology (Oxford). (2018) 57:1235–42. doi: 10.1093/rheumatology/key053
22. Reed, SD, Vollan, TA, and Svec, MA. Pregnancy outcomes in women with rheumatoid arthritis in Washington state. Matern Child Health J. (2006) 10:361–6. doi: 10.1007/s10995-006-0073-3
23. Wallenius, M, Salvesen, K, Daltveit, AK, and Skomsvoll, JF. Rheumatoid arthritis and outcomes in first and subsequent births based on data from a national birth registry. Acta Obstet Gynecol Scand. (2014) 93:302–7. doi: 10.1111/aogs.12324
24. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
25. Zhang, J, and Yu, KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
26. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
27. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
29. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
30. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
31. Gveric-Ahmetasevic, S, Sunjic, SB, Skala, H, Andrisic, L, Stroser, M, Zarkovic, K, et al. Oxidative stress in small-for-gestational age (SGA) term newborns and their mothers. Free Radic Res. (2009) 43:376–84. doi: 10.1080/10715760902783285
32. Mateen, S, Moin, S, Khan, AQ, Zafar, A, and Fatima, N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. (2016) 11:e0152925. doi: 10.1371/journal.pone.0152925
33. García-González, A, Gaxiola-Robles, R, and Zenteno-Savín, T. Oxidative stress in patients with rheumatoid arthritis. Rev Investig Clin. (2015) 67:46–53.
34. Jablonski, KL, Chonchol, M, Pierce, GL, Walker, AE, and Seals, DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. (2011) 57:63–9. doi: 10.1161/HYPERTENSIONAHA.110.160929
35. Lausten-Thomsen, U, Olsen, M, Greisen, G, and Schmiegelow, K. Inflammatory markers in umbilical cord blood from small-for-gestational-age newborns. Fetal Pediatr Pathol. (2014) 33:114–8. doi: 10.3109/15513815.2013.879239
36. Tański, W, Świątoniowska-Lonc, N, Tomasiewicz, A, Dudek, K, and Jankowska-Polańska, B. The impact of sleep disorders on the daily activity and quality of life in rheumatoid arthritis patients – a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2022) 26:3212–29. doi: 10.26355/eurrev_202205_28740
Keywords: rheumatoid arthritis, small for gestational age, systematic review, meta-analysis, maternal rheumatoid arthritis
Citation: Tian L, Zhang Z, Mao Y and Zong M (2023) Association between maternal rheumatoid arthritis and small for gestational age neonates: a systematic review and meta-analysis. Front. Public Health. 11:1075946. doi: 10.3389/fpubh.2023.1075946
Received: 21 October 2022; Accepted: 22 August 2023;
Published: 06 September 2023.
Edited by:
Judie Arulappan, Sultan Qaboos University, OmanReviewed by:
Wenning Fu, Huazhong University of Science and Technology, ChinaCopyright © 2023 Tian, Zhang, Mao and Zong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minru Zong, em9uZ21yQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.