- 1S-3 Research, San Diego, CA, United States
- 2Global Health Policy and Data Institute, San Diego, CA, United States
- 3Global Health Program, Department of Anthropology, University of California, San Diego, San Diego, CA, United States
In 2019, the U.S. Food and Drug Administration (FDA) approved emtricitabine and tenofovir alafenamide (Descovy) as another option for HIV pre-exposure prophylaxis (PrEP) prevention for high-risk adults and adolescents. With the introduction of this new PrEP, millions of current users on emtricitabine and tenofovir disoproxil fumarate (Truvada), another PrEP medication currently used to prevent HIV transmission, have options of whether to continue their current treatment regime or transition to new treatment options. The objective of this study was to conduct a descriptive analysis to characterize user-generated social media conversations on Reddit associated with FDA-approved PrEP prevention treatment options. Key themes identified were associated with perceptions, knowledge, and attitudes associated with the transition of use of different PrEP medications. Data were collected retrospectively and prospectively from the Reddit platform for posts with keywords filtered for HIV, PrEP, and FDA-approved PrEP prevention treatment from October 2020 to December 2020. We chose the Reddit platform based on prior studies that have identified PrEP user conversations and insights on access challenges for specific AIDS communities, such as gays and men who have sex with men (MSM). Reddit posts were then manually annotated using an inductive content coding approach for key themes regarding the transition of use and other emergent themes from user-generated content. Formal coding of text data was conducted with refined codes, and sub-codes created. A total of 3,120 posts were analyzed from Reddit resulting in 315 posts that were coded for PrEP and 105 posts (33.33%) specific to user discussions regarding the transition of PrEP prevention. Overall, users expressed interest in drug switching to Descovy, particularly in the context of poorer adherence or concerns about existing side effects associated with Truvada. Other major themes included discussions about the cost of Descovy, apprehension about side effects in comparison to Truvada, insurance coverage changes, and discussions about the donation of Truvada to other users after transitioning. Among these discussions, topics related to sexual minorities, including MSM, reported concerns when considering a switch in their HIV prevention regime. Understanding the changing public perception associated with the introduction of new HIV prevention is important in the context of market access, patient safety, pharmacovigilance, and health equity, particularly among high-risk populations such as MSM. Results support the use of social media from a digital pharmacovigilance perspective to better understand emerging HIV prevention, treatment, and adherence challenges experienced by patients.
1. Introduction
In 2020, ~37.7 million people were living with human immunodeficiency virus (HIV) globally, and as of 2021, over 28.2 million people were accessing anti-retroviral therapy (ART), representing an increase of more than 20 million people accessing treatment since 2010 (1). This is in large part due to a large reduction in anti-retroviral therapy (ART) prices as well as increases in the availability of treatment options approved for HIV (2, 3). In addition to ARTs, pre-exposure prophylaxis (PrEP) is a highly effective medication, that when taken as prescribed, is highly effective for preventing HIV infection, including for those at risk through sex or injection drug use (4). Importantly, certain sexual and gender minorities (SGMs), including men who have sex with men (MSM) and transgender women, have been disproportionately impacted by HIV infection and may have different attitudes toward PrEP therapy that can impact uptake and adherence (5, 6). Understanding the willingness to use PrEP therapy among these at-risk SGM populations, including in the context of drug transition and switching behavior, is critical to reducing HIV incidence.
In 2012, emtricitabine and tenofovir disoproxil fumarate (brand name Truvada) was approved by the U.S. Food and Drug Administration (FDA) for use in HIV-negative individuals to reduce the likelihood of contracting HIV, in addition to its use to treat active infections (7, 8). Since Truvada's approval as the first drug for HIV prevention using pre-exposure prophylaxis (PrEP), other drugs have also received approval for a PrEP indication in an oral formulation. In 2019, the FDA approved emtricitabine and tenofovir alafenamide (brand name Descovy) for PrEP in at-risk adults and adolescents (9). Truvada is a tenofovir disoproxil fumarate (TDF), and Descovy is a tenofovir alafenamide (TAF).
Although the chemical composition of these therapies is different, studies have found both formulations to be non-inferior to each other (10). Descovy's approval came with reports that its safety profile might be more favorable, with early evidence of fewer side effects compared to other PrEP options. This factor was considered important, as clinicians cited concerns about side effects when prescribing PrEP (11). However, evidence to date does not support that one drug has better safety and efficacy over the other, with a recent meta-analysis finding a difference in viral suppression and bone and renal side effects only when using a boosting agent with TAF (12). Apart from these considerations, cost and access issues are an ongoing concern as these drugs lacked generic treatment options at certain periods of time (e.g., the FDA approved a generic version of Truvada in June 2017) (9, 13). Hence, the potential benefits of increased competition and patent expiry have also been discussed and anticipated (13). Additionally, medical mistrust among SGMs and other minority populations regarding the effectiveness of PrEP may also represent a critical barrier to uptake and adherence (14–19).
Despite active conversation about the similarities, differences, and possible benefits vs. risks of these different FDA-approved PrEP therapy regimes among the HIV/AIDS community, few studies have specifically examined perceptions, attitudes, and behaviors associated with the transition of use for PrEP, though several studies have been published assessing barriers and facilitators to PrEP therapy as discussed on social media (20–23). Furthermore, few studies have examined social media to characterize how willingness to use PrEP may be impacted by perceptions and attitudes about drug transitions, particularly among SGMs as a specific population of interest. This study adds to this growing body of literature on social media and PrEP by conducting a descriptive analysis identifying and analyzing Reddit posts specific to users reporting their lived experiences with transitions of use attitudes for PrEP prevention therapy, while also examining whether these conversations included users who discussed issues related to or specifically self-identified as racial, ethnic, or sexual/gender-identification minorities (e.g., LGBTQ).
2. Method
2.1. Data collection
We used the programming language Python™ to develop a custom programming script to collect publicly available posts from Reddit. Posts were collected from Reddit over a 60-day study period (13 October 2020–11 December 2020) and allowed us to collect both retrospective data from posts that occurred prior to the date of collection and prospective data, posts collected starting on the date of collection. This data collection method and time period were chosen as it was deemed sufficient to collect both past and recent conversations regarding PrEP. Specifically, data were collected by retrieving posts from search results and sub-Reddits filtered for common PrEP and HIV-associated keywords including “HIV,” “PrEP,” “Truvada,” and “Descovy.” These keywords led to additional associated keywords and hashtags identified in Reddit conversations relating to HIV prevention and PrEP that were used for additional data collection. We chose the Reddit platform based on prior studies that have identified PrEP user conversations and insights on access challenges for specific AIDS communities, such as gay and MSM.
2.2. Data analysis
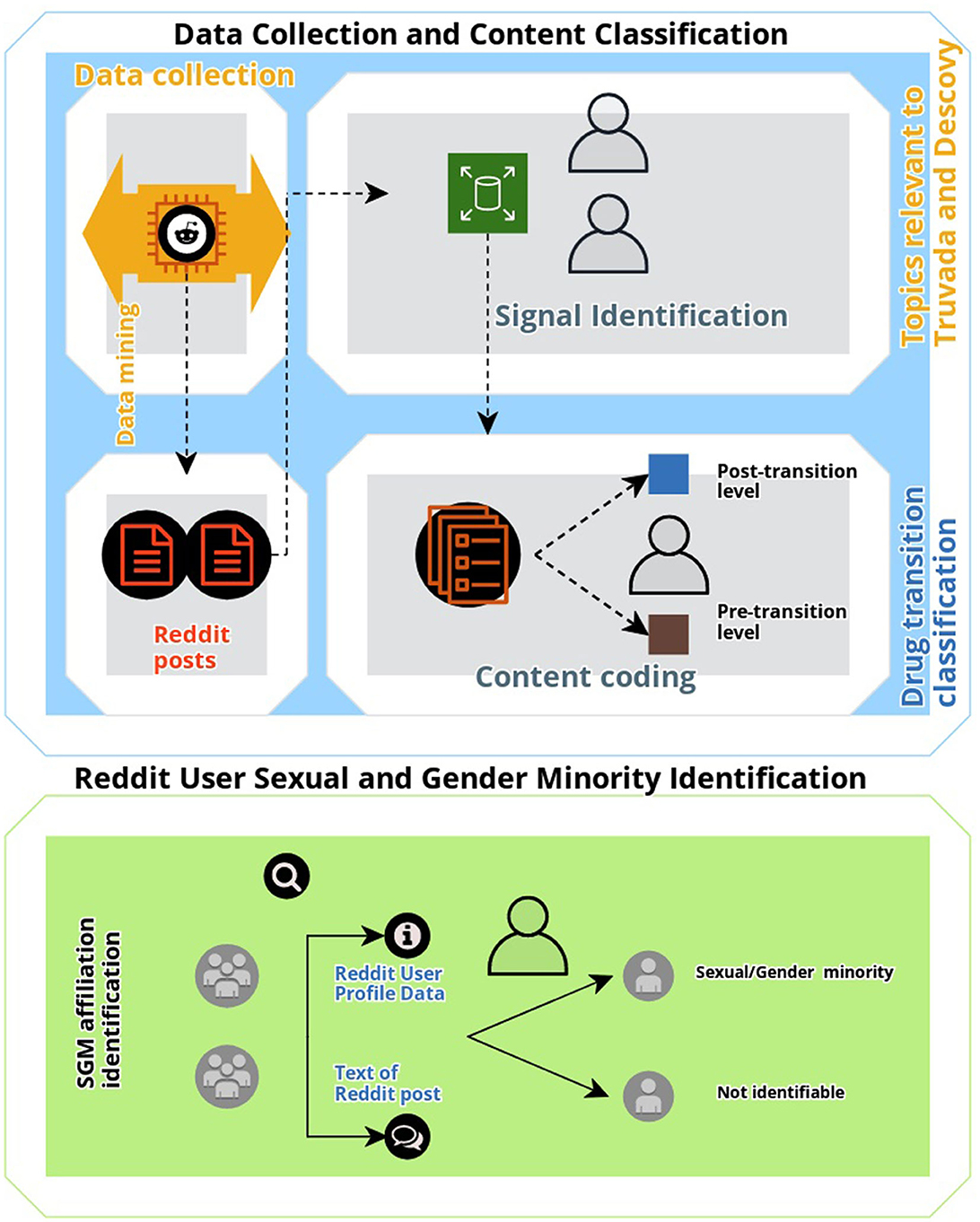
To identify, characterize, and elucidate conversation associated with the two approved PrEP medications, Truvada and Descovy, we manually annotated all collected posts. First, we identified all signal posts. Posts were deemed as “signal” if they were (a) user-generated (i.e., not posted by organizations or media outlets); and (b) conversation discussing topics relevant to Truvada and Descovy accessibility, use and or transition of use, effectiveness, insurance coverage, and associated barriers. Advertisements of the drug, public announcements, and posts not related to Truvada and Descovy were specifically excluded from analysis as the focus of this study was to identify and characterize user-generated posts regarding behavior associated with PrEP, and with a specific focus on drug transitions, not drug promotion or HIV health education and promotion. To classify the content of Reddit posts, a general inductive approach was utilized to code the textual data for descriptive analysis (24). All identified signal posts were reviewed by the first author (HG) and notes were taken on general themes of posts. Formal coding of text data was conducted with refined codes, and sub-codes were created. A final coded dataset was reviewed by the second author (QX), and differences in code definitions and applications were reconciled by the first and second authors. Inductively derived codes (see Table 1) were then included in the analysis if they included content discussing the two FDA-approved PrEP drugs “Truvada” and “Descovy.” A final coded dataset was reviewed by the first, second, and last authors (TKM) to assess whether any differences in code definitions and application occurred with any differences reconciled by consensus on the correct classification. The first and second authors achieved high inter-coder reliability for signal coding (kappa = 95.32). Additionally, the text and metadata (e.g., gay label and rainbow emoji) of Reddit posts associated with PrEP content that was relevant to the themes generated for this study were also coded to determine whether they included comments or discussions related to SGM issues, or whether users specifically self-identified as gay, lesbian, or bisexual (sexual minority status) or whether they self-reported their expression of their gender identity as different from their sex assigned at birth (gender minority status) (25). A visual summary of the study methodology used is shown in Figure 1.
3. Results
3.1. Overview
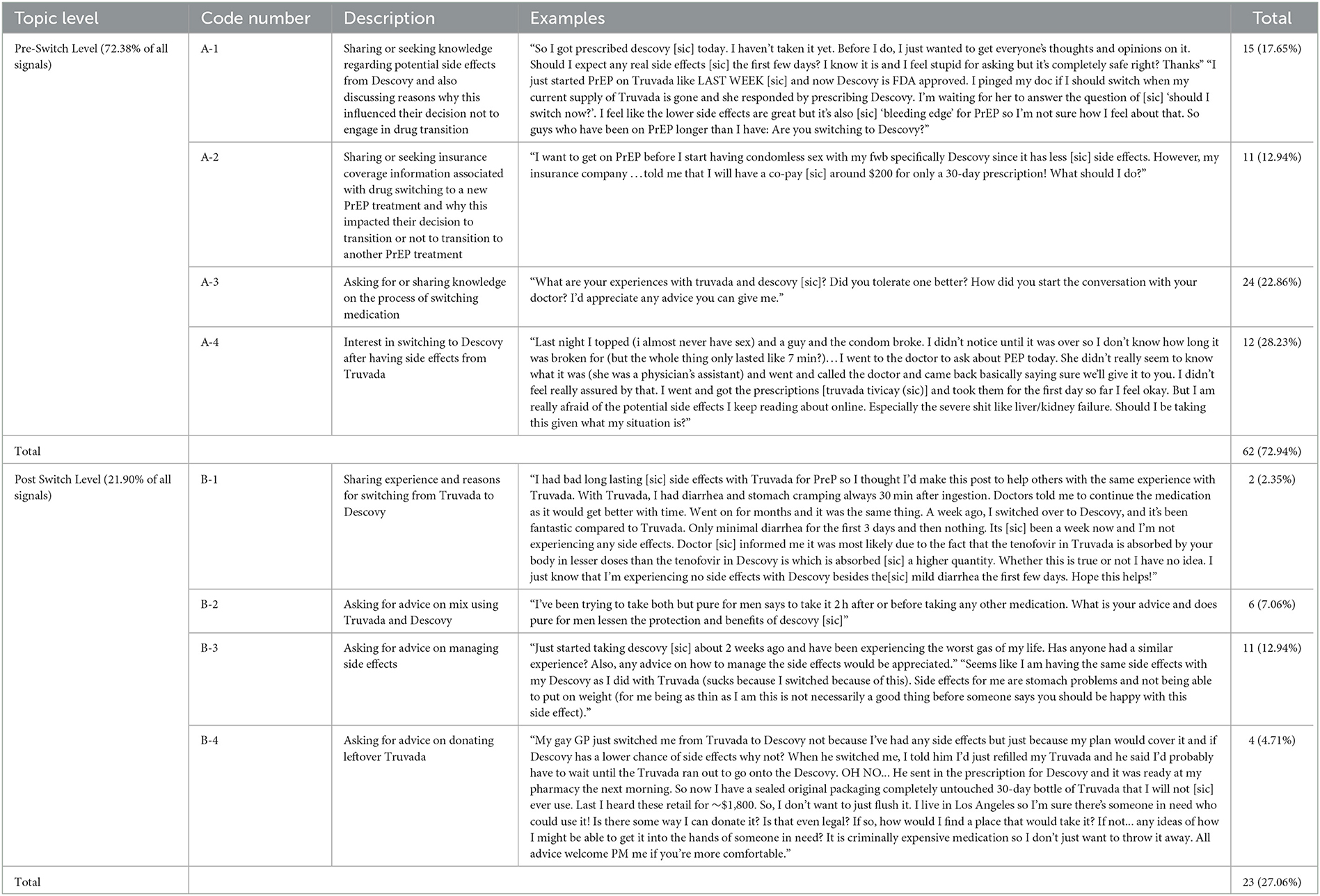
We collected a total of 2,349 unique Reddit posts over the study period. Descriptive analysis and manual annotation of all collected posts identified 85 (4.47% or the entire dataset) signal posts. Posts that did not meet our inclusion criteria for this study but were nevertheless relevant to PrEP facilitators and barriers included posts discussing differences between post-exposure prophylaxis (PEP) and PrEP therapy, confusion regarding PrEP therapy, experiences regarding healthcare providers' lack of PrEP knowledge and training, and comments believing that Descovy advertisements only targeted certain minority populations were excluded. Based on the publicly available text in Reddit posts, the largest representation from a minority group were for members associated with the sexual minority community (95.29%, n = 81), specifically gay and MSM. A summary of parent and sub-code book results and corresponding de-identified examples are reported in Table 1.
3.2. Data analysis
According to our data analysis, all signal posts were categorized into two main parent-level topics: (i) pre-transition: posts regarding users who currently take Truvada, users recognizing a new PrEP medication has been approved, and users sharing or seeking knowledge regarding the differences between Truvada and Descovy and (ii) post-transition: sharing the experience of switching from Truvada to Descovy, or asking questions associated with the transition experience. In the context of PrEP drug transition, all discussions detected concerned a pathway of transition or switching from Truvada (pre-transition) to Descovy (post-transition).
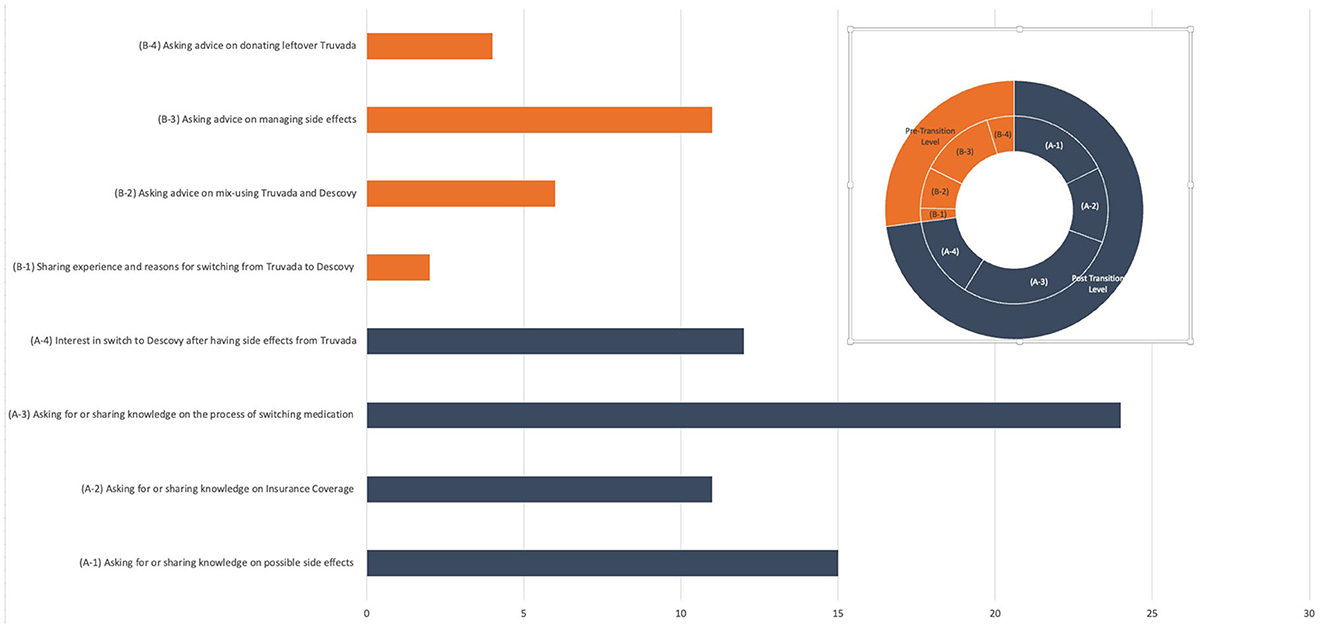
Among all signal posts, 62 posts (72.94% of total signal posts) were classified in the pre-transition parent topic (please see Figure 2 for the visual depiction of themes). We observed four main themes among these posts: conversations sharing or seeking knowledge on how to switch from Truvada to Descovy (A-3) that had the highest number and percentage of posts of all topics (n = 24, 22.86%); followed by topic (A-1) associated with users sharing or seeking knowledge on potential side effects from Descovy and also discussing reasons why this influenced their decision of not to engage in drug transition (n = 15, 17.65%); topic (A-4) included posts in which users expressed interest in switching to Descovy because of the purported side effects experienced from Truvada (n = 12, 28.23%); and topic (A-2) focused on sharing or seeking insurance coverage information associated with drug switching to a new PrEP treatment and how this impacted their decision to transition or not to transition to another PrEP treatment (n = 11,12.94%).
Among posts classified in post-transition parent topics (n = 23, 27.06%), we also observed four main themes. A major theme (B-3) that emerged was the discussion of side effect management after switching to Descovy (n = 11, 12.94%). Another topic (B-2) included discussions regarding mixing Truvada and Descovy medications (i.e., primarily focused on questions by users regarding how they should use (co-use) remaining Truvada medication after switching to and initiating Descovy) (n = 6, 7.07%). Topic (B-4) contained posts regarding users seeking information on how they can donate their remaining Truvada to other people who might benefit from prevention treatment access (n = 4, 4.71%). A final sub-topic (B-1) included general discussion of the experience of switching medications and why users switched to Descovy.
4. Discussion
Our study conducted a descriptive analysis of over 2,000 Reddit posts and found that just under 5% included specific discussions about transitions of use between Truvada and Descovy, with the vast majority of these posts originating from user-generated comments or posts related to SGM issues or from users self-identifying as members of the LGBTQ community, specifically gay and MSM populations. Key insights generated from these discussions include knowledge seeking and information sharing by users to help navigate decisions about transitioning to new PrEP therapy (pre-transition) and concerns that arose after switching from Truvada to Descovy (post-transition).
Our results add to insights generated by prior studies that have examined social media for clues regarding barriers and facilitators associated with PrEP, including specifically among gay and MSM populations. For example, a 2018 systematic review examined both peer-review studies and online posts to identify barriers and facilitators to PrEP among MSM, detecting six overarching categories that included online sources acting as facilitators to PrEP uptake and concerns about perceived side effects (20). A 2021 study specifically examined characteristics of PrEP-related content on Instagram for Truvada, including discussion by users about side effects (21). A recent 2022 study analyzed user perceptions and attitudes toward PrEP across multiple social media platforms (Twitter, YouTube, Instagram, Tumblr, and Reddit) and identified specific barriers to PrEP access including among minority online users (22). Finally, another 2022 study specifically examined conversations from Reddit users on PrEP from 2014 to 2019 and identified discussions about PrEP initiation and side effects as well as other themes (23).
Adding to these prior studies that examined social media for HIV and PrEP topics, this study was able to identify specific lived experiences regarding transitions of use for PrEP therapy, a topic subject to possible social stigma as it relates to HIV status, sexual orientation, and HIV-related risk behavior. However, due to Reddit's relative anonymity as an online platform and the ability to up and downvote content, it appears to be a platform where users may be more willing to discuss stigmatizing topics (26). Specifically, we observed that Reddit users actively shared their lived experiences with PrEP to aid in online peer-to-peer decision-making about the benefits and risks of PrEP therapy, including in the context of facilitating the transition of use or initiation of new HIV therapy. Importantly, users who may lack sufficient information to make informed decisions about PrEP therapy options may utilize these online communities as primary health information-seeking sources, even before or in the absence of consulting with a healthcare professional.
Furthermore, discussion of the impact of perceived side effects of different PrEP medications was prominent in the drug transition of use decisions, along with the discussion of access (e.g., insurance coverage) and cost. An additional consideration not specifically detected in this study but likely influencing the transition of use among users was the financial incentives associated with switching PrEP therapy. Descovy's regulatory approval for PrEP therapy occurred in 2019, just over 1 year prior to the generic availability of Truvada. At its introduction to the market, Descovy was offered with large commercial discounts which resulted in it launching at a 12% lower average net price than Truvada (27). These financial incentives led to changes in PrEP use. Specifically, within the first 9 months of it entering the market, nearly 30% of patients switched from Truvada to Descovy (28). In addition to the initial lower costs, patients may also prefer transitioning from Truvada to Descovy due to early reports that Descovy was less toxic and had fewer side effects, despite these claims since being refuted (10, 29). New themes that emerged from this study also included questions about the co-use of PrEP therapy and ways to donate unused medicines to other patients, unique in the context of examining behaviors associated with the transition of use for HIV/AIDS drugs.
Importantly, these findings provide insights that are important to post-market surveillance and pharmacovigilance efforts attempting to better understand perceptions of uptake, risk, and safety for PrEP among a community of predominantly gay and MSM users at higher risk for HIV. However, this study may not provide an extensive list of barriers and facilitators to accessing and adhering to PrEP therapy, as many people who contemplate HIV prevention or treatment may not openly discuss the unique complications they experience due to the stigmatization associated with this topic whether online or offline. Results can help inform how patients and specific gay and MSM populations in English-speaking countries experience and react to perceived adverse events and side effects and how it may impact the HIV care continuum of initiating, adhering to, and potentially transitioning between PrEP in preventing transmission (30). Importantly, the HIV burden remains high among African, Asian, and other non-English speaking countries. As such, most PrEP users were reported as residing in Africa in 2020 (31).
Hence, future studies should adopt methodologies used in this study for non-English language social media posts to better understand the unique barriers and facilitators in regions representing the largest HIV burden and greatest PrEP use, while also examining topics not explored in this study, such as the targeting of PrEP product advertising or outreach toward specific SGM populations. Future studies should also further validate results generated from social media with other sources of patient experiences (e.g., real-world data, prescribing and reimbursement information, and electronic health records) and integrate these novel data sources into a more proactive system of interdisciplinary pharmacovigilance to ensure better uptake and adherence to PrEP. In particular, the use of digital mixed methods that combine social listening approaches with in-depth qualitative data (e.g., focus groups) and robust quantitative data (e.g., surveys) designed specifically for at-risk SGM populations can better triangulate insights into PrEP barriers that can inform the development of future interventions and patient outreach.
5. Limitations
This study is primarily exploratory and descriptive in nature and has certain limitations. First, we only collected data from Reddit and limited our analysis to keywords and terms in the English language. Hence, the findings are not generalizable to all social media users who discuss transitioning between PrEP therapy, including those in other countries with higher HIV burdens but who are underrepresented in the population of Reddit users. Additionally, this study may represent an underestimate of people's experience with PrEP therapy and may not provide an extensive list of barriers to treatment due to the social stigma associated with HIV and discussing HIV-related care, including via online communities. Our collected and analyzed data sample size was also relatively small, which could lead to sampling bias. Hence, future studies should expand data collection and analysis approaches to different phrases and keywords associated with an individual's HIV-related risk behavior to obtain a more representative corpus of social media conversations and increase the sample size of data that can be analyzed. In addition, Reddit is a social media platform. There is a potential bias in using social media data including population biases, behavioral biases, content biases, and linking biases. Future studies should consider triangulating results from social listening approaches used in this study with other data sources (e.g., focus groups and survey instruments). Future studies should also expand data collection to additional platforms, languages, and specific keywords associated with HIV exposure and risk behavior to generate a more representative corpus of PrEP-related social media conversations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All information collected during this study was available in the public domain, did not include any deleted posts, and the study did not involve any interaction with users. Any user indefinable information was removed from study results and any racial and/or ethnic identifiers were presented as aggregated results to ensure anonymity. This study has been approved by WCG Institutional Review Board's (WCGIRB's). WCG IRB is registered with the Office for Human Research Protections (OHRP) and FDA as IRB00000533.
Author contributions
HG, QX, TJM, JL, and TKM jointly conceived the study, drafted the study, conducted data collection and analysis, and wrote and agreed to the final version of this manuscript.
Acknowledgments
The authors thank Cortni Bardier for the useful discussion, input, and assistance during the conduct of the study.
Conflict of interest
HG, QX, TJM, JL, and TKM were employees of the startup company S-3 Research LLC.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HIV, Human immunodeficiency virus; PrEP, Pre-exposure prophylaxis; FDA, The U.S. Food and Drug Administration; MSM, Men have sex with men; ART, Anti-retroviral therapy; TDF, Tenofovir disoproxil fumarate; TAF, Tenofovir alafenamide; AIDS, Acquired immunodeficiency syndrome; LGBTQ, Lesbian, gay, bisexual, transgender, queer; PEP, Post-exposure prophylaxis; SGM, Sexual and gender minority.
References
1. Global HIV & AIDS statistics-−2020 fact sheet. UNAIDS. Available online at: https://www.unaids.org/en/resources/fact-sheet (accessed March 5, 2021).
2. Sullivan PS, Giler RM, Mouhanna F, Pembleton ES, Guest JL, Jones J, et al. Trends in the use of oral emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against HIV infection, United States, 2012–2017. Ann Epidemiol. (2018) 28:833–40. doi: 10.1016/j.annepidem.2018.06.009
3. Hoen E't, Berger J, Calmy A, Moon S. Driving a decade of change: HIV/AIDS, patents and access to medicines for all. J Int Aids Soc. (2011) 14:15. doi: 10.1186/1758-2652-14-15
4. Pre-Exposure Prophylaxis (PrEP). HIV Risk and Prevention. HIV/AIDS. CDC. Available online at: https://www.cdc.gov/hiv/risk/prep/index.html (accessed May 10, 2023).
5. Schoenberg P, Edwards OW, Merrill L, Martinez CA, Stephenson R, Sullivan PS, et al. Willingness to use and preferences for long-acting injectable PrEP among sexual and gender minority populations in the southern United States, 2021–2022: cross-sectional study. J Int Aids Soc. (2023) 26:e26077. doi: 10.1002/jia2.26077
6. de Aguiar Pereira CC, Torres TS, Luz PM, Hoagland B, Farias A, Brito JDU, et al. Preferences for pre-exposure prophylaxis (PrEP) among sexual and gender minorities: a discrete choice experiment in Brazil. Lancet Reg Health Am. (2023) 19:100432. doi: 10.1016/j.lana.2023.100432
7. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New Engl J Med. (2010) 363:2587–99. doi: 10.1056/NEJMoa1011205
8. Truvada Medication Information Sheet for Patients. Available online at: https://www.cdc.gov/hiv/pdf/PrEP_GL_Patient_Factsheet_Truvada_English.pdf (accessed May 24, 2022).
9. Blackwell CW, Castillo HL. Human immunodeficiency virus pre-exposure prophylaxis: use of emtricitabine/tenofovir alafenamide. J Nurse Pract. (2021) 17:673–6. doi: 10.1016/j.nurpra.2021.01.002
10. Mayer KH, Molina J-M, Thompson MA, Anderson PL, Mounzer KC, Wet JJD, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. (2020) 396:239–54. doi: 10.1016/S0140-6736(20)31065-5
11. D'Angelo AB, Westmoreland DA, Carneiro PB, Johnson J, Grov C. Why are patients switching from tenofovir disoproxil fumarate/emtricitabine (truvada) to tenofovir alafenamide/emtricitabine (descovy) for pre-exposure prophylaxis? AIDS Patient Care STDS. (2021) 35:327–34. doi: 10.1089/apc.2021.0033
12. Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad. (2018) 4:72–9. doi: 10.1016/S2055-6640(20)30248-X
13. Kay ES, Pinto RM. Is insurance a barrier to HIV preexposure prophylaxis? clarifying the issue. Am J Public Health. (2019) 110:e1–4. doi: 10.2105/AJPH.2019.305389
14. Kimball D, Rivera D, Gonzales M, Blashill AJ. Medical mistrust and the PrEP cascade among latino sexual minority men. AIDS Behav. (2020) 24:3456–61. doi: 10.1007/s10461-020-02916-z
15. D'Avanzo PA, Bass SB, Brajuha J, Gutierrez-Mock L, Ventriglia N, Wellington C, et al. Medical mistrust and PrEP perceptions among transgender women: a cluster analysis. Behav Med. (2019) 45:143–52. doi: 10.1080/08964289.2019.1585325
16. Wiginton JM, Eaton LA, Watson RJ, Maksut JL, Earnshaw VA, Berman M. Sex-Positivity, medical mistrust, and PrEP conspiracy beliefs among HIV-negative cisgender black sexual minority men in Atlanta, Georgia. Arch Sex Behav. (2022) 51:2571–81. doi: 10.1007/s10508-021-02174-7
17. Jaiswal J, Halkitis PN. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behav Med. (2019) 45:79–85. doi: 10.1080/08964289.2019.1619511
18. Teng F, Sha Y, Fletcher LM, Welsch M, Burns P, Tang W. Barriers to uptake of PrEP across the continuum among transgender women: a global scoping review. Int J STD AIDS. (2023) 34:299–314. doi: 10.1177/09564624231152781
19. Tekeste M, Hull S, Dovidio JF, Safon CB, Blackstock O, Taggart T, et al. Differences in medical mistrust between black and white women: implications for patient–provider communication about PrEP. AIDS Behav. (2019) 23:1737–48. doi: 10.1007/s10461-018-2283-2
20. Hannaford A, Lipshie-Williams M, Starrels JL, Arnsten JH, Rizzuto J, Cohen P, et al. The use of online posts to identify barriers to and facilitators of HIV pre-exposure prophylaxis (PrEP) among men who have sex with men: a comparison to a systematic review of the peer-reviewed literature. AIDS Behav. (2018) 22:1080–95. doi: 10.1007/s10461-017-2011-3
21. Walsh-Buhi E, Houghton RF, Lange C, Hockensmith R, Ferrand J, Martinez L. Pre-exposure prophylaxis (PrEP) information on instagram: content analysis. JMIR Public Health Surveill. (2021) 7:e23876. doi: 10.2196/23876
22. Xu Q, Nali MC, McMann T, Godinez H, Li J, He Y, et al. Unsupervised machine learning to detect and characterize barriers to pre-exposure prophylaxis therapy: a multiplatform social media study. JMIR Infodemiology. (2022) 2:e35446. doi: 10.2196/35446
23. Loosier PS, Renfro K, Carry M, Williams SP, Hogben M, Aral S. Reddit on PrEP: posts about pre-exposure prophylaxis for HIV from Reddit users, 2014–2019. AIDS Behav. (2022) 26:1084–94. doi: 10.1007/s10461-021-03463-x
24. Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. (2006) 27:237–46. doi: 10.1177/1098214005283748
25. Terminology. DASH CDC. Available online at: https://www.cdc.gov/healthyyouth/terminology/sexual-and-gender-identity-terms.htm (accessed May 10, 2023).
26. Amaya A, Bach R, Keusch F, Kreuter F. New data sources in social science research: things to know before working with Reddit data. Soc Sci Comput Rev. (2021) 39:943–60. doi: 10.1177/0894439319893305
27. Dickson S, Gabriel N, Hernandez I. Estimated changes in price discounts for tenofovir-inclusive HIV treatments following introduction of tenofovir alafenamide. AIDS. (2022) 36:2225–7. doi: 10.1097/QAD.0000000000003401
28. Hoover KW, Zhu W, Wiener J, Huang Y-LA. Trends in Truvada and Descovy Prescriptions for PrEP in the United States, 2014–2020. Available online at: https://natap.org/2021/CROI/croi_201.htm (accessed December 15, 2022).
29. Gupta SK, Post FA, Arribas JR, Eron JJ, Wohl DA, Clarke AE, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. (2019) 33:1455–65. doi: 10.1097/QAD.0000000000002223
30. HIV. Available online at: https://www.who.int/data/gho/data/themes/hiv-aids (accessed December 15, 2022).
31. Global State of PrEP. Available online at: https://www.who.int/groups/global-prep-network/global-state-of-prep (accessed December 15, 2022).
Keywords: PrEP, HIV/AIDS, drug switching, pharmacovigilance, infoveillance, infodemiology, minority health, social media
Citation: Godinez H, Xu Q, McMann TJ, Li J and Mackey TK (2023) Analysis of online user discussions on Reddit associated with the transition of use between HIV PrEP therapy. Front. Public Health 11:1073813. doi: 10.3389/fpubh.2023.1073813
Received: 18 October 2022; Accepted: 05 June 2023;
Published: 28 June 2023.
Edited by:
Mohammad Asif Sherwani, Independent Researcher, Birmingham, AL, United StatesReviewed by:
Suhail Muzaffar, University of Alabama at Birmingham, United StatesArtur Queiroz, Northwestern Medicine, United States
Copyright © 2023 Godinez, Xu, McMann, Li and Mackey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy Ken Mackey, tkmackey@ucsd.edu
 Hector Godinez1
Hector Godinez1