- 1Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, National Center for Respiratory Medicine, China-Japan Friendship Hospital, Beijing, China
- 2Capital Medical University, China-Japan Friendship School of Clinical Medicine, Beijing, China
- 3Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 4Department of Laboratory Medicine, China-Japan Friendship Hospital, Beijing, China
Background: Immunocompromised patients with severe community-acquired pneumonia (SCAP) warrant special attention because they comprise a growing proportion of patients and tend to have poor clinical outcomes. The objective of this study was to compare the characteristics and outcomes of immunocompromised and immunocompetent patients with SCAP, and to investigate the risk factors for mortality in these patients.
Methods: We conducted retrospective observational cohort study of patients aged ≥18 years admitted to the intensive care unit (ICU) of an academic tertiary hospital with SCAP between January 2017 and December 2019 and compared the clinical characteristics and outcomes of immunocompromised and immunocompetent patients.
Results: Among the 393 patients, 119 (30.3%) were immunocompromised. Corticosteroid (51.2%) and immunosuppressive drug (23.5%) therapies were the most common causes. Compared to immunocompetent patients, immunocompromised patients had a higher frequency of polymicrobial infection (56.6 vs. 27.5%, P < 0.001), early mortality (within 7 days) (26.1 vs. 13.1%, P = 0.002), and ICU mortality (49.6 vs. 37.6%, P = 0.027). The pathogen distributions differed between immunocompromised and immunocompetent patients. Among immunocompromised patients, Pneumocystis jirovecii and cytomegalovirus were the most common pathogens. Immunocompromised status (OR: 2.043, 95% CI: 1.114–3.748, P = 0.021) was an independent risk factor for ICU mortality. Independent risk factors for ICU mortality in immunocompromised patients included age ≥ 65 years (odds ratio [OR]: 9.098, 95% confidence interval [CI]: 1.472–56.234, P = 0.018), SOFA score [OR: 1.338, 95% CI: 1.048–1.708, P = 0.019), lymphocyte count < 0.8 × 109/L (OR: 6.640, 95% CI: 1.463–30.141, P = 0.014), D-dimer level (OR: 1.160, 95% CI: 1.013–1.329, P = 0.032), FiO2 > 0.7 (OR: 10.228, 95% CI: 1.992–52.531, P = 0.005), and lactate level (OR: 4.849, 95% CI: 1.701–13.825, P = 0.003).
Conclusions: Immunocompromised patients with SCAP have distinct clinical characteristics and risk factors that should be considered in their clinical evaluation and management.
1. Introduction
Severe community-acquired pneumonia (SCAP) is a common disease in the intensive care unit (ICU), with high mortality rates ranging from 25 to > 50% (1–3). Immunocompromised patients with SCAP warrant special consideration because this population has been growing progressively over recent decades, and an estimated 18–36% of patients with community-acquired pneumonia (CAP) are immunocompromised (4, 5). Among patients with pneumonia, immunocompromised patients experience more severe complications that progress to severe pneumonia (6, 7) and worse outcomes (5).
The clinical characteristics and outcomes of SCAP in immunocompetent patients are well-documented, but few studies have reported on immunocompromised patients with SCAP in intensive care unit (ICU) settings. Most studies of immunocompromised patients with community-acquired pneumonia (CAP) are small, descriptive, and restricted to specific pathogens (such as influenza or bacterial pneumonia) (6, 8), or specific subtypes of immunocompromised conditions (such as human immunodeficiency virus [HIV] or cancer) (9, 10), which limit the generalizability of the conclusions. Delay in initiation of appropriate empiric antibiotic therapy is a known risk factor for worse clinical outcomes, and the etiology of SCAP in immunocompromised patients exhibits different epidemiology, with a greater probability of co-infections; therefore, identifying the causes of SCAP among immunocompromised patients is important. Moreover, immunocompromised patients have mostly been excluded in the published CAP guidelines (11–13) and the characteristics and outcomes of these patients with SCAP are not yet fully understood.
This study aimed to compare the characteristics and outcomes of immunocompromised and immunocompetent patients with SCAP and investigated the risk factors for mortality in immunocompromised patients.
2. Methods
2.1. Design and population
This single-center, retrospective cohort study was conducted in patients with SCAP, aged ≥ 18 years who were admitted to the ICU of a tertiary academic hospital from January 2017 to December 2019. Patients were excluded if they (1) were suspected of having hospital-acquired pneumonia (14); (2) permanently resided in a nursing home; or if (3) the initial diagnosis of SCAP was not confirmed during their ICU stay.
This study was approved by the institutional review board of the China-Japan Friendship Hospital (2019-80-K52) and was performed in accordance with the Helsinki Declaration. The requirement for informed consent was waived because this non-interventional study collected data from previous electronic medical records and did not involve personal privacy or commercial interests.
2.2. Study definitions
SCAP was defined as meeting either one major criterion or at least three minor criteria of the Infectious Diseases Society of America/American Thoracic Society criteria (11). Immunosuppression was defined based on consensus, determined by meeting one of the following criteria (15): primary immune deficiency disease; active malignancy; receiving cancer chemotherapy; HIV infection with CD4 T-lymphocyte count < 200 cells/μL or percentage < 14%; solid organ transplantation; hematopoietic stem cell transplantation; receiving corticosteroid therapy with a prednisone dose of 20 mg or equivalent daily for ≥14 days or a cumulative dose >700 mg; receiving biologic immune modulators; or receiving disease-modifying anti-rheumatic or other immunosuppressive drugs.
Microbiological tests were performed within 48 h of ICU admission in all patients, using bronchoalveolar lavage fluid (BALF) or endotracheal aspirates. Microbiological tests included bacterial and fungal smear and culture, acid-fast stain, and real-time polymerase chain reaction for cytomegalovirus (CMV), Pneumocystis jirovecii (PJ), influenza virus, respiratory syncytial virus, adenovirus, Legionella pneumophila, Chlamydia pneumoniae, and Mycoplasma pneumoniae. Metagenomic next-generation sequencing (mNGS) was performed, if necessary, at the clinician's discretion (more details see Additional file 1).
Pathogens were identified by clinicians based on microbiological tests, clinical manifestations, and chest radiology findings. Atypical pathogens included Legionella, Mycoplasma, and Chlamydia (16). Polymicrobial infection was defined as having more than one type of pathogen diagnosed by clinicians within 48 h of ICU admission.
2.3. Data collection and outcomes
Clinical data, including demographics, comorbidities, season of onset, vital signs, fraction of inspired oxygen (FiO2), respiratory support, arterial blood gas analyses, Acute Physiology and Chronic Health Evaluation (APACHE) II scores, Sequential Organ Failure Assessment (SOFA) scores, laboratory results on ICU admission, pathogens detected within 48 h of ICU admission, and clinical outcomes were extracted from the electronic medical record system. The clinical outcomes included the requirement for invasive mechanical ventilation (IMV), IMV duration, length of ICU stay, mortality within 7 days, and ICU mortality.
2.4. Statistical analyses
Continuous variables were expressed as means and standard deviations or medians and interquartile ranges. Categorical variables were presented as frequencies and percentages. Differences between characteristics, testing results, pathogens detected, treatments, and outcomes of immunocompetent and immunocompromised patients were tested for statistical significance using t-tests for relatively symmetrically distributed continuous variables, Wilcoxon rank-sum test for asymmetrically distributed continuous variables, and Pearson's chi-square test for categorical variables. Two-tailed P < 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS software, version 22 (IBM Corp., Armonk, NY, United States).
To assess for potential confounders, clinically important variables such as age, lymphocyte count, and CD4 cell count that demonstrated possible statistical associations in the univariate analysis (P < 0.1) were transformed from continuous variables into categorical variables according to clinical reference values and standards.
To identify the risk factors of ICU mortality, variables with P < 0.1 in the univariate analyses were entered into a multivariable logistic regression model, using stepwise backward selection based on the likelihood ratio. Variable entry was set at P < 0.05, and variable removal at P > 0.1.
3. Results
3.1. Study population
Of the 457 patients with SCAP who were screened, 64 were excluded based on the exclusion criteria, leaving a total of 393 patients in the analysis, including 274 (69.7%) immunocompetent and 119 (30.3%) immunocompromised patients. The three most common immunocompromising conditions were corticosteroid therapy (61/119, 51.2%), immunosuppressive therapy (28/119, 23.5%), and active malignancy (16/119, 13.4%) (Supplementary Table S1).
3.2. Clinical characteristics and outcomes
Compared to immunocompetent patients, immunocompromised patients included significantly more females (45.4 vs. 30.3%, P = 0.004) and patients with chronic lung disease (31.9 vs. 20.8%, P = 0.018) (Table 1). The season of disease onset differed significantly (P = 0.009) according to immunocompromised status and was mainly in the spring and autumn (49.6%) among immunocompromised patients and the winter among immunocompetent patients (42.3%). The white blood cell (9.1 vs. 10.2, P = 0.030), neutrophil (7.9 vs. 8.9, P = 0.005), lymphocyte (0.5 vs. 0.7, P = 0.044), platelet (150.0 vs. 175.0, P = 0.038), and CD4 T cell (201.5 vs. 286.0, P = 0.001) counts; and hemoglobin (104.0 ± 21.5 vs. 115.6 ± 25.0, P < 0.001), albumin (29.0 vs. 31.0, P < 0.001), and procalcitonin (0.8 vs. 1.2, P = 0.020) levels on ICU admission were significantly lower in immunocompromised patients than in immunocompetent patients (Supplementary Table S2). Other baseline laboratory results, SOFA scores, and APACHE II scores were similar between patients in the two groups (Table 1). The mean time from SCAP onset to ICU admission in immunocompetent and immunocompromised patients was 8.0 and 7.0 days, respectively (P = 0.617).
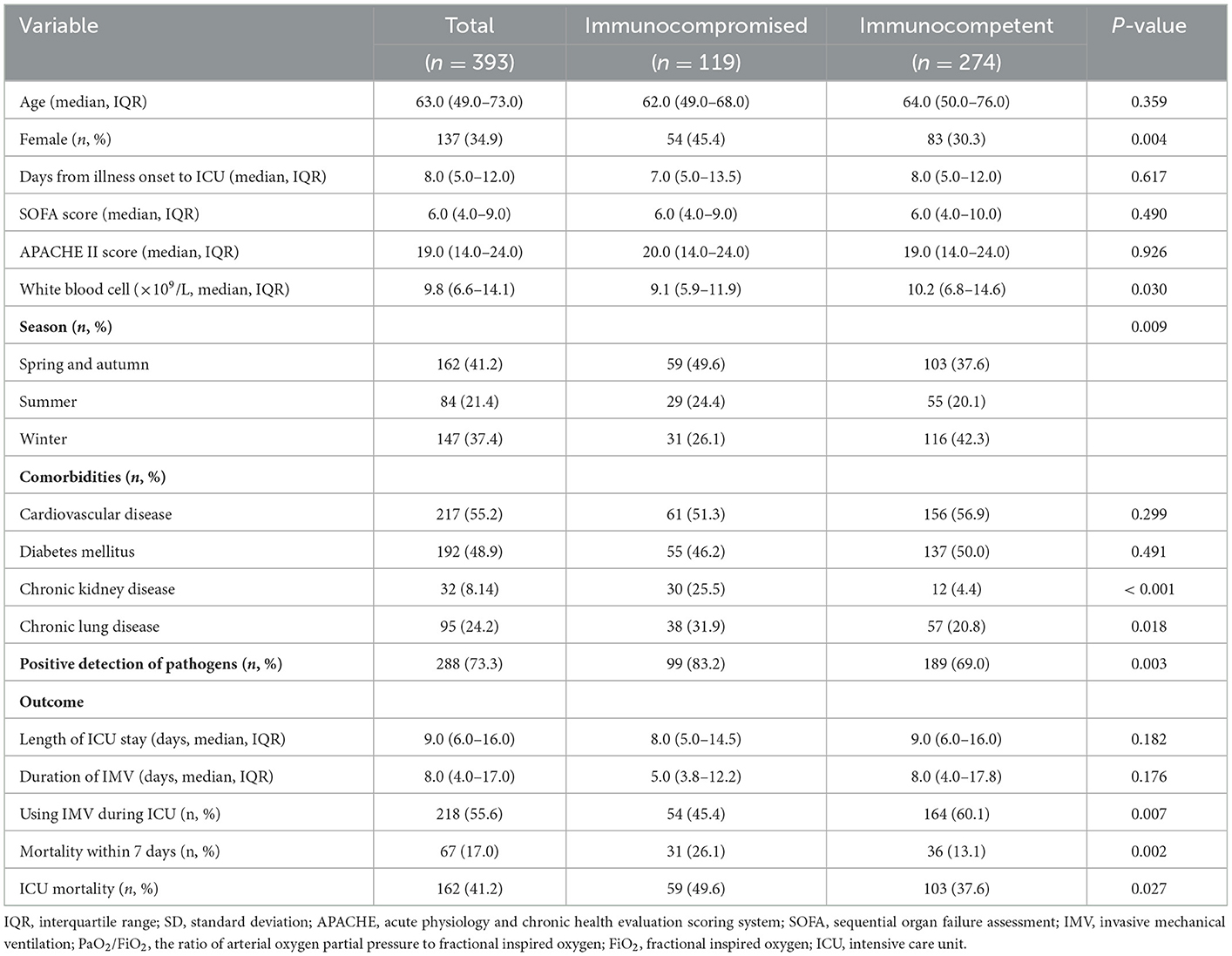
Table 1. The clinical characteristics and outcomes of patients with severe community-acquired pneumonia.
The use of IMV during ICU stay was less frequent in immunocompromised patients (54/119, 45.4%) than in immunocompetent patients (164/274, 60.1%) (P = 0.003); however, the duration of IMV (5.0 vs. 8.0 days; P = 0.176) and ICU stay (8.0 vs. 9.0 days; P = 0.182) were similar between the two groups (Table 1). The rates of mortality within 7 days (26.1 vs. 13.1%, P = 0.002) and ICU mortality (49.6 vs. 37.6%, P = 0.027) were higher in immunocompromised patients (Table 1).
3.3. Pathogens in patients with SCAP
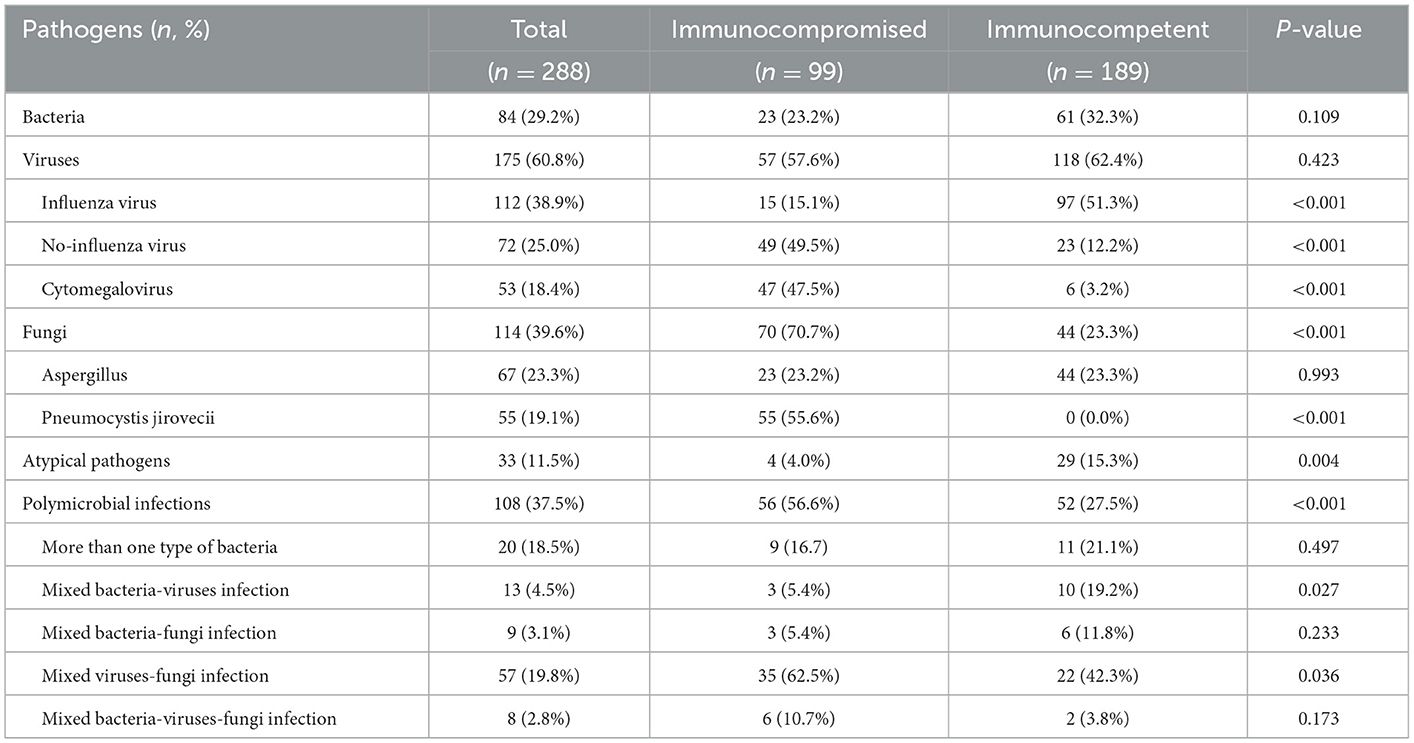
Pathogens were identified in 83.2% (99/119) of immunocompromised patients and 69.0% (189/274) of immunocompetent patients (P = 0.003) (Table 1). Immunocompromised patients had fewer atypical pathogen infections than immunocompetent patients (4.0 vs. 15.3%, P = 0.004) (Table 2). Influenza virus infection was more prevalent in immunocompetent patients than in immunocompromised patients (51.3 vs. 15.1%, P < 0.001). However, immunocompromised patients had a higher prevalence of infection caused by viruses other than influenza virus (49.5 vs. 12.2%, P < 0.001) and cytomegalovirus (47.5 vs. 3.2%, P < 0.001) than immunocompetent patients (Table 2). Fungal infections were more prevalent in immunocompromised patients (70.7 vs. 23.3%, P < 0.001) (Table 2). Immunocompromised patients had a higher frequency of polymicrobial infections (56.6 vs. 27.5%, P < 0.001), especially mixed viral-fungal infections (62.5 vs. 42.3%, P = 0.036) (Table 2).
The details of the pathogens identified are shown in Figure 1 and Supplementary Table S3. The five most frequent pathogens identified in immunocompromised patients were PJ (55/99, 56%), CMV (47/99, 47%), Aspergillus (23/99, 23%), Staphylococcus aureus (9/99, 9%), and influenza A virus (9/99, 9%) (Figure 1). Influenza A virus was the most common pathogen (82/189, 43%) in immunocompetent patients. PJ, nontuberculous mycobacteria (2/99, 2%), and Nocardia spp. (2/99, 2%) were detected only in immunocompromised patients. Adenovirus (10/189) and Streptococcus pneumoniae (9/189) were detected only in immunocompetent patients (Supplementary Table S3).
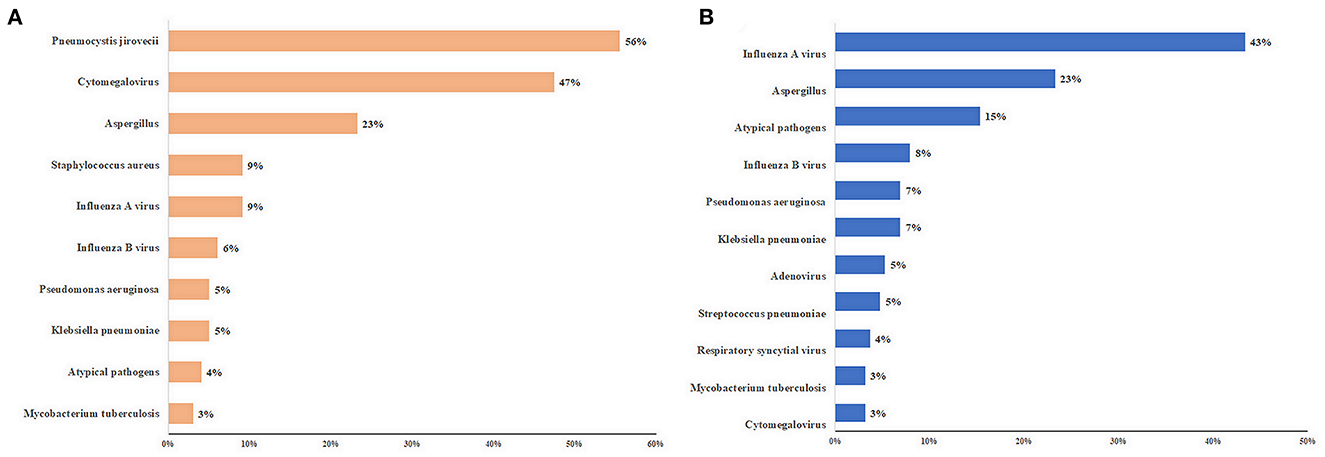
Figure 1. Top 10 pathogens of patients with severe community-acquired pneumonia. (A) immunocompromised patients; (B) immunocompetent patients. The length of the colored bars and numbers behind them indicate the proportion of each pathogen, calculated using their positive number as the numerator and the number of patients with positive pathogen results as the denominator.
3.4. Risk factors for ICU mortality
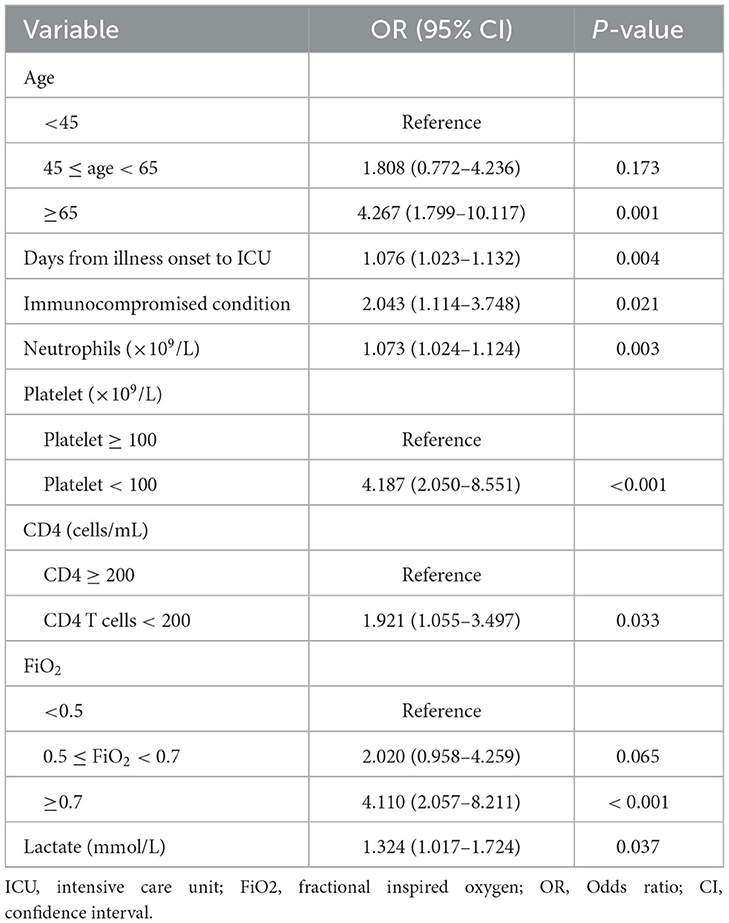
Among immunocompromised patients, the univariate analysis showed that 11 variables were significantly associated with ICU mortality, including age, SOFA score, lymphocyte count, platelet count, D-dimer level, fibrinogen level, PaO2/FiO2, FiO2 group, lactate level, CD4 cell count, and chronic kidney disease (Table 3, Supplementary Table S4). In addition to these variables, the APACHE II score, neutrophil count, and IMV on ICU admission were included in the multivariable logistic regression model (Supplementary Table S4). The final multivariable logistic regression model found that age ≥65 years, SOFA score, lymphocyte count < 0.8 × 109/L, D-dimer level, 0.5 ≤ FiO2 < 0.7, FiO2 > 0.7, and serum lactate level (were independent risk factors for ICU mortality in immunocompromised patients (Table 4).
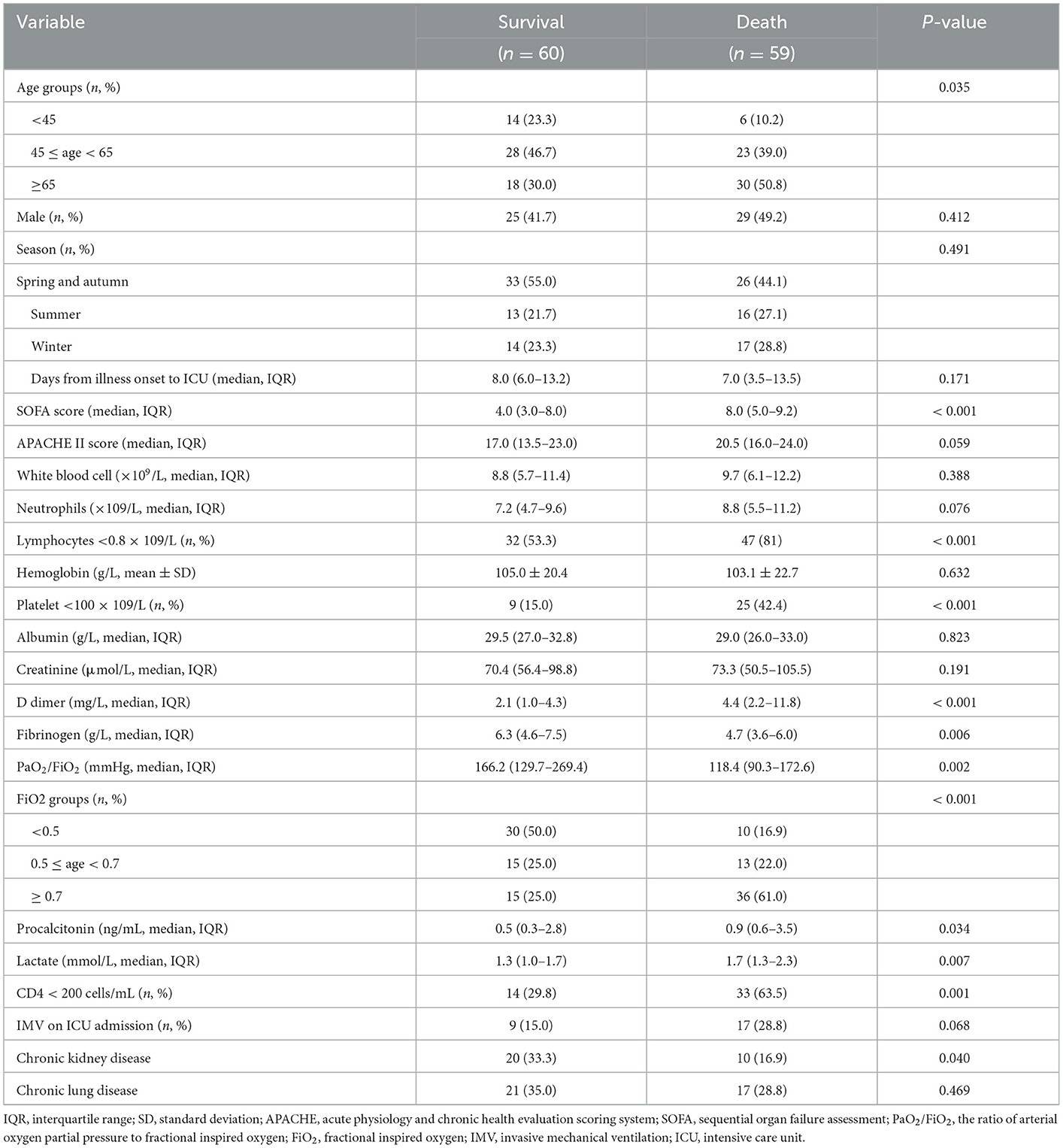
Table 3. Comparison of clinical characteristics between survival and death immunocompromised patients with severe community-acquired pneumonia.
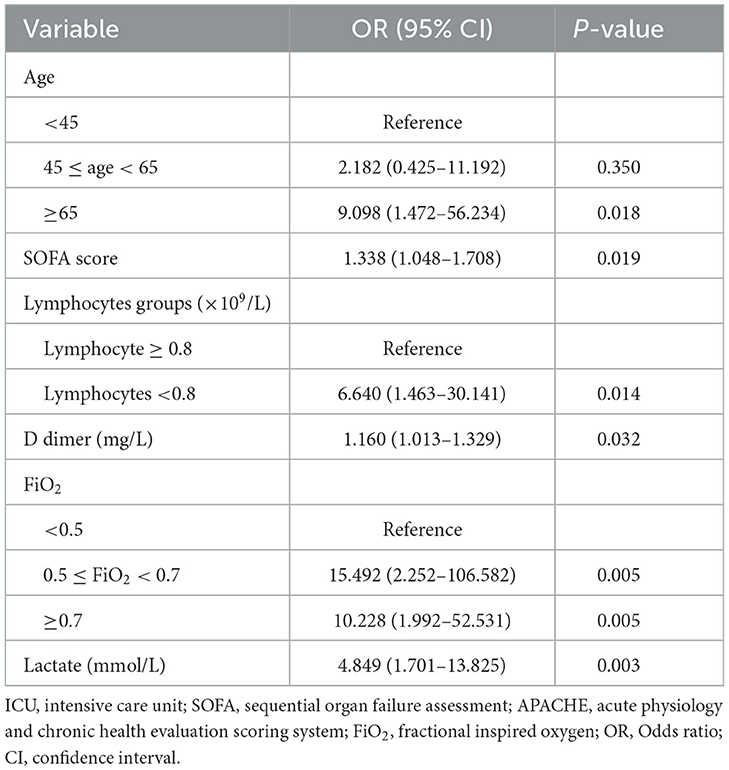
Table 4. Risk factors for ICU mortality in immunocompromised patients with severe community-acquired pneumonia.
The univariate analysis of ICU mortality in immunocompetent patients revealed 13 variables associated with an increased risk of ICU mortality (Supplementary Table S5). In the multivariable analysis, only 3 factors were independent risk factors for ICU mortality in immunocompetent patients (Supplementary Table S6): days from illness onset to ICU admission (OR: 1.101, 95% CI: 1.039–1.166, P = 0.001), platelet count < 100 × 109/L (OR: 2.729, 95% CI: 1.191–6.256, P = 0.018), and FiO2 > 0.7 (OR: 3.372, 95% CI: 1.433–7.932; P = 0.005). Only FiO2 > 0.7 was also a risk factor for ICU mortality in immunocompromised patients (OR: 3.372 vs. 10.228 in immunocompetent and immunocompromised patients, respectively).
In the multivariable analysis including all patients, immunocompromised status (OR: 2.043, 95% CI: 1.114–3.748, P = 0.021) age ≥65 years, days from illness onset to ICU admission, neutrophil count, platelet count < 100 × 109/L, CD4 T cell count < 200 cells/μL, FiO2 > 0.7, and lactate level were independent risk factors for ICU mortality (Table 5). Among them, three risk factors were the same as those for immunocompromised patients: age ≥ 65 years (OR: 4.267 vs. 9.098), FiO2 > 0.7 (OR: 4.110 vs. 10.228), and serum lactate level (OR: 1.324 vs. 4.849).
4. Discussion
This study found that, among patients with SCAP in an ICU, immunocompromised patients had worse clinical outcomes than immunocompetent patients and a large variation in clinical characteristics and pathogen distribution. Additionally, we identified the risk factors of ICU mortality among immunocompromised and immunocompetent patients, which are important for early risk evaluation and may help in developing treatment regimens for these patients. To our knowledge, this study is the first to compare risk factors for mortality among immunocompetent and immunocompromised patients with SCAP.
In this cohort, immunocompromised patients accounted for 30.3% of all patients with SCAP, which is consistent with previous reports (3–5, 17). Although up to one-third of patients with CAP admitted to hospitals worldwide are immunosuppressed, few studies have been conducted among immunocompromised patients, especially those with chronic steroid use and immunosuppressive therapy, and few guidelines are available for managing SCAP in immunocompromised patients. In this study, the two most common immunocompromising conditions were corticosteroid therapy and immunosuppressive therapy. We used a broad definition of immunocompromised status so that our results have broad applicability.
Lower lymphocyte counts, hemoglobin levels, albumin levels, and higher incidence of chronic kidney disease were found in immunocompromised patients, which is consistent with a previous study (18). Compared to immunocompetent patients, immunocompromised patients were more likely to be female, have chronic lung disease, and have lower white blood cell, neutrophil, and platelet counts and procalcitonin levels, which differs from the findings of previous studies (5, 19).
In this study, pathogens were detected in 83.2% of immunocompromised and 69.0% of immunocompetent patients, with more polymicrobial infections in immunocompromised patients than in immunocompetent patients. Our results revealed PJ, CMV, and Aspergillus were the three most common pathogens in immunocompromised patients. Influenza A virus was the most common pathogen in immunocompetent patients. Over the past decade, Streptococcus pneumoniae, respiratory viruses, Haemophilus influenzae, Staphylococcus aureus, and atypical organisms have been frequently identified in patients with SCAP (20). However, few studies have assessed the pathogens among immunocompromised patients with CAP. Bacterial pneumonia accounts for one-third of cases of acute respiratory failure among immunocompromised patients (8, 17). Sousa et al. (5) reported that Streptococcus pneumoniae was the most frequent pathogen identified (65.6%), with no differences between immunocompromised and non-immunocompromised patients. In immunocompromised patients with CAP, the most common respiratory pathogens, in addition to the common pathogens of non-immunocompromised patients, include CMV, PJ, Enterobacteriaceae, and Mycobacterium spp. (21). In this study, a higher proportion of patients had a pathogen identified, and the pathogen distribution differed from those identified in previous studies. These differences may be explained by the choice of microbiological tests and the study population. First, in this study, we employed not only conventional microbiological tests but also mNGS, which has higher sensitivity (22, 23). A previous study found that mNGS may be a useful technique for detecting mixed pathogens in immunocompromised patients with SCAP (24). Second, all included patients completed at least one round of comprehensive microbiological tests within 48 h of ICU admission. Patients were routinely tested for PJ and CMV using BALF. BALF is useful for detecting pathogens in immunocompromised patients with SCAP; the more immunocompromised the patient, the greater the potential benefit (21). Moreover, we used a new, broader definition of immunocompromised status in our study (21), which included a low CD4 count. This could also explain the different pathogen distribution reported in the present study because different immunocompromising conditions may be associated with different pathogen susceptibility (4, 8, 18, 21). Another possible explanation of why our results differed from those of previous studies is that the median time from illness onset to ICU admission was 8 days, and most patients had prior antibiotic exposure before ICU admission, which might have resulted in a lower bacteria detection rate.
Immunocompromising conditions are reportedly associated with mortality in patients with influenza (6, 7), CAP (5, 19), and community-acquired respiratory virus infections (18). Our results are consistent with those of previous studies showing that immunocompromised patients with SCAP had higher early mortality (within 7 days) and ICU mortality. Moreover, immunocompromising conditions and CD4 count < 200 cells/μL were the two independent risk factors for ICU mortality in patients with SCAP, which can add important evidence to previous information.
The multivariable logistic regression analysis of ICU mortality in immunocompromised and immunocompetent patients revealed significantly different risk factors between the two groups; FiO2 > 0.7 was the only common risk factor. Clinicians should consider these different risk factors for early diagnosis and management of SCAP. Few studies have examined the risk factors for poor outcomes in immunosuppressed patients with CAP or severe pneumonia, and previous studies have had inconsistent results (6, 10, 25, 26). Some independent risk factors for ICU mortality in immunocompromised patients with SCAP, such as age, SOFA score, FiO2 > 0.7, and D-dimer and lactate levels, have been reported previously (26–29). Lymphocyte count < 0.8 × 109/L was also a risk factor for these patients. Chen et al. (6) found that baseline lymphocyte counts of 0.6 × 109/L were independently associated with 30-day mortality in immunocompromised patients with influenza-related pneumonia. Lymphocytes are closely related to the immune function in pneumonia and can be recruited to the respiratory tract and reduced in circulation. Guo et al. (30) reported that lymphocyte count ≤ 0.8 × 109/L is one of the predictors of mortality in patients with viral pneumonia. Moreover, PJ and CMV are the most common pathogens in immunocompromised patients. Previous studies have identified lymphocyte count as a risk factor that can influence the mortality of PJ (31) and viral pneumonia (19).
Our study has several limitations. First, it was a small, single-center study and thus susceptible to selection bias. Half of the immunocompromised patients received corticosteroid therapy, and so the immunocompromised patients may not be representative of all potentially eligible immunocompromised patients. Second, given its retrospective design, pathogen tests and diagnoses were conducted based on the clinician judgment and ICU routine, without standardized criteria. Moreover, most patients had prior antibiotic exposure before ICU admission, which might have resulted in the low detection rate of bacteria. Finally, our results are limited to patients with SCAP that necessitated ICU admission and may not be applicable to all patients with SCAP.
5. Conclusion
Compared to immunocompetent patients with SCAP, immunocompromised patients with SCAP have distinct clinical characteristics and pathogen distributions. Immunocompromised status is an independent risk factor for ICU mortality in patients with SCAP. These findings can be used to guide clinicians in evaluating and managing immunocompromised patients with SCAP in the ICU setting.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by China-Japan Friendship Hospital (2019-80-K52). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
QZ provided the study concept, design, and contributed to subsequent versions. XW, TS, YC, TZ, YL, SG, and YZ collected the data. YL and TS performed the statistical analyses. TS and XW drafted the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81870072), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2022-I2M-JB-016), and the National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1070581/full#supplementary-material
References
1. Haessler S, Guo N, Deshpande A, Zilberberg MD, Lagu T, Lindenauer PK, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. (2022) 50:1063–71. doi: 10.1097/CCM.0000000000005498
2. Nair GB, Niederman MS. Updates on community acquired pneumonia management in the ICU. Pharmacol Ther. (2021) 217:107663. doi: 10.1016/j.pharmthera.2020.107663
3. Torres A, Chalmers JD, Dela Cruz CS, Dominedo C, Kollef M, Martin-Loeches I, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. (2019) 45:159–71. doi: 10.1007/s00134-019-05519-y
4. Di Pasquale MF, Sotgiu G, Gramegna A, Radovanovic D, Terraneo S, Reyes LF, et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis. (2019) 68:1482–93. doi: 10.1093/cid/ciy723
5. Sousa D, Justo I, Dominguez A, Manzur A, Izquierdo C, Ruiz L, et al. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin Microbiol Infect. (2013) 19:187–92. doi: 10.1111/j.1469-0691.2012.03765.x
6. Chen L, Han X, Li Y, Zhang C, Xing X. The severity and risk factors for mortality in immunocompromised adult patients hospitalized with influenza-related pneumonia. Ann Clin Microbiol Antimicrob. (2021) 20:55. doi: 10.1186/s12941-021-00462-7
7. Collins JP, Campbell AP, Openo K, Farley MM, Cummings CN, Hill M, et al. Outcomes of immunocompromised adults hospitalized with laboratory-confirmed influenza in the United States, 2011-2015. Clin Infect Dis. (2020) 70:2121–30. doi: 10.1093/cid/ciz638
8. Van de Louw A, Mirouse A, Peyrony O, Lemiale V, Azoulay E. Bacterial pneumonias in immunocompromised patients. Semin Respir Crit Care Med. (2019) 40:498–507. doi: 10.1055/s-0039-1696961
9. Figueiredo-Mello C, Naucler P, Negra MD, Levin AS. Prospective etiological investigation of community-acquired pulmonary infections in hospitalized people living with HIV. Medicine (Baltimore). (2017) 96:e5778. doi: 10.1097/MD.0000000000005778
10. Certan M, Garcia Garrido HM, Wong G, Heijmans J, Grobusch MP, Goorhuis A. Incidence and predictors of community-acquired pneumonia in patients with hematological cancers between 2016 and 2019. Clin Infect Dis. (2022) 75:1046–53. doi: 10.1093/cid/ciac005
11. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An official clinical practice guideline of the american thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
12. Cao B, Huang Y, She DY, Cheng QJ, Fan H, Tian XL, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. (2018) 12:1320–60. doi: 10.1111/crj.12674
13. Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections–full version. Clin Microbiol Infect. (2011) 17 (Suppl 6):E1–59. doi: 10.1111/j.1469-0691.2011.03602.x
14. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. (2016) 63:e61–e111. doi: 10.1093/cid/ciw504
15. Ramirez JA, Musher DM, Evans SE, Dela Cruz C, Crothers KA, Hage CA, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. (2020) 158:1896–911. doi: 10.1016/j.chest.2020.05.598
16. Arnold FW, Summersgill JT, Ramirez JA. Role of atypical pathogens in the etiology of community-acquired pneumonia. Semin Respir Crit Care Med. (2016) 37:819–28. doi: 10.1055/s-0036-1592121
17. Azoulay E, Russell L, Van de Louw A, Metaxa V, Bauer P, Povoa P, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. (2020) 46:298–314. doi: 10.1007/s00134-019-05906-5
18. Rachow T, Lamik T, Kalkreuth J, Kurze S, Wagner K, Stier P, et al. Detection of community-acquired respiratory viruses in allogeneic stem-cell transplant recipients and controls-A prospective cohort study. Transpl Infect Dis. (2020) 22:e13415. doi: 10.1111/tid.13415
19. Li LJ, Liu YM, Wang YM, Zhou F, Li H, Xing XQ, et al. Clinical characteristics and prognosis of long-term glucocorticoid users with community-acquired pneumonia. Zhonghua Yi Xue Za Zhi. (2018) 98:738–43. doi: 10.3760/cma.j.issn.0376-2491.2018.10.005
20. Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia. Lancet. (2021) 398:906–19. doi: 10.1016/S0140-6736(21)00630-9
21. Hill AT. Management of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. (2020) 158:1802–3. doi: 10.1016/j.chest.2020.08.003
22. Xie F, Duan Z, Zeng W, Xie S, Xie M, Fu H, et al. Clinical metagenomics assessments improve diagnosis and outcomes in community-acquired pneumonia. BMC Infect Dis. (2021) 21:352. doi: 10.1186/s12879-021-06039-1
23. Qu J, Zhang J, Chen Y, Huang Y, Xie Y, Zhou M, et al. Aetiology of severe community acquired pneumonia in adults identified by combined detection methods: a multi-centre prospective study in China. Emerg Microbes Infect. (2022) 11:556–66. doi: 10.1080/22221751.2022.2035194
24. Sun T, Wu X, Cai Y, Zhai T, Huang L, Zhang Y, et al. Metagenomic next-generation sequencing for pathogenic diagnosis and antibiotic management of severe community-acquired pneumonia in immunocompromised adults. Front Cell Infect Microbiol. (2021) 11:661589. doi: 10.3389/fcimb.2021.661589
25. Kuang ZS, Yang YL, Wei W, Wang JL, Long XY Li KY, et al. Clinical characteristics and prognosis of community-acquired pneumonia in autoimmune disease-induced immunocompromised host: a retrospective observational study. World J Emerg Med. (2020) 11:145–51. doi: 10.5847/wjem.j.1920-8642.2020.03.003
26. Yang L, He D, Huang D, Zhang Z, Liang Z. Development and validation of nomogram for hospital mortality in immunocompromised patients with severe pneumonia in intensive care units: a single-center, retrospective cohort study. Int J Gen Med. (2022) 15:451–63. doi: 10.2147/IJGM.S344544
27. Celikhisar H, Dasdemir Ilkhan G, Arabaci C. Prognostic factors in elderly patients admitted to the intensive care unit with community-acquired pneumonia. Aging Male. (2020) 23:1425–31. doi: 10.1080/13685538.2020.1775192
28. Song H, Moon HG, Kim SH. Efficacy of quick sequential organ failure assessment with lactate concentration for predicting mortality in patients with community-acquired pneumonia in the emergency department. Clin Exp Emerg Med. (2019) 6:1–8. doi: 10.15441/ceem.17.262
29. Li J, Zhou K, Duan H, Yue P, Zheng X, Liu L, et al. Value of D-dimer in predicting various clinical outcomes following community-acquired pneumonia: a network meta-analysis. PLoS ONE. (2022) 17:e0263215. doi: 10.1371/journal.pone.0263215
30. Guo L, Wei D, Zhang X, Wu Y, Li Q, Zhou M, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. (2019) 10:2752. doi: 10.3389/fmicb.2019.02752
Keywords: clinical characteristics, immunocompromised status, intensive care unit, community-acquired pneumonia, risk factor
Citation: Wu X, Sun T, Cai Y, Zhai T, Liu Y, Gu S, Zhou Y and Zhan Q (2023) Clinical characteristics and outcomes of immunocompromised patients with severe community-acquired pneumonia: A single-center retrospective cohort study. Front. Public Health 11:1070581. doi: 10.3389/fpubh.2023.1070581
Received: 15 October 2022; Accepted: 25 January 2023;
Published: 15 February 2023.
Edited by:
Rabbani Syed, King Saud University, Saudi ArabiaReviewed by:
Li Weng, Peking Union Medical College Hospital (CAMS), ChinaSilvia Terraneo, Santi Paolo e Carlo Hospital, Italy
James John, Sathyabama Institute of Science and Technology, India
Copyright © 2023 Wu, Sun, Cai, Zhai, Liu, Gu, Zhou and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyuan Zhan,  ZHJ6aGFucXlAMTYzLmNvbQ==
ZHJ6aGFucXlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaojing Wu1†
Xiaojing Wu1† Ting Sun
Ting Sun Sichao Gu
Sichao Gu Qingyuan Zhan
Qingyuan Zhan