- 1College of Pharmacy, Keimyung University, Daegu, Republic of Korea
- 2Department of Health Sciences, Dongduk Women’s University, Seoul, Republic of Korea
Although there is evidence that mercury (Hg) exposure may be a potential risk factor for cardiovascular disease (CVD), few nationwide epidemiological researches have analyzed the association between blood Hg concentration and serum high-sensitivity C-reactive protein (hs-CRP) level as a biomarker of CVD. The present population-based national study was performed with data from the 2016–2017 National Health and Nutrition Examination Survey. In the total sample of 3,773 adults aged ≥20 years, the serum hs-CRP concentrations were 1.03 mg/L among participants in the lowest quartile of blood Hg level and 1.18 mg/L among those in highest quartile. The trend for the prevalence of a risky (>1.0 mg/L) hs-CRP level (moderate risk and high risk) was significantly related to an increased quartile blood Hg concentration. After adjustment for confounders, participants with the highest quartiles of blood Hg had increased odds of a risky (>1.0 mg/L) hs-CRP level (adjusted odds ratio = 1.34; 95% confidence interval, 1.02–1.77) compared with those with the lowest quartile of blood Hg. These findings demonstrate that a high blood Hg level increases the concentration of serum hs-CRP, a sensitive marker of chronic low-grade inflammation, and imply that the increased body burden associated with high blood Hg is a potential risk factor in the development of many inflammatory diseases, including CVD.
Introduction
Cardiovascular disease (CVD) is the leading cause of disability and death worldwide, and the numbers of CVD cases and deaths continue to rise (1). In Korea, although the prevalence has been declining in recent years, CVD is still the second main cause of mortality (2). In addition to traditional risk factors for CVD, such as hypertension, diabetes, obesity, and high salt consumption, there are growing concerns about environmental toxicants, such as toxic metals and persistent organic pollutants, as a potential risk factor for CVD (3, 4). In particular, potentially toxic metals are widely distributed in the ambient air, drinking water, and foods; thus, the adverse health effects of toxic metal exposure are of great concern to many population groups globally (5).
Among metals, mercury (Hg) is a toxic metal, with known harmful effects on human health following exposure (6). The general population may be exposed to Hg through environmental or occupational exposure sources; additionally, consumption of fish/seafood is the most common route of exposure to methylmercury (MeHg), a highly toxic form of Hg (7). Hg exposure is recognized as an important public health issue, as Hg is reported primarily as a metal that causes neurodevelopmental toxicity in neonates and children (8, 9). However, there is a growing body of epidemiological and experimental evidence indicating that Hg exposure may increase the risk of adverse cardiovascular outcomes (10–12).
Although the mechanisms supporting the association between Hg exposure and CVD prevalence have not been elucidated in detail, there are some reports that Hg exposure impairs the activity of selenoproteins and promotes lipid peroxidation, as well as chronic inflammation (13). As chronic inflammation is the critical mechanism in the pathogenesis of CVD (14) and serum high-sensitivity C-reactive protein (hs-CRP) is an indicator of inflammation (15–18), hs-CRP is considered to be a biomarker of CVD risk (19).
The identification of environmental risk factors influencing the incidence of CVD in the general population is important for the reduction of disability and premature death from CVD. However, few studies have examined the association between blood concentration of Hg and the hs-CRP level, as a sensitive marker of CVD risk, in the general Korean population. Therefore, the present study was conducted to evaluate this association in Korean adults using data from the Korea National Health and Nutrition Examination Survey (KNHANES).
Materials and methods
Study population
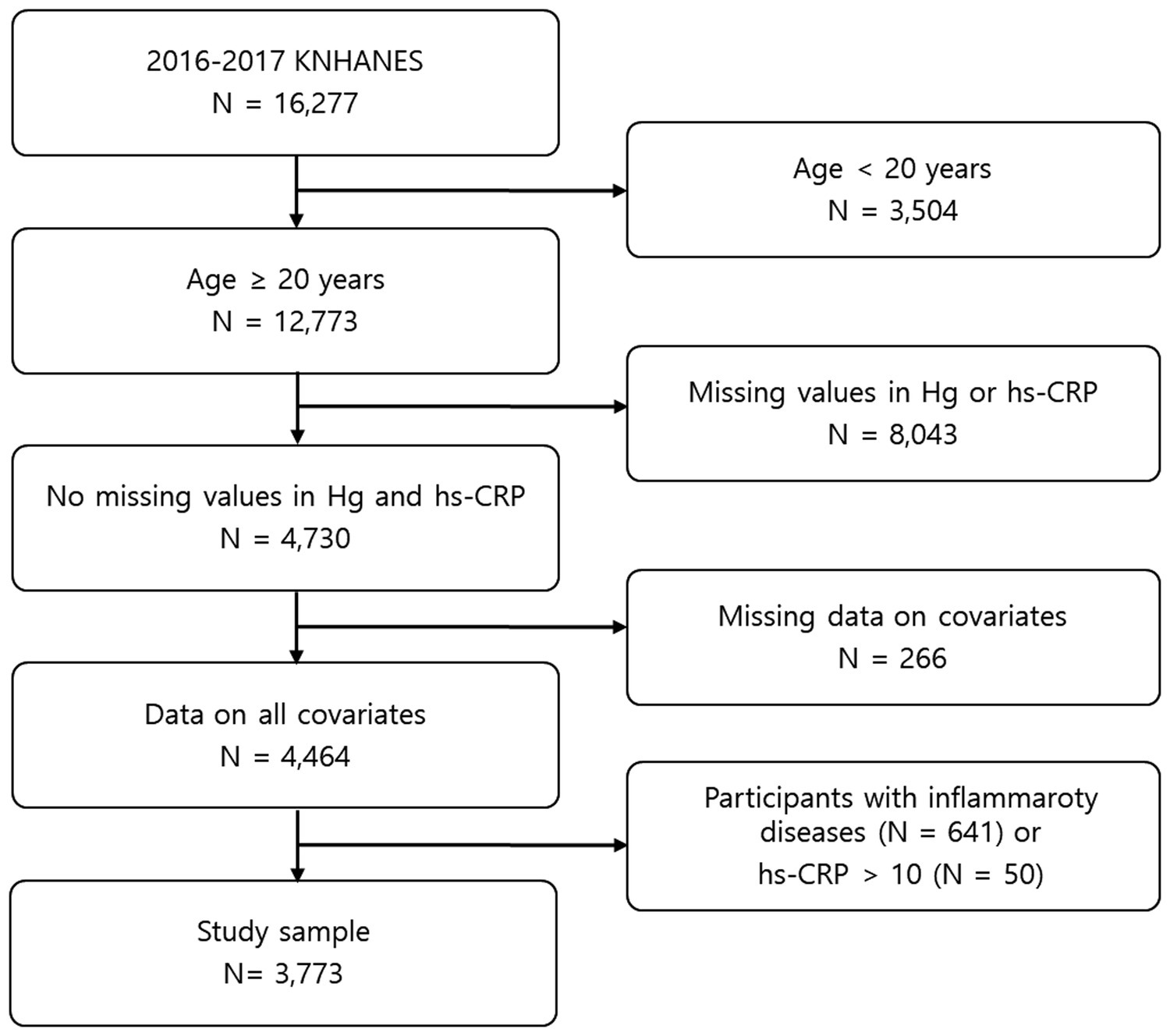
This study was carried out based on the data obtained from the 2016–2017 KNHANES, provided by the Korea Disease Control and Prevention Agency. The KNHANES is performed by applying a stratified multi-stage cluster sampling design, with proportional allocation based on the national census register, to acquire a nationally representative sample of the general Korean population. Because inflammatory conditions can affect the hs-CRP level, participants with thyroid disease, which may induce inflammation, and participants with inflammatory diseases such as asthma and arthritis were excluded (n = 641). In addition, because hs-CRP levels higher than 10 mg/L are likely indicative of infection, participants with a hs-CRP > 10 mg/L were excluded (n = 50). Ultimately, a sample of 3,773 adults aged ≥20 years was analyzed in this study (Figure 1). The protocol of this study was approved by the Korean Ministry of Health and Welfare, and the research was conducted in accordance with the principles of the Declaration of Helsinki. All study participants provided informed consent.
Data collection
In the KNHANES, a questionnaire was used to obtain data on the sociodemographic characteristics of the participants, including sex, age, education level, income, cigarette smoking status, and alcohol drinking status. Education level was classified as less than high school, high school, and more than high school. Income was obtained by dividing the total household income by the square root of the household size. Data on alcohol drinking were acquired by asking questions related to alcohol drinking habits (average alcohol drinking frequency) in the month before the interview. Cigarette smokers and alcohol drinkers were defined as respondents who reported current cigarette smoking and alcohol drinking at least once a month, respectively. Based on the measured height and weight of the participant, the body mass index (BMI) was calculated by using weight in kilograms (kg) divided by the square of height in meters (m2). Then, participants were classified as underweight (BMI < 18.5 kg/m2), normal weight (18.5 kg/m2 ≤ BMI < 23.0 kg/m2), overweight (23.0 kg/m2 ≤ BMI < 25.0 kg/m2), and obese (BMI ≥ 25.0 kg/m2) according to the World Health Organization’s criteria for Asian individuals. Total Hg in blood was measured using a cold-vapor atomic absorption spectrometric method and a dedicated Hg analyzer (M-6000A; CETAC Technologies, Omaha, NE, United States). The details of the Hg analysis procedures have been published elsewhere (20). For the analysis of serum hs-CRP, a COBAS analyzer (Roche Diagnostics, Basel, Switzerland) and an immunoturbidimetric assay (Roche Cardiac C-Reactive Protein High Sensitive Reagent Kit) were used. The analytical measurement range of hs-CRP assay was 0.1–20 mg/L. In this study, moderate-to-high risk hs-CRP levels (>1 mg/L), as specified by the American Heart Association (21), were defined as risky hs-CRP levels.
Statistical analysis
To account for the sample population studied, we calculated frequencies and percentages for sociodemographic variables. In addition, geometric means with 95% confidence intervals (CIs) for blood Hg concentration were calculated by taking the anti-log of the arithmetic mean of log-transformed values. Based on a normal probability distribution, we improved the approximation of a normal distribution using the geometric mean. The Mantel–Haenszel chi-squared test was used to test the significance of differences for categorical variables. Multivariate logistic regression analysis was performed to estimate odds ratios (ORs) with 95% CIs for risky hs-CRP status [vs. low risk (hs-CRP < 1.0 mg/L)] among quartiles of Hg concentration, after adjusting for possible confounding variables such as age, BMI, education level, income, cigarette smoking, and alcohol drinking. The Cochran-Armitage test was used to determine the presence of a significant linear trend in the prevalence of a risky hs-CRP level across the Hg quartiles. The statistical analyses were conducted, taking into account the survey design, and appropriate statistical procedures in SAS (e.g., “surveylogistic”) were applied with weighted data. All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, United States).
Results
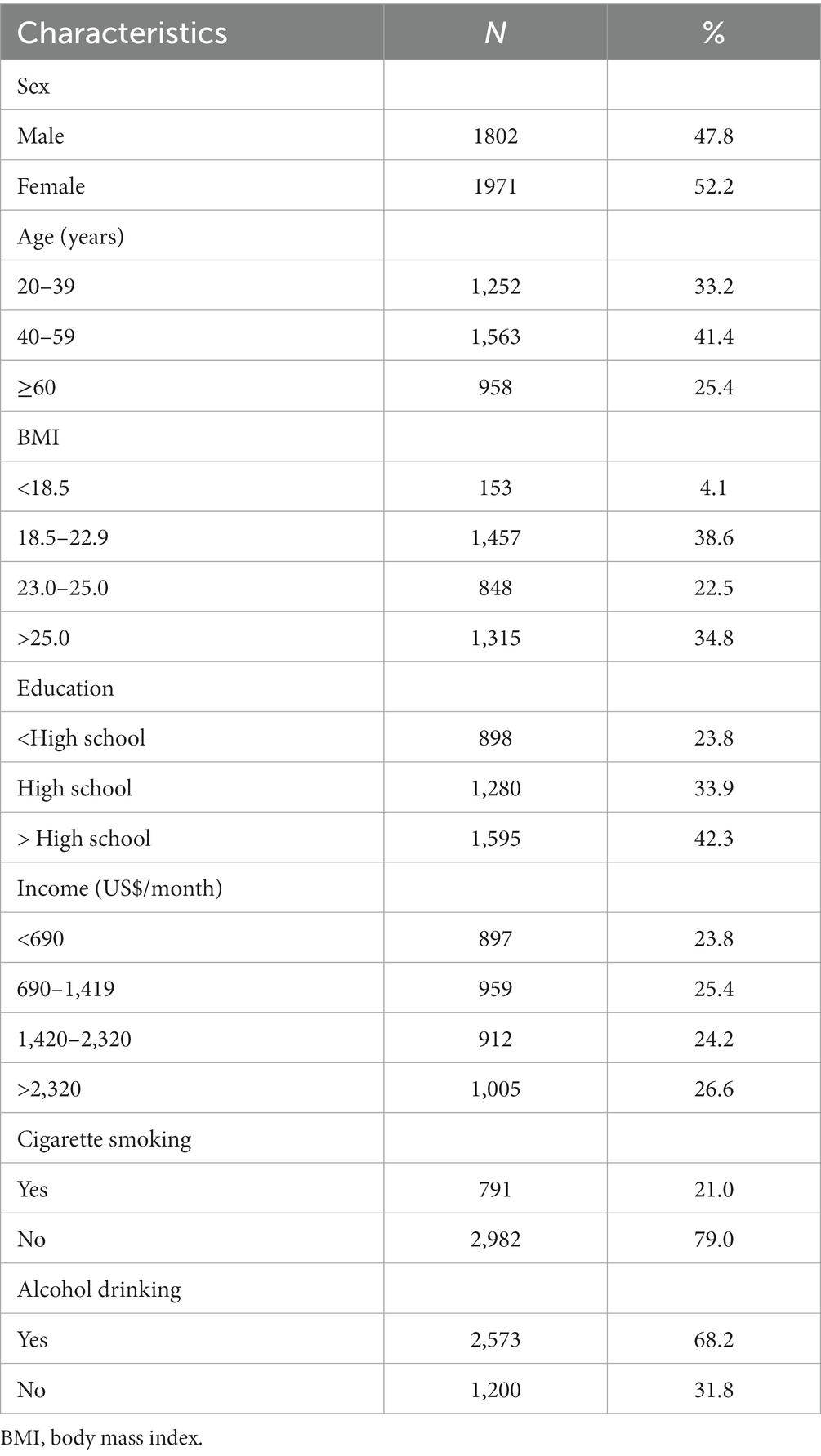
The study sample consist of 3,773 adults aged ≥20 years; their sociodemographic characteristics are presented in Table 1. The average age of the study participants was 48.3 years, and the mean BMI was 23.9 kg/m2.
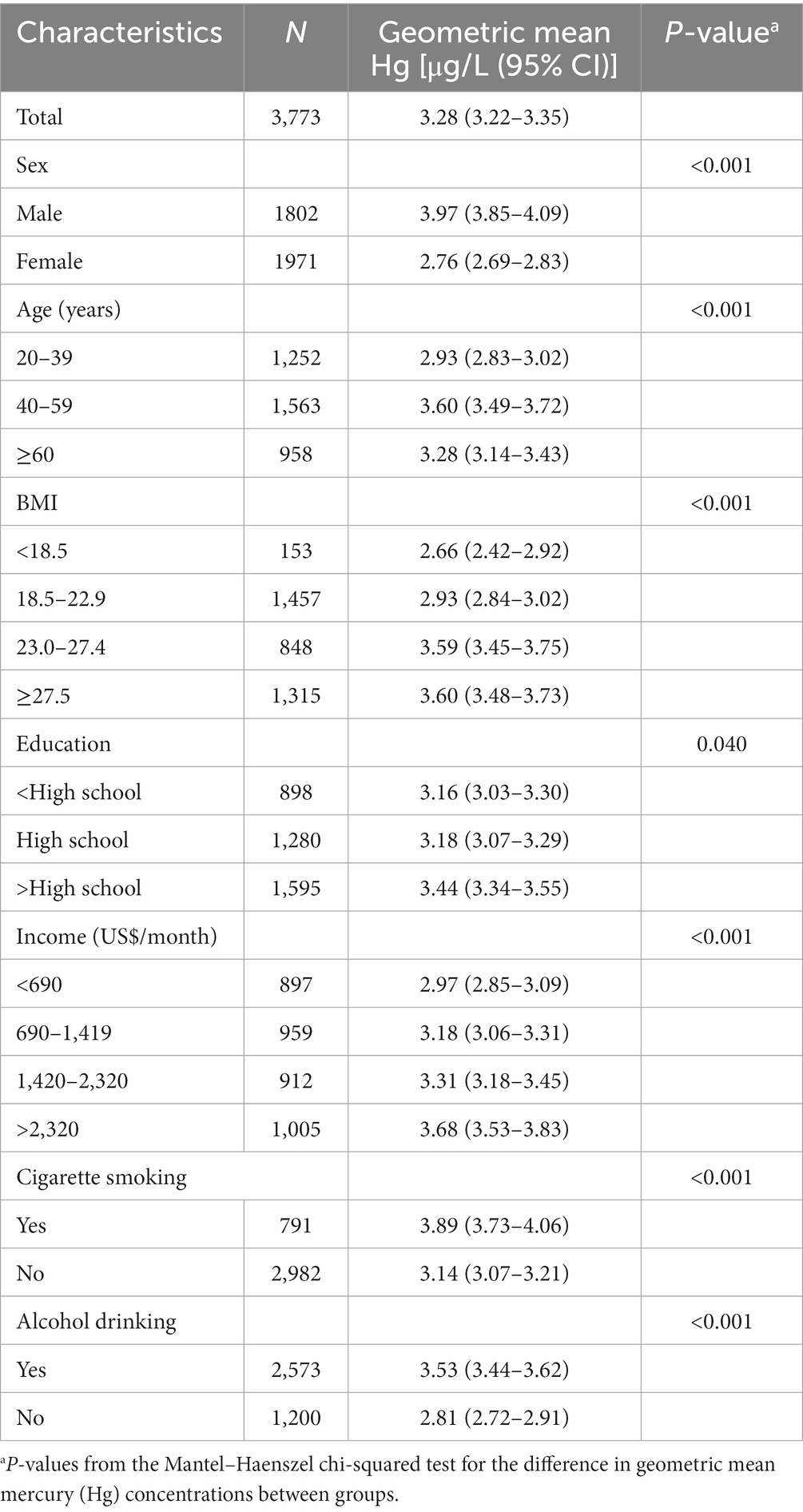
The geometric mean blood Hg level is presented according to the participants’ demographic characteristics in Table 2. Of the 3,773 participants, the geometric mean blood Hg level was 3.28 μg/L; the Hg concentration in men was significantly higher than that in women (p < 0.001). The geometric mean blood Hg level increased with BMI, education level, and income (p < 0.05). In addition, age, cigarette smoking, and alcohol drinking were significantly associated with blood Hg (p < 0.001).
The serum hs-CRP level was significantly associated with an increased quartile of blood Hg concentration (p for trend = 0.019; Figure 2). The serum hs-CRP concentration was 1.18 (95% CI, 1.08–1.27) mg/L among participants having the highest quartile Hg compared to 1.03 (95% CI, 0.94–1.11) mg/L among those having the lowest quartile Hg.
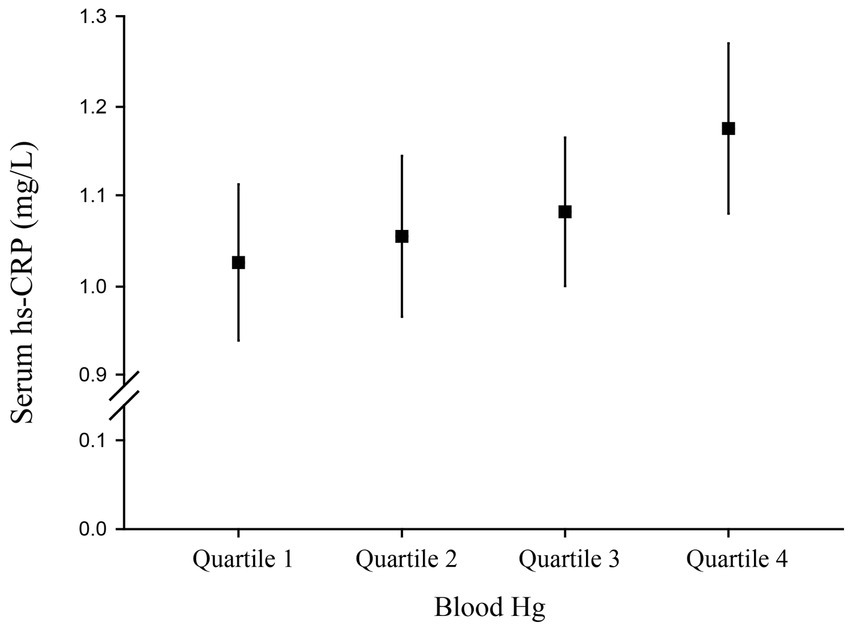
Figure 2. Serum high-sensitivity C-reactive protein (hs-CRP) level (95% confidence interval) by quartile of blood Hg.
Table 3 shows the prevalences and ORs for the associations of having a risky hs-CRP level (moderate-to-high level) with respect to quartiles of Hg concentration. The prevalence of a having a hs-CRP level > 1.0 mg/L was higher among participants in the higher quartile Hg concentration: 26.1% among participants with the lowest quartile Hg (<2.14 μg/L), and 32.0% among those with the highest quartile Hg (> 4.89 μg/L) (p = 0.003). The crude and adjusted ORs (Models 1 and 2) for a risky hs-CRP level was positively correlated with an increasing quartile Hg level (p < 0.01). The crude OR of a risky hs-CRP level in the highest quartile Hg group (blood Hg level > 4.89 μg/L) relative to the reference group (blood Hg level < 2.14 μg/L) was 1.56 (95% CI, 1.23–1.99), and the fully adjusted OR for a risky hs-CRP level was 1.34 (95% CI, 1.02–1.77) (Model 2).
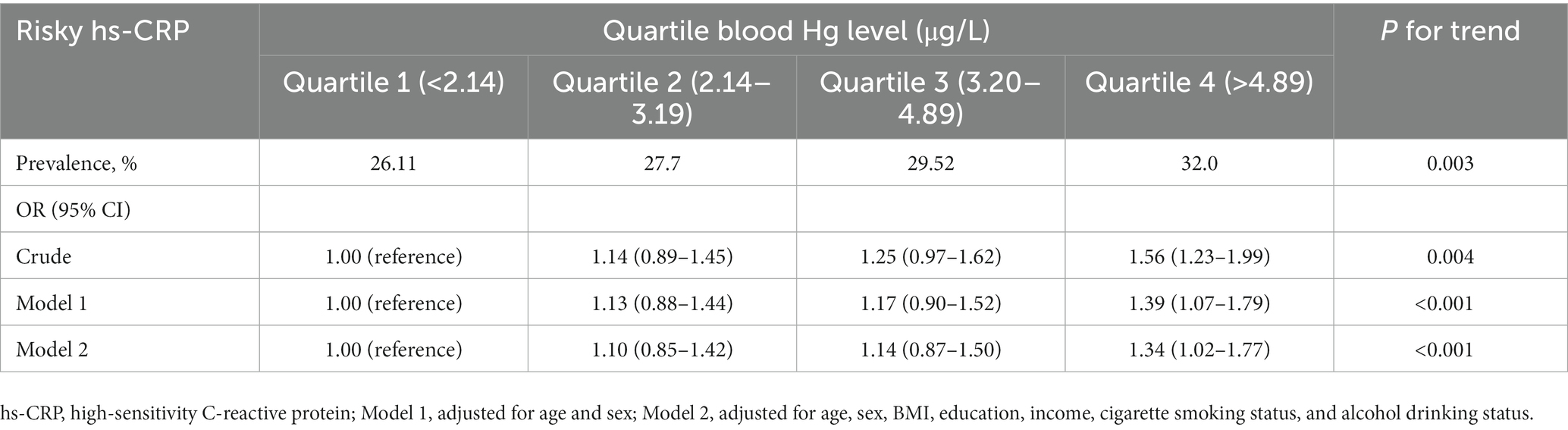
Table 3. Prevalences and adjusted odds ratios (95% confidence intervals) of a risky hs-CRP level by quartile of blood Hg in Korean adults aged ≥20 years.
Discussion
Hg is considered to be one of the most widespread toxic environmental pollutants, and exposure to Hg can occur from both natural and artificial sources (22). Humans are exposed to Hg through several sources including environmental pollutants, contaminated seafood, metallic Hg-containing dental amalgam, and ethyl-Hg-containing vaccines (23, 24). Hg has possible toxic effects on the nervous, digestive, and immune systems, as well as renal function (25, 26). However, accumulating evidence suggests that exposure to Hg is contribute to the risk of CVD and related outcomes, including hypertension and ischemic heart disease (IHD) (11, 12, 27). A meta-analysis of 14 studies reported that chronic exposure to Hg was associated with an increased risk of fatal/nonfatal IHD and all-cause mortality (28). However, in a cohort study of adults in the United States (US), blood Hg level was not significantly related to the risk of all-cause or CVD-related mortality (29). Another population-based cohort study conducted in Greenland also reported no association between blood Hg and the risk of developing CVD (30). Therefore, a potential biomarker associated with CVD could be used to better understand the causal relationship between Hg exposure and CVD prevalence.
Low-grade inflammation is a known contributor to the pathogenesis of several chronic diseases, including CVD, and the use of hs-CRP as a sensitive marker for low-grade inflammation has been investigated extensively (31–33). Several cohort studies have reported that an elevated hs-CRP level is independently associated with an increased incidence of major cerebrovascular and cardiovascular outcomes (34, 35). Similarly, many recent studies have shown that the hs-CRP level is positively correlated with CV risk in various clinical settings (36–39).
In this study, an increase in the blood Hg concentration was significantly associated with an increase in the hs-CRP concentration. Acute exposure to Hg from dental amalgam was reported to have no significant effect on the level of C-reactive protein (CRP) (40), but the association between blood Hg and the level of CRP has been reported in some epidemiological studies (41, 42). In a study of premenopausal women (mean age, 27.4 years) in the US, although it was not statistically significant, a positive association was observed between Hg concentration and hs-CRP level with an adjusted beta coefficient of 0.02 (41). In addition, in the NHANES 2005–2006 study conducted with US participants aged 16–49 (mean age, 34 years), the beta coefficient between Hg and CRP levels was positive in a fully adjusted multiple linear regression model in women, but not in men (42). These two previous studies of US participants showed a positive correlation between Hg and CRP levels only in women and did not reach statistical significance. In our study of Korean adults, a high level of Hg exposure significantly increased the hs-CRP concentration and the frequency of having a risky (> 1.0 mg/L) hs-CRP level. Specifically, participants with the highest quartile of blood Hg were more likely than those with the lowest quartile to have an hs-CRP level > 1.0 mg/L (adjusted OR = 1.34; 95% CI, 1.02–1.77); the cutoff value is associated with the CVD risk. The mean age of our study population was 48.3 years; the difference between the OR values found in our study and those reported in the US studies may be due, at least in part, to the difference in the average age of the study subjects. Given that the geometric mean of blood Hg concentration was much higher in Korean participants than in US participants (3.28 μg/L vs. 1.05 μg/L), the blood Hg level may also have affected the statistical significance of the results. Additionally, differences in race and ethnicity between Korean and US participants likely influenced the findings.
Few epidemiological studies have examined the associations between body burden of Hg and hs-CRP level. To the best of our knowledge, this study is the first to elucidate the relationship between Hg exposure and hs-CRP level, as a highly sensitive cardiovascular biomarker, using population-based survey data on Korean adults aged ≥20 years. However, there are several limitations to this study. First, given the complexity of potential factors affecting serum hs-CRP concentrations, additional factors, including oxidative stress and inflammatory comorbidities, which were not fully considered in the model of this study, could have significant effects on serum hs-CRP (43). Therefore, future studies should consider factors such as antioxidant food or supplement consumption; the use of anti-inflammatory drugs; and the prevalence of inflammatory diseases such as cancer, inflammatory bowel disease, chronic kidney disease, and type 2 diabetes. Second, several sociodemographic factors, including alcohol drinking and cigarette smoking, were derived from self-reporting and therefore may have introduced misclassification or recall bias. Third, although we adjusted for potential confounders in the analysis, several confounding factors, such as vitamins and trace elements that are associated with inflammatory markers and may interact with Hg, were not adjusted for in the study. Despite these limitations, our study findings are important in view of a nationwide study that found an association between body burden of Hg and the potential for chronic low-grade inflammation that can lead to CVDs. Due to the limitation of the cross-sectional design of our study, prospective cohort studies are warranted to elucidate a causal relationship between Hg exposure and cardiovascular markers such as hs-CRP.
Conclusion
In the present study using nationally representative survey data on Korean adults aged ≥20 years, an increase in blood Hg concentration increased the low-grade inflammatory response, as reflected in the hs-CRP level. The results of this study show that Hg exposure may increase chronic inflammation, which in turn may lead to an increased risk of inflammatory diseases such as CVD, type 2 diabetes, and cancer.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://knhanes.kdca.go.kr/knhanes/eng/index.do.
Ethics statement
The studies involving human participants were reviewed and approved by Korean Ministry of Health and Welfare. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KK: conceptualization, methodology, investigation, and writing–review and editing. HP: investigation, data curation, and writing–original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Keimyung University Research Grant of 2019.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pickersgill, SJ, Msemburi, WT, Cobb, L, Ide, N, Moran, AE, Su, Y, et al. Modeling global 80-80-80 blood pressure targets and cardiovascular outcomes. Nat Med. (2022) 28:1693–9. doi: 10.1038/s41591-022-01890-4
2. Kim, HC. Epidemiology of cardiovascular disease and its risk factors in Korea. Glob Health Med. (2021) 3:134–41. doi: 10.35772/ghm.2021.01008
3. GBD. Risk factor collaborators. Global, regional, and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2017) 2018:1923–94. doi: 10.1016/S0140-6736(18)32225-6
4. Landrigan, PJ, Fuller, R, Acosta, NJR, Adeyi, O, Arnold, R, Basu, NN, et al. The lancet commission on pollution and health. Lancet. (2018) 391:462–512. doi: 10.1016/S0140-6736(17)32345-0
5. Briffa, J, Sinagra, E, and Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. (2020) 6:e04691. doi: 10.1016/j.heliyon.2020.e04691
6. Ha, E, Basu, N, Bose-O’Reilly, S, Dórea, JG, McSorley, E, Sakamoto, M, et al. Current progress on understanding the impact of mercury on human health. Environ Res. (2017) 152:419–33. doi: 10.1016/j.envres.2016.06.042
7. Driscoll, CT, Mason, RP, Chan, HM, Jacob, DJ, and Pirrone, N. Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol. (2013) 47:4967–83. doi: 10.1021/es305071v
8. Wu, J, Ying, T, Shen, Z, and Wang, H. Effect of low-level prenatal mercury exposure on neonate neurobehavioral development in China. Pediatr Neurol. (2014) 51:93–9. doi: 10.1016/j.pediatrneurol.2014.03.018
9. Wang, J, Wu, W, Li, H, Cao, L, Wu, M, Liu, J, et al. Relation of prenatal low-level mercury exposure with early child neurobehavioral development and exploration of the effects of sex and DHA on it. Environ Int. (2019) 126:14–23. doi: 10.1016/j.envint.2019.02.012
10. Arbi, S, Bester, MJ, Pretorius, L, and Oberholzer, HM. Adverse cardiovascular effects of exposure to cadmium and mercury alone and in combination on the cardiac tissue and aorta of Sprague-Dawley rats. J Environ Sci Health A Tox Hazard Subst Environ Eng. (2021) 56:609–24. doi: 10.1080/10934529.2021.1899534
11. Genchi, G, Sinicropi, MS, Carocci, A, Lauria, G, and Catalano, A. Mercury exposure and heart diseases. Int J Environ Res Public Health. (2017) 14:74. doi: 10.3390/ijerph14010074
12. Solenkova, NV, Newman, JD, Berger, JS, Thurston, G, Hochman, JS, and Lamas, GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. (2014) 168:812–22. doi: 10.1016/j.ahj.2014.07.007
13. Virtanen, JK, Rissanen, TH, Voutilainen, S, and Tuomainen, TP. Mercury as a risk factor for cardiovascular diseases. J Nutr Biochem. (2007) 18:75–85. doi: 10.1016/j.jnutbio.2006.05.001
14. van der Heijden, CDCC, Bode, M, Riksen, NP, and Wenzel, UO. The role of the mineralocorticoid receptor in immune cells in cardiovascular disease. Br J Pharmacol. (2022) 179:3135–51. doi: 10.1111/bph.15782
15. Yin, WJ, Yu, LJ, Wu, L, Zhang, L, Li, Q, Dai, FC, et al. Adequate 25(OH)D moderates the relationship between dietary inflammatory potential and cardiovascular health risk during the second trimester of pregnancy. Front Nutr. (2022) 9:952652. doi: 10.3389/fnut.2022.952652
16. Li, K, Huang, T, Zheng, J, Wu, K, and Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. PLoS One. (2014) 9:e88103. doi: 10.1371/journal.pone.0088103
17. Farzaneh-Far, R, Harris, WS, Garg, S, Na, B, and Whooley, MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: the heart and soul study. Atherosclerosis. (2009) 205:538–43. doi: 10.1016/j.atherosclerosis.2008.12.013
18. Kalogeropoulos, N, Panagiotakos, DB, Pitsavos, C, Chrysohoou, C, Rousinou, G, Toutouza, M, et al. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta. (2010) 411:584–91. doi: 10.1016/j.cca.2010.01.023
19. Ridker, PM, Hennekens, CH, Buring, JE, and Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. (2000) 342:836–43. doi: 10.1056/NEJM200003233421202
20. Park, H, and Kim, K. Association of blood mercury concentrations with atopic dermatitis in adults: a population-based study in Korea. Environ Res. (2011) 111:573–8. doi: 10.1016/j.envres.2011.02.003
21. Ridker, PM. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. (2003) 108:e81–5. doi: 10.1161/01.CIR.0000093381.57779.67
22. Paduraru, E, Iacob, D, Rarinca, V, Rusu, A, Jijie, R, Ilie, OD, et al. Comprehensive review regarding mercury poisoning and its complex involvement in Alzheimer's disease. Int J Mol Sci. (2022) 23:1992. doi: 10.3390/ijms23041992
23. Bjørklund, G, Dadar, M, Mutter, J, and Aaseth, J. The toxicology of mercury: current research and emerging trends. Environ Res. (2017) 159:545–54. doi: 10.1016/j.envres.2017.08.051
24. Bamonti, F, Guzzi, G, and Ferrero, ME. Cracked mercury dental amalgam as a possible cause of fever of unknown origin: a case report. J Med Case Rep. (2008) 2:72. doi: 10.1186/1752-1947-2-72
25. World Health Organization. Mercury and health (2017). Geneva: World Health Organization. Available online at: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (Accessed July 20, 2022).
26. Ekawanti, A, and Krisnayanti, BD. Effect of mercury exposure on renal function and hematological parameters among artisanal and small-scale gold miners at Sekotong, West Lombok. Indonesia J Health Pollut. (2015) 5:25–32. doi: 10.5696/2156-9614-5-9.25
27. Hu, XF, Singh, K, and Chan, HM. Mercury exposure, blood pressure, and hypertension: A systematic review and dose-response meta-analysis. Environ Health Perspect. (2018) 126:2863. doi: 10.1289/EHP2863
28. Hu, XF, Lowe, M, and Chan, HM. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ Res. (2021) 193:110538. doi: 10.1016/j.envres.2020.110538
29. Sun, Y, Liu, B, Rong, S, Zhang, J, Du, Y, Xu, G, et al. Association of seafood consumption and mercury exposure with cardiovascular and all-cause mortality among US adults. JAMA Netw Open. (2021) 4:e2136367. doi: 10.1001/jamanetworkopen.2021.36367
30. Larsen, TJ, Jørgensen, ME, Larsen, CVL, Dahl-Petersen, IK, Rønn, PF, Bjerregaard, P, et al. Whole blood mercury and the risk of cardiovascular disease among the Greenlandic population. Environ Res. (2018) 164:310–5. doi: 10.1016/j.envres.2018.03.003
31. Barcena, ML, Aslam, M, Pozdniakova, S, Norman, K, and Ladilov, Y. Cardiovascular inflammaging: mechanisms and translational aspects. Cells. (2022) 11:1010. doi: 10.3390/cells11061010
32. Banik, SD, Avila-Nava, A, Lugo, R, Chim Aké, R, and Gutiérrez Solis, AL. Association between low-grade inflammation and hyperuricemia in adults with metabolic syndrome in Yucatán México. Can J Diabetes. (2022) 46:369–74. doi: 10.1016/j.jcjd.2021.11.010
33. Sharif, S, Van der Graaf, Y, Cramer, MJ, Kapelle, LJ, de Borst, GJ, Visseren, FLJ, et al. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc Diabetol. (2021) 20:220. doi: 10.1186/s12933-021-01409-0
34. Liu, Y, Zhang, C, Jiang, L, Xu, L, Tian, J, Zhao, X, et al. Relationship between high-sensitivity C-reactive protein and long-term outcomes in elderly patients with 3-vessel disease. Angiology. (2022) 73:60–7. doi: 10.1177/00033197211021195
35. Dykun, I, Clark, D 3rd, Carlo, J, Lincoff, AM, Menon, V, Nissen, SE, et al. Longitudinal high-sensitivity C-reactive protein and longer-term cardiovascular outcomes in optimally-treated patients with high-risk vascular disease. Am J Cardiol. (2022) 181:1–8. doi: 10.1016/j.amjcard.2022.06.061
36. Toss, H, Lindahl, B, Siegbahn, A, and Wallentin, L. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease: FRISC study group Fragmin during instability. Cor. Artery Dis. Circ. (1997) 96:4204–10. doi: 10.1161/01.cir.96.12.4204
37. Nordenskjöld, AM, Johansson, N, Sunnefeldt, E, Athlin, S, and Fröbert, O. Prevalence and prognostic implications of myocardial injury in patients with influenza. Eur Heart J Open. (2022) 2:oeac051. doi: 10.1093/ehjopen/oeac051
38. Huo, J, Wu, Z, Jiang, H, and Zhang, H. Multi-slice computed tomography angiography imaging diagnosis of lower extremity arteriosclerosis in patients with hypertension and its correlation with the level of high-sensitivity C-reactive protein. Comput Math Methods Med. (2022) 2022:8208. doi: 10.1155/2022/1768208
39. Maierean, S, Webb, R, Banach, M, and Mazidi, M. The role of inflammation and the possibilities of inflammation reduction to prevent cardiovascular events. Eur Heart J Open. (2022) 2:oeac039. doi: 10.1093/ehjopen/oeac039
40. Sandborgh-Englund, G, Geijersstam, E, and Loftenius, A. Rapid communication: acute exposure to mercury from dental amalgam does not affect the levels of C-reactive protein or interleukin-6 in peripheral blood. J Toxicol Environ Health A. (2003) 66:495–9. doi: 10.1080/15287390306352
41. Pollack, AZ, Mumford, SL, Sjaarda, L, Perkins, NJ, Malik, F, Wactawski-Wende, J, et al. Blood lead, cadmium and mercury in relation to homocysteine and C-reactive protein in women of reproductive age: a panel study. Environ Health. (2017) 16:84. doi: 10.1186/s12940-017-0293-6
42. Emanuele, E, and Meliker, J. Seafood intake, polyunsaturated fatty acids, blood mercury, and serum C-reactive protein in US National Health and nutrition examination survey (2005–2006). Int J Environ Health Res. (2017) 27:136–43. doi: 10.1080/09603123.2017.1292495
Keywords: cardiovascular disease, high-sensitivity C-reactive protein, inflammation, KNHANES, mercury
Citation: Kim K and Park H (2023) Association of mercury exposure with the serum high-sensitivity C-reactive protein level in Korean adults. Front. Public Health. 11:1062741. doi: 10.3389/fpubh.2023.1062741
Edited by:
Zengwu Wang, Peking Union Medical College, ChinaReviewed by:
Renying Xu, Shanghai Jiao Tong University, ChinaGianpaolo Guzzi, Italian Association for Metals and Biocompatibility Research, Italy
Copyright © 2023 Kim and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kisok Kim, kimkisok@kmu.ac.kr
 Kisok Kim
Kisok Kim Hyejin Park2
Hyejin Park2