- Department of Pharmacy, Guangxi Academy of Medical Sciences and the People's Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, China
Background: Sugemalimab is a newly developed inhibitor of programmed death ligand 1 (PD-L1). As a first-line treatment for metastatic non-small-cell lung cancer (NSCLC), sugemalimab plus chemotherapy (Sugema-Chemo) has been proven effective. Still, its cost-effectiveness has not yet been determined. The objective of this study was to assess the cost-effectiveness of Sugema-Chemo from a health care perspective in China.
Methods: A partitioned survival model was used. According to the GEMSTONE-302 trial, the clinical characteristics and outcomes of the patients were obtained. The outcomes were costs, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratio (ICER), incremental net health benefits (INHB) and incremental net monetary benefits (INMB). The robustness of the model was further evaluated, as well as subgroup analyses. When the ICER was lower than the willingness to pay (WTP) threshold ($38,017/QALY or $86,376/QALY, defined as three times the per capita gross domestic product value of the general region and Beijing), the cost-effectiveness of Sugema-Chemo was assumed for general regions or Beijing.
Results: Compared with chemotherapy alone, Sugema-Chemo resulted in an incremental gain of 0.82 QALYs, an incremental gain of 1.26 life-years, as well as an average increase cost of $72,472. The ICER was $88,744/QALY. Model outcomes were susceptible to average body weight and cost of sugemalimab. Sugema-Chemo was cost-effective at a WTP threshold of 86,376/QALY if the average body weight was <62.44 kg or if the price of sugemalimab was <$2.996/mg. As well, Sugema-Chemo was also cost-effective when the cost of sugemalimab was <$1.839/mg for a WTP threshold of $38,017/QALY. Sugema-Chemo had a probability of > 50% being considered cost-effective in most subgroups at the $86,376/QALY threshold. However, Sugema-Chemo did not achieve cost-effectiveness (0%) in any of the subgroups when WTP was set at $38,017/QALY.
Conclusion: Sugema-Chemo might not be cost-effective in patients with metastatic NSCLC in China. In deciding between Sugema-Chemo and chemotherapy alone, it is essential to consider both the body weight of patients and the price of sugemalimab. A price reduction of sugemalimab under the National Healthcare Security Administration may be an effective measure to improve the cost-effectiveness of the drug.
Introduction
Lung cancer is one of the most common types of cancer, and it is the leading cause of cancer death worldwide (1). Nearly 85% of lung cancers are non-small cell lung cancers (NSCLC) (2). Squamous and non-squamous lung cancers account for 25–30% and 70–75% of all cases of NSCLC, respectively (1, 3). NSCLC is generally diagnosed at an advanced stage and has a poor prognosis for most patients (4). Platinum-based chemotherapy has traditionally been recommended as first-line treatment for patients with advanced NSCLC without targetable genetic alterations (5). However, there was a limited survival benefit associated with these interventions and the median overall survival (OS) was <1 year for these interventions (6). There is an urgent need for novel interventions to improve survival rates in advanced NSCLC, considering its prevalence and poor outcomes (4, 6).
In the past few years, immune checkpoint inhibitors (ICIs) have demonstrated promising benefits and a favorable safety profile, so they are rapidly incorporated into the standard treatment for advanced NSCLC (7). Sugemalimab is a humanized monoclonal antibody that inhibits programmed death ligands (8). As first-line therapy for patients with metastatic NSCLC, sugemalimab plus chemotherapy (Sugema-Chemo) significantly prolonged progression-free survival (PFS) in the GEMSTONE-302 randomized phase 3 trial compared to chemotherapy alone, as well as increasing response rates (8). Accordingly, Sugema-Chemo is a promising first-line treatment option for metastatic NSCLC. Sugemalimab, in combination with pemetrexed and carboplatin, has been approved in China for treating non-squamous NSCLC, and for patients with squamous NSCLC (8, 9).
Despite this, due to the relatively high cost of the combination therapy (Sugema-Chemo), it is urgent to perform a cost-effectiveness analysis of Sugema-Chemo compared to chemotherapy for treating NSCLC. Therefore, the purpose of the present study was to investigate the cost-effectiveness of Sugema-Chemo as first-line therapy for metastatic NSCLC in comparison with chemotherapy alone from a health care perspective in China.
Materials and methods
Patients and intervention
This study was conducted following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) (10). Patients with metastatic NSCLC enrolled in the GEMSTONE-302 study were the target population (8). GEMSTONE-302 is designed to enroll patients with metastatic non-squamous or squamous NSCLC who are at least 18 years of age without known EGFR sensitizing mutations, ALK, ROS1, or RET fusions and who have not received any prior systemic therapy for metastatic disease. The study enrolled individuals who received sugemalimab 1,200 mg once every 3 weeks or placebo, in combination with carboplatin and paclitaxel for patients with squamous NSCLC, or combination with carboplatin and pemetrexed for patients with non-squamous NSCLC. The maintenance treatment for squamous NSCLC consisted of sugemalimab or placebo, and the maintenance treatment for non-squamous NSCLC consisted of sugemalimab or placebo.
Model structure
We conducted an economic evaluation and used a partitioned survival model that considers three mutually exclusive health states: progression-free survival (PFS), progressive disease (PD), and death (11). Both treatment arms had a 15-year time horizon, and more than 98% of patients died during this period. The cycle length was 1-week. Based on clinical results from the GEMSTONE-302 trial, the proportions of patients with OS and PFS were established in the model (8). A portion of the OS curve was evaluated for the proportion of patients still alive; the portion of the PFS curve was evaluated for the proportion of patients living with PFS, and the difference between OS and PFS curves was evaluated for the proportion of patients living with PD. This study did not require or obtain an institutional review board review or informed consent because data were obtained from the literature and open databases.
Clinical data inputs
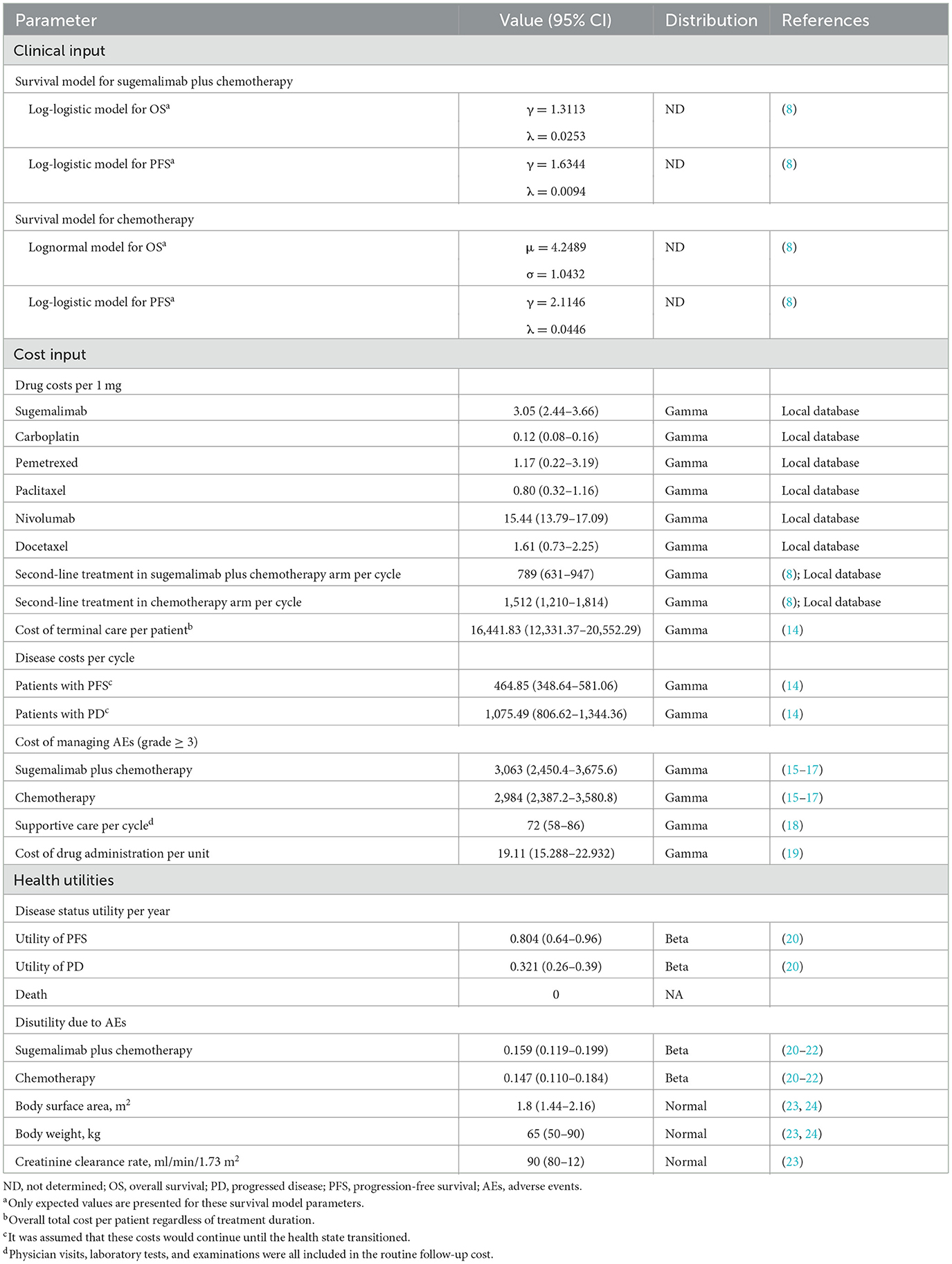
According to Guyot et al.s' algorithm, the OS and PFS of the GEMSTONE-302 trial were extrapolated beyond the follow-up period of the trial using the OS and PFS data obtained from the trial (12). To obtain the individual patient data points, the Kaplan-Meier (K-M) survival curves for OS and PFS were calculated using GetData Graph Digitizer version 2.26 (13). After calculating these data points, we fitted them with parametric survival functions: exponential, Weibull, gamma, lognormal, Gompertz, log-logistic, and generalized gamma. Afterward, the best-fit parametric models for the reconstructed K-M survival curves were selected based on Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). Sugema-Chemo and chemotherapy survival functions and parameterized models are shown in Table 1, while goodness-of-fit results are shown in Supplementary Table 1. Specifically, Log-logistic was selected to fit the PFS K-M curves of Sugema-Chemo or chemotherapy, and the OS K-M curves of Sugema-Chemo. Lognormal was selected to fit the OS K-M curves of chemotherapy alone (Supplementary Figure 1). The key clinical input data are listed in Table 1.
Cost inputs
Several direct medical costs have been evaluated, including those associated with obtaining drugs, the cost of supportive care, the cost of terminal care, and the cost of adverse events (AEs) (Table 1). We obtained the prices in Chinese Yuan and translated them into US dollars using the exchange rate of 2021 (1 US dollar = 6.37 Chinese Yuan) (25). Based on the standard fee database, the drug costs were determined. We assume that the average body surface area (BSA), weight, and creatinine clearance rate (CCR) were 1.80 m2, 65 kg, and 90 ml/min/1.73 m2. Those assumption were used to calculate the median dosage of chemotherapy and sugemalimab (23, 24). In addition, the costs for managing Grade ≥ 3 AEs were calculated by multiplying the rates contained in the randomized controlled trial, and the management costs were derived from the literature (Supplementary Table 2) (15–17). Approximately 141 patients (84%) with radiographic progression in the Sugema-Chemo group and 99 patients (86%) with radiographic progression in the chemotherapy group would receive subsequent treatment in the GEMSTONE-302 trial. Based on the lack of detailed information collected in the preliminary trial, we adopted subsequent treatment strategies recommended by the National Comprehensive Cancer Network (NCCN) (26) and the Chinese Society of Clinical Oncology (CSCO) guidelines (27, 28) (Table 1). According to NCCN and CSCO guidelines, nivolumab or docetaxel were used as the subsequent treatment strategies for NSCLC. Based on the published literature, we determined costs related to disease (14), subsequent supportive care (18), terminal care (14), and drug administration (19) during the study.
Utility inputs
There was a range of health utility scores between 0 (death) and 1 (perfect health). Because health utilities for PFS and PD were not included in GEMSTONE-302, we have adopted health utilities from the clinical literature (20). In relation to NSCLC, the utilities of PFS and PD were 0.804 and 0.321, respectively (20). Additionally, disutility values associated with AEs were determined based on the literature (Supplementary Table 2) (20–22).
Base-case analysis
We calculated an incremental cost-effectiveness ratio (ICER), which is the incremental cost per quality-adjusted life year (QALY) gained. We calculated ICERs based on two willingness to pay (WTP) thresholds in consideration of the imbalance in economic development among Chinese socioeconomic regions: three times the per capita gross domestic product (GDP) value of China in 2021 ($38,017/QALY) for general regions, and three times Beijing's per capita GDP value in 2021 ($86,376/QALY) for affluent regions (29). Costs and utility outcomes were discounted at a rate of 5% annually (30). Moreover, we calculated the incremental net health benefit (INHB) as well as the incremental monetary benefit (INMB) (31).
Sensitivity analysis
We conducted a one-way sensitivity analysis in this study in order to identify significantly sensitive variables and evaluate the robustness of the results. Several variables, such as costs and utilities, were subjected to one-way sensitivity analyses, and the uncertainty of each variable was calculated using 95% confidence intervals reported in the literature or estimated by assuming a 20% variation from the fundamental variables (Table 1). Monte Carlo simulations were used to conduct a probabilistic sensitivity analysis with 10,000 iterations. Three distributions were assigned to the parameters in the model: gamma, log-normal, and beta distributions. Gamma distributions were assigned to the cost parameters, log-normal distributions to the hazard ratios (HRs), and beta distributions to the proportions and probabilities. We then generated a cost-effectiveness acceptability curve to illustrate the possibility that Sugema-Chemo or chemotherapy could be considered a cost-effective option at different WTP levels in terms of per QALYs gained.
Subgroup analysis
We performed a subgroup analysis in order to determine whether different characteristics of patients contribute to the uncertainty of outcomes for each of the subgroups obtained from the GEMSTONE-302 by various HR for PFS (8). In this study, statistical analyses were carried out using R (version 4.0.5, 2021, R Foundation for Statistical Computing) with hesim and heemod packages.
Results
Base-case analysis
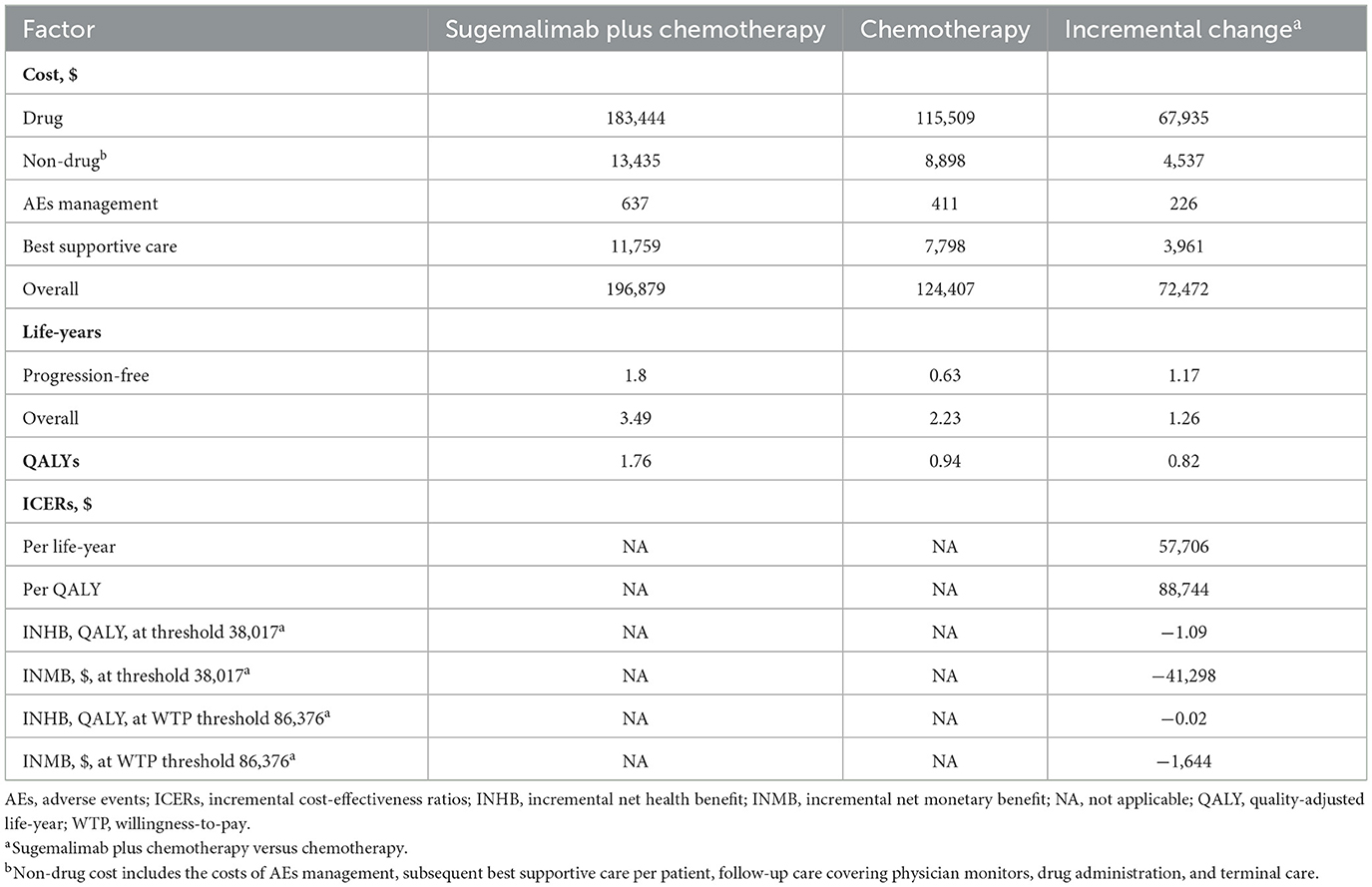
The Sugema-Chemo combination resulted in 0.82 QALYs gain and 1.26 overall life-years gain for patients with NSCLC in the base-case analysis, at additional costs of $72,472 compared to chemotherapy alone, which corresponded to an ICER of $88,744/QALY. In addition, at the $38,017/QALY WTP threshold, Sugema-Chemo had an INHB and an INMB of −1.09 QALYs and –$41,298, respectively, compared to chemotherapy alone. Furthermore, Sugema-Chemo had an INHB of −0.02 QALYs and an INMB of -$1,644 when the WTP threshold was set at $86,376/QALY (Table 2).
Sensitivity analysis
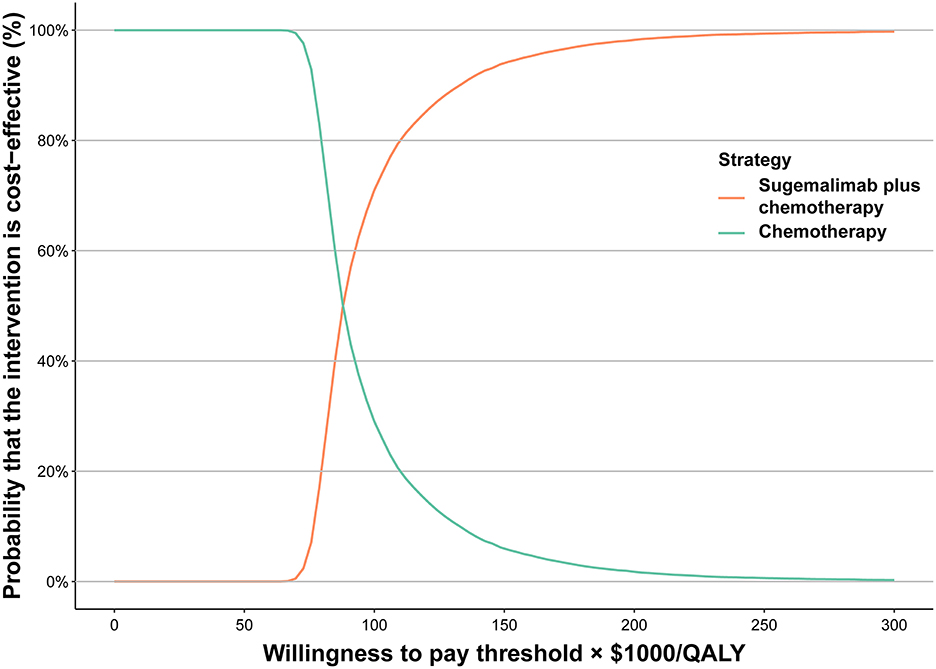
According to the one-way sensitivity analysis, the model outcome was largely driven by the average body weight, the utility for PFS, the cost of sugemalimab, and HR for OS (Sugema-Chemo vs. chemotherapy). There was a marginal relationship between the remaining parameters and outcomes (Supplementary Figure 2). We also examined the relationship between these key variables and the ICER between Sugema-Chemo and chemotherapy alone. Sugema-Chemo was cost-effective when the average body weight was lower than 62.44 kg, the utility of PFS exceeded 0.826, Sugemalimab was purchased at <$2.996/mg, or the HR for OS exceeded 0.711 for a WTP threshold of 86,376/QALY. Otherwise, chemotherapy was preferred. The results also demonstrated that Sugema-Chemo may be cost-effective at the WTP threshold of $38,017/QALY when sugemalimab costs <$1.839/mg; otherwise, chemotherapy was preferred (Supplementary Figure 3). Based on the cost-effectiveness acceptability curve, Sugema-Chemo has a 60% probability to be considered cost-effective, at the WTP threshold of $86,376/QALY. Nevertheless, Sugema-Chemo did not have a chance of being considered cost-effective if the WTP threshold was $38,017/QALY (Figure 1).
Subgroup analysis
Variations in the HR for PFS were used for the subgroup analysis. Compared to chemotherapy, the Sugema-Chemo group demonstrated a more significant reduction in death risk. Further, Sugema-Chemo was not significantly superior to chemotherapy in improving PFS in patients with ECOG performance status 0 and with liver metastases. Most subgroups, except for the patients with brain metastases, were likely to consider Sugema-Chemo cost-effective at the WTP threshold of $86,376/QALY. In all subgroups evaluated, Sugema-Chemo was not cost-effective (0%) at a WTP threshold of $38,017/QALY (Figure 2).
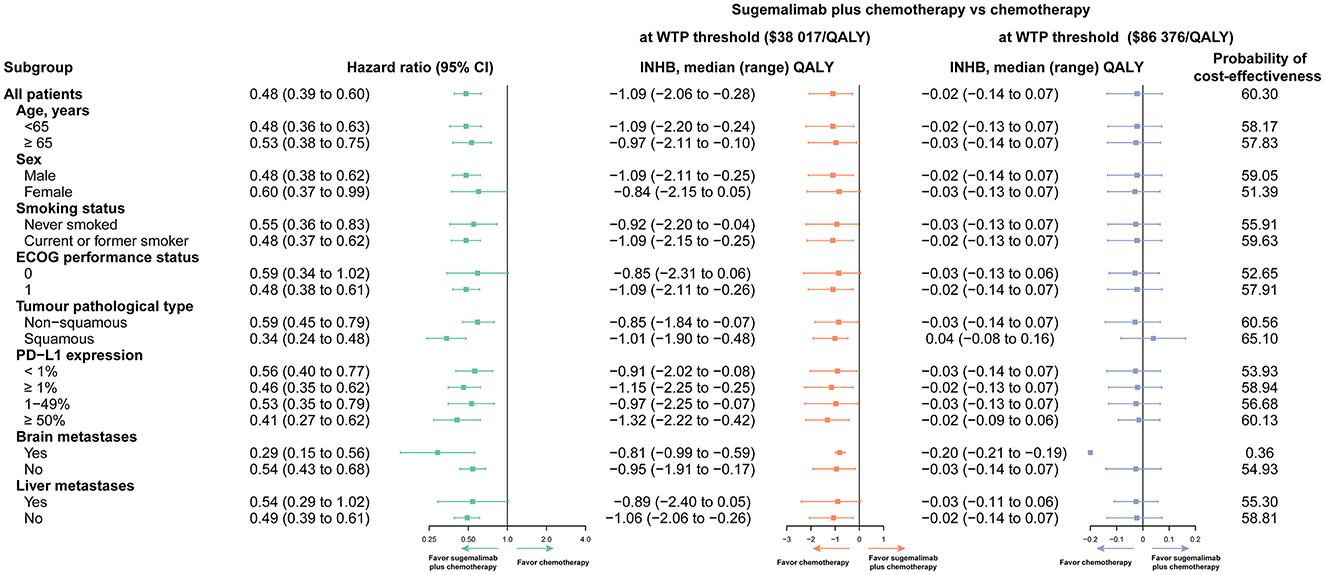
Figure 2. Subgroup analyses obtained by varying the hazard ratios (HRs) for progression-free survival (PFS). INHB, incremental net health benefit; WTP, willingness to pay; QALY, quality-adjusted life-year; PD-L1, programmed cell death ligand 1.
Discussion
This study is the first to compare the cost-effectiveness of Sugema-Chemo to chemotherapy alone in patients with metastatic NSCLC in China. In the GEMSTONE-302 trial, Sugema-Chemo statistically extended PFS in patients with metastatic NSCLC compared with chemotherapy alone. Nevertheless, due to the high price of sugemalimab, physicians and patients are uncertain about which is more beneficial. It is urgent to conduct a cost-effectiveness analysis to evaluate the efficacy and cost of Sugema-Chemo.
With a large population and rapid development, China is one of the most populous countries in the world. Therefore, the imbalance between economic development at the provincial level is therefore an objective fact. Taking into consideration regional economic disequilibrium, the WTP threshold for general regions and affluent regions of China was set at $38,017/QALY and $86,376/QALY, respectively. The combined treatment of Sugema-Chemo may not be a cost-effective alternative to chemotherapy alone, as the ICER of $88,744/QALY is higher than the WTP threshold of $38,017/QALY. There was, however, a 60% probability that this ICER would be considered a cost-effective option if it approached the WTP threshold of $86,376/QALY. One-way sensitivity analysis and probabilistic sensitivity analysis indicate that the results of this model are robust. An analysis of the sensitivity of the model indicated that it was susceptible to the average body weight, as well as the utility for PFS, cost of sugemalimab, and HR for OS. With a WTP threshold of 86,376/QALY, Sugema-Chemo was cost-effective if the average body weight was <62.44 kg, or the price was <$2.996/mg. In addition, for a WTP threshold of $38,017/QALY, Sugema-Chemo is cost-effective if the price of sugemalimab is <$1.839/mg; otherwise, chemotherapy is the preferred option.
As far as we know, since the official establishment of the National Healthcare Security Administration (NHSA) in May 2018, there have been several rounds of negotiations with pharmaceutical companies on the price of cancer drugs, aiming to relieve the medical burden of cancer patients through national strategic procurement (32). The NHSA in China has made a great effort to negotiate drug prices with pharmaceutical companies, with the result that the prices of many anticancer drugs have been reduced by 30–70% (32). Considering the circumstances, it is unlikely that a rise in the price of sugemalimab. On the contrary, if negotiations for sugemalimab are conducted, the cost of sugemalimab is highly likely to decrease. As a result, our findings indicate that Sugema-Chemo can provide adequate first-line treatment for patients with advanced NSCLC within an appropriate price range in a cost-effective manner. The NHSA negotiation will be the most effective approach for optimizing the allocation of medical resources in China for an extended period, providing patients with better health services at low costs (33).
This study has several advantages worth highlighting. First, to our knowledge, this is the first study to examine the cost-effectiveness of Sugema-Chemo combination therapy in treating metastatic NSCLC using a partitioned survival model based on the latest published GEMSTONE-302 trial. Second, at current prices, Sugema-Chemo combination is unlikely to be an attractive cost-effective option over chemotherapy alone. However, clinical trial results indicate that it increases OS and PFS in patients with metastatic NSCLC. Third, the patient population evaluated in the trial was Chinese, which means that race did not influence on the results. Additionally, the partitioned survival model did not require assumptions for the transition of patients between health states but made it possible to directly partition patients into different health states based on the K-M curves. Last, the present study analyzed the economic outcomes of 18 subgroups evaluated by the GEMSTONE-302 trial. Physicians, patients, and policymakers may benefit from the economic results of the subgroups.
There have some limitations in the analysis. The parameter distributions fitted to the K-M curves were assumed to be effective outcomes that exceeded the follow-up period of the GEMSTONE-302 trial, leading to uncertainty in the model outputs. A sensitivity analysis revealed that this finding is generally robust, indicating that this limitation may not be a significant factor. It is also important to note that there is inherent uncertainty when extrapolating PFS and OS over the longer term. Second, the clinical data included in the model have been derived from the results of GEMSTONE-302 trial. Therefore, any biases within the trial may have affected the results of the trial in terms of cost and effectiveness. Accordingly, the characteristics of patients with NSCLC included in the GEMSTONE-302 study were generally strict. Additionally, clinical trial participants tend to adhere to their treatment regimens more closely than patients in real-world practice. Moreover, with a median follow-up of 8.6 months (IQR 6.1–11.4), the preplanned interim analysis of the GEMSTONE-302 trial and the prediction of cost-effectiveness could potentially be altered if more follow-up data are available. Third, the values of utility and disutility were derived from published literature, some of the data were not obtained from Chinese populations, and bias was not distinguished due to of different treatment strategies (20). Based on the data, NSCLC utility values in China were higher than those in other countries.
Conclusion
Based on the health care perspective in China, this study indicates that Sugema-Chemo may not be cost-effective as the first-line treatment for metastatic NSCLC patients at a WTP threshold of $38,017 or $86,376/QALY and under current drug pricing. Sengemalimab may be economically advantageous if its price is reduced substantially. If individual treatments are tailored based on the factors contributing to the economic outcome, the economicoutcomes may be improved. In treating patients with metastatic NSCLC, the results of this study may assist clinicians in choosing appropriate treatments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Gathering and analyzing all data: YL. Concept and design: XLia, YL, and XC. Drafting and statistical analysis: YL and XLia. Funding: XC. Technical and material support and supervision: HL and XLiu. Data interpretation and critical revision of the manuscript: all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82160763).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1054405/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. (2019) 69:363–85. doi: 10.3322/caac.21565
3. Chouaid C, Bensimon L, Clay E, Millier A, Levy-Bachelot L, Huang M, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of Pd-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer. (2019) 127:44–52. doi: 10.1016/j.lungcan.2018.11.008
4. Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. (2016) 9:1023–8. doi: 10.2147/ott.S100685
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 4. (2022). Available online at: https://www.nccn.org/Guidelines/ (accessed May 23, 2022).
6. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. (2019) 322:764–74. doi: 10.1001/jama.2019.11058
7. Zhang Q, Tang L, Zhou Y, He W, Li W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: current understanding in characteristics, diagnosis, and management. Front Immunol. (2021) 12:663986. doi: 10.3389/fimmu.2021.663986
8. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (Gemstone-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. (2022) 23:220–33. doi: 10.1016/s1470-2045(21)00650-1
9. Dhillon S, Duggan S. Sugemalimab: first approval. Drugs. (2022) 82:593–9. doi: 10.1007/s40265-022-01693-4
10. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. (2022) 25:3–9. doi: 10.1016/j.jval.2021.11.1351
11. Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Making. (2017) 37:427–39. doi: 10.1177/0272989x16670617
12. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
13. Getdata Graph Digitizer (2022). Available online at: http://getdata-graph-digitizer.com (accessed June 12, 2022).
14. Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Ejzykowicz F, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr Med Res Opin. (2019) 35:1241–56. doi: 10.1080/03007995.2019.1571297
15. Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen H, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS ONE. (2012) 7:e32530. doi: 10.1371/journal.pone.0032530
16. Wong W, Yim YM, Kim A, Cloutier M, Gauthier-Loiselle M, Gagnon-Sanschagrin P, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS ONE. (2018) 13:e0196007. doi: 10.1371/journal.pone.0196007
17. Zheng H, Xie L, Zhan M, Wen F, Xu T, Li Q. Cost-effectiveness analysis of the addition of bevacizumab to chemotherapy as induction and maintenance therapy for metastatic non-squamous non-small-cell lung cancer. Clin Transl Oncol. (2018) 20:286–93. doi: 10.1007/s12094-017-1715-1
18. Gu X, Zhang Q, Chu YB, Zhao YY, Zhang YJ, Kuo D, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. (2019) 127:84–9. doi: 10.1016/j.lungcan.2018.11.029
19. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. (2018) 6:124. doi: 10.1186/s40425-018-0440-9
20. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. (2017) 13:e195–203. doi: 10.1111/ajco.12477
21. Freeman K, Connock M, Cummins E, Gurung T, Taylor-Phillips S, Court R, et al. Fluorouracil plasma monitoring: systematic review and economic evaluation of the My5-Fu assay for guiding dose adjustment in patients receiving fluorouracil chemotherapy by continuous infusion. Health Technol Assess. (2015) 19:1–321, v–vi. doi: 10.3310/hta19910
22. Konidaris G, Paul E, Kuznik A, Keeping S, Chen CI, Sasane M, et al. Assessing the value of cemiplimab for adults with advanced cutaneous squamous cell carcinoma: a cost-effectiveness analysis. Value Health. (2021) 24:377–87. doi: 10.1016/j.jval.2020.09.014
23. Zhu C, Xing XX, Wu B, Liang G, Han G, Lin CX, et al. Cost-effectiveness analysis of camrelizumab plus chemotherapy vs. chemotherapy alone as the first-line treatment in patients with iiib-iv non-squamous non-small cell lung cancer (NSCLC) without Egfr and Alk alteration from a perspective of health-care system in China. Front Pharmacol. (2021) 12:735536. doi: 10.3389/fphar.2021.735536
24. Wu B, Chen H, Shen J, Ye M. Cost-effectiveness of adding Rh-endostatin to first-line chemotherapy in patients with advanced non-small-cell lung cancer in China. Clin Ther. (2011) 33:1446–55. doi: 10.1016/j.clinthera.2011.09.016
25. China Bo,. Foreign Exchange Rate. (2022). Available online at: https://www.boc.cn/sourcedb/whpj/ (accessed June 24, 2022)
26. National Comprehensive Cancer Network. NCCN Guidelines for Treatment of Cancer by Site: Non-Small Cell Lung Cancer. Version 3. (2022). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed June 9, 2022).
27. Park K, Vansteenkiste J, Lee KH, Pentheroudakis G, Zhou C, Prabhash K, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. (2020) 31:191–201. doi: 10.1016/j.annonc.2019.10.026
28. Zhou C, Wang J, Wang B, Cheng Y, Wang Z, Han B, et al. Chinese experts consensus on immune checkpoint inhibitors for non-small cell lung cancer (2020 version). Zhongguo Fei Ai Za Zhi. (2021) 24:217–35. doi: 10.3779/j.issn.1009-3419.2021.101.13
29. Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. (2004) 7:518–28. doi: 10.1111/j.1524-4733.2004.75003.x
30. Zhang Q, Wu P, He X, Ding Y, Shu Y. Cost-effectiveness analysis of camrelizumab vs. placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front Oncol. (2021) 11:790373. doi: 10.3389/fonc.2021.790373
31. Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. (2021) 4:e210037. doi: 10.1001/jamanetworkopen.2021.0037
32. Administration NHS,. Notice of the National Healthcare Security Administration on Bringing 17 Kinds of Anticancer Drugs into the Category B of National Basic Medical Insurance, Industrial Injury Insurance Maternity Insurance Drug List. (2018). Available online at: http://www.nhsa.gov.cn/art/2018/10/10/art_19_397.html (accessed April 12, 2022).
Keywords: sugemalimab, partitioned survival model, non-small cell lung cancers, chemotherapy, cost-effectiveness
Citation: Liang X, Chen X, Li H, Liu X and Li Y (2023) Sugemalimab plus chemotherapy vs. chemotherapy for metastatic non-small-cell lung cancer: A cost-effectiveness analysis. Front. Public Health 11:1054405. doi: 10.3389/fpubh.2023.1054405
Received: 26 September 2022; Accepted: 09 February 2023;
Published: 27 February 2023.
Edited by:
Simiao Chen, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Sarayut Lucine Geater, Prince of Songkla University, ThailandGeorge Gourzoulidis, Health Through Evidence, Greece
Copyright © 2023 Liang, Chen, Li, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li,  bGl5YW4yMDEwMjAxMEBvdXRsb29rLmNvbQ==
bGl5YW4yMDEwMjAxMEBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work
 Xueyan Liang
Xueyan Liang Xiaoyu Chen
Xiaoyu Chen Huijuan Li
Huijuan Li Yan Li
Yan Li