- 1Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Pharmacy, Sichuan University, Chengdu, China
Background and purpose: Breast cancer is a rapidly raising healthcare problem worldwide. DESTINY-Breast04 demonstrated that trastuzumab deruxtecan (T-Dxd) had a survival advantage comparing to the physician's choice of chemotherapy for patients with HER2-low metastatic breast cancer. But at the same time, this expensive novel treatment also brought an economic burden. This study assessed the cost-effectiveness of T-Dxd based on results of DESTINY-Breast04 from the perspective of Chinese healthcare system.
Materials and methods: A three-state partitioned-survival model [progression-free survival (PFS), progressive disease (PD) and death] based on data from DESTINY-Breast04 and Chinese healthcare system was used to estimate the incremental cost-effectiveness ratio (ICER) of T-Dxd vs. the physician's choice of chemotherapy for HER2-low metastatic breast cancer. Costs, quality-adjusted life-years (QALYs) and the ICER in terms of 2022 US$ per QALY gained were calculated for both hormone receptor–positive cohort and all patients. One-way and probabilistic sensitivity analyses were performed to assess the model robustness.
Results: Compared with the physician's choice of chemotherapy, T-Dxd increased costs by $104,168.30, while gaining 0.31 QALYs, resulting in an ICER of $336,026.77 per QALY in all patients. The costs of T-Dxd and the utility of PFS were the crucial factors in determining the ICER. In the hormone receptor–positive cohort, the ICER was lower than that in all patients, with the ICER of $274,905.72 per QALY. The ICER was much higher than the commonly accepted willingness-to-pay threshold ($357,96.83 per QALY).
Conclusion: T-Dxd as second- or subsequent-line treatment is not a cost-effective treatment option for HER2-low metastatic breast cancer from the perspective of the Chinese healthcare system.
1. Introduction
The burden of breast cancer is increasing rapidly. In 2020, there was an estimated 2.26 million new cases of breast cancer, making it the most commonly diagnosed cancer globally, surpassing even lung cancer. Breast cancer also created 684,996 deaths worldwide, ranking fifth among all cancer-related deaths (1). The age-standardized incidence and mortality rate of breast cancer have significantly increased in China during the past decade, putting a great burden on Chinese healthcare and economic system (2). Human epidermal growth factor receptor 2 (HER2)-low breast cancer, defined as HER2 immunohistochemistry (IHC) 1+ or IHC 2+ and insituhybridization (ISH)-negative, accounts for 40–50% of all breast cancers (3, 4). Previous HER2-targeted therapies remarkably improved clinical outcomes of HER2 positive breast cancer, but have failed to provide prognosis benefit in patients with HER2-low breast cancer. There is limited treatment option for progressed HER2-low breast cancer refractory to standard treatment, and patients often have to receive palliative chemotherapy. Therefore, creating effective new treatments for HER2-low breast cancer is of great clinical significance (5).
Trastuzumab deruxtecan (T-Dxd) is an antibody–drug conjugate (ADC) composed of trastuzumab and a topoisomerase I inhibitor through a tetrapeptide-based cleavable linker (6). Unlike many other HER2-targeted therapies, T-Dxd is also effective in HER2-low breast cancer due to its bystander effect (7, 8). The superiority of T-Dxd over traditional single-agent chemotherapy in patients with HER2-low breast cancer who had received one or two previous lines of treatment was demonstrated in DESTINY-Breast04 (9). Based on DESTINY-Breast04, the US Food and Drug Administration approved T-Dxd for patients with unresectable or metastatic HER2-low breast cancer who have received prior chemotherapy in the metastatic setting. The National Comprehensive Cancer Network (NCCN) also recommended T-Dxd as the preferred second-line therapy for HER2-low breast cancer (10).
However, while T-Dxd demonstrated survival advantage in DESTINY-Breast04, it is extremely expensive for both patients and insurance payers. As such, we sought to evaluate the cost-effectiveness of T-Dxd for advanced HER2-low breast cancer from the Chinese healthcare system perspective.
2. Methods
2.1. Patients and treatment
In the base case analysis, a hypothetical cohort was generated using the clinical information collected from DESTINY-Breast04 (9). The trial included a total of 557 HER2-low metastatic breast cancer patients, of whom 373 were randomly assigned to receive T-Dxd 5.4 mg/kg every 3 weeks (T-Dxd group) while 184 were assigned to the physician's choice of chemotherapy (chemotherapy group) when their breast cancer progressed after one or two previous lines of chemotherapy. 331 (88.7%) T-Dxd group patients and 163 (88.6%) chemotherapy group patients, respectively, were qualified for the hormone receptor–positive cohort. Treatment for chemotherapy group comprised of five regimens: capecitabine (20.1%), eribulin (51.1%), gemcitabine (10.3%), paclitaxel (8.2%), or nab-paclitaxel (10.3%). Overall survival (OS) and progression-free survival (PFS) were evaluated in the hormone receptor–positive cohort and in all patients.
2.2. Model structure and assumptions
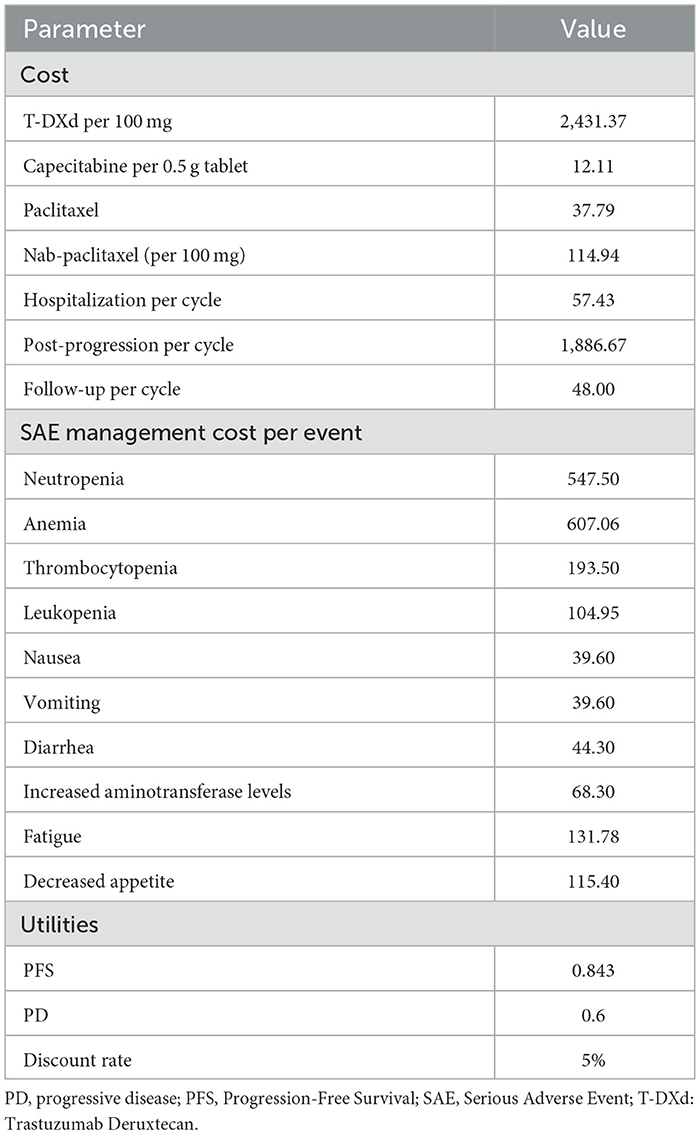
A partitioned-survival model was constructed by Treeage Pro Suite 2019 (Treeage Software, Inc., MA, USA) from the perspective of the Chinese healthcare system. The model included three mutually exclusive health states: PFS, progressive disease (PD) and death. The initial state was assumed to be PFS, and patients could remain in the PFS state or move to PD or death state during each cycle (Figure 1). We assumed that the cycle length was 1-month based on the time span of disease duration and progression. Patients with metastatic HER2-low breast cancer refractory to standard therapies have poor prognosis; the median overall survival ranged from 11.1 to 29.4 months (8, 9, 11). The population in the PSM model had received one or two previous lines of chemotherapy, and the median overall survival in DESTINY-Breast04 was less than two years (9). Therefore, a 5-year time horizon was selected for the model. The annual discount rates for costs and outcomes were set at 5% as recommended by guidelines, and discount rates of 0 and 8% were explored in scenario analyses (12). The threshold of willingness to pay (WTP) was assumed to be three times the Chinese per Gross Domestic Product per capita (GDP) according to WHO guideline (13). As a result, $357,96.83/quality-adjusted life-year (QALY) was set according to per capita GDP of China 2021 released by National Bureau of Statistics. All costs were converted into US dollars, with an exchange rate of $1 = ¥6.7863 (17 Aug 2022).
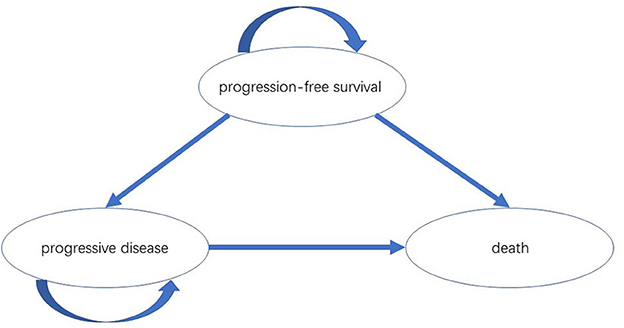
Figure 1. The partitioned-survival model simulated three health states: PFS, PD and death. PFS, progression-free survival; PD, progressive disease.
2.3. Clinical parameters from DESTINY-Breast04
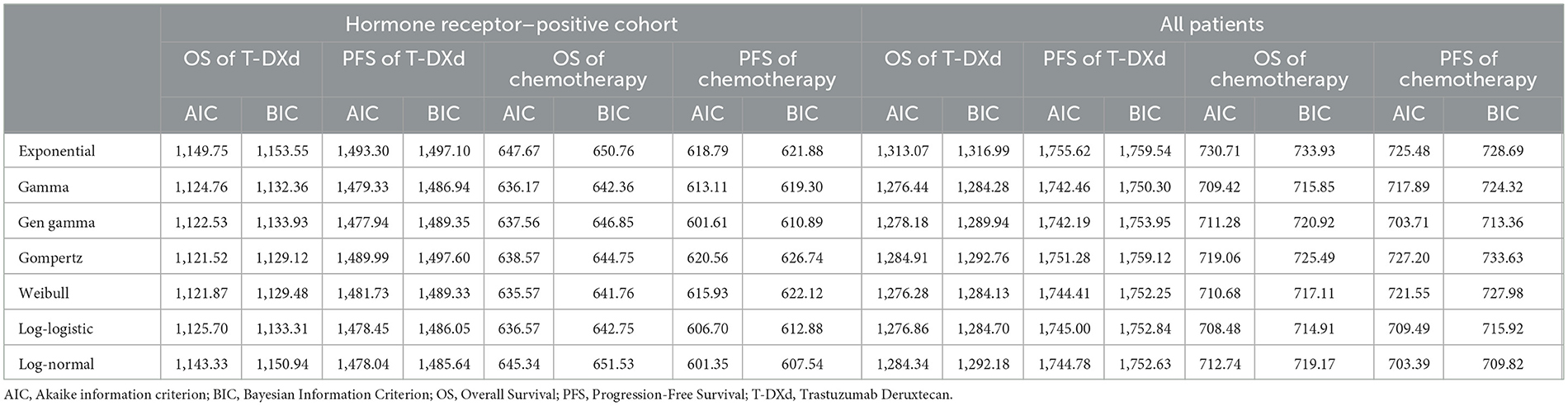
Clinical data on efficacy and safety were obtained from DESTINY-Breast04. Survival parameters were obtained by digitizing the Kaplan– Meier (KM) curve (OS, PFS) of DESTINY-Breast04. Individual patient data were reconstructed using the method described by Guyot et al. (14). KM curves up to the end of follow-up period were followed by simulative curves generated from best-fit parametric distributions. Different parameter distributions (Exponential, Gamma, Gen gamma, Gompertz, Weibull, Log-logistic, Log-normal) were applied to fit the reconstructed OS and PFS curves. The best-fit parametric distributions were selected based on Akaike information criterion (AIC), Bayesian information criterion (BIC) and visual inspection. The IC values for all models were shown in Table 1.
In the hormone receptor–positive cohort, Weibull distribution was selected to fit the KM curves for OS of both T-Dxd and chemotherapy group; for PFS, Gen gamma and Log-normal distribution were chosen for T-Dxd and chemotherapy group, respectively. Among all patients, Weibull distribution and Log-logistic distribution were found to fit the OS curve of the T-Dxd and the chemotherapy group, respectively; Gamma distributions and Log-normal distributions were selected to fit the PFS curve of the T-Dxd and the chemotherapy group, respectively. The original and the fitting curves were shown in Figure 2.
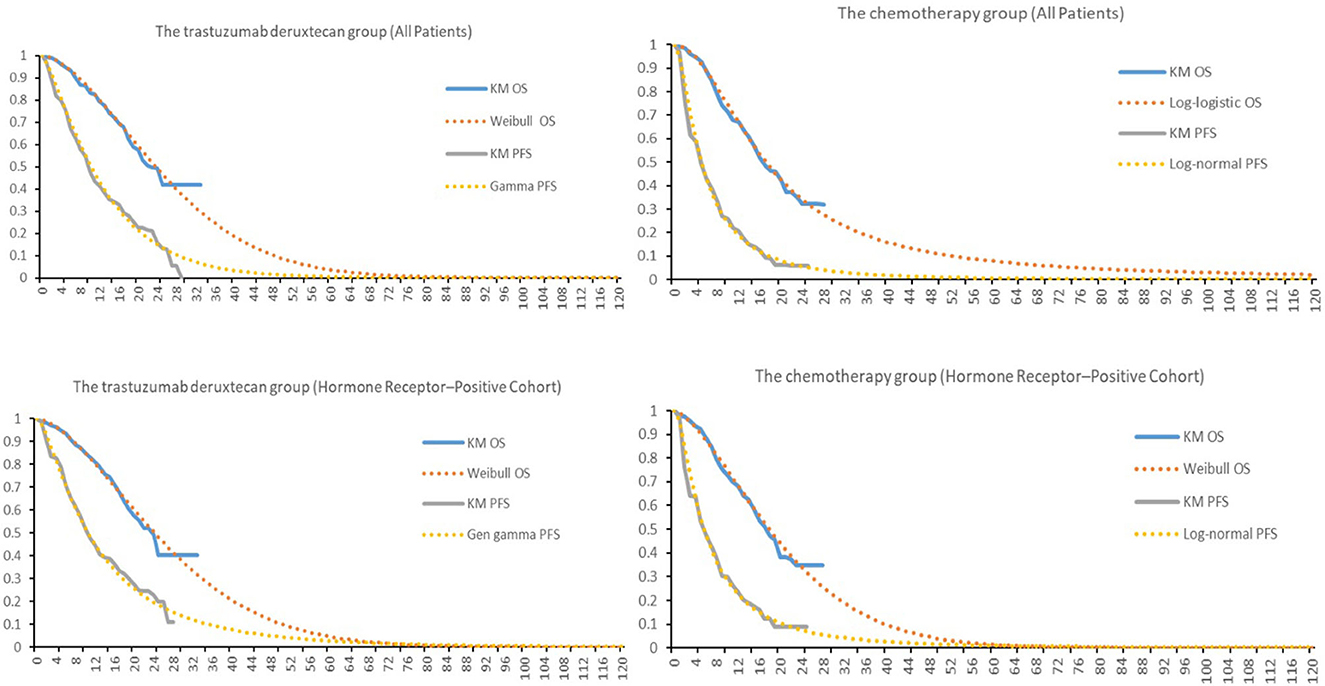
Figure 2. Kaplan-Meier survival for the trastuzumab deruxtecan and chemotherapy groups in DESTINY-Breast04 and the fitting curves. OS, overall survival; PFS, progression-free survival.
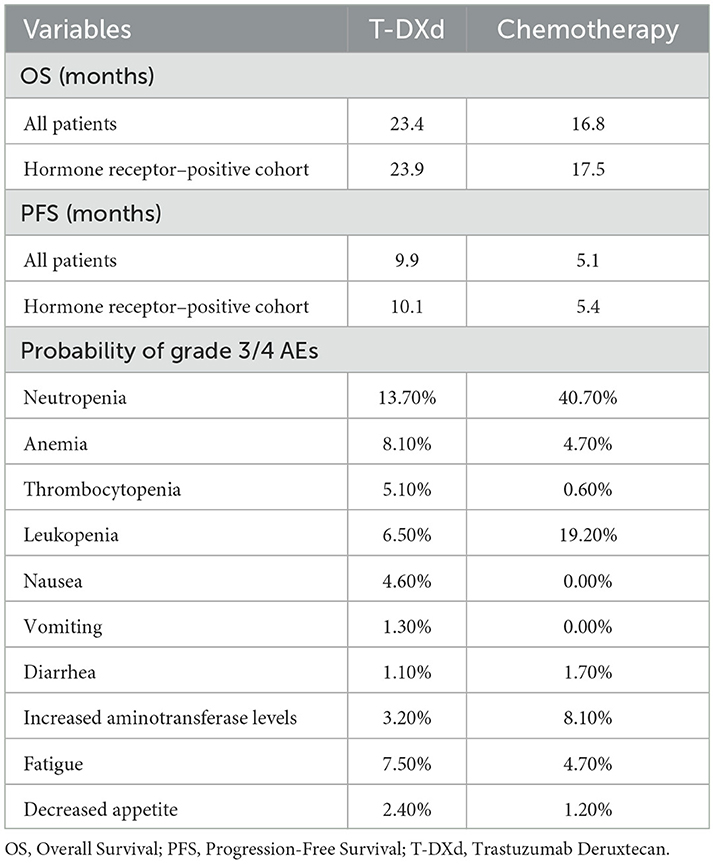
The incidence of adverse events (AEs) required to estimate the management cost of AEs was obtained from DESTINY-Breast04, more details ware shown Table 2. As quality-of-life data was not collected in DESTINY-Breast04, health state utility scores were derived from previously published literature. The utility values of PFS state, PD state and death were 0.843, 0.60 and 0, respectively (15).
2.4. Cost estimates
Direct medical costs consisted of drug treatment costs, AEs treatment costs, follow-up costs, hospital service costs, and best supportive care (BSC) costs, were estimated from the perspective of the Chinese healthcare system. Resource costs except for the drug treatment costs were obtained from Chinese studies.
Destiny-break04 did not provide a subsequent treatment plan for patients whose diseases progressed on T-Dxd or physician's choice of chemotherapy; according to the guideline, BSC is recommended for these patients as they have already received two lines of therapy (16). Costs related to subsequent BSC were derived from published literatures (17). The dosages of chemotherapy agents and T-Dxd were calculated based on standard human body surface area of 1.72 m2 and a standard female bodyweight of 55 kg, respectively (18). Although T-Dxd is yet to be approved for Chinese market, it became available in Hainan's Boao Lecheng International Medical Tourism Pilot Zone in February 2022. For this study, the price for T-Dxd in Chinese market was set with reference to the marketing price of T-Dxd in Boao. Prices of other drugs used in this study were calculated based on the median winning prices of the bid-winning products on https://www.yaozh.com/.
DESTINY-Breast04 reported data on incidences of adverse events (AEs). Only the costs related to managing grade 3 or higher AEs were included for this study; grade 1–2 AEs were considered manageable within standard patient monitoring. The costs of managing grade 3–5 AEs were derived from previously published economic studies (18–23). Detailed information was shown in Table 3.
2.5. Sensitivity analysis
One-way sensitivity analysis and probabilistic sensitivity analyses (PSA) were performed to examine the potential influence on the results. In one-way sensitivity analysis, the most parameters of costs and utilities were varied at a range of ± 20% of their baseline value, and the range of discount rate was from 0 to 8%. Since T-Dxd has not been approved in Chinese Mainland, the price of trastuzumab deruxtecan may decrease sharply in the future. Therefore, the minimum cost of T-Dxd was set to a 50% decrement from the baseline value. The One-way sensitivity analysis results were presented in a tornado diagram. A PSA was performed by using Monte Carlo simulation of 1,000 iterations to assess the robustness of the estimated cost-effectiveness ratio. Gamma and Beta distributions were adopted for costs and utilities, respectively. The results of the PSA were represented by an acceptable curve and incremental cost-effectiveness scatter plot.
3. Results
3.1. Base-case analysis
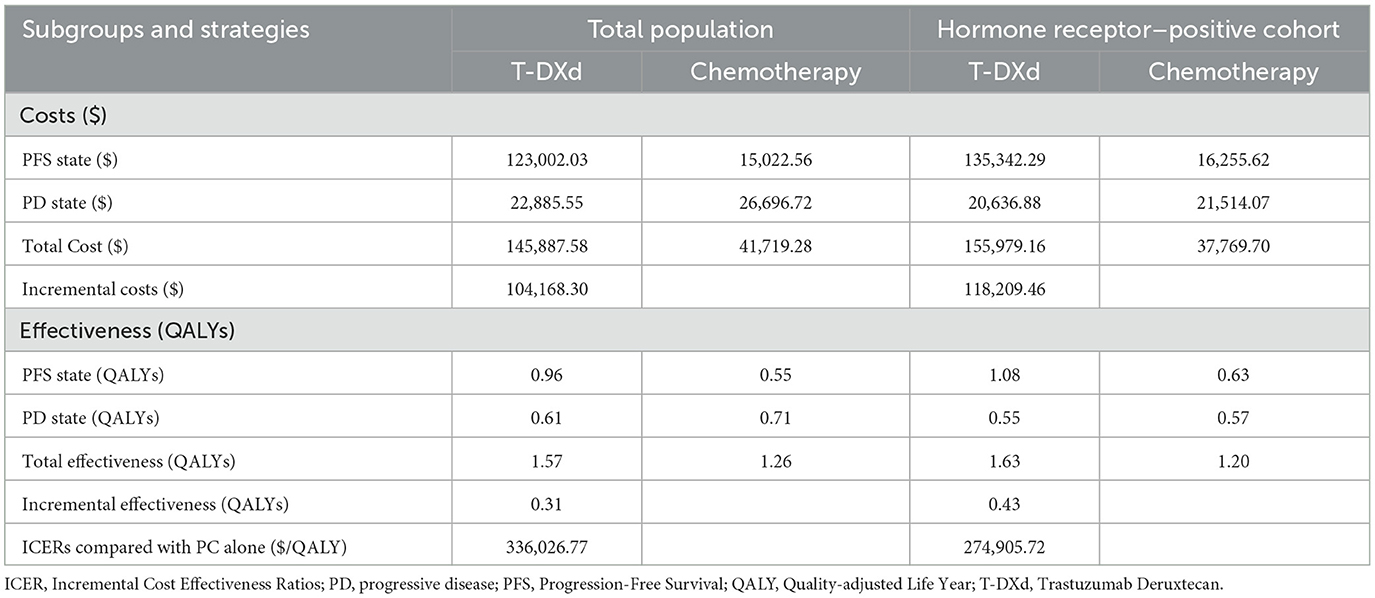
In the base-case analysis, among all patients, the total cost was $145,887.58 for the T-Dxd group and $41,719.28 for the chemotherapy group. The overall QALYs in the T-Dxd group were higher than that in the chemotherapy group (1.57 QALYs vs. 1.26 QALYs). The incremental cost-effectiveness ratio (ICER) was $336,026.77 per QALY, which was more than 9 times the WTP threshold for cost-effectiveness ($357,96.83 per QALY in China). In the hormone receptor–positive cohort, the T-Dxd group comprised even higher QALY. The T-Dxd group cost $118,209.46 more than the chemotherapy group while providing additional 0.43 QALYs, leading to an ICER of $274,905.72 per QALY in the hormone receptor–positive cohort. The details are listed in Table 4.
3.2. Sensitivity analysis
The results of the one-way sensitivity analysis were shown in Figure 3. In both the hormone receptor–positive cohort and all patients, the cost of T-Dxd and the utility of PFS were the most influential factors on the results. In addition, the cost of chemotherapy and the utility of PD had moderate impact on ICER. Other parameters such as discount rate, costs of PD, AEs, hospitalization and follow-up had minor impact on the robustness of the cost-effectiveness analysis. More details were shown in Figure 3.
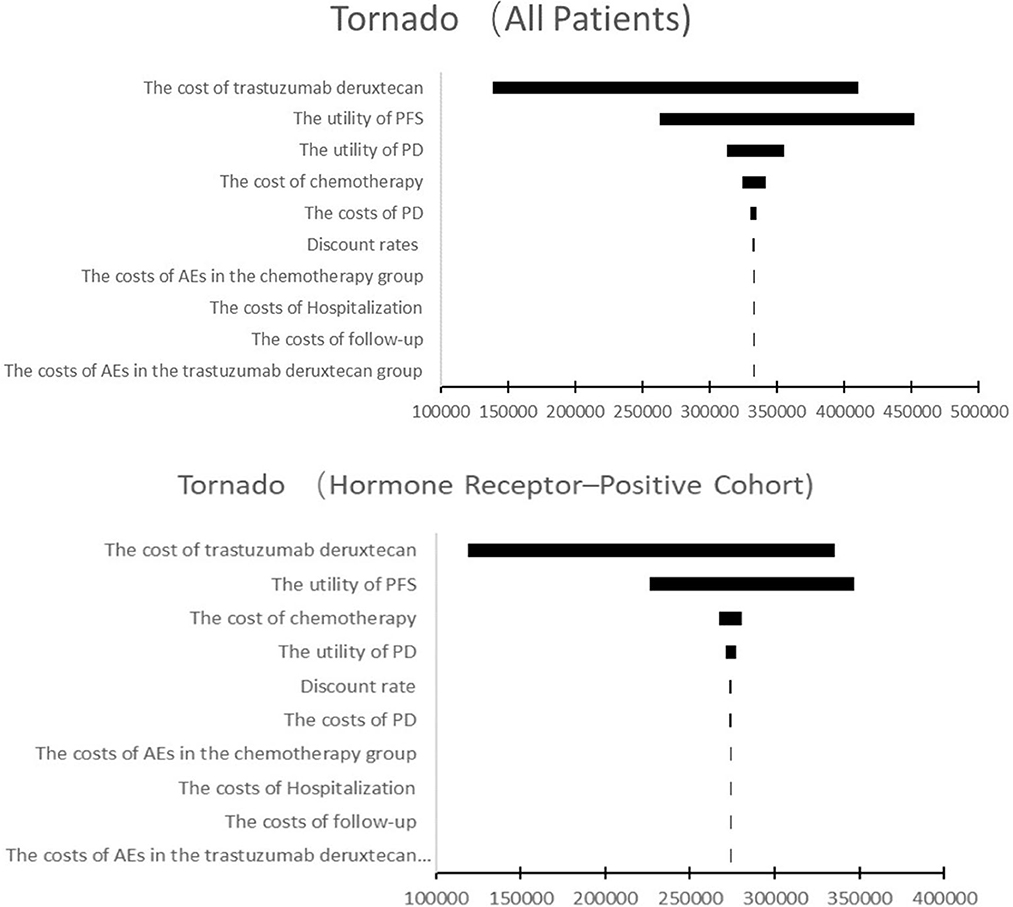
Figure 3. Tornado diagram of one-way sensitivity analysis. This summarizes the results of one-way sensitivity analysis, listing influential parameters in descending order according to their effect on the ICER over the variation of each parameter value. PFS, progression-free survival; PD, progressive disease; AE, adverse event.
T-Dxd would not be cost-effective unless the threshold of the CEA sharply raise to about $170,000–$225,000 per QALY (Figure 4), which seems impossible as China's GDP cannot reach this level in the short term. The PSA suggested that compared with chemotherapy, the probability of T-Dxd being cost-effective was 0% at the WTP threshold of $35,796.83/QALY in both all patients and the hormone receptor–positive cohort (Figure 5). The results of PSA demonstrated that the T-Dxd had no economic advantage over the traditional chemotherapy in China in the near future.
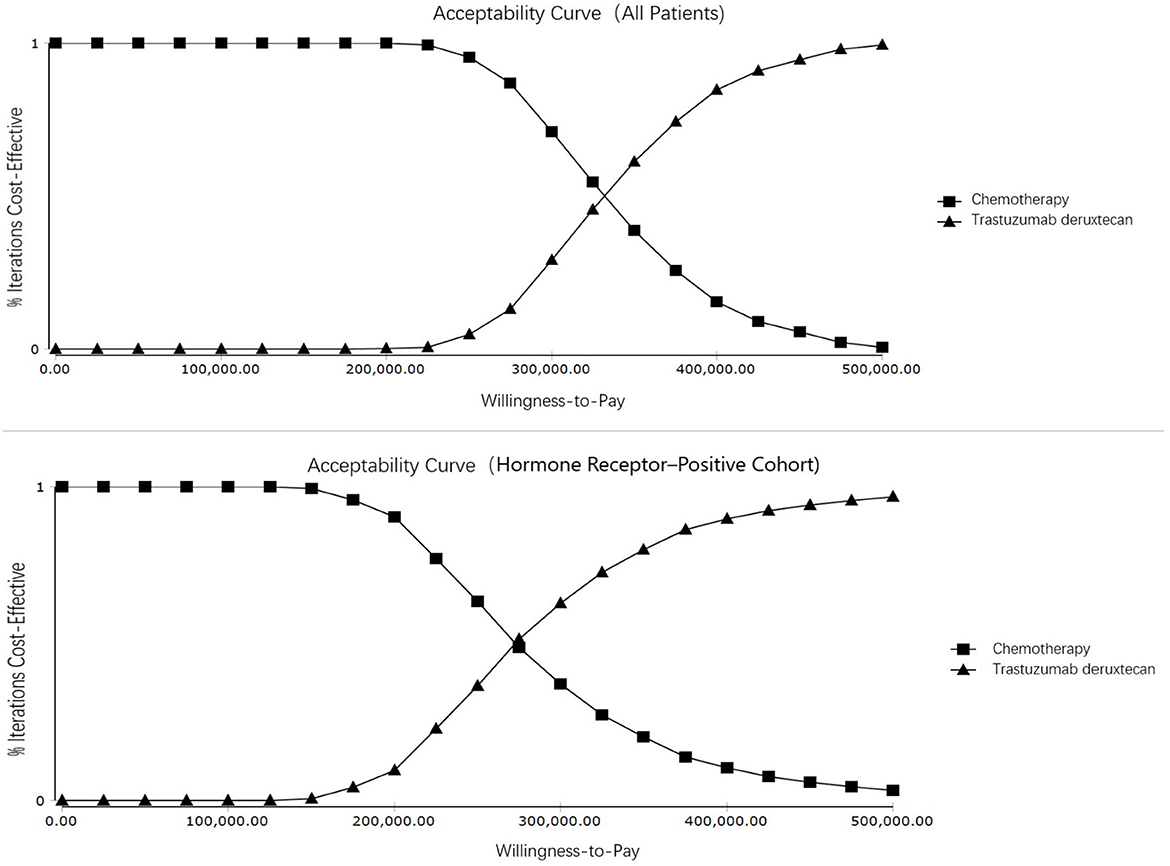
Figure 4. Cost-effectiveness acceptability curves. Cost-effectiveness acceptability curves show the probability of each treatment strategy being cost-effective at different willingness-to-pay thresholds.
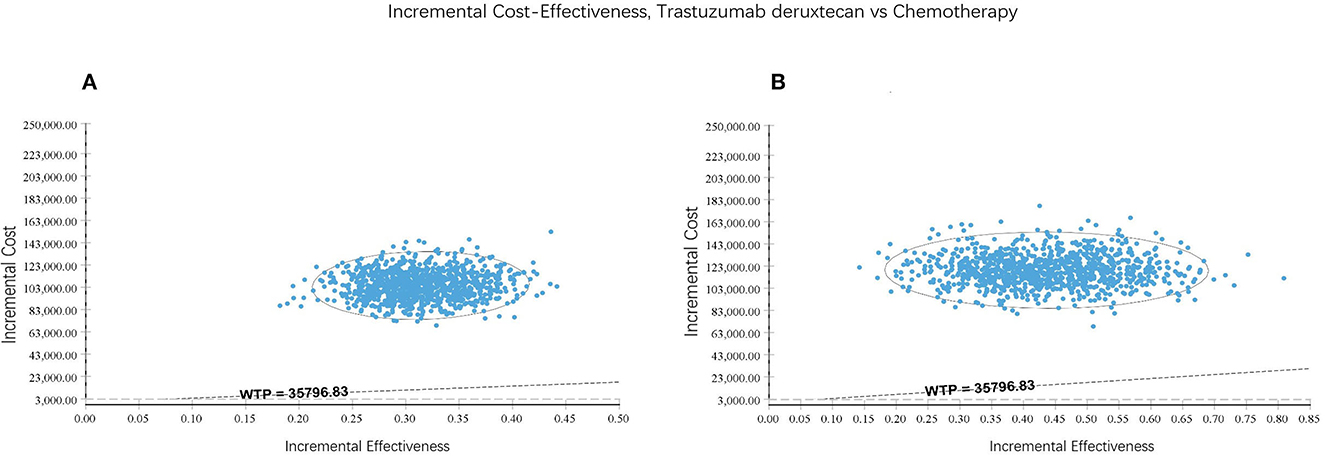
Figure 5. Incremental cost-effectiveness scatter plot reflected the variation and concentration of the incremental cost-effectiveness ratio values in probabilistic sensitivity analysis. (A) All patients. (B) Hormone receptor-positive cohort.
4. Discussion
DESTINY serial studies were launched since the approval of T-Dxd. DESTINY-Breast−02, 03 and 04 studies discovered positive results in T-Dxd groups, changing treatment paradigms in breast cancer (9, 24, 25). As a novel therapy, T-Dxd was associated with high economic burden; therefore, pharmacoeconomic research based on DESTINY trials was warranted to evaluate its cost-effectiveness (26–29). Previously, Zhu et al. conducted a Markov decision-analytic model to evaluate the cost-effectiveness of T-DXd for HER2-low metastatic breast cancer in the United States; their study demonstrated that T-DXd was not cost-effective for patients with HER2-low advanced breast cancer comparing to chemotherapy in the United States. However, by December 2022, there has been no pharmacoeconomic evaluation based on DESTINY-Breast04 from the perspective of Chinese healthcare system. In this study, we proved that T-Dxd was not cost-effective for advanced HER2-low breast cancer compared with chemotherapy from the perspective of Chinese healthcare system using a three-state partitioned-survival model. The price of T-Dxd had highest impact on the ICER, which also aligns with the result from Zhu et al.
In 2013, The State Council officially approved the establishment of Hainan Boao Lecheng International Medical Tourism Pilot Zone, making Boao Lecheng the only area in mainland China that can market drugs that have been approved abroad but not yet marketed in mainland China. The price of T-Dxd was set at the marketing price in Boao for this study, but it may substantially decrease in the next few years as with anticipation of national approval by 2023. At present, anti-tumor drugs must go through national medical insurance negotiations to enter the Chinese medical insurance formulary. In 2022, the average price reduction of 67 drugs upon entering the national medical insurance formulary was 61.71%. In the previous 3 years, the price reductions were 56.7, 60.7, and 53.8% respectively through negotiations led by the National Healthcare Security Administration. Considering the price of T-Dxd may drastically decreased when it enters the Chinese medical insurance formulary, the minimum cost of T-Dxd was set to a 50% decrement from the baseline value in the one-way sensitivity analysis. However, even with the 50% price decrease, the resulting ICER of $162,768.63 per QALY was still much higher than the preset WTP.
WTP is a critical parameter to determine whether the treatment is cost-effective. When the ICER was lower than the WTP, the treatment was considered to be favorably cost-effective. Currently, the WHO standard of WTP setting at 1–3 times GDP per capita is still widely used (30, 31). However, some studies have suggested that three times of GDP per capita is too high for WTP (32, 33). For patients at end of life, the National Institute of Health and Clinical Excellence (NICE) have raised the WTP threshold for life-extending treatments that are not considered cost-effective with conventional WTP (34). At present, there is lack of effective treatment for HER2-low metastatic breast cancer refractory to standard treatment. The expected survival of these patients is < 24 months, and T-Dxd could extend their survival time by more than 3 months comparing to single-agent chemotherapy. Therefore, we chose a high WTP threshold based on the NICE standard. But even if a high WTP is set, the results of this study showed that T-Dxd is still not cost-effective. Additionally, due to a series of new policies such as national centralized drug procurement and national medical insurance negotiations, the prices of anti-cancer drugs in China have greatly reduced in recent years. Therefore, in addition to the predicted price reduction of T-Dxd, the cost of alternative chemotherapy is also expected to decline, which may trigger the ICER to increase even higher. The results of PSA demonstrated that T-Dxd had no chance in practice to be cost-effective at the current payment threshold in China.
There are some limitations with this model-based cost-effectiveness analysis. Imprecise estimates and assumptions were made where it was necessary. First, the one-way sensitivity analysis showed the assumed cost of T-Dxd had significant impact on the results, but the T-Dxd price may drastically fluctuate in the next few years upon national approval. Secondly, DESTINY-Breast04 did not provide the information about the utility scores of the PFS and PD, thus the utility value referenced in this study was not based on Chinese population. Moreover, DESTINY-Breast04 only reported the AE rates for all patients. We hypothesized that the AE incidences were similar among the hormone receptor–positive cohort and all patients, thereby the cost of AEs was estimated based on the AE incidences of all patients. As of December 2022, T-Dxd has not yet been approved for marketing in Chinese mainland; therefore, we performed model-based cost-effectiveness analyses based on the RCT DESTINY-Breast04, the results of which may deviate from real world experience. As a result, imprecise estimates and assumptions were inevitable. The robustness was measured using sensitivity analysis and the results of sensitivity analyses showed that the results were stable.
In conclusion, although T-Dxd in previously treated HER2-low advanced breast cancer showed excellent clinical efficacy, the results of our study suggested that T-Dxd, comparing with single agent chemotherapy, was not cost effective from the perspective of the Chinese healthcare system. Another drug of the ADC class called T-DM1 proactively reduced its price by 50% after T-Dxd filed its application for marketing, therefore T-Dxd may need to mark down its price by a huge degree to appear cost-effective.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Data curation: MZ, ZH, and HZ. Formal analysis: MZ, XX, and FW. Methodology: TX and FW. Writing—original draft: MZ and ZH. Writing—review and editing: FW. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. (2021) 41:1183–94. doi: 10.1002/cac2.12207
3. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. (2020) 38:1951–62. doi: 10.1200/JCO.19.02488
4. Kurozumi S, Padilla M, Kurosumi M, Matsumoto H, Inoue K, Horiguchi J, et al. HER2 intratumoral heterogeneity analyses by concurrent HER2 gene and protein assessment for the prognosis of HER2 negative invasive breast cancer patients. Breast Cancer Res Treat. (2016) 158:99–111. doi: 10.1007/s10549-016-3856-2
5. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast Cancer, Version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:691–722. doi: 10.6004/jnccn.2022.0030
6. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, A Novel HER2-targeting ADC with a novel DNA topoisomerase i inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. (2016) 22:5097–108. doi: 10.1158/1078-0432.CCR-15-2822
7. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. (2016) 107:1039–46. doi: 10.1111/cas.12966
8. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. (2020) 38:1887–96. doi: 10.1200/JCO.19.02318
9. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
10. National Comprehensive Cancer Network. Breast Cancer, Version 4.2022. (2022). Avialable online at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed August 21, 2022).
11. Aogi K, Iwata H, Masuda N, Mukai H, Yoshida M, Rai Y, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. (2012) 23:1441–8. doi: 10.1093/annonc/mdr444
12. Liu GG, Hu S, Wu J, Wu J, Dong Z, Li H. China Guidelines for Pharmacoeconomic Evaluations. (2020). Available online at: https://www.ispor.org/docs/default-source/heor-resources-documents/pe-guidelines/chinaguidelines-for-pharmacoeconomicevaluations-2020.pdf?sfvrsn=446b6f6_3 (accessed August 23, 2022).
13. Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. (2016) 94:925–30. doi: 10.2471/BLT.15.164418
14. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
15. Paracha N, Thuresson PO, Moreno SG, MacGilchrist KS. Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev Pharmacoecon Outcomes Res. (2016) 16:549–59. doi: 10.1080/14737167.2016.1222907
16. Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. (2021) 32:1475–95. doi: 10.1016/j.annonc.2021.09.019
17. Rui M, Shi F, Shang Y, Meng R, Li H. Economic evaluation of cisplatin plus gemcitabine versus paclitaxel plus gemcitabine for the treatment of first-line advanced metastatic triple-negative breast cancer in china: using markov model and partitioned survival model. Adv Ther. (2020) 37:3761–74. doi: 10.1007/s12325-020-01418-7
18. Wang H, Wang Y, Gong R, Geng Y, Li L. Cost-effectiveness of pertuzumab and trastuzumab as a first-line treatment of HER2-positive metastatic breast cancer in China. Annal Palliative Med. (2021) 10:11382–93. doi: 10.21037/apm-21-2412
19. Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen H, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS ONE. (2012) 7:e32530. doi: 10.1371/journal.pone.0032530
20. Liubao P, Xiaomin W, Chongqing T, Karnon J, Gannong C, Jianhe L, et al. Cost-effectiveness analysis of adjuvant therapy for operable breast cancer from a Chinese perspective: doxorubicin plus cyclophosphamide versus docetaxel plus cyclophosphamide. PharmacoEconomics. (2009) 27:873–86. doi: 10.2165/11314750-000000000-00000
21. Lang Y, Dong D. Cetuximab plus chemotherapy versus chemotherapy alone in recurrent or metastatic head and neck squamous cell carcinoma: a cost-effectiveness analysis. Cancer Manag Res. (2020) 12:11383–90. doi: 10.2147/CMAR.S272149
22. Wang H, Zeng C, Li X, Wang Y, Li X, Ge W. Cost-utility of afatinib and gefitinib as first-line treatment for EGFR-mutated advanced non-small-cell lung cancer. Fut Oncol. (2019) 15:181–91. doi: 10.2217/fon-2018-0692
23. Dranitsaris G, Yu B, King J, Kaura S, Zhang A. Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in metastatic breast cancer: a cost-utility analysis from a Chinese health care perspective. ClinicoEcon Outcomes Res. (2015) 7:249–56. doi: 10.2147/CEOR.S82194
24. Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. (2022) 386:1143–54. doi: 10.1056/NEJMoa2115022
25. Hurvitz SA. DESTINY-changing results for advanced breast cancer. N Engl J Med. (2022) 387:75–6. doi: 10.1056/NEJMe2206661
26. Zhu Y, Liu K, Wang M, Wang K, Zhu H. Trastuzumab deruxtecan versus trastuzumab emtansine for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: A cost-effectiveness analysis. Breast. (2022) 66:191–8. doi: 10.1016/j.breast.2022.10.010
27. Yang J, Han J, Zhang Y, Muhetaer M, Chen N, Yan X. Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine for HER2-positive breast cancer. Front Pharmacol. (2022) 13:924126. doi: 10.3389/fphar.2022.924126
28. Wang J, Yi Y, Wan X, Zeng X, Peng Y, Tan C. Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer in the USA. Adv Ther. (2022) 39:4583–93. doi: 10.1007/s12325-022-02273-4
29. Zhu Y, Liu K, Zhu X, Qin Q, Zhu H. Trastuzumab deruxtecan versus chemotherapy for patients with HER2-low advanced breast cancer: a US-based cost-effectiveness analysis. Front Pharmacol. (2022) 13:1025243. doi: 10.3389/fphar.2022.1025243
30. Leech AA, Kim DD, Cohen JT, Neumann PJ. Use and misuse of cost-effectiveness analysis thresholds in low- and middle-income countries: trends in cost-per-DALY studies. Value Health. (2018) 21:759–61. doi: 10.1016/j.jval.2017.12.016
31. Kazibwe J, Gheorghe A, Wilson D, Ruiz F, Chalkidou K, Chi YL. The use of cost-effectiveness thresholds for evaluating health interventions in low- and middle-income countries from 2015 to 2020: a review. Value Health. (2022) 25:385–9. doi: 10.1016/j.jval.2021.08.014
32. Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. (2016) 19:929–35. doi: 10.1016/j.jval.2016.02.017
33. Ochalek J, Wang H, Gu Y, Lomas J, Cutler H, Jin C. Informing a cost-effectiveness threshold for health technology assessment in China: a marginal productivity approach. PharmacoEconomics. (2020) 38:1319–31. doi: 10.1007/s40273-020-00954-y
Keywords: cost-effectiveness, breast cancer, HER2-low, trastuzumab deruxtecan, chemotherapy
Citation: Zhan M, Huang Z, Xu T, Xu X, Zheng H and Wu F (2023) Cost-effectiveness analysis of trastuzumab deruxtecan in patients with HER2-low advanced breast cancer based on DESTINY-Breast04. Front. Public Health 11:1049947. doi: 10.3389/fpubh.2023.1049947
Received: 21 September 2022; Accepted: 19 June 2023;
Published: 30 June 2023.
Edited by:
Nick Verhaeghe, Ghent University, BelgiumReviewed by:
Uygar Halis Tazebay, Gebze Technical University, TürkiyeHaiying Ding, University of Chinese Academy of Sciences, China
Jiaxing Zhang, Guizhou Provincial People's Hospital, China
Copyright © 2023 Zhan, Huang, Xu, Xu, Zheng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengbo Wu, d2ZiX3NjQDE2My5jb20=
 Mei Zhan
Mei Zhan Zijia Huang
Zijia Huang Ting Xu
Ting Xu Xinyi Xu
Xinyi Xu Hanrui Zheng
Hanrui Zheng Fengbo Wu
Fengbo Wu