- 1College of Public Health, Shanghai University of Medicine & Health Sciences, Shanghai, China
- 2Shanghai Key Laboratory of Maternal Fetal Medicine, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, China
- 3Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada
Objective: Postpartum hemorrhage (PPH) is the leading cause of maternal morbidity and mortality. Identifying women who are at high risk of PPH is crucial for implementing early preventive and interventive strategies. This study aimed to examine whether there is an association between the use of in vitro fertilization (IVF) /intracytoplasmic sperm injection (ICSI) and increased risk of PPH.
Method: This retrospective cohort study was conducted using medical record data from women who delivered at a tertiary hospital in Shanghai, China, between January 1, 2013 and April 30, 2019. Logistic regression analysis was used to estimate the associations between the use of IVF/ICSI and the risk of PPH.
Results: A total of 153,765 pregnant women were included, of which 6,484 conceived through IVF/ICSI and147,281 conceived naturally. The incidence of PPH was 1.9% in this cohort. The incidence of PPH in women who conceived through IVF/ICSI was significantly higher than those in women who conceived naturally (3.4% vs. 1.7%, p < 0.01). The use of IVF/ICSI was associated with an increase in the amount of postpartum blood loss. Compared to women who conceived naturally, the average amount of postpartum blood loss increased by 42.1 mL (β = 42.1, 95% CI, 38.2–46.0) for women who conceived through IVF/ICSI. In addition, women who conceived through IVF/ICSI were at higher risk of maternal PPH. The adjusted odds ratio (OR) of PPH in women who conceived through ART was 2.7 (OR = 2.7, 95% CI, 2.3–3.1).
Conclusion: Our findings demonstrated that women who conceived through IVF/ICSI were at higher risk of PPH and suggested to obstetricians and midwives to identify and implement early preventative strategies for PPH among pregnant women who conceived through IVF/ICSI.
Introduction
In recent years, extensive evidence has suggested a global upward trend in infertility (1–3). Reproductive-aged couples increased their use of assisted reproductive technology (ART), such as in-vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), to treat infertility for the past 30 years (4, 5).
With the increasing success rate of ART, more and more attention has been paid to the safety of ART treatment (5). It is well recognized that the use of ART increases the incidence of adverse birth outcomes, including preterm birth (PTB) (6, 7), low birth weight (LBW) (8–10) and some birth defects (11–13). Regarding maternal health outcomes, some recent studies reported that women who underwent ART were at increased risk of pregnancy complications, such as gestational hypertension, preeclampsia and gestational diabetes (GDM) compared with women who conceived spontaneously (14, 15).
Postpartum hemorrhage (PPH) is one of the most common maternal complications of pregnancy. It is the leading cause of maternal morbidity and mortality, and has posed critical health challenges globally (16–18). However, only three studies with different diagnostic criteria of PPH have examined the associations of ART treatment with the risk of maternal PPH, and those three studies all demonstrated that pregnant women who underwent ART were at higher risk of PPH (14, 19, 20). In this study, we examined the associations between the use of IVF/ICSI and risk of maternal PPH among a large cohort of 153,765 Chinese pregnant women.
Methods
Study participants
Data in this retrospective cohort study were derived from the electronic medical record system of the Shanghai First Maternity and Infant Hospital (one of the largest prenatal care providers in Shanghai, China, with about 25,000 deliveries per year). The hospital’s electronic medical record system included data on maternal demographic characteristics, reproductive history, pregnancy complications and pregnancy outcomes. We used data of all women who gave birth between January 1, 2013 and April 30, 2019. After excluding multiple deliveries to the same woman, women with stillbirth, undergoing labor induction, delivery with fetal anomalies or women with missing data (N = 16,911), 153,765 pregnancies were included in this analysis. This study was approved by the Ethics Committee of the Shanghai First Maternity and Infant Hospital. Individual consent was waived with permission from the ethics committee because all data were deidentified.
Exposure and outcome definitions
Blood loss was measured gravimetrically after each delivery as follows. Conical drapes were placed at the end of the bed to collect blood lost during and after each vaginal delivery. During cesarean section, a nurse counted the number of blood soaked cotton gauzes and assessed the blood collected in a container. Nurses also measured blood loss during ward rounds. Two specific outcomes were identified: a continuous outcome that measured the volume of postpartum blood loss; and a binary outcome of PPH or not. Specifically, we estimated the effect of the use of IVF/ICSI on those two outcomes: (1) postpartum blood loss (in mL), defined as the amount of postpartum bleeding within 24 h of delivery; (2) PPH, defined as blood loss ≥500 mL for vaginal delivery or blood loss ≥1,000 mL for cesarean section. This definition was based on guidelines from the Chinese Society of Obstetrics and Gynecology and Chinese Medical Association (21).
The use of ART is hereby defined as the use of technology, including in vitro treatment of human oocytes or sperm, to assist human reproduction in the treatment of infertility (22, 23). These treatments include in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).
Covariate variables
In all analyses, we adjusted for maternal demographic factors, pregnancy-related complications and reproductive history that have been suggested to be associated with postpartum bleeding (24–27). Those covariate variables included maternal age at delivery, parity, gravidity, hypertensive disorders in pregnancy (HDP), GDM, mode of delivery, prolonged second stage of labor, intra-amniotic infection, gestational age at delivery, newborn birthweight, and placental abnormality (placental abruption, placenta previa, low-lying placenta, placenta accrete and increta).
Statistical analysis
We used two different approaches to estimate the associations between the use of IVF/ICSI and two specific outcomes. First, we used ordinary least squares (OLS) regression analysis to evaluate the association of IVF/ICSI with the amount of postpartum blood loss (continuous variable), and then we used logistic regression analysis to assess the association of IVF/ICSI with the binary outcome of PPH (blood loss ≥500 mL following a vaginal delivery or blood loss ≥1,000 mL following cesarean section).
We subsequently performed stratified analyses to explore whether the associations between the use of IVF/ICSI and two postpartum bleeding outcomes differed according to mode of delivery (vaginal delivery or cesarean section) or maternal age at delivery (<35 years or ≥ 35 years). As a sensitivity analysis to examine the impact of pregnancy complications, we replicated all models restricted to women without HDP or GDM. In another sensitivity analysis, we replicated all models restricted to women without placental abnormality in order to evaluate the impact of placental abnormality. All estimates were performed using the STATA 14. A 2-sided p value less than 0.05 was considered statistically significant.
Results
Characteristics of the participants
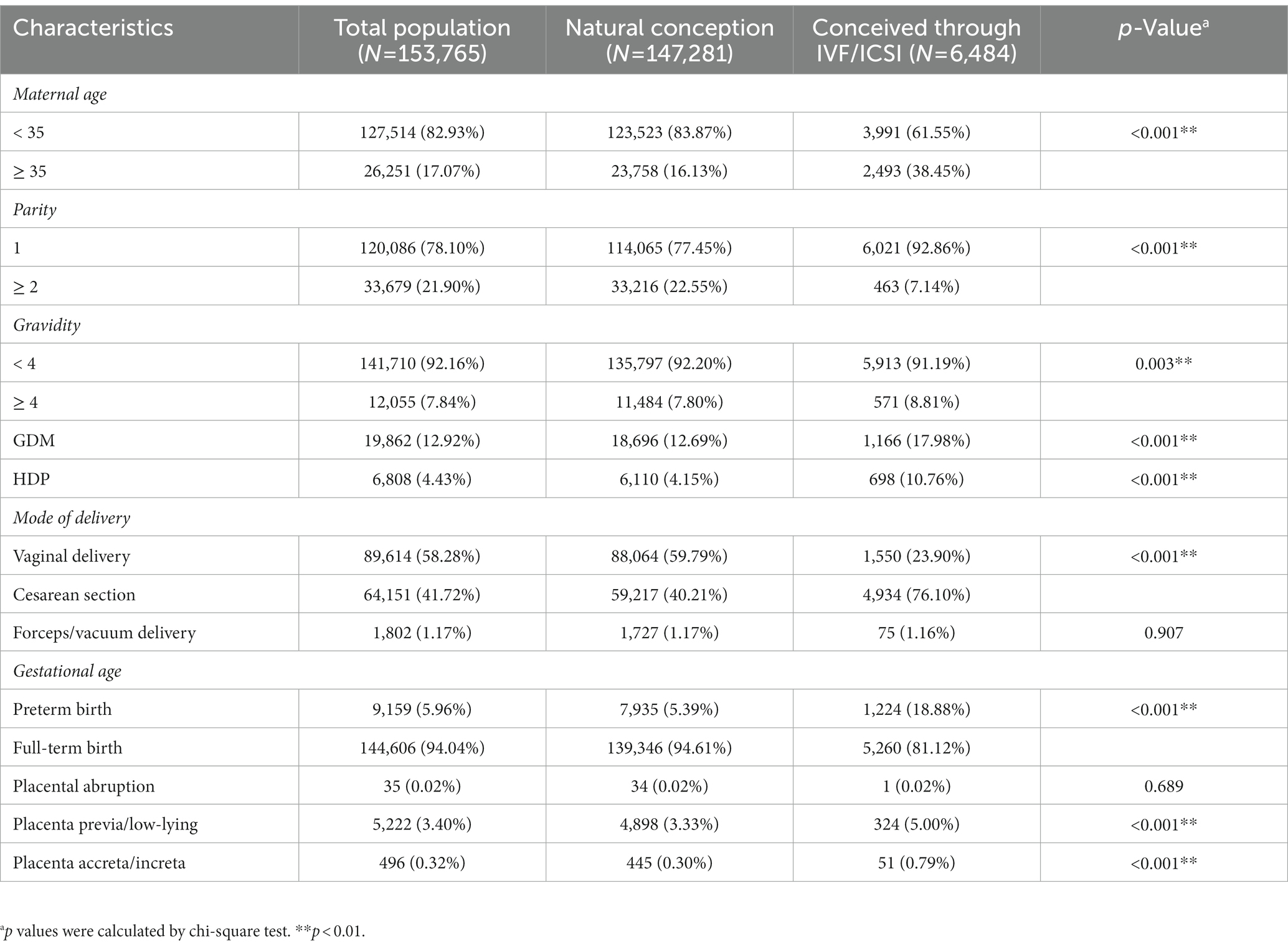
The overall cohort included 153,765 women, of whom 6,484 (4.22%) conceived through IVF/ICSI. The general characteristics of the participants are summarized in Table 1. The majority of women (82.93%) were aged less than 35 years old. The proportion of women above the age of 35 was 2.4-fold higher among women who conceived through IVF/ICSI than those in women who conceived naturally (38.45% vs. 16.13%, p < 0.01). Compared to women who conceived naturally, those who conceived through IVF/ICSI were more likely to be primiparous (92.86% vs. 77.45%, p < 0.01), more likely to have GDM (17.98% vs. 12.69%, p < 0.01) or HDP (10.76% vs. 4.15%, p < 0.01) and more like to have placental abnormality including placenta previa/low-lying (5.00% vs. 3.33%, p < 0.01) and placenta accreta/increta (0.79% vs. 0.30%, p < 0.01).
Distributions of the amount of postpartum blood loss or PPH
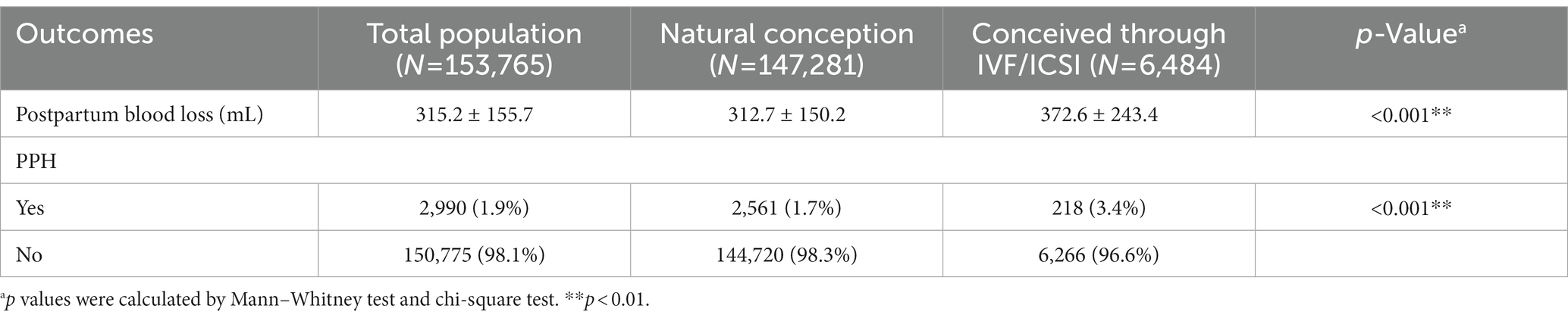
The amount of postpartum blood loss within 24 h of delivery was measured. As shown in Table 2, the mean amount of postpartum blood loss was 315.2 (155.7) mL. Analyses of potential differences in the amount of postpartum blood loss between the two groups showed that the amount of postpartum blood loss was significantly higher in women who conceived through IVF/ICSI than those in women who conceived naturally (372.6 vs. 312.7, p < 0.01).
According to the diagnostic criteria for maternal PPH, we identified 2,990 cases of PPH and the incidence of PPH was 1.9% in this cohort. The incidence of PPH in women who conceived through IVF/ICSI was significantly higher than those in women who conceived naturally (3.4% vs. 1.7%, p < 0.01).
Associations of IVF/ICSI with the amount of postpartum blood loss or PPH
Table 3 showed the associations between the use of IVF/ICSI and the amount of postpartum blood loss. The use of IVF/ICSI was associated with a significant increase in the amount of postpartum blood loss. Compared to women who conceived naturally, the average amount of postpartum blood loss increased by 42.1 mL (β = 42.1, 95% CI, 38.2–46.0) for women who conceived with the use of IVF/ICSI.
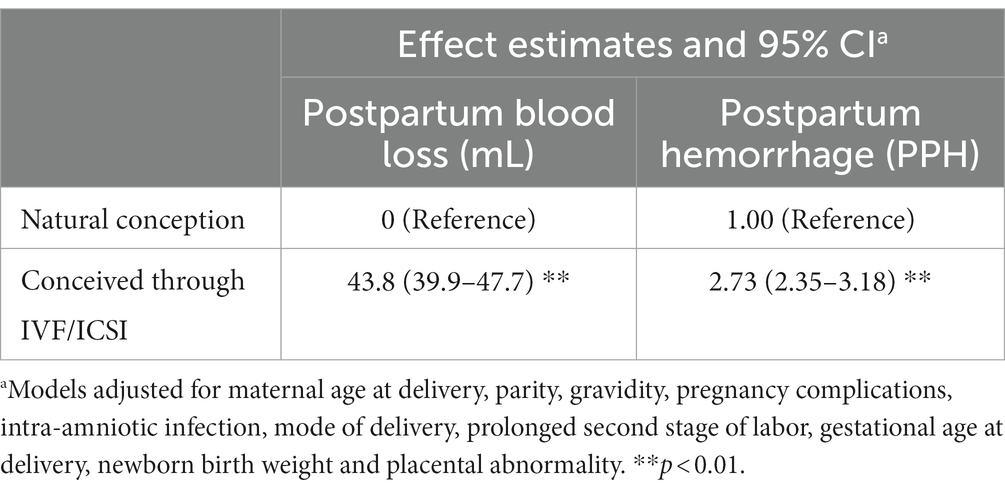
Table 3. Adjusted associations between the use of IVF/ICSI and the amount of postpartum blood loss or risk of PPH.
We subsequently examined the associations between the use of IVF/ICSI and risk of PPH. As shown in Table 3, the use of IVF/ICSI was found to be associated with increased odds of PPH. Compared to women who conceived naturally, the odds of PPH were doubled in women who conceived through IVF/ICSI and the adjusted OR was 2.7 (OR = 2.7, 95% CI, 2.3, 3.1).
Stratified and sensitive analysis
Table 4 showed the associations of IVF/ICSI with postpartum hemorrhage stratified by mode of delivery (vaginal delivery or cesarean section) or maternal age at delivery (< 35 years or ≥ 35 years). The associations of IVF/ICSI with risk of PPH were generally similar across different modes of delivery or different maternal age (overlapping confidence intervals). While we found maternal age at delivery modified the associations of IVF/ICSI with the amount of postpartum blood loss or PPH, and the observed associations were stronger among women aged less than 35 years old.
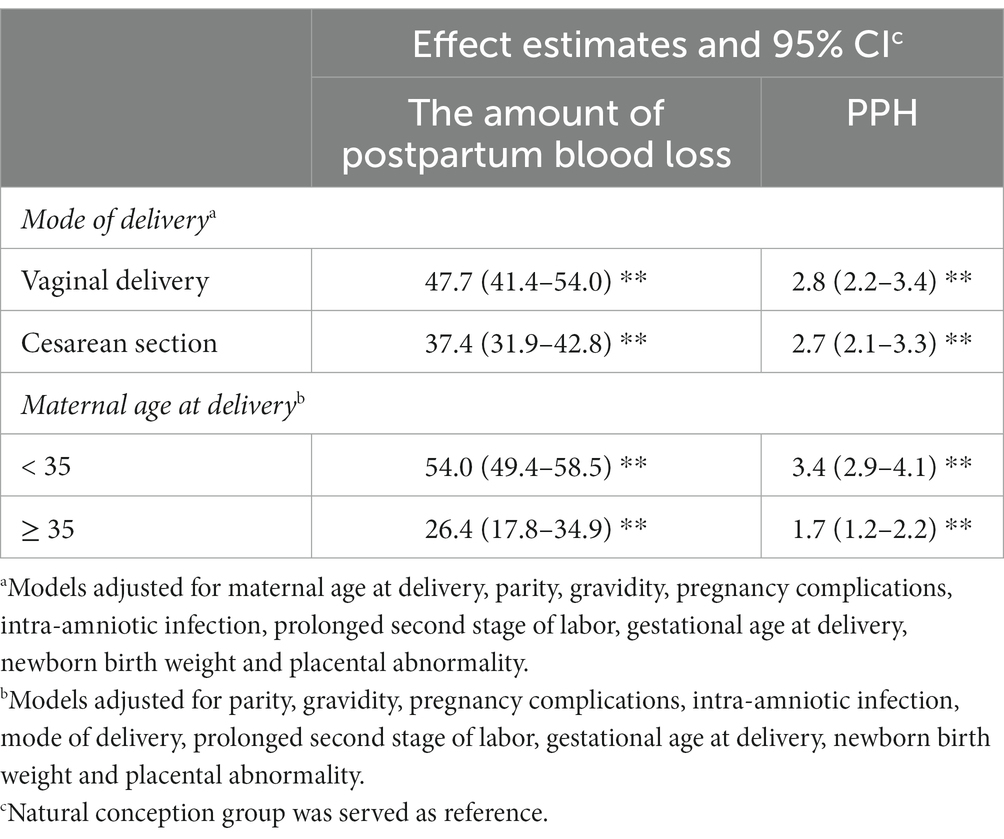
Table 4. Adjusted associations between the use of IVF/ICSI and the amount of postpartum blood loss or risk of PPH stratified by mode of delivery or maternal age at delivery.
In sensitivity analyses, when restricting all analysis to women without pregnancy complications, the associations of IVF/ICSI with postpartum blood loss or PPH were generally similar with those observed in all participants (Supplementary Table S1). Further, restricting the analysis to women without placental abnormality yielded generally similar results with those observed in all participants (Supplementary Table S2).
Discussion
PPH, especially severe PPH, contributes substantially to maternal morbidity, causing >50% of all events of severe maternal morbidity (28). Studies have shown an increasing trend in PPH, but the causes for the increase are still uncertain (29, 30). In this large retrospective cohort of 153,765 Chinese pregnant women, we compared PPH between women who conceived through IVF/ICSI and corresponding controls who conceived naturally. We found a significant increase in the amount of postpartum blood loss and higher risk of PPH among women who conceived through IVF/ICSI. Our findings suggest that women who conceived through IVF/ICSI should be allocated to delivery hospitals where adequate preventive and treatment measures are available for PPH.
Globally, an increasing proportion of pregnancies are conceived by ART, and therefore, more concerns are paid on the possible iatrogenic side effects of the treatment (31). We are aware that three previous studies have examined the associations between the use of ART and PPH (14, 19, 20). In one study conducted in Victoria, Australia, Healy DL et al., matched ART clinic data to birth certificate data for single births and found obstetric hemorrhages were more common in women who conceived through ART (20). In another study, Nyfløt Lt et al., used a case–control design of 3,123 women at the Oslo University Hospital and Drammen Hospital in Norway and observed an increased risk of severe PPH in women who conceived through ART procedures (19). Our results were consistent with those of previous studies.
Our stratified analysis indicated greater effects of IVF/ICSI on postpartum hemorrhage in younger women. It seems advanced maternal age protected against postpartum bleeding. However, it is difficult to draw this conclusion based on current evidence. Older women have reduced uterine vascularity, which may decrease PPH risk (32, 33). However, reduced uterine vascularity may also pose an increased risk for thinner endometrium and abnormal placentation, which in turn increases the risk of PPH. Our study suggests that it is imperative to implement intensive preventions and interventions (early cord clamping, controlled cord traction and continuous uterine massage) for younger pregnant women who conceived through IVF/ICSI.
Although the risk of PPH was found to be increased in women who conceived through IVF/ICSI, whether the increased risk was due to IVF/ICSI treatment themselves or maternal factors associated with infertility, was still unclear. In the study by Hayashi M et al., the authors reported that women who conceived through ART procedures were at increased risk for several adverse obstetric and perinatal outcomes, regardless of the type of ART procedure used, and concluded that maternal factors associated with infertility may contribute to the adverse outcomes rather than the ART treatment themselves (34). However, this study only focused on intrauterine insemination (IUI) and IVF and was unable to identify ICSI cycles. While a large proportion of women undergoing ICSI may have no maternal factors related to infertility. In another study by Romundstad LB et al., within unselected population, the authors found that placenta previa occurred 6 times more often in singleton pregnancies after ART compared with naturally conceived pregnancies. Further, by comparing consecutive pregnancies, where the mother conceived spontaneously in one pregnancy and after ART in the other, they still observed a nearly 3-fold higher risk of placenta previa in pregnancy following ART (31). They concluded that the higher risk of adverse pregnancy outcomes is most likely due to a combination of ART treatment and maternal factors associated with infertility.
Previous studies have shown that ART procedures may interfere with the formation of the maternal-fetal interface and disrupt the placentation (31, 34). In our results, placental abnormalities including placenta previa/low-lying (5.0% vs. 3.3%, p < 0.01) and placenta accrete/increta (0.79% vs. 0.30%, p < 0.01) are more likely to occur in women who conceived through ART. Those placental abnormalities are known risk factors of PPH (35–37). Additionally, several maternal factors including endometriosis, adenomyosis and hysteromyoma that associated with infertility are also known risk factors of PPH (38–40). Therefore, factors in women requiring ART procedures to conceive may also contribute to the increased risk of PPH. We speculated that the higher risk of PPH among pregnant women who conceived through ART is most likely due to a combination of placental abnormalities and maternal factors related to infertility.
It seems that the difference in blood loss (42 mL) between IVF/ICSI and the natural conception groups is not clinically significant. However, it has significant clinical implications for woman whose postpartum blood loss are close to 500 mL (vaginal delivery) or 1,000 mL (cesarean section), since a very small changes in postpartum blood loss may be enough to pass the threshold of PPH. To our knowledge, this is the largest study to examine the associations of ART with PPH. Given PPH is the leading cause of maternal morbidity and mortality, our findings may have important public health implications. However, there are also some limitations that warrant attention. First, although all participants were from one of the largest prenatal care providers in Shanghai, China, we could not generalize our findings to other countries due to the differences in race and ethnicity, and socio-economic status across different countries. Second, ART and infertility are sensitive issues, most participants did not report the exactly type of ART they used and the underlying infertility diagnosis. Therefore, we could not examine whether the increased risk of PPH among women conceived through ART procedures was due to ART treatment themselves or maternal factors associated with infertility. Third, we were unable to differentiate women with infertility but using other fertility treatments to conceive. These women would inevitably be included in the natural conceived group, which would bias the results toward null. Fourth, our data were derived from the hospital’s electronic medical record. Due to data inaccessibility, we could not adjust for some unmeasured confounders which may lower the certainty of the observed associations. Finally, this study is only an observation and the involvement of ART in any causal pathway of PPH has not been proven. Further studies are needed to explore the underlying mechanisms.
Conclusion
We found that women who conceived through ART procedures were at increased risk of PPH. Our findings suggest that obstetricians and midwives identify and implement early preventative strategies for PPH among pregnant women who conceived through ART. Decision-makers need to pay more attention to the issue of PPH as the trend will increase if the rate of infertility continues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Shanghai First Maternity and Infant Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
XL conceptualized and designed the study, reviewed and revised the initial manuscript. DT carried out the statistical analyses and drafted the initial manuscript. PC provided technical support for statistical analyses. YC and XF helped acquire the data and assisted in the interpretation of results. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Shanghai (20ZR1444000) and the Shanghai Hospital Development Center, Promotion and optimal management program for diagnosis and treatment of municipal hospitals (SHDC22022230).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1016457/full#supplementary-material
References
1. Inhorn, MC, and Patrizio, P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. (2015) 21:411–26. doi: 10.1093/humupd/dmv016
2. Boivin, J, Bunting, L, Collins, JA, and Nygren, KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. (2007) 22:1506–12. doi: 10.1093/humrep/dem046
3. Kumar, N, and Singh, AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. (2015) 8:191–6. doi: 10.4103/0974-1208.170370
4. Sunderam, S, Kissin, DM, Zhang, Y, Jewett, A, Boulet, SL, Warner, L, et al. Assisted reproductive technology surveillance-United States, 2018. MMWR Surveill Summ. (2022) 71:1–19. doi: 10.15585/mmwr.ss7104a1
5. Kushnir, VA, Barad, DH, Albertini, DF, Darmon, SK, and Gleicher, N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. (2017) 15:6. doi: 10.1186/s12958-016-0225-2
6. Bu, Z, Zhang, J, Hu, L, and Sun, Y. Preterm birth in assisted reproductive technology: an analysis of more than 20, 000 singleton newborns. Front Endocrinol (Lausanne). (2020) 11:558819. doi: 10.3389/fendo.2020.558819
7. Cavoretto, P, Candiani, M, Giorgione, V, Inversetti, A, Abu-Saba, MM, Tiberio, F, et al. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound Obstet Gynecol. (2018) 51:43–53. doi: 10.1002/uog.18930
8. Centers for Disease C, Prevention. Assisted reproductive technology and trends in low birthweight--Massachusetts, 1997-2004. MMWR Morb Mortal Wkly Rep. (2009) 58:49–52.
9. Reig, A, and Seli, E. The association between assisted reproductive technologies and low birth weight. Curr Opin Obstet Gynecol. (2019) 31:183–7. doi: 10.1097/GCO.0000000000000535
10. Chang, HY, Hwu, WL, Chen, CH, Hou, CY, and Cheng, W. Children conceived by assisted reproductive technology prone to low birth weight, preterm birth, and birth defects: a cohort review of more than 50,000 live births During 2011-2017 in Taiwan. Front Pediatr. (2020) 8:87. doi: 10.3389/fped.2020.00087
11. Liberman, RF, Getz, KD, Heinke, D, Luke, B, Stern, JE, Declercq, ER, et al. Assisted reproductive technology and birth defects: effects of subfertility and multiple births. Birth Defects Res. (2017) 109:1144–53. doi: 10.1002/bdr2.1055
12. Hansen, M, Kurinczuk, JJ, Milne, E, de Klerk, N, and Bower, C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. (2013) 19:330–53. doi: 10.1093/humupd/dmt006
13. Zhao, J, Yan, Y, Huang, X, and Li, Y. Do the children born after assisted reproductive technology have an increased risk of birth defects? A systematic review and meta-analysis. J Matern Fetal Neonatal Med. (2020) 33:322–33. doi: 10.1080/14767058.2018.1488168
14. Lei, LL, Lan, YL, Wang, SY, Feng, W, and Zhai, ZJ. Perinatal complications and live-birth outcomes following assisted reproductive technology: a retrospective cohort study. Chin Med J. (2019) 132:2408–16. doi: 10.1097/CM9.0000000000000484
15. Luke, B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol. (2017) 217:270–81. doi: 10.1016/j.ajog.2017.03.012
16. Knight, M, Callaghan, WM, Berg, C, Alexander, S, Bouvier-Colle, MH, Ford, JB, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the international postpartum hemorrhage collaborative group. BMC Pregnancy Childbirth. (2009) 9:55. doi: 10.1186/1471-2393-9-55
17. Andrikopoulou, M, and D'Alton, ME. Postpartum hemorrhage: early identification challenges. Semin Perinatol. (2019) 43:11–7. doi: 10.1053/j.semperi.2018.11.003
18. Soyer, P, Dohan, A, Dautry, R, Guerrache, Y, Ricbourg, A, Gayat, E, et al. Transcatheter arterial embolization for postpartum hemorrhage: indications, technique, results, and complications. Cardiovasc Intervent Radiol. (2015) 38:1068–81. doi: 10.1007/s00270-015-1054-y
19. Nyflot, LT, Sandven, I, Oldereid, NB, Stray-Pedersen, B, and Vangen, S. Assisted reproductive technology and severe postpartum haemorrhage: a case-control study. BJOG. (2017) 124:1198–205. doi: 10.1111/1471-0528.14471
20. Healy, DL, Breheny, S, Halliday, J, Jaques, A, Rushford, D, Garrett, C, et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod. (2010) 25:265–74. doi: 10.1093/humrep/dep376
21. Obstetrics Subgroup CSoO, Gynecology CMA. Obstetrics subgroup Chinese Society of O, gynecology Chinese medical a. [guideline of prevention and treatment about postpartum hemorrhage (2014)]. Zhonghua Fu Chan Ke Za Zhi. (2014) 49:641–6.
22. Zegers-Hochschild, F, Adamson, GD, Dyer, S, Racowsky, C, de Mouzon, J, Sokol, R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. (2017) 32:1786–801. doi: 10.1093/humrep/dex234
23. Datta, S, Kodali, BS, and Segal, S. The Obstetric Anesthesia Handbook. 5th ed. New York: Springer (2010).
24. Tan, J, Qi, YN, Zhang, J, Wang, W, Zhang, GT, Zou, K, et al. The mediation effect of multiple gestations on the association between in vitro fertilisation and severe maternal morbidities: a retrospective cohort study. BMJ Open. (2019) 9:e022670. doi: 10.1136/bmjopen-2018-022670
25. Pinborg, A, Loft, A, Rasmussen, S, Schmidt, L, Langhoff-Roos, J, Greisen, G, et al. Neonatal outcome in a Danish national cohort of 3438 IVF/ICSI and 10,362 non-IVF/ICSI twins born between 1995 and 2000. Hum Reprod. (2004) 19:435–41. doi: 10.1093/humrep/deh063
26. Oyelese, Y, and Ananth, CV. Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol. (2010) 53:147–56. doi: 10.1097/GRF.0b013e3181cc406d
27. Neary, C, Naheed, S, McLernon, DJ, and Black, M. Predicting risk of postpartum haemorrhage: a systematic review. BJOG. (2021) 128:46–53. doi: 10.1111/1471-0528.16379
28. Zwart, JJ, Richters, JM, Ory, F, de Vries, JI, Bloemenkamp, KW, and van Roosmalen, J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. BJOG. (2008) 115:842–50. doi: 10.1111/j.1471-0528.2008.01713.x
29. Callaghan, WM, Kuklina, EV, and Berg, CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. (2010) 202:353.e1. doi: 10.1016/j.ajog.2010.01.011
30. Kramer, MS, Berg, C, Abenhaim, H, Dahhou, M, Rouleau, J, Mehrabadi, A, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. (2013) 209:449.e1. doi: 10.1016/j.ajog.2013.07.007
31. Romundstad, LB, Romundstad, PR, Sunde, A, von During, V, Skjaerven, R, and Vatten, LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. (2006) 21:2353–8. doi: 10.1093/humrep/del153
32. Lao, TT, Sahota, DS, Cheng, YK, Law, LW, and Leung, TY. Advanced maternal age and postpartum hemorrhage -risk factor or red herring? J Matern Fetal Neonatal Med. (2014) 27:243–6. doi: 10.3109/14767058.2013.807240
33. Naeye, RL. Maternal age, obstetric complications, and the outcome of pregnancy. Obstet Gynecol. (1983) 61:210–6.
34. Hayashi, M, Nakai, A, Satoh, S, and Matsuda, Y. Adverse obstetric and perinatal outcomes of singleton pregnancies may be related to maternal factors associated with infertility rather than the type of assisted reproductive technology procedure used. Fertil Steril. (2012) 98:922–8. doi: 10.1016/j.fertnstert.2012.05.049
35. Dahlke, JD, Mendez-Figueroa, H, Maggio, L, Hauspurg, AK, Sperling, JD, Chauhan, SP, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. (2015) 213:76.e1. doi: 10.1016/j.ajog.2015.02.023
36. Chen, C, Liu, X, Chen, D, Huang, S, Yan, X, Liu, H, et al. A risk model to predict severe postpartum hemorrhage in patients with placenta previa: a single-center retrospective study. Ann Palliat Med. (2019) 8:611–21. doi: 10.21037/apm.2019.09.04
37. Borovac-Pinheiro, A, Ribeiro, FM, and Pacagnella, RC. Risk factors for postpartum hemorrhage and its severe forms with blood loss evaluated objectively -a prospective cohort study. Rev Bras Ginecol Obstet. (2021) 43:113–8. doi: 10.1055/s-0040-1718439
38. Vannuccini, S, Clifton, VL, Fraser, IS, Taylor, HS, Critchley, H, Giudice, LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. (2016) 22:104–15. doi: 10.1093/humupd/dmv044
39. Nirgianakis, K, Kalaitzopoulos, DR, Schwartz, ASK, Spaanderman, M, Kramer, BW, Mueller, MD, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod Biomed Online. (2021) 42:185–206. doi: 10.1016/j.rbmo.2020.09.023
Keywords: assisted reproductive technology, postpartum hemorrhage, retrospective cohort study, pregnant women, in vitro fertilization
Citation: Tang D, Cheng Y, Feng X, Li X and Coyte PC (2023) The use of IVF/ICSI and risk of postpartum hemorrhage: A retrospective cohort study of 153,765 women in China. Front. Public Health. 11:1016457. doi: 10.3389/fpubh.2023.1016457
Edited by:
Matthew J. Blitz, Donald and Barbara Zucker School of Medicine at Hofstra-Northwell, United StatesReviewed by:
Simona Zaami, Department of Anatomical, Histological, Medical, Legal and Locomotor Apparatus Sciences, Faculty of Pharmacy and Medicine, Sapienza University of Rome, ItalyNathan A. Keller, Hofstra University, United States
Copyright © 2023 Tang, Cheng, Feng, Li and Coyte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaocui Li, c2lzaTExMTNAMTYzLmNvbQ==
 Di Tang
Di Tang Yufeng Cheng2
Yufeng Cheng2 Xiaocui Li
Xiaocui Li Peter C. Coyte
Peter C. Coyte