
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 24 October 2022
Sec. Public Health and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.996447
This article is part of the Research Topic Assessment Methods in Human Nutrition and Metabolism for the Monitoring of Non-Communicable Chronic Diseases View all 16 articles
 Xiaoyu Chen1
Xiaoyu Chen1 Peipei Han1†
Peipei Han1† Xiaoyan Zhu2†
Xiaoyan Zhu2† Peiyu Song3
Peiyu Song3 Yinjiao Zhao3
Yinjiao Zhao3 Hui Zhang3
Hui Zhang3 Chen Yu4
Chen Yu4 Jianying Niu5
Jianying Niu5 Wei Ding6
Wei Ding6 Junli Zhao7
Junli Zhao7 Liming Zhang8
Liming Zhang8 Hualin Qi9
Hualin Qi9 Suhua Zhang10
Suhua Zhang10 Qi Guo1*
Qi Guo1*Background: Malnutrition, dynapenia, and sarcopenia are prevalent conditions among patients with maintenance hemodialysis (MHD). They are related to numerous adverse health outcomes. The aim of this study was to compare the effect of three nutritional screening tools on predicting the risk of dynapenia and sarcopenia in patients with MHD.
Methods: From July 2020 to April 2021, a total of 849 patients with MHD were enrolled at seven different healthcare facilities in Shanghai, China in this multi-center cross-sectional study. Geriatric nutritional risk index (GNRI), malnutrition inflammation score (MIS), and creatinine (Cr) index were used for nutritional assessment. The cutoff values of muscle mass and strength to define dynapenia, pre-sarcopenia, and sarcopenia were based on the consensus by the Asia Working Group of Sarcopenia in 2019.
Results: Among 849, almost 60% were malnourished with the majority suffering from dynapenia (27.7%), followed by sarcopenia (22.7%), and pre-sarcopenia (6.2%).The area under the receiver–operating characteristic curve for GNRI was 0.722 [95% confidence interval (CI) = 0.684–0.760] and 0.723 (95% CI = 0.663–0.783) in predicting sarcopenia and pre-sarcopenia. The GNRI [odds ratio (OR) =6.28, 95% CI: 4.05–9.73], MIS (OR =1.91, 95% CI: 1.31–2.78), and the Cr index (OR =2.73, 95% CI: 1.71–4.34) were all significantly associated with the risk of sarcopenia. More importantly, the sarcopenia predictability of the GNRI appears greater than the MIS and Cr index, while MIS was similar to the Cr index. Similarly, the superiority of GNRI prediction was also found in pre-sarcopenia, but not in dynapenia.
Conclusion: All the three nutritional screening tools were significantly associated with an increased risk of sarcopenia. The sarcopenia predictability of the GNRI was greater than the MIS and Cr index.
Sarcopenia is a clinical condition characterized by an aged-related decrease in skeletal muscle mass and low muscle strength and/or physical performance (1). Muscle mass and muscle strength have been discussed together since 2010 when the European Working Group on Sarcopenia in Older People (EWGSOP) defined pre-sarcopenia and sarcopenia (2). Evidence is accumulating that both low muscle quantity and quality might relate to adverse clinical outcomes, including increased hospitalization, poorer quality of life, and increased mortality in patients with maintenance hemodialysis (MHD) (3–5). Interestingly, however, several recent studies suggest that the loss of muscle strength without low muscle mass as dynapenia, is an important risk for mortality in patients with MHD (6). Hence, there is a great interest in correctly differentiating the loss of muscle mass from strength. In fact, this deviation between the association of muscle mass with muscle strength and clinical adverse outcomes is a matter of interest and debate in the international scientific community (7, 8).
The patients with MHD are related to a range of causes of muscle mass and function, such as decreased physical activity and nutrition intake, hormone dysfunction, and chronic inflammation (9). Among these, malnutrition is a significant risk factor for the development of sarcopenia (10). A wide variety of nutritional screening markers and tools are available to assess the nutritional status of patients with MHD (11), such as body mass index (BMI), serum creatinine (Cr), and serum albumin. These markers are insufficient when used alone, so many clinicians usually use them in combination in clinical practice. The malnutrition–inflammation score (MIS) (12) is a valid diagnostic tool for evaluating nutritional status in patients with MHD. However, the MIS is time-consuming and cumbersome, because it requires a subjective evaluation. Indeed, several simple and completely objective nutritional screening tools can also be used to evaluate the nutritional risk of patients with MHD. The Cr index and geriatric nutritional risk index (GNRI) are recommended as the simple risk indexes for nutritional status assessment among patients with MHD (13). These two markers are objective and do not need special skills or experience but instead can easily calculated from the results of routine blood tests obtained at the bedside. Previous reports have reported the association between the nutritional marks and sarcopenia among the hospitalized older adults (14) and kidney transplant recipients (15). The conclusions are inconsistent as different nutritional indicators were used. To date, there are no research on the association between dynapenia and nutritional status in the dialysis population, so further study is needed.
In addition, few studies have compared the usefulness and predictive ability of these three nutritional indexes (MIS, GNRI, and Cr index) regarding dynapenia, pre-sarcopenia and sarcopenia in this population. It is clinically important to directly compare these three indexes in the hemodialysis population comprising a relatively large number of patients with MHD. Thus, the aim of this study was to evaluate the ability of the three nutritional screening tools (MIS, GNRI, and Cr index) to identify dynapenia, pre-sarcopenia, and sarcopenia in patients with MHD.
A multi-center cross-sectional study was conducted in Shanghai between July 2020 and April 2021and included seven hemodialysis centers. The inclusion were (1) age ≥ 18 years, (2) receiving maintenance hemodialysis for at least 3 months, (3) able to provide informed consent, while the exclusion criteria included (1) missing data on diagnostic criteria for sarcopenia, (2) did not complete date on the nutrition assessments, (3) clinical instability (presented with an infection, pulmonary edema, amputated limb, or malignancy), and (4) unable to communicate with the researchers or refusal to participate in this study. We excluded patients who: (1) refused to undergo body composition examinations (n = 11); (2) unable to complete the handgrip strength test because of hand disability (n = 2); (3) unable to walk due to disability or complete wheelchair dependence and unable to perform gait speed tests (n = 6); and 4) absence of results of relevant nutritional blood tests (n = 12). The remaining 849 patients were included in the final analytic sample (Figure 1). The Ethics Committee of Shanghai University of Medicine and Health Sciences approved this study (number 2019-A4-2621-19-201001-03-12010419771113601X), and all of the patients provided written informed consent to take part in this study. These methods were implemented in accordance with the principles of the Declaration of Helsinki.
All the patients were invited to a face-to-face interview to answer a standardized questionnaire. Covariates included socio-demographic characteristics and chronic disease status. Demographic characteristics comprised of age, sex, post-dialysis weight, dialysis vintage, and education level. The International Physical Activity Questionnaire (IPAQ) (16) was used to evaluate the physical activity. Comorbidity was assessed using the Charlson Comorbidity Index (CCI), which accounted for multiple comorbidity by creating a summation score based on 19 comorbidity conditions (17). Moreover, all the blood samples were taken prior to dialysis. The dialysis quality was assessed by fractional clearance index for urea (Kt/V), which was monitored in every dialysis treatment using the OCM® (On-line Clearance Monitor; Fresenius Medical Care, Bad Homburg, Germany).
A direct segmental multi-frequency bioelectrical impedance analysis (BIA; InBody S10; Biospace, Seoul, Korea) was used to measure muscle mass (18). Low muscle mass was defined as skeletal muscle index (SMI) lower than 7.0 and 5.7 kg/m2 in man and women, respectively. The dynamometer (GRIP-D; Takei Ltd, Niigata, Japan) on the non-fistula hand before a dialysis session to evaluate the muscle strength.
For patients with an indwelling dialysis catheter, we used the dominant hand to test muscle strength. Low muscle strength was defined as handgrip strength < 28 or < 18 kg in males and females. Usual gait speed was measured to assess poor-physical performance, which was less than 1.0 m/s in both males and females. All the patients were measured for these tests using the same measurement by a physical therapist at each facility on dialysis day before dialysis treatment.
Dynapenia was defined as patients with decreased muscle strength and normal muscle mass (8). A low SMI with normal handgrip strength and normal gait speed were to defined pre-sarcopenia (2). Sarcopenia was diagnosed based on 2019 new version of the Asian Working Group for Sarcopenia (AWGS) criteria. These criteria included both low muscle mass and low muscle strength and/or poor-physical performance (1).
The Malnutrition–Inflammation Score (MIS) is a comprehensive scoring system that is associated with expected hospitalization rates, mortality rates, and nutritional measures of patients with MHD (12). The 10 items about the patient's with MHD nutritional and functional state were included in the MIS. Each component of the MIS has four severity levels, ranging from 0 (normal) to 3 (severely abnormal). The total score ranges from 0 to 30. The higher score suggests a more severe degree of malnutrition status.
In the several previous studies, Geriatric Nutritional Risk Index (GNRI) has been shown to be an important predictor of morbidity and mortality and associated with a variety of nutrition-related markers (19, 20). The GNRI was calculated using the following equation (21):
Ideal body weight was defined as having a body mass index (BMI) value of 22 kg/m2.
Creatinine (Cr) index was calculated based on known sex differences as follows (13, 22):
Independent t-test (numeric variables) or by chi-square test (categorical variables) were used to compared patients with and without sarcopenia. Numeric variables with a normal distribution are expressed as the mean ± SD, while with a non-normal distribution are expressed as the median, with the 25–75% interquartile ranges given in parentheses. Categorical variables used an absolute number and proportions. The receiver operating characteristic curve (ROC) was a graph of sensitivity plotted against (1–specificity) overall possible diagnostic cut points. The cutoff points of dynapenia, pre-sarcopenia and sarcopenia were determined by the maximal Youden's index, which was calculated as (sensitivity + specificity −1) and the greatest combination of sensitivity and specificity. Logistic regression analyses were used to assess the relationships between three nutritional screening tools (MIS, GNRI, and Cr index) and dynapenia, pre-sarcopenia, or sarcopenia. Adjusted model included age, sex, Kt/V, IPAQ, CCI, Cr, PTH, and phosphorus. We further used Harrell's C-statistics to confirm which nutritional markers were best for identifying dynapenia, pre-sarcopenia, and sarcopenia. P < 0.05 was considered statistically significant. IBM SPSS Statistics v26.0 (SPSS Inc., Chicago, IL, United States) was used to perform all the statistical analyses.
Among 849 participants (520 men, 329 women; mean age 61.4 ±12.6 years), and there were 235 (27.7%) patients with dynapenia, followed by 193 (22.7%) patients with sarcopenia, and 53 (6.2%) with pre-sarcopenia, respectively. Table 1 shows the socioeconomic and health-related characteristics of patients with MHD stratified by sarcopenic status. Age, post-dialysis weight, BMI, Kt/v, handgrip strength, SMI, gait speed, IPAQ, nutritional factors (MIS, GNRI, and Cr index), CCI, and laboratory parameters (albumin, Cr, PTH, and phosphorus) significantly differed between groups (P < 0.05, Table 1).
The ROC curves were created to quantify sensitivity, specificity, areas under the ROC curves (AUC), and optimal cutoff points of three nutritional screening tools. Table 2 shows that the AUC of GNRI toward sarcopenia and pre-sarcopenia were lager than Cr index and MIS. The AUC of MIS, GNRI, and Cr index toward sarcopenia were 0.640, 0.722, and 0.700, respectively (Figure 2), while pre-sarcopenia was 0.517, 0.723, and 0.558, respectively. Meanwhile, the cutoff points of GNRI toward pre-sarcopenia and sarcopenia were 103 and 102.4, respectively.
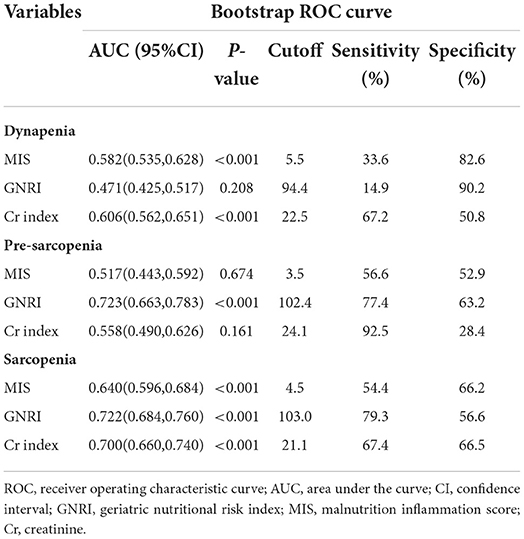
Table 2. Receiver operating characteristic curve for the nutritional factors to estimate the probability of dynapenia, pre-sarcopenia, and sarcopenia.

Figure 2. Receiver operating characteristic (ROC) curve for geriatric nutritional risk index (GNRI), malnutrition inflammation score (MIS), and Creatinine (Cr) index according to sarcopenia.
The relationships between the three nutritional screening tools and dynapenia, pre-sarcopenia, and sarcopenia are shown in Table 3. After adjustment for age, sex, Kt/V, IPAQ, CCI, Cr, PTH, and phosphorus, a significantly higher risk of sarcopenia was found in patients in poor-nutrition categories identified by GNRI [odds ratio (OR) = 6.28, 95%CI: 4.05, 9.73) than Cr index (OR = 2.73, 95%CI: 1.71, 4.34) and MIS (OR = 1.91, 95%CI: 1.31, 2.78). The OR for pre-sarcopenia was higher in Cr index in pre-sarcopenia (OR = 5.49, 95% CI: 1.88, 16.02) than GNRI (OR = 5.29, 95% CI: 2.69, 10.39). However, the OR for dynapenia was slightly higher in MIS (OR = 2.12, 95% CI: 1.42, 3.17) than GNRI (OR = 1.71, 95% CI: 1.02, 2.86), and Cr index (OR = 1.73, 95% CI: 1.09, 2.73).
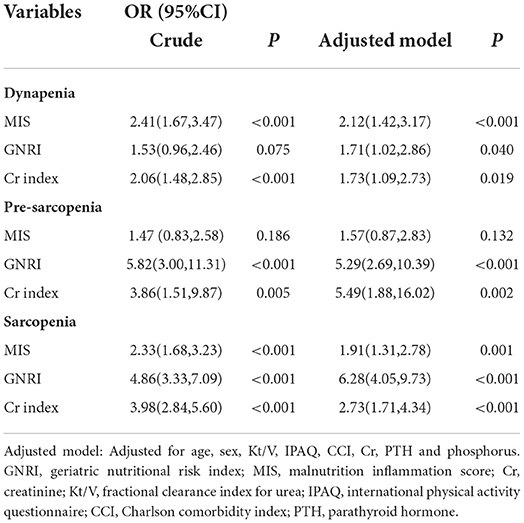
Table 3. Multivariable logistic regression analysis evaluating nutritional factors associated with dynapenia, pre-sarcopenia, and sarcopenia.
Harrell's C statistic were used to evaluate the discrimination of each nutritional screening tool. The Harrell's C statistics of the GNRI and Cr index were significantly higher than the MIS (GNRI vs. MIS: P = 0.001; GNRI vs. Cr index: P = 0.001), and there was no significant difference between the MIS and the Cr index in the values of the Harrell's C statistic for sarcopenia (P = 0.436, Table 4). Pre-sarcopenia also showed the similar results; however, there were no significant difference among the three nutritional screening tools in the values of the Harrell's C statistic for dynapenia (Table 4).
Our study suggested that each of the three nutritional screening tools was significantly associated with an increased risk of dynapenia, pre-sarcopenia, and sarcopenia in Chinese patients with MHD. Moreover, the sarcopenia predictability of the GNRI appears greater than Cr index and MIS, while Cr index similar to the MIS. Thus, GNRI is considered a useful tool for predicting sarcopenia in this group.
In our study, the prevalence of sarcopenia was 22.7%, which is consistent with the previously reported sarcopenia prevalence in dialysis patients (20.0%) (6), as well as the results of a recent systematic analysis of 30 studies in dialysis patients (sarcopenia prevalence = 28.5%) (23). Based on the cut-off values of muscle mass and function recommended by existing guidelines for the diagnosis of sarcopenia in the general population, the prevalence of sarcopenia in hemodialysis patients ranges from 4 to 68% (6, 23–25). This wide range is partly representative of the lack of recognized definition for diagnosing low muscle mass and low muscle strength (24). On the other hand, sarcopenia, as defined in the healthy population, may not apply to dialysis population. Therefore, more research is needed in the future to determine the appropriate diagnostic cut-point values for sarcopenia in the dialysis population.
Consistently, previous studies found that malnutrition was significantly associated with sarcopenia in patients with MHD (26). With regard to the indicators used for nutritional assessment, studies have shown that the GNRI (27) and MIS (10) were associated with sarcopenia in patients with MHD. However, the relationship between the Cr index and sarcopenia remains scarce. Furthermore, the GNRI has the best discrimination to predict sarcopenia, while the Cr index similar to the MIS. However, Beberashvili et al. (28) reported that MIS is more comprehensive than GNRI in monitoring of nutritional status in patients with MHD. In addition, another study found that lower GNRI and Cr index values were both independently and equally associated with an increased risk of all-cause mortality in a multivariable-adjusted model (13). Differences of sample size, dialysis vintage, or population characteristics could explain this inconsistent finding. Our findings and hypotheses warrant further confirmation.
In our study, the cut-off value for estimating GNRI for sarcopenia was 103. In a previous study of Japanese dialysis patients, the cut-off value for GNRI was 90 when the outcome was mortality (19). The cut-off value in our study for sarcopenia was slightly higher, possibly due to the lower prevalence of malnutrition among patients in this population. The reason may be that universal health insurance system in Shanghai of China allows all the dialysis patients to treat complications early, and it should be recognized that many patients may be not malnourished due to their relative financial wealth. In addition, several research on the effectiveness of the GNRI in patients with MHD have shown that the GNRI is more sensitive and specific than other assessment tools in predicting mortality (29). Nutritional screening tool should be simple, fast, and reproducible (intra-rater and interrater reliability) and sufficiently discriminating. MIS is time-consuming and cumbersome because it requires a subjective evaluation. Moreover, GNRI requires only two parameters while the Cr index needs four parameters, making GNRI easier. The simpler of the two would be more practical and useful in the clinical setting, which is consistent with our results. Hence, GNRI may be used as a screening indicator based on medical record information in place of complex diagnostic criteria described in the consensus for estimating sarcopenia in patients with MHD.
This study has important implications for clinical practice and future research. First, it highlights that screening poor nutrition status is usually highly predictive of sarcopenia in patients with MHD. Second, it is strongly recommended to maintain sufficient calorie and protein intake especially physical therapy to avoid or reverse sarcopenia (26). However, efforts to increase food intake in patients with MHD with sarcopenia often are unsuccessful because of the persistent anorexia arising from sarcopenia-related inflammatory status (30). Finally, physical therapy may increase appetite in the short term by improving the patient's mood and increase metabolic rate in the long term by activating muscle metabolism.
Some limitations should be considered in our study. First, it is not possible to elucidate clear causal associations between three nutritional screening tools and sarcopenia in the patients with MHD because this study is a cross-sectional design. Second, the prevalence of malnutrition in this population is relatively low and few patients had severe malnutrition. Future studies with longitudinal designs are required to confirm the causal association between different nutritional screening tools and sarcopenia.
In conclusion, MIS, GNRI, and Cr indexes were almost equally and significantly associated with an increased risk of dynapenia, pre-sarcopenia, and sarcopenia. The sarcopenia predictability of the GNRI was greater than the Cr index and MIS, while the Cr index similar to MIS. Our findings provide further evidence for the selection of the nutritional screening tool to predict sarcopenia in patients with MHD. If further confirmed, GNRI as a simple tool could be used to identify patients with MHD at high risk of sarcopenia. Multiple interventions targeting these high-risk patients and implementing early intensification can help reduce their risk of sarcopenia and other clinical complications in the future.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai University of Medicine and Health Sciences. The patients/participants provided their written informed consent to participate in this study.
XC, PH, and QG: study concept and design. PS, YZ, HZ, CY, JN, WD, and JZ: acquisition, analysis, and interpretation of data. XC and PH: drafting of the work. XZ, LZ, HQ, SZ, and QG: critical revision of the manuscript. All the authors contributed to the article and approved the submitted version.
This work was supported by the funding of the National Natural Science Foundation of China (82172552), Shanghai Sailing Program (22YF1417900), the Clinical Trial Incubation Program From Tongji Hospital of Tongji University [ITJ(ZD)1808], and the Minhang District Medical Characteristic Specialty Construction Project (2020MWTZA01).
We are grateful to all the medical workers at the multi-center dialysis for their generous technical assistance and guidance. We also thank all the study participants for their participation and cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7. doi: 10.1016/j.jamda.2019.12.012
2. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
3. Kim JK, Kim SG, Oh JE, Lee YK, Noh JW, Kim HJ, et al. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis. Korean J Intern Med. (2019) 34:599–607. doi: 10.3904/kjim.2017.083
4. Macedo C, Amaral TF, Rodrigues J, Santin F, Avesani CM. Malnutrition and sarcopenia combined increases the risk for mortality in older adults on hemodialysis. Front Nutr. (2021) 8:721941. doi: 10.3389/fnut.2021.721941
5. Mori K, Nishide K, Okuno S, Shoji T, Emoto M, Tsuda A, et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. (2019) 20:105. doi: 10.1186/s12882-019-1271-8
6. Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. (2014) 9:1720–8. doi: 10.2215/CJN.10261013
7. Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM, et al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. (2016) 90:53–66. doi: 10.1016/j.kint.2016.02.025
8. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. (2012) 67:28–40. doi: 10.1093/gerona/glr010
9. Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. (2013) 24:337–51. doi: 10.1681/ASN.2012010047
10. Vettoretti S, Caldiroli L, Armelloni S, Ferrari C, Cesari M, Messa P, et al. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced CKD. Nutrients. (2019) 11:1378. doi: 10.3390/nu11061378
11. Roij DE, Van Zuijdewijn CL, Ter Wee PM, Chapdelaine I, Bots ML, Blankestijn PJ, et al. A Comparison of 8 nutrition-related tests to predict mortality in hemodialysis patients. J Ren Nutr. (2015) 25:412–9. doi: 10.1053/j.jrn.2015.02.005
12. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MHA. malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. (2001) 38:1251–63. doi: 10.1053/ajkd.2001.29222
13. Yamada S, Yamamoto S, Fukuma S, Nakano T, Tsuruya K, Inaba M, et al. Geriatric nutritional risk index (GNRI) and creatinine index equally predict the risk of mortality in hemodialysis patients: J-DOPPS. Sci Rep. (2020) 10:5756. doi: 10.1038/s41598-020-62720-6
14. Rasheedy D, El-Kawaly WH. The accuracy of the geriatric nutritional risk index in detecting frailty and sarcopenia in hospitalized older adults. Aging Clin Exp Res. (2020) 32:2469–77. doi: 10.1007/s40520-020-01492-5
15. Kosoku A, Uchida J, Nishide S, Kabei K, Shimada H, Iwai T, et al. Association of sarcopenia with phase angle and body mass index in kidney transplant recipients. Sci Rep. (2020) 10:266. doi: 10.1038/s41598-019-57195-z
16. Bassett DR. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1396. doi: 10.1249/01.MSS.0000078923.96621.1D
17. Liu H, Song B, Jin J, Liu Y, Wen X, Cheng S, et al. Length of stay, hospital costs and mortality associated with comorbidity according to the charlson comorbidity index in immobile patients after ischemic stroke in China: a national study. Int J Health Policy Manag. (2022) 11:1780–7. doi: 10.34172/ijhpm.2021.79
18. Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, et al. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol. (2015) 10:1192–200. doi: 10.2215/CJN.08550814
19. Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, et al. Geriatric nutritional risk index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant. (2010) 25:3361–5. doi: 10.1093/ndt/gfq211
20. Takahashi H, Inoue K, Shimizu K, Hiraga K, Takahashi E, Otaki K, et al. Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J Ren Nutr. (2017) 27:201–6. doi: 10.1053/j.jrn.2016.12.005
21. Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. (2008) 87:106–13. doi: 10.1093/ajcn/87.1.106
22. Arase H, Yamada S, Yotsueda R, Taniguchi M, Yoshida H, Tokumoto M, et al. Modified creatinine index and risk for cardiovascular events and all-cause mortality in patients undergoing hemodialysis: the Q-Cohort study. Atherosclerosis. (2018) 275:115–23. doi: 10.1016/j.atherosclerosis.2018.06.001
23. Shu X, Lin T, Wang H, Zhao Y, Jiang T, Peng X, et al. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:145–58. doi: 10.1002/jcsm.12890
24. Lamarca F, Carrero JJ, Rodrigues JC, Bigogno FG, Fetter RL, Avesani CM, et al. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging. (2014) 18:710–7. doi: 10.1007/s12603-014-0505-5
25. Yoowannakul S, Tangvoraphonkchai K, Vongsanim S, Mohamed A, Davenport A. Differences in the prevalence of sarcopenia in haemodialysis patients: the effects of gender and ethnicity. J Hum Nutr Diet. (2018) 31:689–96. doi: 10.1111/jhn.12555
26. Inaba M, Okuno S, Ohno Y. Importance of considering malnutrition and sarcopenia in order to improve the QOL of elderly hemodialysis patients in Japan in the Era of 100-year life. Nutrients. (2021) 13:2377. doi: 10.3390/nu13072377
27. Lee H, Kim K, Ahn J, Lee DR, Lee JH, Hwang SD, et al. Association of nutritional status with osteoporosis, sarcopenia, and cognitive impairment in patients on hemodialysis. Asia Pac J Clin Nutr. (2020) 29:712–23. doi: 10.6133/apjcn.202012_29(4).0006
28. Beberashvili I, Azar A, Sinuani I, Kadoshi H, Shapiro G, Feldman L, et al. Comparison analysis of nutritional scores for serial monitoring of nutritional status in hemodialysis patients. Clin J Am Soc Nephrol. (2013) 8:443–51. doi: 10.2215/CJN.04980512
29. Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Sandbank J, et al. Geriatric nutritional risk index, muscle function, quality of life and clinical outcome in hemodialysis patients. Clin Nutr. (2016) 35:1522–9. doi: 10.1016/j.clnu.2016.04.010
Keywords: dynapenia, geriatric nutritional risk index, hemodialysis, nutritional screening tool, pre-sarcopenia, sarcopenia
Citation: Chen X, Han P, Zhu X, Song P, Zhao Y, Zhang H, Yu C, Niu J, Ding W, Zhao J, Zhang L, Qi H, Zhang S and Guo Q (2022) Comparison of three nutritional screening tools for detecting sarcopenia in patients with maintenance hemodialysis. Front. Public Health 10:996447. doi: 10.3389/fpubh.2022.996447
Received: 17 July 2022; Accepted: 26 September 2022;
Published: 24 October 2022.
Edited by:
Falak Zeb, University of Sharjah, United Arab EmiratesReviewed by:
Iftikhar Alam, Bacha Khan University, PakistanCopyright © 2022 Chen, Han, Zhu, Song, Zhao, Zhang, Yu, Niu, Ding, Zhao, Zhang, Qi, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Guo, Z3VvcWlqcEBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.